INTRODUCTION
Diabetes is a significant contributor to morbidity and mortality through its associated micro- and macrovascular complications (Dabelea et al., 2017; Graves and Donaghue, 2020). Type 1 diabetes, an autoimmune disease recognized by its early onset in childhood or adolescence, has become more prevalent in recent years (Dabelea, 2018; Hamman et al., 2014). Although diabetic patients currently have increased awareness of the importance of strict glycemic control, chronic vascular complications are still an ongoing burden for type 1 diabetic youths (Dabelea et al., 2017; Libby et al., 2005).
Streptozotocin (STZ) is an antibiotic that causes selective destruction of pancreatic islet β-cells and is widely used to induce type 1 diabetes in experimental animals (Furman, 2021). The STZ-diabetic model mimics the main features of human diabetes and its associated vascular complications (Kara et al., 2022; Lim et al., 2022; Wei et al., 2003).
A plethora of mechanisms may underly diabetic vascular complications. These include, but are not limited to, sustained hyperglycemia, oxidative vascular damage, glycation end products (AGEs) accumulation, diminished bioavailability of vascular nitric oxide (NO), and increased systemic and vascular tissue inflammation (Ceriello, 2006; Devaraj et al., 2007; Graves and Donaghue, 2020; Satoh et al., 2005; Wu et al., 2006).
Patients with type 1 diabetes are extremely vulnerable to microvascular issues such as retinopathy and nephropathy (Libby et al., 2005). Nephropathy can result in end-stage renal disease (ESRD) (Kuhad and Chopra, 2009) and is characterized by elevation of protein and albumin in urine, renal hypertrophy, glomerulosclerosis, decrease in the GFR, and eventually tubulointerstitial fibrosis (Mogensen, 1995; Soldatos and Cooper, 2008).
One of the key causes in the onset and progression of nephropathy, which in turn sets off a number of inflammatory variables that cause renal fibrogenesis, is the activation of the renal renin-angiotensin system (Aggarwal et al., 2017; Giacchetti et al., 2005). Currently, the major goals of employing ACE inhibitors and angiotensin receptor blockers (ARBs) in diabetes are to delay the onset of diabetic nephropathy (Aggarwal et al., 2017; Giacchetti et al., 2005; Ruggenenti et al., 2010).
However, these drugs are not able to provide stable and full renoprotection for diabetic patients (Benigni et al., 2003; Bilous et al., 2009; Mauer et al., 2009). Therefore, more effective alternative therapies for patients with diabetic nephropathy are warranted.
Losartan (LOS) is a potent, orally active, and highly specific ARB drug. It showed protective influences against nephropathy (Manni et al., 2012; Murali and Goyal, 2001; Volpini et al., 2003; Yao et al., 2018) and ameliorated endothelial dysfunction (Ateyya et al., 2018; Sleem et al., 2014) in diabetic rats. LOS also prevented the progression of early diabetic nephropathy, reduced the incidence of ESRD, and improved endothelial function in type 2 diabetic patients (Brenner et al., 2001; Cheetham et al., 2001; Weil et al., 2013).
Montelukast (MONT) is an FDA-approved antiasthmatic drug for children and adolescents. MONT is a leukotriene receptor antagonist that selectively blocks the cysteinyl leukotriene 1 (CysLT1) receptor (Nayak, 2004). Several studies showed that MONT may mitigate experimental tissue injury by diminishing oxidative stress and inflammation (El-Boghdady et al., 2017; Khodir et al., 2014; Saad et al., 2014; Said and Bosland, 2016). MONT protected renal tissues against ischemia/reperfusion injury by attenuating oxidative stress and reducing the generation of inflammatory mediators (Sener et al., 2006). Moreover, MONT diminished renal damage in rats with unilateral ureteral obstruction (Otunctemur et al., 2015) and lipopolysaccharide-challenged rats (Khodir et al., 2014) via its antioxidant and anti-inflammatory potential.
Interestingly, MONT prevented early diabetic retinopathy in mice by inhibiting proinflammatory leukotriene generation and superoxide accumulation (Bapputty et al., 2019). However, the renoprotective and vasculoprotective effects of MONT in diabetic rats were not investigated.
In this research, the beneficial effects of MONT on renal and aortic tissues of STZ-diabetic rats were compared to those brought about by the ARB drug Losartan.
MATERIALS AND METHODS
Drugs and chemicals
MONT was purchased from Sigma Chemicals (St. Louis, MO). LOS was obtained from Amriya Pharmaceutical Industries (Cairo, Egypt). Before giving either medication to rats, it was suspended in 0.5% carboxymethyl cellulose (CMC). STZ and all other chemicals were from Sigma Chemicals.
Animals
Male Sprague-Dawley rats (weighing 200–250 g) were placed in a temperature-controlled environment (23°C–2°C) with a 12-hour light–dark cycle. Animals had unrestricted access to food pellets and water. The Mansoura University Faculty of Pharmacy’s Research Ethics Committee approved experimental protocols that complied with national and international NIH standards for using animals in research.
Experimental protocol
To induce diabetes, rats were given a single intraperitoneal injection of STZ at a dose of 50 mg/kg in ice-cold saline (Amin et al., 2020). Rats with hyperglycemia levels more than 250 mg/dl 48 hours after STZ treatment were deemed diabetic and enrolled in the study. To lower mortality, insulin (4 IU/kg, subcutaneous, twice weekly) was administered to all diabetic rats. Insulin administration limits excessive hyperglycemia and diminishes extreme body weight loss (Alderson et al., 2004).
Diabetic rats were randomly distributed into three groups (n = 8 each), as follows: STZ, received 0.5% CMC (3 ml/ kg/day, orally); STZ-LOS, received LOS (25 mg/kg/day, orally); and STZ-MONT, received MONT (10 mg/kg/day, orally). Drug administration started 2 weeks after the induction of diabetes and continued till the end of the experiments (10 weeks). Doses of LOS and MONT were selected based on former rat studies (Abdel-Raheem and Khedr, 2014; Gad et al., 2017; Khodir et al., 2014; Manni et al., 2012; Sleem et al., 2014). Age-matched normal rats (n = 8) received the vehicle of drug administration (0.5% CMC, 3 ml/kg/day, orally) and served as the Control group. On day 70, whole blood, serum specimens, and 24-hour urine outputs were obtained from rats. Rats were also euthanized, and the kidney and aorta were taken out, cleaned with ice-cold saline, dried off, and weighed. They were then used for tissue homogenate preparation (1:10 w/v in 0.9% NaCl, pH 7.4) and histopathological examinations. Moreover, vascular contractile responsiveness of isolated aortic rings to PE was assessed. The experimental protocol is summarized in Figure 1.
Body weight change and kidney mass index
By deducting each rat’s ultimate body weight (at day 70) from its starting body weight (on day 1), the change in rat weight was computed. To determine kidney mass index, kidney weight was standardized to body weight.
