INTRODUCTION
Plants have been utilized worldwide to cure several diseases since time immemorial and as per World Health Organization, about 80% of individuals globally still use them (Tugume and Nyakoojo, 2019). The herbal-based medicine could be a novel approach to the replacement of synthetic drugs like aspirin, orlistat, ibuprofen, and others due to their adverse effect of the later on human health (Jagessar, 2020; Maqbool et al., 2019). Plant-based medications are safe, and efficacious owing to the presence of diverse phytoconstituents (Tugume and Nyakoojo, 2019; WHO, 2013). In this context, Allium fistulosum L. can be utilized as a useful herbal drug candidate to treat various human diseases. Allium fistulosum commonly known as Welsh onion, is a member of the family Amaryllidaceae. It is grown in Asia, including Korea, Japan, China, and Vietnam (Lee et al., 2018).
Allium fistulosum is a commonly used vegetable crop in various Eastern countries including Japan, Korea, and China (Sung et al., 2018). Western countries have used Welsh onion as traditional medicine against flu, abdominal pain, constipation, headache, arthritis, gastrointestinal disorders, parasitic infestations, cardiac diseases (Sung et al., 2018), colon diseases (Chang et al., 2016), as well as against hypercholesterolemia (Bhat and Nagandram, 2001). In traditional Chinese medicine, Welsh onion is recommended against renal de?ciency, dizziness, and cold (Lai et al., 2010), and is also used for the common cold in Japan (Bhat and Nagandram, 2001). Further, plant parts like flowers, leaves, stems, and roots have been used to lower blood pressure, treat cancer, inhibit bacterial strains, and resist viruses, also stem and roots are used as antipyretic and also against the common cold in China (Zhai et al., 2015). The bulbs are used to enhance vision in China (Longe, 2005). Subsequently, the plant is used as an immunity booster in Japan (Ueda et al., 2012). Interestingly, A. fistulosum congee (A. fistulosum and Oryza sativa mixture) was found effective in treating numerous COVID-19 patients at an early stage (Hsu et al., 2020). The major bioactive components of the plant include phenolic imidates, cinnamic acid derivatives, phenolic glycoside (Zolfaghari et al., 2021), flavonoids (Choi et al., 2017; Zolfaghari et al., 2021), furostanol saponins (Lai et al., 2010), welsonins A1 (Nohara et al., 2016), Onionins A1 (Nohara et al., 2017), fructans, polymers of fructose (Lee et al., 2012), and phenylpropenoic acid amides (Park, 2011). Several pharmacological activities have been reported for extracts and isolated compounds of Welsh onion like anti-arthritis, anti-inflammatory, antimicrobial, anti-oxidant, anti-obesity, anti-tumor, anti-viral, and effects on the immune system as proven by animal and cellular models. In this review, we have endeavored to critically assess the scientific information of A. fistulosum which includes traditional uses, salient botanical features, bioactive composition, and pharmacological activities which might be used to develop a potential plant-based drug for the management of diverse ailments.
INCLUSION AND EXCLUSION CRITERIA
In light of existing literature, this study was drafted utilizing various online databases, such as PubMed, Google Scholar, Web of Science, and Science Direct. Different keywords used were A. fistulosum, Welsh onion, bioactive composition, phytochemistry, ethnomedicine, biological potential, clinical trials, mechanistic insights, mechanism of action, and others. Full-length, peer-reviewed, and English-language papers have been included in this study. The replicated records, conference proceedings, editorials, and also papers not indexed in Scopus and WOS were excluded.
 | Figure 1. Salient botanical features of A. fistulosum (Source: Patanjali Herbal Museum). [Click here to view] |
BOTANICAL DESCRIPTION OF A. fistulosum
The main botanical aspects of A. fistulosum are depicted in Figure 1 for correct identification. Bulbs solitary or clustered, cylindric, 1–2 cm in diameter. Leaves sub-equaling, persistent, 2–6; blade terete, fistulose, 10–40 × 1–2.5 cm. Scape solitary, erect, fistulose, 15–70 × 0.8–2.5 cm. Umbel persistent, erect, compact, 50–100-flowered; spathe bracts persistent, 1–2. Flowers 6–9 mm; tepals erect, yellowish-white; stamens long-exserted; anthers white to yellow; pollen white; ovary crestless; style linear; stigma capitate. Seed coat shining (Eflora-Flora of China, 2023).
ETHNOMEDICINAL IMPORTANCE OF A. fistulosum
Ethnomedicines are the traditional heritage used to treat ailments in diverse ethnic cultures. The ethnic group has transferred this knowledge orally over the years to their future generations. Allium fistulosum is used by different communities in various countries, such as China, Taiwan, Japan, India, etc. for treating diseases related to the respiratory system, gastrointestinal system, cancer, arthritis, skin disorders, and others (Table 1).
In China, A. fistulosum has been extensively employed against various illnesses. In terms of plant parts, whole plant, and roots are recommended for the treatment of the majority of ailments. According to ethnomedicinal records, this plant has been used to treat disorders of the respiratory system, digestive system, arthritis, cephalgia, and other diverse ailments (Table 1).
BIOACTIVE COMPOSITION OF A. fistulosum
Plants serve as a reservoir for bioactive compounds, they have a diverse range of chemical structures and are associated with the medicinal potential of plants (Balkrishna et al., 2021; Sharma et al., 2021, 2022; Sonam et al., 2021). The bioactivity, functionality, quantity, and applications of various plant-derived chemical components are affected by a variety of internal and external factors, such as geographical location, altitude, climate change, temperature, seasonal variations, etc. Allium fistulosum also have rich bioactive composition, some of which are shown in Figure 2. Several researchers have reported a variety of bioactive compounds from A. fistulosum. Vlase et al. (2013) reported the flavonoids as isoquercitrin, kaempferol, quercetol, quercitrin; phenolic compounds as ferulic acids, p-coumaric acid; sterols as β-sitosterol, campesterol, stigmasterol and allicin from whole plant. Along with these compounds steroidal sapogenins, such as yuccagenin (Kim et al., 1991), cinnamic acid amide (Seo et al., 2011), typheramide, alfrutamide (Park, 2011), fistuloimidates (Zolfaghari et al., 2021), and Onionins A1, A2, and A3 (Nohara et al., 2017), were also reported in A. fistulosum. Subsequently, Choi et al. (2017) documented several flavonoids, such as kaempferol, quercetin, p-coumaric, and ferulic acid, from the aerial parts. Bulbs were found to be rich in welsonins A1 (Nohara et al., 2016), coumaran derivatives, cinnamic acid amides, and hydroxy phenol (Hwang et al., 2020). The leaves contain flavonoids, saponins, steroids (Husori et al., 2016), and β-sitosterol (Liu et al., 2020). Tigu et al. (2021) reported that the leaves also contain alliin, allicin, 4-hydroxybenzoic, and p-coumaric acid (Tigu et al., 2021). Terada et al. (2006) reported fistulosin from the roots of A. fistulosum. Lastly, seeds contain tianshic acid, p-hydroxybenzoic acid, vanillic acid, daucosterol (Sang et al., 2002), fistulosaponins A–F, and protogracillin (Lai et al., 2010).
