INTRODUCTION
Adverse drug reactions (ADRs) are now an economic and health system burden across countries (Liao et al., 2019; Plumpton et al., 2016). ADR causes hospitalization, an increase in medical and drug expenses, a prolonged length of stay in the hospital, and even a poorer prognosis for patients (Liao et al., 2019; Mejía et al., 2020). The causes of ADR differed between populations. Inappropriate prescribing, age, polypharmacy, and length of therapy were factors related to ADR occurring in patients (Assiri et al., 2018; Davies et al., 2020; Tamma et al., 2017).
Previous systematic reviews found that the prevalence of adverse drug events (ADEs) in hospitalized inpatients was around 2.6%, with about 1.3% of ADEs being preventable and caused by medication errors (Gates et al., 2018). Drugs causing preventable ADEs in pediatric patients are commonly anti-infective agents (Alghamdi et al., 2019). Another systematic review of ADR prevalence was done in geriatric patients. The prevalence of ADR in geriatric patients ranged from 5.8% to 46.3% (Alhawassi et al., 2014).
The prevalence of ADR among countries is varied. In Europe, some countries collected patient ADR reports under 10% a year in 2017–2018, namely, Austria, Belgium, Greece, Latvia, Portugal, and Spain, while in Lithuania, Germany, and Finland, the ADR reported by patients ranged from 12% to 21% in 2017–2018. The highest ADR report in Europe came from Ireland and Estonia, which were at 36% in the same year (Valinciute-Jankauskiene and Kubiliene, 2021). The prevalence of ADR that caused hospital admission in the US was 0.4% in 2013–2014, and that was related to anticoagulants, antibiotics, and antidiabetic agents (Shehab et al., 2016). Despite the risk that Asian people will experience some adverse events due to genetic risk association (Yu-Hor Thong, 2020), the prevalence of ADR in Asian countries, particularly in Indonesia, remains unknown. Information regarding ADR only came from China, where researchers evaluated the ADR in COVID-19 patients, which reached 37.8% among hospitalized patients (Sun et al., 2020).
ADR has become a serious health risk influencing treatment outcomes in patients. Previous systematic reviews have not addressed this issue in specific countries, such as Indonesia. The aim of this systematic review was to provide reliable information regarding the prevalence of ADRs in Indonesia.
METHODS
Search strategy and information sources
We performed this systematic review following the PRISMA statement guideline (2020 updated), as seen in Figure 1 about the flow diagram of study selection (Page et al., 2021), but we did not register this review in PROSPERO. The databases used in this review were PubMed and Google Scholar. In the PubMed database, we used the keywords “side effect” or “ADRs” or “adverse event” and “Indonesia.” For specifying and limiting the result of the search, we used the filters “human,” “last 10 years” for a time range, and “full text” and “free full text” availability and filtered the types of documents searched to the only case report, clinical study, clinical trial (phase I, II, III, IV, and controlled), multicenter study, observational study, and randomized controlled trial (RCT), while in the Google Scholar database, the keywords were “side effect” or “ADRs” or “adverse drug reaction” and “Indonesia” and “patient” and “actual” and not “potential.” We also narrowed the search results by including the keyword “journal” rather than “review.” The filter used selected a range of years from 2011 until 2021 and excluded the citation of documents.
Study selection and eligibility criteria
The search process was performed by the first author (L. M.), and the selection process was performed by all the authors (L. M. and A. Y.) by individual peer review. Agreement between reviewers in the selection process was reached through discussion, and selected articles were determined by the agreement of the reviewers. The following criteria were required for inclusion in this review: 1) research articles using RCT, quasi-experimental studies, observational studies (cohort, case-control, and cross-sectional), clinical trials, and case reports or series; 2) to be published between 2011 and 2021; 3) to be published in English or Indonesian language, while the exclusion criteria are as follows: 1) research that has been published as an undergraduate thesis repository; 2) study protocol. All articles fulfilled the criteria before going through further selection for abstract and content. The inclusion criteria used for selecting the articles by abstract and content were as follows: 1) the study population is patients, not healthy people; 2) the measurements measure actual ADR of drugs, not potential ones. The content was excluded based on the following criteria: 1) measured effect of medical interventions, vaccines, herbal remedies, or anything other than drugs; 2) study located outside of Indonesia. Except for a reference manager, Mendeley, no automated tools were used in the selection and eligibility determination processes.
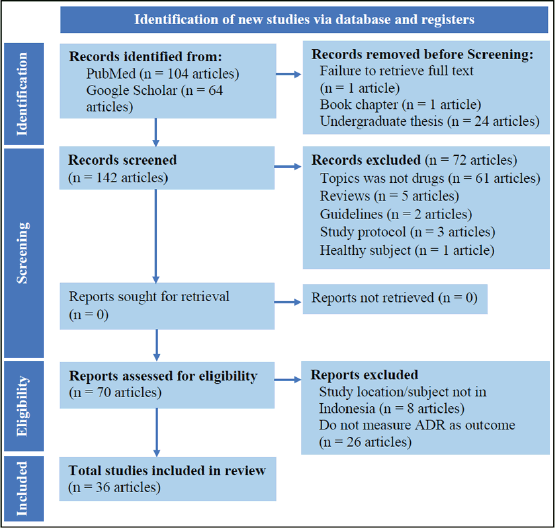 | Figure 1. PRISMA diagram (2020) of studies selection. [Click here to view] |
Quality assessment and risk of bias
The quality assessment and risk of bias processes were performed by all the authors using a checklist based on the type of article. Articles that contain RCT and clinical trial methods were assessed using CONSORT 2010 guidelines (Cuschieri, 2019b; Schulz et al., 2010), while articles using observational studies were assessed using the STROBE checklist (Cuschieri, 2019a). Another type of article, such as a case report or a case series, was assessed using the CARE guidelines (Riley et al., 2017), and non-randomized methodology-containing articles were assessed using MINORS (Slim et al., 2003). All authors used the checklist individually and then discussed the agreement score between authors for each article.
Data items, collection, and synthesis
Data collection performed using a Microsoft Excel spreadsheet consisted of demographic information about the articles and the results of the articles. Demographic information recorded included authors, year of publications, year and duration of the study (data collection) performed, location of the study, and sample size. The results of the studies extracted were the name of the drugs causing ADR, the percentage or prevalence of the ADR, the population of the study, and how the ADR was assessed. The prevalence of ADR in percentage and the list of drugs causing ADR were the effects measured for the overall result from the articles. Data were synthesized into tables and descriptively discussed for further explanation.
RESULTS
During literature searching on September 21–23, 2021, we found 168 articles from two databases (104 articles from PubMed and 64 articles from Google Scholar). Article identification, screening, and eligibility assessment resulted in 36 articles being included in this review, as seen in Figure 1. Most of the exclusion articles (61 articles) were caused by topics not assessing ADR in medications used, such as vaccines or medical interventions. Another reason for the articles’ exclusion (26 articles) was that the articles did not measure ADR as an outcome of the study and only measured the effectiveness of the drugs.
