INTRODUCTION
The increasing incidence of chemoresistance to the existing drugs leads to a constant quest for new drug candidates. Secondary metabolites derived from natural products are still considered propitious sources of new drugs, including antimicrobial and anticancer agents. In the last four decades, 26.9% of the approved drugs are reported to be inspired by natural products (Newman and Cragg, 2020). In 2021, 8% of the FDA-approved drugs originated from natural compounds (de la Torre and Albericio, 2022). Particularly, endophytic fungi are one of the prospective natural resources of prolific novel bioactive compounds that attracted many scientists to explore their hidden pharmacological potential.
Endophytic fungi belong to microorganisms that asymptomatically inhabit the inner tissue of plants without causing any harmful physiological effect on their host (Sarkar et al., 2021). Numerous studies have reported various chemical constituents of fungal endophytes origin, which demonstrated wide-ranging pharmacological activities. As an example, alkaloids shearilicine together with other indole diterpenoid derivatives from Penicillium sp. ZO-R1-1, an endophytic fungus isolated from the Indonesian medicinal plant, ginger (Zingiber officinale), showed strong cytotoxic activity when tested against a series of cancer cell lines (Ariantari et al., 2019). Moreover, alkaloids didymellanosine and ascomylactam C isolated from Terminalia catappa-derived endophytic fungus, Didymella sp. IEA-3B.1, revealed prominent antibacterial activity when tested against both sensitive and resistant strains of Gram-positive bacteria (Staphylococcus aureus, Enterococcus faecalis, and Enterococcus faecium), as well as Gram-negative Acinetobacter baumannii with the presence of 0.1 µM of colistin, with minimum inhibition concentration (MIC) values ranging from 3.1 to 6.3 µM (Ariantari et al., 2020). Furthermore, in our recent study on endophytic fungus, Fusarium sp. BZCB-CA, from a Chinese medicinal plant, Bothriospermum chinense, rubrofusarin was isolated along with lateropyrone and other new fusaristatin derivatives. Among them, rubrofusarin showed promising cytotoxic effects against Ramos and Jurkat cancer cell lines with IC50 values of 6.3 and 6.2 µM, respectively (Ariantari et al., 2021).
In the continuation of our search for talented endophytic fungi capable of producing bioactive secondary metabolites, we explored endophytic fungi associated with the medicinal plant, Antidesma bunius (L.) Spreng, and investigated their antimicrobial and cytotoxic activities. Antidesma bunius (L.) Spreng (family Euphorbiaceae) is a tropical tree commonly found in the Southeast Asia region. Traditionally, the Asian natives harness the leaves to treat snakebite or syphilis. The Filipinos used its fruits to treat hypertension, loss of appetite, diabetes, or digestion disturbance (Lim, 2012). Barks and fruits of A. bunius were also used by Indians to ameliorate any infection-related symptoms or diarrhea (Panda et al., 2017). Pharmacological studies on leaves, barks, and fruits of A. bunius revealed its bioactivity as antibacterial (Zaman et al., 2018), antioxidant (Krongyut and Sutthanut, 2019), cytotoxic (Geronimo et al., 2020), and antidiabetic (Aksornchu et al., 2020; Mauldina et al., 2017; Ratnadewi et al., 2020). Phytochemical investigation on different parts of A. bunius indicated the presence of triterpenoids (Mauldina et al., 2017), polyphenols (Jorjong et al., 2015), flavonoids and vitamin C (Islary et al., 2017), and anthocyanins (Chamnansilpa et al., 2020), which contributed to its wide range of bioactivities. However, no previous reports on endophytic fungi were associated with A. bunius or the investigation into their hidden pharmacological potential so far.
In this study, we reported six endophytic fungi isolated from A. bunius leaves, i.e., Penicillium steckii AAB-01, Nemania bipapillata AAB-02, Xylaria feejeensis AAB-03, Hypomontagnella monticulosa AAB-04, Daldinia eschscholtzii AAB-05, and Phyllosticta capitalensis AAB-06, as well as antibacterial activity and cytotoxicity against breast cancer cells of the resulting fungal extracts. Furthermore, identification of secondary metabolites of antibacterial and cytotoxic fungal extracts was carried out. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is one of the widely used techniques for the separation and identification of crude extract containing a mixture of unknown fungal secondary metabolites, which provides the exact mass measurement of molecular ions of any peaks detected in the LC system. This technique is also proven as a powerful tool for a wide range of analysis given the advantage of easy sample preparation, high sensitivity, and suitability for various less volatile metabolites (Mishra et al., 2022). Therefore, in an attempt to identify the main secondary metabolites of bioactive fungal extracts which may contribute to their antibacterial and cytotoxicity, LC-MS/MS technique was applied in this study.
MATERIALS AND METHODS
Sample collection and isolation of endophytic fungi
The fresh and healthy leaves of A. bunius were collected from Tabanan, Bali-Indonesia in July 2021. Isolation of endophytic fungi from the leaves of A. bunius was carried out following the isolation procedure described previously (Kjer et al., 2010). In brief, leaves were washed with running tap water for 3 minutes. Leaves were then subjected to surface sterilization by immersing in 70% EtOH for 2 minutes and allowed to dry. Sterile samples were cut into small pieces (1 × 1 cm) and then inoculated on an agar plate containing isolation media. For the isolation of endophytic fungi, a medium containing malt extract, Bacto agar, and chloramphenicol in demineralized water was used. Agar plates containing inoculated samples were incubated at room temperature for several days until fungal growth was observed. For the fungal purification, each fungal colony with a different morphological appearance was transferred to a new agar plate containing medium without the presence of chloramphenicol. Agar plates containing fungal colonies were allowed at room temperature to obtain pure fungal isolates. For long-term fungal maintenance, a medium containing malt extract, Bacto agar, yeast extract, and glycerol in demineralized water was used.
Identification of endophytic fungi
Following the isolation procedure of endophytic fungi from the host plant, A. bunius leaves, the morphology of fungal colonies was observed. Morphological observation included the appearance of color, texture, shape, elevation, and margin of fungal colonies. A colony with different morphological characteristics was isolated and regarded as an individual isolate. Hyphal tips of the pure endophytic strain were then observed under the microscope.
Furthermore, molecular identification through comparison of the DNA sequence of the internal transcribed spacer (ITS) region was performed to identify the species of the fungal isolates using ITS1 (5’-TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) as the forward and reverse primers, respectively. Before PCR, the fungal genomic DNA was extracted using Quick-DNA Fungal/Bacterial Miniprep Kit (Zymo Research) according to the manufacturer’s protocol. The extracted DNA was then amplified by PCR (Thermocycler Labcycler 48) in a prepared mixture containing 0.5 µl of ITS1 primer, 0.5 µl of ITS4 primer, 0.5 µl of DNA polymerase, 0.5 µl of dNTP, 0.5 µl of DMSO, 5 µl of 5× buffer, 7.5 µl of extracted fungal genomic DNA as a template, and sterile demineralized water up to 25 µl. For DNA amplification, the thermocycler was set under the following condition: predenaturation at 95°C for 1.5 minutes, followed by 35 cycles of denaturation at 95°C, annealing at 56°C, and extension at 72°C; each cycle was run for 1 minute and then ended by a final extension at 72°C for 15 minutes. Subsequently, the amplicon was analyzed by electrophoresis using 1% agarose gel in TAE 1× (Tris-acetate-EDTA) buffer at 75 volts for 45 minutes. The PCR products were submitted to 1st BASE for sequencing analysis. The probable identity of the fungi was investigated by comparing the obtained nucleotide sequences to the previously submitted sequences in National Center for Biotechnology Information (NCBI) GenBank using the Basic Local Alignment Search Tool (BLAST) program for nucleotides. The desired reference sequences and the endophytic fungal sequences were pairwise aligned by the MUSCLE method. The phylogenetic tree was reconstructed using MEGA version 11.0.11 software by the neighbor-joining method with 1,000 replication bootstraps.