Glycemia and renal function
Glycated hemoglobin A1c (HbA1c) levels were assessed in whole blood samples using a kit from Biosystems (Spain). Rat urine and/or serum were examined to find the levels of creatinine, urea, albumin, and total albumin using commercial kits from Biodiagnostic (Giza, Egypt). Creatinine and urea clearance, indices of GFR, were determined using serum and urine concentrations of creatinine and urea, respectively, as previously mentioned (Bazzano et al., 2015).
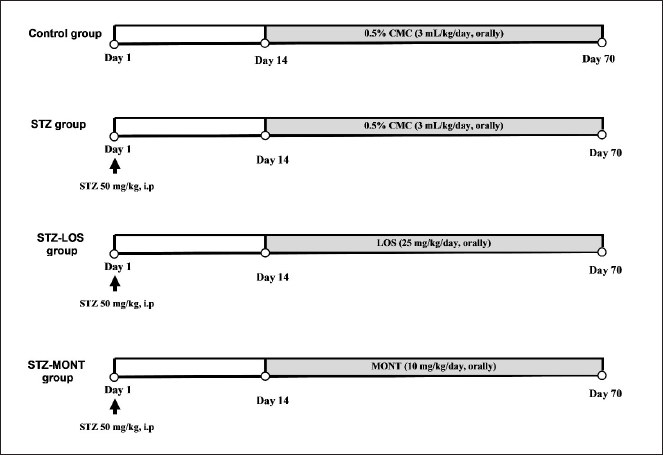 | Figure 1. An illustration of the experimental protocol. CMC, carboxymethyl cellulose; LOS, Losartan; MONT, montelukast; STZ, streptozotocin. [Click here to view] |
Oxidative stress parameters
Superoxide dismutase (SOD) activity levels in tissues were measured spectrophotometrically (Marklund, 1985). Moreover, renal and aortic tissue levels of malondialdehyde (MDA), measured as thiobarbituric acid reactive species (Ohkawa et al., 1979), and reduced glutathione (GSH), assessed as acid-soluble sulfhydryl compounds (Ellman, 1959), were quantified.
Total nitrite/nitrate (NOx)
Using a colorimetric kit from R&D Systems (catalog number KGE001, Minneapolis, USA), a commercially available product, tissue NOx concentrations were evaluated. The assay is based on using reductase enzyme to reduce the nitrate content to nitrite. Using the Griess reaction, the total nitrite was measured as a colored azo-dye product that absorbs light at 540 nm (Bories and Bories, 1995).
Tumor necrosis factor-α (TNF-α) and TGF-β1 levels
Following the manufacturer’s instructions, the levels of TGF-1 and TNF- in tissue homogenates were assessed using rat ELISA kits (catalog numbers BMS623-3 and KRC3011, respectively, from Thermo Fisher Scientific, MA, USA).
Aortic ring responsiveness to PE
Vascular reactivity of isolated aortic rings to PE [10−7–10−5 M] was assessed, as previously described (Shawky et al., 2019).
Histopathological analyses
Specimens of the kidney and aorta were embedded in paraffin, fixed in 10% buffered formalin, and cut into 4-μm slices. Periodic acid-Schiff (PAS) and/or Masson’s trichrome were then used to stain them. On PAS-stained renal tissues, renal glomerulosclerosis was semiquantitatively graded on a scale from 0 to 4. (Benigni et al., 2003). Furthermore, using ImageJ software, percentage areas of collagen deposition within the glomeruli and interstitium were evaluated on Masson’s trichrome-stained renal sections (National Institutes of Health, USA) (Shang et al., 2013). ImageJ software was also used to determine aortic media thickness.
Statistics
The data are shown as means ± SEM. One-way analysis of variance (ANOVA) followed by Tukey-Kramer post hoc tests was used to compare the group means. The histopathological scores were analyzed using Kruskal-Wallis followed by Dunn’s post hoc test. Cumulative concentration-response curves for aortic ring reactivity to PE were fitted using nonlinear regression analysis. GraphPad Prism was used to create all graphs and statistical analyses (San Diego, CA). Statistical significance was set at p < 0.05 (Amin et al., 2022; Meyerholz et al., 2019).
RESULTS
Body weight, kidney relative weight, and glycemic levels
STZ rats had significantly lower body weights (p ? 0.0001 vs. control rats). Both LOS and MONT prevented diabetes-induced body weight loss (p ? 0.0001 vs. STZ group). However, STZ-LOS and STZ-MONT rats still showed significantly lower weight gains than the control group (p ? 0.0001).
Moreover, STZ rats exhibited significantly higher kidney relative weights than the control group (by 108%, p < 0.0001). MONT, but not LOS, significantly diminished diabetic renal hypertrophy (by 23%, p < 0.01 vs. untreated STZ group).
Expectedly, there were significant increases in diabetic levels of serum glucose (by 362%, p ? 0.0001) and glycated HbA1c (by 43%, p ? 0.0001) when compared to those of control rats. LOS and MONT treatments significantly reduced serum glucose levels by 23.02% (p < 0.001) and 19.67% (p ? 0.01), respectively, relative to untreated STZ glycemic levels. However, MONT achieved better long-term glycemic control than LOS, as indicated by near-normal levels of HbA1c in STZ-MONT rats (p < 0.0001 vs. STZ and STZ-LOS groups).These results are shown in Table 1.
Renal function
STZ rats exhibited significantly lower levels of serum albumin (by 24.15%, p < 0.001) and significantly higher levels of serum creatinine (by 193%, p < 0.001), blood urea (by 133%, p < 0.0001), and urinary protein (by 206%, p < 0.0001) when compared to control levels. These findings indicate an impaired renal function in STZ-diabetic rats. In line with this, they also showed diminished clearance rates of creatinine (by 64.6%, p< 0.01) and urea (by 75.8%, p < 0.05) compared to those of control rats.
Both MONT and LOS comparably restored renal function parameters in STZ rats to near-normal levels. These findings are presented in Table 2.
Renal histological changes
To determine the amount of collagen deposition in the study groups’ renal tissues, Masson’s trichrome was used as a stain (Fig. 2A). When compared to control rats, the kidneys of STZ-diabetic animals exhibited interstitial fibrosis, segmental glomerulosclerosis, and vascular tuft atrophy. STZ-LOS kidneys exhibited partially resolved glomerular lesions with residual mild segmental glomerular mesangial expansion and no interstitial fibrosis. On the other hand, STZ-MONT rats demonstrated perfect renal histology with no evidence of glomerular lesions or interstitial fibrosis.
PAS-stained renal tissues are shown in Figure 2B. Focal segmental glomerulosclerosis and shrinkage of the vascular glomerular tuft were also observed in diabetic kidneys. STZ-LOS rats showed normal glomerular histology with mild shrinkage tuft and mild focal tubular atrophy. Renal specimens from the STZ-MONT group showed normal histology with no evidence of glomerular lesions or tubular atrophy.
Quantification of renal fibrosis areas in kidneys and renal glomerulosclerosis scores are presented in Figure 2C and D, respectively.