Study characteristics
Studies included in this systematic review were varied: fifteen studies were RCT (Aditianingsih et al., 2019; Beardsley et al., 2016; Hustrini et al., 2019; Jian Cheng et al., 2015; Kang et al., 2015; Krentel et al., 2021; Pasaribu et al., 2013; Rahardjo et al., 2018; Ralph et al., 2013; Somasetia et al., 2014; Supali et al., 2021; Taylor et al., 2019; Wagenaar et al., 2017; Yuri et al., 2016; Yusuf et al., 2021), eleven studies were observational cohort or cross-sectional (Budiman et al., 2019; Emral et al., 2017; Kurniawati et al., 2020; Lorensia and Amalia, 2015; Padoli and Norontoko, 2018; Pranoto et al., 2015; Rudijanto et al., 2018; Setiawati and Pohan, 2013; Setiyaningrum and Pramantara, 2019; Sulaiman et al., 2014; Supraptia et al., 2014), six studies were clinical trials (Dian et al., 2018; Do et al., 2020; Herardi et al., 2020; Kwak et al., 2017; Menzies et al., 2018; Tjitra et al., 2012), three studies were quasi-experimental design (Ahmed et al., 2019; Diallo et al., 2018; Weil et al., 2019), and only one study was a case series (Nataprawira et al., 2021). Twenty-three studies were located in one or more cities in Indonesia (Aditianingsih et al., 2019; Ahmed et al., 2019; Budiman et al., 2019; Dian et al., 2018; Herardi et al., 2020; Hustrini et al., 2019; Kurniawati et al., 2020; Lorensia and Amalia, 2015; Nataprawira et al., 2021; Padoli and Norontoko, 2018; Pasaribu et al., 2013; Pranoto et al., 2015; Rahardjo et al., 2018; Ralph et al., 2013; Rudijanto et al., 2018; Setiawati and Pohan, 2013; Setiyaningrum and Pramantara, 2019; Somasetia et al., 2014; Sulaiman et al., 2014; Supali et al., 2021; Supraptia et al., 2014; Tjitra et al., 2012; Yuri et al., 2016) , and another thirteen studies were multicenter in some countries including Indonesia (Beardsley et al., 2016; Diallo et al., 2018; Do et al., 2020; Emral et al., 2017; Weil et al., 2019; Jian Cheng et al., 2015; Kang et al., 2015; Krentel et al., 2021; Kwak et al., 2017; Menzies et al., 2018; Taylor et al., 2019; Wagenaar et al., 2017; Yusuf et al., 2021). Most of the studies were performed from 2011 to 2018, but five studies were held before 2011 (Ralph et al., 2013; Setiawati and Pohan, 2013; Somasetia et al., 2014; Sulaiman et al., 2014; Tjitra et al., 2012), and eight studies did not mention the year of the conducted study clearly in the articles (Emral et al., 2017; Jian Cheng et al., 2015; Kang et al., 2015; Krentel et al., 2021; Nataprawira et al., 2021; Padoli and Norontoko, 2018; Rahardjo et al., 2018; Supali et al., 2021). Three biggest-sample-size studies were as follows: a quasi-experimental multicenter study by Weil et al. (2019) in 5 countries including Indonesia which counts 26,836 patients with lymphatic filariasis to assess the safety of triple-drug combination (ivermectin with diethylcarbamazine and albendazole) versus two-drug combinations (diethylcarbamazine and albendazole) (Weil et al., 2019); an RCT study on Asian race by Kang et al. (2015) which compared the use of ticagrelor and clopidogrel in acute coronary syndrome patients; clinical trial conducted in 9 countries including Indonesia by Menzies et al. (2018) about the use of rifampin or isoniazid for adults with tuberculosis. On the other hand, studies that have the least patients were as follows: a case series of 6 patients by Nataprawira et al. (2021) about the recurrence of anti-tuberculosis drug-induced hepatotoxicity and a cross-sectional study by Padoli and Norontoko (2018) (about self-acceptance and side effect in 30 patients who underwent chemotherapy for breast cancer in Surabaya, Indonesia. The complete characteristics of the studies are presented in Table 1.
Risk of bias in studies
Quality assessment was performed by all authors independently, and the consensus was derived from the discussion after scores were obtained. Most RCT articles have complied with the CONSORT checklist, especially for the title and abstract, introduction, discussion, and other (trial number registry, availability of protocol, and funding) sections, but in the Results section, there were still some articles that did not provide all the parts expected in the checklist. The unseen parts were typically randomization and blinding for clinical trial articles or changes in trial design and outcome following the announced protocol. A checklist was used for assessing the quality of observational articles. Almost all articles except the one written by Rudijanto et al. (2018) did not comply with the stated research design in the title, and most of the articles did not mention the funding source. All the quasi-experimental articles comply with most of the checklist in MINORS, and the only case report also complies with the checklist in CARE except for additional information such as the patient’s perspective and informed consent. All articles were included in the data extraction process, regardless of whether various points in the checklist were found or not. Figure 2 depicts the complete data of the articles’ quality assessment results.
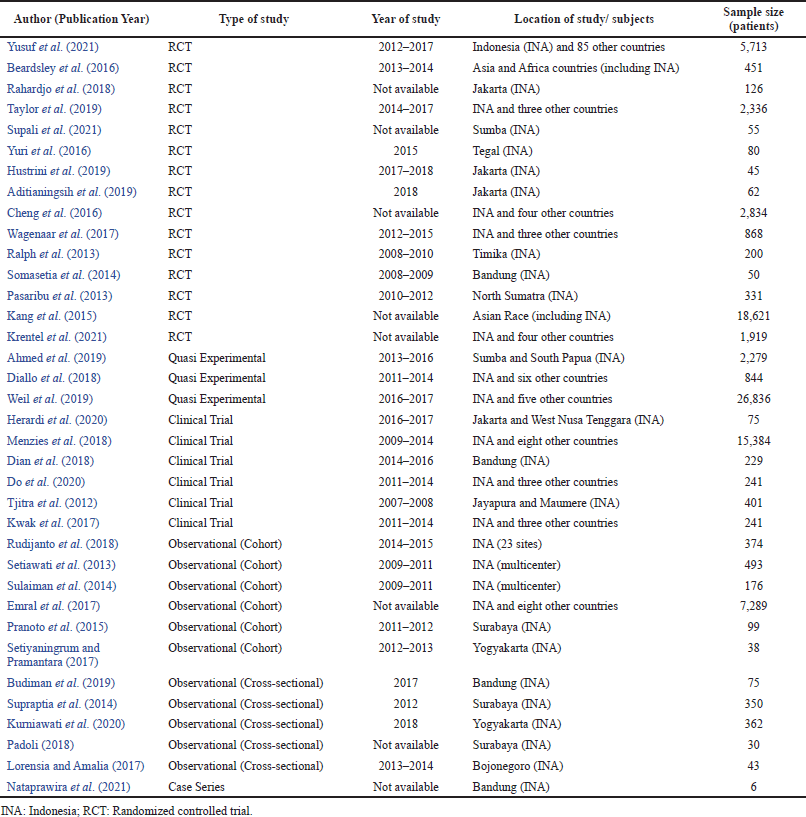 | Table 1. Study characteristics. [Click here to view] |
Prevalence of ADR in Indonesia
The prevalence of ADR in Indonesia ranged from 0.9% to 99% based on drug use, duration, and doses of therapy. In cancer patients, the prevalence of side effects was found to be higher (Do et al., 2020) than in any other disease. The use of anti-tuberculosis drugs is also related to high side effect prevalence (85%), especially in rifampin therapy (Dian et al., 2018). The use of antiretrovirals in HIV patients has a prevalence of approximately 45.33% (Budiman et al., 2019), whereas the use of antiviral agents in hepatitis B patients has a prevalence of approximately 8.3% (Sulaiman et al., 2014). Insulin users reported various prevalence of side effects, ranging from 11.2% to 67.5% (Rudijanto et al., 2018). Asthma patients have a prevalence of 20%–23% of side effects after bronchodilator use (Jian Cheng et al., 2015). In general, the use of antibiotic treatment in various infections showed that the prevalence of side effects ranged from 0.9% to 18.4% (Ahmed et al., 2019; Krentel et al., 2021; Wagenaar et al., 2017). Table 2 explains the complete data on the prevalence of side effects according to the drug used and disease diagnosis in patients.