Fermentation and extraction
For the fermentation, each fungal isolate was cut into small pieces (1 × 1 cm). Each fungal isolate was inoculated on 2 Erlenmeyer flasks (1 l) containing sterile rice medium. Each flask contains 100 g of rice and 110 ml of distilled water. Fungal cultivation was done at room temperature for 3–4 weeks in daylight conditions until the fungal mycelia entirely covered the rice media. At the end of cultivation, each fermentation flask was soaked with 500 ml ethyl acetate followed by agitation on the shaker at 150 rpm for 6–8 hours. The resulting liquid extract was separated from mycelia residue through vacuum filtration. The solvent was removed by a vacuum rotary evaporator to yield crude ethyl acetate extract. The crude extract was then partitioned by liquid-liquid extraction between methanol containing 10% water and n-hexane. The aqueous phase was dried in vacuo and the obtained methanolic extract was subjected to antimicrobial, toxicity, and cytotoxicity assays as well as LC-MS/MS analysis.
Antimicrobial assay
Antimicrobial potency was evaluated according to the Clinical and Laboratory Standards Institute (CLSI) protocol (Clinical and Laboratory Standards Institute, 2018). The antimicrobial activity of the tested extracts is expressed as MIC in microgram per milliliter. MIC is the lowest concentration of the tested extract to restrain the growth of a particular microorganism. MIC value is determined from microbial cultures upon the treatment with a series concentration of the tested extract employing the microdilution technique (Rajashekara et al., 2020; Rajashekara et al., 2022).
In brief, the methanolic extract of each fungal isolate was dissolved in DMSO prior to the test. Next, each fungal extract was serially diluted in the 96-microwell plates to the resulting concentrations ranging from 1,000 to 1.95 µg/ml. The test was done in triplicates. For the antibacterial assay, each extract was tested against S. aureus ATCC 6538, Staphylococcus epidermidis ATCC 12228, and Propionibacterium acnes ATCC 1223, while an antifungal assay was performed against Candida albicans ATCC 10231. Chloramphenicol and ketoconazole were used as positive controls in this assay, while a medium containing 1% DMSO was included as a negative control.
Toxicity
The brine shrimp lethality test (BSLT) was performed according to the modified protocol (Niksic et al., 2021). Artemia salina eggs (Supreme Plus®) were used for this assay. Artificial seawater salt (Himedia®) was used for hatching A. salina eggs and performing the assay. Test solutions were prepared in artificial seawater and diluted to achieve final tested concentrations of 62.5, 125, 250, 500, and 1,000 μg/ml. Brine shrimp larvae were prepared by adding A. salina eggs (20 mg) in a brine incubator filled with 300 ml artificial seawater (9.5 g artificial seawater salt in 300 ml distilled water). The eggs were illuminated and aerated for 24 hours until they hatched to be mature nauplii. For the toxicity assay, ten mature nauplii were added to the vials, each containing tested fungal extract solution with the aforementioned tested concentration. As a negative control, nauplii were also added to artificial seawater containing 0.5% DMSO. Each treatment was done in triplicates and all treatments were illuminated for 24 hours. The dead nauplii in each vial were observed using a magnifying glass and were counted. The mean mortality of A. salina nauplii was then calculated. The LC50 value of each tested methanolic extract was determined using probit analysis of concentration vs. mortality with SPSS version 26.
Cytotoxicity against breast cancer cells
Breast cancer cells MCF-7 and the triple-negative breast cancer (TNBC), 4T1 cell lines, were originally obtained from Masashi Kawaichi, NAIST, and maintained by Cancer Chemoprevention Research Center, UGM under the American Type Culture Collection (ATCC) protocol. Cells were cultured in DMEM medium (#31800022, Gibco Life Technologies, CA, USA) complemented with fetal bovine serum (FBS) (10% v/v) (#10270-106, Gibco Life Technologies, CA, USA), penicillin, and streptomycin (10,000 units/ml Penicillin and 10,000 μg/ml streptomycin) (#15140-148, Gibco Life Technologies). The confluence cells were harvested using trypsin-EDTA (#25200-056, Gibco Life Technologies) and regrown in the well plate for further experiment.
For the cytotoxicity test, we used the modified CCK-8 assay to assess the cytotoxic activity of the tested extracts. The cells (2.5 × 103/well) were dispensed overnight in a 96-well plate (#3599, Corning Incorporated-Life Sciences, Wujiang, Jiangsu). The next day, cells were exposed to a series of concentrations of each tested extract ranging from 4 to 500 μg/ml. After 24 hours, the medium was removed and replaced with 100 μl of PBS solution. Then, 10 μl of CCK-8 (Dojindo laboratories) solution was added to each well. Afterward, the plate was incubated for 4 hours in a 5% CO2 incubator. The absorbance was measured at 450 nm using a microplate reader. The IC50 values were obtained from the calculation of linear regression of concentrations vs. cell viability.
Identification of secondary metabolites by LC-MS/MS
Identification of secondary metabolites from bioactive fungal extracts by LC-MS/MS analysis was performed on ACQUITY UPLC® H-Class unit (Waters, USA) equipped with a C18 column (1.8μm 2.1 × 100 mm) and a Xevo G2-S QTOF mass spectrometer (Waters, USA), at the column temperature of 50°C. For the UPLC separation, water containing 5 mM ammonium formic (A) and acetonitrile containing 0.05% formic acid (B) were used as a mobile phase in a stepwise gradient, at the flow rate of 0.2 ml/minute for 23 minutes. The mass spectrum was acquired using electrospray ionization in a positive mode and a mass range of 50–1,200 m/z. For data acquisition, MassLynx software version 4.1 was used.
RESULTS
Isolation and identification of endophytic fungi
A total of six fungal isolates documented as AAB-01, AAB-02, AAB-03, AAB-04, AAB-05, and AAB-06 were isolated from A. bunius leaves. Each isolate showed distinct morphological characteristics on agar media used in this study. The macroscopic and microscopic appearance of fungal isolates was depicted in Figure 1.
According to the morphological characteristics, the conidia of two fungal isolates (AAB-01 and AAB-03) were observed, while the remaining isolates were Mycelia sterilia with no spore observed. Based on the conidia observation, AAB-01 and AAB-03 can be classified into Penicillium and Xylaria genera, respectively. Nevertheless, due to the lack of conidia in most of the endophytic fungal isolates, identification of those isolates based on morphological characteristics could not be done. Therefore, molecular identification based on the comparison of the sequence of ITS1-5.8S-ITS4 rDNA was performed to identify the species of endophytic isolates.
PCR amplification of the ITS rDNA region generated a single band of DNA segment with a size between 500 and 750 base pairs (bp), as shown in Figure 2. Following sequencing analysis, the NCBI’s BLAST program was used to compare the sequences and identify the predicted taxon. The sequences of the isolates can be classified into six different species with good similarity percentages (> 99%) and E values (Table 1). Moreover, the phylogenetic analysis suggested that each isolate lineage was split up into six distinct primary clades with good bootstrap support (100%) as depicted in Figure 3.
Sequence analysis showed that AAB-01 isolate was identified as Penicillium steckii with 99.82% similarity to P. steckii (MT582790.1). Phylogenetic analysis supported this finding with 100% bootstrap support. Meanwhile, AAB-02 isolate was identified as Nemania bipapillata with similarity of 99.81% to N. bipapillata (ON514551.1) and was supported with high bootstrap support (100%). Moreover, AAB-03 isolate showed sequence similarity of 99.63% with Xylaria feejeensis (KJ767108.1) and bootstrap support of 100% on phylogenetic analysis. Sequence comparison revealed that AAB-04 had high sequence similarity (99.82%) with Hypomontagnella monticulosa (KJ774047.1), in line with its phylogenetic analysis which showed bootstrap support of 100%. Furthermore, AAB-05 isolate had high sequence homology (100%) with the species of Daldinia eschscholtzii (KC895542.1) which was corroborated by 100% bootstrap support on phylogenetic analysis. AAB-06 isolate was deduced as Phyllosticta capitalensis based on its high sequence similarity (99.67%) to this species (MK396601.1), as well as bootstrap support of 100% in phylogenetic analysis.
