INTRODUCTION
Nuclear receptors (NRs) have advanced remarkably in modern biomedical research and drug discovery (Mazaira et al., 2018). Hollenberg et al. (1985) first discovered NRs as the receptors of the most familiar hormones, including 17β-estradiol, and corticosteroids/thyroid hormone (TH) (Holzer et al., 2017). NRs play a significant role in physiological processes such as metabolism, osmoregulation, cell growth, immune response, enzymatic activities, development, and reproduction. The NRs superfamily comprises over 500 members and approximately 50 transcription factors (Alexander et al., 2017; Burris et al., 2013). NRs are an essential component of cellular physiology, which has attracted pharmaceutical interest. They serve as a target to treat diseases such as cancer, diabetes, inflammation, atherosclerosis, and endocrine disorders (Alexander et al., 2017; Burris et al., 2013). For example, estrogen receptors (ERs) antagonists treat breast cancer. Also, peroxisome proliferator-proliferator receptors (PPARs) agonists treat type 2 diabetes mellitus (T2DM) and hyperlipidemia. Furthermore, the role of NRs in the circadian rhythm, cholesterol transport, and autoimmune disorders has taken utmost significance in treating infectious diseases (Khorasanizadeh and Rastinejad, 2016; Leopold Wager et al., 2019).
NRs allow bone cells, such as osteoblasts, osteoclasts, and osteocytes, to sense their dynamic nature and respond to the development of bones and remodeling. Deficiency in specific NRs ligands can lead to bone loss; for example, estrogen loss in postmenopausal women can cause osteoporosis and increase the risk of bone fracture. On the other hand, glucocorticoids (GCs) could hamper one’s health (Zuo and Wan, 2017). NRs classically act in three steps, i) repression, ii) derepression, and iii) transcriptional activation (Robinson-Rechavi et al., 2003). The binding of NRs hormone to specific DNA sequences, termed hormone response elements (HRE), is responsible for the abovementioned functions (Escriva et al., 2000). A significant objective of ongoing medication advancement programs has been to deliver ligands that display agonist or antagonist activity in the tissue in a receptor-particular way. The point is to lessen adverse reactions and, on account of certain malignant growths (Robinson-Rechavi et al., 2003). NRs require multiple protein-protein interactions to regulate their target gene expression (Robinson-Rechavi et al., 2003). Hence, the engagement with co-regulators offers functional flexibility to NRs (McKenna and O’Malley, 2002).
NR CLASSIFICATION
NRs were grouped into three subfamilies based on their DNA-binding properties (Laudet et al., 1992). Later, in 2004, it was divided into four subfamilies based on their DNA-binding properties and heterodimer priorities (Novac and Heinzel, 2004; Porter et al., 2019). These are class I-steroid hormone receptors that primarily form ligand-induced homodimers. Receptors belonging to class I bind to DNA target sequences arranged in inverted repeats or palindromes. The second class (class II) is retinoid X receptors (RXRs) and thyroid receptors (TRs). The receptor belongs to class II heterodimerized and binds to symmetrical response elements. The third class (class III) is homodimerized and binds primarily to direct repeats. The fourth class (class IV) attaches to the core site of DNA as monomers (Novac and Heinzel, 2004; Porter et al., 2019). In addition to this, based on the amino acid and dipeptide composition, NR classification has also been further designed into TH (TR, RAR, ROR), HNF4-like (HNF4, RXR, TLL, Coup, USP), Fushi tarazu-F1 like (SFI, FTF, FTZ-F1), and estrogen-like [ER, ERR, GC receptor (GR), mineralocorticoid receptor (MR), progesterone receptor (PR), androgen receptor (AR)] (Bhasin and Raghava, 2004). To date, the most acceptable one is the classification that classified NR into seven subfamilies. This classification numbered NR from zero to six based on the endogenous ligands that bind to specific receptors (Weikum et al., 2018).
OVERVIEW OF NR’S STRUCTURE
The general structure of NRs comprises five domains. These are the N-terminal domain (NTD), also known as the A/B domain, the DNA binding domain (DBD) or C domain, the hinge region (HRe) or D domain, the ligand-binding domain (LBD) or E domain, and the C-terminal domain (CTD) or F domain (Fig. 1) (Porter et al., 2019). These domains play a vital role in receptor biology. NRs mass might vary but generally between 66 and 100 kDa (Weikum et al., 2018). Unlike DBD, NTD is a highly variable region and least conserved in size and sequence. The NTD contains the activation function (AF) regions (Weikum et al., 2018). These AF regions are responsible for transcriptional activation. AF is transcriptionally active and mediates ligand-independent activation function-1 (AF-1). AF-1 is the term used to distinguish them from the well-known AF-2 domain found at the C terminus of LBD. The AF-1 region can initiate transcription independent of LBD or synergize with the AF-2 (ligand-dependent) region to produce maximal receptor function (Onate et al., 1998). AF-1 is a poorly structured activation domain that belongs to a broad category of intrinsically active compared to AF-2 (Almlöf et al., 1998, 1997). The NTD region completely lacks conservation within the NRs subfamilies: PPARs (PPARα, PPARβ, PPARγ), ERs (ERα and ERβ) / ERRs (ERRα, ERRβ, and ERRγ) suggesting the functional roles of the NTD is highly receptor-specific (Gadaleta and Magnani, 2013).
 | Figure 1. The general structure of NRs representing five domains. [Click here to view] |
DBD is adjacent to NTD and is the central, most highly conserved domain of NRs (Gadaleta and Magnani, 2013). DBD also has conserved zinc fingers that are accountable for binding to cognate HRE (Zhang et al., 2004). Additionally, zinc fingers within the DBD are responsible for setting NRs apart from other DNA-binding proteins (Mangelsdorf et al., 1995) and interacting with histone deacetylases 3 and 4 (Franco et al., 2003). Zinc fingers contain a carboxy-terminal extension that provides DNA response elements and protein binding interfaces (Hart, 2002). Zinc finger motifs permit the specific recognition of short, imperfect inverted repeats of DNA (for steroid receptors) or direct repeats (for RXR-heterodimeric receptors) (Weatherman et al., 1999). According to nuclear magnetic resonance and crystallographic research, four invariable cysteines in each zinc finger organize tetrahedrally on zinc ions. Both zinc finger modules fold together to produce a compact and interdependent structure. Zinc fingers contain the “P box” and “D box.” These boxes are required to dimerize and discriminate core DNA recognition motifs. They are also responsible for direct interaction and binding specificity (Aranda and Pascual, 2001). The antiquated relationship between LBD and DBD has been demonstrated to be a profoundly influential association (Mangelsdorf et al., 1995). DBD is required for gene activation, while other molecular regions in the carboxyl and amino terminus enhance its AF (Carson-Jurica et al., 1990). DBD interacts with DNA sequences that are palindromic or direct repeats (six nucleotide segments in different configurations) or response elements (Fernandez, 2018).
LBD, the third region of NRs, was discovered in 1995 and contained -300 amino acids (Bain et al., 2007). This region shows diversity in sequence but exhibits certain similarities in its overall design. This region functions in a ligand-specific manner (Mahajan and Samuels, 2000). Generally, LBDs are 12α helices arranged in a three-layered antiparallel sandwich (van der Vaart and Schaaf, 2009). The “front” and “back” sheets are made up of helices that are parallel to one another. The middle sheet of helices runs across the two outer sheets and only takes up space in the domain’s upper half. The lower section of the domain is relatively devoid of protein, which forms an internal cavity for small-molecule ligands in most NR LBDs (Franco et al., 2003). This domain is required for efficient homo/heterodimerization and interaction with transcriptional co-factors and components of the general transcriptional machinery (Uppenberg et al., 1998). In this region, mutational investigation of ERs led to the identification of activation function-2 (AF-2). AF-2 is a highly conserved subregion inside the LBD’s distal carboxyl terminus and is required for transcriptional activation (AF-2) (Glass et al., 1997). Many pharmaceutical companies are developing LBD ligands to limit or modify hormone-dependent processes such as infertility, bone loss, tumor, and skin diseases (Egner, 2002).
HRe is also known as the DBD’s C-terminal extension. This region has about 25 amino acids and has a low similarity and resemblance to other NRs (Pawlak et al., 2012; Tetel et al., 1997). It is flexible, least conserved, and vulnerable to protease cleavage. It connects or hinges between the DBD and LBD for proper DNA binding and dimerization (Haelens et al., 2007). HRe also plays a role in harboring nuclear localization signals and comprises residues that abolish NRs interaction with the NR compressor when the mutation occurs (Aranda and Pascual, 2001). Though this domain is regarded as a passive polypeptide linker in the initial stage, it is a hotspot for post-translational modifications that exert translocation, DNA binding, and transactivation (Weikum et al., 2018).
F domain or CTD is present at the C-terminus of ER alpha and beta but not in all the NRs. It is one of the least conserved regions and varies in size (19–80 residues) (Skafar and Zhao, 2008). Though varying in length, the F domain plays a common function by affecting the transcriptional activation, dimerization, interactions with other proteins, and stabilizing agonist or antagonist-bound conformations in the NRs LBD (Patel and Skafar, 2015). The LBD was the least conserved region among other domains. Alternative mRNA splicing has been reported to occur in this region, resulting in complete or partial loss of LBD. The formation of these splice variants further influences the canonical receptors by functioning as dominant-negative inhibitors (van der Vaart and Schaaf, 2009).
NRS AS DRUG TARGETS
Drugs targeting NRs are explored as therapeutic targets and are among the most widely and commercially successful. Tamoxifen and raloxifene for ERs in breast cancer, thiazolidinediones for PPARs in type 2 diabetes, mifepristone for PRs in ovulation, and dexamethasone for GRs in inflammatory disorders are a few examples (Burris et al., 2013; Rastinejad et al., 2013). A few drugs targeting NRs are Cytosporone B, 6-mercaptopurine, Celastrol, and Amoitone B. These compounds have been reported to possess antitumor properties in-vitro and in-vivo (Zhang et al., 2018). In their study, Bertilsson et al. (1998)have shown that the search for other unknown/unexplored NR could be discovered using a few standard methods, viz., PCR or yeast two-hybrid interactions. Their findings demonstrated an hidden markov model (HMM) profile-based search strategy for identifying novel orphan NRs in expressed sequence tag databases. Using this technology, the authors discovered a previously unknown human NR called hPAR, which is effectively triggered by a group of pregnanes and a group of clinically used drugs known to stimulate the activation of human CYP3A enzymes selectively. As a result, it was concluded that hPAR would most likely intervene in a novel signal pathway important for CYP3A gene expression regulation, with possible implications for the therapeutic evaluation of drug reactions that are beneficial to humans (Bertilsson et al. 1998).
NRs play a significant role in the gut’s intestinal functions like absorption of nutrients and transport, water secretion/absorption, and gut-to-liver communication and regulation of the gut microbiome (Ning et al., 2019). Recently, several members of NRs have been validated as a therapeutic target in patients with IBD regulating gut physiology that is ERβ, GR, FXR, PPARγ, PXR, RARα, vitamin D receptor (VDR), HNF4α, or NR2F6 (Klepsch et al., 2019). In line with this, a study on mice deficient in NR2f6 was reported to be highly susceptible to dextran sodium sulfate-induced colitis characterized by the increased loss of weight, colonic tissue destruction, and immune cell infiltration. Taken together, NR2f6 alters intestinal permeability and decreases Muc2 expression (Klepsch et al., 2018). Similarly, the deletion of VDR in mice exaggerates colitis and colon tumorigenesis (Takada and Makishima, 2016). Interestingly, scientists have extended the role of AR from prostate cancer to T2DM. It has been demonstrated that AR deficiency in males is associated with T2DM and vice versa in females. The activation of AR in males has shown a beneficial effect in preventing T2DM. This is corroborated by preventing visceral fat accumulation and promoting insulin sensitivity in adipose tissue, skeletal muscle, and liver, regulating energy homeostasis in the liver and improving β-cell functions (Navarro et al., 2015).
DIABETES MELLITUS
According to WHO estimates from 2014, 422 million people worldwide have diabetes, with the majority living in low- and middle-income countries. According to WHO 2021, diabetes is accountable for 1.6 million mortality per year. Over the last 10 years, the number of incidents and the prevalence of diabetes has steadily increased. Type 1 diabetes mellitus (T1DM) affects 1.1 million children and adolescents aged 14–19 years, according to the International Diabetes Federation (IDF) report. Every year, over 4 million people die from chronic hyperglycemia. Additionally, it is expected that by 2025, India, China, and the United States will have the most significant percentage of diabetic patients (King et al., 1998). Diabetes is a group of metabolic diseases characterized by chronic hyperglycemia and glucose intolerance resulting from defects in insulin secretion, insulin action, or both (Kharroubi, 2015). Diabetes is a dangerous and potentially fatal disease marked by thirst, polyuria, blurred eyesight, and weight loss. The most severe clinical signs are ketoacidosis or a non-ketotic hyperosmolar condition, which can lead to dehydration and coma. Commonly, diabetes is categorized into T1DM, T2DM, gestational diabetes mellitus (GDM), prediabetes, and idiopathic diabetes. T1DM (due to autoimmune β-cell destruction, usually leading to total insulin deficiency) occurs at any age but most often happens in children and young adults. Family history plays a role, but only in about 10%–15% of people of this type (Gottlieb, 2004). T2DM is characterized by insulin resistance due to insulin secretory deficiency. GDM is diagnosed in the second or third trimester of pregnancy that is not overt diabetes before gestation. Prediabetes refers to blood glucose levels higher than usual but not yet high enough to be diagnosed as type 2 diabetes. In other words, prediabetes also refers to impaired fasting glucose, impaired glucose tolerance, or glycated hemoglobin (A1C) of 6.0%–6.4%. Although not everyone with prediabetes will develop type 2 diabetes, people are at a greater risk for developing diabetes and severe comorbidities (Gottlieb, 2004). Patients with idiopathic diabetes tend to have permanent insulinopenia and are also vulnerable to episodic ketoacidosis. A few cases with type 1 diabetes fall into this category (Zuo and Wan, 2017). Other types of diabetes, such as monogenic diabetes syndromes (like neonatal diabetes and maturity-onset diabetes of the young), diseases of the exocrine pancreas (such as cystic fibrosis and pancreatitis, hemochromatosis), and drug-or chemical-induced diabetes (as with GCs, neuroleptics, HIV/AIDS treatment, or after organ transplantation) (Petersmann et al., 2018).