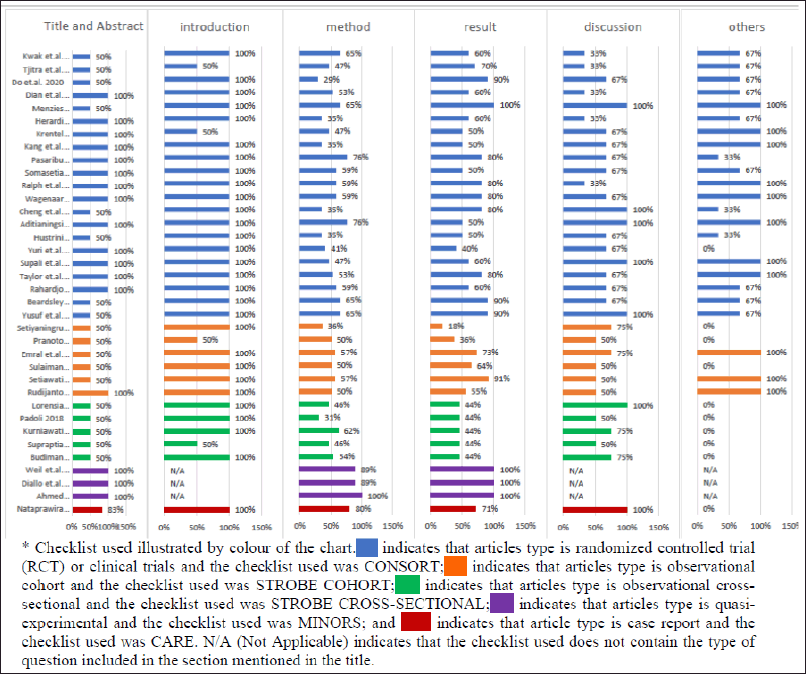 | Figure 2. Percentage of quality assessment checklist * completion for articles included. [Click here to view] |
Drug-related side effects in patients
The distribution of side effects based on the system organ affected is presented in Table 3. The antineoplastic agent was known to have various side effects from gastrointestinal (GI) to skin disorders especially radotinib and imatinib (Do et al., 2020; Kwak et al., 2017). Cardiovascular agents were found to have the most respiratory-disorders-related side effects, namely, cough, more than any other agents, with research focusing on angiotensin-converting enzyme inhibitors (ACEIs) such as lisinopril and captopril; calcium-channel blockers such as amlodipine, nifedipine, and diltiazem; angiotensin II receptor blockers such as candesartan and irbesartan. Insulin research has typically focused on its ability to cause hypoglycemia, with results ranging from 6.06% to 974% (Emral et al., 2017; Pranoto et al., 2015; Rudijanto et al., 2018). Anti-infection drugs, such as anti-parasites, antivirus, antimalaria, anti-tuberculosis, and antibiotics, are known to cause GI side effects, central nervous system (CNS) abnormalities, and skin disorders (Ahmed et al., 2019; Budiman et al., 2019; Diallo et al., 2018; Dian et al., 2018; Herardi et al., 2020), and anesthesia commonly causes GI disorders and general disorders such as fever and fatigue (Aditianingsih et al., 2019; Yuri et al., 2016), while bronchodilators mostly cause tachycardia, which is included in cardiovascular disorders (Jian Cheng et al., 2015; Lorensia and Amalia, 2015). Anti-inflammation showed higher side effects in CNS disorders (Lorensia and Amalia, 2015; Wagenaar et al., 2017), and anticholinergics had the highest side effect (22%), causing dry mouth (Rahardjo et al., 2018). Some nutrition and electrolyte agents were also assessed for their side effects since they were used to overcome pathologic situations such as hypertonic sodium lactate solution (HSL) and ringer lactate in shock in dengue fever (Somasetia et al., 2014), while L-arginine and vitamin D were used in tuberculosis patients as adjunctive therapies (Ralph et al., 2013). A detailed type of side effect caused by specific drugs listed in Table 3 is described in Supplementary Material.