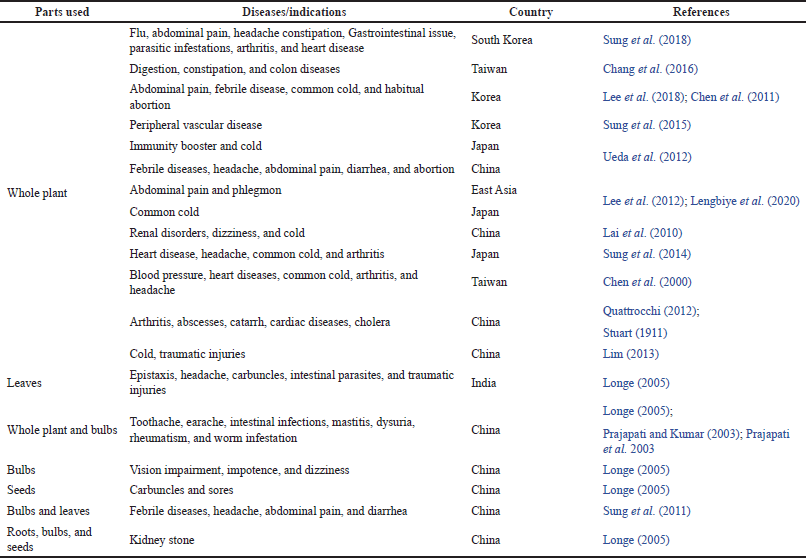 | Table 1. Ethnomedicinal uses of A. fistulosum across the globe. [Click here to view] |
PHARMACOLOGICAL PROFILE OF A. fistulosum: MECHANISTIC INSIGHTS
In light of existing literature, several studies were found with the pharmacological properties of A. fistulosum extracts and compounds. These pharmacological studies provided scientific evidence for some ethnomedicinal uses of this plant. A brief overview of the biological activities of A. fistulosum and mechanistic insights into these activities have been depicted in Table 2 and Figure 3, respectively.
Analgesic activity
The ethanol extract (200, 400, and 600 mg/kg) of A. fistulosum demonstrated analgesic activity in the hot plate, tail flick, and acetic acid-induced writhing in adult Albino mice. The administration of extract and standard aspirin (300 mg/kg) led to a significant (p < 0.01) increase in latency time at 4 hours in both the hot plate and tail flick test as compared to the control. Likewise, the writhing response stimulated by acetic acid was found to be remarkably inhibited by the extract (600 mg/kg) and standard diclofenac sodium (5 mg/kg) with 50% and 46% inhibition, respectively (Nazir et al., 2022).
Anthelmintic activity
The aqueous and ethanolic extracts (50, 100, and 200 mg/ml) of A. fistulosum leaves showed dose-dependent and significant anthelmintic effect with decreased paralysis and death time of Ascaris lumbricoides when compared with pyrantel pamoate (3 and 6 mg/ml) used as standard drug. It was concluded that both extracts were more efficient than the standard (Husori et al., 2016).
Anti-allergic activity
Allium fistulosum leaves ethanolic extract (200–1,000 μg/ml) exerted anti-allergic activity on calcium ionophore A23187 stimulated mast cell line (RBL-2H3) by notably reducing β-hexosaminidase and indicating a suppressive effect on the degranulation of RBL-2H3 cells (Jippo et al., 2022).
Anti-arthritis activity
The aqueous extract of A. fistulosum with rice porridge revealed significant anti-arthritis activity against monoiodoacetate-induced ovariectomized female rats. The extract increased the body weight, bone mineral density, food intake, lean body mass, and fat mass for 11 weeks and also showed enhancement in collagen deposition score and knee joint gap. Whereas, the extract showed a reduction in peri-uterine, retroperitoneum, visceral fats, serum alkaline, insulin, glucose, homeostasis model assessment estimate of insulin resistance (HOMA-IR), triglyceride, phosphatase activity, areas under the curves, swelling knee score, leg limping score, right hind paws weight distribution, treadmill running velocity, matrix metalloproteinases (MMP-1 & 13) expressions of messenger ribonucleic acid (mRNA), serum interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) concentrations, as well as tibial surface damage score (Yang et al., 2019). Thus, it was concluded that plant has a healing property to cure women’s osteoarthritis.
 | Figure 2. Representative bioactive composition of A. fistulosum (Structure source: https://pubchem.ncbi.nlm.nih.gov). [Click here to view] |
Anti-glycation activity
Advanced glycation end products (AGEs) are a complex class of substances that can be exogenously or endogenously in nature and have a pathogenic role in the onset and progression of oxidative stress-mediated diseases, such as atherosclerosis, diabetes, and neurological conditions (Vistoli et al., 2013). The ethanolic extract (100 μl) of Welsh onion exhibited a significant anti-glycation effect by inhibiting the fluorescent AGEs formation with 27.9%, as compared with positive control; aminoguanidine at 20 mM (75.9%) when monitored after 2 weeks (Ramkissoon et al., 2013). Thus, the Welsh onion can be employed in the management of diverse complications.
Anti-cancer activity
The compounds; N-caffeoyltyramine and isorhamnetin 3-O-galtopyranoside from Welsh onion exhibited an anti-cancer effect on breast cancer (MCF-7) cell line (IC50 94.4 μg/ml) whereas, other compounds showed weak effectiveness with >100 μg/ml (Zolfaghari et al., 2021). Besides, welsonins A1 (50 μM) of A. fistulosum bulbs significantly attenuated the interleukin-10 (IL-10)-induced cluster of differentiation 163 (CD163) expression in human monocyte-derived macrophages, indicating its potential to suppress tumor-cell proliferation through inhibition of macrophages polarization (Nohara et al., 2016). Furthermore, the ethanolic leaves extract inhibited the cancerous cells (MCF-7, DLD-1, SK-MES-1, and MDA-MB-231) with IC50 values of 2.124%, 2.464%, 3.353%, and 5.819%, respectively (Tigu et al., 2021). The aqueous ethanol extract of Welsh onion stem with varied nitrogen (N) levels (N1 and N2 at 130 and 260 kg/ha) showed anti-cancer activities by significantly decreasing the viability of HepG2 and HeLa cell lines. This activity is attributed to the fact that total phenols and flavonoids are significantly affected by N fertilizers (Zhao et al., 2021). The Onionin A1 of A. fistulosum significantly inhibited the IL-10-induced CD163 expression (M2 macrophage marker), tumor culture supernatant-induced CD163 overexpression, and also, reversed the interleukin-12 (IL-12) downregulation and IL-10 upregulation in ovarian cancer (SKOV3) cell. Moreover, Onionin A1 inhibited the cell-cell interactions between macrophages and suppressed the protumoral macrophage function. Simultaneously, Onionin A1 significantly suppressed?the tumor weight and lung metastasis in osteosarcoma (LM-8) cell-bearing C3H mice along with suppression in?tumor?progression of SKOV-3-bearing C57B6 mice (Nohara et al., 2017). Likewise, ethanolic and aqueous extracts (cold and hot) of A. fistulosum were investigated for anti-cancer activity against colon carcinoma (CT-26) cells using a mouse model. The hot-aqueous extract significantly inhibited tumor growth and increased the apoptotic index more than other extracts. Moreover, hot-aqueous extract highly suppressed both cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) activities and the levels of IL-6 and TNF-α than other tested extracts. Similarly, hot-aqueous extract displayed a better reduction of cellular markers that were involved in proliferation (cyclin D1 and c-Myc), angiogenesis (vascular endothelial growth factor and hypoxia-inducible factor 1-alpha), and tumor invasion [MMP-9 and intercellular adhesion molecule (ICAM-1)] (Arulselvan et al., 2012).