Renal oxidative status
Chronic STZ diabetes elicited a significant increase in renal MDA (by 59%, p < 0.001, Fig. 3A) and significant reductions in renal levels of GSH (by 53.27%, p < 0.0001, Fig. 3B), SOD activity (by 68.00%, p < 0.0001, Fig. 3C), and NOx (by 19.16%, p < 0.0001, Fig. 3D) relative to corresponding levels in the control group. Treatment with MONT and LOS attenuated diabetes-induced renal oxidative stress, suggesting the antioxidant properties of both drugs in diabetic rats (Fig. 3A–D).
Renal cytokine levels
Type 1 diabetic rats showed significantly higher concentrations of TNF-α (by 347%, p < 0.0001, Fig. 4A) and TGF-β1 (by 128%, p < 0.0001, Fig. 4B) than corresponding levels in the control group.
MONT treatment significantly reduced both renal TNF-α (by 38.5%, p < 0.05) and TGF-β1 (by 49.3%, p < 0.0001) relative to nontreated STZ levels. While LOS failed to lessen renal TNF-α (p > 0.05 vs. STZ group), it was able to diminish renal TGF-β1 concentrations (by 33.9%, p < 0.01) compared to the model STZ group.
Aortic reactivity to PE and NOx levels
STZ aortas exhibited significantly higher responses (Emax values) and enhanced sensitivity (pEC50 values) to PE-induced contractility in comparison to control aortic rings (Fig. 5A). In contrast to the STZ-LOS group, aortic rings from the STZ-MONT group showed significantly diminished Emax of contractile responsiveness to PE compared to those of untreated STZ aortas.
Diabetic aortas showed a significant reduction in NOx concentrations (by 53.1%, p < 0.01, Fig. 5B) compared to control aortic levels. MONT treatment, but not LOS, was able to restore altered aortic NOx in STZ rats to almost control levels.
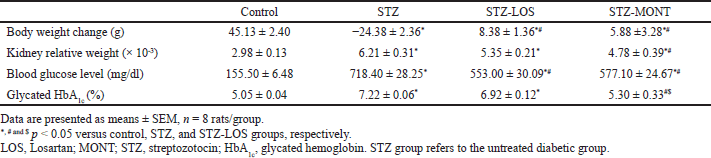 | Table 1. Effect of MONT treatment (10 mg/kg/day) on body weight, kidney relative weight, and glycemic levels in STZ-diabetic rats. [Click here to view] |
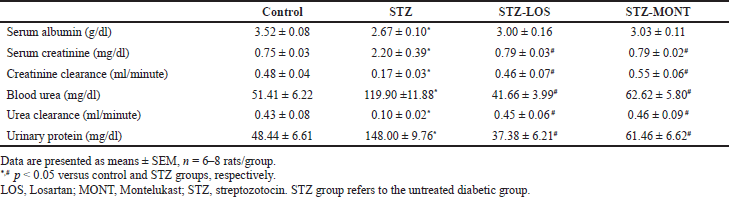 | Table 2. Effect of MONT treatment (10 mg/kg/day) on renal dysfunction in STZ-diabetic rats. [Click here to view] |
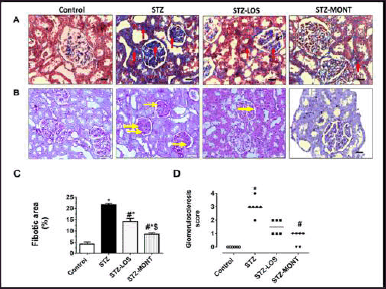 | Figure 2. Effect of MONT treatment (10 mg/kg/day) on renal histological alterations in STZ-diabetic rats. A. Masson’s trichrome-stained sections of the renal medulla (200× magnification; scale bar:50 µm). Red arrows denote collagen deposition in the glomeruli and interstitial spaces. B. PAS-stained sections of the renal cortex (200 × magnification; scale bar:50 µm). Yellow arrows show sclerotic lesions. C. Percentage fibrosis area. Data are presented as means ± SEM, n = 6 rats/group. D. Scatter plots of renal glomerulosclerosis scores in the study groups. *, # and $ p < 0.05 versus control, STZ, and STZ-LOS groups, respectively. LOS, Losartan; MONT, Montelukast; PAS, Periodic acid-Schiff; STZ, streptozotocin. STZ group refers to the untreated diabetic group. [Click here to view] |
Aortic oxidative stress
Both MONT and LOS treatments significantly reduced aortic MDA levels in STZ rats (by 72.9 % and 69.8%, respectively, p < 0.0001, Fig. 6A) relative to model STZ levels. Moreover, STZMONT and STZ-LOS aortas showed near-normal levels of SOD activity as compared to significantly diminished aortic SOD levels in STZ rats (Fig. 6C). However, MONT, but not LOS, was able to restore aortic GSH to almost control levels (p < 0.0001 vs. STZ group and p < 0.01 vs. STZ-LOS group, Fig. 6B).
Aortic medial thickness and TGF-β1 levels
Figure 7A demonstrates PAS-stained aortic sections from the study groups. In contrast to control aortas, STZ aortic walls exhibited structural alterations, including increased thickness of the medial layer (quantified in Fig. 7B) and disorganized appearance of elastic fibers. STZ-MONT rats significantly showed reduced thickness of the medial layer compared to model STZ and STZ-LOS groups (Fig. 7A and B). There were no significant differences in terms of aortic TGF-β1 levels in the experimental groups (Fig. 7C).
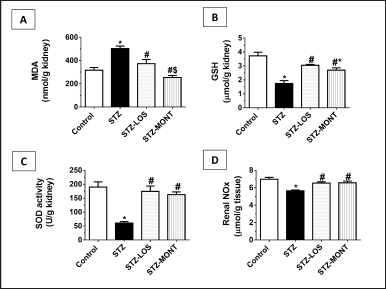 | Figure 3. Effect of MONT treatment (10 mg/kg/day) on renal oxidative stress in STZ-diabetic rats. Data are presented as means ± SEM, n = 6–8 rats/group. *, # and $ p < 0.05 versus control, STZ, and STZ-LOS groups, respectively. LOS, Losartan; MONT, Montelukast; STZ, streptozotocin; MDA, malondialdehyde; GSH, reduced glutathione; SOD, superoxide dismutase; NOx, total nitrate/nitrite. STZ group refers to the untreated diabetic group. [Click here to view] |
A. Representative microimages of aortic tissues from the study groups (PAS-stain, 400× magnification). Yellow double-sided arrows denote the thickness of the aortic medial layer. B. Aortic media thickness. C. Aortic TGF-β1 levels. Data are presented as means ± SEM, n = 6 rats/group. *, # and $ p < 0.05 versus control, STZ, and STZ-LOS groups, respectively. LOS, Losartan; MONT, Montelukast; STZ, streptozotocin; TGF-β1, transforming growth factor-β1; PAS, periodic acid-Schiff. STZ group is the diabetic group without treatment.
DISCUSSION
This work showed that chronic administration of the CysLT1 receptor antagonist MONT to STZ-diabetic rats elicited comparable reno- and vasculoprotective influences to the ARB blocker LOS. Primarily, MONT ameliorated diabetic hyperglycemia and renal functions, diminished renal interstitial fibrosis and glomerulosclerosis, reduced aortic medial thickness, and attenuated aortic hypercontractility to PE in diabetic animals. These effects are possibly mediated via diminishing renal and aortic tissue oxidative stress and inflammation.
