INTRODUCTION
Physiological bone remodeling plays a pivotal role in coordinating structural bone integrity, as well as preserving bone mass and systemic mineral homeostasis. This process involved a delicate balance between bone resorption by osteoclasts and bone formation by osteoblasts (Kenkre and Bassett, 2018; Kim and Koh, 2019). Excessive bone resorption or reduced bone formation causes an imbalance of this coupling process, leading to bone diseases such as osteoporosis and osteopetrosis (Lombardi and Delvin, 2022; Terkawi et al., 2022). Therefore, direct communication between osteoblasts and osteoclasts is essential for consenting activation signals through cell-cell contact, cytokines secretion, hormone signaling pathway, and nuclear receptor (NR) pathway, and regulating cell differentiation and activities (Kim and Koh, 2019; Weivoda et al., 2020).
Various endogenous (hormones, growth factors, and cytokines) and exogenous (nutrients, drugs, and phytoestrogens) regulators are vital in their direct actions toward bone development, growth, and maintenance (Kang et al., 2021; Macías et al., 2021; Zhou et al., 2021). These regulators act as ligands of NRs, a distinct set of DNA transcription factors that modulate gene expression in bone cells, particularly osteoblast and osteoclast cells at specific stages (Imai et al., 2013; Lee and Park, 2018). NRs bind to DNA response elements in specific regulatory regions of target genes that markedly lead to respective ligand signaling pathways. Following binding specific cognate ligands, NRs modulate the expression of specific target genes at the transcription level (Frigo et al., 2021; Jin et al., 2015; Li et al., 2022a, 2022b, 2022c). The physiology of NRs has been extensively studied via the development of novel genetic manipulation and experimental animal models in which certain NR genes were mutated in specific cell types.
NRs are specific targets of genes that can bind to synthetic ligands (drugs or chemical compounds), which can be effectively used to attenuate the initiation and development of bone diseases, particularly in the case of estrogen deficiency-induced osteoporosis in postmenopausal women (Burr and Phipps, 2022; Li et al., 2022a, 2022b, 2022c). For instance, selective estrogen receptor modulators (SERMs) and non-steroidal compounds are exogenous partial estrogen receptor (ER) agonists which are clinically proven for osteoporosis treatment. The effects of SERMs on bone metabolism are similar to endogenous estrogen. SERMs have been shown to subtly bind to ERs and subsequently to estrogen response elements (EREs) to activate or repress the transcriptional activation of estrogen target genes. SERMs could extraordinarily act as agonists or antagonists in specific target genes and their transcriptional regulation produces unique physiologic effects on bone (Goldstein, 2022).
Isoflavones are flavonoid compounds acting as phytoestrogens owing to their structural similarity to 17-β estradiol (Jiang et al., 2013). In Asian countries, soy foods enriched with isoflavones include tofu, tempeh, miso, natto, cheonggukjang, kinema, hawaijar, and tungrymbai, while in Western countries, isoflavones are mainly found in dairy substitutes, such as soy milk, soy cheese, and soy yogurt. The most recognized isoflavones are genistein, daidzein, S-equol, biochanin A, and coumestrol (do Prado et al., 2022; Kim, 2021; Mutha et al., 2021). Soy is the main dietary source of isoflavones, the most prevalent type of phytoestrogen. Genistein and daidzein, the two primary isoflavones found in soybean as D-glycosides, are not physiologically active. These glycosides are first hydrolyzed by bacterial glucosidases in the digestive tract to produce the equivalent bioactive aglycones, genistein, and daidzein, which are then absorbed into the bloodstream. Although some genistein and daidzein are available in plasma as an aglycone, plasma conjugates genistein and daidzein for the most part (never glucoside). Daidzein, genistein, and o-desmethylangolensin (O-DMA) can all be extensively metabolized in the digestive system producing dihydrodaidzein, equol, and p-ethyl phenol (Chen et al., 2018, 2022a; Gonzales et al., 2015). Notably, S-equol is the most prevalent and active metabolite of daidzein found in the digestive tract. All rodents make equol, but only 30% of people can convert daidzein to equol in their bodies (Pawlowski et al., 2015).
Pharmacokinetic studies have proved that healthy adults absorb isoflavones quickly and efficiently (Chen et al., 2022a, 2022b). The aglycones in phytoestrogen-rich foods typically take 4–7 hours to reach plasma concentrations after consumption (Chen et al., 2022a, 2022b; K?ížová et al., 2019). According to research conducted by Chang and Choue (2013) on South Korean women, soy-based diets with a high isoflavone aglycone content are more efficient at increasing plasma isoflavone levels. For this point, bioavailability increases as the percentage of aglycones increases. Therefore, isoflavone aglycone-rich foods such as fermented soybeans promote beneficial impacts in improving human health (do Prado et al., 2022). Moreover, isoflavones are reported to have numerous health benefits such as reducing the risk of menopausal syndrome (Chen and Chen, 2021), cardiovascular diseases (Bara?ska et al., 2021; Sathyapalan et al., 2018), cancer (Aboushanab et al., 2021; Fan et al., 2022), neurodegenerative diseases (Li et al., 2022a, 2022b, 2022c), osteoporosis (Zheng et al., 2016), and diabetes mellitus (Laily et al., 2022). These beneficial effects arise from the estrogen-like structure of isoflavones such as genistein (Elsayed et al., 2022), daidzein (Mayo et al., 2019), S-equol (Wang et al., 2014), and 8-prenylgenistein (8-PG) (Li et al., 2019a, 2019b).
In recent times, most isoflavones have been enormously employed to prevent estrogen-deficient bone loss. Due to their structural similarity to 17-β estradiol, they have binding affinities to ER and may exert estrogenic activities as either an estrogen agonist or antagonist. Many experimental studies demonstrated the ability of isoflavones to inhibit the loss of bone mineral density (BMD). In vitro, in vivo, and human studies have demonstrated the bone protective properties of isoflavones by increasing osteoblasts number, trabecular thickness, and osteocalcin (OCN) level, as well as diminishing osteoclasts number and C-telopeptide of type I collagen level (Bellavia et al., 2021; Cao et al., 2022; Zheng et al., 2016). However, the potential underlying mechanism of isoflavones at the upstream transcriptional level is not well established.
Efforts to identify the roles of isoflavones in the upstreaming of genetic pathways have generated new insights into the mechanism of action that is critical for ideal drug targets in regulating proper bone remodeling. It is now clear that other NRs such as androgen receptor (AR), peroxisome proliferator-activated receptor (PPAR), and estrogen-related receptor (ERR) are also the targets of isoflavones’ biological actions. Isoflavones manifest their biological action by binding to NRs, modulating their transcriptional responses, and entering the signaling pathways regulated by endogenous receptor ligands (Maldonado-Rojas et al., 2021). This is highly warranted to develop better therapeutic options for bone-related disorders. Therefore, this review highlights recent study findings to better understand the unique nature of isoflavones’ actions on the regulation of NRs involved in bone remodeling.
DATA SOURCES AND SEARCHES
This review was based on data obtained from PubMed, Google Scholar, and EBSCOhost Medline databases from their inception to November 2022. Special attention was given to the mechanisms of particular isoflavones on NRs in the regulation of bone remodeling (summarized in Table 1).