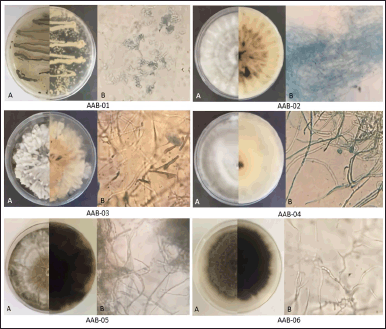 | Figure 1. The appearance of endophytic fungi isolated from the leaves of A. bunius on media consisting of malt extract, yeast extract, glycerol, and Bacto agar in demineralized water. (A) Macroscopic morphology of each fungal isolate from the front (half left) and reverse (half right) sides. (B) Microscopic morphology of each fungal isolate (400× magnification). [Click here to view] |
All of the isolated fungal species can be differentiated into three classes of taxon, of which P. steckii belong to Eurotiomycetes; P. capitalensis belong to Dothideomycetes; and N. bipapillata, X. feejeensis, H. monticulosa, and D. eschscholtzii belong to Sordariomycetes. All isolates were classified as a member of the subphylum of Saccharomyceta.
Antimicrobial activity
We further evaluate the antibacterial activity of all methanolic extracts afforded from A. bunius-derived endophytic fungi. The MIC values of each fungal methanolic extract against the tested microorganisms are displayed in Table 2. All of the tested extracts showed moderate to weak activity against S. aureus ATCC 6538 with MIC values ranging from 125 to 500 μg/ml, except for H. monticulosa AAB-04 extract which showed no activity up to the tested concentration of 1,000 µg/ml. Among the active extracts, P. steckii AAB-01 extract showed the highest inhibition against S. aureus ATCC 6538 and S. epidermidis ATCC 12228 with MIC values of 125 and 250 µg/ml, whereas N. bipapillata AAB-02 had weaker inhibition against these bacteria with MIC values of 500 µg/ml. Similarly, X. feejeensis AAB-03, D. eschscholtzii AAB-05, and P. capitalensis AAB-06 showed mild inhibition only against S. aureus ATCC 6538. All fungal extracts were found inactive against P. acnes ATCC 1223 and C. albicans ATCC 10231.
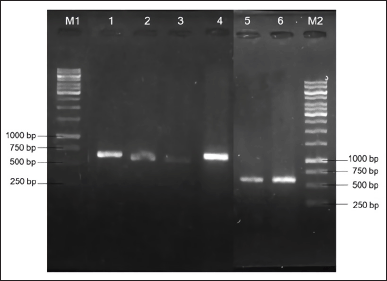 | Figure 2. PCR results of amplified ITS rDNA region from six fungal isolates displayed on agarose gel electrophoresis. M1: 100 bp DNA ladder for lane 1-4; 1: AAB-06 (~550 bp); 2: AAB-03 (~500 bp); 3: AAB-02 (~500 bp); 4: AAB-01 (~550 bp); 5: AAB-05 (~600 bp); 6: AAB-04 (~600 bp); M2: 100 bp DNA ladder for lane 5 and 6. [Click here to view] |
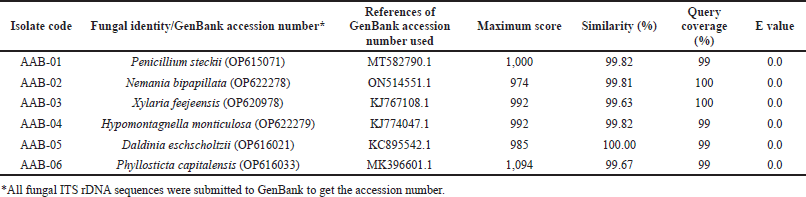 | Table 1. The molecular identification of the endophytic fungi isolated from the leaves of A. bunius. [Click here to view] |
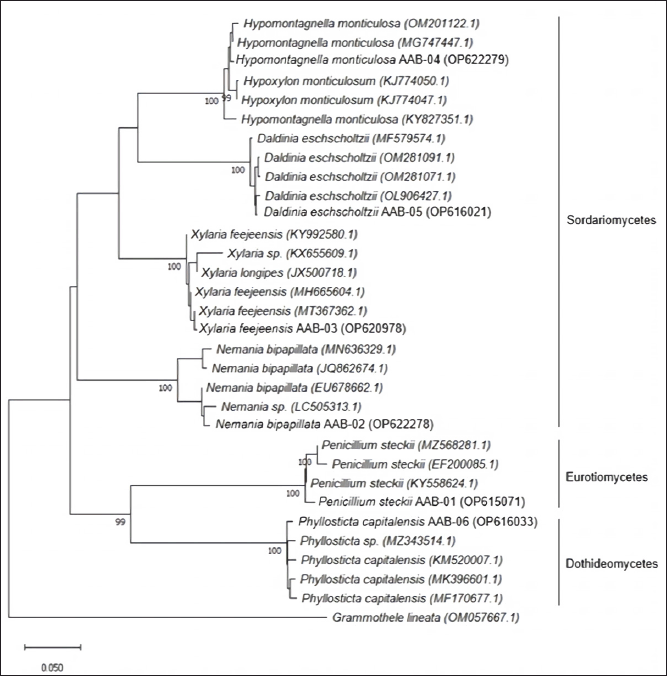 | Figure 3. Phylogenetic tree of endophytic fungi obtained from A. bunius leaves according to the analysis of ITS region. [Click here to view] |
Brine shrimp lethality test (BSLT)
The result of toxicity screening employing BSLT as displayed in Table 3 showed that X. feejeensis AAB-03, P. steckii AAB-01, D. eschscholtzii AAB-05, and N. bipapillata AAB-02 extracts were moderately toxic against A. salina nauplii with LC50 values ranging from 114 to 243 µg/ml. Meanwhile, H. monticulosa AAB-04 and P. capitalensis AAB-06 extracts had no toxicity against A. salina nauplii up to the tested concentration of 1,000 μg/ml.
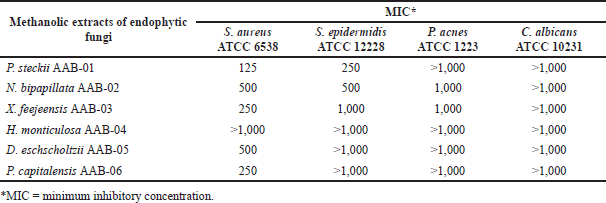 | Table 2. MIC values (µg/ml) of methanolic extracts of A. bunius-derived endophytic fungi. [Click here to view] |
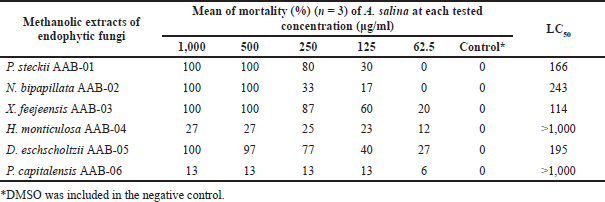 | Table 3. LC50 values (µg/ml) of the tested fungal extracts toward A. salina nauplii after 24-hour treatment. [Click here to view] |
Cytotoxic test against breast cancer cells
We performed the cytotoxic assay on estrogen receptor-expressing breast cancer cells, namely MCF-7 and the TNBC, 4T1 cells. This test is based on the percentage of cells that are still alive after being treated for 24 hours with the tested extracts. Living cells have dehydrogenase enzymes that can hydrolyze NAD to NADH, resulting in the color changes of WST-8 to orange soluble WST-formazan, which can be quantified by spectrophotometer. Upon the exposure of the tested extracts on MCF-7 and 4T1 cells, the viability of the treated cells decreased in a dose-dependent manner (Figure 4). A substantial decrease of MCF-7 cells viability was observed upon the treatment with P. steckii AAB-01 extract, whereas the treatment on 4T1 cells with D. eschscholtzii AAB-05 extract up to the tested concentration of 200 µg/ml led to the lowest cells viability.