Of all the different forms of diabetes, T2DM is globally burdened and rapidly increasing, affecting people of all ages (Al-Rifai et al., 2019). It is reported that 90%–95% of patients suffer from T2DM (Galicia-Garcia et al., 2020). T2DM prevalence in the adult population has nearly doubled in the last decade, from 4.7% in 1980 to 8.5% in 2014. The global burden of T2DM in adults aged 20–79 is expected to go up to 629 million in 2045 (Al-Rifai et al., 2019). In the UK, the prevalence of T2DM was estimated at 4.5 million in 2016. According to audits, males are more likely to get T2DM than females, with men contributing to 56% of all adult diabetics. T2DM is linked with pancreatic, liver, and kidney cancer (Man Chu, 2018). According to the IDF, 415 million people worldwide have diabetes, with 91% suffering from T2DM. Those with diabetes have a greater cardiovascular disease (CVD) prevalence rate than adults without diabetes. T2DM can shorten life expectancy by up to 10 years. Other studies (Einarson et al., 2018) found that, along with rising T2DM incidence, the associated risk of CVD due to T2DM increased from 5.4% in 1952–1974 to 8.7% between 1975 and 1998 in the Framingham Heart Study (Einarson et al., 2018).
T2DM is also called non–insulin-dependent diabetes/type 2 diabetes/adult-onset diabetes. Patients suffering from this type are resistant to insulin, have relative (rather than absolute) insulin deficiency, and do not need insulin treatment to survive. There are probably several causes of this kind of diabetes (Galicia-Garcia et al., 2020). Although the specific etiology of T2DM is unknown, most people suffering from T2DM are obese. These patients develop insulin resistance in the peripheral tissues, hyperglycemia, and ketoacidosis (rarely occurring). In addition to obesity, other risk factors are age, women having a history of GDM, and also in people with hypertension and dyslipidemia. Patients suffering from T2DM are also associated with cognition and moderate cognitive impairment. Altogether, T2DM impairs cerebral health, structural rigidity, and brain activity (Tiwari and Kumar, 2018). T2DM undergoes undiagnosed for several years because the hyperglycemia develops slowly and, at the initial stages, is often not severe enough for the patient to observe any of the symptoms of diabetes.
Nonetheless, patients are at higher risk of macrovascular and microvascular complications. Macrovascular complications include cerebrovascular diseases (heart attacks, stroke, insufficient blood flow to legs) and peripheral vascular and cardiovascular disease. Microvascular complications include retinopathies (blindness), nephropathy, neuropathy with the incidence of ulcers in the foot, Charcot joints, amputation, and autonomic and sexual dysfunction. Insulin levels in individuals with this type of diabetes may appear normal or increased. As a result, insulin secretion in these people is impaired and is inadequate to counteract insulin sensitivity (Tiwari and Kumar, 2018).
Diabetes-related NRs
NRs are hormone-sensing transcription factors that convert changes in gene expression to nutritional or endocrine signals (Schupp and Lazar, 2010). Endogenous ligands for NRs include bile acids, phospholipids, steroid hormones, THs, retinoids, and vitamin D (Table 1) (Delerive et al., 2000; Schupp and Lazar, 2010).
The endogenous ligands of ERs are: estrone (E1), estradiol (E2), and estriol (E3) and have also been shown to bind diverse compounds with varying activity (Nelson and Habibi, 2013). ERα and ERβ are the most widely studied ERs. These receptors play a significant role in reproduction. They are expressed in other species, such as zebrafish and sea bream (Bardet et al., 2002). Both zebrafish (Menuet et al., 2002) and sea bream (Muñoz-Cueto et al., 1999) have receptors that are homologous to the human ERα (Green et al., 1986). AR endogenous ligands include testosterone and the more active form, dihydrotestosterone. Reproductive, immunologic, cardiovascular, musculoskeletal, neurological, and hemopoietic systems rely on AR for their development and maintenance (Davey and Grossmann, 2016). PRs are of two types: PRA and PRB. Both these receptors are activated in response to hormonal stimuli such as progesterone (Banner et al., 1992). Progesterone act on various tissues such as the uterus, mammary gland, brain, and bone and exerts diverse functions in these tissues (Graham and Clarke, 1997).
Bile acid is the endogenous ligand for FXR (Wang et al., 1999). FXR regulates bile acid and glucose metabolism, inflammatory reactions, barrier function, bacterial translocation prevention in the digestive organs (Ding et al., 2015), and cholesterol homeostasis (Wang et al., 1999). FXR also prevents cardiovascular risk factors such as central obesity, glucose intolerance, high cholesterol, and hypertension (Lefebvre et al., 2009). For VDRs, an endogenous ligand is 1,25-dihydroxy vitamin D3 (1,25-(OH)2D3) (Teske et al., 2016). The activation of VDR initiates the autocrine and endocrine processes that are involved in gene expression and calcium absorption regulation (Heaney, 2008), the normal functioning of bones (Tanaka and Deluca, 1974), and the immune system (Haug et al., 1998).
 | Table 1. Few examples of NRs and their endogenous ligands. [Click here to view] |
THR’s endogenous ligands include 3,5,3’,5’-tetraiodo-l-thyronine (T4) and 3,5,3’-triiodo-l-thyronine (T3). These hormones are vital for mammals’ average growth, development, and homeostasis. They also treat obesity, hyperlipidemia, depression, osteoporosis (Ye et al., 2003), cardiac diseases, and cancer. They also improve cognitive function (Brent, 2012), regulate skeletal growth (Kim and Mohan, 2013), and maintain adult brain function (Schroeder and Privalsky, 2014). While any dysfunction in this receptor, such as mutations, leads to resistance to THs, which further causes thyroid cancer, pituitary tumor, dwarfism, and metabolic abnormality (Cheng et al., 2010).
In mice, 9-cis-13,14-dihydroretinoic acid was reported to be the endogenous RXR ligand (Rühl et al., 2018). All-trans retinoic acid is the principal endogenous ligand for RAR. It is formed by the enzymatic oxidation of dietary vitamin A (di Martino and Welch, 2019). It is beneficial in treating osteoporosis, autoimmune disorders, asthma, cancer, diabetes, and obesity (Solt et al., 2010). From the experimental and clinical approach, it has been evidenced that vitamin A (retinol) and its active derivatives (i.e., the retinoids) exert various effects on vertebrate embryos. These effects include body shaping and organogenesis, tissue regeneration, cell growth, differentiation, apoptosis (Mark et al., 2009), visual functions (Kam et al., 2012), acute myeloid leukemia therapy (di Martino and Welch, 2019), and inflammation (Mihály et al., 2013). For REV-ERB, the endogenous ligand is haem/heme (Raghuram et al., 2007). Heme comprises an iron atom and a heterocyclic tetrapyrrole ring structure known as porphyrin. Several heme-containing proteins, such as hemoglobin, use this as a prosthetic group. Furthermore, the capacity of heme to organize an iron atom inside its structure and promote reduction-oxidation (redox) reactions is essential for the function of these proteins (Sawicki et al., 2015).
15d-PGJ2 appears to be a promising PPARγ endogenous ligand. Two linoleate derivatives, 9- and 13-HODEare possible PPARγ ligands among the oxidized lipids. OEA is an endogenous ligand with a higher affinity for PPARα (Lathion et al., 2006). Its primary role is discovered in many diseases such as adipogenesis and storage of fat and eventually obesity and associated metabolic diseases such as hypercholesterolemia, insulin sensitivity, T2DM; and cardiovascular disease including high blood pressure (Heikkinen et al., 2007), inflammation (Zieleniak et al., 2008), gastrointestinal cancer (Necela and Thompson, 2008), and suppress colon carcinogenesis (Thompson, 2007). LXR has two isoforms, LXRα and LXRβ. LXRα is predominantly found in the liver and gut, while LXRβ is found throughout the body. Endogenous ligands for LXR consist of cholesterol derivatives such as 22(R)-hydroxycholesterol, 24(S)-hydroxycholesterol, and 24(S)-25-epoxycholesterol. These ligands bind to both LXR isoforms and increase their transcriptional activity (Schulman, 2010). The involvement of NRs in diabetes has been studied since the discovery of PPAR agonists. Other NRs that act as potential targets for T2DM are discussed in Table 2.
Peroxisome proliferator-proliferator receptors
PPARs are the most widely studied NRs. PPAR subtypes, namely PPARα, γ, -β/δ, are members of the NR family that regulate glucose and lipid metabolism. PPARα agonist increases lipoprotein lipase activity and speeds up the process of β-oxidation (Wilding, 2012). Insulin sensitivity, blood pressure, and fatty acid (FA) uptake have all been demonstrated to improve when PPAR agonists are used (Ahmadian et al., 2013). When used alone, rosiglitazone, pioglitazone, and troglitazone have been found to cause obesity, bone resorption, heart failure, and bladder cancer (Ma et al., 2012). Troglitazone was also taken off the market due to its liver damage. However, using a dual PPAR agonist that activates PPARα and PPARγ has improved lipid profile and glycemic management. Remarkable, this dual activation displays fewer side effects. Such an approach has emerged as one of the most promising strategies for treating T2DM (Wei et al., 2013). Another approach includes a combination of PPAR agonists and dipeptidyl peptidase 4 (DPP4) inhibitors. This strategy improved glycemia by increasing the expression of insulin receptor substrate (IRS)-2 and protecting pancreatic β-cells (Hirukawa et al., 2015).
 | Table 2. NRs and potential ligands reported from preclinical studies for T2DM. [Click here to view] |
Growing evidence shows that PPARγ activation in adipocytes makes a significant contribution to its insulin-sensitizing effects by repressing the release of insulin-desensitizing factors like non-esterified fatty acids (NEFAs), tumor necrosis factor-α, leptin, interleukin-6, and resistin, and stimulating the release of insulin-sensitizing factors like adiponectin and visfatin (Kanatani et al., 2007). Thiazolidinediones are progressively being demonstrated to reduce proteinuria and postpone the course of diabetic nephropathy, depending on or independent of glycemic management, in addition to their impact on insulin resistance and T2DM (Zheng and Guan, 2007). PPARγ has been linked to various renoprotective effects, including improved insulin resistance, reduced blood glucose, decreased rates of circulating NEFAs, and insulin-desensitizing cytokines, increased plasma adiponectin, lower blood pressure, and direct renal actions (Kollerits et al., 2007). Recently, intraperitoneal administration of sulfonylhydrazone derivative, LASSBio-1773, showed hypoglycemic efficacy in a streptozocin-induced diabetic pain model, lowering heat hyperalgesia and mechanical allodynia and demonstrating potential new targets in T2DM (Zapata-Sudo et al., 2016).
Liver X receptors
The LXR is an NR that regulates triglycerides, fat metabolism, and inflammation. LXR also prevents the production of acellular capillaries caused by diabetes. Sirtuin-1 and LXR regulate retinal cholesterol metabolism and inflammation in diabetic retinas (Hammer et al., 2017). The role of LXRs in diabetes has been correlated with the identification of their endogenous ligands, namely 22(R)-hydroxycholesterol, 24(S)-hydroxycholesterol, and 24(S),25-epoxycholesterol. The activation of LXRs increases the transcription of genes such as GLUT4 and insulin genes (Schulman, 2010) enhances glucose uptake and normalizes glycemia in mice with T2DM and insulin resistance (Baranowski, 2008). Interestingly, LXRβ is expressed in β-cells, and its ligands act as insulin sensitizers (Schulman, 2010). Additionally, LXR activation increased the transcription ABCA1 gene. ABCA1 participates in cholesterol efflux to acceptor proteins on pre β high-density lipoprotein (HDL), a process of reverse cholesterol transport. The loss of ABCA1 correlates with low HDL levels and increases the risk of T2DM (Fig. 2) (Haase et al., 2015).