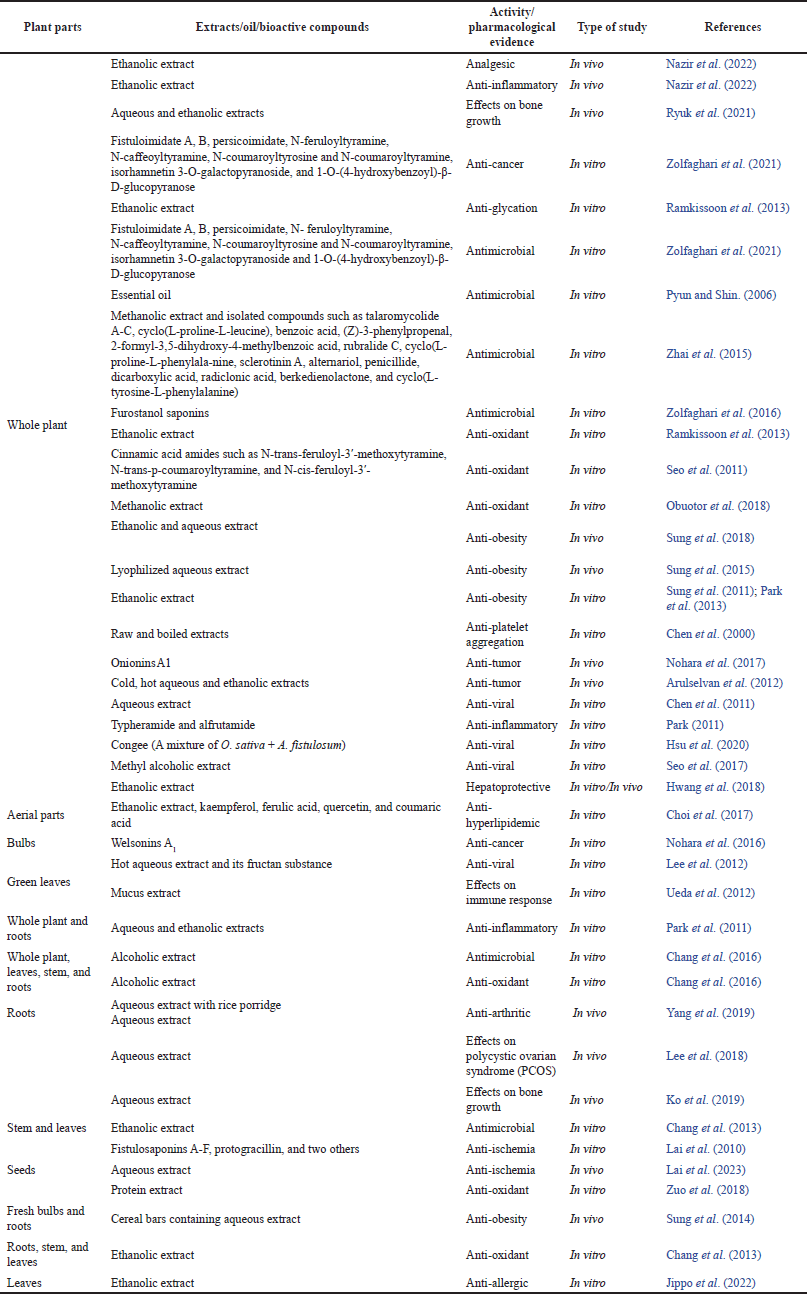 | Table 2. Pharmacological profile of A. fistulosum extracts and compounds. [Click here to view] |
Anti-hyperlipidemic activity
The ethanolic extract, p-coumaric acid, quercetin, ferulic acid, and kaempferol from Welsh onion were investigated for anti-hyperlipidemic activity against delipidated serum (DLPS; a mixture of mevalonate and rosuvastatin)-treated HepG2 cells. The extract significantly reduced the low-density lipoprotein receptor (LDLR), pro-protein convertase subtilisin/kexin type 9 (PCSK9), hepatocyte nuclear factor 1α, sterol regulatory element-binding protein-2 (SREBP-1c) gene expressions, as well as, LDLR protein expressions without affecting the cell viability. Moreover, the extract protected the lysosomal degradation of LDLR protein in the presence of bafilomycin A1 (lysosomal inhibitor) and blocked the protein synthesis of LDLR. Subsequently, all compounds maintained the LDLR level while quercetin, p-coumaric acid, and kaempferol significantly attenuated the PCSK9 level under conditions of lipid depletion. The tested samples can be effective in lowering hypercholesterolemia in humans (Choi et al., 2017).
Anti-ischemia activity
The fistulosaponins (A–F) and protogracillin (0.5–100 μM) of A. fistulosum seeds displayed an anti-ischemia effect against hypoxia/reoxygenation (H/R)-stimulated injury in human umbilical vein endothelial cell (HUVEC). The tested compounds significantly protected HUVEC from injury and increased the survival of H/R-treated cells, where fistulasaponin A was highly resistant to injury even better than positive control dioscin (Lai et al., 2010). Allium fistulosum aqueous seed extract (15, 150, and 300 mg/kg) exerted a protective effect on acute myocardial ischemia in Sprague Dawley rats. Extract and positive control xingling granules (900 mg/kg) significantly decreased serum creatine kinase levels (p < 0.05), lactate dehydrogenase levels, and infarcted area (p < 0.01) as compared with the saline group (Lai et al., 2023). Thus, the Welsh onion showed a beneficial effect on cardiac function in rat model of acute myocardial ischemia.