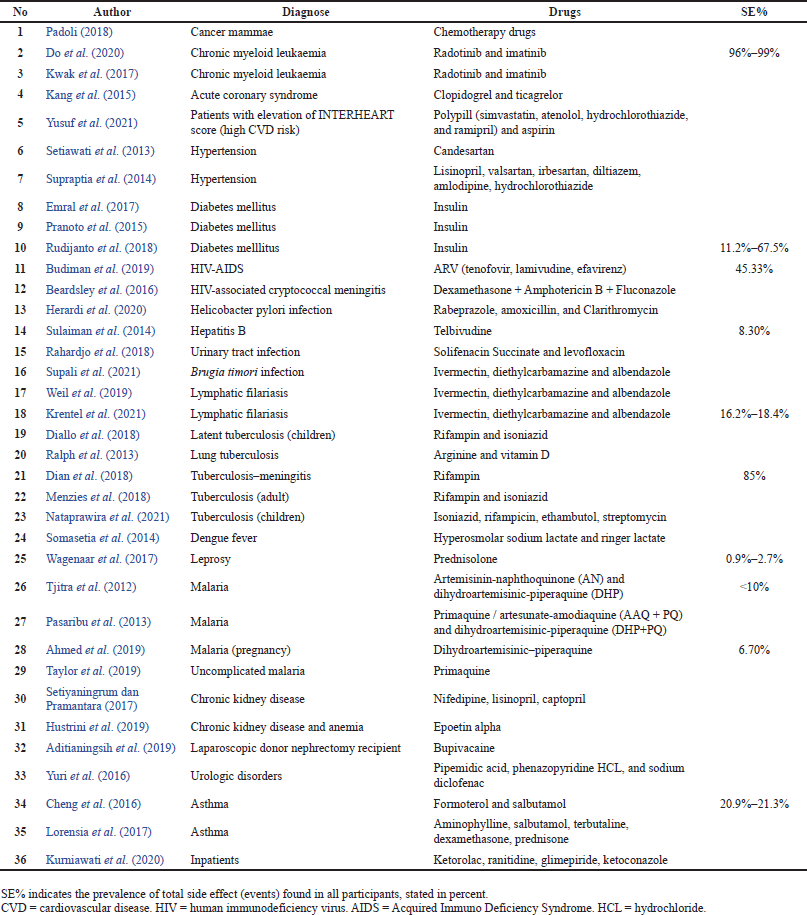 | Table 2. Result of data extraction. [Click here to view] |
DISCUSSION
Countries commonly underreport ADR prevalence. Despite the healthcare facility setting, a review of 12 countries found that the prevalence of underreporting ADR was 94% at the median (Hazell and Shakir, 2006). Healthcare professionals in Indonesia can report ADR by submitting a yellow form to the National Agency of Drug and Food Control as a voluntary report (Biswas, 2013). The prevalence of ADR in Indonesia is commonly higher with chemotherapy drugs such as those reported in radotinib and imatinib. A clinical trial found that the ADR of this combination happened in 96% to 99% of patients. Hematologic abnormalities were one of the ADRs commonly reported in imatinib users, but liver-related abnormalities tended to occur more in radotinib users (Do et al., 2020). Transient dose interruption or reduction has been suggested for the management of ADR-related radotinib and imatinib (Kwak et al., 2017). Insulin was the second most common drug causing ADRs in Indonesia, with hypoglycemia rates ranging from 6.06% to 97.4% (Emral et al., 2017; Pranoto et al., 2015; Rudijanto et al., 2018). These studies varied in sample size and research design. A prospective study with 99 samples showed that the hypoglycemia event occurred in only 6.06% of patients receiving insulin. This study performed monitoring of blood glucose levels every 2 weeks, and the research had a relatively higher risk of bias in reporting compared to the two other studies included in this review related to hypoglycemia incidence (Pranoto et al., 2015). Two other studies found that hypoglycemia occurred more frequently in the prospective observation phase than in the retrospective observation phase. Prospective studies showed the prevalence of hypoglycemia to be about 67%–97%, while in the retrospective phase, the event only occurred in 33%–72% of patients using insulin. These studies used a self-assessment questionnaire recorded in patient diaries to assess hypoglycemia events, and in the study by Emral et al. (2017) the events were confirmed by checking blood glucose levels (Emral et al., 2017; Rudijanto et al., 2018).
Various cardiovascular agents have been reported in some studies as having ADR outside the cardiovascular system. ACEIs such as lisinopril and captopril both cause coughing in the respiratory system. Calcium channel blockers are known to cause a general disorder, namely, edema (pedal or general edema), and are found in amlodipine, nifedipine, and diltiazem users (Setiyaningrum and Pramantara, 2019; Supraptia et al., 2014). Antiplatelet therapy showed various side effects, including respiratory, cardiovascular, and CNS disorders, but hematology disorders were only found in clopidogrel and ticagrelor (adenosine diphosphate receptor inhibitors) users and not in aspirin users (Kang et al., 2015; Yusuf et al., 2021). One study of a polypill containing 40 mg of simvastatin, 100 mg of atenolol, 25 mg of hydrochlorothiazide, and 10 mg of ramipril showed similar side effects to those of the separate drugs used alone in patients with high cardiovascular disease risk (Yusuf et al., 2021).
The combination of anti-parasite drugs, diethylcarbamazine, and albendazole with or without ivermectin, resulted in a low prevalence of side effects (under 22%) when used in filariasis patients. These studies were large multicenter studies held in five countries, including Indonesia. A study by Supali et al. (2021) performed in Sumba, Indonesia, in 55 patients infected with Brugia timori produced similar results in terms of side effects from the combination of drugs. Headache, dizziness, and drowsiness were common side effects in the CNS area, while general disorders’ side effects commonly appeared as muscle, joint, or low back pain. These combinations also produce GI side effects such as abdominal pain, nausea, and vomiting. Only in the ivermectin add-on group, there was a side effect of cough (Krentel et al., 2021; Supali et al., 2021; Weil et al., 2019;).
The study of antimalarial agents is commonly held in Indonesia since this disease is easily found in some places in Indonesia. Three studies included in this review were located only in Indonesia, while one study was multicenter in Indonesia, Ethiopia, Vietnam, and Afghanistan. Studies about malaria in Indonesia were conducted in Sumatra, Papua, Sumba, and Nusa Tenggara. Primaquine, piperaquine, artesunate, amodiaquine, and dihydroartemisinin were observed for their side effects along with their efficacy. Antimalaria showed a higher prevalence of side effects in the CNS (headache and dizziness) and GI disorders (nausea, vomiting, and diarrhea), while primaquine monotherapy showed the occurrence of fever (general disorder) and dark urine (renal and electrolyte disorder), which were not found in combination therapy of antimalaria agent side effects (Ahmed et al., 2019; Pasaribu et al., 2013; Taylor et al., 2019; Tjitra et al., 2012).
Rifampin and isoniazid, which were used as first choices in tuberculosis treatment, had similar patterns of side effects. Both have the potential to cause a skin rash or pruritus as well as hepatotoxicity. Disorders such as purpura, thrombocytopenia, anemia, and leukopenia were found in rifampin users, but only leukopenia also happened in isoniazid users. Treatment with rifampin also showed GI side effects such as nausea, vomiting, diarrhea, and abdominal pain, which were not found in the isoniazid group (Diallo et al., 2018; Dian et al., 2018; Menzies et al., 2018; Nataprawira et al., 2021). Other antibiotics used in various infections such as levofloxacin in urinary tract infections (Rahardjo et al., 2018), pipemidic acid in urologic infections (Yuri et al., 2016), and amoxicillin and clarithromycin in Helicobacter pylori infection (Herardi et al., 2020) showed similar side effects in GI disorders, namely, nausea. The most common side effects of these antibiotics were allergy, fever, and fatigue. Headache and sleepiness were side effects reported in amoxicillin-clarithromycin and levofloxacin users, while other side effects, namely, dry mouth, only happened in levofloxacin users (Herardi et al., 2020; Rahardjo et al., 2018; Yuri et al., 2016).
