INTRODUCTION
Streptococcus anginosus group (SAG) is classified under the category of Gram-positive streptococci, common commensals in the upper airway, gastrointestinal, and progenitive pathways consisting of three well-defined species: S. intermedius, S. constellatus, and S. anginosus (Fazili et al., 2017). As a commensal/microbiota, this microorganism cannot cause any pathogenic infection in an immunocompetent individual. Nevertheless, when certain risk factors exist, the colonized SAG can induce noninvasive infections independently. On the other hand, it can cause quick and aggressive infections in a sterile environment by entering through the bloodstream and serosal cavities, ultimately causing infection in the tissues and organs of different systems (Jiang et al., 2020). Additionally, it also tends to cause pyogenic infections with abscess formation. SAG was formerly thought to cause oral cavity infections, but subsequent case reports have revealed that it can also cause infections of other body parts, including the skin, soft tissue, lungs, and liver. The fact that these opportunistic pathogens could cause invasive infections is not well known. This case report highlights the challenge of interpreting microbiological analysis in forensic practice, mainly when commensal organisms were grown in the culture.
CASE REPORT
In August 2021, a 41-year-old male was brought in dead to the forensic department by the police. A legal order to perform an autopsy examination was issued (Pol 61) by the police to ascertain the cause of death. As a routine practice, history was obtained from the next of kin, and a complete autopsy was performed by the forensic pathologist at 12 hours postmortem interval (PMI) to determine the cause of death of the deceased. The PMI was established from rectal temperature and was consistent with the time of death pronounced by a paramedic at the scene.
Autopsy findings
At autopsy, the body was that of a customarily built male with a Body Mass Index (BMI) of 20.4. Pallor was seen on the lower palpebrae conjunctiva and finger nailbeds. Sclera showed a tinge of jaundice. The external autopsy examination revealed that no fatal injuries were found on the body. The internal autopsy examination showed no pathology changes in the head and neck regions. In the thoracic region, no rib fracture was seen. Both pleura cavities were intact and clear. There was dense adhesion between the posterior surface of the upper portion of the right-sided pulmonary tree to the right parietal pleura. Both lungs were congested and edematous upon cut section. No consolidation was felt. The pericardium contained 250 ml of purulent fluid with strands of stringy pale fibrin between the visceral and parietal pericardium (Fig. 1). The heart weighed 230 g and appeared dusky and flabby. The coronaries were patent and typically arose from the aorta. All the valves showed no calcifications or vegetation. The myocardium was healthy, and no area of fibrosis or hemorrhage.
The peritoneum was filled with 300 ml of straw-colored fluid. There was hepatomegaly (1,872 g) with multiple variable sizes of liver abscesses. The largest was 7 × 6 × 3 cm, and the smallest was 3 × 2 × 2 cm (Fig. 2). A stent was found in the pancreatic duct (Fig. 3). Pancreatic tissue showed no evidence of hemorrhage or necrosis grossly. Both kidneys were unremarkable, and the remaining abdominal organs appeared normal.
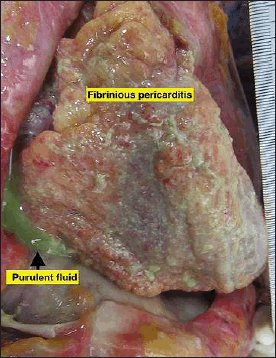 | Figure 1. The pericardium contained 250 ml of purulent fluid with strands of stringy pale fibrin between the visceral and parietal pericardium. [Click here to view] |
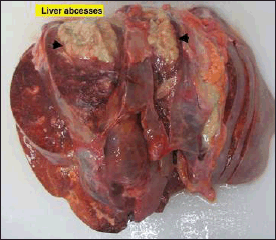 | Figure 2. The liver showed hepatomegaly (1,872 g) with multiple variable-sized liver abscesses. The largest was 7 × 6 × 3 cm, and the smallest was 3 × 2 × 2 cm. [Click here to view] |
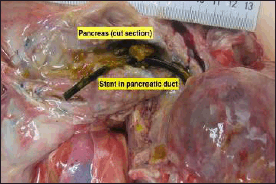 | Figure 3. The pancreatic duct is the presence of a stent in the lumen. [Click here to view] |
Laboratory investigations
The microbiology specimens were taken by an aseptic technique to minimize contamination. The heart and liver tissue cultures revealed S. anginosus, whilst pericardial and peritoneal fluids showed mixed growth of S. anginosus and other microorganisms. On the other hand, the blood culture and sensitivity grew Bacillus species (aerobic) and Staphylococcus hominis (anaerobic). The biochemistry and toxicology analyses were all within the normal range.