ROLES OF NRs IN BONE REMODELING
NRs, a superfamily comprising 48 members in humans, are activated by lipophilic ligands including steroid hormones, thyroid hormones, lipophilic vitamins, and cholesterol metabolites (Gustafsson, 2016). They are mainly composed of a DNA-binding domain (DBD), a ligand-binding domain (LBD), an N-terminal domain, and a variable C-terminal domain. The binding of ligands causes conformational changes in the NR that initiate binding to chromatin within the nucleus. NRs subsequently bind to their responsive elements in the promoters and other regulatory regions (co-regulators) of target genes to intricately orchestrate an appropriate gene expression. Following binding to co-regulators, NRs exhibit their transcription by stimulation (co-activators) or repression (co-repressors) of transcription. In addition, ligands serve as agonists or antagonists, leading to up- or downregulation of target genes. NRs are involved in all key biological processes including development, cell growth, and differentiation, metabolism, immunity, reproduction, circadian rhythm, and behavior control (Gopi et al., 2021; Gustafsson, 2016; Papageorgiou et al., 2021). However, failure of translational regulation of NRs in terms of mutations, misfolding, or alteration of the ligand-signaling pathway can lead to numerous diseases such as obesity, diabetes, osteoporosis, and cancer (Anbalagan et al., 2012; Wang et al., 2017).
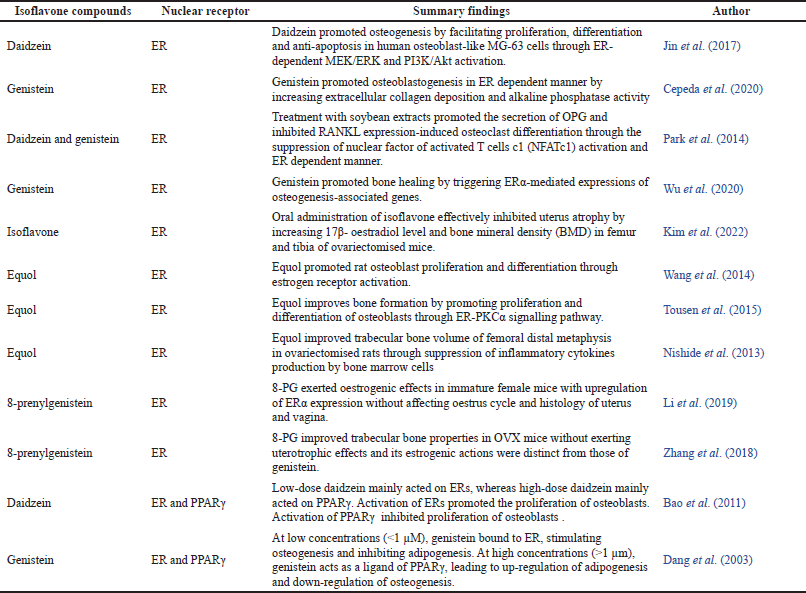 | Table 1. Summary findings of isoflavones on modulation of bone remodelling through nuclear receptor mediated pathway. The parameter for these findings are statistically significant (p < 0.05). [Click here to view] |
The members of the steroid receptors include the ER (α and β), AR, glucocorticoid receptor, and progesterone receptor. Vitamin D, thyroid hormone receptor (TR), PPAR (α, δ/β, and γ), and liver X receptor are classified as non-steroid receptors (Gopi et al., 2021; Gustafsson, 2016; Papageorgiou et al., 2021). Orphan receptors that lack endogenous ligands include ERRs (α, β, and γ) (Tanida, 2022). For orphan receptors, once their endogenous ligands are discovered, these receptors are called “adopted orphans” (de Vera, 2018; Tanida, 2022).
In bone-related disorder treatment especially osteoporosis, many researchers have focused on the ability to selectively modulate the receptors, which led to the preferable drug targets including SERMs (Clarke, 2020) and selective AR modulators (SARMs) that target steroid receptors (Solomon et al., 2019; Xie et al., 2022), selective PPAR modulators that aim at the non-steroid receptor (Liu et al., 2015; Marciano et al., 2015), and selective NR modulators (Sturm’s) that target orphan receptors (Gallet and Vanacker, 2010; Kim et al., 2019; Zuo and Wan, 2017). Selective drug targets can be discovered by activating specific NRs with a specific set of target genes initiated by a specific ligand, which causes allosteric conformational changes in the NR. Herein, the NRs subfamilies that display pivotal roles in bone remodeling are discussed, particularly in promoting bone deposition and inhibiting bone resorption.
Estrogen receptor
ERα and ERβ are two steroid receptors whose ligand-activated transcription factors are detected by immunohistochemistry in osteoblasts, osteocytes, and osteoclasts. Both ERs regulate bone deposition and resorption through ligand or non-ligand-dependent nuclear mechanism or membrane-associated (DNA-independent) mechanism (Jiang et al., 2021; Khalid and Krum, 2016). They can transduce the physiological functions of estrogen, particularly on bone metabolism. Following binding to estrogen, activated ERs are translocated into the nucleus. They then transcriptionally regulate an appropriate gene expression by associating with estrogen response elements (EREs) or binding to non-ligand ER of other DNA transcription factors and subsequent associated binding in ER/specificity protein and ER/activating protein 1 (Fuentes and Silveyra, 2019; Palaniappan et al., 2019).
Previous studies demonstrated that ER deletion in osteoclast lineage initiates more loss of trabecular than cortical bone, showing that ER indirectly inhibits osteoclast differentiation and resorption (Nicks et al., 2016; Melville et al., 2014). Interestingly, ERα has been reported to be expressed more than ERβ and ameliorated the progression of fracture healing in ovariectomized (OVX) rats by promoting osteoblast proliferation (Haffner-Luntzer et al., 2018; Melville et al., 2015). The level of estrogen ERα was elevated during bone restoration by eliciting chromosomal osteogenesis-related gene expressions such as Runx2, alkaline phosphatase (ALP), and OCN, which promote osteoblast maturation (Almeida et al., 2012). In addition, an immunohistochemistry study demonstrated that ERα and ERβ produced opposite effects with ERα highly expressed in cortical bone and ERβ highly expressed in trabecular bone (Bord et al., 2001).
A pioneering study in the 2000s used ER knockout murine models that are completely deficient of ERα and/or ERβ (ERα −/− and/or ERβ −/−) to study the roles of each receptor in bone by measuring BMD and cortical thickness. Findings showed that the deletion of both receptors (ERα −/− and ERβ −/−) caused a reduction of bone turnover and trabecular bone volume in both genders. The deletion of ERα (ERα −/−) was associated with declined bone turnover but increased bone volume of trabecular bone for both genders. Meanwhile, the deletion of ERβ (ERβ −/−) produced similar results as ERα but only occurred in females (Sims et al., 2002). To understand the roles of estrogen and its corresponding receptor on bone turnover and its underlying mechanism, gonadectomy was performed in the knockout mice. It was shown that estrogen treatment in orchidectomized ERα–/– mice failed to prevent bone loss. In contrast, estrogen treatment impeded ovariectomy and orchidectomy-induced bone loss in ERβ –/– mice. Collectively, these studies demonstrated that ERα is the central player of estrogen effects on bone (Sims et al., 2003).
SERMs are synthetic pharmacological compounds with less estrogen steroidal structure and exhibiting a tertiary structure. They can bind to ERs to produce estrogen’s beneficial effects on bone and the cardiovascular system without producing adverse effects on the uterus or mammary glands after menopause (Burr and Phipps, 2022). Conceptually, SERMs differentially express multiple genes and the transcription activity is regulated by ER. The expression of agonist or antagonist activity by SERMs genes was determined by either co-activators or co-repressors’ preferential binding to SERM/ER NR transcription complex (Puranik et al., 2019).