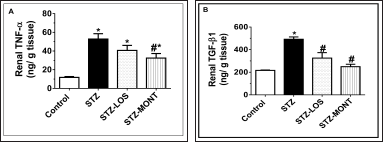 | Figure 4. Effect of MONT treatment (10 mg/kg/day) on renal levels of TNF-α (A) and TGF-β1 (B) in STZ-diabetic rats. Data are presented as means ± SEM, n = 6 rats/group. * and # p < 0.05 versus control and STZ groups, respectively. LOS, Losartan; MONT, Montelukast; STZ, streptozotocin; TNF-α, tumor necrosis factor-α; TGF-β1, transforming growth factor-β1. STZ group refers to the untreated diabetic group. [Click here to view] |
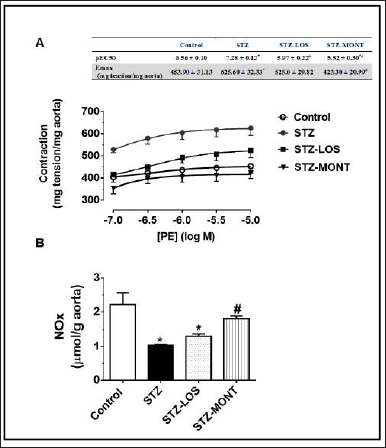 | Figure 5. Effect of MONT treatment (10 mg/kg/day) on aortic contractility to PE (A) and NOx levels (B) in STZ-diabetic rats. Data are presented as means ± SEM, n = 6 rats/group. * and # p < 0.05 versus control and STZ groups, respectively. pEC50, -log EC50 (the required effective concentration to achieve 50% of the maximal contractile response); Emax, the maximum response; PE, phenylephrine; LOS, Losartan; MONT, Montelukast; STZ, streptozotocin; NOx, total nitrate/nitrite. STZ group is the diabetic group without treatment. [Click here to view] |
In this research, a rat STZ-diabetic model was used to investigate the effect of MONT on vascular complications. This model is robustly used to investigate the pathogenesis of diabetic vascular complications (Amin et al., 2022; Kara et al., 2022; Lim et al., 2022; Sleem et al., 2014), as it simulates many aspects of human diabetes (Wei et al., 2003). The duration of experimental protocol was selected based on several studies which reported that diabetic rats developed nephropathy and renal fibrosis within 8 weeks following STZ administration (Jia et al., 2019; Mestry et al., 2017). LOS was used as a standard renoprotective agent in this research. LOS showed protective influences against nephropathy (Manni et al., 2012; Murali and Goyal, 2001; Volpini et al., 2003; Yao et al., 2018) and ameliorated endothelial dysfunction (Ateyya et al., 2018; Sleem et al., 2014) in diabetic rats. ARB drugs have been shown to offer significant renal benefits in ameliorating nephropathy and slowing the progression of renal failure in diabetic patients (Wang et al., 2018). LOS also reduced the incidence of ESRD in type 2 diabetic patients (Brenner et al., 2001). MONT and LOS treatments were started 2 weeks following the induction of diabetes to give diabetic kidneys sufficient time to recuperate from the modest nephrotoxic effects of STZ, as previously observed (Kraynak et al., 1995).
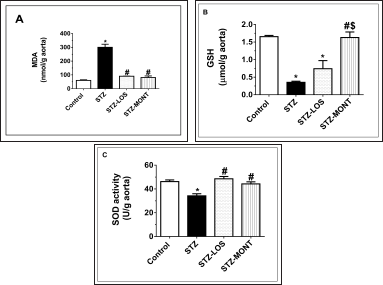 | Figure 6. Effect of MONT treatment (10 mg/kg/day) on aortic oxidative stress in STZ-diabetic rats. Data are presented as means ± SEM, n = 6 rats/group. *, # and $ p < 0.05 versus control, STZ, and STZ-LOS groups, respectively. LOS, Losartan; MONT, Montelukast; STZ, streptozotocin; MDA,malondialdehyde; GSH, reduced glutathione; SOD, superoxide dismutase. STZ group is the diabetic group without treatment. [Click here to view] |
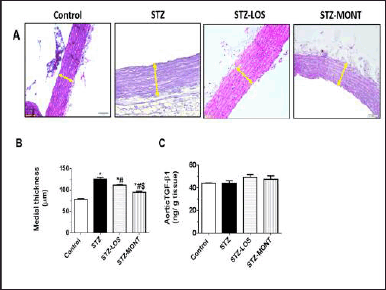 | Figure 7. Effect of MONT treatment (10 mg/kg/day) on the aortic medial thickness and TGF-β1 levels in STZ-diabetic rats. [Click here to view] |
Both LOS and MONT reduced random blood glucose levels in diabetic animals. However, only MONT treatment was able to significantly reduce glycated HbA1c values, an index of chronic hyperglycemia, compared to levels in untreated STZ rats. This may explain, at least in part, why MONT could significantly lessen diabetic relative kidney weight as high blood glucose levels in diabetic rats were associated with increased kidney weight (Rasch and Dorup, 1997). Supporting these findings, LOS administration reduced diabetic hyperglycemia by only ~17% and failed to alter hypoinsulinemia in STZ-diabetic rats (Murali and Goyal, 2001). MONT diminished fasting blood glucose and normalized insulin levels in rats with metabolic syndrome (Ibrahim et al., 2014). A 6-week MONT therapy in type 2 diabetic patients (n = 6) elicited reductions, albeit insignificant, in HbA1c compared with baseline levels (Faul et al., 2009). It was reported that MONT promoted glucose-stimulated insulin secretion (GSIS) in a dose-dependent manner in pancreatic MIN6 β-cells (Guo et al., 2018), an effect that might explain the lowering effect of MONT on fasting glycemia and HbA1c levels in the present work. Impaired GSIS in β-cells have been associated with STZ-induced diabetes (Delaney et al., 1995).
Renal injury was evident in STZ rats, which showed increased kidney mass index, proteinuria, elevated blood urea, and diminished serum albumin levels compared to control animals. Increased proteinuria is a marker of glomerular damage, which indicates GFR decline (Marques et al., 2022). Reduced urea and creatinine clearances in the STZ group supported altered GFR in diabetic animals (Matboli et al., 2017; Zhang et al., 2017). Both MONT and LOS improved assessed parameters of renal function to comparable levels. In line with these findings, MONT showed renoprotective effects in models of acute kidney injury (Abdel-Raheem and Khedr, 2014; Khodir et al., 2014; Sener et al., 2006).