Histology findings
Pathologic tissues and representative organs, including skin, were taken for microscopic examination. The liver tissue showed an area of necrotic hepatocytes with abundant inflammatory infiltrates, predominantly neutrophils, lymphocytes, plasma cells, and Kupffer cells. The portal triad showed increased lymphocytic infiltrates and bile duct hyperplasia. The adjacent intact liver tissue shows marked sinusoidal congestion and no evidence of parasitic infiltration or malignancy. The pericardium and heart tissues showed generalized infiltrations, predominantly neutrophils at the superficial epicardial layers, with proof of mesothelial cell metaplasia. The myocardium and endocardium showed no ischemia or myocarditis changes. The lungs section showed multifocal infiltration by neutrophils, lymphocytes, and plasma cells within the edematous alveoli and no evidence of microabscess or granulomatous lesion. Pancreatic tissue with a stent shows foci of lymphocytic infiltrates in the background of fibrosis, and patchy lymphocytic infiltrates are seen at the peripancreatic fat area. No evidence of fat necrosis and neutrophilic infiltrates in the area adjacent to the stent. The skin, kidneys, spleen, adrenal, and brain were congested.
DISCUSSION
It is a routine forensics practice that history was taken from the next of kin. The history obtained was often incomplete since the next of kin and the deceased did not live together. Due to this limitation, the history obtained is occasionally unreliable. This could lead to the wrong approach to managing the autopsy and incorrectly handling the necessary ancillary tests. However, this problem can be tackled by performing a complete autopsy. The burden of responsibilities lies on a forensic pathologist to conscientiously analyze the case before reaching a reasonable conclusion on the cause of death.
The clinical significance of these SAG strains in causing pulmonary infection and empyema has only recently been identified. According to previous literature, SAG strains can enter the thoracic cavity through aspiration of oral discharge, direct impregnation following injury or surgery, and spread from adjacent bordering focus points of infection such as subphrenic abscesses and hematogenic propagation (Hocken and Dussek, 1985; Okada et al., 2013).
The case reports of S. anginosus pericarditis are limited; moreover, this is an autopsy-based diagnosis describing a documented infection with purulent pericarditis furnishing manifold liver abscesses. In this case, pericarditis was caused by local spread from the nearby liver abscess. It has been postulated that SAG commensal gains access to the liver via the earlier stenting procedure in the pancreas. This was supported by the microscopic examination of the adjacent pancreatic tissue that showed chronic inflammatory changes, indicating that the tissue response to infection occurred for quite some time. The age of the tissue response is further confirmed by retrospective inquiry to the next of kin regarding the date of previous surgery. Since the liver abscess was located adjacent to the heart, this commensal may have reached the pericardial sac by direct transmission from the liver abscess. Nowadays, purulent pericarditis has grown scarce.
After everything, it is a crucial impediment to lung infections such as pneumonia. In this case, the abdomen and chest were principally taken in, and most infections originated from S. anginosus, which accords with formerly published research reports (Claridge et al., 2001; Junckerstorff et al., 2014). It is interesting to understand the immune response of the deceased. In this case report, although the autopsy findings revealed significant pathologic changes in the affected organs, the history elicited from the next of kin failed to address any illness symptoms on the deceased. Age, cytokines, and their antagonists play substantial factors in host immune responses to infection and inflammatory stimuli. Catania et al. (1997) described that in elderly patients, the plasma concentration of the interleukin-1 (IL-1) receptor antagonist and soluble tumor necrosis factor-alpha receptor were high; therefore, febrile reactions toward underlying infection are inhibited (Cartmell et al., 2001; Catania et al., 1997; Stefferl et al., 1996). Older patients with pneumonia present with considerably fewer symptoms than younger patients. El-Solh et al. (2001)reported that 44% of very old patients with severe pneumonia presented with fever (Li et al., 2015).