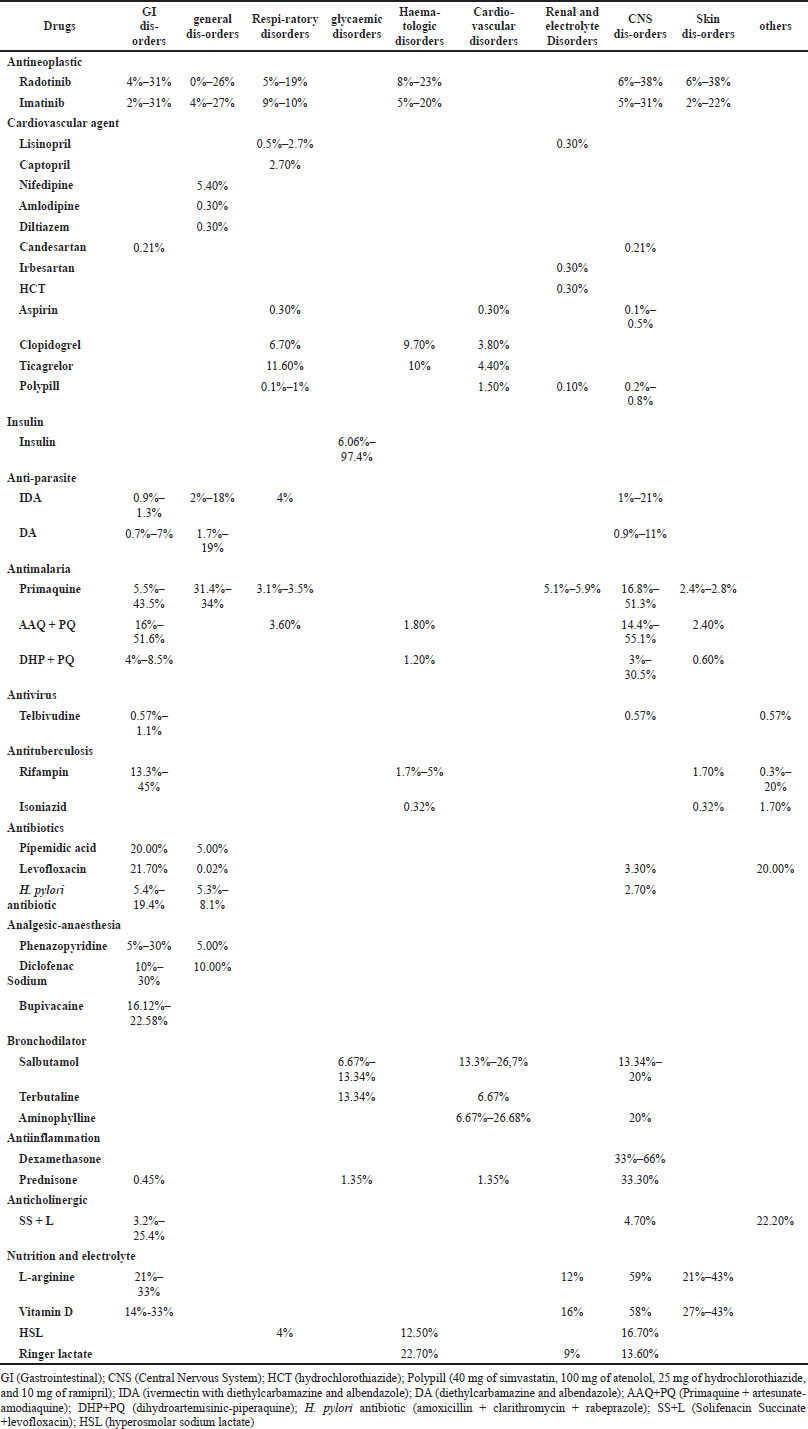 | Table 3. Prevalence of side-effects based on drug—system organ categories. [Click here to view] |
Analgesics and anesthesia such as phenazopyridine, sodium diclofenac, and bupivacaine induce fever and GI disorders such as nausea, vomiting, and abdominal pain. The prevalence of GI disorders and their side effects was higher (up to 22%) than the incidence of fever (5%–10%) (Aditianingsih et al., 2019; Yuri et al., 2016). Telbivudine, an antiviral used in hepatitis B patients, is also known to have a similar incidence of causing nausea, myotoxicity, and neuropathy (0.57%) but a slightly higher incidence of abdominal pain (1.1%) (Sulaiman et al., 2014). Dexamethasone, as an anti-inflammatory agent, was found to cause headaches in patients with a prevalence ranging from 33% to 66% (Beardsley et al., 2016), while prednisone also caused hyperglycemia, hypertension, and peptic ulcer (Lorensia and Amalia, 2015; Wagenaar et al., 2017).
The high prevalence of cardiovascular side effects in bronchodilators (salbutamol, terbutaline, and aminophylline) was regarded as due to their ability to induce tachycardia and chest pain, while their ability to cause headaches and glycemic control disorders was limited (Jian Cheng et al., 2015; Lorensia and Amalia, 2015). Solifenacin succinate, an anticholinergic used for urinary tract infection treatment, was found to have a high side effect of dry mouth (22.2%), but a low incidence of causing constipation and sleepiness. Its side effect of causing nausea was also high (25.4%) because of the treatment combination with levofloxacin (Rahardjo et al., 2018). Side effects occur not only in drug treatment but also in nutrition and electrolyte treatments in patients. The use of L-arginine and vitamin D as adjudicative treatments for patients with lung tuberculosis showed a high prevalence of joint pain and rash (more than 40%) (Ralph et al., 2013), while HSL and ringer lactate used in dengue fever resuscitation were found to induce dissemination, intravascular coagulation, and encephalopathy (Somasetia et al., 2014).
The prevalence of various drugs, nutrients, and electrolytes among patients in Indonesia produces a wide range of percentage results. This could be due to differences in sample sizes between studies and how widely drugs are evaluated. This review still has limitations in producing a single incidence for every drug stated in the study because of those reasons. A future review should be conducted to investigate the other side of ADR prevalence in Indonesia, such as the detection method used to determine ADR or specific populations. Reports in Indonesia should specify a similar technique of survey and calculation of sample size to produce an accurate annual ADR prevalence report.
This review has some limitations related to the methodology used. The first limitation is that other studies that were published outside of the chosen database may still be excluded from this review. The second one is that we could not differentiate serious ADR from mild or minor ones in this review because of limited data and analysis methods. The third result of this review also could not generate the global prevalence of ADR or other countries’ prevalence of ADR because we used various types of study design. Finally, because this study only gives a glance at ADR prevalence in Indonesia, there is a need for an annual national report of ADR in Indonesia with similar survey and calculation techniques to produce more accurate data on ADR prevalence. Future research with a bigger database and more specific analysis should be done to achieve this goal.
CONCLUSION
The prevalence of ADR in Indonesia ranged from 0.9% to 99% based on drug use, duration, and doses of therapy. The antineoplastic agents, radotinib and imatinib, were known to have various side effects, from GI to skin disorders. Cardiovascular agents (lisinopril, captopril, amlodipine, nifedipine, diltiazem, candesartan, irbesartan, hydrochlorothiazide, and antiplatelets) related to respiratory disorders have a side effect of cough. Anti-infection drugs such as anti-parasites, antivirus, antimalaria, anti-tuberculosis, and antibiotics usually cause GI side effects, CNS abnormalities, and skin disorders. Analgesics and anesthesia are frequently responsible for GI disorders as well as general illnesses like fever and fatigue. Other drugs, such as insulin, have been linked to hypoglycemia, while bronchodilators have been linked to tachycardia, anti-inflammatories have been linked to CNS disorders, and anticholinergics have been linked to dry mouth.