Following statistical analysis, the extract produced by P. steckii AAB-01 showed the highest cytotoxic effect against MCF-7 cells with an IC50 value of 35 µg/ml (Table 4), while it possessed lower inhibition against 4T1 cells (IC50 = 98 µg/ml). In contrast, D. eschscholtzii AAB-05 extract possessed strong inhibition against 4T1 cells with an IC50 value of 11 µg/ml, despite its relatively weak cytotoxic effect on MCF-7 cells (IC50 = 271 µg/ml). Extract of N. bipapillata AAB-02 revealed weak activity against MCF-7 cells, with no inhibition found against 4T1 cells. Meanwhile, X. feejeensis AAB-03 extract showed no activity against both tested cancer cells.
Identification of secondary metabolites by LC-MS/MS
LC-MS/MS analysis was carried out in our attempt to identify major secondary metabolites from bioactive methanolic extracts of P. steckii AAB-01 and D. eschscholtzii AAB-05. The total ion chromatogram (TIC) of the methanolic extract of P. steckii AAB-01 showed 16 peaks with retention time (tR) at 1.28, 1.83, 4.45, 4.86, 5.01, 5.94, 6.09, 6.29, 6.36, 6.58, 7.06, 7.34, 8.40, 8.51, 9.17, and 11.81 minutes (Figure 5A). Three dominant peaks appeared at tR of 4.45, 4.86, and 6.29 minutes which were assigned as peaks 1–3, respectively. Peaks 1–3 had prominent pseudomolecular ion signals at m/z 176.0713 [M+H]+ (Figure 5B), 241.0978 [M+H]+ (Figure 5C), and 211.0870 [M+H]+ (Figure 5D), attributed to the molecular formula C10H9NO2, C14H12N2O2, and C13H10N2O, respectively. For data interpretation, NIST/KnowItAll mass spectral libraries (WILEY) were used in combination with published literature, leading to the tentative identification of peaks 1–3 as indole acetic acid (IAA), O-acetylharmol, and oxindole I, respectively (Figure 7A–C). Meanwhile, LC-MS/MS data of methanolic extracts of D. eschscholtzii AAB-05 displayed 10 peaks at tR of 1.30, 3.85, 4.11, 4.88, 5.39, 5.78, 6.95, 7.73, 9.04, and 12.33 minutes (Figure 6A). Two major peaks at tR of 4.88 and 12.33 minutes were assigned as peaks 1 and 2. Peaks 1 and 2 showed pronounced pseudomolecular ion signals at m/z 249.1237 [M+H]+ (Figure 6B) and 304.3000 [M+H]+ (Figure 6C), corresponding to the molecular formula C13H16N2O3 and C21H37N, respectively. The molecular structures of these peaks were tentatively designated as 6-hydroxymelatonin and 2-methyl-6-pentadecylpyridine (Figure 7D–E).
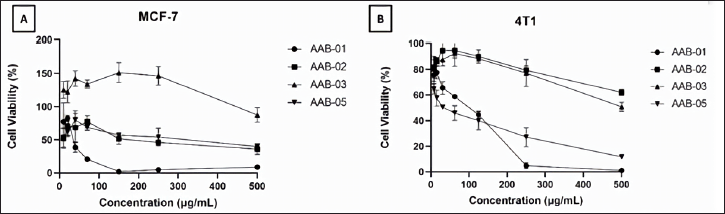 | Figure 4. Cytotoxic effect of methanolic extracts from endophytic fungal strains P. steckii AAB-01, N. bipapillata AAB-02, X. feejeensis AAB-03, and D. eschscholtzii AAB-05 toward MCF-7 cells (A) and 4T1 cells (B). The MCF-7 cell lines were inoculated in 96-well plates and treated with extract for 24 h. Cell viability was determined by using a CCK-8 kit which contains WST (water-soluble tetrazolium salt) as described in the methods. The cytotoxicity of the extract was expressed by percent cell viability (mean ± SD of three experiments). [Click here to view] |
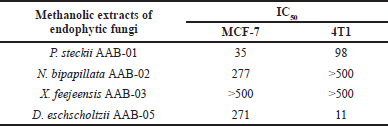 | Table 4. IC50 values (µg/ml) of the tested fungal extracts against MCF-7 and 4T1 breast cancer cells. [Click here to view] |
DISCUSSION
Species identification of fungi can be achieved through morphology and/or molecular biology. However, relying solely on the morphological approach in a species-level identification is error-prone due to hybridization (Hughes et al., 2013), unrecognized cryptic speciation (Perez et al., 2013), or highly phenotypic plasticity (Alster et al., 2021; Slepecky et al., 2009) of the fungi being studied. Therefore, DNA sequence-based identification is done to accurately characterize fungal species (Raja et al., 2017). The sequence of nuclear ribosomal markers, for instance, nuclear ribosomal large subunit (nrLSU-26S or 28S), nuclear ribosomal small subunit (nrSSU-18S), and ITS region, is commonly analyzed in fungal species identification. Among them, ITS is considered an official DNA barcoding marker for species-level determination of fungi due to its simplicity in DNA amplification, being broadly used and fast-evolving and having large barcode gap (Schoch et al., 2012). Thus, molecular identification based on the comparison of the ITS1+4 sequences was able to confirm the species identity of endophytic fungal isolates in this study as Penicillium steckii AAB-01, Nemania bipapillata AAB-02, Xylaria feejeensis AAB-03, Hypomontagnella monticulosa AAB-04, Daldinia eschscholtzii AAB-05, and Phyllosticta capitalensis AAB-06.
To the best of our knowledge, this is the first report on endophytic fungi from the host plant, A. bunius, although bacterial endophytes from this plant were reported before (Indrawati et al., 2020). Penicillium steckii was isolated before as an endophytic fungus associated with mangroves (Chen et al., 2021a; Chen et al., 2021b) and banana leaves (Zakaria and Aziz, 2018). However, this strain was repeatedly reported from marine sources, such as sediment samples (Wu et al., 2021), sponges, and deep-sea coral (Hu et al., 2022; Shin et al., 2016; Yao et al., 2021). Nemania bipapillata was recently reported as an endophyte of Vaccinium dunalianum Wight leaves along with P. capitalensis (Fan et al., 2020). It was also found in a mutualistic relationship with Diospyros crassiflora (Douanla-Meli and Langer, 2012). Meanwhile, an endophytic X. feejeensis was isolated previously from various host plants, such as an Euphorbiaceae plant, Sapium macrocarpum (García-Méndez et al., 2016), and from medicinal plants Hintonia latiflora (Rivera-Chávez et al., 2015) and Eryngium foetidum (Siriwach et al., 2011). The genus Phyllosticta is known to inhabit a wide host range (Zhu et al., 2021). Particularly, P. capitalensis was found inhabiting the healthy leaves of Tibouchina granulosa (Golias et al., 2020), Loropetalum chinense (Zhu et al., 2021), and Hippobroma longiflora (Widjajanti et al., 2021). Furthermore, Daldinia eschscholtzii is known as a wood-inhabiting endophyte, which is most commonly distributed in the tropical region (Stadler et al., 2014). However, this strain was also found in healthy leaves of Asteraceae plant, Tridax procumbens (Mishra et al., 2020), as well as the medicinal plant Pogostemon cablin (Liu et al., 2019) in previous studies. Hypomontagnella was clustered previously within the genus Hypoxylon (Lambert et al., 2019). Despite the limited reports on H. monticulosa, this endophytic strain was reported before from the host plant Zingiber griffithii Baker, collected from North Sumatera, Indonesia (Lutfia et al., 2021), in addition to our finding on H. monticulosa AAB-04 from A. bunius leaves.