Oxidative stress is a central contributor to the pathogenesis of diabetic nephropathy. Renal tissues in STZ rats exhibited significantly elevated levels of the lipid peroxidation marker MDA and diminished contents of the antioxidant components, reduced glutathione, and SOD activity. These findings agree with other studies (Qi et al., 2020; Tang et al., 2020). Sustained diabetic hyperglycemia was shown to enhance ROS generation and attenuate antioxidant mechanisms via the glycation of scavenging enzymes (Ha and Kim, 1999). Both MONT and LOS treatments mitigated renal oxidative stress in STZ rats. Previously, MONT enhanced the antioxidant capacity of renal tissues in rats with experimental sepsis (Coskun et al., 2011; Khodir et al., 2014). Moreover, MONT diminished the nephrotoxic effects of methotrexate in rats via the mitigation of renal oxidative stress (Abdel-Raheem and Khedr, 2014). Furthermore, MONT lessened MDA and boosted reduced glutathione levels in renal tissues of rats with renal ischemia/reperfusion injury (Sener et al., 2006). MONT attenuated kidney damage in rats with unilateral ureteral obstruction via its antioxidant effects (Otunctemur et al., 2015). MONT also reduced interleukin Il-1β-induced oxidative stress in chondrocytes (Li et al., 2021).
NOx levels were significantly lower in diabetic renal tissues compared to control tissues, which may be attributed to glomerular eNOS uncoupling (Alaofi, 2020; Satoh et al., 2005). NO regulates several vital processes in the kidney, including glomerular and medullary hemodynamics, renal blood flow, GFR, and mesangial matrix accumulation. NO is also involved in the tubuloglomerular feedback response and regulation of the extracellular fluid volume (Kone, 1997). Both LOS and MONT returned renal NOx levels to near the normal range.
Diabetes-associated oxidative damage results in the enhancement of the formation of inflammatory mediators and proinflammatory cytokines (Alaofi, 2020). Renal tissue contents of TNF-α and TGF-β1 were significantly elevated in STZ animals compared with those in control rats. Similar findings were revealed in other studies (Alaofi, 2020; Ko et al., 2008). Inflammatory markers were significantly elevated in type 1 diabetic patients who harbored macrovascular complications (Schram et al., 2003).
TNF-α is cytotoxic to mesangial cells, which directly causes kidney failure (Bertani et al., 1989; Ortiz et al., 1995). TNF-α also boosts the production of ROS which disrupts the glomerular protein permeability barrier (McCarthy et al., 1998). Numerous studies demonstrated that MONT decreased renal TNF-α (Abdel-Raheem and Khedr, 2014; Khodir et al., 2014) and plasma proinflammatory cytokine levels (Sener et al., 2006). TGF-β1, a profibrotic cytokine, stimulates glomerular hypertrophy, proteinuria, and extracellular matrix buildup. Consequently, it is crucial to the emergence and development of diabetic nephropathy (Zhao et al., 2020). MONT was more effective than LOS in lowering increased kidney levels of TNF-α and TGF-β1 in diabetic rats.
Aortas from STZ rats exhibited enhanced responsiveness to PE-induced contractility. Vascular irregularities and endothelial dysfunction are greatly attributed to enhanced oxidative stress and reduced NO bioavailability in diabetic vessels (Ateyya et al., 2018; Ceriello, 2006; Liu et al., 2014; Satoh et al., 2005). In line with this, the diabetic aorta showed diminished NO bioavailability, reduced antioxidant levels of SOD and GSH, and increased concentrations of MDA compared to those of control animals. Structurally, the medial layer of diabetic aortas showed increased medial thickening and atypical elastic fiber organization, which indicated vascular hypertrophic alterations. Several studies reported similar findings (Baluchnejadmojarad and Roghani, 2008; Elbe et al., 2014; Fukuda et al., 2005; Jandeleit-Dahm et al., 2000; Sleem et al., 2014; Xavier et al., 2003).
Aortic medial layer thickening is a characteristic aspect of arterial wall remodeling in diabetic patients (Astrand et al., 2007; Frost and Beischer, 1998). It is regarded as an independent risk factor for vascular complications in patients with diabetes (Harrington et al., 2010). Increased vascular smooth muscle cell proliferation is the cause of these structural anomalies in the vessels (Ruiz et al., 2006) as well as a lower medial elastin content (Salum et al., 2014). The indifferent aortic TGF-β1 contents between the groups in this study may be a sign that extracellular matrix deposition in the diabetic aorta has not changed at this stage of the disease’s development as previously reported (Akhtar et al., 2014; Salum et al., 2012).
The abnormal functional alteration in the aortic reactivity to PE and the structural changes in STZ rats was attenuated to a greater extent by MONT than by LOS. MONT was also able to restore aortic NOx levels and showed greater antioxidant activity than LOS on diabetic aortic tissues. Therefore, MONT ability to ameliorate vascular dysfunction may be related to its effects on NO levels and vascular oxidative stress. Moreover, CysLT1 receptors were reported to mediate vasoconstrictor effects on diabetic aortas (Hardy et al., 2001).
In conclusion, according to the current research, MONT treatment for experimental STZ diabetes resulted in vasculoprotective and renoprotective effects that were comparable to or even greater than those caused by LOS treatment. MONT administration might provide a prospective treatment option for diabetic individuals who do not receive the full benefit of ARB medication for kidney and vascular protection. Multiple mechanisms may mediate MONT effects in the current investigation, including attenuation of inflammation and oxidative stress. Glycemic management is improved, and diabetic rats’ kidney and blood arteries directly benefit. It should be investigated in the future whether MONT-mediated effects also occur in people and other disease models.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research was funded by Mansoura University, Mansoura, Egypt and Dubai Pharmacy College for Girls, Dubai, United Arab Emirates.
ETHICAL APPROVALS
The protocol was approved by the Research Ethics Committee at Dubai Pharmacy College for Girls, Dubai, United Arab Emirates (Reference # FEC/FD/2020/02).
