INTRODUCTION
Nanosponges (NS) describe insoluble solid porous matter with typical nanometric porosity and lofty absorption with complexation properties. It is synthesized using organic or inorganic compounds (Caldera, 2017; David, 2010). This is a novel class of tiny sponges with a structure shown in Figure 1 that is as practical as the dimension of a virus, with numerous voids in which it can be packed with a drug and target the tumor cell by linking a particular chemical linker. The NS is considered a three-dimensional or scaffold-structured network (Kaulitzki, 2013; Shringirishi et al., 2014). The polymer in the NS has an extended length of the polyester backbone and cross-linking agents that resemble microscopic grappling hooks to clip various parts of the polymer together. When the polyester is reacted with the cross-linker, it forms a spherically shaped particle constructed with a pouch that can be used to hold therapeutic molecules. Polyester degrades gradually in the body since it is biodegradable. Using different cross-linkers will alter the length and cavity of the NS (Bolmal, 2013). At this point, the polymer blocks the drug inside the fissure as the core. In alginate NS, numerous pores with a sponge-like structure can carry the drug molecules (Dai et al., 2009). NS has the potential to capture poorly soluble drugs and optimizing delivery. Because of its wide range of diameters (1 μm or less) and the cavities variable polarity, it can be created synthetically by changing the crosslinker ratio (Tejashri et al., 2013).
It serves as a vehicle for delivering proteins, enzymes, vaccines, antibodies, and lipophilic and hydrophilic compounds to improve the low solubility of poorly water-soluble drugs (Trotta et al., 2006, 2012). It can disguise unpleasant flavors and solidification of the substances as they change from a liquid to a solid (Jilsha and Vidya, 2013). This innovative technology is five times more successful in potential delivering drugs for breast cancer than traditional approaches, it has the potential to completely change how many diseases are treated (David, 2010). They move towards and bind with a tumor cell’s surface, wherever they are affixed superficially, and thus start discharging effectively the drug in a measured and predictable manner (Bolmal, 2013; David, 2010; Shivani and Kranthi, 2015). Unlike other drug delivery systems that release a maximum of their drug rapidly and uncontrollably after reaching their targeted site (known as the burst effect), NS releases the drug slowly, when it reaches the target site. After intravenous distribution, the mononuclear phagocyte system (MPS), which is predominantly made up of Kupffer cells in the liver pairing the phagocytic activity and recognizes nanodrug carriers, including liposomes, as foreign particles and promptly eliminates them. The MPS is cumulatively affected by multiple injections of particulate drug transporters, which compromises this crucial host defence system which is less possible in NS (Allen, 1988). NS can remove the organic froth from water (Taka et al., 2017). Moreover, NS exhibit a notable benefit above conventional nanoparticles: indeed, it is readily regenerable by a variety of processes, such as washing with efficient and environmentally solvents, employing relatively inert hot gases for stripping, mild heating, or changing pH or ionic strength. NS has already been used in a variety of application domains for all these qualities, including the cosmetic and pharmaceutical industries, flower cultivation, and polymer flame retardancy (Alongi et al., 2010; Boscolo et al., 2010).
The followings are some of the topics on the review of NS, Cyclodextrin (CD)-based NS: A critical review (Sherje et al., 2017), The application of NS to cancer drug delivery (Trotta et al., 2014), NS: a potential nanocarrier for novel drug delivery-a review (Shringirishi et al., 2014), Evolution of CD NS (Caldera, 2017).
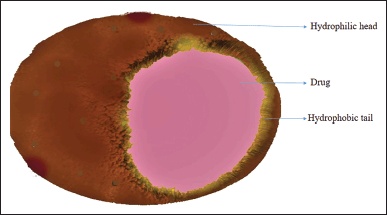 | Figure 1. Structure of NS. [Click here to view] |
Chemicals used for the synthesis
Polymers and cross-linkers are the chief ingredients in the formulation of NS. The polymer used can influence the content and development of NS. It can bind to specific ligands, and an agent such as crosslinker will rely on the polymer structure and the drug formulation (Singh and Monika, 2022). Polymers are frequently used in the production of medications with prolonged release and cutting-edge polymeric delivery systems, including stimuli-responsive polymeric systems (Raval et al., 2019). The crosslinking agent determines the space dimensions of NSs that can accommodate drug loading (Selvamuthukumar et al., 2012). The chemicals used in the preparation method are listed below (Table 1).
Recent discoveries include the NS, organic or inorganic materials constructed of three-dimensional networks of spherical particles through a porous medium with cavities a few nanometers across and various dimensions (1 μm or less). The porous NS particles are nontoxic, will not solubilize in organic solvents and will stable up to 300°C. NS formulations can be stabilized at temperatures of up to 130°C and a pH range of 1 to 11. It can be extended to release the medications with continuous action for 12 to 24 hours, examples of which are titanium or other metal-oxide-based, silicon-based, carbon-coated metallic, hyper-cross-linked polystyrene and CD-based NS. NS suspended in water but does not chemically degrade in it, which was then used as a transport fluid (David, 2010). It dissolves in water to create clear solutions, opalescent suspensions and can be recovered through direct thermal desorption, solvent extraction, microwave treatment, and ultrasonic treatment. Nanosized colloidal carriers, which were created specifically for the delivery of drugs. According to Singireddy and Subramanian (2016); the low water solubility and poor absorption of quercetin can be enhanced by increasing the dissolution rate of encapsulating in CD NS for delayed drug delivery for particular tissue applications (Singireddy and Subramanian, 2016). NS can provide sustainable prolonged release as well as enhance the drug molecules bioavailability, thus modifying the pharmacokinetic parameters (Tejashri et al., 2013).
CDs form complexes with active pharmaceutical ingredient(s) to improve water solubility, conceal undesirable properties, reduce adverse effects, and improve photostability and stability. Additionally, CDs have been demonstrated to regulate the release of specific active substances. CD-based NSs are hyper-crosslinked systems created by crosslinking several CD molecules using substances like carbonyl or carboxyl chemicals. Crosslinked polymers are the resultant systems and exhibit remarkable features including chemical inclusion or absorption, swelling, and efficient active agent release. The NS delivers high efficient drug loading capacity compared to other structured nanocarriers and has tremendous potential for addressing issues with bioavailability, solubility, the pre-regulated sustained release of a variety of pharmacologically active therapeutic agents and stability. Additionally, the developed NSs can be incorporated into common dosage forms such as ointments, gels, creams, lotions, and powders for therapeutic purposes. Prior research has revealed that CD-based carbamate NS can bind to organic molecules and are used to filter water. This type of NS can simultaneously store both the non-polar organic chemicals and cations because it encompasses a free polar carboxylic acid group. According to previous studies, CD-based NS acts as a delivery system for drugs like paclitaxel, acyclovir, curcumin, camptothecin and itraconazole (Torne et al., 2013; Hariri, 2014; Lembo et al., 2013; Mognetti et al., 2012; Swaminathan et al., 2010a, 2010b; Torne et al., 2010). Furthermore, it has been observed that NS can improve the solubility and bioavailability of various compounds, such as resveratrol and tamoxifen (Lembo et al., 2013; Osmani et al., 2015).
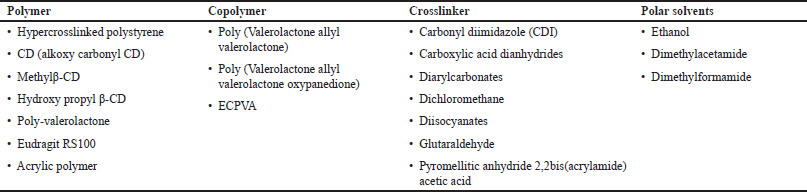 | Table 1. Chemicals in the preparation of NS. [Click here to view] |
Patient pliability during dosage form administration and subsequent repeated-dose therapy regimen is a crucial difficulty in vaginal drug delivery. Gels have essential advantages over conventional formulations, including adaptability, safety, high bioavailability, and cost-effectiveness. Patients tolerate gels better than any other dose form and vaginal therapy can be greatly enhanced with drug delivery systems like keeping the medication at the specifically employed target site for a long period. In situ gelling drug delivery systems change the rheological characteristics of the polymer platform in reaction to stimuli, releasing pharmaceuticals in response to environmental signals. Hence, these systems can be designed to adequately cover the entire vagina and keep the formulation adhered on to the mucosal tissue. A reversible form of gelation may take place at physiological temperature and this thermoreversible behavior of Pluronic F 127 gel is caused by a conversion of its micellar characteristics as a function of both the difference in the polymer concentration and ambient temperature. Such systems provide the vagina several advantages, including great spreadability, simplicity of application at body temperatures below sol-gel temperature, rheological structuring, and the ability to deliver therapeutic substances topically. As a result, vaginal retention time at body temperature is improved.
Hyper-cross-linked CD-based NS can be made from CD , either by themselves or in blends with appropriate proportions of linear dextrin, and cross-linked with the appropriate cross-linking agent. Chemical linkers enable preferential connection of NS to the target site. With various medications, they produce both the inclusion and non-inclusion complexes. Their crystal structure greatly influences the complexation of NS with medicines. The degree of crystallization seems to have a major impact on the loading capacity of NS. Different drug loading capabilities have been observed for para-crystalline NS. As drug carriers, intriguing results have already been attained by utilizing a compound of an active carbonyl molecule, such as carbonyldimiidazole, triphosgene, diphenyl carbonate (DPC), or organic dianhydrides. Overall, this results in the production of spherical particles containing hydrophobic cavities and hydrophilic channels that can trap drug molecules. Several interconnected voids make up a single NS system, a non-collapsible structure that can store various chemicals. NS will offer a perfect drug release profile and a longer retention period at the specific target site profile of encapsulated drug moieties for improved and good efficient therapy to treat various cancers, including breast and colon cancer. The current review was undertaken for the drug delivery of NS, preparation methods, characterization, mechanism of drug release, and applications.