An ideal SERM can thus be utilized for the treatment and prevention of breast cancer (Makar et al., 2020) and osteoporosis (Goldstein, 2022) or to provide relief of hot flashes and other menopause symptoms (Mehedintu et al., 2021). The classical SERM, tamoxifen, is a selective ER blocker in the breast and is an effective agent for treating breast cancer (Cha et al., 2021; Slana? et al., 2021). It has also been indicated to prevent bone loss (Genant, 2011) and provide cardioprotective benefits (Ebrahimi et al., 2020). However, it is associated with a significantly higher incidence of venous thromboembolic events (Lin et al., 2018). The newer SERMs including raloxifene (Nagai et al., 2018), lasofoxifene (Cummings et al., 2010), and bazedoxifene (Cho et al., 2021) have been approved as osteoprotective agents in postmenopausal women with a favorable uterine and breast safety profile. However, they produce adverse effects such as hot flashes and symptoms of vaginal atrophy including dyspareunia (Pinkerton and Thomas, 2014).
Estrogen-related receptors
ERRs, termed ERRα, ERRβ, and ERRγ, belong to the subfamily of orphan NRs that act as transcription factors. As ERRs are orphan NRs, no natural ligands have been identified for them. ERRs have a similar molecular structure to that of other NRs and act as ligand-independent transcription factors (Goher and Elgendy, 2021). Their transcriptional activities are regulated by post-translational modification or the availability of transcriptional co-regulators (Goher and Elgendy, 2021; Huss et al., 2015). ERRs recruit co-regulators to modulate gene expression transcription and play a part in various physiological functions including energy metabolism, embryonic stem cell pluripotency, bone metabolism, and cancer progression (Huang and Sun, 2021; Ranhotra, 2018). In terms of ERRs activities on bone metabolism, ERRα and ERRγ are potential targets to protect against bone loss (Bonnelye, 2016; Feng et al., 2022; Gallet and Vanacker, 2010).
In vitro experiments have shown that ERRα was strongly expressed in mesenchymal cell commitment, and when upregulated, they promote early osteoblast and adipogenic differentiation. Several findings pointed to the role of ERRα as a switch that suppresses the differentiation of MSCs into osteoblasts of the bone formation pathway while favoring the adipocytic pathway (Bonnelye, 2016; Gallet and Vanacker, 2010). ERRα constructively modulates the key proteins in osteoblastogenesis including Runt-related transcription factor (Runx2), osteopontin (OPN), and OCN transcription. This regulation is dependent on respective PPAR, gamma coactivator-1 alpha (PGC-1α), and PGC-1β expression levels (Chen et al., 2022a, 2022b; Feng et al., 2022; Kammerer et al., 2013; Wang and Wang, 2013). The prominent role of ERRα in osteoblast differentiation was underlined by a demonstration that ERRα acted as a transcriptional activator of Runx2-I in the presence of PGC-1α and as a transcriptional repressor of Runx2-I in the presence of PGC-1β (Kammerer et al., 2013). ERRα has also been found to interact mutually with PGC-1α and increase OCN promoter activity (Wang and Wang, 2013). In addition, high ERRα expression has been found in the ossification zones (long and flat bones) during mouse embryonic development, suggesting that this receptor may promote endochondral and intramembranous ossifications (Bonnelye, 2022).
There is evidence that ERRα may also regulate osteoclastogenesis activity (Bae et al., 2017; Kim et al., 2021; Yang and Wan, 2019). A study by Yang and Wan (2019) verified that ERRα deletion disrupted bone hemopoiesis, as seen in ERRα knockout mice which exhibited osteopetrosis due to decreased bone resorption and high bone mass. Since ERRα is an orphan receptor, it can bind to any synthetic compounds including cholesterol. Cholesterol has been identified as a potential agonist to modulate ERRα activities and stability (Casaburi et al., 2018). Cholesterol binding to ERRα synergistically promoted downstream osteoclastogenesis. Furthermore, the study by Wei et al. (2016) revealed that cholesterol enhanced the interaction between ERRα and PGC-1α in osteoclasts, thus promoting bone resorption activity. Following these study findings, several other studies reported low BMD in dyslipidemia patients (Kim et al., 2013) and post-menopausal women with high lipid profiles (Yang et al., 2018). Thus, dyslipidemia could accelerate the bone resorption process and lead to bone-related disorders such as osteopenia and osteoporosis.
Accordingly, statins are the most prescribed cholesterol-lowering drugs that block 3- hydroxy-3-mthylglutaryl-coenzyme A reductase activity and inhibit the synthesis of mevalonate, the precursor of cholesterol (Zhang et al., 2021). Besides their cardioprotective properties, statins produce pleiotropic osteoprotective effects, which affect bone formation rather than bone resorption (Murphy et al., 2020). The lipophilic structure of statin and its capability of modulating the transcriptional activity of ERRα in bones have been reported. Statins have been shown to act as an endogenous agonist of ERRα to suppress osteoclastogenesis by decreasing the free cholesterol bioavailability (Casaburi et al., 2018; Wei et al., 2016). Concomitantly, statin has been found to inhibit the receptor of nuclear factor κβ ligand (RANKL) in macrophages, which caused a reduction in free cholesterol and prevented ERRα from stimulating osteoclastogenesis (Climent et al., 2021). The osteoprotective effects of statin was also associated with increased expression of the bone morphogenetic protein-2 gene that promotes osteoblast differentiation (Kuwahara et al., 2022; Li et al., 2022a, 2022b, 2022c). Congruously, in human studies, BMDdecreases with an increase in statin dose (Fadheel and Naser, 2022; Zheng et al., 2020). Thus, these findings implied the importance of ERRα in the pathogenesis of osteoporosis, leading to enormous interest in this protein as a novel therapeutic target.
Peroxisome proliferator-activated receptor-γ (PPAR-γ)
PPARs are adopted orphan NRs, which consist of three isoforms, PPAR-α, PPAR-γ, and PPAR-δ/β (Grygiel-Górniak, 2014; Palomer et al., 2018). In the DNA-dependent pathway, PPARs form heterodimers with retinoid X receptors and are associated with peroxisome proliferator response elements in the promoter of their target genes (Kilu et al., 2021). PPAR-γ has been markedly established as the master regulator of adipocyte differentiation, which plays a role in adipogenic and lipogenic pathways (Ma et al., 2018). However, PPAR-γ activities have also been addressed in osteoblasts (Li et al., 2019a, 2019b), osteoclasts (Guo et al., 2019), and chondrocytes (Chen et al., 2015). In these cells, PPAR-γ suppressed bone formation and stimulated bone resorption by favoring adipogenesis (Guo et al., 2019; Li et al., 2019a, 2019b). PPAR-γ agonists such as thiazolidinediones (TZDs) (Ahsan, 2019), lobeglitazone (Rocha et al., 2020), and pioglitazone (Tomlinson et al., 2022) are potent treatments of type II diabetes but may cause adverse effects of increased fracture risk. The PPAR-γ activated by TZDs causes disproportionate bone remodeling, which led to increased bone resorption and decreased bone formation. The use of selective PPAR-γ modulators can reduce the harmful effects of the PPAR-γ on bones (Wei and Wan, 2011).