Estrogen receptors
ERs consist of mainly three isoforms, ERα, ERβ, and GPER1. Among these, ERβ has a crucial role in diabetes prevention. ERβ is involved in adenosine triphosphate (ATP)-sensitive potassium (KATP) channel blockade. This effect occurs through the induction of 17β-estradiol (E2) in ERα-/- and ERβ-/- mice. Similarly, agonist activation of ERβ by diarylpropionitrile (DPN) lowered the activity of KATP in a cGMP/PKG-dependent manner in β-cells of wild type (WT) but not in ERβ-/- mice. Consequently, this leads to increased glucose-induced intracellular calcium [Ca2+]I signals and induced insulin release (Soriano et al., 2009). KATP channels are complexes formed by the hetero-octameric and co-assembly of four Kir6.2 subunits called pore-forming subunits and four sulfonylurea receptor1 (SUR1) subunits called regulatory subunits. KCNJ11 and ABCC8 are the genes that code for both these two subunits. KATP channels are crucial in physiological and pathophysiological states (Remedi and Nichols, 2016). KATP converts metabolic status into electrical activity and processes as a metabolic sensor by sensing the metabolic changes in the pancreatic β-cells. This event occurs due to KATP channel inhibition by ATP and activation by magnesium-ADP (Mg-ADP) (Szeto et al., 2018). In diabetes mellitus, where normal glucose oxidation and a rise in [ATP]:[ADP] ratio fails to take place, inhibition or reduction of KATP channels is necessary because this helps in membrane depolarization and is critical for voltage-dependent Ca2+ channels opening. This further allows calcium entry into the cells and increases [Ca2+] concentration. Consequently, this is essential for insulin vesicles-plasma membrane fusion and subsequent insulin release (Remedi and Nichols, 2016). Hence, selective ERβ agonists could help increase insulin release (Fig. 3). In contrast, in vivo study of postmenopausal (performed via bilateral ovariectomy) diabetes-induced vascular dysfunction (VD) in rats, selective ERβ agonists, and DPN did not show any improvement in impaired glycemia, VD, oxidative stress (OS), or inflammation (Bansal and Chopra, 2014).
 | Figure 2. Mechanism of action of LXRs in diabetes. [Click here to view] |
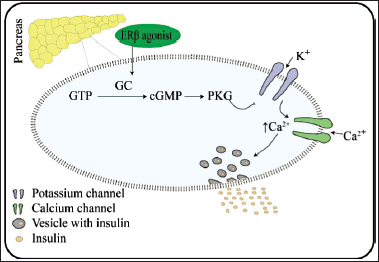 | Figure 3. Mechanism of action of ERs in diabetes. [Click here to view] |
Androgen receptor
ARs have been found in different tissues and are involved in both central and peripheral physiology and insulin action (Diamanti-Kandarakis et al., 2019). Androgens have a bidirectional effect on glucose homeostasis in males and females (Navarro et al., 2015). Excess androgen in women, as observed in polycystic ovarian syndrome, can impair insulin levels and lead to excess body weight, adiposity dysfunction, and metabolic syndrome (Diamanti-Kandarakis et al., 2019). The link between testosterone and diabetes, on the other contrary, has emerged as a significant risk factor for T2DM in the male population. In contrast to women, multiple studies in the last decade have found that androgen depletion in men is associated with an increased prevalence of T2DM (Yao et al., 2018). Several reports have shown that testosterone therapy improved visceral obesity, insulin resistance, glycemic control, and lipid profile in hypogonadism males with T2DM (Li et al., 2020). Also, testosterone administration has been shown to protect β-cell from apoptosis and stress-induced accelerated senescence. These effects of testosterone have been linked with the activation of the AR/PI3K/AKT pathway (Divakar et al., 2017; Dong et al., 2020). The activation of AR/PI3K/AKT could be the reason for the anti-inflammatory effect of testosterone in hypogonadism men with T2DM (Fig. 4) (Navarro et al., 2016; Yamaguchi et al., 2019). Altogether, the activation of AR in the male sex contributes to reversed impaired glucose tolerance in the skeletal muscle and decreased cholesterol in obese men (Pal et al., 2019). Since testosterone can improve the β-cell function of the pancreas, improve insulin resistance, and reduce inflammatory milieu, targeting the androgen pathway could be a novel therapeutic option for developing anti-diabetic drugs.
Vitamin D receptor
VDR is a nonsteroidal NR. VDR heterodimerized with retinoid X receptor to transcribe genes (Lei et al., 2020). Apart from immune cells, VDR is also found in the liver, muscle fibers, adipocytes, and islets of the pancreas and may improve insulin sensitivity upon activation. In both T1DM and T2DM models, it has been found that VDR expression is decreased, suggesting that a decrease in VDR expression is a potential risk for diabetes development (Morró et al., 2020). Recently, the potential role of VDR agonists and the bromodomain-containing protein 9 (BDCP9) inhibitor (iBDCP9) in partially restoring β-cell function and ameliorating hyperglycemia has been reported. The activation of VDR prevents the activities of inflammatory genes, while the use of iBDCP9 prolongs the activation and enhances the activity of VDR. In physiological condition and at the chromatin level, BDCP9 bind with acetylated lysine 91 residue of VDR and inhibit its activity (Wei et al., 2018). Further, mechanistic insight into the anti-inflammatory activity of VDR activation has been linked with the inhibition of inhibitor of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) alpha (IκBα) kinase (IκK), a key signaling component of NF-κB. Consequently, this improves insulin sensitivity (Dong et al., 2020) and could be one of the mechanisms for preserving β-cells, reducing inflammation, and preventing Streptozotocin (STZ)-induced diabetes (Morró et al., 2020).
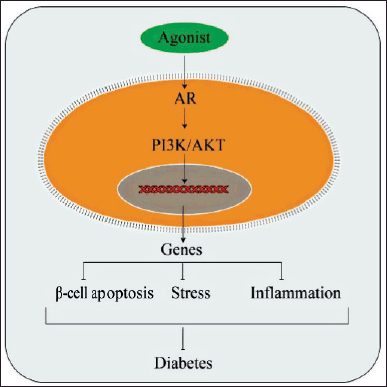 | Figure 4. Mechanism of action of AR activation in diabetes. [Click here to view] |
Progesterone receptors
PR is a steroidal receptor of two isoforms, PRA and PRB (Gao and Nawaz, 2002). Using an animal model, progesterone and PRs have been linked with the pathogenesis of gestational diabetes (Picard et al., 2002). When compared to the vehicle-treated group, female db/db mice treated with progesterone exhibited a significant rise in glucose levels (320 vs. 215 mg/dl, p < 0.002) after 9 weeks of treatment (Picard et al., 2002). The increase in glucose levels is correlated with the decreased GLUT4 protein when rats are treated with progesterone (Campbell and Febbraio, 2002). While treatment with progesterone antagonist RU486 (30 mg/kg) for 2 weeks significantly reduces glucose levels. Further, using PR-knockout (PR−/−) female mice, the study reported that this group improved in glucose tolerance, secreted more insulin, and showed increased β-cell numbers when compared to the WT. In a similar study, female PR−/− mice improved β-cell functions such as insulin production and lower blood glucose levels (De Sa et al., 2021).
GC receptor
GCs are stress hormones that are produced by the adrenal cortex. Besides stress-related processes, GCs are also involved in glucose homeostasis, adipocyte formation, and inflammation regulation. Clinically, GCs such as dexamethasone, betamethasone, and hydrocortisone are employed to manage acute and chronic inflammatory diseases (Li and Cummins, 2022). However, an excess of GCs is linked to the development of hyperglycemia, insulin resistance, and abdominal obesity (Bauerle and Harris, 2016; Li and Cummins, 2022). Furthermore, these conditions were associated with risk factors such as hypertension, obesity, coronary heart disease, single nucleotide polymorphisms, impaired renal functions, cytomegalovirus infection, smoking, race and ethnicity, concomitant immunosuppressive, and hyperlipidemia (Li and Cummins, 2022). The pathophysiology behind the involvement of GCs in diabetes includes multi-organs crosstalk. These organs are the brain, skeletal muscle, liver, adipose tissue, pancreas, and bone.
In the brain, particularly in the arcuate nucleus of the hypothalamus, GCs increase the transcription and functional activities of neuropeptide Y-agouti-related peptide neurons leading to increased appetite and leptin resistance (Li and Cummins, 2022). GCs cause muscle atrophy in skeletal muscle by decreasing glucose uptake and protein synthesis and increasing protein degradation. In the liver, GCs cause liver steatosis and hepatic insulin resistance. Liver steatosis is caused by the synergetic effects of GCs and insulin that stimulate the uptake of NEFAs by hepatocytes and triglyceride synthesis in the liver. Insulin resistance causes GCs-mediated increased enzyme expression and activity that participates in gluconeogenesis. In adipose tissue, GCs decrease glucose but increase lipid uptake and storage (Li and Cummins, 2022). GCs also increase adipogenesis, de novo lipogenesis, and triglyceride synthesis. In the pancreas, long-term exposure to GCs interferes with insulin synthesis and secretion and causes β-cell apoptosis. In bone, GCs inhibit the expression of osteocalcin, a protein secretes by osteoblast cells and promotes the activity of β-cell in secreting insulin. Hence, the inhibition of osteocalcin is indirectly linked to inhibiting insulin secretion (Li and Cummins, 2022). From a perspective, inhibition of GR could be beneficial in managing and treating diabetes.
TH receptors
THs regulate body temperature, appetite, and sympathetic activity. THs also regulate metabolic rate through its metabolite (Teixeira et al., 2020). The work which pioneered a link between hyperthyroidism and diabetes worsening was carried out byColler and Huggins (1927). However, such a study conflicts with other studies. Recent studies have shown that insulin resistance occurs in a hypothyroidism state due decrease in GLUT2 gene expression and activity and missense variation in TH (Thr92Ala) (Mohammed Hussein and AbdElmageed, 2021). Results from these conflicting studies suggest a bidirectional role of thyroids in diabetes (Teixeira et al., 2020). In that, both decreased (hyperthyroidism) and decreased (hypothyroidism) thyroid levels cause insulin resistance, impaired glucose tolerance (Wang, 2013), and high blood pressure (influencing adiposity). These bidirectional effects could result from genomic and non-genomic action of THs on the vasculature and in the hearts (Teixeira et al., 2020). Hence, from these studies, the role of THR in diabetes is inconclusive.
Reverse erb A (REV-ERB A)
The clock gene REV-ERB is an element of the biological clock’s molecular machinery and has multiple metabolic activities in several organs, including the endocrine pancreas (Vieira et al., 2012). REV-ERB is a transcription factor that controls lipids and glucose homeostasis, metabolism, adipocyte, and muscle development, and mitochondrial activity. In the endocrine pancreas, REV-ERB regulates glucose, insulin release, and pancreatic cell proliferation (Vieira et al., 2012). REV-ERB is also implicated in inhibiting inflammation by preventing the phosphorylation of IκK and nuclear translocation of the NF-κB subunit, p65. Consequently, this leads to the suppression of expression and secretion of pro-inflammatory markers (Guo et al., 2019) that act as vital pro-diabetogenic stressors of T2DM (Brown et al., 2022). In contrast to the REV-ERB activation, other studies (Brown et al., 2022) demonstrated that REV-ERB inhibition in β-cell provides partial protection from inflammation and glucotoxicity-induced β-cell failure (Brown et al., 2022). Hence, from these studies, the role of REV-ERB in diabetes is inconclusive.
Mineralocorticoid receptor
Enhanced renin-angiotensin-aldosterone system activation has been associated with diabetes. Evidence suggests that aldosterone-binding MR may have a role in obesity and diabetes-related VD (Bender et al., 2013; Schäfer et al., 2013). In addition, regardless of race, blood pressure, or body weight, mineralocorticoid activity is associated with glucose intolerance. Increasing MR activity may indicate the typical relationship between obesity, high blood pressure, hyperlipidemia, and insulin resistance. The contribution of MR to the pathogenesis of diabetes involves a decreased transcription of the IR gene and increased degradation of IRS. MR also influences adipocyte production and increases OS and inflammation (Garg and Adler, 2012).
OTHER THERAPEUTIC TARGETS IN T2DM
Current T2DM treatments aim to increase pancreatic β-cell production and decrease insulin resistance. Other treatment includes lifestyle counseling, oral anti-hyperglycemic medications, and/or insulin secretion (Esther Chan et al., 2012). Currently approved therapies for T2DM are metformin, sulfonylureas, meglitinides, alpha-glucosidase inhibitors, thiazolidinediones, glucagon-like peptide1 (GLP-1) receptor agonists, DPP4 inhibitors, and sodium-glucose co-transporter-2 inhibitors. These therapies, in some cases, are ineffective in maintaining long-term glycemic control and display severe side effects. Problems related to sulfonylureas and meglitinides include loss of effectiveness, hypoglycemia, and weight gain. Also, metformin, alpha-glycosidase inhibitors, and GLP-1 receptor agonists have gastrointestinal side effects. At the same time, weight gain and heart failure are possible side effects of thiazolidinediones (Marín-Peñalver et al., 2016). A few examples of the targets for T2DM are discussed in Table 3.
G Protein-coupled receptors (GPCRs)
GPCRs are the most diverse and widely studied transmembrane receptors. These cell surface receptors are targeted for a wide range of diseases. In diabetes, these receptors may be divided into two main groups. One group increases insulin secretion and maintains β-cell mass; the other acts on adipose tissue and skeletal muscle. The second group contributes to glycogen storage and inhibiting glycogenolysis and gluconeogenesis in the liver. Other GPCRs are present in gastrointestinal enteroendocrine cells (GECs), the hypothalamus, and the gastrointestinal tract (GIT). These GPCRs are targeted to induce incretin secretion, decrease appetite, and reduce glucose after food intake (Atanes and Persaud, 2019). Of these, GPCRs that are targeting on induce incretin secretion have gained attention in recent times. A few examples of GPCRs targeting in diabetes are GLP1R, GIPR, GPR119, free FA receptor 1 (FFAR1) (GPR40), FFAR4 (GPR120), and the bile acid receptor GPBAR1 (TGR5). GLP1 analogs such as exenatide, lixisenatide, and liraglutide target GLP1 receptors, bromocriptine target dopamine D2 receptors, and pramlintide target calcitonin/RAMP receptors are at the forefront GPCRs that have been approved for the treatment of diabetes (Atanes and Persaud, 2019).