CONFLICTS OF INTEREST
The authors declared they have no conflicts of interest.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdel-Raheem IT, Khedr NF. Renoprotective effects of montelukast, a cysteinyl leukotriene receptor antagonist, against methotrexate-induced kidney damage in rats. Naunyn Schmiedebergs Arch Pharmacol, 2014; 387(4):341–53. CrossRef
Aggarwal N, Kare PK, Varshney P, Kalra OP, Madhu SV, Banerjee BD, Yadav A, Raizada A, Tripathi AK. Role of angiotensin converting enzyme and angiotensinogen gene polymorphisms in angiotensin converting enzyme inhibitor-mediated antiproteinuric action in type 2 diabetic nephropathy patients. World J Diabetes, 2017; 8(3):112–9. CrossRef
Akhtar R, Cruickshank JK, Zhao X, Walton LA, Gardiner NJ, Barrett SD, Graham HK, Derby B, Sherratt MJ. Localized micro- and nanoscale remodelling in the diabetic aorta. Acta Biomater, 2014; 10(11): 4843–51. CrossRef
Alaofi AL. Sinapic acid ameliorates the progression of streptozotocin (stz)-induced diabetic nephropathy in rats via nrf2/ho-1 mediated pathways, Front Pharmacol, 2020; 111119. CrossRef
Alderson NL, Chachich ME, Frizzell N, Canning P, Metz TO, Januszewski AS, Youssef NN, Stitt AW, Baynes JW, Thorpe SR. Effect of antioxidants and ace inhibition on chemical modification of proteins and progression of nephropathy in the streptozotocin diabetic rat. Diabetologia, 2004; 47(8):1385–95. CrossRef
Amin FM, Shehatou GGS, Nader MA, Abdelaziz RR. Piperine mitigates aortic vasculopathy in streptozotocin-diabetic rats via targeting txnip-nlrp3 signaling. Life Sci, 2022; 121275. CrossRef
Astrand H, Ryden-Ahlgren A, Sundkvist G, Sandgren T, Lanne T. Reduced aortic wall stress in diabetes mellitus. Eur J Vasc Endovasc Surg, 2007; 33(5):592–8. CrossRef
Ateyya H, Nader MA, El-Sherbeeny NA. Beneficial effects of rosiglitazone and losartan combination in diabetic rats. Can J Physiol Pharmacol, 2018; 96(3):215–20. CrossRef
Baluchnejadmojarad T, Roghani M. Chronic administration of genistein improves aortic reactivity of streptozotocin-diabetic rats: mode of action. Vascul Pharmacol, 2008; 49(1):1–5. CrossRef
Bapputty R, Talahalli R, Zarini S, Samuels I, Murphy R, Gubitosi-Klug R. Montelukast prevents early diabetic retinopathy in mice. Diabetes, 2019; 68(10):2004–15. CrossRef
Bazzano T, Restel TI, Porfirio LC, Souza AS, Silva IS. Renal biomarkers of male and female wistar rats (rattus norvegicus) undergoing renal ischemia and reperfusion. Acta Cir Bras, 2015; 30(4):277–88. CrossRef
Benigni A, Zoja C, Corna D, Zatelli C, Conti S, Campana M, Gagliardini E, Rottoli D, Zanchi C, Abbate M, Ledbetter S, Remuzzi G. Add-on anti-tgf-beta antibody to ace inhibitor arrests progressive diabetic nephropathy in the rat. J Am Soc Nephrol, 2003; 14(7):1816–24. CrossRef
Bertani T, Abbate M, Zoja C, Corna D, Perico N, Ghezzi P, Remuzzi G. Tumor necrosis factor induces glomerular damage in the rabbit. Am J Pathol, 1989; 134(2):419–30.
Bilous R, Chaturvedi N, Sjolie AK, Fuller J, Klein R, Orchard T, Porta M, Parving HH. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Ann Intern Med, 2009; 151(1):11-20, W3–4. CrossRef
Bories PN, Bories C. Nitrate determination in biological fluids by an enzymatic one-step assay with nitrate reductase. Clin Chem, 1995; 41(6 Pt 1):904–7. CrossRef
Brenner BM, Cooper ME, De Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med, 2001; 345(12):861–9. CrossRef
Ceriello A (2006). Oxidative stress and diabetes-associated complications. Endocr Pract, 2006; 12 Suppl 160–2. CrossRef
Cheetham C, O’driscoll G, Stanton K, Taylor R, Green D. Losartan, an angiotensin type i receptor antagonist, improves conduit vessel endothelial function in type ii diabetes. Clin Sci (Lond), 2001; 100(1):13–7. CrossRef
Coskun AK, Yigiter M, Oral A, Odabasoglu F, Halici Z, Mentes O, Cadirci E, Atalay F, Suleyman H. The effects of montelukast on antioxidant enzymes and proinflammatory cytokines on the heart, liver, lungs, and kidneys in a rat model of cecal ligation and puncture-induced sepsis. SciWorld J, 2011; 111341–56. CrossRef
Dabelea D. Diabetes in youth-looking backwards to inform the future: kelly west award lecture 2017. Diabetes Care. 2018; 41(2):233–40. CrossRef
Dabelea D, Stafford JM, Mayer-Davis EJ, D’agostino R, Jr., Dolan L, Imperatore G, Linder B, Lawrence JM, Marcovina SM, Mottl AK, Black MH, Pop-Busui R, Saydah S, Hamman RF, Pihoker C. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017; 317(8):825–35. CrossRef
Delaney CA, Dunger A, Di Matteo M, Cunningham JM, Green MH, Green IC. Comparison of inhibition of glucose-stimulated insulin secretion in rat islets of langerhans by streptozotocin and methyl and ethyl nitrosoureas and methanesulphonates. Lack of correlation with nitric oxide-releasing or o6alkylating ability. Biochem Pharmacol, 1995; 50(12):2015–20. CrossRef
Devaraj S, Cheung AT, Jialal I, Griffen SC, Nguyen D, Glaser N, Aoki T. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes, 2007; 56(11):2790–6. CrossRef
El-Boghdady NA, Abdeltawab NF, Nooh MM (2017). Resveratrol and montelukast alleviate paraquat-induced hepatic injury in mice: modulation of oxidative stress, inflammation, and apoptosis. Oxid Med Cell Longev, 2017; 20179396425. CrossRef
Elbe H, Vardi N, Orman D, Taslidere E, Yildiz A. Ameliorative effects of aminoguanidine on rat aorta in streptozotocin-induced diabetes and evaluation of alpha-sma expression. Anadolu Kardiyol Derg, 2014; 14(8):679–84. CrossRef
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys, 1959; 82(1):70–7. CrossRef
Faul JL, Wilson SR, Chu JW, Canfield J, Kuschner WG. The effect of an inhaled corticosteroid on glucose control in type 2 diabetes. Clin Med Res, 2009; 7(1-2):14–20. CrossRef
Frost D, Beischer W. Determinants of carotid artery wall thickening in young patients with type 1 diabetes mellitus. Diabet Med, 1998; 15(10):851–7. CrossRef
Fukuda G, Khan ZA, Barbin YP, Farhangkhoee H, Tilton RG, Chakrabarti S. Endothelin-mediated remodeling in aortas of diabetic rats. Diabetes Metab Res Rev, 2005; 21(4):367–75. CrossRef
Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc, 2021; 1(4):e78. CrossRef
Gad AM, El-Raouf OMA, El-Sayeh BM, Fawzy HM, Abdallah DM. Renoprotective effects of montelukast in an experimental model of cisplatin nephrotoxicity in rats, J Biochem Mol Toxicol. 2017; 31(12). CrossRef
Giacchetti G, Sechi LA, Rilli S, Carey RM. The reninangiotensin-aldosterone system, glucose metabolism and diabetes. Trends Endocrinol Metab, 2005; 16(3):120–6. CrossRef