ACKNOWLEDGMENTS
This research was sponsored by the Center for Education Financial Services (Puslapdik) in which the first author is an awardee and the fund was raised by Indonesia Endowment Fund for Education (LPDP). The authors would like to thank Sebelas Maret University and Jenderal Soedirman University as the places where authors work and get ideas.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
All authors reported no potential conflicts of interest relevant to this article.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aditianingsih D, Pryambodho, Anasy N, Tantri AR, Mochtar CA. A randomized controlled trial on analgesic effect of repeated Quadratus Lumborum block versus continuous epidural analgesia following laparoscopic nephrectomy. BMC Anesthesiol, 2019; 19(1):1–11; doi:10.1186/s12871-019-0891-7 CrossRef
Ahmed R, Poespoprodjo JR, Syafruddin D, Khairallah C, Pace C, Lukito T, Maratina SS, Asih PBS, Santana-Morales MA, Adams ER, Unwin VT, Williams CT, Chen T, Smedley J, Wang D, Faragher B, Price RN, Ter Kuile FO. Efficacy and safety of intermittent preventive treatment and intermittent screening and treatment versus single screening and treatment with dihydroartemisinin–piperaquine for the control of malaria in pregnancy in Indonesia: a cluster-randomised, open-la. Lancet Infect Dis, 2019; 19(9):973–87; doi:10.1016/S1473-3099(19)30156-2 CrossRef
Alghamdi AA, Keers RN, Sutherland A, Ashcroft DM. Prevalence and nature of medication errors and preventable adverse drug events in paediatric and neonatal intensive care settings: a systematic review. Drug Saf, 2019; 42:1423–36; doi:10.1007/s40264-019-00856-9 CrossRef
Alhawassi TM, Krass I, Bajorek BV, Pont LG. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging, 2014; 9:2079–86; doi:10.2147/CIA.S71178 CrossRef
Assiri GA, Shebl NA, Mahmoud MA, Aloudah N, Grant E, Aljadhey H, Sheikh A. What is the epidemiology of medication errors, error-related adverse events and risk factors for errors in adults managed in community care contexts? A systematic review of the international literature. BMJ Open, 2018; 8(5):19101; doi:10.1136/bmjopen-2017-019101 CrossRef
Beardsley J, Wolbers M, Kibengo FM, Ggayi ABM, Kamali A, Cuc NTK, Binh TQ, Chau NVV, Farrar J, Merson L, Phuong L, Thwaites G, Van Kinh N, Thuy PT, Chierakul W, Siriboon S, Thiansukhon E, Onsanit S, Supphamongkholchaikul W, Chan AK, Heyderman R, Mwinjiwa E, van Oosterhout JJ, Imran D, Basri H, Mayxay M, Dance D, Phimmasone P, Rattanavong S, Lalloo DG, Day JN. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med, 2016; 374(6):542–54; doi:10.1056/nejmoa1509024 CrossRef
Biswas P. Pharmacovigilance in Asia. J Pharmacol Pharmacother, 2013; 4(Suppl 1):S7–19; doi:10.4103/0976-500X.120941 CrossRef
Budiman B, Hartantri Y, Susandi E, Agustanti N. Low body mass index as a risk factor for antiretroviral drug-related liver injury among HIV patients. Acta Med Indones, 2019; 51(3):214–9.
Cuschieri S. (2019a); doi:10.4103/sja.SJA_559_18 CrossRef
Cuschieri S. The CONSORT statement. Saudi J Anaesth, 2019b; 13(5):S27–30; doi:10.4103/sja.SJA_559_18 CrossRef
Davies LE, Spiers G, Kingston A, Todd A, Adamson J, Hanratty B. Adverse outcomes of polypharmacy in older people: systematic review of reviews. J Am Med Dir Assoc, 2020; 21(2):181–7; doi:10.1016/j.jamda.2019.10.022 CrossRef
Diallo T, Adjobimey M, Ruslami R, Trajman A, Sow O, Obeng Baah J, Marks GB, Long R, Elwood K, Zielinski D, Gninafon M, Wulandari DA, Apriani L, Valiquette C, Fregonese F, Hornby K, Li PZ, Hill PC, Schwartzman K, Benedetti A, Menzies D. Safety and side effects of Rifampin versus isoniazid in children. N Engl J Med, 2018; 379(5):454–63; doi:10.1056/nejmoa1714284 CrossRef
Dian S, Yunivita V, Ganiem AR, Pramaesya T, Chaidir L, Wahyudi K, Achmad TH, Colbers A, Te Brake L, Van Crevel R, Ruslami R, Aarnoutse R. Double-blind, randomized, placebo-controlled phase II dose-finding study to evaluate high-dose Rifampin for tuberculous meningitis. Antimicrob Agents Chemother, 2018; 62(12):e01014–18; doi:10.1128/AAC CrossRef
Do YR, Kwak JY, Kim JA, Kim HJ, Chung JS, Shin HJ, Kim SH, Bunworasate U, Choi CW, Zang DY, Oh SJ, Jootar S, Reksodiputro AH, Lee WS, Mun YC, Kong JH, Caguioa PB, Kim H, Park J, Kim DW. Long-term data from a phase 3 study of radotinib versus imatinib in patients with newly diagnosed, chronic myeloid leukaemia in the chronic phase (RERISE). Br J Haematol, 2020; 189; 303–12; doi:10.1111/bjh.16381 CrossRef
Emral R, Pathan F, Cortés CAY, El-Hefnawy MH, Goh SY, Gómez AM, Murphy A, Abusnana S, Rudijanto A, Jain A, Ma Z, Mirasol R. Self-reported hypoglycemia in insulin-treated patients with diabetes: results from an international survey on 7289 patients from nine countries. Diabetes Res Clin Pract, 2017; 134:17–28; doi:10.1016/j.diabres.2017.07.031 CrossRef
Gates PJ, Meyerson SA, Baysari MT, Lehmann CU, Westbrook JI. Preventable adverse drug events among inpatients: a systematic review. Pediatrics, 2018; 142(3):20180805. Available via www.aappublications.org/news
Hazell L, Shakir SAW. Under-reporting of adverse drug reactions?: a systematic review. Drug Saf, 2006; 29(5):385–96; doi:10.2165/00002018-200629050-00003 CrossRef
Herardi R, Syam AF, Simadibrata M, Setiati S, Darnindro N, Abdullah M, Makmun D. Comparison of 10-day course of triple therapy versus 14-day course for eradication of Helicobacter pylori infection in an Indonesian population: double-blinded randomized clinical trial. Asian Pac J Cancer Prev, 2020; 21(1):19–24; doi:10.31557/APJCP.2020.21.1.19 CrossRef
Hustrini NM, Siregar P, Setiawati A, Nugroho P. Clinical comparison of renogen, a biosimilar epoetin-a, with the originator, eprex, in chronic kidney disease anemia in Indonesia: a preliminary study. Acta Med Indones, 2019; 51(3):230–7.