Noteworthily, homozygous PPAR-γ-deficient embryonic stem cells failed to differentiate into adipocytes but displayed an increased number of osteoblasts (Akune et al., 2004). The deletion of PPAR-γ in mesenchymal progenitors’ cells has also been shown to improve BMD, bone volume, trabecular bone number, and osteoblasts cell number (Cao et al., 2020). In addition, PPAR-γ deletion in osteoclast and endothelial cells increased bone mass due to reduced osteoclast differentiation (Zou et al., 2016). Physiologically, it is worth noting that PPAR-γ could act as a pro-osteoclastogenesis regulator (Cao et al., 2020; Li et al., 2019a, 2019b; Zou et al., 2016). Therefore, the molecular mechanisms of PPAR-γ that link adipogenic signaling molecules and osteoclast differentiation need to be elucidated. This is important to determine if PPAR-γ modulators may provide therapeutic strategies for the treatment of metabolic diseases especially cardiovascular disease and osteoporosis (Muruganandan et al., 2020)
Androgen receptor
AR is a ligand-inducible transcription factor and a member of the nuclear steroid TR gene superfamily. Androgen hormone specifically binds to AR which causes conformational changes in AR and the recruitment of specific promoter elements. This is followed by transcription activation or repression of various target genes. Since AR is on chromosome X, the deficiency of AR mostly affects males (Davey and Grossmann, 2016; Levine and Garabedian 2014). Androgen is also essential for conversion to estrogen by aromatase activity; therefore, androgen is competent for activating both ERs and ARs expression (Bianchi et al., 2021; Rosati et al., 2021).
Osteoblasts and osteocytes in bone tissues express AR to modulate several gene expressions that encode various growth factors and cytokines to control bone remodeling (Chen et al., 2019; Gong et al., 2021). AR in osteoblasts is stimulated by estrogen, androgen, and 1,25-dihydroxy vitamin D to accelerate osteoblast proliferation, differentiation, and synthesis of extracellular matrix protein to initiate mineralization (Chen et al., 2019; Chinetti and Neels, 2021). AR that presents on osteocytes has a pivotal role in improving skeletal integrity and bone quality (Sinnesael et al., 2012). Impaired AR signaling can lead to irregular bone cell activities (Russell et al., 2012). A study by Kawano et al. (2003) revealed that the upregulation of RANKL expression in osteoblasts of AR-deficient mice augmented osteoclastogenesis . Furthermore, AR activation in osteoblasts inhibited bone resorption in the cancellous compartment (Sinnesael et al., 2015). These findings also showed that osteoclasts in AR knockout (ARKO) mice did not show changes in their proliferation and differentiation and there were no changes in bone microarchitecture (Kawano et al., 2003; Sinnesael et al., 2015). These two findings evidently illustrated that osteoclast function is mainly regulated by estrogens and ER, not AR.
To outline the physiological roles of AR on bone metabolism, AR transgenic and ARKO models were designed through the deletion of exon 3 of AR, which encode the 2nd zinc finger of the DBD (Chang et al., 2013). In ARKO mice, the DNA-binding-dependent AR pathway is abolished but the non-DNA pathway remains functional (Rana et al., 2014). The clinical phenotype of ARKO mice is consistent with hypogonadism in human males, with high-fat mass but low bone and muscle mass (Rana et al., 2014; Sebo et al., 2021). Previous studies showed that ARKO murine displayed osteopenia, retarded growth curves, and increased trabecular bone resorption. Since AR affects both osteoblast and osteoclast cells, mature osteoblast ARKO and mature osteoclast ARKO were developed. The study by Kawano et al. (2003) revealed that AR deletion in mature osteoblasts increased RANKL expression, followed by enhanced osteoclast differentiation. This has been further supported by subsequent studies demonstrating that deletion of exon 3 of the AR gene in osteoblast cells led to pronounced trabecular bone loss in adult male mice (Notini et al., 2007; Wu et al., 2019;). On the other hand, Jardi et al. (2019) demonstrated that 46-week-old neuronal ARKO mild mice displayed pronounced loss of cortical thickness and strength. The study concluded that AR in neurons retains bone mass and strength in aging mice.
SARMs are anabolic steroids that bind to AR by a DNA-dependent pathway and display pronounced tissue selectivity. Examples of SARMs currently available are ostarine and andarine (Solomon et al., 2019). The understanding of AR interactions with various co-activators and co-suppressors is therefore crucial.
Interaction of isoflavones to ER
Upon ingestion, the isoflavones are deconjugated to their respective aglycone in the gastrointestinal tract. These aglycones are extensively metabolized during absorption to become glucuronidated and/or sulfated conjugates before entering the bloodstream (Chen et al., 2018, 2022a, 2022b; Gonzales et al., 2015). These conjugates have been demonstrated to be agonistic ligands of both ER-LBD and ER-LBD to stimulate ER-LBD-coregulator interaction and exhibit their transcription (Morito et al., 2002). Examples of these conjugates include dihydrodaidzein, dihydrogenistein, equol, and O-DMA (Gaya et al., 2016; Islam et al., 2015). Isoflavones’ ER-binding abilities have the ability to induce intracellular signaling processes, which are crucial for cellular growth (Lee et al., 2013).
Isoflavones and estradiol are competitively binding on ERs. According to Beekmann et al. (2015) ’s findings, isoflavone aglycones were less effective than E2 at activating the LBDs of ERα and ERβ, and genistein was more effective than daidzein. This is consistent with other studies showing that genistein and daidzein have a lower affinity for binding to ERα and ERβ than E2 (Jiang et al., 2013) and that they can induce ERα and ERβ-mediated gene transcription as well as cell proliferation at concentrations higher than E2 with genistein frequently being more potent than daidzein (Islam et al., 2015). ERβ-LBD was shown to be more responsive to genistein activation than ERα-LBD (Beekmann et al., 2015). This is consistent with observations in the literature that show that genistein preferentially binds to and activates ERβ over ERα during transcription (Jiang et al., 2013).
ERs are widely distributed in reproductive organs, particularly the uterus and breast. The affinity of 17β-estradiol for ERα and ERβ receptors is equal, whereas the affinity of isoflavones for ERβ receptor is higher (Lee et al., 2021; Mbachu et al., 2020). When a phytoestrogen binds to a receptor, the receptor may be partially activated (have an agonistic impact) or become less activated (have an antagonistic effect), depending on the effect of the estrogen molecule being displaced by the phytoestrogen (Khan et al., 2022; Wang et al., 2021). Researchers are interested in tissue-specific phytoestrogens because estrogenic (agonist) activity in some tissues can help maintain BMDand enhance blood lipid profiles while antiestrogenic (antagonist) activity in reproductive tissues can help lower the risk of hormone-related tumors (such as those of the breast, uterus, and prostate) (Hsieh et al., 2018). Isoflavones can also exert a biphasic scheme by acting as estrogen agonists at low concentrations but as an antagonist at high concentrations (Erguc et al., 2021; Manayi, 2021). For instance, isoflavones inhibit the growth of breast cancer cells at higher doses while stimulating the growth of positive ER breast cancer cells at low concentrations (Martinkovich et al., 2014).
EFFECTS OF ISOFLAVONES ON BONE REMODELING THROUGH ER PATHWAY
Various in vitro and in vivo studies on bone remodeling demonstrated that isoflavones positively stimulate osteoblastic bone formation and inhibit osteoclastic bone resorption (Fig. 1). Daidzein and genistein have been found to stimulate osteoblastogenesis in rat and human osteoblast cells. These isoflavones facilitated osteoblast proliferation, differentiation, and anti-apoptosis via the activation of phosphoinositide 3-kinase/protein kinase B or PKB (PI3K/Akt) in an ER-dependent manner (Cepeda et al., 2020; Jin et al., 2017). The osteoblastogenic effects of these isoflavones were confirmed with increased levels of osteoblast differentiation markers such as ALP, type 1 collagen, bone sialoprotein, OPN, and OCN (Cepeda et al., 2020). On the other hand, daidzein and genistein exerted anti-resorptive effects by increasing the osteoprotegerin (OPG) level and decreasing the RANKL level. This was achieved through the suppression of the nuclear factor of activated T cells c1 expression in the ER-dependent manner (Park et al., 2014).