Anti-inflammatory activity
The typheramide and alfrutamide compounds of Welsh onion potently inhibited the cyclooxygenases-1 (COX1) enzyme even greater than aspirin (COX1 inhibitor), while also inhibiting the lipoxygenases-15 and COX 2 enzymes. However, typheramide is slightly more potent than COX 2-speci?c inhibitor named NS-398 (Park, 2011). On the other hand, A. fistulosum roots aqueous and whole plant ethanolic extracts lowered the mRNA and protein expressions of iNOS, generation of COX-2, and nitric oxide along with, mRNA levels of IL-1β, TNF-α, & IL-6 in lipopolysaccharide (LPS)-stimulated BV2 microglia cells without affecting the cell viability (Park et al., 2011). Subsequently, A. fistulosum ethanolic extract displayed anti-inflammatory properties toward carrageenan-stimulated edema in adult Albino mice. Oral administration with extract (600 mg/kg) and standard ibuprofen significantly reduced edema with 70.27% and 58.10% inhibition, respectively (Nazir et al., 2022). These findings suggested that the plant has the potential to be used for inflammatory disease.
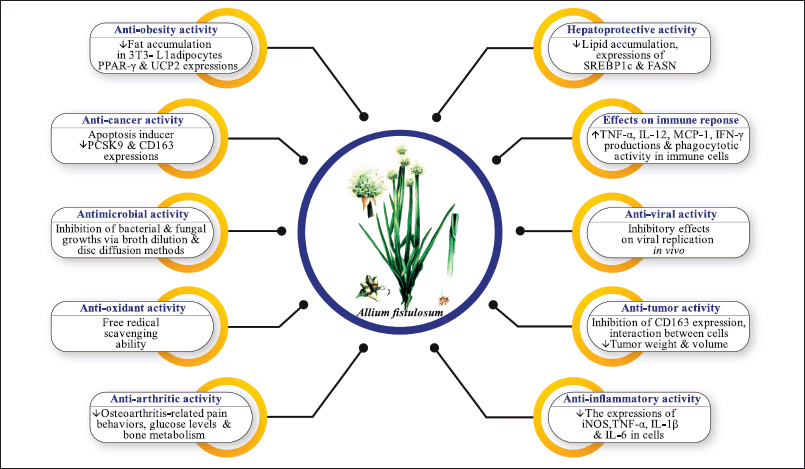 | Figure 3. Mechanistic insights into the biological potential of A. fistulosum. ↑- Increased ↓- Decreased. PPAR-γ: Peroxisome proliferator-activated receptor gamma; CD163: Cluster of differentiation 163; UCP-2: Uncoupling protein-2; ABTS: 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); IL-1β: Interleukin-1β; DPPH: 2,2-diphenyl-1-picrylhydrazyl; IL-6: Interleukin-6; IL-10: Interleukin-10; TNF-α: Tumor necrosis factor-α; IL-12: Interleukin-12; SREBP-1c: Sterol regulatory element-binding protein-2; FASN: fatty acid synthase; MCP-1: Monocyte chemotactic protein-1; IFN-γ: Interferon-γ; CD163: Cluster of differentiation 163; iNOS: Inducible nitric oxide synthase. [Click here to view] |
Antimicrobial activity
The N-coumaroyltyramine from the subterranean part of the Welsh onion showed the highest anti-bacterial effect against Staphylococcus aureus and Escherichia coli. Subsequently, N-feruloyltyramine and N-coumaroyltyrosine attained effectiveness only against S. aureus while, 1-O-(4-hydroxybenzoyl)-β-D-glucopyranose and isorhamnetin 3-O-galactopyranoside were active toward E. coli (Zolfaghari et al., 2021). Similarly, fistulosaponin G-J of A. fistulosum displayed anti-bacterial activity against E. coli and Enterococcus faecalis (Zolfaghari et al., 2016). The methanolic extract along with sclerotinin A, cyclo (L-proline-L-phenylalanine, talaromycolide A-C, cyclo (L-proline-L-leucine), dicarboxylic acid, benzoic acid, 2-formyl-3,5-dihydroxy-4-methylbenzoic acid, (Z)-3-phenylpropenal rubralide C, alternariol, penicillide, radiclonic acid, berkedienolactone, and cyclo (L-tyrosine-L-phenylalanine) of A. fistulosum exhibited anti-bacterial effect against Bacillus megaterium, Bacillus subtilis, Clostridium perfringens, Micrococcus tetragenus and E. coli (Zhai et al., 2015). Likewise, the alcoholic extracts from different parts (root stem and leaf) of A. fistulosum prepared from rice wine (Michiu Tou; 34% and Michiu; 19.5% alcohol) showed antimicrobial ability toward B. subtilis, Pseudomonas aeruginosa, E. coli, and S. aureus where highest potential found with Michiu Tou comparatively Michiu extract (Chang et al., 2016). Also, essential oil from the whole plant exhibited an anti-fungal effect on Trichophyton soudanense, Trichophyton erinacei, and Trichophyton rubrum (Pyun and Shin, 2006). Lastly, the stem and leaves ethanolic extract attained effectiveness toward P. aeruginosa, B. subtilis, E. coli, and S. aureus (Pyun and Shin, 2006).
Anti-obesity activity
Obesity, a metabolic disorder is characterized by an excess accumulation of fats in the body. In this context, several reports revealed the anti-obesity potential of A. fistulosum. The aqueous and ethanolic extracts significantly decreased the lipid accumulation, final body weight, serum level of leptin, weight of adipose tissues, triacylglycerol, alanine transaminase (ALT) levels, and adipocyte area whereas, enhanced the adiponectin level, high-density lipoprotein (HDL)-cholesterol level, adenosine monophosphate-activated protein kinase-α1 and 2 mRNA expressions in liver tissue of high-fat diet (HFD) obese mice. Moreover, ethanolic extract lowered the aspartate transaminase (AST) level, low-density lipoprotein (LDL)-cholesterol, food efficiency ratio, total cholesterol, and peroxisome proliferator-activated receptor gamma (PPAR-γ) mRNA expression, whereas, enhanced uncoupling protein 2 mRNA expression. Thus, extracts can be an ingredient of functional food to control obesity in people (Sung et al., 2018). Similarly, lyophilized whole plant extract (aqueous) significantly reduced the body, liver weight, food efficiency, serum triglyceride, leptin, glucose, total and LDL cholesterol, AST, ALT, creatinine, mRNA uncoupling protein-2 (UCP-2) and adiponectin levels, adipocyte size and fat accumulation while, enhanced the HDL-cholesterol, mRNA PPAR-γ, and leptin levels in HFD obese mice (Sung et al., 2015). Likewise, cereal bars containing A. fistulosum fresh bulbs and roots aqueous extract significantly attenuated the body weight gain, food ef?ciency ratio, subcutaneous and visceral fats, liver, adipocytes area, and other lipid parameters whereas, increased the β3-adrenoreceptors, UCP-2, and PPAR-γ mRNA expressions in HFD obese mice (Sung et al., 2014). Whole plant ethanolic extract significantly attenuated weight gain, triglycerides, total cholesterol, leptin, adiponectin, white adipose tissue area, mRNA expression of SREBP1c, PPARγ and FAS, LDL-cholesterol, and white adipose tissue weight in HFD mice (Sung et al., 2011). In the in vitro study, the extract reduced the intracellular lipid accumulation and free glycerol release by 36.7% and 84%, respectively in 3T3-L1 adipocytes (Park et al., 2013). As a result, the plant might be useful in treating metabolic disorders linked to obesity, such as hyperlipidemia and insulin resistance.