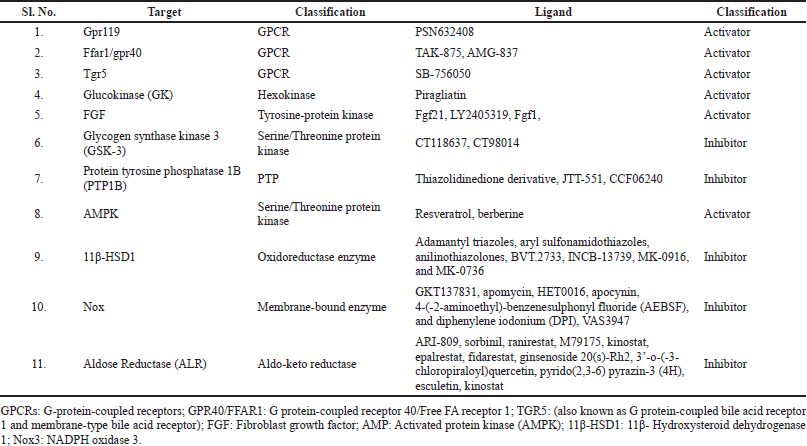 | Table 3. Other molecular targets and potential ligands reported from preclinical studies for T2DM. [Click here to view] |
G Protein-coupled receptor 119 (GPR119)
GPR119 is a Gs-coupled receptor expressed in GECs, GIT, and pancreatic β-cells. It plays a crucial role in regulating energy balance and body weight. GPR119 coupled to Gs in response to lipid metabolites such as lysophosphatidylethanolamine, palmitoylethanolamide, oleoylethylamide, and OEA. Upon activation, GPR119 transduced the signal by increasing cGMP levels and cAMP response element-binding protein (CREB) activation leading to glucose-stimulated insulin secretion (GSIS) from β-cells (Atanes and Persaud, 2019; Oliveira de Souza et al., 2021). GPR119 regulates glucose uptake in two ways: directly at the β-cell level and indirectly by releasing incretin hormones. Unfortunately, GPR119 activation has a negative impact on the heart and liver (Ritter et al., 2016). Additionally, GPR119 knockout mice in β-cells failed to show glucose tolerance, and insulin secretory responsiveness remains unaffected (Atanes and Persaud, 2019). Perhaps these could be the additional reasons for the failure of GPR119 agonists in clinical trials apart from the lack of efficacy and pharmacodynamic and pharmacokinetic limitations. Hence, a better promising approach, such as a combination therapy of GPR119 agonist and DPP-4 inhibitor or other standard regiments, could prove the beneficial effect of GPR119 agonists in T2DM patients (Ansarullah et al., 2013).
Free FA receptor 1
FFAR1/GPR40isa new therapeutic target for T2DM that regulates insulin secretion. It is also present in pancreatic β-cells, and its regulation occurs by activating medium and long-chain FAs (Itoh et al., 2003). As a receptor for medium and long-chain free FAs, FFAR1 plays a vital function in insulin release in the presence of rising glucose levels. As a result, using particular agonists to target FFAR1 is a viable strategy to improve insulin secretion in T2DM patients. Selective agonists of FFAR1 increase cytosolic calcium, activate protein kinase C isoforms and enhance insulin signaling (Bailey, 2012). Many FFAR1 agonists, such as TAK-875 and AMG-837, have been pulled off the market due to their hepatotoxicity. Compound 11K (GPCR40/FFAR1 agonist containing 3,5-dimethylisoxazole) possessed a promising target. Also, novel agents such as yhhu4488, structurally different from other GPCR40 agonists, lowered serum glucose levels and improved β-cell function in T2DM (Guo et al., 2015).
Takeda G protein-coupled receptor 5 (TGR5)
TGR5 activation in GECs enhances GLP-1 release and maintains blood glucose homeostasis by enhancing GSIS. TGR5 activation also suppresses glucagon release, delaying gastric emptying time, inducing satiety, and improving glucose disposal in the peripheral tissues (Donnelly, 2012). During energy expenditure, TGR5 also engages in brown fat and skeletal muscle metabolism (Watanabe et al., 2006). TGR5 agonists, for instance: SB-756050, have shown promise as a T2DM treatment (Duan et al., 2015) but have a negative role on the heart and gall bladder (Duan et al., 2012).
Glucokinase
GK is an isoenzyme that is involved in glucose metabolism to glucose-6-phosphate. GK does so by phosphorylating glucose in β-cells, causing insulin release. GK activators act by attaching to the allosteric site of the GK enzyme. Piragliatin, for example, has been shown to have an immediate glucose-reducing impact in T2DM patients with no significant adverse effects. It has also been used to treat obesity-related issues. The only drawbacks of GK activators are the increased hepatic glycogen storage deposition of lipids in the liver and muscles. However, because combination therapy is already widely practiced, GK activators’ combined pancreatic and hepatic effects may be more successful than currently available oral anti-diabetic drugs (Chu et al., 2020).
Fibroblast growth factors (FGFs)
FGFs are a group of growth factors that are involved in the repair and regeneration of tissues. Originally, FGF was identified as a protein responsible for promoting fibroblast proliferation. In humans, a total of 22 FGFs have been identified (FGF1-14 and FGF16-23), out of which 18 are FGF receptor (FGFR) ligands, and 4 (FGF11, FGF12, FGF13, and FGF14) do not bind to FGFR as FGF homologous factors (Yun et al., 2010). Based on sequence homology and phylogeny, these 22 FGFs are further grouped into 6 subfamilies: i) FGF1 and FGF2; ii) FGF3, FGF7, FGF10, FGF22; iii) FGF4, FGF5, and FGF6; iv) FGF8, FGF17, and FGF18; v) FGF9, FGF16, and FGF20; and vi) FGF19, FGF21, and FGF23. Classically, FGFs are considered paracrine factors, while recently, FGF19 and FGF21-22 actin an endocrine manner. These three FGFs (FGF19, FGF21-22) regulate cholesterol, bile acid, vitamin D, glucose, and phosphate homeostasis (Beenken and Mohammadi, 2009). Certain FGFs influence metabolism, energy homeostasis, and adipogenesis making them promising therapeutic targets for obesity and obesity-related cardiometabolic illness.
Fibroblast growth factor 21
FGF21 increases glucose uptake, which lowers lipid content in skeletal muscle (Mashili et al., 2011). In addition, it stimulates FA metabolism, reduces triglyceride levels, and prevents growth hormone (GH) signaling in the liver (Camporez et al., 2013), decreased lipolysis, improved glucose uptake, and adiponectin release in white adipose tissue (Lee et al., 2014). FGF21 also stimulates glucose uptake in brown adipose tissue (Hondares et al., 2011), enhancing peripheral insulin sensitivity. FGF21 also aids in the maintenance of insulin secretion and cell growth of pancreatic islets, and it primarily enhances β-cell survival and GSIS and inhibits GH signaling (Wente et al., 2006). According to clinical studies, LY2405319 improves lipid profiles by reducing harmful cholesterol levels, low-density lipoprotein (LDL), and triglycerides while increasing good cholesterol levels, HDL (Gaich et al., 2013). More research into novel FGF21 analogs and their side effects is needed, as this could be a chance to provide a favorable impact in the treatment of T2DM.
Fibroblast growth factor 1
FGF1 is a prototype of the FGF protein family with autocrine/paracrine signaling function upon binding to heparan sulfate proteoglycans. FGF1 is the standard FGFR ligand. It can connect and trigger the other splice variants of four tyrosine kinase FGF receptors (FGFRs 1–4). Studies have shown that the FGF1 knockout mouse exhibited glucose intolerance, representing a new target in T2DM. The mechanism improves skeletal muscle insulin-dependent glucose absorption while suppressing hepatic glucose synthesis, improving glycemic control (Suh et al., 2014). Recent studies show that FGF1 administration to db/db mice significantly reduced blood glucose levels and strengthened autophagy dysfunction and diabetes-induced liver steatosis, fibrosis, and apoptosis. Mechanistically, FGF1 exhibits these effects by inhibiting OS and endoplasmic reticulum stress (Xu et al., 2020). Additionally, FGF1 has been shown to prevent lipolysis and hepatic glucose production by activating FGFR1/PED4D leading to the inhibition of the cAMP-PKA pathway (Sancar et al., 2022).
GS kinase-3
GSK-3 is a widely expressed serine/threonine-protein kinase that is considered to be cytosolic in origin. However, it was also reported in mitochondria, nuclei, and other subcellular compartments. It is the principal GS regulator and plays a significant role in its regulation. Mammal GSK-3 exists as two isoforms, GSK-3α and GSK-3β, which are 51 and 46 kDa, respectively. Their functional domain is 98% identical in sequencing, but their N- and C-terminal sections differ. Despite significant similarities, GSK-3α knock-out animals show lower weight gain and improved glucose and insulin sensitivity. For this reason, GSK-3α has been associated with the development of T2DM when compared to GSK-3β (MacAulay and Woodgett, 2008). Other studies revealed that GSK-3β’s role in the development of T2DM was associated with the induction of inflammation through the activation of signal transducer and activator of transcription 3 (STAT3). This activation of STAT3 occurs through its phosphorylation at tyrosine 705. To further confirm the role of GSK-3β/STAT3-mediated inflammation, lithium was used to inhibit GSK-3β. The use of lithium abolished the inflammatory markers by phosphorylation of GSK-3β at serine 9 (inactive GSK-3β). The inhibition of GSK-3β also correlates with improved glucose-induced insulin secretion, decreased hyperglycemia, and increased pancreatic insulin content (Pitasi et al., 2020). Other GSK-3 inhibitors, such as CT118637 and CT98014 have also enhanced insulin signaling (MacAulay and Woodgett, 2008). Although GSK-3 represents a potential target in TD2M, developing an inhibitor(s) specific to GSK-3 isoforms poses a challenging step (Gupte et al., 2022).
Protein tyrosine phosphatase 1B
PTP1B is an enzyme that is negatively participating in the insulin cascade. PTP1B interrupts insulin signaling by dephosphorylation of IR and IRS (Rocha et al., 2021). This is further demonstrated by PTP1B-KO mice that resist high-fat diet-induced obesity and enhanced insulin sensitivity (Klaman et al., 2000). A strong relationship between insulin sensitivity and PTP1Bat the clinical level has been demonstrated (Cheung et al., 1999). Other than insulin signaling disruption, PTP1B is a negative leptin signaling regulator. Leptin is a hormone mainly expressed in adipose tissue-leptin functions by increasing tolerance to dietary fats and thereby maintaining FA homeostasis. When leptin signaling is disrupted, lipid content is increased in tissues such as the liver and muscle, leading to prolonged lipid accumulation in these tissues and favoring lipotoxicity. Mechanistically, leptin binds to the obesity receptor and causes Janus kinase 2 (JAK2) phosphorylation. Further, this leads to the activation of downstream intracellular signaling crucial for FA homeostasis. PTP1B disrupts leptin signaling by inhibiting JAK2 phosphorylation and causing leptin resistance (Rocha et al., 2021). Hence, PTP1B inhibitors such as compound 3e (thiazolidinedione derivative), JTT-551, and CCF06240 have been employed to manage and treat diabetes. These inhibitors improve insulin sensitivity, improve glucose tolerance and suppress weight gain (Fukuda et al., 2010).
Activated protein kinase (AMPK)
AMPK enhances glucose transport, FA metabolism, GLUT4 translocation, and mitochondrial biogenesis in skeletal muscle and liver. AMPK promotes lipolysis in adipose tissue by phosphorylating AMPK downstream targets (acetyl-CoA carboxylase). AMPK inhibits FA production and lipolysis (Steinberg and Kemp, 2009). Hence, AMPK activation is a prospective T2DM target. Natural substances such as resveratrol and berberine activate AMPK, but they are not safe for the elderly due to protein synthesis inhibition (Drummond et al., 2009). In light of these findings, more research into innovative AMPK activation techniques for treating T2DM is needed.
11β- Hydroxysteroid dehydrogenase 1·(11β-HSD1)
11βHSD1, a GC generating enzyme in the liver and adipocytes, facilitates the intra-cellular regeneration of activated GCs (cortisol, corticosterone) from inert 11-keto forms (Cooper and Stewart, 2009). Overexpression of GCs inhibits glucose uptake in peripheral tissue by blocking GLUT4 translocation, resulting in insulin resistance via reducing pancreatic β-cell insulin production. Upregulation of 11β -HSD1 in adipocytes and excess GCs have been linked to T2DM (Wamil and Seckl, 2007). Insulin sensitivity in skeletal muscle has been reported to be improved by 11β-HSD1 blockers (Morgan et al., 2009). Many new 11-HSD1 inhibitors have been identified, including adamantly-triazoles, aryl sulfonamidothiazoles, anilinothiazolones, BVT.2733, INCB-13739, MK-0916, and MK-0736. This help reduce body weight without producing hypoglycemia and improve cardiovascular disease (Hughes et al., 2008), and total cholesterol, LDL, and triglyceride reductions in hyperlipidemia patients (Rosenstock et al., 2010). According to numerous studies, 11β-HSD1 inhibitors are efficacious and potential targets for T2DM, and their possible mechanism should be identified in other tissues and considered for further clinical evaluation.