Method of preparation
Solvent method
This method uses chemicals such as a polymer [β-CD, ethyl cellulose (EC), alginates, eudragit, polyvinyl alco (PVA)] and a suitable solvent, particularly aprotic polar solvents like dimethylformamide and dimethylsulfoxide, were mixed to form a mixture (Prabhu et al., 2020). Then the mixture, add a sufficient quantity of cross-linker (Dimethyl carbonate and CDI), preferably to a cross-linked/polymer with the determined molar ratio of 4 to 16 for the formulation. The reaction is conducted for 1 to 48 hours at temperatures ranging from 10°C to the solvent’s reflux temperature. The finished product is made by mixing the cooled solution with a significant amount of bidistilled water. Filtration is used to recover the product under vacuum, and long-term Soxhlet extraction is used to purify it further. Finally, the end product was dehydrated under a vacuum and was used to minimize the size using a mechanical mill to create a uniform powder (Lala et al., 2011; Trotta et al., 2006). This method is diagrammatically explained in Figure 2.
Ultrasound-assisted synthesis
NS is formed in this technical method by sonicating the polymers with the cross-linkers without using a solvent. When made using this technique, the NS is spherical and homogeneous in size. A flask was filled with the polymer and the cross-linker at a certain molar ratio. After that, it was held for 5 hours under continuous effective sonication in an ultrasound bath device filled with water that had been heated to the temperature up to 90°C. The non-reacted polymer was then effectively removed by adding too much water, then by using ethanol it was extracted for a long-time. It is possible to obtain NS with a narrow particle size distribution by high-pressure homogenization technique. Finally, the product was then desiccated at 25°C under a vacuum. Figure 3 explains the method (Alongi et al., 2010; Ansari et al., 2011; Dhavala and Tenneti, 2017; Lala et al., 2011; Shende et al., 2012).
 | Figure 2. Solvent method. [Click here to view] |
β-CD that are hyper cross-linked
In this method, the drug carrier for delivering the drug was β-CD. NS was prepared by means of reacting β-CD and employing a cross-linker. Its Synthesis can be in the nature of neutral or acidic forms and have a mean diameter of lesser than 1 µm, but fractions of particles below 500 nm can be picked out (Davankov et al., 1996; Setijadi et al., 2009). Figure 4 represents the β-CD NS.
Solvent evaporation method
NS can be formed using EC and PVA. Here, dichloromethane, an organic solvent, was used to dissolve the dispersed phase EC and then it was thoroughly mixed with the PVA aqueous solution, the continuous aqueous phase. The reaction is then continued via magnetic mixing for 5 hours. Then finally after filtration, the product was dried for 24 hours at 40°C in an oven (Swaminathan et al., 2010a, 2010b). This procedure is explained in Figure 5.
Melt method
The crosslinker and β-CDs are fused when using the melting technique. The remaining fixings are finely homogenized and added to a 250 ml jar preheated to 100°C. The reaction is then carried out for 5 hours by magnetic mixing. The reaction mixture is allowed to cool, after which the result is broken down and repeatedly washed well with suitable solvents, i.e., ethanol, to eliminate the excipients and byproducts that have not reacted completely (Sharma et al., 2022). These blank NS’s were then encapsulated (Rao and Bhingole, 2015). This method is illustrated in Figure 6.
Bubble electrospinning
A syringe, syringe pump, high-voltage power, and a grounded collector are the main components of a standard electrospinning setup, as outlined in several pieces of literature. However, the amount of nanofibers production is one of the key restrictions that restrict their applicability. PVA can also be utilized as a polymer in the bubble electrospinning technique. The solution of polymer (10%) was organized by adding distilled water, it was then stirred at 80°C–90°C for 2 hours to produce a one-phase mixture. The polymer solution was then allowed to cool before making NS fibers (Yang et al., 2009). The process is illustrated in Figure 7.
Synthesis by the use of microwave radiation
Compared to traditionally manufactured nanoparticles, the loading capacity of CD NS made with microwave assistance was doubled. This group described the use of microwave technology as a very effective, straightforward, repeatable, scalable, and affordable approach to creating CD NS within a short period.
 | Figure 3. Ultrasound-assisted method. [Click here to view] |
 | Figure 4. β-CD. [Click here to view] |
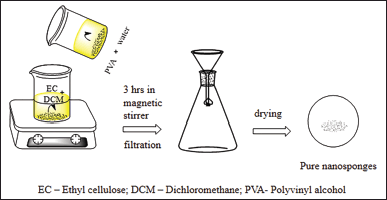 | Figure 5. Solvent evaporation method. Ec-Ethyl cellulose; DCM-Dichloromethiane; PVA-Polyvinyl alcohl. [Click here to view] |
 | Figure 6. Melt method. [Click here to view] |
 | Figure 7. Bubble electro spinning method. [Click here to view] |
Zainuddin et al. (2017) developed a microwave-mediated approach to create CD NS to increase the bioavailability of rilpivirine hydrochloride. This group optimized the solvent volume, the crosslinker, and the polymer-to-watt power ratio while using DPC as the crosslinker. In the investigation, paracrystalline CD NS was developed, which significantly increased the oral bioavailability of the medication rilpivirine hydrochloride (Zainuddin et al., 2017).
This microwave irradiation synthesis approach for CD-based NS drastically speeds up response time. The crystallinity of these NS is excellent. Compared to conventional heating methods, microwave synthesis of NS produced a consistent particle size distribution and uniform crystallinity while reducing reaction time by four. Singireddy et al. (2016) tested the advantages of the technique of microwave-assisted heating over conventional heating when synthesizing CD-based NSs. The outcomes results demonstrated that the model drug’s capacity to retain drugs was doubled by NSs produced using microwave assistance. The NSs produced via microwave synthesis have an elevated level of complexity, a limited size distribution, and a high degree of crystallinity. When microwave-assisted heating, reaction durations were dramatically reduced, and reaction products were significantly enhanced (Singireddy et al., 2016). Utilizing microwave irradiation for synthesis has the advantage of precisely providing direct energy to the targeted molecules. Thus, the real impact is visible as the reaction progresses (Zainuddin et al., 2017).
Quasi-emulsion solvent method
The polymer of different ratios was used to assemble the NS in various quantities. To prepare the inner phase, Eudragit RS 100 is mixed with the relevant solvent. The drug was dissolved using ultrasound at 35°C and given in a solution form. This internal phase acts as an emulsifier when combined with the PVA-containing external phase. The formulated mixture is agitated for 3 hours at the speed of 1,000–2,000 rpm at ambient room temperature and then dried for 12 hours in hot-air oven at 40°C (Eldose et al., 2015).
Experimental design
The NS preparation formulas are optimized using a statistical method experimental design (Box–Behnken method in the Design–Expert version 9.0.1; Stat-Ease, Inc., Minneapolis, MN). Design-Expert DX 8.0.7.1 was used to optimize the formulation, using 23 complete factorial designs, the administration of two drugs with CD cross-linked to artemether (ART) and lumefantrine (LUM) NS for synergistic effects was optimized. Impact of independent factors on dependent variables at various levels and a complete factorial design. The data from the ANOVA test demonstrated the model’s applicability with a p-value of less than 0.05.
The considerable impact of hydroxyl propyl (HP) β-CD, β-CD, and CDI on entrapment efficiency (EE) and particle size was demonstrated by 23 complete factorial designs. Figure 8 depicts particle size, EE of ART using HP-CD, and EE of LUM using a 3D response surface curve that shows the impact of independent factors on each (Pawar and Shende, 2020a). The Design Expert® (10.0.0.1) program was carried out for a statistical design to develop the Risedronate Sodium-loaded NS formulation. The quantity of EC (X1) and the percentage of PVA (X2), were independent variables that were employed at low, medium, and high levels. The particle size (Y1) and percent of EE were two dependent variables they examined in relation to independent factors (EE, Y2). According to the ANOVA analysis, the p-value declined beneath 0.05, demonstrating that the model fitting was significant (Pandya et al., 2019).
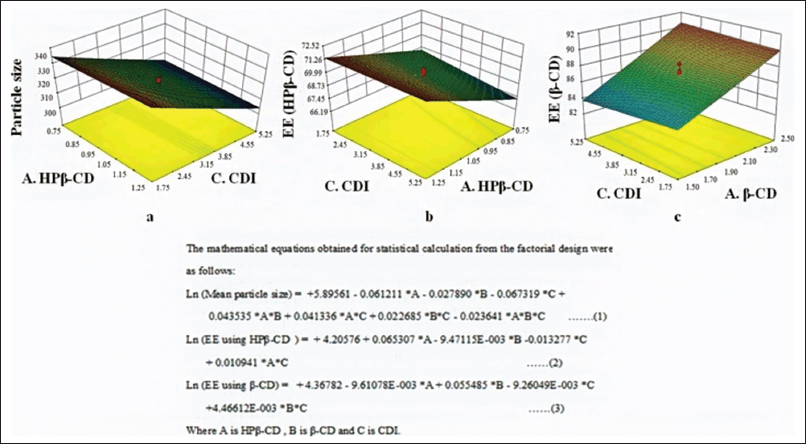 | Figure 8. Particle size, EE of ART using HP-CD, and EE of LUM. [Click here to view] |
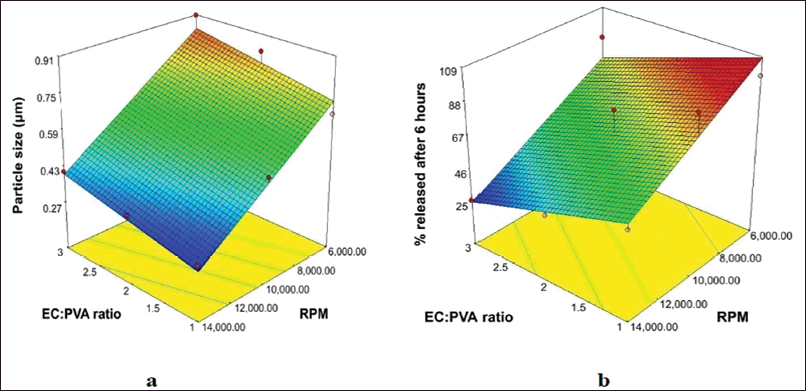
According to Aldawsari et al. (2015) the effect of two studied variables such as EC:PVA ratio which was represented as X1 and stirring rate which was represented as X2 on the specified in vitro characteristics of all the prepared formulations with response variables like to be particle size (Y1) and percentage of drug released after 6 hours (Y2) were statistically analyzed by using Design-Expert® Software Version 7.0.0 (Stat-Ease Inc, Minneapolis, MN). The mean percentage of lemongrass oil released after 6 hours (Y2) was in Figure 9 (Aldawsari et al., 2015).