Jian Cheng Q, Huang SG, Zhi Chen Y, Lin JT, Zhou X, Chen BY, Feng YL, Ling X, Sears MR. Formoterol as reliever medication in asthma: a post-hoc analysis of the subgroup of the RELIEF study in East Asia Sears 8 and on behalf of the RELIEF Asia study investigators. BMC Pulm Med, 2015; doi:10.1186/s12890-015-0166-0 CrossRef
Kang HJ, Clare RM, Gao R, Held C, Himmelmann A, James SK, Lim ST, Santoso A, Yu CM, Wallentin L, Becker RC. Ticagrelor versus clopidogrel in Asian patients with acute coronary syndrome: a retrospective analysis from the platelet inhibition and patient outcomes (PLATO) trial. Am Heart J, 2015; 169(6):899–905; doi:10.1016/j.ahj.2015.03.015 CrossRef
Krentel A, Basker N, de Rochars MB, Bogus J, Dilliott D, Direny AN, Dubray C, Fischer PU, Ga AL, Goss CW, Hardy M, Howard C, Jambulingam P, King CL, Laman M, Lemoine JF, Mallya S, Robinson LJ, Samuela J, Schechtman KB, Steer AC, Supali T, Tavul L, Weil GJ. A multicenter, community-based, mixed methods assessment of the acceptability of a triple drug regimen for elimination of lymphatic filariasis. PLoS Negl Trop Dis, 2021; 15(3):1–27; doi:10.1371/journal.pntd.0009002 CrossRef
Kurniawati F, Yasin NM, Dina A, Atana S, Hakim SN, Farmasi F, Mada UG, Sarjana P, Studi P, Farmasi F, Mada UG, Universitas A, Mada G. Kajian Adverse Drug Reactions Terkait Interaksi Obat di Bangsal Rawat Inap Rumah Sakit Akademik UGM [Study on adverse drug reactions related to drug interactions on medical ward teaching hospital UGM]. J Manag Pharm Pract, 2020; 10(4), 297–308. CrossRef
Kwak JY, Kim SH, Joong Oh S, Young Zang D, Kim H, Kim JA, Rok Do Y, Joon Kim H, Seong Park J, Won Choi C, Sik Lee W, Mun YC, Hyun Kong J, Seop Chung J, Shin HJ, Kim DY, Park J, Won Jung C, Bunworasate U, Comia NS, Jootar S, Reksodiputro AH, Caguioa PB, Lee SE, Kim DW. Cancer therapy: clinical phase III clinical trial (RERISE study) results of efficacy and safety of radotinib compared with imatinib in newly diagnosed chronic phase chronic myeloid leukemia. Clin Cancer Res, 2017; 23(23):7180–8;doi:10.1158/1078-0432.CCR-17-0957 CrossRef
Liao PJ, Mao CT, Chen TL, Deng ST, Hsu KH. Factors associated with adverse drug reaction occurrence and prognosis, and their economic impacts in older inpatients in Taiwan: a nested case-control study. BMJ Open, 2019; 9(5):26771; doi:10.1136/bmjopen-2018-026771 CrossRef
Lorensia A, Amalia RA. Studi farmakovigilans pengobatan asma pada pasien rawat inap di suatu rumah sakit di Bojonegoro. J Ilmiah Manuntung, 2015; 1(1):8–18. Available via https://www.jurnal.stiksam.ac.id/index.php/jim/article/view/5
Mejía G, Saiz-Rodríguez M, Gómez de Olea B, Ochoa D, Abad-Santos F. Urgent hospital admissions caused by adverse drug reactions and medication errors—a population-based study in Spain. Front Pharmacol, 2020; 11:1; doi:10.3389/fphar.2020.00734 CrossRef
Menzies D, Adjobimey M, Ruslami R, Trajman A, Sow O, Kim H, Obeng Baah J, Marks GB, Long R, Hoeppner V, Elwood K, Al-Jahdali H, Gninafon M, Apriani L, Koesoemadinata RC, Kritski A, Rolla V, Bah B, Camara A, Boakye I, Cook VJ, Goldberg H, Valiquette C, Hornby K, Dion MJ, Li PZ, Hill PC, Schwartzman K, Benedetti A. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med, 2018; 379(5):440–53; doi:10.1056/nejmoa1714283 CrossRef
Nataprawira HM, Aliyannissa A, Febrianti SA. Unusual recurrence of antituberculosis drug-induced hepatotoxicity in children: a case series. Am J Case Rep, 2021; 22(1):1–4; doi:10.12659/AJCR.930828 CrossRef
Padoli FD, Norontoko DA. Penerimaan Diri dan Efek Samping Kemoterapi pada Klien Kanker Payudara yang Menjalani Kemoterapi di Rumah Sakit Onkologi Surabaya. J Keperawatan, 2018; XI(1):24–34.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg, 2021; 88:1–11; doi:10.1016/j.ijsu.2021.105906 CrossRef
Pasaribu AP, Chokejindachai W, Sirivichayakul C, Tanomsing N, Chavez I, Tjitra E, Pasaribu S, Imwong M, White NJ, Dondorp AM. A randomized comparison of dihydroartemisinin-piperaquine and artesunate-amodiaquine combined with primaquine for radical treatment of Vivax malaria in Sumatera, Indonesia. J Infect Dis, 2013; 208(11):1906–13; doi:10.1093/infdis/jit407 CrossRef
Plumpton CO, Roberts D, Pirmohamed M, Hughes DA. A systematic review of economic evaluations of pharmacogenetic testing for prevention of adverse drug reactions. PharmacoEconomics, 2016; 34(8):771–93; doi:10.1007/s40273-016-0397-9 CrossRef
Pranoto A, Novida H, Prajitno JH, Tjokroprawiro A. Safety and efficacy in early insulin initiation as comprehensive therapy for patients with type 2 diabetes in primary health care centers. Acta Med Indones, 2015; 47(2):104–10.
Rahardjo HE, Syahputra FA, Islianti PI, Matondang FA. Efficacy of additional solifenacin succinate therapy for storage symptoms in females with uncomplicated lower urinary tract infection: the SOLUTION randomized controlled trial. Acta Med Indones, 2018; 50(3):200–7.
Ralph AP, Waramori G, Pontororing GJ, Kenangalem E, Wiguna A, Tjitra E, Sandjaja, Lolong DB, Yeo TW, Chatfield MD, Soemanto RK, Bastian I, Lumb R, Maguire GP, Eisman J, Price RN, Morris PS, Kelly PM, Anstey NM. L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial. PLoS ONE, 2013; 8(8); doi:10.1371/journal.pone.0070032 CrossRef
Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, Kiene H, Helfand M, Altman DG, Sox H, Werthmann PG, Moher D, Rison RA, Shamseer L, Koch CA, Sun GH, Hanaway P, Sudak NL, Kaszkin-Bettag M, Carpenter JE, Gagnier JJ. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol, 2017; 89(January 2020):218–35; doi:10.1016/j.jclinepi.2017.04.026 CrossRef
Rudijanto A, Saraswati MR, Yunir E, Kumala P, Puteri HH, Mandang VV. Indonesia cohort of IO HAT study to evaluate diabetes management, control, and complications in retrospective and prospective periods among insulin-treated patients with type 1 and type 2 diabetes. Acta Med Indones, 2018; 50(1):26–37.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ (Online), 2010; 340(7748):698–702; doi:10.1136/bmj.c332 CrossRef
Setiawati A, Pohan T. Safety and effectiveness of candesartan and candesartan/HCT fixed dose combination in patients with hypertension. Acta Med Indones, 2013; 45(3):193–201.