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox)
The critical enzyme (Nox) produces reactive oxygen species (ROS) and causes OS. This is often associated with T2DM and its major cardiovascular complications (Asmat et al., 2016; Brown et al., 2021). In humans, four isoforms of NoXs have been identified; these are NoX1, NoX2, NoX4, and NoX5. Out of these, NoX1, NoX2, and NoX5 overexpression play a significant role in the pathogenesis of T2DM by the generation of O2−. At the same time, NoX4 overexpression is considered to play a more physiological role by increased H2O2 production rather than a pathological role (Brown et al., 2021). In pancreatic β-cells, the overexpression of these NoX isoforms has a negative regulator on insulin secretory responses. This effect occurs due to ROS generation and adenylate cyclase/cAMP/PKA signaling inhibition (Li et al., 2012). Additionally, gene polymorphism (C242T) in p22phox, an essential subunit of NoXs, has been shown to correlate positively with endothelial dysfunction in T2DM (Osmenda et al., 2021). In line with these findings, inhibitors of NoXs have a positive effect on diabetes-related cardiovascular disorders. GKT137831, apomycin, HET0016, apocynin, AEBSF, and DPI, VAS3947 are specific Nox inhibitors that prevent OS (Gray et al., 2013). Hence, further studies should focus on discovering novel NADPH oxidase inhibitors and also clarify their exact mechanism of action by in-vivo studies for T2DM.
Aldose reductase
ALR is an NADPH-dependent enzyme that catalyzes the transfer of D-glucose to D-sorbitol utilizing reductant (NADPH) in the polyol pathway (Brownlee, 2001). ALR converts glucose to sorbitol (a generally non-metabolizable sugar) in patients with hyperglycemia via the active polyol pathway. When intracellular D-sorbitol concentrations rise, NADPH is consumed at greater levels, resulting in an osmotic pressure imbalance that produces peripheral neuropathy, cataract, nephropathy, and angiopathy problems (Hotta et al., 2001). Increased sorbitol via the polyol cascade causes myo-inositol depletion and decreased Na/K+ ATPase activity, resulting in loss of nerve function and vascular disease. ARI-809, sorbinil, ranirestat, M79175, kinostat, epalrestat, and fidarestat are ALR inhibitors that have been reported and tested (Kador et al., 2016; Zhu, 2013). Many more compounds as ALR inhibitors have recently been identified, including pyrido(2,3-6), pyrazin-3 (4H), compound 9c, and compound 1a, as well as flavonoids, phenols, terpenes, and alkaloids (Han et al., 2016; Saito et al., 2016) ginsenoside 20(s)-Rh2, 3’-o-(-3-chloropiraloyl)quercetin, esculetin, and kinostat. All of these compounds showed positive effects. In-vivo, kinostat and M79175 showed favorable outcomes. However, due to its unacceptable toxicity in clinical trials, it has not been employed in diabetic retinopathy to delay the formation of sugar cataracts in diabetic patients (Fatmawati et al., 2014; Kim et al., 2016).
CONCLUSION
T2DM is the most common type of diabetes, causing a global burden. Recently, it has been demonstrated that NRs deficiency, namely AR (in males), ERβ, LXR, VDR, REV-ERB A, PPAR, and NRs overexpression, PR, GR, and MR are associated with T2DM. Therefore, the activation of AR (in males), ERβ, and LXR and the inactivation of PR, GR, and MR could be a potential strategy for managing and treating T2DM. Several recent studies have shown great interest in AR activation for T2DM. These studies, however, focus on the uses of AR’s endogenous ligands, such as testosterone and Dihydrotestosterone (DHT). Particularly in men, the use of these agents is controversial because they are androgenic and could potentially promote prostate cancer development. Since AR activation is required in men with T2DM, the search for modulators that are cell-specific, non-steroidal, and non-androgenic is the need of the hour. For this reason, we are currently working on identifying selective AR modulators (SARMs) that are cell-specific. Importantly, we focus on plant-derived compounds because they offer fewer side effects. These compounds are currently under development for pancreatic β-cell regeneration since β-cell apoptosis has been reported in most cases of T2DM. Further, we are working on targeting pancreatic β-cell of the male population since clinical data on T2DM shows a positive correlation with androgen deficiency. The successful development of SARMs could revolutionize the harmful impact of T2DM on males.
ACKNOWLEDGEMENT
The authors would like to thank the Indian Council of Medical Research (ICMR), Department of Health Research-Ministry of Health and Family Welfare for supporting this project under the division “Senior Research Fellow” Award No- 2021-11508-F1. The authors would also like to thank the Department of Science and Technology—Fund for Improvement of Science and Technology Infrastructure in Universities and Higher Educational Institutions (DST-FIST), New Delhi, for their infrastructure support to our department.
AUTHOR CONTRIBUTION
Deepa initiated the conception, design, and data collection. Deepa also wrote and prepared the entire manuscript. Emdormi contributed to data collection, diagrammatic illustration, and manuscript preparation. Soumya contributed to data collection. Divakar contributed to manuscript suggestions and corrections. Divakar also contributed to proofreading the whole manuscript. All the authors approved the final manuscript for submission and publication.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. Pparγ signaling and metabolism: the good, the bad and the future. Nat Med, 2013; 19(5):557–66. https://doi.org/10.1038/nm.3159 CrossRef
Al-Rifai RH, Majeed M, Qambar MA, Ibrahim A, Alyammahi KM, Aziz F. Type 2 diabetes and pre-diabetes mellitus: a systematic review and meta-analysis of prevalence studies in women of childbearing age in the middle east and North Africa, 2000-2018. Syst Rev, 2019; 8(1):1–32; https://doi.org/10.1186/s13643-019-1187-1 CrossRef
Alexander SPH, Cidlowski JA, Kelly E, Marrion N V., Peters JA, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA. The concise guide to pharmacology 2017/18: nuclear hormone receptors. Br J Pharmacol, 2017; 174(1):S208–24; https://doi.org/10.1111/bph.13880 CrossRef
Almlöf T, Gustafsson JA, Wright AP. Role of hydrophobic amino acid clusters in the transactivation activity of the human glucocorticoid receptor. Mol Cell Biol, 1997; 17(2):934–45; https://doi.org/10.1128/mcb.17.2.934 CrossRef
Almlöf T, Wallberg AE, Gustafsson JÅ, Wright APH. Role of important hydrophobic amino acids in the interaction between the glucocorticoid receptor τ1-core activation domain and target factors. Biochemistry, 1998; 37(26):9586–94; https://doi.org/10.1021/bi973029x CrossRef
Ansarullah, Lu Y, Holstein M, DeRuyter B, Rabinovitch A, Guo Z. 2013; 8(1):e53345; https://doi.org/10.1371/journal.pone.0053345 CrossRef
Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev, 2001; 81(3):1269–304; https://doi.org/10.1152/physrev.2001.81.3.1269 CrossRef
Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress—a concise review. Saudi Pharm J, 2016; 24(5):547–53; https://doi.org/10.1016/j.jsps.2015.03.013 CrossRef
Atanes P, Persaud SJ. GPCR targets in type 2 diabetes. GPCRs Struct Funct Drug Discov, 2019; (25)10:367–91; https://doi.org/10.1016/B978-0-12-816228-6.00018-0 CrossRef
Bailey CJ. Could FFAR1 assist insulin secretion in type 2 diabetes? Lancet, 2012; 379(9824):1370–1; https://doi.org/10.1016/S0140-6736(12)60165-2 CrossRef
Bain DL, Heneghan AF, Connaghan-Jones KD, Miura MT. Nuclear receptor structure: implications for function. Annu Rev Physiol, 2007; 69:201–20; https://doi.org/10.1146/annurev.physiol.69.031905.160308 CrossRef
Banner CD, Goos-Nilsson A, Sjövall J, Gustafsson JÅ, Rafter JJ. A method for characterization of endogenous ligands to orphan receptors belonging to the steroid hormone receptor superfamily-isolation of progesterone from pregnancy plasma using progesterone receptor ligand-binding domain. Anal Biochem, 1992; 200:163–70; https://doi.org/10.1016/0003-2697(92)90293-G CrossRef
Bansal S, Chopra K. Distinct role of estrogen receptor-alpha and beta on postmenopausal diabetes-induced vascular dysfunction. Gen Comp Endocrinol, 2014; 206:51–9; https://doi.org/10.1016/j.ygcen.2014.06.013 CrossRef
Baranowski M. Biological role of liver X receptors. J Physiol Pharmacol, 2008; 59:31–55.
Bardet PL, Horard B, Robinson-Rechavi M, Laudet V, Vanacker JM. Characterization of oestrogen receptors in zebrafish (Danio rerio). J Mol Endocrinol, 2002; 28(3):153–63; https://doi.org/10.1677/jme.0.0280153 CrossRef
Bauerle KT, Harris C. Glucocorticoids and diabetes mellitus. Mo Med, 2016; 113:378–83.
Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov, 2009; 8:235–253 https://doi.org/10.1038/nrd2792
Bender SB, McGraw AP, Jaffe IZ, Sowers JR. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes, 2013; 62:313–9; https://doi.org/10.2337/db12-0905 CrossRef
Bertilsson G, Heidrich J, Svensson K, Åsman M, Jendeberg L, Sydow-Bäckman M, Ohlsson R, Postlind H, Blomquist P, Berkenstam A. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci USA, 13; 95(21):12208–13 https://doi.org/10.1073/pnas.95.21.12208 CrossRef
Bhasin M, Raghava GPS. Classification of nuclear receptors based on amino acid composition and dipeptide composition. J Biol Chem, 2004; 279(22):23262-6; https://doi.org/10.1074/jbc.M401932200 CrossRef
Brent GA. Mechanisms of thyroid hormone action. J Clin Invest, 2012; 122(9):3035–43 https://doi.org/10.1172/JCI60047 CrossRef
Brown MR, Laouteouet D, Delobel M, Villard O, Broca C, Bertrand G, Wojtusciszyn A, Dalle S, Ravier MA, Matveyenko AV, Costes S. The nuclear receptor REV-ERBα is implicated in the alteration of β-cell autophagy and survival under diabetogenic conditions. Cell Death Dis, 2022; 13:1–12; https://doi.org/10.1038/s41419-022-04767-z CrossRef
Brown OI, Bridge KI, Kearney MT. Nicotinamide adenosine dinucleotide phosphate oxidases in glucose homeostasis and diabetes-related endothelial cell dysfunction. Cells, 2021; 10:2315; https://doi.org/10.3390/cells10092315
Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature, 2001; 13; 414(6865):813–20 https://doi.org/10.1038/414813 CrossRef
Burris TP, Solt LA, Wang Y, Crumbley C, Banerjee S, Griffett K, Lundasen T, Hughes T, Kojetin DJ, Perez DM. Nuclear receptors and their selective pharmacologic modulators. Pharmacol Rev, 2013; 65(2):710–78 https://doi.org/10.1124/pr.112.006833
Campbell SE, Febbraio MA. Effect of the ovarian hormones on GLUT4 expression and contraction-stimulated glucose uptake. Am J Physiol Endocrinol Metab, 2002; 282(5):E1139–46 https://doi.org/10.1152/ajpendo.00184.2001 CrossRef
Camporez JPG, Jornayvaz FR, Petersen MC, Pesta D, Guigni BA, Serr J, Zhang D, Kahn M, Samuel VT, Jurczak MJ, Shulman GI. Cellular mechanisms by which FGF21 improves insulin sensitivity in male mice. Endocrinology, 2013; 154(9):3099–109. https://doi.org/10.1210/en.2013-1191 CrossRef
Carson-Jurica MA, Schrader WT, O’Malley BW. Steroid receptor family: structure and functions. Endocr Rev, 1990; 11(2):201–20. https://doi.org/10.1210/edrv-11-2-201 CrossRef
Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev, 2010; 31(2):139–70. https://doi.org/10.1210/er.2009-0007 CrossRef
Cheung A, Kusari J, Jansen D, Bandyopadhyay D, Kusari A, Bryer-Ash M. Marked impairment of protein tyrosine phosphatase 1B activity in adipose tissue of obese subjects with and without type 2 diabetes mellitus. J Lab Clin Med, 1999; 134(2):115–23. https://doi.org/10.1016/S0022-2143(99)90115-4 CrossRef
Chu C, Gao X, Li X, Zhang X, Ma R, Jia Y, Li D, Wang D, Xu F. Involvement of estrogen receptor-α in the activation of Nrf2-antioxidative signaling pathways by silibinin in pancreatic β-cells. Biomol Ther, 2020; 28:163–71; https://doi.org/10.4062/biomolther.2019.071 CrossRef
Coller FA, Huggins CB. Effect of hyperthyroidism upon diabetes mellitus striking improvement in diabetes mellitus from thyroidectomy. Ann Surg, 1927; 86:877–84; https://doi.org/10.1097/00000658-192712000-00009 CrossRef
Cooper MS, Stewart PM. 11β-hydroxysteroid dehydrogenase type 1 and its role in the hypothalamus-pituitary-adrenal axis, metabolic syndrome, and inflammation. J Clin Endocrinol Metab, 2009; https://doi.org/10.1210/jc.2009-1412 CrossRef
Davey RA, Grossmann M. Androgen receptor structure, function and biology: from bench to bedside. Clin Biochem Rev, 2016; 37(1):3–15.