The analysis of microbiology tissue culture in forensic practices is challenging. The presence of the microorganism in the autopsy tissue culture does not necessarily indicate genuine infections. To conclude that death is due to infections, it must incorporate the gross pathological changes of the organs, supported by vital reactions in the microscopic examination and the presence of the pathogen in the tissue culture. The presence of the microorganism after death can be regarded as normal postmortem changes. The earlier studies stated that two salient theories explain bacterial magnification in postmortem blood and tissue cultures either via (i) agonal spread or (ii) postmortem bacterial transmigration. In the former, it is best understood that the bacteria can invade the bloodstream after death during the agonal period because the systemic circulation is de-escalating or artificially sustained during resuscitation. In 1916, this concept was first introduced and was later supported by other researchers (Fredette, 1916; Koneman and Davis, 1974; Riedel, 2014). On the contrary, this concept was argued by other researchers in 1975. They disagreed and published their findings on bacteremia and postmortem microbiology in children suffering from burn wounds (Smith, 1975).
On the other hand, in the latter, the action by which the microorganism migrates from mucosal surfaces and tissues into the blood flow after circulation has halted is used to describe the term by multiple studies “postmortem bacterial transmigration” (Morris et al., 2007; Riedel 2014; Saegeman et al., 2009). The process has first described this process in 1904 (Gradwohl, 1904) and was subsequently supported by findings in other studies (Carpenter and Wilkins, 1964; Epstein and Kugel, 1929; Rose and Hockett, 1971). A few studies revealed bacterial transmigration in animal studies and in vitro experiments combined with the verification provided by observing the autopsy series (Burn, 1934; Kellerman et al., 1976). Although only a few historical data exist in support of the concept of agonal spread is merely theoretical, the theory of bacterial transmigration has far more evidence in the most scientific and medical literature (Tuomisto et al., 2013). Kellerman and his colleagues reported that bacteria could migrate through the intact human intestinal wall within 12–15 hours after death based on in vitro experiments (Kellerman et al., 1976).
Even though death has been categorized as natural death due to infection, conscientious interpretation of microbiological analysis in autopsy is vital. This case report highlights the infection caused by a commensal and this autopsy-based diagnosis may benefit the clinician. Ignoring the potential of a commensal may lead to deteriorating illness and even death in a patient.
CONCLUSION
It has been reported that SAG possesses the potential to generate pyogenic infections irrespective of age because of its opportunistic pathogenic quality. The capability of distinguishing between true-positive culture findings and postmortem transmigration and/or contaminant exists in considerable defiance to microbiologists and pathologists. This case exemplifies the importance of a careful approach in dealing with the commensal pathogen. An understanding of postmortem microbiology interpretation is crucial to analyze a case comprehensively. Finding a commensal organism in a postmortem sample is not unusual, and the correlation with gross and histology findings is critical for determining the cause of death with certainty. Though history in forensic practice is just “hearsay,” it should not be excluded and must be illicit retrospectively.
ACKNOWLEDGMENT
The authors would like to thank the Director General of Health Malaysia for the permission to publish this article.
AUTHOR CONTRIBUTIONS
NAR is the first author that prepared this article and assisted in the autopsy. SAMS, a forensic pathologist, led the autopsy. The corresponding author MH and author RA supervised this preparation of articles.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
FUNDING
There is no funding to report.
INFORMED CONSENT
The anonymity of the subject and confidentiality were well preserved. Written informed consent was obtained from the next of kin of the deceased for the publication of this case report and accompanying images. The next of kin were informed regarding the findings after the postmortem and the usage of the tissues for this case report remains anonymous.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Burn CC. Experimental studies of post-mortem bacterial invasion in animals. J Infect Dis, 1934; 54(3):388–94. CrossRef
Carpenter HM, Wilkins RM. Autopsy bacteriology: review of 2,033 cases. Arch Pathol, 1964; 77:73–81.
Cartmell T, Luheshi GN, Hopkins SJ, Rothwell NJ, Poole S. Role of endogenous interleukin-1 receptor antagonist in regulating fever induced by localised inflammation in the rat. J Physiol, 2001; 531(Pt 1):171–80; doi: 10.1111/j.1469-7793.2001.0171j.x CrossRef
Catania A, Airaghi L, Motta P, Manfredi MG, Annoni G, Pettenati C, Brambilla F, Lipton JM. Cytokine antagonists in aged subjects and their relation with cellular immunity. J Gerontol A Biol Sci Med Sci, 1997; 52(2): B93–7; doi: 10.1093/gerona/52a.2.b93 CrossRef
Claridge JE 3rd, Attorri S, Musher DM, Hebert J, Dunbar S. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (“Streptococcus milleri group”) are of different clinical importance and are not equally associated with abscess. Clin Infect Dis, 2001; 32(10):1511–5; doi: 10.1086/320163 CrossRef
El-Solh AA, Sikka P, Ramadan F, Davies J. Etiology of severe pneumonia in the very elderly. Am J Respir Crit Care Med, 2001; 163(3 Pt 1):645–51; doi: 10.1164/ajrccm.163.3.2005075 CrossRef
Epstein EZ, Kugel MA. The significance of post-mortem bacteriological examination. J Infect Dis, 1929; 44(4):327–34. CrossRef
Fazili T, Riddell S, Kiska D, Endy T, Giurgea L, Sharngoe C, Javaid W. Streptococcus anginosus group bacterial infections. Am J Med Sci, 2017; 354(3):257–61; doi: 10.1016/j.amjms.2017.05.011 CrossRef
Fredette JW. Bacteremias in the agonal period. J Lab Clin Med, 1916; 2:180–8.