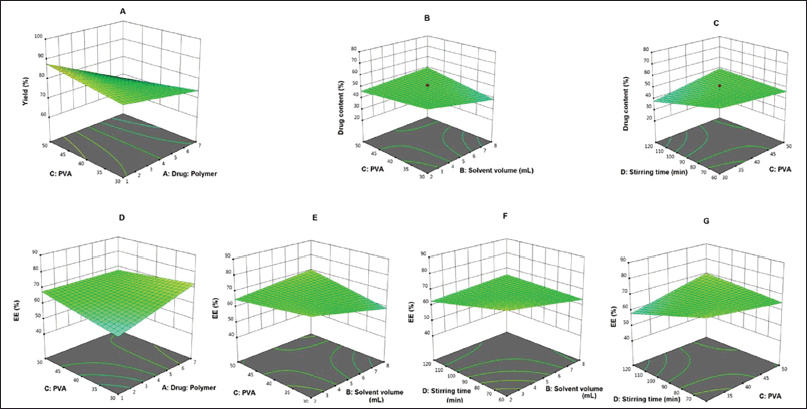
In order to ascertain the effects of four different independent variables at their predetermined levels on the percentage yield, drug content, and EE for the formation of colloidal NS that was loaded with hesperetin, (Quality by design) was developed according to a 42 factorial design. Nineteen trials were developed and 3D surface response plots are used to explain the impact of various independent variable factors on the responses Y1–Y3. An intimate match between experimental and predicted values suggests that the design was successful in analyzing and optimizing the formulations. Figure 10 represents the 3D impacts of variables on the yield, amount of drug, and effectiveness of entrapment of hesperetin-loaded colloidal NS (Rodrigues et al., 2022).
Drug loading
These NS must be reduced to less than 500 nm particle size. Then a colloidal segment is produced by centrifuging the suspension. The uncomplexed drug was separated from the suspensions as a residue below the colloidal supernatant by centrifuging them at 3,000 rpm for 10 minutes. Then, Using a lyophilizer, the resulting supernatant was freeze-dried (Selvamuthukumar et al., 2012). The development of the drug’s complex depends critically on this solid, freeze-dried form of NS, and the drug loading range (62.8 ± 0.22 to 73.1 ± 0.39) (Solunke et al., 2019) of crystalline NS is greater than those of paracrystalline NS. The drug loading occurs as a mechanical mixture in weak crystalline NS (Indira, 2012; Lala et al., 2011). The drugs that have been administered in NS formulation are listed in the table below. (Table 2).
In vitro drug release mechanism from NS and pharmacokinetics
The tiny vehicle’s encapsulated active ingredient travels freely out of the tiny vehicle due to the open nature of the nanosponge until equilibrium is reached. The equilibrium changes after local application, leading to the unsaturation of active ingredient-containing vehicles. As a result, until the vehicle is entirely dried up or absorbed, the flow of active drug contained in the vehicles begin traveling toward the stratum corneum of the skin. Over time, the NS kept on the stratum corneum’s surface, constantly releasing active ingredients to the skin, resulting in pseudo-zero-order kinetics (Lembo et al., 2018; Sadhasivam et al., 2020; Swaminathan et al., 2016).
 | Figure 9. Particle size and percentage released after 6 hours of lemongrass oil. [Click here to view] |
 | Figure 10. 3D response surface curve illustrating the effect of independent variables on yield (A), drug content (B and C) of hesperetin loaded colloidal NS. [Click here to view] |
Release kinetics
The in-vitro release tests for NS are performed using a rotating multi-compartment cell with a dialysis membrane. Drug-loaded NS complexes in distilled water are present in the donor phase. The receptor phase is then completely removed, diluted with water, and examined by a UV-visible spectrophotometer at predetermined intervals. Also, USP Apparatus II can be used in several cases depending on the formulation (Mathew et al., 2013). To further understand the mechanism of drug release from the NS, the release data was analyzed. NSs’ in vitro release data were fitted to models using zero-order, first-order, Higuchi, Korsmeyer-Peppas, Kopcha, and Makoid-Banakar coefficients (Asad et al., 2014; Fontana et al., 2019; Higuchi, 1963; Jyoti et al., 2016). Software like GraphPad Prism could be used for data analysis. The software determines the nonlinear function’s and the parameters for the nonlinear function that best matches the experimental data (Matencio et al., 2020).
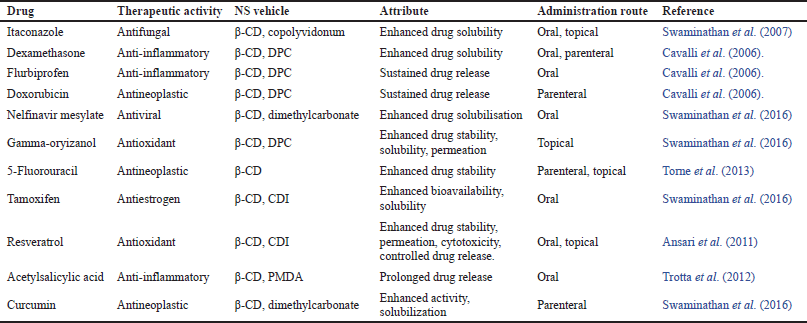 | Table 2. Drugs captured in NS. [Click here to view] |
TYPES OF NS
Titanium-based NS (Guo et al., 2008), silicon-based NS (Bryant, 2013), hypercross-linked polystyrene NS (Davankov et al., 1996), and CD-based NS (Swaminathan et al., 2010a, 2010b). CD-based NS are further classified as carbamate NS, carbonated NS, ester NS, and polyamidoamine NS (Madhuri et al., 2010; Shringirishi et al., 2015). Nonenzymatic hydrolysis seems to have a much lower impact on naturally occurring α-CD, β-CD, and γ-CD than it does on linear oligosaccharides. Moreover, human salivary and pancreatic amylases are unable to hydrolyze α-CD and γ-CD. Due to this property, CD drug conjugates function normally in the upper gastrointestinal tract until they reach the colon. Large amounts of macrobiotics, primarily bacteriaides, in the colon prevent CD from fermenting into tiny saccharides, resulting in fast drug release (Shahiwala, 2020).
Evaluation of NS
The characterization parameters for NS formulations are given in Table 3.
Solubility studies
The phase solubility method, proposed by Higuchi and Connors, was the method most frequently used to investigate inclusion complexation The impact of NS on the solubility of drugs was investigated in these studies. Complexation level is determined via phase solubility diagrams. An Erlenmeyer flask is used to determine the solubility studies in this method. For this, the flask containing drugs with an aqueous solution with varying concentrations of NS was agitated with a mechanical shaker at 37°C. When the suspension had attained a balanced state, a 3,000-dalton molecular filter was used to clarify it. The drug content in the solution was ascertained using high-performance liquid chromatography (HPLC) analysis (Shringirishi et al., 2014).
Efficiency of loading and entrapment
Before being analyzed with a UV spectrophotometer or HPLC procedures, a weighed quantity of loaded NS complexes must be dissolved in a suitable solvent, broken up using a sonicator, and then adequately diluted (Patel and Oswal, 2012; Shobhana and Suma, 2017). The following formula can be used to calculate the loading efficiency of NS.
ADC = Actual drug content,
LE = Loading Efficiency,
TDC = Theoretical drug content.
Production yield (PY)
The PY can be calculated by calculating the initial weight of the raw materials and the final weight of the NS (Patel and Oswal, 2012; Shobhana and Suma, 2017).
PMN = Practical mass of NS,
PY = Production yield,
TM = Theoretical mass (polymer + drug).
Microscopy studies
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are employed to examine the microscopic properties of the drug and drug/NS complex. Fresh ingredients and products from various crystallization phases combine to generate inclusion complexes, which may be seen under an electron microscope (Caldera, 2017; Challa et al., 2005; Singh et al., 2010; Swaminathan et al., 2010a, 2010b).
Polydispersity and particle size
Using a 90 Plus particle sizer and the MAS OPTION particle sizing program, one can use dynamic light scattering to calculate the particle size. The polydispersity index (PDI) and mean diameter may be computed using this data. The PDI measures variance or dispersion within the particle size distribution. A sample is considered monodisperse if the PDI value is lower, whereas a sample is considered polydisperse if the PDI value is higher and displays a wider range of particle sizes. The polydisperse index of dispersion is given in Table 4. To calculate PDI, use the following equation:
 | Table 3. Characterization parameters for NS formulations. [Click here to view] |
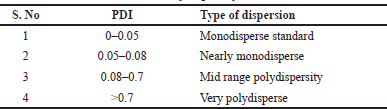 | Table 4. Polydispersity index. [Click here to view] |
Whereas on the particle size data sheet, (d) is the width of the distribution marked by SD, and (d Avg) is the average particle size denoted by MV (nm) (Schärtl, 2007).
The following methods can be used to measure particle size: SEM, TEM, Atomic Force Microscopy, and Freeze Fracture Electron Microscopy (Rao et al., 2012).
Zeta potential
Surface charge is measured by the zeta potential. An additional electrode in particle size measurement equipment can be used to determine it (Swaminathan et al., 2010a, 2010b). After being diluted with 0.1 mol/l KCl, NS samples were put in an electrophoretic cell with an electric field of roughly 15 V/cm for zeta potential analysis.
Thermo-analytical methods
The three most frequently employed techniques with thermograms are peak broadening, peak shifting, and the emergence and disappearance of certain peaks. Thermal degradation of the NS can be monitored with these thermo-analytical methods. Melting, evaporation, breakdown, oxidation, or polymorphic transition are some examples of this deterioration The complex formed is due to the modifications in the drug substance (Patel, 2014; Singh et al., 2010).