Delerive P, Furman C, Teissier E, Fruchart JC, Duriez P, Staels B. Oxidized phospholipids activate PPARα in a phospholipase A2-dependent manner. FEBS Lett, 2000; 471(1):34–8. https://doi.org/10.1016/S0014-5793(00)01364-8 CrossRef
De Sa PM, Qadir MMF, Mauvais-Jarvis F. 226-LB: elimination of the progesterone receptor in ß cells improves ß-cell function and glucose tolerance during pregnancy and diet-induced insulin resistance in female mice. Diabetes, 2021; 70:226–LB; https://doi.org/10.2337/db21-226-lb CrossRef
Diamanti-Kandarakis E, Pappalou O, Kandaraki EA. The role of androgen excess on insulin sensitivity in women. Front Horm Res, 2019; 53:50–64; https://doi.org/10.1159/000494902 CrossRef
Ding L, Yang L, Wang Z, Huang W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B, 2015; 5(2):135–44. https://doi.org/10.1016/j.apsb.2015.01.004
Divakar S, Saravanan K, Karthikeyan P, Elancheran R, Kabilan S, Balasubramanian KK, Devi R, Kotoky J, Ramanathan M. Iminoenamine based novel androgen receptor antagonist exhibited anti-prostate cancer activity in androgen independent prostate cancer cells through inhibition of AKT pathway. Chem Biol Interact, 2017; 275:22–34. https://doi.org/10.1016/j.cbi.2017.07.023 CrossRef
Dong B, Zhou Y, Wang W, Scott J, Kim KH, Sun Z, Guo Q, Lu Y, Gonzales NM, Wu H, Hartig SM, York RB, Yang F, Moore DD. Vitamin D receptor activation in liver macrophages ameliorates hepatic inflammation, steatosis, and insulin resistance in mice. Hepatology, 2020; 71:1559–74; https://doi.org/10.1002/hep.30937 CrossRef
Donnelly D. The structure and function of the glucagon-like peptide-1 receptor and its ligands. Br J Pharmacol, 2012; 166(1):27–41. https://doi.org/10.1111/j.1476-5381.2011.01687.x
Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol, 2009; 106(4):1374–84. https://doi.org/10.1152/japplphysiol.91397.2008 CrossRef
Duan H, Ning M, Chen X, Zou Q, Zhang L, Feng Y, Zhang L, Leng Y, Shen J. Design, synthesis, and antidiabetic activity of 4-phenoxynicotinamide and 4-phenoxypyrimidine-5-carboxamide derivatives as potent and orally efficacious TGR5 agonists. J Med Chem, 2012; 55(23):10475–89. https://doi.org/10.1021/jm301071h CrossRef
Duan H, Ning M, Zou Q, Ye Y, Feng Y, Zhang L, Leng Y, Shen J. Discovery of intestinal targeted TGR5 agonists for the treatment of type 2 diabetes. J Med Chem, 2015; 58(8):3315–28. https://doi.org/10.1021/jm500829b CrossRef
Egner U. Structural analysis of the GR ligand-binding domain. Ernst Schering Res Found Workshop, 2002; (40):341–56. https://doi.org/10.1007/978-3-662-04660-9_19 CrossRef
Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol, 2018; 17(1):83. https://doi.org/10.1186/s12933-018-0728-6 CrossRef
Escriva H, Delaunay F, Laudet V. Ligand binding and nuclear receptor evolution. BioEssays, 2000; 22(8):717–27. https://doi.org/10.1002/1521-1878(200008)22:8<717:AID-BIES5>3.0.CO;2-I CrossRef
Esther Chan HW, Ashan B, Jayasekera P, Collier A, Ghosh S. A new class of drug for the management of type 2 diabetes: sodium glucose co-transporter inhibitors: “Glucuretics.” Diabetes Metab Syndr Clin Res Rev, 2012; 6(4):224–8. https://doi.org/10.1016/j.dsx.2012.08.003 CrossRef
Fatmawati S, Ersam T, Yu H, Zhang C, Jin F, Shimizu K. 20(S)-Ginsenoside Rh2 as aldose reductase inhibitor from Panax ginseng. Bioorg Med Chem Lett, 2014; 24(18):4407–4409. https://doi.org/10.1016/j.bmcl.2014.08.009 CrossRef
Fernandez EJ. Allosteric pathways in nuclear receptors—potential targets for drug design. Pharmacol Ther, 2018; 183:152–159. https://doi.org/10.1016/j.pharmthera.2017.10.014
Franco PJ, Li G, Wei LN. Interaction of nuclear receptor zinc finger DNA binding domains with histone deacetylase. Mol Cell Endocrinol, 2003; 206(1-2):1–12. https://doi.org/10.1016/S0303-7207(03)00254-5 CrossRef
Fukuda S, Ohta T, Sakata S, Morinaga H, Ito M, Nakagawa Y, Tanaka M, Matsushita M. Pharmacological profiles of a novel protein tyrosine phosphatase 1B inhibitor, JTT-551. Diabetes Obes Metab, 2010; 12(4):299–306. https://doi.org/10.1111/j.1463-1326.2009.01162.x CrossRef
Gadaleta RM, Magnani L. Nuclear receptors and chromatin: an inducible couple. J Mol Endocrinol, 2013; 52:R137–49; https://doi.org/10.1530/JME-13-0170 CrossRef
Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, Moller DE. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab, 2013; 18(3):333–40. https://doi.org/10.1016/j.cmet.2013.08.005 CrossRef
Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci, 2020; 21(17):6275. https://doi.org/10.3390/ijms21176275 CrossRef
Gao X, Nawaz Z. Progesterone receptors—animal models and cell signaling in breast cancer: role of steroid receptor coactivators and corepressors of progesterone receptors in breast cancer. Breast Cancer Res, 2002; 4(5):182–6. https://doi.org/10.1186/bcr449 CrossRef
Garg R, Adler GK. Role of mineralocorticoid receptor in insulin resistance. Curr Opin Endocrinol Diabetes Obes, 2012; 19:168–75; https://doi.org/10.1097/MED.0b013e3283533955 CrossRef
Glass CK, Rose DW, Rosenfeld MG. Nuclear receptor coactivators. Curr Opin Cell Biol, 1997; 9(2):222–32. https://doi.org/10.1016/S0955-0674(97)80066-X
Gottlieb PA. Type 1 diabetes. Endocrinol Metab Clin North Am, 2004; 33:2004; https://doi.org/10.1016/S0889-8529(03)00102-6 CrossRef
Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev, 1997; 18:502–19; https://doi.org/10.1210/edrv.18.4.0308 CrossRef
Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, De Haan JB, Koulis C, El-Osta A, Andrews KL, Chin-Dusting JPF, Touyz RM, Wingler K, Cooper ME, Schmidt HHHW, Jandeleit-Dahm KA. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation, 2013; 127(18):1888–1902. https://doi.org/10.1161/CIRCULATIONAHA.112.132159 CrossRef
Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature, 1986; 320(6058):134–9 https://doi.org/10.1038/320134a0 CrossRef
Guo D, Zhu Y, Sun H, Xu X, Zhang S, Hao Z, Wang G, Mu C, Ren H. Pharmacological activation of REV-ERBα represses LPS-induced microglial activation through the NF-κB pathway. Acta Pharmacol Sin, 2019; 40:26–34; https://doi.org/10.1038/s41401-018-0064-0 CrossRef
Guo DY, Li DW, Ning MM, Dang XY, Zhang LN, Zeng LM, Hu Y, Leng Y. Yhhu4488, a novel GPR40 agonist, promotes GLP-1 secretion and exerts anti-diabetic effect in rodent models. Biochem Biophys Res Commun, 2015; 466(4):740–7 https://doi.org/10.1016/j.bbrc.2015.09.130 CrossRef
Gupte M, Tousif S, Lemon JJ, Cora AT, Umbarkar P, Lal H. Isoform-specific role of GSK-3 in high fat diet induced obesity and glucose intolerance. Cells, 2022; 11:559; https://doi.org/10.3390/cells11030559 CrossRef
Haase CL, Tybjerg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. HDL cholesterol and risk of type 2 diabetes: a mendelian randomization study. Diabetes, 2015; 64:3328–33; https://doi.org/10.2337/db14-1603 CrossRef
Haelens A, Tanner T, Denayer S, Callewaert L, Claessens F. The hinge region regulates DNA binding, nuclear translocation, and transactivation of the androgen receptor. Cancer Res, 2007; 67(9):4514–23. https://doi.org/10.1158/0008-5472.CAN-06-1701 CrossRef
Hammer SS, Beli E, Kady N, Wang Q, Wood K, Lydic TA, Malek G, Saban DR, Wang XX, Hazra S, Levi M, Busik JV, Grant MB. The mechanism of diabetic retinopathy pathogenesis unifying key lipid regulators, sirtuin 1 and liver X receptor. EBioMedicine, 2017; 22:181–90; https://doi.org/10.1016/j.ebiom.2017.07.008 CrossRef
Han Z, Hao X, Ma B, Zhu C. A series of pyrido[2,3-b[pyrazin-3(4 H)-one derivatives as aldose reductase inhibitors with antioxidant activity. Eur J Med Chem, 2016; 121:308–317. https://doi.org/10.1016/j.ejmech.2016.05.036 CrossRef
Hart SM. Modulation of nuclear receptor dependent transcription. Biol Res, 2002; 35(2):295–303. https://doi.org/10.4067/S0716-97602002000200021 CrossRef
Haug CJ, Aukrust P, Haug E, Mørkrid L, Müller F, Frøland SS. Severe deficiency of 1,25-dihydroxyvitamin D3 in human immunodeficiency virus infection: association with immunological hyperactivity and only minor changes in calcium homeostasis. J Clin Endocrinol Metab, 1998; 83(11):3832–3838 https://doi.org/10.1210/jcem.83.11.5270 CrossRef
Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol, 2008; 3:1535–41; https://doi.org/10.2215/CJN.01160308 CrossRef
Heikkinen S, Auwerx J, Argmann CA. PPARgamma in human and mouse physiology. Biochim Biophys Acta, 2007; (8):999–1013. CrossRef
Hirukawa H, Kaneto H, Shimoda M, Kimura T, Okauchi S, Obata A, Kohara K, Hamamoto S, Tawaramoto K, Hashiramoto M, Kaku K. Combination of DPP-4 inhibitor and PPARγ agonist exerts protective effects on pancreatic β-cells in diabetic db/db mice through the augmentation of IRS-2 expression. Mol Cell Endocrinol, 2015; 413:49–60; https://doi.org/10.1016/j.mce.2015.06.010 CrossRef
Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature, 1985; 318(6047):635–41; https://doi.org/10.1038/318635a0 CrossRef
Holzer G, Markov G V., Laudet V. Evolution of nuclear receptors and ligand signaling: toward a soft key–lock model?. Curr Top Dev Biol, 2017; 125:1–38; https://doi.org/10.1016/bs.ctdb.2017.02.003 CrossRef
Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, Villarroya F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem, 2011; 286(15):12983–90; https://doi.org/10.1074/jbc.M110.215889 CrossRef
Hotta N, Toyota T, Matsuoka K, Shigeta Y, Kikkawa R, Kaneko T, Takahashi A, Sugimura K, Koike Y, Ishii J, Sakamoto N. Clinical efficacy of fidarestat, a novel aldose reductase inhibitor, for diabetic peripheral neuropathy: a 52-week multicenter placebo-controlled double-blind parallel group study. Diabetes Care, 2001; (10):1776–82; https://doi.org/10.2337/diacare.24.10.1776 CrossRef
Hughes KA, Webster SP, Walker BR. 11-Beta-hydroxysteroid dehydrogenase type 1 (11β-HSD1) inhibitors in type 2 diabetes mellitus and obesity. Expert Opin Investig Drugs, 2008; 17:481–96; https://doi.org/10.1517/13543784.17.4.481 CrossRef
Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature, 2003; 422(6928):173–6; https://doi.org/10.1038/nature01478 CrossRef
Kador PF, Wyman M, Oates PJ. Aldose reductase, ocular diabetic complications and the development of topical Kinostat®. Prog Retin Eye Res, 2016; 54:1–29; https://doi.org/10.1016/j.preteyeres.2016.04.006 CrossRef
Kam RKT, Deng Y, Chen Y, Zhao H. Retinoic acid synthesis and functions in early embryonic development. Cell Biosci, 2012; 2(1):11; https://doi.org/10.1186/2045-3701-2-11 CrossRef
Kanatani Y, Usui I, Ishizuka K, Bukhari A, Fujisaka S, Urakaze M, Haruta T, Kishimoto T, Naka T, Kobayashi M. Effects of pioglitazone on suppressor of cytokine signaling 3 expression: potential mechanisms for its effects on insulin sensitivity and adiponectin expression. Diabetes, 2007; 56(3):795–803; https://doi.org/10.2337/db06-1039 CrossRef
Kharroubi AT. Diabetes mellitus: the epidemic of the century. World J Diabetes, 2015; 6:850–867; https://doi.org/10.4239/wjd.v6.i6.850 CrossRef
Khorasanizadeh S, Rastinejad F. Visualizing the architectures and interactions of nuclear receptors. Endocrinology, 2016; 157:4212–21; https://doi.org/10.1210/en.2016-1559 CrossRef
Kim CS, Kim J, Lee YM, Sohn E, Kim JS. Esculetin, a coumarin derivative, inhibits aldose reductase activity in vitro and cataractogenesis in galactose-fed rats. Biomol Ther (Seoul), 2016; 24(2):178–83; https://doi.org/10.4062/biomolther.2015.101 CrossRef