More remarkably, in in vivo studies, genistein was capable of promoting fracture healing by stimulating osteoblast maturation via an ERα-dependent mechanism (Wu et al., 2020). This suggested that genistein has the potential to be developed as drug therapy for osteoporosis and osteoporotic fracture. Additionally, Kim et al. (2022) showed that oral administration of isoflavone to OVX mice increased 17β-estradiol level and effectively inhibited uterus atrophy and promoted BMD of femora and tibiae. In line with these findings, isoflavone was found to enhance the ratio of serum OPG/RANKL in OVX mice, conceivably improving bone remodeling (Kim et al., 2022).
Previous studies reported that equol, the active metabolite of daidzein, showed greater affinity for ERβ and has a longer half-life and greater bioavailability than daidzein and genistein (Mayo et al., 2019). Equol promoted the proliferation and differentiation of osteoblasts through the ER -protein kinase C alpha (ER-PKCα) signaling pathway, suggesting its ability to improve bone formation (Wang et al., 2014). In addition, Tousen et al. (2015) indicated that equol was more efficient than daidzein to increase the BMD of growing females by enhancing bone formation without affecting the weight of reproductive organs. Furthermore, equol was revealed to improve the femoral trabecular bone volume of OVX rats through suppression of inflammatory cytokines production by bone marrow cells (Nishide et al., 2013).
Prenylated isoflavone is characterized by the presence of a prenylated side chain in the flavonoid skeleton. The substitute for genistein, 8-PG, is found in the flower of hops (Humulus lupulus L.). Multiple in vitro and in vivo studies have regarded 8-PG as one of the potent isoflavones with estrogenic activities. Interestingly, Kretzschmar et al. (2010) demonstrated that 8-PG was able to induce responsive reporter activities in yeast and ALP activities in Ishikawa cells much greater than other phytoestrogens such as genistein, daidzein, and coumestrol. The high estrogenic activity of 8-PG was confirmed in an in vivo study by the upregulation of ERα in bone cells of immature female mice without altering ERα level in the uterus (Li et al., 2019a, 2019b). Intriguingly, prenylated isoflavones displayed an essential role in bone remodeling. 8-PG was reported to improve trabecular bone properties in OVX mice through an ERα-dependent mechanism without exerting uterotrophic effects (Zhang et al., 2018). Moreover, 8-PG displayed a more pronounced ability than naringenin in enhancing osteoblast differentiation and mineralization (Ming et al., 2012). These studies clearly indicated that prenylation modification in the isoflavone compound is essential for inducing osteogenesis.
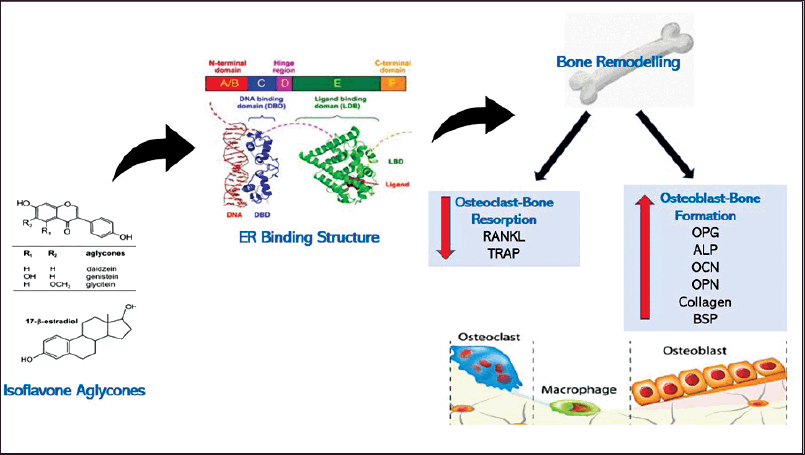 | Figure 1. Isoflavones aglycones bind to either ERα or ERβ, increase osteoblast bone formation markers such as OPG, ALP, OCN, OPN, collagen, and BSP, and reduce osteoclast bone resorption markers such as RANKL and TRAP via ER-dependent signaling pathway. ER: Estrogen receptor; OPG: Osteoprotegerin; ALP: Alkaline phosphatase; OCN: Osteocalcin; OPN: Osteopontin; BSP: Bone sialoprotein; RANKL: Receptor activator of the nuclear factor-κB ligand; OPG: Osteoprotegerin; TRAP: Tartrate-resistant acid phosphatase. [Click here to view] |
The beneficial effects of soy isoflavones on BMD of postmenopausal women have been discussed in a systematic review (Bara?ska et al., 2022).
EFFECTS OF ISOFLAVONES ON BONE REMODELING THROUGH ERR PATHWAY
Suetsugi et al. (2003) demonstrated that the activation of ERRα by genistein and daidzein was comparable to the activation of ERα and ERβ. Note that even though ERRα is structurally similar to ERα, it is not activated by 17β-estradiol. Concomitantly, daidzein reduced lipid deposition in muscle cells through the ERRα pathway and not the ER pathway (Kitamura et al., 2020). The important role of ERRα in bone remodeling is well accepted. ERRα exhibits diverse functions in osteogenesis, especially in regulating osteoblast and osteoclast differentiation (Feng et al., 2022). A study is required to determine the effects of isoflavones on bone remodeling through the ERRα-mediated pathway.
EFFECTS OF ISOFLAVONES ON BONE REMODELING THROUGH PPARY DOWNREGULATION
In a study on murine mesenchymal progenitor cell lines, low concentration of isoflavones stimulated osteogenesis and inhibited adipogenesis. Conversely, a high concentration of isoflavones inhibited osteogenesis and accelerated adipogenesis. It is interesting to note that when ERs were blocked by ICI182780, an ER antagonist, daidzein could activate PPARγ signaling pathway to modulate adipogenesis and osteogenesis signaling pathway (Bao et al., 2011). In line with this, the bone marrow mesenchymal stem cell of PPARγ knockout mice exhibited abolishment of adipogenesis and increased osteoblastogenesis (Cao et al., 2015). Further, genistein at low concentration (<1 µM) was shown to act as an inhibitor of PPARγ by promoting osteogenesis and inhibiting adipogenesis (Dang et al., 2003). The inhibition of PPARγ could also inactivate bone resorption activities (Guo et al., 2019; Li et al., 2018). Thus, blockage of the PPARγ-mediated signaling pathway by isoflavones could increase bone formation and attenuate bone resorption. In summary, the biphasic dose-dependent modulation of osteogenesis and adipogenesis by isoflavones is crucial for the treatment of metabolic disorders and osteoporosis.
EFFECTS OF ISOFLAVONES ON BONE REMODELING THROUGH AR PATHWAY
Isoflavones have been found to bind to ARs (Mahmoud et al., 2011). Multiple studies demonstrated that the binding to AR ligands would promote anabolic AR responses, which maintain muscle mass and bone integrity (Almeida et al., 2017; Chen et al., 2019; Lan et al., 2022). Isoflavones have been shown to downregulate AR expressions to suppress prostate cancer cells (Ajdžanovic et al., 2019; Sivo?ová et al., 2019; Stanis?awska et al., 2022). However, their actions toward bone remodeling via AR have yet to be elucidated.
CONCLUSION
This review offers up-to-date literature on the influence of isoflavones on bone remodeling, especially via osteoclast and osteoblast differentiation. Isoflavones have exemplified NRs regulation on bone osteoprotective effects. The classical steroid receptors recognized as regulators of bone remodeling include estrogen and AR, whereas recent studies have identified new NRs including ERR and PPAR-γ as novel regulators of osteogenesis. These NRs could be therapeutic targets that gain access to the mechanism controlling gene regulation in osteoporosis treatment. Therefore, this review has highlighted the potential of dietary isoflavones to prevent osteoporosis and exert beneficial effects on bone remodeling.