X-ray diffractometry structure analysis
The solid-state inclusion complex was detected by using the Powder X-ray diffractometry technique. Since a liquid drug molecule has no inherent diffraction pattern, it behaves differently from an un-complex NS in terms of its diffraction design pattern. A complex formation is indicated by a variation in the diffraction pattern. If drug compounds are in a solid state, a comparison study between the mechanical combination of the drug/polymer molecules and the assumed complex of the diffractogram must be done (Lee et al., 2011; Liang et al., 2012; Trotta et al., 2012). The diffraction pattern of a physical mixture is typically the result of such factors. A “new” solid phase is produced by distinct diffractograms if each complicated component displays a distinct diffraction pattern. Chemical decomposition and complex formation can be known using diffraction peaks obtained in a mixture of compounds (Shringirishi et al., 2014). The diffraction patterns will alter if the drug and the NS form a complex and change the drug’s crystalline nature. The complex formation of a compound indicates that the existing peaks will become sharper, a few new peaks will appear, and the exact peaks will move (Trotta et al., 2012).
Infra-red spectroscopy
With the aid of infrared spectroscopy, the interactions of NS with pharmaceutical compounds in the solid state are evaluated. When a complex forms, NSs’ peaks often vary relatively slightly. Peaks that could be attributed to the guest molecules’ involved portion are blatantly concealed by the peaks of the spectrum of the NS if the segment of the visitor molecules that was encapsulated in the complex was less than 25%. The technique is used less commonly to locate the inclusion complexes and does not provide as much clarification as other techniques. (Caldera, 2017; Shringirishi et al., 2014). Only certain medications with distinctive peaks, like carbonyl or sulfonyl groups, are eligible for infrared spectroscopy. Studies using infrared spectroscopy expression that hydrogen is an associate of multiple functional groups. This frequently encompasses the oscillation of the group essential to the design of the hydrogen bonds, causing the absorbance peaks to shift to lower frequencies, increase in strength, and broaden. The stretching vibration peaks near the origins of the hydroxyl group undergo the most significant alteration due to the hydrogen bond (Caldera, 2017).
Thin layer chromatography
The Rf values were identified using thin-layer chromatography in this NS complex drug molecule exposed the lower Rf value significantly, supporting the recognition of the complex between the NS and drug molecule (Singh et al., 2010).
Resilience tests
NS’s toughness (viscoelastic properties) can be changed to produce softer or harder beadlets depending on the needs of the final product. The release rate gradually slows down as crosslinking increases. Therefore, by considering the release pattern as a function of crosslinking with time, the resilience of sponges can be studied and enhanced as needed (Tiwari and Bhattacharya, 2022).
In vitro cytotoxicity studies on NS
Pushpalatha et al. (2019) studied curcumin NS and resveratrol NS and their combination were tested for cytotoxicity against Michigan Cancer Foundation-7 (MCF-7) cell lines. (3-(4,5 dimethyl thiazole-2yl)-2,5- diphenyl tetrazolium bromide) (MTT) assay was employed to assess cell viability. Compared to the single treatment of curcumin NS and resveratrol CD NS, In a concentration-dependent manner, the 3:1 mixture of curcumin NS and resveratrol CD NS significantly increased the cytotoxicity impact (p < 0.05). There was no cytotoxicity in blank NS. Improved chemical stability, better solubility, and tiny nanoscale diameter particle size may have aided in the better absorption of active drug molecules into the cells, which leads to increased cytotoxicity at low concentrations. Curcumin CD NS and resveratrol CD NS work together to boost anticancer activity (Pushpalatha et al., 2019).
Kumar et al. (2018) investigated the cytotoxicity of Bakuchi oil in CD-based NS against HaCaT cell lines using the MTT assay. These results showed that treating these cell lines with Bakuchi oil-loaded NS at 320 g/ml caused cytotoxicity with an IC50 of 191.4 g/ml and for blank plain Bakuchi oil with an IC50 of 172.3 g/ml. Although there is no noticeable difference between Bakuchi oil and Bakuchi oil NS in terms of cytotoxicity, this suggests that NS releases enough drugs to cause cell damage. As a result of the MTT experiment, the produced nanoformulation was found to be safer on human skin cells than bakuchi essential oil.
MTT assay was carried out for the determination of the cytotoxicity in the MCF-7 cell line of Paclitaxel NS’s complex. In all doses tested, the paclitaxel complex with NS inhibited cell growth at least 10% better than the free drug equivalent. There is strong evidence from cytotoxicity experiments on MCF-7 cell line cultures that NS may have a role in either blocking the P-glycoprotein and boosting the action of paclitaxel or interfering with cell permeability in some way. However, improving cell permeability appears less feasible, as NS were tested for cytotoxicity in MCF-7 cell line culture and shown to have little effect on cell toxicity (Ansari et al., 2011).
Allahyari et al. (2020) used the MTT test to investigate the in vitro cellular cytotoxicity of bortezomib CD NS and bortezomib free against MCF-7 cells. It may also be deduced from the MTT findings that a blank nanocarrier is non-toxic even at larger doses. As can be observed, Bortezomib exhibited a concentration and time-dependent impact. The persistent release characteristic of CD NS might explain these findings.
Torne et al. (2013) reported by MTT assay to assess the cytotoxicity of Tamoxifen CD-based NS and free Tamoxifen against MCF-7 (epithelial cell line isolated from the breast tissue cells). 96-well titration plates with the MCF-7 cell line were seeded and incubated for adhesion at 37°C in 5% CO2. About 20 l of the drug solution or formulation were added, with increasing Tamoxifen concentrations, a rapid decrease in cell growth was discovered. Tamoxifen NS complex inhibited cell growth better than the free drug.
Dhakar et al. (2019) investigated the percentage suppression of cell viability by resveratrol, and oxyresveratrol-loaded NS against DU-145 prostate cancer cells using the MTT assay. The toxicity of resveratrol and oxyresveratrol NS was greater than that of resveratrol and oxyresveratrol alone. At the highest concentrations, a significant difference (p < 0.05) in cytotoxicity was detected between resveratrol and oxyresveratrol compared with NS complex resveratrol and oxyresveratrol.
The MTT assay against breast cancer cell lines was used by Ahmed et al. (2013) to evaluate the anticancer effects of pure ribociclib and ribociclib NS against cell lines from human breast cancer (MCF-7 and MDA-MB-231). The MTT assay revealed that both the pure medication ribociclib and the tailored ribociclib NS reduced cell viability in a concentration-dependent manner. The ribociclib NS showed a significant reduction in cell viability compared to the pure medication ribociclib. Compared to free medication, the anticancer impact of ribociclib NS was 2.14-fold and 2.79-fold against MDAMB-231 and MCF-7 breast cancer lines, respectively. According to anticancer data, the ribociclib encapsulated in NS demonstrated potential cytotoxicity in cell line at lower doses than the free medication ribociclib. Generally, a high dose of drug with numerous regimens is required to achieve chemotherapeutic effects, which might result in severe or many side effects owing to anticancer drug cell toxicity. The new ribociclib NS with prolonged drug release might minimize both the dose and frequency of medication administration in the future (Ahmed et al., 2022).
Anwer et al. (2022) used the MTT assay to investigate the in-vitro cytotoxicity of pure Abemaciclib suspension and Abemaciclib NS against MCF-7 and MDA-MB-231 (human breast cancer cells). Against MCF-7 and MDA-MB-231 cancer cell lines, the MTT bioassay revealed a concentration-dependent decrease in cell viability for the pure medication and the improved Abemaciclib NS. When comparing to pure drug Abemaciclib and Abemaciclib NS complex, it showed a significant reduction in cell viability displayed action against MCF-7 cells are shown by MTT assay, Abemaciclib NS showed anticancer potential activity against the breast cancer cell lines, likely owing to the enhanced drug release and treat breast cancer as a powerful carrier than the pure Abemaciclib.
Khazaei Monfared et al. (2022) investigated the cytotoxicity of blank CD-based materials. In the two cell lines, NS and pyromellitic dianhydride (PMDA) NS were tested. NS did not cause cytotoxicity in tumor cells at high concentrations (250 g/ml) after 24 hours, demonstrating that some nanoparticles are harmless to tumor cells at specific doses. But tumor cells were cytotoxic to pure drug Nisin Z and also with Nizin Z loaded on both nanoparticles in a dose-dependent manner. The cytotoxicity of free Nisin against MCF-7 cells was not significant. Still, Nisin loaded on both NS significantly reduced cell viability in all different concentrations compared to Nisin (p < 0.0001). At the same time, there were no differences in cytotoxicity between Nisin encapsulated with PMDA NS and CD-NS. Free Nisin, also had enhanced effective cytotoxicity impact against colon-rectal cancer cells than breast cancer cells, which might be because of the changes in Nisin anticancer mechanism effect in these cells.
Toxicological studies
Toxicological testing is essential in determining the safety of medicine and its excipients, also optimizing the best dosage form for the individual. The crucial finding is that hydrolysis resistance and enzymatic degradation determine the fate of parent CDs in the gastrointestinal system. β-CDs show resilience to stomach fluid and pancreatic amylases, allowing them to reach the colon for complete hydrolysis. Clinical research participants and ileostomists participated in the study, and it was discovered that while β-CDs are entirely digested in the colonic microbiota, their breakdown in the alkaline pH of the intestine is insufficient. The enzymatic screening of β-CDs that is in the human colon can be carried out by glucose or maltodextrins. The LD50 of β-CD after oral treatment to rats was determined to be 18.8 g/kg (Irie and Uekama, 1997). The safety regarding NS has already been evaluated in vitro, and testing on many cell lines revealed no cytotoxicity or hemolytic activity. Additionally, the resistance of NSs to chemical degradation in vitro was assessed. When the NSs were subjected to 0.1 N hydrochloric acid, the NS structure began to deteriorate immediately, and only a small number of CD units were released. In contrast, when the NSs were exposed to a virtual environment 0.1N of NaOH, the stability was maintained and there were no visible effects on the NSs. However, it appears that utilizing PMDA as a crosslinker for NS preparation compromises structural integrity in simple solutions, even though the structure is preserved for 24 hours. The initial dosage for the LD50 test was 300 mg/kg, and the results showed that oral delivery had the least amount of toxicity. During the trial period, there were no fatality occurrences in the control or treatment groups at all accessible dosage levels.