Kim HY, Mohan S. Role and mechanisms of actions of thyroid hormone on the skeletal development. Bone Res, 2013; 1(2):146–61; https://doi.org/10.4248/BR201302004 CrossRef
King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care, 1998; (9):1414–31; https://doi.org/10.2337/diacare.21.9.1414 CrossRef
Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB . Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol, 2000; (15):5479–89; https://doi.org/10.1128/mcb.20.15.5479-5489.2000 CrossRef
Klepsch V, Gerner RR, Klepsch S, Olson WJ, Tilg H, Moschen AR,. Nuclear orphan receptor NR2F6 as a safeguard against experimental murine colitis. Gut, 2018; https://doi.org/10.1136/gutjnl-2016-313466 CrossRef
Klepsch V, Moschen AR, Tilg H, Baier G, Hermann-Kleiter N. Nuclear receptors regulate intestinal inflammation in the context of IBD. Front Immunol, 2019; (8):1434–44; https://doi.org/10.3389/fimmu.2019.01070 CrossRef
Kollerits B, Fliser D, Heid IM, Ritz E, Kronenberg F. Gender-specific association of adiponectin as a predictor of progression of chronic kidney disease: the mild to moderate kidney disease study. Kidney Int, 2007; 71(12):1279–86. https://doi.org/10.1038/sj.ki.5002191 CrossRef
Lathion C, Michalik L, Wahli W. Physiological ligands of PPARs in inflammation and lipid homeostasis. Future Lipidol, 2006; 1(2):191–201; https://doi.org/10.2217/17460875.1.2.191 CrossRef
Laudet V, Hanni C, Coll J, Catzeflis F, Stehelin D. Evolution of the nuclear receptor gene superfamily. EMBO J, 1992; (3):1003–13; https://doi.org/10.1002/j.1460-2075.1992.tb05139.x CrossRef
Lee D V., Li D, Yan Q, Zhu Y, Goodwin B, Calle R, Brenner MB, Talukdar S. Fibroblast growth factor 21 improves insulin sensitivity and synergizes with insulin in human adipose stem cell-Derived (hASC) adipocytes. PLoS One, 2014; 9(11):e111767; https://doi.org/10.1371/journal.pone.0111767 CrossRef
Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev, 2009; 89(1):147–91 https://doi.org/10.1152/physrev.00010.2008 CrossRef
Lei M, Liu Z, Guo J. The emerging role of vitamin D and vitamin D receptor in diabetic nephropathy. Biomed Res Int, 2020; 2020:4137268; https://doi.org/10.1155/2020/4137268 CrossRef
Leopold Wager CM, Arnett E, Schlesinger LS. Macrophage nuclear receptors: emerging key players in infectious diseases. PLoS Pathog, 2019; (3):e1007585; https://doi.org/10.1371/journal.ppat.1007585 CrossRef
Li J-X, Cummins CL. Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions. Nat Rev Endocrinol, 2022; 18:1–18; https://doi.org/10.1038/s41574-022-00683-6
Li N, Li B, Brun T, Deffert-Delbouille C, Mahiout Z, Daali Y, Ma XJ, Krause KH, Maechler P. NADPH oxidase NOX2 defines a new antagonistic role for reactive oxygen species and cAMP/PKA in the regulation of insulin secretion. Diabetes, 2012; (11):2842–50; https://doi.org/10.2337/db12-0009 CrossRef
Li SY, Zhao YL, Yang YF, Wang X, Nie M, Wu XY, Mao JF. Metabolic effects of testosterone replacement therapy in patients with type 2 diabetes mellitus or metabolic syndrome: a meta-analysis. Int J Endocrinol, 2020; 2020:4732021; https://doi.org/10.1155/2020/4732021 CrossRef
Ma Y, Wang SQ, Xu WR, Wang RL, Chou KC. Design novel dual agonists for treating type-2 diabetes by targeting peroxisome proliferator-activated receptors with core hopping approach. PLoS One, 2012; (6):e38546; https://doi.org/10.1371/journal.pone.0038546 CrossRef
MacAulay K, Woodgett JR. Targeting glycogen synthase kinase-3 (GSK-3) in the treatment of Type 2 diabetes. Expert Opin Ther Targets, 2008; (10):1265–74; https://doi.org/10.1517/14728222.12.10.1265 CrossRef
Mahajan MA, Samuels HH. A new family of nuclear receptor coregulators that integrate nuclear receptor signaling through CREB-binding protein. Mol Cell Biol, 2000; (14):5048–63; https://doi.org/10.1128/mcb.20.14.5048-5063.2000 CrossRef
Man Chu TS. The relationship between Type 2 diabetes mellitus and risk of prostate cancer: literature review and critical appraisal. J Diabetes Metab, 2018; 9:1–5 https://doi.org/10.4172/2155-6156.1000787 CrossRef
Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RMl. The nuclear receptor superfamily: the second decade. Cell, 1995; (6):835; https://doi.org/10.1016/0092-8674(95)90199-X CrossRef
Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, Cañizo-Gómez FJ del. Update on the treatment of type 2 diabetes mellitus. World J Diabetes, 2016; (17):354; https://doi.org/10.4239/wjd.v7.i17.354 CrossRef
Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal, 2009; 7:e002. https://doi.org/10.1621/nrs.07002 CrossRef
di Martino O, Welch JS. Retinoic acid receptors in acute myeloid leukemia therapy. Cancers (Basel), 2019; (12):1915; https://doi.org/10.3390/cancers11121915 CrossRef
Mashili FL, Austin RL, Deshmukh AS, Fritz T, Caidahl K, Bergdahl K, Zierath JR, Chibalin AV, Moller DE, Kharitonenkov A, Krook A. Direct effects of FGF21 on glucose uptake in human skeletal muscle: implications for type 2 diabetes and obesity. Diabetes Metab Res Rev, 2011; (3):286–97; https://doi.org/10.1002/dmrr.1177 CrossRef
Mazaira GI, Zgajnar NR, Lotufo CM, Daneri-Becerra C, Sivils JC, Soto OB, Cox MB, Galigniana MD. The nuclear receptor field: a historical overview and future challenges. Nucl Recept Res, 2018; 5:101320; https://doi.org/10.11131/2018/101320 CrossRef
McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell, 2002; 5:101320; https://doi.org/10.1016/S0092-8674(02)00641-4 CrossRef
Menuet A, Pellegrini E, Anglade I, Blaise O, Lauder V, Kah O, Pakdel F. Molecular characterization of three estrogen receptor forms in zebrafish: binding characteristics, transactivation properties, and tissue distributions. Biol Reprod, 2002; (6):1881–92; https://doi.org/10.1095/biolreprod66.6.1881 CrossRef
Mihály J, Gericke J, Aydemir G, Weiss K, Carlsen H, Blomhoff R, Garcia J, Rühl R. Reduced retinoid signaling in the skin after systemic retinoid-X receptor ligand treatment in mice with potential relevance for skin disorders. Dermatology, 2013; (4):304–11; https://doi.org/10.1159/000345496 CrossRef
Mohammed Hussein SM, AbdElmageed RM. The relationship between type 2 diabetes mellitus and related thyroid diseases. Cureus, 2021; 13:e20697; https://doi.org/10.7759/cureus.20697 CrossRef
Morgan SA, Sherlock M, Gathercole LL, Lavery GG, Lenaghan C, Bujalska IJ, Laber D, Yu A, Convey G, Mayers R, Hegyi K, Sethi JK, Stewart PM, Smith DM, Tomlinson JW. 11β-hydroxysteroid dehydrogenase type 1 regulates glucocorticoid- induced insulin resistance in skeletal muscle. Diabetes, 2009; (11):2506–15; https://doi.org/10.2337/db09-0525 CrossRef
Morró M, Vilà L, Franckhauser S, Mallol C, Elias G, Ferré T, Molas M, Casana E, Rodó J, Pujol A, Téllez N, Bosch F, Casellas A. Vitamin D receptor overexpression in β-cells ameliorates diabetes in mice. Diabetes, 2020; 69:927–39; https://doi.org/10.2337/db19-0757 CrossRef
Muñoz-Cueto JA, Burzawa-Gérard E, Kah O, Valotaire Y, Pakdel F. Cloning and sequencing of the gilthead sea bream estrogen receptor cDNA. DNA Seq, 1999; (2):75–84; https://doi.org/10.3109/10425179909008421 CrossRef
Navarro G, Allard C, Xu W, Mauvais-Jarvis F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity (Silver Spring), 2015; (4):713–9; https://doi.org/10.1002/oby.21033 CrossRef
Navarro G, Xu W, Jacobson DA, Wicksteed B, Allard C, Zhang G, De Gendt K, Kim SH, Wu H, Zhang H, Verhoeven G, Katzenellenbogen JA, Mauvais-Jarvis F. Extranuclear actions of the androgen receptor enhance glucose-stimulated insulin secretion in the male. Cell Metab, 2016; 23:837–51; https://doi.org/10.1016/j.cmet.2016.03.015 CrossRef
Necela BM, Thompson EA. Pathophysiological roles of PPARγ in gastrointestinal epithelial cells. PPAR Res, 2008; 2008:148687. https://doi.org/10.1155/2008/148687 CrossRef
Nelson ER, Habibi HR. Estrogen receptor function and regulation in fish and other vertebrates. Gen Comp Endocrinol, 2013; 192:15–24; https://doi.org/10.1016/j.ygcen.2013.03.032 CrossRef
Ning L, Lou X, Zhang F, Xu G. Nuclear receptors in the pathogenesis and management of inflammatory bowel disease. Mediators Inflamm, 2019; 0962–9351 https://doi.org/10.1155/2019/2624941 CrossRef
Novac N, Heinzel T. Nuclear receptors: overview and classification. Curr Drug Targets Inflamm Allergy, 2004; (4):335–46; https://doi.org/10.2174/1568010042634541 CrossRef
Oliveira de Souza C, Sun X, Oh D. Metabolic functions of G protein-coupled receptors and β-arrestin-mediated signaling pathways in the pathophysiology of type 2 diabetes and obesity. Front Endocrinol (Lausanne), 2021; 12:715877. https://doi.org/10.3389/fendo.2021.715877 CrossRef
Onate SA, Boonyaratanakornkit V, Spencer TE, Tsai SY, Tsai MJ, Edwards DP, O’Malley BW. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. J Biol Chem, 1998; 273(20):12101–8. https://doi.org/10.1074/jbc.273.20.12101 CrossRef
Osmenda G, Matusik PT, Sliwa T, Czesnikiewicz-Guzik M, Skupien J, Ma?ecki MT, Siedlinski M. Nicotinamide adenine dinucleotide phosphate (nadph) oxidase p22phox subunit polymorphisms, systemic oxidative stress, endothelial dysfunction, and atherosclerosis in type 2 diabetes mellitus. Polish Arch Intern Med, 2021; 131:447–54; https://doi.org/10.20452/pamw.15937 CrossRef
Pal M, Khan J, Kumar R, Surolia A, Gupta S. Testosterone supplementation improves insulin responsiveness in HFD fed male T2DM mice and potentiates insulin signaling in the skeletal muscle and C2C12 myocyte cell line. PLoS One, 2019; 14; https://doi.org/10.1371/journal.pone.0224162 CrossRef
Patel SR, Skafar DF. Modulation of nuclear receptor activity by the F domain. Mol Cell Endocrinol, 2015; 3:298–305; https://doi.org/10.1016/j.mce.2015.07.009 CrossRef
Pawlak M, Lefebvre P, Staels B. General molecular biology and architecture of nuclear receptors. Curr Top Med Chem, 2012; 12(6):486–504. https://doi.org/10.2174/156802612799436641 CrossRef
Petersmann A, Nauck M, Müller-Wieland D, Kerner W, Müller UA, Landgraf R, Nauck M, Freckmann G, Heinemann L, Schleicher E. Definition, classification and diagnostics of diabetes mellitus. J Lab Med, 2018; 42(3):73–79. https://doi.org/10.1515/labmed-2018-0016 CrossRef
Picard F, Wanatabe M, Schoonjans K, Lydon J, O’Malley BW, Auwerx J. Progesterone receptor knockout mice have an improved glucose homeostasis secondary to β-cell proliferation. Proc Natl Acad Sci U S A, 2002; 99:15644–8; https://doi.org/10.1073/pnas.202612199 CrossRef
Pitasi CL, Liu J, Gausserès B, Pommier G, Delangre E, Armanet M, Cattan P, Mégarbane B, Hanak AS, Maouche K, Bailbé D, Portha B, Movassat J. Implication of glycogen synthase kinase 3 in diabetes-associated islet inflammation. J Endocrinol, 2020; 244:133–48; https://doi.org/10.1530/JOE-19-0239 CrossRef
Porter BA, Ortiz MA, Bratslavsky G, Kotula L. Structure and function of the nuclear receptor superfamily and current targeted therapies of prostate cancer. Cancers (Basel), 2019; (12):1852; https://doi.org/10.3390/cancers11121852 CrossRef
Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nat Struct Mol Biol, 2007; 14(12):1207–13. https://doi.org/10.1038/nsmb1344 CrossRef
Rastinejad F, Huang P, Chandra V, Khorasanizadeh S. Understanding nuclear receptor form and function using structural biology. J Mol Endocrinol, 2013; 51(3):T1–T21. https://doi.org/10.1530/JME-13-0173 CrossRef