AUTHORS’ CONTRIBUTIONS
Conceptualization was done by H. A. H. and A. N. S.; material searching was done by H. A. H., N. M., and P. A. J.; original draft preparation was contributed by H. A. H.; writing review and editing were contributed by H. A. H. and A. N. S. All authors have read and agreed to the published version of the manuscript.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
LIST OF ABBREVIATIONS
8-PG, 8-Prenylgenistein; ALP, Alkaline phosphatase; AR, Androgen receptor; BMD, Bone mineral density; ER, Estrogen receptor; ERR, Estrogen-related receptor; NR, Nuclear receptor; OPG, Osteoprotegerin; OVX, Ovariectomized; PPAR, Peroxisome proliferator-activated receptor; RANKL, Receptor activator of nuclear factor-κB ligand; SERM, Selective estrogen receptor modulator.
REFERENCES
Aboushanab SA, Khedr SM, Gette IF, Danilova IG, Kolberg NA, Ravishankar GA, Ambati RR, Kovaleva EG. Isoflavones derived from plant raw materials: bioavailability, anti-cancer, anti-aging potentials, and microbiome modulation. Crit Rev Food Sci Nutr, 2021; 27:1–27. CrossRef
Ahsan W. The journey of thiazolidinediones as modulators of PPARs for the management of diabetes: a current perspective. Curr Pharm Des, 2019; 25(23):2540–54. CrossRef
Ajdžanovic V, Filipovic B, Miljic D, Mijatovic S, Maksimovic-Ivanic D, Miler M, Živanovic J, Miloševic V. Prostate cancer metastasis and soy isoflavones: a dogfight over a bone. EXCLI J, 2019; 18:106.
Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T. PPAR γ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Investig, 2004; 113(6):846–55. CrossRef
Almeida M, Iyer S, Martin-Millan M, Bartell SM, Han L, Ambrogini E, Onal M, Xiong J, Weinstein RS, Jilka RL, O’Brien CA. Estrogen receptor-α signaling in osteoblast progenitors stimulates cortical bone accrual. J Clin Investig, 2012; 123(1):394–404. CrossRef
Almeida M, Laurent MR, Dubois V, Claessens F, O’Brien CA, Bouillon R, Vanderschueren D, Manolagas SC. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev, 2017; 97(1):135–87. CrossRef
Anbalagan M, Huderson B, Murphy L, Rowan BG. Post-translational modifications of nuclear receptors and human disease. Nucl Recept Signal, 2012; 10:e001. CrossRef
Bae S, Lee MJ, Mun SH, Giannopoulou EG, Yong-Gonzalez V, Cross JR, Murata K, Giguère V, van der Meulen M, Park-Min KH. MYC-dependent oxidative metabolism regulates osteoclastogenesis via nuclear receptor ERRα. J Clin Invest, 2017; 127(7):2555–68. CrossRef
Bao L, Zou SE, Zhang SF. Dose-dependent effects of daidzein in regulating bone formation through estrogen receptors and peroxisome proliferator-activated receptor γ. Zhong xi yi jie he xue bao Chin J Integr Med, 2011; 9(2):165–72. CrossRef
Baranska A, Blaszczuk A, Kanadys W, Baczewska B, Jedrych M, Wawryk-Gawda E, Polz-Dacewicz M. Effects of soy protein containing of isoflavones and isoflavones extract on plasma lipid profile in postmenopausal women as a potential prevention factor in cardiovascular diseases: systematic review and meta-analysis of randomized controlled trials. Nutrients, 2021; 13(8):2531. CrossRef
Baranska A, Kanadys W, Bogdan M, Stepien E, Barczynski B, Klak A, Augustynowicz A, Szajnik M, Religioni U. The role of soy isoflavones in the prevention of bone loss in postmenopausal omen: a systematic review with meta-analysis of randomized controlled trials. J Clin Med, 2022; 11(16):4676. CrossRef
Beekmann K, de Haan LH, Actis-Goretta L, Houtman R, van Bladeren PJ, Rietjens IM. The effect of glucuronidation on isoflavone induced estrogen receptor (ER) α and ERβ mediated coregulator interactions. J Steroid Biochem Mol Biol, 2015; 154:245–53. CrossRef
Bellavia D, Dimarco E, Costa V, Carina V, De Luca A, Raimondi L, Fini M, Gentile C, Caradonna F, Giavaresi G. Flavonoids in bone erosive diseases: perspectives in osteoporosis treatment. Trends Endocrinol Metab, 2021; 32(2):76–94. CrossRef
Bianchi VE, Bresciani E, Meanti R, Rizzi L, Omeljaniuk RJ, Torsello A. The role of androgens in women’s health and wellbeing. Pharmacol Res, 2021; 171:105758. CrossRef
Bonnelye E. Estrogen receptor related receptor alpha (ERRα) in skeletal tissues. Endocrinol Metab Syndr, 2016; 5(4). CrossRef
Bonnelye E. Energy metabolism in bone tumors: fuel selection and adaptation. In: Bone sarcomas and bone metastases-from bench to bedside, Academic Press, Cambridge, MA, pp 337–55, 2022. CrossRef
Bord S, Horner A, Beavan S, Compston J. Estrogen receptors α and β are differentially expressed in developing human bone. J Clin Endocrinol Metab, 2001; 86(5):2309–14. CrossRef
Burr DB, Phipps R. Selective estrogen receptor modulators (SERMs). In: Takahashi HE, Burr DB, Yamamoto N (eds.). Osteoporotic fracture and systemic skeletal disorders, Springer, Singapore, pp 399–411, 2002. CrossRef
Cao J, Ding K, Pan G, Rosario R, Su Y, Bao Y, Zhou H, Xu J, McGee Lawrence ME, Hamrick MW, Isales CM. Deletion of PPARγ in mesenchymal lineage cells protects against aging-induced cortical bone loss in mice. J Gerontol A, 2020; 75(5):826–34. CrossRef
Cao J, Ou G, Yang N, Ding K, Kream BE, Hamrick MW, Isales CM, Shi XM. Impact of targeted PPARγ disruption on bone remodeling. Mol Cell Endocrino, 2015; 410:27–34. CrossRef
Cao L, Wang J, Zhang Y, Tian F, Wang C. Osteoprotective effects of flavonoids: evidence from in vivo and in vitro studies. Mol Med Rep, 2022; 25(6):1–9. CrossRef
Casaburi I, Chimento A, De Luca A, Nocito M, Sculco S, Avena P, Trotta F, Rago V, Sirianni R, Pezzi V. Cholesterol as an endogenous ERRα agonist: a new perspective to cancer treatment. Front Endocrinol (Lausanne), 2018; 9:525. CrossRef
Cepeda SB, Sandoval MJ, Crescitelli MC, Rauschemberger MB, Massheimer VL. The isoflavone genistein enhances osteoblastogenesis: signaling pathways involved. J Physiol Biochem, 2020; 76(1):99–110 CrossRef
Cha C, Lee SJ, Hong H, Choi YY, Chung MS. Adverse effects of adjuvant tamoxifen treatment on bone mineral density in premenopausal breast cancer patients: a systematic review and meta-analysis. 2021; e12500. CrossRef
Chang Y, Choue R. Plasma pharmacokinetics and urinary excretion of isoflavones after ingestion of soy products with different aglycone/glucoside ratios in South Korean women. Nut Res Pract, 2013; 7(5):393–9. CrossRef