Anti-oxidant activity
Allium fistulosum was reported to be a strong anti-oxidant evident by various studies. The extracts from different parts (root, stem, and leaf) were prepared in rice wine (Michiu Tou; 34% alcohol and Michiu; 19.5% alcohol) and exhibited an anti-oxidant effect with 6.2–15.5 mmol TE/g in Trolox equivalent antioxidant capacity. Further, extracts showed a scavenging effect with IC50 ranging between 14.6 and 26 μg/ml in 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. The effect of alcohol concentrations on the anti-oxidant profile needs further validation (Chang et al., 2016). The ethanolic extract of A. fistulosum showed an anti-oxidant effect with 1.220 and 0.812 mg ascorbic acid/ml using ferric reducing antioxidant power (FRAP) and DPPH assays, respectively (Ramkissoon et al., 2013). Similarly, protein extract from the seeds of this species showed anti-oxidant potential with IC50 of 1.43, 1.37, 2.17, and 0.006 mg/ml in DPPH, hydroxyl, superoxide radical and chelating assays, respectively as well as, this extract increased the reducing power at 700 nm (Zuo et al., 2018). Additionally, ethanolic extract of the whole plant exhibited an anti-oxidant effect with IC50 of 0.58 and 0.24 mg/ml in DPPH and metal chelating assays. Whereas, absorbance with 3.38 and 6.35 mg equivalent of ascorbic acid/g at 563 nm was observed during FRAP and total phosphomolybdate assays, respectively (Obuotor et al., 2018). Some compounds N-trans-feruloyl-3′-methoxytyramine and N-cis-feruloyl-3′-methoxytyramine from the whole plant showed potent DPPH scavenging activity as compared with N-trans-p-coumaroyltyramine (Seo et al., 2011). The ethanolic extracts of A. fistulosum stem and leaves displayed higher DPPH and 2,2-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) scavenging effects than that of root extract (Chang et al., 2013). Besides, the aqueous ethanol extract of Welsh onion stems with different nitrogen levels (N1 and N2: 0130 and 260 kg/ha) as urea fertilizer showed DPPH and ABTS scavenging effects (Zhao et al., 2021). Thus, A. fistulosum can be used to develop antioxidant-rich food products that will help in the fight against oxidative stress-mediated disorders.
Anti-platelet aggregation activity
The raw and boiled A. fistulosum green leaf and white sheath extracts were evaluated against adenosine diphosphate (ADP)-stimulated platelet aggregation where green extracts significantly and potently suppressed the platelet aggregation and adhesion more than white extracts. In addition, green extracts significantly affected the thromboxane release from platelets and also stimulated the cyclic adenosine monophosphate (cAMP) level. Moreover, ADP stimulation of platelets caused their [Ca2+] elevation, which was suppressed by the green extract (raw) while increased by green extract (boiled). Furthermore, the raw extract also stimulated cAMP levels (Chen et al., 2000). Thus, results revealed that raw extracts inhibited platelet function while boiled extracts activate platelets.
Anti-viral activity
Welsh onion was reported to be broad spectrum anti-viral agent. For instance, A. fistulosum congee (40–80 g with 50 g rice/400 ml water) was evaluated against SARS-COV-2. Administration with congee on day 1 in male patient resulted in sweating, phlegm release, and a decrease from 38.5°C to 37.3°C in body temperature after 2–3 hours. Subsequently next day, more phlegm was discharged and the temperature declined to 36.7°C. Interestingly, only one patient completely recovered in 11 days, while other patients were recovered usually within 3–4 days after consuming congee (Hsu et al., 2020). The hot aqueous extract along with its compound, fructan from leaves showed an effect on influenza A virus (IFV-As)-infected mice, with a significant decrease in virus replication (titer) in bronchoalveolar lavage ?uid (BALF) and lung, followed by increased production of neutralizing antibodies in BALFs. Whereas, only fructan enhanced neutralizing antibodies production in sera of mice. Additionally, from day 3 to day 7, extract and compound both showed a consistent drop in body weight which was recovered after day 9 (Lee et al., 2012). Whole plant aqueous extract attained an effective response on adenovirus (ADV41 and ADV3)-infected human lung carcinoma (A549) cells that exhibited an anti-viral effect closer to its cytotoxic concentration at EC50 value >960 μg/ml (Chen et al., 2011). In contrast, whole plant methyl alcoholic extract significantly reduced the hepatitis A virus titer by 2.07 log after co-treatment at 50 μg/ml (Seo et al., 2017).
Effects on bone growth
The Welsh onion roots aqueous extract increased the longitudinal bone growth by promoting insulin-like growth factor-1 and transforming growth factor signaling, with a significant decrease in body weight gain, retroperitoneal, visceral fat, serum osteocalcin, and total area under the curve, along with a notable increase in fasting serum glucose, insulin, HOMA-IR, serum ALP, femur, tibia bones length, and hypertrophic growth in weanling rats. In addition, the extract markedly improved the survival, alkaline phosphatase (ALP) activity, and mRNA gene expressions of bone morphogenetic protein-2 and Smad4 while, decreased the dickkopf-related protein-1, phosphorylation of insulin receptor substrate-2 and Akt in MG-63 osteoblast cell (Ko et al., 2019). Hence, the longitudinal bone growth of children and adolescents can be achieved by utilizing Welsh onion. Allium fistulosum extracts (aqueous and 30% ethanolic) demonstrated facilitation of bone growth in vitamin D and calcium de?cient C57BL/6 mice. Both extracts (150 and 450 mg/kg) significantly increased bone mineral density (BMD), bone mineral content, and growth in a way that encouraged the development of the osteogenic markers (calcium, collagen type 1, ALP, and osteocalcin) in the bone. Additionally, both extracts also displayed signi?cant alkaline phosphate activities. Thus, A. fistulosum can be used to develop functional foods that will promote bone formation and raise BMD (Ryuk et al., 2021).