In the antibacterial assay, the methanolic extract of P. steckii AAB-01 showed the strongest growth inhibition against the tested Gram-positive bacteria, S. aureus ATCC 6538 and S. epidermidis ATCC 12228. Furthermore, toxicity screening employing BSLT was applied to assess the toxicity of all fungal extracts obtained in this study before the cytotoxicity assay, due to its simplicity, low requirement, rapidness, robustness, and affordability (Hamidi et al., 2014). According to Meyer et al. (1982), LC50 values below 1,000 μg/ml are considered toxic. Following this criterion, only four extracts produced by X. feejeensis AAB-03, P. steckii AAB-01, D. eschscholtzii AAB-05, and N. bipapillata AAB-02 were found moderately toxic against A. salina nauplii and therefore subjected to cytotoxicity test against human breast cancer cell lines. Our investigation further confirmed that P. steckii AAB-01 extract showed the most potent cytotoxic activity against luminal cancer cells (MCF-7), while it possessed lower inhibition against TNBC (4T1) cells. Luminal breast cancer cells represent more than 70% of cases expressing estrogen (ER2+) and/or progesterone (PR+) (Ogba et al., 2014). Estrogen receptors are known to be important regulators of cancer development, proliferation, and apoptosis (Livezey et al., 2018). Regarding these results, whether P. steckii AAB-01 extract modulates ER and/or PR signaling to inhibit cell growth will be an interesting focus for further research. Interestingly, D. eschscholtzii AAB-05 extract, which showed only weak cytotoxicity to MCF-7 cells, exhibited strong cytotoxic activity against TNBC with an IC50 value of 11 µg/mL. TNBC is a highly metastatic breast cancer that is difficult to treat as it does not express proliferative receptors that can be used as targets (Costa and Gradishar, 2017). Therefore, investigation of the mechanism of D. eschscholtzii AAB-05 extract in inhibiting 4T1 cells as well as investigation on cytotoxic compounds from this fungal strain is merit for further research. Overall, although this cytotoxic test has only been performed on two types of breast cancer cells, this is sufficient to illustrate the potential of P. steckii AAB-01 and D. eschscholtzii AAB-05 as a promising source of anticancer agents.
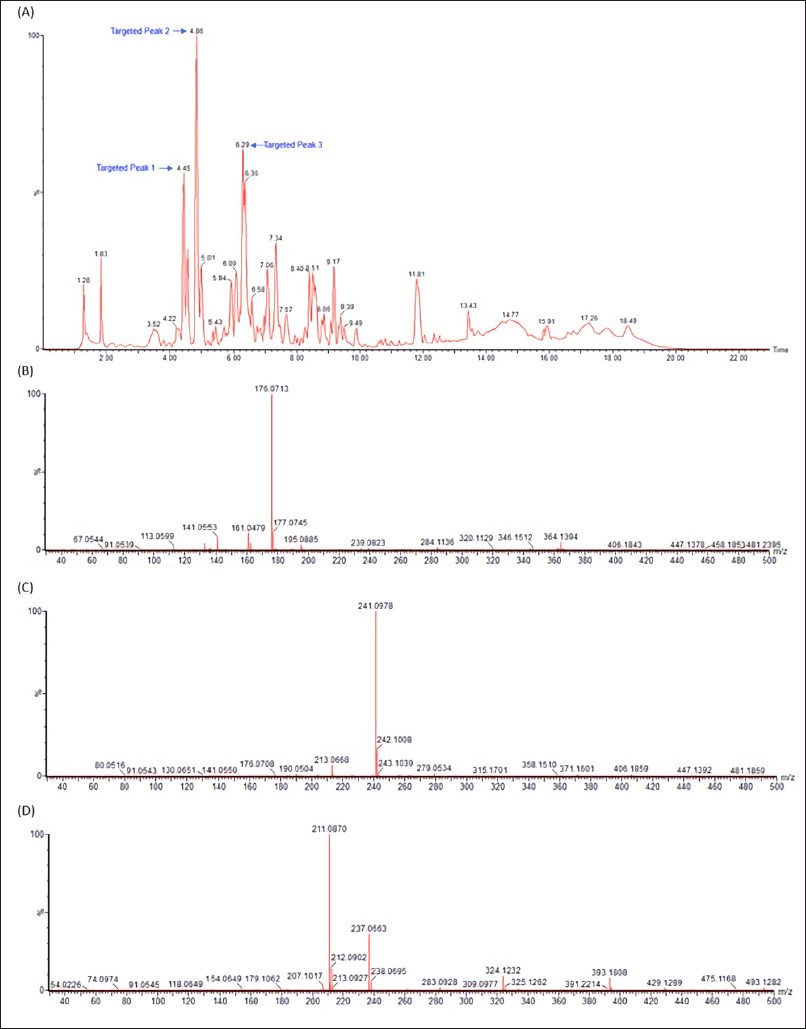 | Figure 5. LC-MS/MS data of methanolic extract of P. steckii AAB-01 (A) and the observed m/z of major peaks at the retention time of 4.45 (Peak 1) (B), 4.86 (Peak 2) (C), and 6.29 (Peak 3) (D) minutes using electrospray ionization (ESI) in a positive mode. [Click here to view] |
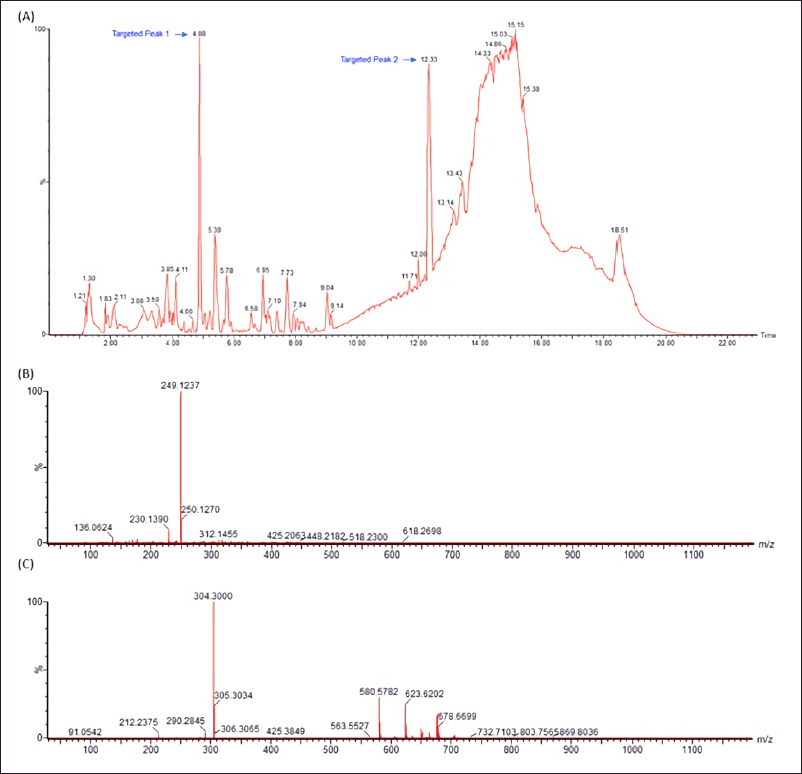 | Figure 6. LC-MS/MS data of methanolic extract of D. eschscholtzii AAB-05 (A) and the observed m/z of major peaks at the retention time of 4.88 (Peak 1) (B) and 12.33 (Peak 2) (C) minutes using electrospray ionization (ESI) in a positive mode. [Click here to view] |
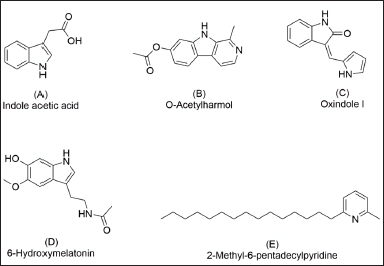 | Figure 7. Chemical structures of secondary metabolites detected in methanolic extracts of P. steckii AAB-01 (A-C) and D. eschscholtzii AAB-05 (D-E) according to the LC-MS/MS data. [Click here to view] |
Identification of main secondary metabolites by LC-MS/MS analysis revealed the presence of IAA, O-acetylharmol, and oxindole I in P. steckii AAB-01 extract, whereas 6-hydroxymelatonin and 2-methyl-6-pentadecylpyridine were suggested as the main metabolites in D. eschscholtzii AAB-05 extract. IAA was repeatedly reported from Penicillium sp. cultures (Ikram et al., 2018; Radhakrishnan et al., 2013; Waqas et al., 2012). IAA produced by endophytic Penicillium sp. is known as capable of enhancing their plant host tolerance in biotic and abiotic stresses (Bilal et al., 2019; Sharma et al., 2021). So far, there are no reports on O-acetylharmol from Penicillium sp.; however, β-carboline type of structures was isolated before from a deep-sea fungus, Trichoderma sp. (Hao et al., 2022). Meanwhile, oxindole type of alkaloids was reported in numerous studies from Penicillium sp. cultures (Hu et al., 2014; Lee et al., 2015; Xiao et al., 2018; Xu et al., 2015). In addition to our findings, previous studies on P. steckii have reported this species as a producer of mycotoxin citrinin (Yao et al., 2021), polyketides (Chen et al., 2021a), including tanzawaic acid derivatives (Malmstrùm et al., 2000), as well as isoquinoline (Yao et al., 2021) and pyrrolyl 4-quinolone alkaloids (Chen et al., 2021b). Polyketide derivatives, citrinin, and isoquinoline alkaloids isolated from P. steckii were found before to have strong antibacterial activity against S. aureus (Chen et al., 2021a; Yao et al., 2021); however, none of these metabolites were detected as main metabolites in P. steckii AAB-01 under study.