Remedi MS, Nichols CG. KATP channels in the pancreas: hyperinsulinism and diabetes. Hyperinsulinism and diabetes. Elsevier, 2016; 199–221. https://doi.org/10.1016/B978-0-12-802002-9.00008-X CrossRef
Ritter K, Buning C, Halland N, Pöverlein C, Schwink L. G protein-coupled receptor 119 (GPR119) agonists for the treatment of diabetes: recent progress and prevailing challenges. J Med Chem, 2016; 59(8):3579–92 https://doi.org/10.1021/acs.jmedchem.5b01198 CrossRef
Robinson-Rechavi M, Garcia HE, Laudet V. The nuclear receptor superfamily. J Cell Sci, 2003; 116(Pt 4):585–6 https://doi.org/10.1242/jcs.00247 CrossRef
Rocha S, Luísa Corvo M, Fernandes E, Freitas M. The emerging target protein tyrosine phosphatase 1B (PTP1B) for type 2 diabetes mellitus management. J Diabetes Clin Res, 2021; 3:99–105. CrossRef
Rosenstock J, Banarer S, Fonseca VA, Inzucchi SE, Sun W, Yao W, Hollis G, Flores R, Levy R, Williams WV, Seckl JR, Huber R. The 11-β-hydroxysteroid dehydrogenase type 1 inhibitor INCB13739 improves hyperglycemia in patients with type 2 diabetes inadequately controlled by metformin monotherapy. Diabetes Care, 2010; 33(7):1516–22. https://doi.org/10.2337/dc09-2315 CrossRef
Rühl R, Krezel W, de Lera AR. 9-Cis-13,14-dihydroretinoic acid, a new endogenous mammalian ligand of retinoid X receptor and the active ligand of a potential new vitamin A category: vitamin A5. Nutr Rev, 2018; 76(12):929–941 https://doi.org/10.1093/nutrit/nuy057 CrossRef
Saito R, Suzuki S, Sasaki K. Pterin-7-carboxamides as a new class of aldose reductase inhibitors. Bioorganic Med Chem Lett, 2016; 26(20):4870–4874. https://doi.org/10.1016/j.bmcl.2016.09.033 CrossRef
Sancar G, Liu S, Gasser E, Alvarez JG, Moutos C, Kim K, van Zutphen T, Wang Y, Huddy TF, Ross B, Dai Y. FGF1 and insulin control lipolysis by convergent pathways. Cell Metab, 2022; 34:171–83; https://doi.org/10.1016/j.cmet.2021.12.004 CrossRef
Sawicki KT, Chang HC, Ardehali H. Role of heme in cardiovascular physiology and disease. J Am Heart Assoc, 2015; 4(1):e001138. https://doi.org/10.1161/JAHA.114.001138 CrossRef
Schäfer N, Lohmann C, Winnik S, Van Tits LJ, Miranda MX, Vergopoulos A, Ruschitzka F, Nussberger J, Berger S, Lüscher TF, Verrey F. Endothelialmineralocorticoid receptor activation mediates endothelial dysfunction in diet-induced obesity. Eur Heart J, 2013; 34:3515–24; https://doi.org/10.1093/eurheartj/eht095 CrossRef
Schroeder AC, Privalsky ML. Thyroid hormones, T3 and T4, in the brain. Front Endocrinol (Lausanne), 2014; 5:40. https://doi.org/10.3389/fendo.2014.00040 CrossRef
Schulman IG. Nuclear receptors as drug targets for metabolic disease. Adv Drug Deliv Rev, 2010; 62:1307–15; https://doi.org/10.1016/j.addr.2010.07.002 CrossRef
Schupp M, Lazar MA. Endogenous ligands for nuclear receptors: digging deeper. J Biol Chem, 2010; 285(52):40409–15. https://doi.org/10.1074/jbc.R110.182451 CrossRef
Skafar DF, Zhao C. The multifunctional estrogen receptor-alpha F domain. Endocrine, 2008; 33(1):1–8. https://doi.org/10.1007/s12020-008-9054-1 CrossRef
Solt LA, Griffin PR, Burris TP. Ligand regulation of retinoic acid receptor-related orphan receptors: implications for development of novel therapeutics. Curr Opin Lipidol, 2010; 21(3):204–11. https://doi.org/10.1097/MOL.0b013e328338ca18
Soriano S, Ropero AB, Alonso-Magdalena P, Ripoll C, Quesada I, Gassner B, Kuhn M, Gustafsson JA, Nadal A. Rapid regulation of KATP channel activity by 17β-estradiol in pancreatic β-cells involves the estrogen receptor β and the atrial natriuretic peptide receptor. Mol Endocrinol, 2009; 23:1973–82; https://doi.org/10.1210/me.2009-0287 CrossRef
Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev, 2009; 89(3):1025–78. https://doi.org/10.1152/physrev.00011.2008 CrossRef
Suh JM, Jonker JW, Ahmadian M, Goetz R, Lackey D, Osborn O, Huang Z, Liu W, Yoshihara E, van Dijk TH, Havinga R. Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature, 2014; 513(7518):436–9. https://doi.org/10.1038/nature13540 CrossRef
Szeto V, Chen NH, Feng ZP, Sun HS. The role of KATP channels in cerebral ischemic stroke and diabetes. Acta Pharmacol Sin, 2018; 39:683–94; https://doi.org/10.1038/aps.2018.10 CrossRef
Takada I, Makishima M. Control of inflammatory bowel disease and colorectal cancer by synthetic vitamin D receptor ligands. Curr Med Chem, 2017; 24(9):868–875. https://doi.org/10.2174/0929867323666161202145509 CrossRef
Tanaka Y, Deluca HF. Role of 1,25 dihydroxyvitamin D3 in maintaining serum phosphorus and curing rickets. Proc Natl Acad Sci U S A, 1974; 71(4):1040–4. https://doi.org/10.1073/pnas.71.4.1040 CrossRef
Teixeira P de F dos S, dos Santos PB, Pazos-Moura CC. The role of thyroid hormone in metabolism and metabolic syndrome. Ther Adv Endocrinol Metab, 2020; 11:204201882091786; https://doi.org/10.1177/2042018820917869 CrossRef
Teske KA, Yu O, Arnold LA. Inhibitors for the vitamin D receptor-coregulator interaction. Vitam. Horm., 2016; 100:45–82. https://doi.org/10.1016/bs.vh.2015.10.002 CrossRef
Tetel MJ, Jung S, Carbajo P, Ladtkow T, Skafar DF, Edwards DP. Hinge and amino-terminal sequences contribute to solution dimerization of human progesterone receptor. Mol Endocrinol, 1997; 11(8):1114–1128 https://doi.org/10.1210/mend.11.8.9963 CrossRef
Thompson EA. PPARγ physiology and pathology in gastrointestinal epithelial cells. Mol Cells, 2007; 24:167–76.
Tiwari K, Kumar D. Recent classification of diabetes mellitus. Int J Innov Sci Technol, 2018; 3(4):52–57 https://doi.org/10.22270/ijist.v3i4.24 CrossRef
Uppenberg J, Svensson C, Jaki M, Bertilsson G, Jendeberg L, Berkenstam A. Crystal structure of the ligand binding domain of the human nuclear receptor PPARγ. J Biol Chem, 1998; 273(47):31108–12. https://doi.org/10.1074/jbc.273.47.31108 CrossRef
van der Vaart M, Schaaf MJM. Naturally occurring C-terminal splice variants of nuclear receptors. Nucl Recept Signal, 2009; 7:e007. https://doi.org/10.1621/nrs.07007 CrossRef
Vieira E, Marroquí L, Batista TM, Caballero-Garrido E, Carneiro EM, Boschero AC, Nadal A, Quesada I. The clock gene Rev-erbα regulates pancreatic β-cell function: modulation by leptin and high-fat diet. Endocrinology, 2012; 153:592–601; https://doi.org/10.1210/en.2011-1595 CrossRef
Wamil M, Seckl JR. Inhibition of 11ß-hydroxysteroid dehydrogenase type 1 as a promising therapeutic target. Drug Discov Today, 2007; 12:504–20; https://doi.org/10.1016/j.drudis.2007.06.001 CrossRef
Wang C. The relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res, 2013; 2013:390534. https://doi.org/10.1155/2013/390534 CrossRef
Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell, 1999; 3(5):543–53. https://doi.org/10.1016/S1097-2765(00)80348-2 CrossRef
Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature, 2006; 439(7075):484–9. https://doi.org/10.1038/nature04330 CrossRef
Weatherman R V., Fletterick RJ, Scanlan TS. Nuclear-receptor ligands and ligand-binding domains. Annu Rev Biochem, 1999; 68:559–81. https://doi.org/10.1146/annurev.biochem.68.1.559 CrossRef
Wei J, Tang Q, Liu L, Bin J. Combination of peroxisome proliferator-activated receptor α/γ agonists may benefit type 2 diabetes patients with coronary artery disease through inhibition of inflammatory cytokine secretion. Exp Ther Med, 2013; 5(3):783–788. https://doi.org/10.3892/etm.2013.891 CrossRef
Wei Z, Yoshihara E, He N, Hah N, Fan W, Pinto AFM, Huddy T, Wang Y, Ross B, Estepa G, Dai Y, Ding N, Sherman MH, Fang S, Zhao X, Liddle C, Atkins AR, Yu RT, Downes M, Evans RM. Vitamin D Switches BAF Complexes to protect β cells. Cell, 2018; 173:1135–49.e15; https://doi.org/10.1016/j.cell.2018.04.013 CrossRef
Weikum ER, Liu X, Ortlund EA. The nuclear receptor superfamily: a structural perspective. Protein Sci, 2018; 27(11):1876–1892. https://doi.org/10.1002/pro.3496
Wente W, Efanov AM, Brenner M, Kharitonenkov A, Köster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J. Fibroblast growth factor-21 improves pancreatic β-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes, 2006; 55(9):2470–8. https://doi.org/10.2337/db05-1435 CrossRef
Wilding JPH. PPAR agonists for the treatment of cardiovascular disease in patients with diabetes. Diabetes Obes Metab, 2012; 14:973–982 https://doi.org/10.1111/j.1463-1326.2012.01601.x
Xu Z, Wu Y, Wang F, Li X, Wang P, Li Y, Wu J, Li Y, Jiang T, Pan X, Zhang X, Xie L, Xiao J, Liu Y. Fibroblast growth factor 1 ameliorates diabetes-induced liver injury by reducing cellular stress and restoring autophagy. Front Pharmacol, 2020; 11:52; https://doi.org/10.3389/fphar.2020.00052 CrossRef
Yamaguchi AV, Costanzo PR, Peuchot VA, Knoblovits P. Testosterone replacement therapy and the risk of hypoglycemia. Case Rep Endocrinol, 2019; 2019; https://doi.org/10.1155/2019/9616125 CrossRef
Yao QM, Wang B, An XF, Zhang JA, Ding L. Testosterone level and risk of type 2 diabetes in men: a systematic review and meta-analysis. Endocr Connect, 2018; 7:220–31; https://doi.org/10.1530/EC-17-0253 CrossRef
Ye L, Li YL, Mellström K, Mellin C, Bladh LG, Koehler K, Garg N, Garcia Collazo AM, Litten C, Husman B, Persson K, Ljunggren J, Grover G, Sleph PG, George R, Malm J. Thyroid receptor ligands. 1. Agonist ligands selective for the thyroid receptor β1. J Med Chem, 2003; 46(9):1580–8. https://doi.org/10.1021/jm021080f CrossRef
Yun YR, Won JE, Jeon E, Lee S, Kang W, Jo H, Jang JH, Shin US, Kim HW. Fibroblast growth factors: biology, function, and application for tissue regeneration. J Tissue Eng, 2010; 2010:218142. https://doi.org/10.4061/2010/218142 CrossRef
Zapata-Sudo G, Nunes IK da C, Araujo JSC, da Silva JS, Trachez MM, da Silva TF, da Costa FP, Sudo RT, Barreiro EJ, Lima LM. Synthesis, solubility, plasma stability, and pharmacological evaluation of novel sulfonylhydrazones designed as anti-diabetic agents. Drug Des Devel Ther, 2016; 10:2869–2879. https://doi.org/10.2147/DDDT.S108327 CrossRef
Zhang L, Wang Q, Liu W, Liu F, Ji A, Li Y. The orphan nuclear receptor 4A1: a potential new therapeutic target for metabolic diseases. J Diabetes Res, 2018; 2018:9363461. https://doi.org/10.1155/2018/9363461 CrossRef
Zhang Z, Burch PE, Cooney AJ, Lanz RB, Pereira FA, Wu J, Gibbs RA, Weinstock G, Wheeler DA. Genomic analysis of the nuclear receptor family: new insights into structure, regulation, and evolution from the rat genome. Genome Res, 2004; 14(4):580–90. https://doi.org/10.1101/gr.2160004 CrossRef
Zheng F, Guan Y. Thiazolidinediones: a novel class of drugs for the prevention of diabetic nephropathy? Kidney Int, 2007; 72(11):1301–3. https://doi.org/10.1038/sj.ki.5002557
Zhu C. Aldose reductase inhibitors as potential therapeutic drugs of diabetic complications. In: Oguntibeju O (Ed.). Diabetes mellitus—insights and perspectives, Intech Open, 2013; https://doi.org/10.5772/54642 CrossRef
Zieleniak A, Wójcik M, Wo?niak LA. Structure and physiological functions of the human peroxisome proliferator-activated receptor γ. Arch Immunol Ther Exp (Warsz), 2008; 56(5):331–45. https://doi.org/10.1007/s00005-008-0037-y CrossRef
Zuo H, Wan Y. Nuclear receptors in skeletal homeostasis. Curr Top Dev Biol, 2017; 125:71–107. https://doi.org/10.1016/bs.ctdb.2017.01.002 CrossRef