Effects on immune response enhancement
The mucus extract from green leaves of A. fistulosum showed significant enhancement of TNF-α and monocyte chemotactic protein-1 (MCP-1) in leukemic monocytes (RAW 264) and IL-12 in macrophage-like J774.1 cells in vitro and also increased the TNF-α, IL-12 and phagocytotic activity in peritoneal cells, LPS-interferon-γ (IFN-γ) production in spleen cells and the activity of natural killer cells of mice in vivo (Ueda et al., 2012). These findings revealed that A. fistulosum can enhance natural immunity and can be used as a major defense against various diseases.
Effects on polycystic ovary syndrome (PCOS)
Allium fistulosum’s roots extract (aqueous) significantly decreased the blood triacylglycerol, luteinizing hormone, number of follicular cysts, and relative mRNA expressions of luteinizing hormone receptor whereas, increased the blood glucose, relative mRNA expressions of Kitl, bone morphogenetic protein, progesterone receptor, estrogen receptor 1, Cyp19a1, restoration of estrogen and plasma testosterone levels in letrozole-induced PCOS female Sprague Dawley rats. Additionally, the extract normalized the estrus cycle as indicated by the presence of epithelial nucleated and corni?ed cells in rats (Lee et al., 2018). Thus, the extensive property of this plant is mediated by normalizing hormonal levels that were altered by PCOS.
Hepatoprotective activity
The ethanolic extract of A. fistulosum significantly reduced the lipid accumulation, mRNA expression of SREBP1c, and fatty acid synthase (FASN) in oleic acid (OA)-induced non-alcoholic fatty liver disease model using HepG2 cells, without affecting the cell viability. Moreover, the extract significantly attenuated body, liver, and epididymal fat weight, food efficiency ratio, and plasma levels of ALP, ALT, and AST in the western diet-fed obese mice (Hwang et al., 2018).
Vasorelaxant activity
The raw and boiled A. fistulosum green leaf and white sheath extracts-induced vasodilatation in norepinephrine (NE)-precontracted aortae of rats, where green extract (<5 × 10−4 g/ml) stimulated vasorelaxation that was completely inhibited in the presence of indomethacin (inhibitor of prostanoid synthesis). Moreover, boiled green and white extracts evoked the vasocontractile responses in NE-precontracted or resting vessel rings, which was abolished by thromboxane A2 (TXA2)-receptor antagonist, SQ29548 as well as, the release of endothelium-derived contracting factor, TXA2 was also stimulated by white extract (Chen et al., 1999).
CLINICAL EVIDENCE OF A. fistulosum’s BIOLOGICAL POTENTIAL
Welsh onion has been proclaimed to have health benefits, several formulations reported the efficacy of its formulations against different diseases but effective targeted constituents have not been focused on a large scale. In this context, some studies highlighted the medicinal efficacy of individual or herbal formulations containing A. fistulosum has been included here. A 29-year-old male patient was diagnosed with wind-cold joined by dampness, characterized by an impaired flow of Lung-Qi. He was given a detoxifying powder formulation (Jing Fang Bai Du San) consisting of A. fistulosum (three pieces), Citrus tangerina (6 g), Schizonepeta (5 g) along with other ingredients. It was observed that after one dose, the patient sweated and recovered from cold and fever with improvement in headache and body pain along with pulse stabilization (Li, 2006). The children (3rd and 25th percentiles in height, aged between 5 and 12 years) had significant enhancement in height after treatment with A. fistulosum root extract (5 g) in a double-blind and placebo-controlled trial (Shim et al., 2021). The clinical studies of the bioactive components of the plant should be futuristic work for researchers to develop novel efficacious remedies which are safe and cost-effective.
CONCLUSION AND FUTURE PERSPECTIVES
In conclusion, the information about medicinal uses, chemical constituents, and pharmacological profile of A. fistulosum is documented in this study. Traditionally, this plant has been used for treating cold, influenza, abdominal pain, cephalgia, ulcers, parasitic infestations, arthritis, and cardiac disorders in several countries. Fistuloimidates, onionins A1, cinnamic acid amides, flavonoids, and fistulosaponins constitute the bioactive composition of A. fistulosum which are reported to be anti-obesity, anti-cancer, anti-oxidant, antimicrobial as well as anti-viral agents. Several pharmacological actions were reported using different plant parts but the active phytochemicals and their mechanistic insights are still obscure. A few clinical trials of A. fistulosum were reported with the plant alone or as an ingredient of herbal formulations. Allium fistulosum as well as its bioactive composition could be developed as a drug-developing candidate after detailed follow-up studies.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to the revered Swami Ramdev for his inspiration. Further, the authors are thankful to the Ministry of AYUSH under Grant-in-Aid for the Establishment of the Centre of Excellence of Renovation and Upgradation of Patanjali Ayurveda Hospital, Haridwar. Mr. Harshit Thakur, a designer at Patanjali Research Institute assisted the authors with the infographics.
FINANCIAL SUPPORT
This research was supported by the Ministry of Ayush, Government of India, under Ayurswasthya Yojana-3988.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Arulselvan P, Wen CC, Lan CW, Chen YH, Wei WC, Yang NS. Dietary administration of scallion extract effectively inhibits colorectal tumor growth: cellular and molecular mechanisms in mice. PLOS One, 2012; 7(9):e44658. CrossRef
Balkrishna A, Rohela A, Kumar A, Kumar A, Arya V, Thakur P, Oleksak P, Krejcar O, Verma R, Kumar D, Kuca K. Mechanistic insight into antimicrobial and antioxidant potential of Jasminum species: a herbal approach for disease management. Plants, 2021; 10(6):1–25.
Bhat GK, Nagandram CR. Sedges and grasses (Dakshina Kannada and Udupi districts). Bishan Singh Mahendra pal Singh, Dehradun, India, 2001. CrossRef
Chang TC, Chang HT, Chang ST, Lin SF, Chang YH, Jang HD. A comparative study on the total antioxidant and antimicrobial potentials of ethanolic extracts from various organ tissues of Allium spp. Food Sci Nutr, 2013; 4:182–90. CrossRef
Chang TC, Jang HD, LinWD, Duan PF. Antioxidant and antimicrobial activities of commercial rice wine extracts of Taiwanese Allium fistulosum. Food Chem, 2016; 190:724–9. CrossRef
Chen CH, Chou TW, Cheng LH, Ho CW. In vitro anti-adenoviral activity of five Allium plants. J Taiwan Inst Chem Eng, 2011; 42(2):228–32. CrossRef
Chen JH, Chen HI, Wang JS, Tsai SJ, Jen CJ. Effects of welsh onion extracts on human platelet function in vitro. Life Sci, 2000; 66(17):1571–9. CrossRef
Chen JH, Tsai SJ, Chen HI. Welsh onion (Allium fistulosum L.) extracts alter vascular responses in rat aortae. J Cardiovasc Pharmacol, 1999; 33(4):515–20. CrossRef
Choi HK, Hwang JT, Nam TG, Kim SH, Min DK, Park SW, Chung MY. Welsh onion extract inhibits PCSK9 expression contributing to the maintenance of the LDLR level under lipid depletion conditions of HepG2 cells. Food Funct, 2017; 8(12):4582–91. CrossRef
Duke JA, Vasquez R. Amazonian ethnobotanical dictionary. CRC Press, Boca Raton, FL, 1994.