Chang C, Yeh S, Lee SO, Chang TM. Androgen receptor (AR) pathophysiological roles in androgen related diseases in skin, metabolism syndrome, bone/muscle and neuron/immune systems: lessons learned from mice lacking AR in specific cells. Nucl Recept Signal, 2013; 11(1):nrs–11001. CrossRef
Chen L, Cao H, Huang Q, Xiao J, Teng H. Absorption, metabolism and bioavailability of flavonoids: a review. Crit Rev Food Sci Nutr, 2022a; 62(28):7730–42. CrossRef
Chen YJ, Chan DC, Lan KC, Wang CC, Chen CM, Chao SC, Tsai KS, Yang RS, Liu SH. PPARγ is involved in the hyperglycemia-induced inflammatory responses and collagen degradation in human chondrocytes and diabetic mouse cartilages. J Orthop Res, 2015; 33(3):373–81. CrossRef
Chen LR, Chen KH. Utilization of isoflavones in soybeans for women with menopausal syndrome: an overview. Int J Mol Sci, 2021; 22(6):3212. CrossRef
Chen H, Fan W, He H, Huang F. PGC-1: a key regulator in bone homeostasis. J Bone Miner Metab, 2022b; 40(1):1–8. CrossRef
Chen JF, Lin PW, Tsai YR, Yang YC, Kang HY. Androgens and androgen receptor actions on bone health and disease: from androgen deficiency to androgen therapy. Cells, 2019; 8(11):1318. CrossRef
Chen L, Teng H, Xie Z, Cao H, Cheang WS, Skalicka-Woniak K, Georgiev MI, Xiao J. Modifications of dietary flavonoids towards improved bioactivity: an update on structure–activity relationship. Crit Rev Food Sci Nutr, 2018; 58(4):513–27. CrossRef
Chinetti G, Neels JG. Roles of nuclear receptors in vascular calcification. Int J Mol Sci, 2021; 22(12):6491. CrossRef
Cho SK, Kim H, Lee J, Nam E, Lee S, Choi YY, Sung YK. Effectiveness of bazedoxifene in preventing glucocorticoid-induced bone loss in rheumatoid arthritis patients. Arthritis Res Ther, 2021; 23(1):1–2. CrossRef
Clarke BL. Effects of estrogens and SERMs on bone metabolism: clinical aspects. In: Osteoporosis, Humana, Cham, Switzerland, pp 239–57, 2020. CrossRef
Climent E, Benaiges D, Pedro-Botet J. Hydrophilic or lipophilic statins? Front Cardiovasc Med, 2021; 8:687585. CrossRef
Cummings SR, Ensrud K, Delmas PD, LaCroix AZ, Vukicevic S, Reid DM, Goldstein S, Sriram U, Lee A, Thompson J, Armstrong RA, Thompson DD, Powles T, Zanchetta J, Kendler D, Neven P, Eastell R. PEARL study investigators. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med, 2010; 362(8):686–96. CrossRef
Dang ZC, Audinot V, Papapoulos SE, Boutin JA, Löwik CW. Peroxisome proliferator-activated receptor gamma (PPARgamma) as a molecular target for the soy phytoestrogen genistein. J Biol Chem, 2003; 278(2):962–7.
Davey RA, Grossmann M. Androgen receptor structure, function and biology: from bench to bedside. Clin Biochem Rev, 2016; 37(1):3. CrossRef
de Vera IMS. Advances in orphan nuclear receptor pharmacology: a new era in drug discovery. ACS Pharmacol Transl Sci, 2018; 1(2):134–7. CrossRef
do Prado FG, Pagnoncelli MG, de Melo Pereira GV, Karp SG, Soccol CR. Fermented soy products and their potential health benefits: a review. Microorganisms, 2022; 10(8):1606. CrossRef
Ebrahimi MN, Khaksari M, Sepehri G, Karam GA, Raji-Amirhasani A, Azizian H. The effects of alone and combination tamoxifen, raloxifene and estrogen on lipid profile and atherogenic index of ovariectomized type 2 diabetic rats. Life Sci, 2020; 263:118573. CrossRef
Elsayed DH, Helmy SA, Dessouki AA, El-Nahla AM, Abdelrazek HM, El-Hak HN. Influence of genistein and diadizine on regularity of estrous cycle in cyclic female Wistar rat: interaction with estradiol receptors and vascular endothelial growth factor. Open Vet J, 2022; 12(5):639–48. CrossRef
Erguc EI, Tascioglu-Aliyev A, Entezari B, Gurer-Orhan H. The role of biotransformation in the activity of endocrine disruptors. Curr Drug Met, 2021; 22(8):628–44.
Fadheel QJ, Naser RT. Assessment of simvastatin effect and compare it’s with combination of calcium plus vitamin D3 in postmenopausal women with osteoporosis. Med J Ahl al-Bayt Univ, 2022; 1(1):21–37. CrossRef
Fan Y, Wang M, Li Z, Jiang H, Shi J, Shi X, Liu S, Zhao J, Kong L, Zhang W, Ma L. Intake of soy, soy isoflavones and soy protein and risk of cancer incidence and mortality. Front Nutr, 2022; 9:847421. CrossRef
Feng C, Xu Z, Tang X, Cao H, Zhang G, Tan J. Estrogen-related receptor α: a significant regulator and promising target in bone homeostasis and bone metastasis. Molecules, 2022; 27(13):3976. CrossRef
Frigo DE, Bondesson M, Williams C. Nuclear receptors: from molecular mechanisms to therapeutics. Essays Biochem, 2021; 65(6):847–56. CrossRef
Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol, 2019; 116:135–70. CrossRef
Gallet M, Vanacker JM. ERR receptors as potential targets in osteoporosis. Trends Endocrinol Metab, 2010; 21(10):637–41. CrossRef
Gaya P, Medina M, Sánchez-Jiménez A, Landete JM. Phytoestrogen metabolism by adult human gut microbiota. Molecules, 2016; 21(8):1034. CrossRef
Genant HK. Bazedoxifene: a new selective estrogen receptor modulator for postmenopausal osteoporosis. Menopause Int, 2011; 17(2):44–9. CrossRef
Goher SS, Elgendy B. Structure-based design of estrogen-related receptors modulators. In: Badr MZ (ed.). Nuclear receptors, Springer, Cham, Switzerland, pp 79–109, 2021. CrossRef
Goldstein SR. Selective estrogen receptor modulators and bone health. Climacteric, 2022; 25(1):56–9. CrossRef
Gong D, Sun Y, Guo C, Sheu TJ, Zhai W, Zheng J, Chang C. Androgen receptor decreases renal cell carcinoma bone metastases via suppressing the osteolytic formation through altering a novel circEXOC7 regulatory axis. Clin Transl Med, 2021; 11(3):e353. CrossRef
Gonzales GB, Smagghe G, Grootaert C, Zotti M, Raes K, Camp JV. Flavonoid interactions during digestion, absorption, distribution and metabolism: a sequential structure–activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab Rev, 2015; 47(2):175–90. CrossRef
Gopi S, Lukose B, Naganathan AN. Diverse native ensembles dictate the differential functional responses of nuclear receptor ligand-binding domains. J Phys Chem B, 2021; 125(14):3546–55. CrossRef
Grygiel-Górniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications - a review. Nutr J, 2014; 13:17 CrossRef