Gradwohl RBH. Importance de l’examen bactériolique pratiqué sur les cadavers. Ann Inst Pasteur (Paris), 1904; 18:767–73.
Hocken DB, Dussek JE. Streptococcus milleri as a cause of pleural empyema. Thorax, 1985; 40(8):626–8; doi: 10.1136/thx.40.8.626 CrossRef
Jiang S, Li M, Fu T, Shan F, Jiang L, Shao Z. Clinical characteristics of infections caused by Streptococcus anginosus group. Sci Rep, 2020; 10(1):9032; doi: 10.1038/s41598-020-65977-z CrossRef
Junckerstorff RK, Robinson JO, Murray RJ. Invasive Streptococcus anginosus group infection-does the species predict the outcome?. Int J Infect Dis, 2014; 18:38–40; doi: 10.1016/j.ijid.2013.09.003 CrossRef
Kellerman GD, Waterman NG, Scharefenberger LF. Demonstration in vitro of post-mortem bacterial transmigration. Am J Clin Pathol, 1976; 66(5):911–5; doi: 10.1093/ajcp/66.5.911 CrossRef
Koneman EW, Davis MA. Post-mortem bacteriology. 3. Clinical significance of microorganisms recovered at autopsy. Am J Clin Pathol, 1974; 61(1):28–40; doi: 10.1093/ajcp/61.1.28 CrossRef
Li W, Ding C, Yin S. Severe pneumonia in the elderly: a multivariate analysis of risk factors. Int J Clin Exp Med, 2015; 8(8):12463–75. Available via https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4612842/pdf/ijcem0008-12463.pdf
Morris JA, Harrison LM, Partridge SM. Practical and theoretical aspects of post-mortem bacteriology. Curr Diagn Pathol, 2007; 13(1):65– 74; doi: 10.1016/j.cdip.2006.07.005 CrossRef
Okada F, Ono A, Ando Y, Nakayama T, Ishii H, Hiramatsu K, Sato H, Kira A, Otabe M, Mori H. High-resolution CT findings in Streptococcus milleri pulmonary infection. Clin Radiol, 2013; 68(6):e331– 7; doi: 10.1016/j.crad.2013.01.019 CrossRef
Riedel S. The value of post-mortem microbiology cultures. J Clin Microbiol, 2014;52(4):1028–33; doi: 10.1128/JCM.03102-13 CrossRef
Rose GW, Hockett RN. The microbiologic evaluation and enumeration of post-mortem specimens from human remains. Health Lab Sci, 1971;8(2):75–8.
Saegeman V, Verhaegen J, Lismont D, Verduyckt B, De Rijdt T, Ectors N. Influence of post-mortem time on the outcome of blood cultures among cadaveric tissue donors. Eur J Clin Microbiol Infect Dis, 2009; 28(2):161–8; doi: 10.1007/s10096-008-0609-0 CrossRef
Smith RF, Linares HA, Jorgensen JH. Bacteremia and postmortem microbiology in burned children. Am J Clin Pathol, 1975; 63(4):502–8; doi: 10.1093/ajcp/63.4.502 CrossRef
Stefferl A, Hopkins SJ, Rothwell NJ, Luheshi GN. The role of TNF-alpha in fever: opposing actions of human and murine TNF-alpha and interactions with IL-beta in the rat. Br J Pharmacol, 1996; 118(8):1919–24; doi: 10.1111/j.1476-5381.1996.tb15625.x CrossRef
Tuomisto S, Karhunen PJ, Vuento R, Aittoniemi J, Pessi T. Evaluation of post-mortem bacterial migration using culturing and real-time quantitative PCR. J Forensic Sci, 2013; 58(4):910–6; doi: 10.1111/15564029.12124 CrossRef