Eflora-Flora of China. Allium fistulosum (L). 2023. Available via http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200027477
Hsu E, Zhu B, Ding Z. Allium fistulosum congee as a home remedy to ward off the corona virus at an early stage. Integr Med Res, 2020; (3):1–2.
Husori D, Bancin DY, Muhaimin BS, Patilaya P. Anthelmintic Activity of ethanolic and aqueous extracts of Allium fistulosum L. leaves on Ascaris lumbricoides. Int J Pharm, 2016; 8(9):1310–3.
Hwang JT, Ryuk JA, Kim HJ, Jung DH, Ko BS. Validation study on the geometric isomers from bulbs of Allium fistulosum and their conversion. Appl Biol Chem, 2020; 63(1):1–2. CrossRef
Hwang JT, Shin EJ, Chung MY, Park JH, Chung S, Choi HK. Ethanol extract of Allium fistulosum inhibits development of non-alcoholic fatty liver disease. Nutr Res Pract, 2018; 12(2):110. CrossRef
Jagessar RC. Plant extracts as an alternative to synthetic medicines. Drug Design Intellect Prop Int J, 2020; 3(3):366–9.
Jippo T, Kobayashi Y, Kitada K, Kitsuda K. Anti-allergic activity of an ethanol extract of bunching onion (Allium fistulosum), a traditional vegetable from Osaka. J Funct Food Health Dis, 2022; 12(3):128–33. CrossRef
Kim CM, Son KH, Kim, SH, Kim HP. Steroidal sapogenin contents in some domestic plants. Arch Pharm Res, 1991; 14:305–10. CrossRef
Ko BS, Ryuk JA, Hwang JT, Zhang T, Wu X, Kim HJ, Park S. Allium fistulosum (Welsh onion) and Portulaca oleracea increase longitudinal bone growth in weanling rats possibly by promoting TGF-β and IGF-1 signaling. J Funct Foods, 2019; 58:151–60. CrossRef
Lai W, Wu Z, Lin H, Li T, Sun L, Chai Y, Chen W. Anti-ischemia steroidal saponins from the seeds of Allium fistulosum. J Nat Prod, 2010; 73(6):1053–7. CrossRef
Lai W, Xu D, Zheng Z, Lu W, Wu Z, Chen W. Protective effect of the seeds of Allium fistulosum extract against acute myocardial ischemia in rats and dogs. J Funct Foods, 2023; 101:105413. CrossRef
Lee JB, Miyake S, Umetsu R, Hayashi K, Chijimatsu T, Hayashi T. Anti-influenza A virus effects of fructan from Welsh onion (Allium fistulosum L.). Food Chem, 2012; 134(4):2164–8. CrossRef
Lee YH, Yang H, Lee SR, Kwon SW, Hong EJ, Lee HW. Welsh onion root (Allium fistulosum) restores ovarian functions from letrozole induced-polycystic ovary syndrome. Nutrients, 2018; 10(10):1430. CrossRef
Lengbiye EM, Mbadiko CM, Falanga CM, Matondo A, Inkoto CL, Ngoyi EM, Mpiana, PT. Antiviral activity, phytochemistry and toxicology of some medically interesting Allium species: a mini review. Int J Pathog Res, 2020; 5(4):64–77.
Li TS. Taiwanese native medicinal plants: phytopharmacology and therapeutic values. CRC Press, Boca Raton, FL, 2006. CrossRef
Lim TK. Edible medicinal and non-medicinal plants. Fruits. Springer Science & Business Media, Houten, The Netherlands, vol. 2, 2013. CrossRef
Liu X, Gao S, Liu Y, Cao B, Chen Z, Xu K. Comparative analysis of the chemical composition and water permeability of the cuticular wax barrier in Welsh onion (Allium fistulosum L.). Protoplasma, 2020; 257:833–40. CrossRef
Longe JL. (Ed.). The Gale encyclopedia of alternative medicine. 2nd edition, Thomson Gale Corporation, Farmington Hills, MI, vol. 4, 2005.
Maqbool M, Dar MA, Gani I, Mir SA, Khan M. Herbal medicines as an alternative source of therapy: a review. World J Pharm Sci, 2019; 3:374–80.
Nazir S, Afroz S, Tauseef H, Afsheen H, Farooqui R, Rizvi A. Phytochemical analysis, safety profile, analgesic, and anti-inflammatory effect of ethanol extract of Allium fistulosum L. Pak Euro J Med Life Sci, 2022; 5(1):135–46. CrossRef
Nohara T, Fujiwara Y, El-Aasr M, Ikeda T, Ono M, Nakano D, Kinjo J. Antitumor Allium sulfides. Chem Pharm Bull, 2017; 65(3):209–17. CrossRef
Nohara T, Fujiwara Y, Ikeda T, Murakami K, Ono M, El-Aasr M, Kinjo J. Two new bicyclic sulfoxides from Welsh onion. J Nat Med, 2016; 70(2):260–5. CrossRef
Obuotor EM, Ola-Mudathir KF, Wahab AA, Moshood AI. Comparative evaluation of antioxidant properties of methanol extracts of Allium cepa bulb, Allium cepa bulb peels and Allium fistulosum. Kragujevac J Sci, 2018; 40:131–41. CrossRef
Park HS, Choi EJ, Lee JH, Kim GH. Evaluation of Allium vegetables for anti-adipogenic, anti-cancer, and anti-inflammatory activities in vitro. J Life Sci, 2013; 5(2):127–32. CrossRef
Park JB. Effects of typheramide and alfrutamide found in Allium species on cyclooxygenases and lipoxygenases. J Med Food, 2011; 14(3):226–31. CrossRef
Park SH, Kim JI, Jeong YK, Choi YH. Extracts of Allium fistulosum attenuates pro-inflammatory action in the lipopolysaccharide-stimulated BV2 microglia cells. J Life Sci, 2011; 21(6):796–804. CrossRef
Prajapati ND, Kumar U. Agro’s: dictionary of medicinal plants. Agrobios, Jodhpur, India, 2003.
Prajapati ND, Purohit SS, Sharma AK, Kumar T. A handbook of medicinal plants: a complete source book. Agrobios, Jodhpur, India, 2003.
Pyun MS, Shin S. Antifungal effects of the volatile oils from Allium plants against Trichophyton species and synergism of the oils with ketoconazole. Phytomedicine, 2006; 13(6):394–400. CrossRef
Quattrocchi U. CRC World dictionary of medicinal and poisonous plants. CRC Press, Boca Raton, FL, vol. 1, 2020.