Furthermore, 6-hydroxymelatonin and 2-methyl-6-pentadecylpyridine were detected in the methanolic extract of D. eschscholtzii AAB-05. Despite having no reports on D. eschscholtzii as a melatonin producer, melatonin analogs were previously afforded from a marine fungus, Penicillium sp. (Yurchenko et al., 2018). Nevertheless, numerous studies revealed that D. eschscholtzii is a versatile producer of polyketides with varied prospective bioactivities as antibacterial (Lin et al., 2019), cytotoxic (Wang et al., 2015), antifibrotic (Zhang et al., 2019), immunosuppressive (Zhang et al., 2011), and antidiabetic (Wutthiwong et al., 2021) agents. Therefore, in-depth chemical and pharmacological investigations of secondary metabolites from A. bunius-derived P. steckii AAB-01 and D. eschscholtzii AAB-05 are noteworthy to further study.
CONCLUSIONS
A total of six endophytic fungi were isolated from A. bunius and were identified as P. steckii AAB-01, N. bipapillata AAB-02, X. feejeensis AAB-03, H. monticulosa AAB-04, D. eschscholtzii AAB-05, and P. capitalensis AAB-06 by the molecular biology protocol. In antibacterial assay, P. steckii AAB-01 extract showed the most potent antibacterial activity against S. aureus ATCC 6538 and S. epidermidis ATCC 12228. Moreover, cytotoxicity against MCF-7 and 4T1 human breast cancer cells revealed the promising cytotoxic effects of P. steckii AAB-01 extract against MCF cells as well as the cytotoxicity of D. eschscholtzii AAB-05 extract against 4T1 cells. The LC-MS/MS analysis suggested the presence of IAA, O-acetylharmol, and oxindole I, as major secondary metabolites of P. steckii AAB-01, while 6-hydroxymelatonin and 2-methyl-6-pentadecylpyridine were detected in D. eschscholtzii AAB-05 extract. Isolation and characterization of bioactive secondary metabolites, in particular for antibacterial and anticancer effects, produced by these strains merits further chemical and pharmacological investigation.
AUTHORS’ CONTRIBUTIONS
Concept and design are done by N.P.A.; data acquisition is carried out by N.P.A., I.P.Y.A.P., N.P.E.K., N.N., and U.M.Z.; data analysis and interpretation are done by N.P.A, I.P.Y.A.P, M.W.P., N.N., and U.M.Z.; drafting manuscript is performed by N.P.A., I.P.Y.A.P., and N.N.; critical revision of the manuscript is done by N.P.A., R.I.J., and E.M.; statistical analysis is performed by P.S.Y., N.P.E.K., and N.N.; funding acquisition is done by N.P.A., M.W.P., and E.M.; material support is done by P.S.Y. All authors have read and agreed to the published version of the manuscript.
FINANCIAL SUPPORT
This research was funded by Udayana University, grant number B/96-153/UN14.4.A/PT01.05/2021. Additional funding by The Directorate General of Higher Education, Ministry of Education, Culture, Research and Technology, The Republic of Indonesia through The World Class Professor Program 2022 is gratefully acknowledged.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aksornchu P, Chamnansilpa N, Adisakwattana S, Thilavech T, Choosak C, Marnpae M, Mäkynen K, Dahlan W, Ngamukote S. Inhibitory effect of Antidesma bunius fruit extract on carbohydrate digestive enzymes activity and protein glycation in vitro. Antioxidants, 2020; 10(1):32. CrossRef
Alster CJ, Allison SD, Johnson NG, Glassman SI, Treseder KK. Phenotypic plasticity of fungal traits in response to moisture and temperature. ISME Commun, 2021; 1(1):1–8. CrossRef
Ariantari NP, Ancheeva E, Wang C, Ma?ndi A, Knedel T-O, Kurta?n T, Chaidir C, Mu?ller WEG, Kassack MU, Janiak C, Daletos G, Proksch P. Indole diterpenoids from an Endophytic Penicillium sp. J Nat Prod, 2019; 82(6):1412–23. CrossRef
Ariantari NP, Ancheeva E, Frank M, Stuhldreier F, Meier D, Gr¨oner Y, Reimche I, Teusch N, Wesselborg S, Mu?ller WEG, Kalscheuer R, Liu Z, Proksch P. Didymellanosine, a new decahydrofluorene analogue, and ascolactone C from Didymella sp. IEA-3B.1, an endophyte of Terminalia catappa. RSC Adv, 2020; 10(12):7232–40. CrossRef
Ariantari NP, Frank M, Gao Y, Stuhldreier F, Kiffe-Delf A-L, Hartmann R, Hofert SP, Janiak C, Wesselborg S, Mu?ller WEG, et al. Fusaristatins D–F and (7S,8R)-(−)-chlamydospordiol from Fusarium sp. BZCB-CA, an endophyte of Bothriospermum chinense. Tetrahedron, 2021; 85:132065 CrossRef
Bilal S, Shahzad R, Khan AL, Al-Harrassi A, Kim CK, Lee I.J. Phytohormones enabled endophytic Penicillium funiculosum LHL06 protects Glycine max L. from synergistic toxicity of heavy metals by hormonal and stress-responsive proteins modulation. J Hazard Mater, 2019; 379:120824. CrossRef
Chamnansilpa N, Aksornchu P, Adisakwattana S, Thilavech T, Makynen K, Dahlan W, Ngamukote S. Anthocyanin-rich fraction from Thai berries interferes with the key steps of lipid digestion and cholesterol absorption. Heliyon, 2020; 6(11):1–6. CrossRef
Chen C-M, Chen W-H, Tao H-M, Yang B, Zhou X-F, Luo X-W, Liu T-H. Diversified polyketides and nitrogenous compounds from the mangrove endophytic fungus Penicillium steckii SCSIO 41025. Chin J Chem, 2021a; 39(8):2132–40. CrossRef
Chen C-M, Chen W-H, Pang X-Y, Liao S-R, Wang J-F, Lin X-P, Yang B, Zhou X-F, Luo X-W, Liu Y-H. Pyrrolyl 4-quinolone alkaloids from the mangrove endophytic fungus Penicillium steckii SCSIO 41025: chiral resolution, consurational assignment, and enzyme inhibitory activities. Phytochemistry, 2021b; 186:1–8. CrossRef
Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 11th edition, Clinical and Laboratory Standards Institute, Wayne, Pennsylvania, PA, 2018..
Costa RLB, Gradishar WJ. Triple-negative breast cancer: Current practice and future directions. J Oncol Pract, 2017; 13(5):301–3. CrossRef
de la Torre BG, Albericio F. The pharmaceutical industry in 2021. An analysis of FDA drug approvals from the perspective of molecules. Molecules, 2022; 27(3):1–15. CrossRef
Douanla-Meli C, Langer E, Diversity and molecular phylogeny of fungal endophytes associated with Diospyros crassiflora. Mycology, 2012; 3(3):175–187.