Setiyaningrum N, Pramantara IDP. Kajian Keamanan Antihipertensi Pasien Gagal Ginjal Kronik Lanjut Usia Di Unit Hemodialisa Rsup Dr. Sardjito Yogyakarta. JFIOnline, 2019; 9(1); doi:10.35617/jfi.v9i1.551 CrossRef
Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013-2014. JAMA J Am Med Assoc, 2016; 316(20):2115–25; doi:10.1001/jama.2016.16201 CrossRef
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (Minors): development and validation of a new instrument. ANZ J Surg, 2003; 73(9):712–6; doi:10.1046/j.1445-2197.2003.02748.x CrossRef
Somasetia DH, Setiati TE, Sjahrodji AM, Idjradinata PS, Setiabudi D, Roth H, Ichai C, Fontaine E, Leverve XM. Early resuscitation of dengue shock syndrome in children with hyperosmolar sodium-lactate: a randomized single-blind clinical trial of efficacy and safety. Crit Care, 2014; 18(5):466; doi:10.1186/s13054-014-0466-4 CrossRef
Sulaiman A, Lesmana LA, Nafrialdi, Helyanna. An observational study to evaluate the safety and efficacy of telbivudine in adults with chronic hepatitis B. Acta Med Indones, 2014; 46(1):38–43.
Sun J, Deng X, Chen X, Huang J, Huang S, Li Y, Feng J, Liu J, He G. Incidence of adverse drug reactions in COVID-19 patients in China: an active monitoring study by hospital pharmacovigilance system. Clin Pharmacol Ther, 2020; 108(4):791–7; doi:10.1002/cpt.1866 CrossRef
Supali T, Djuardi Y, Christian M, Iskandar E, Alfian R, Maylasari R, Destani Y, Lomiga A, Minggu D, Lew D, Bogus J, Weil GJ, Fischer PU. An open label, randomized clinical trial to compare the tolerability and efficacy of ivermectin plus diethylcarbamazine and albendazole vs. diethylcarbamazine plus albendazole for treatment of brugian filariasis in Indonesia. PLoS Negl Trop Dis, 2021; 15(3):1–11; doi:10.1371/journal.pntd.0009294 CrossRef
Supraptia B, Nilamsari WP, Hapsari PP, Muzayana HA, Firdausi H. Permasalahan Terkait Obat Antihipertensi pada Pasien Usia Lanjut di Poli Geriatri RSUD Dr.Soetomo, Surabaya. 2014; 1(2):36–41.
Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Int Med, 2017; 177(9):1308–15; doi:10.1001/jamainternmed.2017.1938 CrossRef
Taylor WRJ, Thriemer K, von Seidlein L, Yuentrakul P, Assawariyathipat T, Assefa A, Auburn S, Chand K, Chau NH, Cheah PY, Dong LT, Dhorda M, Degaga TS, Devine A, Ekawati LL, Fahmi F, Hailu A, Hasanzai MA, Hien TT, Khu H, Ley B, Lubell Y, Marfurt J, Mohammad H, Moore KA, Naddim MN, Pasaribu AP, Pasaribu S, Promnarate C, Rahim AG, Sirithiranont P, Solomon H, Sudoyo H, Sutanto I, Thanh NV, Tuyet-Trinh NT, Waithira N, Woyessa A, Yamin FY, Dondorp A, Simpson AJ, Baird JK, White NJ, Day NP, Price RN. Short-course primaquine for the radical cure of Plasmodium vivax malaria: a multicentre, randomised, placebo-controlled non-inferiority trial. Lancet, 2019; 394(10202):929–38; doi:10.1016/S0140-6736(19)31285-1 CrossRef
Tjitra E, Hasugian AR, Siswantoro H, Prasetyorini B, Ekowatiningsih R, Yusnita EA, Purnamasari T, Driyah S, Salwati E, Yuwarni E, Januar L, Labora J, Wijayanto B, Amansyah F, Dedang TA, Purnama A. Efficacy and safety of artemisinin-naphthoquine versus dihydroartemisinin-piperaquine in adult patients with uncomplicated malaria: a multi-centre study in Indonesia. Malar J, 2012; 11:153. Available via http://www.malariajournal.com/content/11/1/153
Valinciute-Jankauskiene A, Kubiliene L. Adverse drug reaction reporting by patients in 12 european countries. Int J Environ Res Public Health, 2021; 18(4):1–8; doi:10.3390/ijerph18041507 CrossRef
Wagenaar I, Post E, Brandsma W, Bowers B, Alam K, Shetty V, Pai V, Husain S, Sigit Prakoeswa CR, Astari L, Hagge D, Shah M, Neupane K, Tamang KB, Nicholls P, Richardus JH. Effectiveness of 32 versus 20 weeks of prednisolone in leprosy patients with recent nerve function impairment: a randomized controlled trial. PLoS Negl Trop Dis, 2017; 11(10):1–18; doi:10.1371/journal.pntd.0005952 CrossRef
Weil GJ, Bogus J, Christian M, Dubray C, Djuardi Y, Fischer PU, Goss CW, Hardy M, Jambulingam P, King CL, Kuttiat VS, Krishnamoorthy K, Laman M, Lemoine JF, O’Brian KK, Robinson LJ, Samuela J, Schechtman KB, Sircar A, Srividya A, Steer AC, Supali T, Subramanian S. The safety of double- and triple-drug community mass drug administration for lymphatic filariasis?: a multicenter, open-label, cluster-randomized study. PLoS Med, 2019; 16(6):1–20. CrossRef
Yu-Hor Thong B. Current review. 2020; doi:10.5415/apallergy.2020.10.e8 CrossRef
Yuri P, Ali Z, Rasyid N, Birowo P. Effects of pipemidic acid, phenazopyridine HCL and sodium diclofenac on pain perception following endoscopic urological surgery: double-blinded randomized-controlled trial. Acta Med Indones, 2016; 48(3):184–92. CrossRef
Yusuf S, Joseph P, Dans A, Gao P, Teo K, Xavier D, López-Jaramillo P, Yusoff K, Santoso A, Gamra H, Talukder S, Christou C, Girish P, Yeates K, Xavier F, Dagenais G, Rocha C, McCready T, Tyrwhitt J, Bosch J, Pais P. Polypill with or without aspirin in persons without cardiovascular disease. N Engl J Med, 2021; 384(3):216–28; doi:10.1056/nejmoa2028220 CrossRef
SUPPLEMENTARY MATERIAL
Supplementary data can be downloaded from the link [https://japsonline.com/admin/php/uploadss/3993_pdf.pdf]