The NS formulation was administered at a dose of 2,000 mg/kg per body weight, a low-toxicity dose based on recent research findings, and the dose did not suggest any negative impacts that had not been seen (Shende et al., 2015). Significant variations in enzymes like alkaline phosphatase, aspartate aminotransferase, and alanine transaminase are linked to liver damage. A change in cholesterol levels might indicate liver damage because cholesterol and protein are made in the liver. The principal source of cholesterol synthesis and removal, hepatic glycogen, is converted into free glucose by the liver to regulate blood glucose levels. Over the past 28 days, the liver has not changed, suggesting no severe damage has occurred. Changes in biochemical indicators like urea and creatinine are detected as kidney disease. The indicators above remained normal after 28 days of NS formulation treatment. Consequently, the administration of NS formulations did not cause renal or hepatic damage.
Factors influence NS
Types of polymer
The formulation and the performance of the NS structure can be influenced by the nature of the polymer used. The pore diameter size of NS must be in the appropriate particular size to provide accommodations for a drug molecule and for the complexation of the drug with the NS (Ajinkya et al., 2015).
Type of drugs
The nature of drug molecules must meet specific requirements to be complex with NS, including having a molecular weight range between 100 and 400, also having of about fewer than five condensed rings. The drug’s aqueous solubility nature should be less than 10 mg/ml, and should have the melting point of less than 250°C (Vyas et al., 2008).
Temperature
Temperature deviations can also affect the drug or NS complexation. As temperature rises, drug/NS contact forces, such as van der Waals and hydrophobic forces, may weaken, reducing the amount of the apparent stability constant complex (Challa et al., 2005).
Method of preparation technique
Loading the active drug molecule into the NS can disrupt the drug-NS complexation. Skill of a procedure depends on the drug molecule and nature of the polymer. The most active method for drug complexation is freeze drying (Challa et al., 2005).
Degree of substitution
The NS’s capability to form complexes may be exaggerated by the type, number, and position of substituents on the paternal molecule (Challa et al., 2005).
APPLICATION OF NS
NSs are being studied as potential medication delivery systems for treating infectious and cancerous disorders. NS can transport thousands of drug molecules despite being one-third the size of red blood cells. They can hide within the immune system, which uses immune cells to challenge and get rid of foreign substances from the body. It is impossible to distinguish membrane-coated particles from moving red blood cells. Furthermore, white blood cells or leukocyte membrane-shielded particles in circulation withstand macrophage attacks (Patra et al., 2018).
Pharmacokinetic difficulties, low water solubility, and inadequate bioavailability are three main challenges with freshly created chemical entities. When employing normal medicine dose forms, these cause problems. Due to their special ability to entrap both hydrophilic and hydrophobic drugs and release them in a strictly controlled manner, NS can solve these challenges. NS technology in different drug delivery methods is actively being investigated. Potential candidates include antineoplastic drugs, proteins and peptides, volatile oils, and genetic materials. These tiny NS travels throughout the entire body until they reach the appropriate point, at which the spot they bind to the targeted surface and slowly release the drugs in a controlled and sustained manner. The liver, spleen, and lungs are potential targeted sites for this type of drug administration (Vega-Vasquez et al., 2020).
NS is versatile and biocompatible, with several pharmaceutical industry applications. It serves as an excipient in the fabrication of topical dosage forms, suspensions, solid dispersions, pellets, granules, tablets, and capsules. (Indira, 2012). The drugs which are captured in NS are shown in (Table 2). It can serve as a multifunctional carrier for better product presentation, elegance, extended drug release, and better product thermal, physical, and chemical stability. The following use of NS exemplifies the versatility of NS.
Sustained delivery system
Crosslinking β-CD with PMDA, followed by the formation of the insulin NS, was a top-down approach to its formation. The in-vitro release of insulin was minimal at stomach pH below (2%) but maintained at intestinal pH, demonstrating the NS’s pH sensitivity (Appleton et al., 2020). tailor-made active release. As an illustration, the steady release of glipizide from NS (99.71%) after 12 hours (Arvapally et al., 2017; Panda et al., 2015).
The antiviral drug acyclovir is frequently prescribed for herpes simplex virus infections (O’Brien and Campoli-Richards, 2007). Acyclovir can deliver the right concentrations of the drug to the target areas through parenteral or oral administration. Because of acyclovir’s slow and incomplete absorption, the bioavailability is low when it is absorbed in the gastrointestinal tract. So the sustained release is preferred in the form of NS, signifying the hosting of acyclovir within the pores of nanostructures. Initially, the bursting effect was not detected for the formulation, proving that the drug was very strongly adsorbed onto the NS surfaces (Lembo et al., 2013).
Solubility enhancement
An antifungal drug like itraconazole that is classified into a BCS Class II drug with a dissolving rate constrained to poor bioavailability. NS formulation of certain drug increases the solubility over 27-fold. Copolyvidonum was incorporated into the NS’s formulation as an adjunct, and this increased 55-fold. (Swaminathan et al., 2010a, 2010b). By effectively hiding the hydrophobic groups, enhancing the drug’s wetting properties, and also by reducing its crystallinity, the drug’s solubility was improved. (Swaminathan et al., 2007). The nature of NS was to include either lipophilic drugs (dexamethasone or flurbiprofen) or hydrophilic drugs (doxorubicin) and has good solubilization capacity. They significantly enhanced the drugs’ solubility and dissolution rate (Cavalli et al., 2006).
Drug delivery
The spherical shape of NS can be produced in a variety of dosage forms, including topical, parenteral, aerosol, tablets, and capsules. NS are nanometric virus particles (Selvamuthukumar et al., 2012). A Biopharmaceutics Classification System (BCS) Class II antihypertensive medication with a restricted bioavailability dissolution rate is telmisartan (TEL). By using carbonate bonds to cross-link β-CD, TEL β-CD NSs were created. The NS and TEL were integrated. Whereas β-CD NS complexed with TEL was compared to blank TEL, NS complexes of TEL in terms of its total solubility and in vitro dissolution experiments. By using carbonate bonds to cross-link β-CD, TEL β-CD NS were created. The NS and TEL were integrated. The β-CD complex of TEL was compared to plain TEL and NS complexes of TEL in terms of its total solubility and in vitro dissolution experiments. It was reported that the solubility of TEL in the drug-complex was enhanced by 8.53 times better in distilled water. Additionally, it has improved 4.66-fold better in phosphate buffer pH 6.8 and by 3.35-fold in 1 mol HCl. The most potent solubility and in vitro drug release are shown by TEL-NS with NaHCO3. (Rao et al., 2013).
Paclitaxel is an anticancer drug that has poor water solubility. In order to boost the solubility of paclitaxel, the traditional formulation in cremophor EL was replaced with the technique of β-CD-based NSs since cremophor reduces paclitaxel’s ability to penetrate tissue. NS highly enhanced the pharmacological effect of the drug paclitaxel, not only it increase the cytotoxicity greatly, but also the intracellular concentration of paclitaxel is considerably enhanced when associated with plain paclitaxel after 72 hours of incubation (Mognetti et al., 2012). Natural α-CD, β-CD, and γ-CD are unaffected by non-enzymatic hydrolysis more than the linear oligosaccharides. Human salivary and pancreatic amylases do not hydrolyze the α-CD and γ-CD. Due to this characteristic, CD drug conjugates behave the same way in the upper gastrointestinal tract as they do in the colon. Large numbers of microbes, particularly bacteria, break CD down into little saccharides through fermentation, resulting in fast drug release (Swaminathan et al., 2013). The antifungal medication econazole nitrate, which comes in various dosage forms such as cream, ointment, lotion, and solution form, is applied topically to treat the symptoms of skin diseases like superficial candidiasis, dermatophytosis, and other skin infections. Econazole nitrate when applied to the skin, adsorption is minimal, hence an active substance in large concentration must be added for therapy to be effective. In order to create local depots for sustained drug release, econazole nitrate NS was created using the emulsion solvent diffusion technique and loaded onto NS in hydrogel (Sharma et al., 2011). β-CD-based NS is an efficient nanocarrier for the delivery of tamoxifen in the treatment of cancer (Torne et al., 2013).
Protein delivery
The effective development of medicines, especially macromolecular ones like proteins, depends on long-term stability (Klibanov and Jennifer, 2004). However, during lyophilization, proteins can denature irreversibly (or even permanently) and take on conformations that are significantly different from their previous ones. The integrity of the original protein structure throughout the preparation process and enduring storage is thus a significant challenge in the creation of protein formulations. (Schwartz et al., 2006).
The formation of the swellable CD-based poly(amidoamine) NS was achieved by long-term cross-linking of β-CD with either 2,2-bis-acrylamidoacetic acid or a short polyamido-amine chain produced from 2,2-bisacrylamidoacetic acid and 2-methyl piperazine, respectively. The developed β-CD poly(amidoamine) NS was shown to be stable at 300°C and to have a good protein complexation capacity by Swaminathan et al. (2010b).
Enzyme immobilization
For lipases, the issue of enzyme immobilization is particularly important because it increases their stability and controls aspects like enantio selectivity and reaction speeds. (Mateo et al., 2007). Consequently, a new solid backing was in demand for a suitable family of enzymes to constantly grow. The high catalytic performance of Pseudomonas fluorescent lipase adsorbed on a novel type of CD-based nanosponge (Boscolo et al., 2010).
Gas delivery vehicle
Gases are crucial to the diagnosis and treatment of medical conditions. Numerous illnesses, including cancer and inflammation, are linked to the lack of an adequate oxygen source. This condition is said to be hypoxia. Since oxygen delivery methods for topical applications can store and release oxygen slowly for a prolonged period of time, delivering oxygen in the appropriate form and dose in clinical practice is a challenging approach. In order to provide oxygen topically, Cavalli et al. (2010) created formulations for NS that have the capacity to store and release oxygen gradually over time.
Light or deterioration protection agent
A ferulic acid ester combination known as gamma-oryzanol has recently gained a lot of attention due to its potential as a natural antioxidant. It is typically used to stabilize food and pharmaceutical raw materials as well as sunscreen in the cosmetics industry. Because of its high instability and photodegradation, its applicability is restricted. Gamma-oryzanol NS has a superb defence against photodegradation. The gamma-oryzanol-loaded NS was utilized to create a gel and an O/W emulsion (Sapino et al., 2013).