Fan M, Chen X, Luo X, Zhang H, Liu Y, Zhang Y, Wu J, Zhao C, Zhao P. Diversity of endophytic fungi from the leaves of Vaccinium dunalianum. Lett Appl Microbiol, 2020; 71(5):479–89. CrossRef
García-Méndez MC, MacÍas-Ruvalcaba NA, Lappe-Oliveras P, Hernández-Ortega S, MacÍas-Rubalcava ML. Phytotoxic potential of secondary metabolites and semisynthetic compounds from endophytic fungus Xylaria feejeensis strain SM3e-1b isolated from Sapium macrocarpum. J Agric Food Chem, 2016; 64(21):4255–63. CrossRef
Geronimo AJO, Bancual MEJF, Ko KAL, Soliba LML, Ildefonso JJC, Soriano AMB, Tagalog ACMM, Acosta NE, Ang VS, Apigo MA,. Free radical scavenging and in vitro cytotoxic activity of Bugnay (Antidesma bunius) leaves extract against A549 human lung adenocarcinoma and HCT-116 human colorectal cancer cell lines. Indones J Cancer Chemoprevent, 2020; 11(3):124–33. CrossRef
Golias HC, Polonio J, dos Santos Ribeiro MA, Polli A, da Silva AA, Bulla AM, Volpato H, Nakamura CV, Meurer EC, Azevedo JL, Pamphile JA. Tibouchina granulosa (Vell.) Cogn (Melastomataceae) as source of endophytic fungi: Isolation, identification, and antiprotozoal activity of metabolites from Phyllosticta capitalensis. Braz J Microbiol, 2020; 51(2):557–69. CrossRef
Hamidi ?R, Jovanova B, Panovska TK. Toxic?logical evaluation of the plant products using brine shrimp (Artemia salina L.) model. Maced Pharm Bull, 2014; 60(1):9–18. CrossRef
Hao M-J, Chen P-N, Li H-J, Wu F, Zhang G-Y, Shao Z-Z, Liu X-P, Ma W-Z, Xu J, Mahmud T, Lan W-J. β-Carboline alkaloids from the deep-sea fungus Trichoderma sp. MCCC 3A01244 as a new type of anti-pulmonary fibrosis agent that inhibits TGF-β/smad signaling pathway. Front Microbiol, 2022; 13:1–11. CrossRef
Hu X-L, Bian X-Q, Wu X, Li J-Y, Hua H-M, Pei Y-H, Han A-H, Bai J. Penioxalamine A, a novel prenylated spiro-oxindole alkaloid from Penicillium oxalicum TW01-1. Tetrahedron Let, 2014; 55(29):3864–7. CrossRef
Hu XY, Li XM, Wang BG, Meng LH. Uncommon polyketides from Penicillium steckii AS-324, a marine endozoic fungus isolated from deep-sea coral in the magellan seamount. Int J Mol Sci, 2022; 23(11):1–13. CrossRef
Hughes KW, Petersen RH, Lodge DJ, Bergemann SE, Baumgartner K, Tulloss RE, Lickey E, Cifuentes J. Evolutionary consequences of putative intra- and interspecific hybridization in agaric fungi. Mycologia, 2013; 105(6):1577–94. CrossRef
Ikram M, Ali N, Jan G, Jan FG, Rahman IU, Iqbal A, Hamayun M. IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils. PLoS One, 2018; 13(11):e0208150. CrossRef
Indrawati I, Rosiana N, Fitri A, Rizki M, Nada N. Antimicrobial potency from endophytic bacteria of bignay plant (Antidesma bunius (L.) Spreng.) against pathogenic bacteria. World News Nat Sci, 2020; 31:1–8.
Islary A, Sarmah J, Basumatary S. Nutritional value, phytochemicals and antioxidant properties of two wild edible fruits (Eugenia operculata Roxb. and Antidesma bunius L.) from Assam, North-East India. Med J Nutrition Metab, 2017; 10(1):29–40. CrossRef
Jorjong S, Butkhup L, Samappito S. Phytochemicals and antioxidant capacities of Mao-Luang (Antidesma bunius L.) cultivars from Northeastern Thailand. Food Chem, 2015; 181:248–55. CrossRef
Krongyut O, Sutthanut K. Phenolic profile, antioxidant activity, and anti-obesogenic bioactivity of Mao luang fruits (Antidesma bunius L.). Molecules, 2019; 24(22):1–15. CrossRef
Kjer J, Debbab A, Aly AH, Proksch P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat Protoc, 2010; 5(3):479–90. CrossRef
Lambert C, Wendt L, Hladki AI, Stadler M, Sir EB. Hypomontagnella (Hypoxylaceae): a new genus segregated from Hypoxylon by a polyphasic taxonomic approach. Mycol Prog, 2019; 18(1-2):187–201. CrossRef
Lee C, Sohn JH, Jang JH, Ahn JS, Oh H, Baltrusaitis J, Hwang IH, Gloer JB. Cycloexpansamines A and B: spiroindolinone alkaloids from a marine isolate of Penicillium sp. (SF-5292). J Antibiot, 2015; 68(11):715–8. CrossRef
Lim TK. Edible medicinal and non-medicinal plants. Springer Dordrecht, Dordrecht, Netherland, 2012. CrossRef
Lin L, Jiang N, Wu H, Mei Y, Yang J, Tan R. Cytotoxic and antibacterial polyketide-indole hybrids synthesized from indole-3-carbinol by Daldinia eschscholzii. Acta Pharm Sin B, 2019; 9(2):369–80. CrossRef
Liu H-X, Tan H-B, Li S-N, Chen Y-C, Li H-H, Zhang W-M. Two new metabolites from Daldinia eschscholtzii, an endophytic fungus derived from Pogostemon cablin. J Asian Nat Prod Res, 2019; 21(2):150–6. CrossRef
Livezey M, Kim JE, Shapiro DJ. A new role for estrogen receptor A in cell proliferation and cancer: Activating the anticipatory unfolded protein response. Front Endocrinol, 2018; 9:325. CrossRef
Lutfia A, Munir E, Yurnaliza Y, Basyuni M. Chemical analysis and anticancer activity of sesterterpenoid from an endophytic fungus Hypomontagnella monticulosa Zg15SU and its host Zingiber griffithii baker. Heliyon, 2021; 7(2):1–20. CrossRef
Malmstrùm J, Christophersen C, Frisvad JC. Secondary metabolites characteristic of Penicillium citrinum, Penicillium steckii and related species. Phytochemistry, 2000; 54(3):301–9. CrossRef
Mauldina MG, Sauriasari R, Elya B. α-glucosidase inhibitory activity from ethyl acetate extract of Antidesma bunius (L.) Spreng stem bark containing triterpenoids. Pharmacogn Mag, 2017; 13(52):590–94. CrossRef
Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DJ, McLaughlin JL. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med, 1982; 45(5):31–4. CrossRef
Mishra R, Kushveer JS, Khan MIK, Pagal S, Meena CK, Murali A, Dhayalan A, Sarma VV. 2,4-Di-tert-butylphenol isolated from an endophytic fungus, Daldinia eschscholtzii, reduces virulence and quorum sensing in Pseudomonas aeruginosa. Front Microbiol, 2020; 11:1668. CrossRef
Mishra S, Priyanka, Sharma S. Metabolomic insights into endophyte-derived bioactive compounds. Front Microbiol, 2022; 13:835931. CrossRef
Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod, 2020; 83(3):770–803. CrossRef
Niksic H, Becic F, Koric E, Gusic I, Omeragic E, Muratovic S, Miladinovic B, Duric K. Cytotoxicity screening of Thymus vulgaris L. essential oil in brine shrimp nauplii and cancer cell lines. Sci Rep, 2021; 11(1). CrossRef
Ogba N, Manning NG, Bliesner BS, Ambler SK, Haughian JM, Pinto MP, Jedlicka P, Joensuu K, Heikkilä P, Horwitz KB. Luminal breast cancer metastases and tumor arousal from dormancy are promoted by direct actions of estradiol and progesterone on the malignant cells. Breast Cancer Res, 2014; 16(6):1–14. CrossRef