Graves LE, Donaghue KC. Vascular complication in adolescents with diabetes mellitus. Front Endocrinol (Lausanne), 2020; 11370. CrossRef
Guo R, Jiang J, Jing Z, Chen Y, Shi Z, Deng B. Cysteinyl leukotriene receptor 1 regulates glucose-stimulated insulin secretion (gsis). Cell Signal, 2018; 46129–134. CrossRef
Ha H, Kim KH (1999). Pathogenesis of diabetic nephropathy: the role of oxidative stress and protein kinase c. Diabetes Res Clin Pract, 1999; 45(2-3):147–51. CrossRef
Hamman RF, Bell RA, Dabelea D, D’agostino RB, Jr., Dolan L, Imperatore G, Lawrence JM, Linder B, Marcovina SM, Mayer-Davis EJ, Pihoker C, Rodriguez BL, Saydah S. The search for diabetes in youth study: rationale, findings, and future directions. Diabetes Care, 2014; 37(12):3336–44. CrossRef
Hardy G, Stanke-Labesque F, Peoc’h M, Hakim A, Devillier P, Caron F, Morel S, Faure P, Halimi S, Bessard G. Cysteinyl leukotrienes modulate angiotensin ii constrictor effects on aortas from streptozotocininduced diabetic rats. Arterioscler Thromb Vasc Biol, 2001; 21(11):1751–8. CrossRef
Harrington J, Pena AS, Gent R, Hirte C, Couper J. Aortic intima media thickness is an early marker of atherosclerosis in children with type 1 diabetes mellitus. J Pediatr, 2010; 156(2):237–41. CrossRef
Ibrahim MA, Amin EF, Ibrahim SA, Abdelzaher WY, Abdelrahman AM. Montelukast and irbesartan ameliorate metabolic and hepatic disorders in fructose-induced metabolic syndrome in rats. Eur J Pharmacol, 2014; 724204–10. CrossRef
Jandeleit-Dahm K, Hannan KM, Farrelly CA, Allen TJ, Rumble JR, Gilbert RE, Cooper ME, Little PJ. Diabetes-induced vascular hypertrophy is accompanied by activation of na (+)-h (+) exchange and prevented by na (+) -h (+) exchange inhibition. Circ Res, 2000; 87(12):1133–40. CrossRef
Jia Q, Yang R, Liu XF, Ma SF, Wang L. Genistein attenuates renal fibrosis in streptozotocininduced diabetic rats. Mol Med Rep, 2019; 19(1):423–31. CrossRef
Kara Z, Guven B, Onay Besikci A, Yildirim N, Altunay H. Pleiotropic vascular effects of ivabradine in streptozotocin-induced diabetes. Eur J Pharmacol, 2022; 916174551. CrossRef
Khodir AE, Ghoneim HA, Rahim MA, Suddek GM. Montelukast reduces sepsis-induced lung and renal injury in rats. Can J Physiol Pharmacol, 2014; 92(10):839–47. CrossRef
Ko GJ, Kang YS, Han SY, Lee MH, Song HK, Han KH, Kim HK, Han JY, Cha DR. Pioglitazone attenuates diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. Nephrol Dial Transplant, 2008; 23(9):2750–60. CrossRef
Kone BC. Nitric oxide in renal health and disease. Am J Kidney Dis, 1997; 30(3):311–3. CrossRef
Kraynak AR, Storer RD, Jensen RD, Kloss MW, Soper KA, Clair JH, Deluca JG, Nichols WW, Eydelloth RS. Extent and persistence of streptozotocin-induced DNA damage and cell proliferation in rat kidney as determined by in vivo alkaline elution and brdurd labeling assays. Toxicol Appl Pharmacol, 1995; 135(2):279–86. CrossRef
Kuhad A, Chopra K. Attenuation of diabetic nephropathy by tocotrienol: involvement of nfkb signaling pathway. Life Sci, 2009; 84(910):296–301. CrossRef
Li Z, Wang J, Ma Y. Montelukast attenuates interleukin il-1betainduced oxidative stress and apoptosis in chondrocytes by inhibiting cysltr1 (cysteinyl leukotriene receptor 1) and activating klf2 (kruppel like factor 2). Bioengineered, 2021; 12(1):8476–84. CrossRef
Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, Hsueh W, Rewers M, Roberts BT, Savage PJ, Skarlatos S, Wassef M, Rabadan-Diehl C. Report of the national heart, lung, and blood institute-national institute of diabetes and digestive and kidney diseases working group on cardiovascular complications of type 1 diabetes mellitus. Circulation, 2005; 111(25):3489–93. CrossRef
Lim KG, Varatharajan R, Muthuraman A The attenuating effect of beta-carotene on streptozotocin induced diabetic vascular dementia symptoms in rats. Molecules, 2022; 27(13). CrossRef
Liu LL, Yan L, Chen YH, Zeng GH, Zhou Y, Chen HP, Peng WJ, He M, Huang QR. A role for diallyl trisulfide in mitochondrial antioxidative stress contributes to its protective effects against vascular endothelial impairment. Eur J Pharmacol, 2014; 72523–31. CrossRef
Manni ME, Bigagli E, Lodovici M, Zazzeri M, Raimondi L. The protective effect of losartan in the nephropathy of the diabetic rat includes the control of monoamine oxidase type a activity. Pharmacol Res, 2012; 65(4):465–71. CrossRef
Marklund SL (1985). Superoxide dismutase isoenzymes in tissues and plasma from new zealand black mice, nude mice and normal balb/c mice. Mutat Res, 1985; 148(1-2):129–34. CrossRef
Marques F, Reis J, Godinho I, Pereira M, Fernandes P, Jorge S, Lopes JA, Gameiro J. Impact of early proteinuria reduction in glomerular disease and decline of kidney function: a retrospective cohort. J Clin Med, 2022; 11(19). CrossRef
Matboli M, Eissa S, Ibrahim D, Hegazy MGA, Imam SS, Habib EK. Caffeic acid attenuates diabetic kidney disease via modulation of autophagy in a high-fat diet/streptozotocin- induced diabetic rat. Sci Rep, 2017; 7(1):2263. CrossRef
Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med, 2009; 361(1):40–51. CrossRef
Mccarthy ET, Sharma R, Sharma M, Li JZ, Ge XL, Dileepan KN, Savin VJ. Tnf-alpha increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J Am Soc Nephrol, 1998; 9(3):433–8. CrossRef
Mestry SN, Dhodi JB, Kumbhar SB, Juvekar AR. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by punica granatum linn. Leaves extract. J Tradit Complement Med, 2017; 7(3):273–80. CrossRef
Meyerholz DK, Tintle NL, Beck AP (2019). Common pitfalls in analysis of tissue scores. Vet Pathol, 2019; 56(1):39–42. CrossRef
Mogensen CE. Microalbuminuria in prediction and prevention of diabetic nephropathy in insulin-dependent diabetes mellitus patients. J Diabetes Complications, 1995; 9(4):337–49. CrossRef
Murali B, Goyal RK. Effect of chronic treatment with losartan on streptozotocin induced diabetic nephropathy. Clin Exp Hypertens, 2001; 23(7):513–20. CrossRef
Nayak A. A review of montelukast in the treatment of asthma and allergic rhinitis. Expert Opin Pharmacother. 2004; 5(3):679–86. CrossRef
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 1979; 95(2):351–8. CrossRef
Ortiz A, Bustos C, Alonso J, Alcazar R, Lopez-Armada MJ, Plaza JJ, Gonzalez E, Egido J. Involvement of tumor necrosis factor-alpha in the pathogenesis of experimental and human glomerulonephritis. Adv Nephrol Necker Hosp, 1995; 2453–77.