SARS-CoV-2 inhibition
The NS permit using biocompatible nanomaterial to treat and prevent various severe diseases like SARS COVID-19, and the Zika virus (Singh and Chauhan, 2021; Yang et al., 2021). Severe acute respiratory syndrome is caused by the spike glycoprotein which is an ‘S’ protein (SARS-CoV-2) virus that mediates cellular interaction and entrance. It interacts with human angiotensin-converting enzyme two receptors as well as glycosaminoglycans like heparin. ACE2-containing NS were made using polymeric cores encased in plasma membranes which are made from human lung epithelial type II cells or macrophages (Xu et al., 2022). In order to trap and neutralize SARS-CoV-2 through natural cellular receptors, cell membrane-coated nanoparticles (cellular NS) mimic the host cells. This results in a comprehensive antiviral approach (Ai et al., 2021).
Diagnostic tool
Numerous diagnostic items are produced, often using β-CD. CD NSs are the best option because of their excellent biocompatibility, prolonged blood circulation, homogenous uniform size distribution for permeability, and simplicity of access to the target (Gidwani and Vyas, 2015).
Cosmetics
In the cosmetics sector, NSs are used in a variety of ways. NSs offer a fair level of protection for photosensitive cosmetic components. It can slow down and extend the time that volatile oils are released, dude. Sweating also produces a foul body odor that can be absorbed. It may gently remove volatile compounds, giving oral cosmetics a long-lasting fresh sensation. It may also be used to provide a lasting effect in products like rouge and lipsticks (Trotta, 2011)
PATENTS
New patents in NSs were recently submitted, authorized, and used to improve the making process more efficiently. The patents cover toxin absorption agents, growth preservation, enzyme release, and biocatalyst investigations, demonstrating promise as anticancer agents. As stated below in (Table 5), the authorities have also granted patents that will assist in shifting demand to NSs as a breakthrough pharmaceutical delivery technology (Tiwari and Bhattacharya, 2022).
MARKETED FORMULATIONS OF NS
There are several NS-based formulations on the market and some drugs like prostavastin, brexin, glymesason, mena-gargle, and other formulations are in clinical studies (Pawar and Shende, 2020b). Some of the marketed formulations of NS are listed in Table 6.
TRENDS FOR THE FUTURE
The development of nanoscale NS has sparked a revolution in medical research. Using technology to reach the nanoscale has started a trend in medical research. Because of the enhanced therapeutic results when a targeted and drug release-controlled mechanism was implemented, the drug toxicity is decreased. The importance of NS in medicine and nanotechnology development cannot be overstated. It might be used as a basic water filter in the future. Lowering production costs is a crucial concern, which calls for creating innovative polymers, cross-linkers, and new manufacturing techniques.
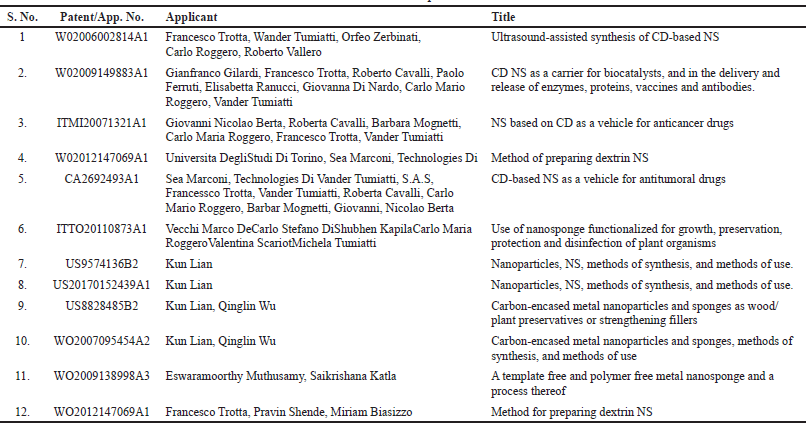 | Table 5. Recent patents. [Click here to view] |
 | Table 6. List of some marketed formulations of NS. [Click here to view] |
They play a crucial role in downstream production due to their unique characteristics, necessitating substantial investigation. Drug release is influenced by their features such as particle diameter, synthesis, crystallinity, porosity, and crosslinking intensity of the particles. Up to this point, ultrasound-assisted method of synthesis and the traditional methodology have been the preparation methods that have been most frequently described. However, novel techniques like solvent evaporation and bubble electro-spinning are additionally being updated and developed. The new trends supporting mass production quickly include higher yields, cost-effective manufacturing, and repeatability. The recent trends that will help mass production quickly include higher yields, cost-effective manufacturing, and repeatability (Ahmed et al., 2013).
CONCLUSION
NS are an effective carrier for the delivery system, such as target drug delivery, sustained delivery, and improved effectiveness of several pharmacological medicines as a treatment. NS are flexible drug carrier structures by making complexes of inclusion and non-inclusion as they carry hydrophilic (gentamicin) and hydrophobic (dexamethasone) drugs. NS can transport drugs via a number of routes, like oral, topical, rectal, and parenteral, in an expectable manner to the marked site. β-CD NS has excellent applications in different specific drug delivery systems. Moreover, it increases the solubility of biopharmaceutical class II drugs. This review discusses NS, including their synthesis methods, numerous varieties, characterization methods, formulation creation, and applications such as in vitro cytotoxicity studies, patents filed in this field, and some commercial formulations. Together with these recent approvals, the area of NS drug delivery continues to make breakthroughs that improve human health. A continuous effort to discover new drugs through the NS has been made to overcome that improved delivery and increase the potential therapeutic effects of drugs. The pharmaceutical companies will offer outstanding support as the safety and efficacy of drugs administered by NS can be demonstrated. Several assessment processes are used to evaluate the product’s structural and chemical integrity. They will benefit several disciplines in the future, and their breadth of use will extend as this field’s study progresses. As a result, scientists are concentrating their efforts on a novel component of medication development.
ACKNOWLEDGMENTS
The authors thank the authorities of Annamalai University, Chidambaram, Tamilnadu, for funding the research and providing the other resources such as the internet, a library, and further technical assistance necessary to write this review article. V. Mahalekshmi, a research scholar currently pursuing her PhD under the guidance and supervision of Dr V. Parthasarathy, Professor, Department of Pharmacy, Annamalai University, AnnamalaiNagar, Tamil Nadu, India, wrote the current review article. Additional support is from S.A. Raja Pharmacy College, Tirunelveli, Tamil Nadu.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ahmed MM, Fatima F, Alali A, Kalam MA, Alhazzani K, Bhatia S, Alshehri S, Ghoneim MM. Ribociclib-loaded ethylcellulose-based nanosponges: formulation, physicochemical characterization, and cytotoxic potential against breast cancer. Adsorp Sci Technol, 2022. CrossRef
Ahmed RZ, Patil G, Zaheer Z. Nanosponges–a completely new nano-horizon: pharmaceutical applications and recent advances. Drug Dev Ind Pharm, 2013; 39(9):1263–72. CrossRef
Ai X, Wang D, Honko A, Duan Y, Gavrish I, Fang RH, Griffiths A, Gao W, Zhang L. Surface glycan modification of cellular nanosponges to promote SARS-CoV-2 inhibition. J Am Chem Soc, 2021; 143(42):17615–21. CrossRef
Ajinkya K, Kendre P, Pande V. Scaffold based drug delivery system: a special emphasison nanosponges. Int J Pharm Drug Anal, 2015; 3(4):98–104.
Aldawsari HM, Badr-Eldin SM, Labib GS, El-Kamel AH. Design and formulation of a topical hydrogel integrating lemongrass-loaded nanosponges with an enhanced antifungal effect: in vitro/in vivo evaluation. Int J Nanomed, 2015; 10:893. CrossRef
Allahyari S, Valizadeh H, Roshangar L, Mahmoudian M, Trotta F, Caldera F, Jelvehgari M, Zakeri-Milani P. Preparation and characterization of cyclodextrin nanosponges for bortezomib delivery. Expert Opin Drug Deliv, 2020; 17(12):1807–16. CrossRef
Allen TM. Toxicity of drug carriers to the mononuclear phagocyte system. Adv Drug Deliv Rev, 1988; 2(1):55–67. CrossRef
Alongi J, Merima P, Alberto F, Francesco T. Novel flame retardants containing cyclodextrin nanosponges and phosphorus compounds to enhance EVA combustion properties. Polym Degrad Stab, 2010; 95(10):2093–100. CrossRef
Ansari KA, Vavia PR, Trotta F, Cavalli R. Cyclodextrin-based nanosponges for delivery of resveratrol: in vitro characterisation, stability, cytotoxicity and permeation study. AAPS PharmSciTech, 2011; 12(1):279–86. CrossRef
Anwer MK, Fatima F, Ahmed MM, Aldawsari MF, Alali AS, Kalam MA, Alshamsan A, Alkholief M, Malik A, Az A, Al-Shdefat R. Abemaciclib-loaded ethylcellulose based nanosponges for sustained cytotoxicity against MCF-7 and MDA-MB-231 human breast cancer cells lines. Saudi Pharm J, 2022; 30(6):726–34. CrossRef
Appleton SL, Tannous M, Argenziano M, Muntoni E, Rosa AC, Rossi D, Caldera F, Scomparin A, Trotta F, Cavalli R. Nanosponges as protein delivery systems: insulin, a case study. Int J Pharm, 2020; 590:119888. CrossRef
Arvapally S, Harini M, Harshitha G, Arun Kumar A. Formulation and in vitro evaluation of glipizide nanosponges. Am J Pharmtech Res, 2017; 7:341–61.
Asad M, Bashir S, Mahmood T, Nazir I, Imran M, Karim S. Fabrication and characterization of gliclazide loaded microcapsules. Braz Arch Biol Technol, 2014; 57:874–81. CrossRef
Bolmal UB. Recent advances in nanosponges as drug delivery system. Int J Pharm Sci Nanotechnol, 2013; 6(1):1934–44. CrossRef
Boscolo B, Trotta F, Ghibaudi E. High catalytic performances of Pseudomonas Fluorescens lipase adsorbed on a new type of cyclodextrin-based nanosponges. J Mol Catal B Enzym, 2010; 62(2):155–61. CrossRef
Bryant JL. Silicon nanosponge particle (12) United States patent. United States Patent 2013; 2(12).