Ramkissoon JS, Mahomoodally MF, Ahmed N, Subratty AH. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac J Trop Med, 2013; 6(7):561–9. CrossRef
Ryuk JA, Kim HJ, Hwang JT, Ko BS. Effect of Allium fistulosum extracts on the stimulation of longitudinal bone growth in animal modeling diet-induced calcium and vitamin D deficiencies. Appl Sci, 2021; 11(17):1–3. CrossRef
Sang S, Lao A, Wang Y, Chin CK, Rosen RT, Ho CT. Antifungal constituents from the seeds of Allium fistulosum L. J Agric Food Chem, 2002; 50(22):631821. CrossRef
Seo DJ, Lee M, Jeon SB, Park H, Jeong S, Lee BH, Choi C. Antiviral activity of herbal extracts against the hepatitis A virus. Food Control, 2017; 72:9–13. CrossRef
Seo GW, Cho JY, Moon JH, Park KH. Isolation and identification of cinnamic acid amides as antioxidants from Allium fistulosum L. and their free radical scavenging activity. Food Sci Biotechnol, 2011; 20(2):555. CrossRef
Sharma K, Verma R, Kumar D, Nepovimova E, Ku?a K, Kumar A, Raghuvanshi D, Dhalaria R, Puri S. Ethnomedicinal plants used for the treatment of neurodegenerative diseases in Himachal Pradesh, India in Western Himalaya. J Ethnopharmacol, 2022; 293:115318
Sharma S, Kumari A, Dhatwalia J, Guleria I, Lal S, Upadhyay N, Kumar V, Kumar A. Effect of solvents extraction on phytochemical profile and biological activities of two Ocimum species: a comparative study. J Appl Res Med Aromat Plant, 2021; 25:100348. CrossRef
Shim SB, Ko BS, Ryuk JA, Lee JH, Lee HB, Ha KC, Lee, HL Randomized, double-blind, and placebo-controlled human trial to evaluate the efficacy and safety of Allium fistulosum L. root extract on improvement of child height growth: study protocol. Korean J Pediatr, 2021; 35(2):11–20.
Sonam KA, Kumar V, Guleria I, Sharma M, Kumar A, Alruways MW, Khan N, Raina R. Antimicrobial potential and chemical profiling of leaves essential oil of Mentha species growing under North-West Himalaya conditions. J Pure Appl Microbiol, 2021; 15(4):2229–43. CrossRef
Stuart GA. Chinese materiamedica Part-1: vegetable kingdom. Gordon Press, Gordon, NE, 1911.
Sung YY, Kim DS, Kim SH, Kim HK. Aqueous and ethanolic extracts of Welsh onion, Allium fistulosum, attenuate high-fat diet-induced obesity. BMC Complement Altern Med, 2018; 18(1):1–1. CrossRef
Sung YY, Kim SH, Kim DS, Park SH, Yoo BW, Kim HK. Nutritional composition and anti-obesity effects of cereal bar containing Allium fistulosum (Welsh onion) extract. J Funct Foods, 2014; 6:428–37. CrossRef
Sung YY, Kim SH, Yoo BW, Kim HK. The nutritional composition and anti-obesity effects of an herbal mixed extract containing Allium fistulosum and Viola mandshurica in high-fat-diet-induced obese mice. BMC Complement Altern Med, 2015; 15(1):1–9. CrossRef
Sung YY, Yoon T, KiM SJ, Yang WK, KiM HK. Anti-obesity activity of Allium fistulosum L. extract by down-regulation of the expression of lipogenic genes in high-fat diet-induced obese mice. Mol Med Rep, 2011; 4(3):431–5.
Terada Y, Arisawa M, Nishida A. Synthesis of the putative structure of fistulosin using the ruthenium-catalyzed cycloisomerization of diene. J Org Chem, 2006; 71(3):1269–72. CrossRef
Tigu AB, Moldovan CS, Toma VA, Farca? AD, Mo? AC, Jurj A, Pârvu M. Phytochemical analysis and in vitro effects of Allium fistulosum L. and Allium sativum L. extracts on human normal and tumor cell lines: a comparative study. Molecules, 2021; 26(3):574. CrossRef
Tugume P, Nyakoojo C. Ethno-pharmacological survey of herbal remedies used in the treatment of paediatric diseases in Buhunga parish, Rukungiri District, Uganda. BMC Complement Altern Med, 2019; 19(1):1–10. CrossRef
Ueda H, Takeuchi A, Wako T. Activation of immune responses in mice by an oral administration of bunching Onion (Allium fistulosum) mucus. Biosci Biotechnol Biochem, 2013; 77(9):1809–13. CrossRef
Vistoli G, De Maddis D, Cipak A, Zarkovic N, Carini M, Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic Res, 2013; 47(sup1):3–27. CrossRef
Vlase L, Parvu M, Parvu EA, Toiu A. Phytochemical analysis of Allium fistulosum L. and A. ursinum L. Dig J Nanomater Biostructures, 2013; 8(1): 457–67.
WHO. Traditional medicine strategy, 2014-2023. 2013. Available via https://apps.who.int/iris/bitstream/handle/10665/92455/9789241506090_eng.pdf (Accessed 21 December 2022).
Yang HJ, Kim MJ, Qiu JY, Zhang T, Wu X, Jang DJ, Park S. Rice porridge containing Welsh onion root water extract alleviates osteoarthritis-related pain behaviors, glucose levels and bone metabolism in osteoarthritis-induced ovariectomized rats. Nutrients, 2019; 11(7):1503. CrossRef
Zhai MM, Niu HT, Li J, Xiao H, Shi YP, Di DL, Wu QX. Talaromycolides A-C, novel phenyl-substituted phthalides isolated from the green Chinese Onion-derived fungus Talaromyces pinophilus AF-02. J Agric Food Chem, 2015; 63(43):9558–64. CrossRef
Zhao C, Wang Z, Cui R, Su L, Sun X, Borras-Hidalgo O, Zhao L. Effects of nitrogen application on phytochemical component levels and anticancer and antioxidant activities of Allium fistulosum. PeerJ, 2021; 9:e11706. CrossRef
Zolfaghari B, Yazdiniapour Z, Sadeghi M, Akbari M, Troiano R, Lanzotti V. Cinnamic acid derivatives from Welsh onion (Allium fistulosum) and their antibacterial and cytotoxic activities. Phytochem, 2021; 32(1):84–90.
Zolfaghari B, Yazdiniapour Z, Sadeghi M, Troiano R, Lanzotti, V. Furostanol saponins from the bulbs of Welsh onion, Allium fistulosum L. Planta Med, 2016; 82(18):1584–90. CrossRef
Zuo M, Liu XX, Liu D, Zhao HY, Xuan LL, Jiang WX, Li WZ. Extraction, characterization and antioxidant activity in vitro of proteins from Semen Allii Fistulosi. Molecules, 2018; 23(12):1–12. CrossRef