Otunctemur A, Ozbek E, Cakir SS, Dursun M, Cekmen M, Polat EC, Ozcan L, Somay A, Ozbay N. Beneficial effects montelukast, cysteinyl-leukotriene receptor antagonist, on renal damage after unilateral ureteral obstruction in rats. Int Braz J Urol, 2015; 41(2):279–87. CrossRef
Qi SS, Zheng HX, Jiang H, Yuan LP, Dong LC. Protective effects of chromium picolinate against diabetic-induced renal dysfunction and renal fibrosis in streptozotocin-induced diabetic rats. Biomolecules, 2020; 10(3). CrossRef
Rasch R, Dorup J. Quantitative morphology of the rat kidney during diabetes mellitus and insulin treatment. Diabetologia, 1997; 40(7):802–9. CrossRef
Ruggenenti P, Cravedi P, Remuzzi G (2010). The raas in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol, 2010; 6(6):319–0. CrossRef
Ruiz E, Gordillo-Moscoso A, Padilla E, Redondo S, Rodriguez E, Reguillo F, Briones AM, Van Breemen C, Okon E, Tejerina T. Human vascular smooth muscle cells from diabetic patients are resistant to induced apoptosis due to high bcl-2 expression. Diabetes, 2006; 55(5):1243–51. CrossRef
Saad MA, Abdelsalam RM, Kenawy SA, Attia AS. Montelukast, a cysteinyl leukotriene receptor-1 antagonist protects against hippocampal injury induced by transient global cerebral ischemia and reperfusion in rats. Neurochem Res, 2014; 40(1):139–50. CrossRef
Said MM, Bosland MC. The anti-inflammatory effect of montelukast, a cysteinyl leukotriene receptor-1 antagonist, against estradiol-induced nonbacterial inflammation in the rat prostate. Naunyn Schmiedebergs Arch Pharmacol, 2016; 390(2):197–205. CrossRef
Salum E, Butlin M, Kals J, Zilmer M, Eha J, Avolio AP, Arend A, Aunapuu M, Kampus P. Angiotensin ii receptor blocker telmisartan attenuates aortic stiffening and remodelling in stz-diabetic rats. Diabetol Metab Syndr, 2014; 657. CrossRef
Salum E, Kampus P, Zilmer M, Eha J, Butlin M, Avolio AP, Podramagi T, Arend A, Aunapuu M, Kals J. Effect of vitamin d on aortic remodeling in streptozotocin-induced diabetes. Cardiovasc Diabetol, 2012; 1158. CrossRef
Satoh M, Fujimoto S, Haruna Y, Arakawa S, Horike H, Komai N, Sasaki T, Tsujioka K, Makino H, Kashihara N. Nad(p)h oxidase and uncoupled nitric oxide synthase are major sources of glomerular superoxide in rats with experimental diabetic nephropathy. Am J Physiol Renal Physiol, 2005; 288(6):F1144–52. CrossRef
Schram MT, Chaturvedi N, Schalkwijk C, Giorgino F, Ebeling P, Fuller JH, Stehouwer CD, Study EPC. Vascular risk factors and markers of endothelial function as determinants of inflammatory markers in type 1 diabetes: the eurodiab prospective complications study. Diabetes Care, 2003; 26(7):2165–73. CrossRef
Sener G, Sehirli O, Velioglu-Ogunc A, Cetinel S, Gedik N, Caner M, Sakarcan A, Yegen BC (2006). Montelukast protects against renal ischemia/reperfusion injury in rats. Pharmacol Res, 2006; 54(1):65–71. CrossRef
Shang G, Gao P, Zhao Z, Chen Q, Jiang T, Zhang N, Li H. 3,5-diiodo-l-thyronine ameliorates diabetic nephropathy in streptozotocininduced diabetic rats. Biochim Biophys Acta, 2013; 1832(5):674–84. CrossRef
Shawky NM, Shehatou GSG, Suddek GM, Gameil NM. Comparison of the effects of sulforaphane and pioglitazone on insulin resistance and associated dyslipidemia, hepatosteatosis, and endothelial dysfunction in fructose-fed rats. Environ Toxicol Pharmacol, 2019; 6643–54. CrossRef
Sleem M, Taye A, El-Moselhy MA, Mangoura SA. Combination therapy with losartan and l-carnitine protects against endothelial dysfunction of streptozotocin-induced diabetic rats. Eur J Pharmacol, 2014; 74410–7. CrossRef
Soldatos G, Cooper ME. Diabetic nephropathy: important pathophysiologic mechanisms. Diabetes Res Clin Pract, 2008; 82 Suppl 1S75–9. CrossRef
Tang L, Li K, Zhang Y, Li H, Li A, Xu Y, Wei B. Quercetin liposomes ameliorate streptozotocin-induced diabetic nephropathy in diabetic rats. Sci Rep. 2020; 10(1):2440. CrossRef
Volpini RA, Da Silva CG, Costa RS, Coimbra TM. Effect of enalapril and losartan on the events that precede diabetic nephropathy in rats. Diabetes Metab Res Rev, 2003; 19(1):43–51. CrossRef
Wang K, Hu J, Luo T, Wang Y, Yang S, Qing H, Cheng Q, Li Q. Effects of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers on all-cause mortality and renal outcomes in patients with diabetes and albuminuria: a systematic review and meta-analysis. Kidney Blood Press Res, 2018; 43(3):768–79. CrossRef
Wei M, Ong L, Smith MT, Ross FB, Schmid K, Hoey AJ, Burstow D, Brown L. The streptozotocin-diabetic rat as a model of the chronic complications of human diabetes. Heart Lung Circ, 2003; 12(1): 44–50. CrossRef
Weil EJ, Fufaa G, Jones LI, Lovato T, Lemley KV, Hanson RL, Knowler WC, Bennett PH, Yee B, Myers BD, Nelson RG. Effect of losartan on prevention and progression of early diabetic nephropathy in american indians with type 2 diabetes. Diabetes, 2013; 62(9):3224–1. CrossRef
Wu X, Zha D, Xiang G, Zhang B, Xiao SY, Jia R. Combined mmf and insulin therapy prevents renal injury in experimental diabetic rats. Cytokine, 2006; 36(5-6):229–36. CrossRef
Xavier FE, Davel AP, Rossoni LV, Vassallo DV. Time-dependent hyperreactivity to phenylephrine in aorta from untreated diabetic rats: role of prostanoids and calcium mobilization. Vascul Pharmacol, 2003; 40(1):67–76. CrossRef
Yao Y, Li Y, Zeng X, Ye Z, Li X, Zhang L. Losartan alleviates renal fibrosis and inhibits endothelial-to-mesenchymal transition (emt) under high-fat diet-induced hyperglycemia. Front Pharmacol, 2018; 91213. CrossRef
Zhang S, Xu H, Yu X, Wu Y, Sui D. Metformin ameliorates diabetic nephropathy in a rat model of low-dose streptozotocin-induced diabetes. Exp Ther Med, 2017; 14(1):383–90. CrossRef
Zhao, Zhao L, Zou Y, Liu F. Transforming growth factor-beta1 in diabetic kidney disease. Front Cell Dev Biol, 2020; 8:187. CrossRef