Caldera F. Evolution of cyclodextrin nanosponges. Int J Pharm, 2017; 531(2):470–79. CrossRef
Cavalli R, Akhter AK, Bisazza A, Giustetto P, Trotta F, Vavia P. Nanosponge formulations as oxygen delivery systems. Int J Pharm, 2010; 402(1–2):254–57. CrossRef
Cavalli R, Francesco T, Wander T. Cyclodextrin-based nanosponges for drug delivery. J Incl Phenom Macrocycl Chem, 2006; 56(1–2):209–13. CrossRef
Challa R, Alka A, Javed A, Khar RK. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech, 2005; 6(2):329–57. CrossRef
Dai M, Zheng X, Xu X, Kong X, Li X, Guo G, Luo F, Zhao X, Wei YQ, Qian Z. Chitosan-alginate sponge: preparation and application in curcumin delivery for dermal wound healing in rat. J Biomed Biotechnol, 2009; 2009:595126; doi:10.1155/2009/595126. CrossRef
Davankov VA, Ilyin MM, Tsyurupa MP, Timofeeva GI, Dubrovina LV. From a dissolved polystyrene coil to an intramolecularly-hyper-cross-linked Nanosponge. Macromolecules, 1996; 29(26):8398–403. CrossRef
David FS. Nanosponge drug delivery system more effective than direct injection. Phys Org, 2010:2–4.
Dhakar NK, Caldera F, Bessone F, Cecone C, Pedrazzo AR, Cavalli R, Dianzani C, Trotta F. Evaluation of solubility enhancement, antioxidant activity, and cytotoxicity studies of kynurenic acid loaded cyclodextrin nanosponge. Carbohydr Polym, 2019; 224:115168. CrossRef
Dhavala PB, Tenneti VSVK. An interesting nanosponges as a nanocarrier for novel drug delivery?: a review. Int J Pharm Med Res, 2017; (5):1–7.
Eldose A, Twinkle P, Honey S, Twinkle Z, Jain H, Umesh U. Nanosponge: a novel nano drug carrier. J Adv Res Pharm Biol Sci, 2015; 1(7):01–6. CrossRef
Fontana RM, Milano N, Barbara L, Di Vincenzo A, Gallo G, Meo PL. Cyclodextrin-calixarene nanosponges as potential platforms for pH-dependent delivery of tetracycline. ChemistrySelect, 2019; 4(33):9743–7. CrossRef
Gidwani B, Vyas A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. BioMed Res Int, 2015; 2015:1–15. CrossRef
Guo L, Gao G, Liu X, Liu F. Preparation and characterization of TiO2 nanosponge. Mater Chem Phys, 2008; 111(2–3):322–25. CrossRef
Hariri G. Development, optimization and evaluation of tumor-specific nanosponge drug delivery systems as chemotherapeutics. Doctoral dissertation, Graduate School of Vanderbilt University, Nashville, TN, 2014.
Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci, 1963; 52(12):1145–9. CrossRef
Indira B. Nanosponges?: a new era in drug delivery?: review. J Pharm Res, 2012; 5(11):5293–96.
Irie T, Uekama K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J Pharm Sci, 1997; 86(2):147–62. CrossRef
Jilsha G, Vidya V. Nanosponges: a novel approach of drug delivery system. Int J Pharm Sci Rev Res, 2013; 19(2):119–23.
Jyoti P, Tulsi B, Popin K, Chetna B. An innovative advancement for targeted drug delivery: nanosponges. Indo Glob J Pharm Sci, 2016; 6(2):59–64. CrossRef
Kaulitzki S. Nanosponges. What are nanosponges?? Nanosponge structure. News-Medical.Net, vol. 8(5). pp 336–40, 2013.
Khazaei Monfared Y, Mahmoudian M, Cecone C, Caldera F, Zakeri-Milani P, Matencio A, Trotta F. Stabilization and anticancer enhancing activity of the peptide nisin by cyclodextrin-based nanosponges against colon and breast cancer cells. Polymers (Basel), 2022; 14(3):594. CrossRef
Klibanov AM, Jennifer AS. On the relationship between conformations and stability in solid pharmaceutical protein formulations. Biotechnol Lett, 2004; 26(14):1103–6. CrossRef
Kumar S, Trotta F, Rao R. Encapsulation of babchi oil in cyclodextrin-based nanosponges: physicochemical characterization, photodegradation, and in vitro cytotoxicity studies. Pharmaceutics, 2018; 10(4):169. CrossRef
Lala R, Thorat A, Gargote CS. Current trends in β-cyclodextrin based drug delivery systems. Int J Res Ayur Pharm, 2011; 2:1520–6.
Lee CL, Chao YJ, Chen CH, Chiou HP, Syu CC. Graphite-nanofiber-supported porous Pt-Ag nanosponges: synthesis and oxygen reduction electrocatalysis. Int J Hydrog Energy, 2011; 36(23):15045–51. CrossRef
Lembo D, Swaminathan S, Donalisio M, Civra A, Pastero L, Aquilano D, Vavia P, Trotta F, Cavalli R. Encapsulation of acyclovir in new carboxylated cyclodextrin-based nanosponges improves the agent’s antiviral efficacy. Int J Pharm, 2013; 443(1–2):262–72. CrossRef
Lembo D, Trotta F, Cavalli R. Cyclodextrin-based nanosponges as vehicles for antiviral drugs: challenges and perspectives. Nanomedicine, 2018; 13(5):477–80. CrossRef
Liang W, Yang C, Nishijima M, Fukuhara G, Mori T, Mele A, Castiglione F, Caldera F, Trotta F, Inoue Y. Cyclodextrin nanosponge-sensitized enantio differentiating photoisomerization of cyclooctene and 1,3-cyclooctadiene. Beilstein J Org Chem, 2012; 8:1305–11. CrossRef
Madhuri S, Sunil KP, Alok M, Shashi A, Poonam Y, Amita V. Nanospheres: a novel approach for targeted drug delivery system. Int J Pharm Sci Rev Res, 2010; 5(3):84–8.
Matencio A, Dhakar NK, Bessone F, Musso G, Cavalli R, Dianzani C, García-Carmona F, López-Nicolás JM, Trotta F. Study of oxyresveratrol complexes with insoluble cyclodextrin based nanosponges: developing a novel way to obtain their complexation constants and application in an anticancer study. Carbohydr Polym, 2020; 231:115763. CrossRef
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol, 2007; 40(6):1451–63. CrossRef
Mathew F, Nair SS, Nair KG, Soman A, Alias M, Joseph J, Varghese N. A review on targeted drug delivery through nanosponge. Int J Univers Pharm Bio Sci, 2013; (2):285–97.
Mognetti B, Barberis A, Marino S, Berta G, De Francia S, Trotta F, Cavalli R. In vitro enhancement of anticancer activity of paclitaxel by a cremophor free cyclodextrin-based nanosponge formulation. J Incl Phenom Macrocycl Chem, 2012; 74(1–4):201–10. CrossRef
Naga SJ, Nissankararao S, Bhimavarapu R, Sravanthi SL, Vinusha K. Nanosponges: a versatile drug delivery system. Int J Pharm Life Sci, 2013; 4(8):2920–5.
O’Brien JJ, Campoli-Richards DM. Acyclovir an updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drug Eval, 2007; 309(37):1–4. CrossRef
Osmani AM, R Bhosale R, Hani U, Vaghela R, Kulkarni PK. Cyclodextrin based nanosponges: impending carters in drug delivery and nanotherapeutics. Curr Drug Ther, 2015; 10(1):3–19. CrossRef
Pandya KD, Shah NV, Gohil DY, Seth AK, Aundhia CJ, Patel SS. Development of risedronate sodium-loaded nanosponges by experimental design: optimization and in vitro characterization. Indian J Pharm Sci, 2019; 81(2):309–16. CrossRef
Panda S, Vijayalakshmi SV, Pattnaik S, Swain RP. Nanosponges: a novel carrier for targeted drug delivery. Int J PharmTech Res, 2015; 8(7):213–24.
Patel B. An assessment on preparations, characterization, and poles apart appliances of nanosponge. Int J PharmTech Res, 2014; 6(7):2092–101.
Patel EK, Oswal RJ. Nanosponge and microsponges: a novel drug delivery system. Int J Res Pharm Chem, 2012; 2(2):237–44.
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol, 2018; 16(1):1–33. CrossRef
Pawar S, Shende P. Dual drug delivery of cyclodextrin cross-linked artemether and lumefantrine nanosponges for synergistic action using 23 full factorial designs. Colloids Surf A Physicochem Eng Asp, 2020a; 602:125049. CrossRef
Pawar S, Shende P. A comprehensive patent review on β-cyclodextrin cross-linked nanosponges for multiple applications. Recent Pat Nanotechnol, 2020b; 14(1):75–89. CrossRef
Prabhu PP, Mehta CH, Nayak UY. Nanosponges-revolutionary approach: a review. Res J Pharm Technol, 2020; 13(7):3536–44. CrossRef
Pushpalatha R, Selvamuthukumar S, Kilimozhi D. Cyclodextrin nanosponge based hydrogel for the transdermal co-delivery of curcumin and resveratrol: development, optimization, in vitro and ex vivo evaluation. J Drug Deliv Sci Technol, 2019; 52:55–64. CrossRef
Rao M, Bajaj A, Khole I, Munjapar G, Trott F. In vitro and in vivo evaluation of β-cyclodextrin-based nanosponges of telmisartan. J Incl Phenom Macrocycl Chem, 2013; 77(1–4):135–45. CrossRef
Rao MR, Bajaj AN, Pardeshi AA, Aghav SS. Investigation of nanoporous colloidal carrier for solubility enhancement of cefpodoxime proxetil. J Pharm Res, 2012; 5(5):2496–9.