Panda SK, Padhi L, Leyssen P, Liu M, Neyts J, Luyten W. Antimicrobial, anthelmintic, and antiviral activity of plants traditionally used for treating infectious disease in the similipal biosphere reserve, Odisha, India. Front Pharmacol, 2017; 8:658. CrossRef
Perez KE, Foltz MJ, Volk TJ. Molecular phylogeny and morphology reveal three new species of Cantharellus within 20 m of one another in Western Wisconsin, USA. Mycologia, 2013; 105(2):447–61. CrossRef
Radhakrishnan R, Shim KB, Lee BW, Hwang CD, Pae SB, Park CH, Kim SU, Lee CK, Baek IY. IAA-producing Penicillium sp. NICS01 triggers plant growth and suppresses Fusarium sp.-induced oxidative stress in sesame (Sesamum indicum L.). J Microbiol Biotechnol, 2013; 23:856–63. CrossRef
Raja HA, Miller AN, Pearce CJ, Oberlies NH. Fungal identification using molecular tools: a primer for the natural products research community. J Nat Prod, 2017; 80(3):756–70. CrossRef
Rajashekara S, Reena D, Mainavi MV, Sandhya LS, Baro U. Biological isolation and characterization of Catharanthus roseus (L.) G. Don methanolic leaves extracts and their assessment for antimicrobial, cytotoxic, and apoptotic activities. BMC Complement Med Ther, 2022; 22(1):328. CrossRef
Rajashekara S, Shrivastava A, Sumhitha S, Kumari, S. Biomedical applications of biogenic zinc oxide nanoparticles manufactured from leaf extracts of Calotropis gigantea (L.) Dryand. BioNanoSci, 2020; 10:654–71. CrossRef
Ratnadewi AAI, Rahayu LD, Rochman J, Susilowati, Nugraha AS, Siswoyo TA. Revealing anti-diabetic potency of medicinal plants of Meru Betiri National Park, Jember–Indonesia. Arab J Chem, 2020; 13(1):1831–6. CrossRef
Rivera-Chávez J, Figueroa M, González MDC, Glenn AE, Mata R. α-Glucosidase inhibitors from a Xylaria feejeensis associated with Hintonia latiflora. J Nat Prod, 2015; 78(4):730–735. CrossRef
Sarkar S, Dey A, Kumar V, Batiha GE, El-Esawi MA, Tomczyk M, Ray P. Fungal endophyte: an interactive endosymbiont with the capability of modulating host physiology in myriad ways. Front Plant Sci, 2021; 12:701800. CrossRef
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA, Chen W, Fungal Barcoding Consortium. Nuclear ribosomal ITS region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci USA, 2012; 109(16):6241–6. CrossRef
Sharma H, Rai AK, Chettri R, Nigam PS. Bioactivites of Penicillium citrinum isolated from a medicinal plant Swertia chirayita. Arch Microbiol, 2021; 203(8): 5173–82. CrossRef
Shin HJ, Pil GB, Heo SJ, Lee H-S, Lee JS, Lee Y-J, Lee J, Wo HS. Anti-inflammatory activity of tanzawaic acid derivatives from a marine-derived fungus Penicillium steckii 108YD142. Mar Drugs, 2016; 14(1):14. CrossRef
Siriwach R, Kinoshita H, Kitani S, Igarashi Y, Pansuksan K, Panbangred W, Nihira T. Xylaropyrone, a new γ-pyrone from the endophytic fungus Xylaria feejeensis MU18. J Antibiot (Tokyo), 2011; 64(2):217–9. CrossRef
Slepecky RA, Starmer WT. Phenotypic plasticity in fungi: a review with observations on Aureobasidium pullulans. Mycologia, 2009; 101(6):823–32. CrossRef
Stadler M, Læssøe T, Fournier J, Decock C, Schmieschek B, Tichy H-V, Peršoh DA. Polyphasic taxonomy of Daldinia (Xylariaceae). Stud Mycol, 2014; 77:1–143. CrossRef
Wang G, Fan JY, Zhang WJ, Hua CP, Chen CJ, Yan W, Ge HM, Jiao RH, Tan RX. Polyketides from mantis-associated fungus Daldinia eschscholzii IFB-TL01. Chem Biodivers, 2015; 12(9):1349–55. CrossRef
Waqas M, Khan AL, Kamran M, Hamayun M, Kang S-M, Kim Y-H, Lee I-J. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules, 2012; 17(9):10754–73. CrossRef
Widjajanti H, Muharni, Nurnawati E, Tripuspita V. The potency of endophytic fungi isolated from Hippobroma longiflora (L) G. Don as an antioxidant sources. In: Proceedings of IOP Conference Series: Earth and Environmental Science, 2021 Oct 23–24, Banjarbaru City, Indonesia. CrossRef
Wu X-Z, Huang W-J, Liu W, Mándi A, Zhang Q, Zhang L, Zhang W, Kurtán T, Yuan C-S, Changsheng Z. Penicisteckins A-F, isochroman-derived atropisomeric dimers from Penicillium steckii HNNU-5B18. J Nat Prod, 2021; 84(11):2953–60. CrossRef
Wutthiwong N, Suthiphasilp V, Pintatum A, Suwannarach N, Kumla J, Lumyong S, Maneerat T, Charoensup R, Cheenpracha S, Limtharakul T, Pyne SG, Laphookhieo S. Daldiniaeschsone A, a rare tricyclic polyketide having a chromone unit fused to a δ-lactone and its symmetrical biphenyl dimer, daldiniaeschsone B, from an endophytic fungus Daldinia eschscholtzii SDBR-CMUNKC745. J Fungi, 2021; 7(5):358. CrossRef
Xiao JA, Cheng XL, Li YC, He YM, Li JL, Liu ZP, Xia PJ, Su W, Yang H. Palladium-catalysed ring-opening [3 + 2]-annulation of spirovinylcyclopropyl oxindole to diastereoselectively access spirooxindoles. Org Biomol Chem, 2018; 17(1):103–7. CrossRef
Xu X, Zhang X, Nong X, Wei X, Qi S. Oxindole alkaloids from the fungus Penicillium commune DFFSCS026 isolated from deep-sea-derived sediments. Tetrahedron, 2015; 71(4):610–15. CrossRef
Yao G, Chen X, Zheng H, Liao D, Yu Z, Wang Z, Chen J. Genomic and chemical investigation of bioactive secondary metabolites from a marine-derived fungus Penicillium steckii P2648. Front Microbiol, 2021; 12:1–10. CrossRef
Yurchenko EA, Menchinskaya ES, Pislyagin EA, Trinh PTH, Ivanets EV, Smetanina OF, Yurchenko AN. Neuroprotective activity of some marine fungal metabolites in the 6-hydroxydopamin- and paraquat-induced parkinson’s disease models. Mar Drugs, 2018; 16(11):457. CrossRef
Zakaria L, Aziz WNW. Molecular identification of endophytic fungi from banana leaves (Musa spp.). Trop Life Sci Res, 2018; 29(2):201–11. CrossRef
Zaman S, Islam M, Koly S, Faisal T, Rakib K. Evaluation of cytotoxicity and antibacterial activities of methanolic extract of Antidesma bunius (Linn.) (Family Euphorbiaceae) Leaf. J Adv Med Pharm Sci, 2018; 16(2):1–7. CrossRef
Zhang YL, Zhang J, Jiang N, Lu YH, Wang L, Xu SH, Wang W, Zhang GF, Xu Q, Ge HM, Ma J, Song YC, Tan RX. Immunosuppressive polyketides from mantis-associated Daldinia eschscholzii. J Am Chem Soc, 2011; 133(15):5931–40. CrossRef
Zhang AH, Jiang N, Wang XQ, Tan RX. Galewone, an anti-fibrotic polyketide from Daldinia eschscholzii with an undescribed carbon skeleton. Sci Rep, 2019; 9(1):14316. CrossRef
Zhu X, Liu Y, Hu Y, Lv X, Shi Z, Yu Y, Jiang X, Feng F, Xu J. Neuroprotective activities of constituents from Phyllosticta capitalensis, an endophyte fungus of Loropetalum chinense var. rubrum. Chem Biodivers, 2021; 18(8):e2100314. CrossRef