Rao MR, Bhingole RC. Nanosponge-based pediatric-controlled release dry suspension of gabapentin for reconstitution. Drug Dev Ind Pharm, 2015; 41(12):2029–36. CrossRef
Raval N, Kalyane D, Maheshwari R, Tekade RK. Copolymers and block copolymers in drug delivery and therapy. In: Tekade RK (ed.). Basic fundamentals of drug delivery, Academic Press, Cambridge, MA, pp 173–201, 2019. CrossRef
Rodrigues K, Nadaf S, Rarokar N, Gurav N, Jagtap P, Mali P, Ayyanar M, Kalaskar M, Gurav S. QBD approach for the development of hesperetin loaded colloidal nanosponges for sustained delivery: in-vitro, ex-vivo, and in-vivo assessment. OpenNano, 2022; 7:100045. CrossRef
Rogoši? M, Mencer HJ, Gomzi Z. Polydispersity index and molecular weight distributions of polymers. Eur Polym J, 1996; 32(11):1337–44. CrossRef
Sadhasivam J, Sugumaran A, Narayanaswamy D. Nano sponges: a potential drug delivery approach. Res J Pharm Technol, 2020; 13(7):3442–8. CrossRef
Saokham P, Muankaew C, Jansook P, Loftsson T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules, 2018; 23(5):1161 CrossRef
Sapino S, Carlotti ME, Cavalli R, Ugazio E, Berlier G, Gastaldi L, Morel S. Photochemical and antioxidant properties of gamma-oryzanol in beta cyclodextrin-based nanosponges. J Incl Phenom Macrocycl Chem, 2013; 75(1–2):69–76. CrossRef
Schärtl W. Light scattering from polymer solutions and nanoparticle dispersions. Springer Science & Business Media, Berlin, Germany, 2007.
Schwartz D, Susan S, Wolfgang F. Integrity and stability studies of precipitated RhBMP-2 microparticles with a focus on ATR-FTIR measurements. Eur J Pharm Biopharm, 2006; 63(3):241–48. CrossRef
Selvamuthukumar S, Anandam S, Krishnamoorthy K, Rajappan M. Nanosponges: a novel class of drug delivery system-review. J Pharm Pharm Sci, 2012; 15(1):103–11. CrossRef
Setijadi E, Tao L, Liu J, Jia Z, Boyer C, Davis TP. Biodegradable star polymers functionalized with β-cyclodextrin inclusion complexes. Biomacromolecules, 2009; 10(9):2699–707. CrossRef
Shahiwala A. Cyclodextrin conjugates for colon drug delivery. J Drug Deliv Sci Technol, 2020; 55(2019):101448. CrossRef
Sharma, Renuka, Roderick B, Walker, Kamla, Pathak. Evaluation of the kinetics and mechanism of drug release from econazole nitrate nanosponge loaded carbapol hydrogel. Indian J Pharm Educ Res, 2011; 45(1):25–31.
Shende P, Kulkarni YA, Gaud RS, Deshmukh K, Cavalli R, Trotta F, Caldera F. Acute and repeated dose toxicity studies of different β-cyclodextrin-based nanosponge formulations. J Pharm Sci, 2015; 104(5):1856–63. CrossRef
Shende PK, Trotta F, Gaud RS, Deshmukh K, Cavalli R, Biasizzo M. Influence of different techniques on formulation and comparative characterization of inclusion complexes of ASA with β-cyclodextrin and inclusion complexes of ASA with PMDA cross-linked β-cyclodextrin nanosponges. J Incl Phenom Macrocycl Chem, 2012; 74(1–4):447–54. CrossRef
Sherje AP, Dravyakar BR, Kadam D, Jadhav M. Cyclodextrin-based nanosponges: a critical review. Carbohydr Polym, 2017; 173:37–49. CrossRef
Shivani S, Kranthi KP. Nanosponges -novel emerging drug delivery system: a review. Int J Pharm Sci Res, 2015; 6(2):529–40.
Shobhana N, Suma R. Nanosponges: a boon to field of pharmacy. Indo Am J Pharm Res, 2017; 7(02):7780–8.
Shringirishi M, Prajapati SK, Mahor A, Alok S, Yadav P, Verma A. Nanosponges: a potential nanocarrier for novel drug delivery-a review. Asian Pac J Trop Dis, 2014; 4(S2):S519–26. CrossRef
Shringirishi M, Prajapati SK, Mahor A, Alok S, Yadav P, Verma A. Nanosponges?: a potential nanocarrier for novel drug delivery-a review. Asian Pac J Trop Dis, 2015; 4(S2):S519–26. CrossRef
Singh A, Chauhan CS. Nanosponges: blooming ndds in the future perspective. Int J Pharm Sci Rev Res, 2021; 70(2):213–6. CrossRef
Singh S, Monika K. Nanosponges as emerging carriers for drug delivery. Sys Rev Pharm, 2022; 13(1):55–62.
Singh R, Nitin B, Jyotsana M, Hiremath SN. Characterization of cyclodextrin inclusion complexes—a review. J Pharm Sci Technol, 2010; 2(3):171–83.
Singireddy A, Pedireddi SR, Nimmagadda S, Subramanian S. Beneficial effects of microwave assisted heating versus conventional heating in synthesis of cyclodextrin based nanosponges. Mater Today Proc, 2016; 3(10):3951–9. CrossRef
Singireddy A, Subramanian S. Cyclodextrin nanosponges to enhance the dissolution profile of quercetin by inclusion complex formation. Part Sci Technol, 2016; 34(3):341–6. CrossRef
Solunke RS, Borge UR, Murthy K, Deshmukh MT, Shete RV. Formulation and evaluation of gliclazide nanosponges. Int J Appl Pharm, 2019; 11(6):181–9. CrossRef
Swaminathan S, Cavalli R, Trotta F, Ferruti P, Ranucci E, Gerges I, Vavia PR. In vitro release modulation and conformational stabilization of a model protein using swellable polyamidoamine nanosponges of β-cyclodextrin. J Incl Phenom Macrocycl Chem, 2010a; 68(1):183–91. CrossRef
Swaminathan S, Cavalli R, Trotta F. Cyclodextrin-based nanosponges: a versatile platform for cancer nanotherapeutics development. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2016; 8(4):579–601. CrossRef
Swaminathan S, Francesco T, Satyen T. Formulation of betacyclodextrin based nanosponges of itraconazole. J Incl Phenom Macrocycl Chem, 2007; (57):89–94. CrossRef
Swaminathan S, Pastero L, Serpe L, Trotta F, Vavia P, Aquilano D, Cavalli R. Cyclodextrin-based nanosponges encapsulating camptothecin: physicochemical characterization, stability and cytotoxicity. Eur J Pharm Biopharm, 2010b; 74(2):193–201. CrossRef
Swaminathan S, Vavia PR, Trotta F, Cavalli R, Tumbiolo S, Bertinetti LC, Salvatore. Structural evidence of differential forms of nanosponges of beta-cyclodextrin and its effect on solubilization of a model drug. J Incl Phenom Macrocycl Chem, 2013; 76(1–2):201–11. CrossRef
Taka AL, Pillay K, Mbianda XY. Nanosponge cyclodextrin polyurethanes and their modification with nanomaterials for the removal of pollutants from waste water: a review. Carbohydr Polym, 2017; 159:94–107. CrossRef
Tejashri G, Bajaj A, Jain D. Cyclodextrin based nanosponges for pharmaceutical use: a review. Acta Pharm, 2013; 63(3):335–58. CrossRef
Tiwari K, Bhattacharya S. The ascension of nanosponges as a drug delivery carrier: preparation, characterization, and applications. J Mater Sci Mater Med, 2022; 33(3):1–21. CrossRef
Torne SJ, Ansari KA, Vavia PR, Trotta F, Cavalli R. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded nanosponges. Drug Deliv, 2010; 17(6):419–25. CrossRef
Torne S, Darandale S, Vavia P, Trotta F, Cavalli R. Cyclodextrin-based nanosponges: effective nanocarrier for tamoxifen delivery. Pharm Dev Technol, 2013; 18(3):619–25. CrossRef
Trotta F. Cyclodextrin nanosponges and their applications. In: Bilensoy E (ed.). Cyclodextrins in pharmaceutics, cosmetics, and biomedicine: current and future industrial applications, Torino, Italy, pp 323–42, 2011. CrossRef
Trotta F, Cavalli R, Tumiatti W, Zerbinati O, Rogero C, Vallero R. Ultrasound-assisted synthesis of cyclodextrin-based nanosponges. Eur Patent Spec, 2006; 99(19):1–8.
Trotta F, Dianzani C, Caldera F, Mognetti B, Cavalli R. The application of nanosponges to cancer drug delivery. Expert Opin Drug Deliv, 2014; 11(6):931–41. CrossRef
Trotta F, Marco Z, Roberta C. Cyclodextrin-based nanosponges as drug carriers. Beilstein J Org Chem, 2012; 8:2091–99. CrossRef
Vega-Vasquez P, Mosier NS, Irudayaraj J. Nanoscale drug delivery systems: from medicine to agriculture. Front Bioeng Biotechnol, 2020; 8:79. CrossRef
Vyas A, Shailendra S, Swarnlata S. Cyclodextrin based novel drug delivery systems. J Incl Phenom Macrocycl Chem, 2008; 62(1–2):23–42. CrossRef
Xu X, Zhang J, Liu S, Wang C, Wang H, Fan H, Tong Y, Liu H, Zhou D. New advances in nanomaterial-based antiviral strategies. Small Struct, 2022; 3. CrossRef
Yang R, He J, Xu L, Yu J. Bubble-electrospinning for fabricating nanofibers. Polymer, 2009; 50(24):5846–50. CrossRef
Yang CY, Liao TC, Shuai HH, Shen TL, Yeh JA, Cheng CM. Micropatterning of mammalian cells on inorganic-based nanosponges. Biomaterials, 2012; 33(20):4988–97. CrossRef
Zainuddin R, Zaheer Z, Sangshetti JN, Momin M. Enhancement of oral bioavailability of anti-HIV drug rilpivirine HCl through nanosponge formulation. Drug Dev Ind Pharm, 2017; 43(12):2076–84. CrossRef