Guo L, Chen K, Yuan J, Huang P, Xu X, Li C, Qian N, Qi J, Shao Z, Deng L, He C. Estrogen inhibits osteoclasts formation and bone resorption via microRNA-27a targeting PPARγ and APC. J Cell Physiol, 2019; 234(1):581–94. CrossRef
Gustafsson JA. Historical overview of nuclear receptors. J Steroid Biochem Mol Biol, 2016; 157:3–6. CrossRef
Haffner-Luntzer M, Kovtun A, Lackner I, Mödinger Y, Hacker S, Liedert A, Tuckermann J, Ignatius A. Estrogen receptor α- (ERα), but not ERβ-signaling, is crucially involved in mechanostimulation of bone fracture healing by whole-body vibration. Bone, 2018; 110:11–20. CrossRef
Hsieh CJ, Hsu YL, Huang YF, Tsai EM. Molecular mechanisms of anticancer effects of phytoestrogens in breast cancer. Curr Protein Pept Sci, 2018; 19(3):323–32. CrossRef
Huang WY, Sun PM. Estrogen receptor-associated receptor α and peroxisome proliferator-activated receptor γ in metabolism and disease. Mol Med Rep, 2021; 23(2):1. CrossRef
Huss JM, Garbacz WG, Xie W. Constitutive activities of estrogen-related receptors: transcriptional regulation of metabolism by the ERR pathways in health and disease. Biochim Biophys Acta Mol Basis Dis, 2015; 1852(9):1912–27. CrossRef
Imai Y, Youn MY, Inoue K, Takada I, Kouzmenko A, Kato S. Nuclear receptors in bone physiology and diseases. Physiol Rev, 2013; 93(2):481–523. CrossRef
Islam MA, Bekele R, Vanden Berg JH, Kuswanti Y, Thapa O, Soltani S, van Leeuwen FR, Rietjens IM, Murk AJ. Deconjugation of soy isoflavone glucuronides needed for estrogenic activity. Toxicol In Vitro, 2015; 29(4):706–15. CrossRef
Jardí F, Kim N, Laurent MR, Khalil R, Deboel L, Schollaert D, van Lenthe GH, Decallonne B, Carmeliet G, Claessens F, Vanderschueren D. Androgen receptor in neurons slows age-related cortical thinning in male mice. J Bone Miner Res, 2019; 34(3):508–19. CrossRef
Jiang Y, Gong P, Madak-Erdogan Z, Martin T, Jeyakumar M, Carlson K, Khan I, Smillie TJ, Chittiboyina AG, Rotte SC, Helferich WG, Katzenellenbogen JA, Katzenellenbogen BS. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB J, 2013; 27(11):4406. CrossRef
Jiang X, Randhawa SB, Kagan R. Estrogen and estrogen analogs for prevention and treatment of osteoporosis. In: Marcus and feldman’s osteoporosis, Academic Press, Cambridge, MA, pp 1711–9, 2021. CrossRef
Jin Z, Li X, Wan Y. Minireview: nuclear receptor regulation of osteoclast and bone remodeling. Mol Endocrinol, 2015; 29(2):172–86. CrossRef
Jin X, Sun J, Yu B, Wang Y, Sun WJ, Yang J, Huang SH, Xie WL. Daidzein stimulates osteogenesis facilitating proliferation, differentiation, and antiapoptosis in human osteoblast-like MG-63 cells via estrogen receptor-dependent MEK/ERK and PI3K/Akt activation. Nutr Res, 2017; 42:20–30. CrossRef
Kammerer M, Gutzwiller S, Stauffer D, Delhon I, Seltenmeyer Y, Fournier B. Estrogen receptor α (ERα) and estrogen related receptor α (ERRα) are both transcriptional regulators of the Runx2-I isoform. Mol Cell Endocrinol, 2013; 369(1–2):150–60. CrossRef
Kang F, Yi Q, Gu P, Dong Y, Zhang Z, Zhang L, Bai Y. Controlled growth factor delivery system with osteogenic-angiogenic coupling effect for bone regeneration. J Orthop Translat, 2021; 31:110–25. CrossRef
Kawano H, Sato T, Yamada T, Matsumoto T, Sekine K, Watanabe T, Nakamura T, Fukuda T, Yoshimura K, Yoshizawa T, Aihara KI. Suppressive function of androgen receptor in bone resorption. Proc Natl Acad Sci, 2003; 100(16):9416–21. CrossRef
Kenkre JS, Bassett JHD. The bone remodelling cycle. Ann Clin Biochem, 2018; 55(3):308–27. CrossRef
Khalid AB, Krum SA. Estrogen receptors alpha and beta in bone. Bone, 2016; 87:130–5. CrossRef
Khan MZ, Uzair M, Nazli A, Chen JZ. An overview on estrogen receptors signaling and its ligands in breast cancer. Eur J Med Chem, 2022; 8:114658. CrossRef
Kilu W, Merk D, Steinhilber D, Proschak E, Heering J. Heterodimer formation with retinoic acid receptor RXRα modulates coactivator recruitment by peroxisome proliferator-activated receptor PPARγ. J Biol Chem, 2021; 297(1):100814. CrossRef
Kim IS. Current perspectives on the beneficial effects of soybean isoflavones and their metabolites for humans. Antioxidants, 2021; 10(7):1064. CrossRef
Kim HJ, Kim BK, Ohk B, Yoon HJ, Kang WY, Cho S, Seong SJ, Lee HW, Yoon YR. Estrogen-related receptor γ negatively regulates osteoclastogenesis and protects against inflammatory bone loss. J Cell Physiol, 2019; 234(2):1659–70. CrossRef
Kim B, Koh J. Coupling factors involved in preserving bone balance. CellMol Life Sci, 2019; 76(7):1243–53. CrossRef
Kim YH, Nam GE, Cho KH, Choi YS, Kim SM, Han BD, Han KD, Lee KS, Park CH, Kim DH. Low bone mineral density is associated with dyslipidemia in South Korean men: the 2008-2010 Korean national health and nutrition examination survey. Endocr J, 2013; 60(10):1179–89. CrossRef
Kim H, Oh B, Park-Min KH. Regulation of osteoclast differentiation and activity by lipid metabolism. Cells, 2021; 10(1):89. CrossRef
Kim SI, Park SH, Na W, Shin YC, Oh MS, Sim YE, Zheng Y, Kim AH, Kang IJ, Kang YH. Dietary collagen hydrolysates retard estrogen deficiency-induced bone loss through blocking osteoclastic activation and enhancing osteoblastic matrix mineralization. Biomedicines, 2022; 10(6):1382. CrossRef
Kitamura K, Erlangga JS, Tsukamoto S, Sakamoto Y, Mabashi-Asazuma H, Iida K. Daidzein promotes the expression of oxidative phosphorylation- and fatty acid oxidation-related genes via an estrogen-related receptor α pathway to decrease lipid accumulation in muscle cells. J Nutr Biochem, 2020; 77:108315. CrossRef
Kretzschmar G, Zierau O, Wober J, Tischer S, Metz P, Vollmer G. Prenylation has a compound specific effect on the estrogenicity of naringenin and genistein. J Steroid Biochem Mol Biol, 2010; 118:1–6. CrossRef
K?ížová L, Dadáková K, Kašparovská J, Kašparovský T. Isoflavones. Molecules, 2019; 24(6):1076. CrossRef
Kuwahara M, Akasaki Y, Goto N, Kurakazu I, Sueishi T, Toya M, Uchida T, Tsutsui T, Hirose R, Tsushima H, Nakashima Y. Fluvastatin promotes chondrogenic differentiation of adipose-derived mesenchymal stem cells by inducing bone morphogenetic protein 2. BMC Pharmacol Toxicol, 2022; 23(1):1–9. CrossRef
Laily WN, Wati DA, Ayu RN, Pratiwi AR. The correlation between consumption levels of isoflavones and fiber sources with HbA1c levels in patients with type 2 diabetes mellitus at Dr. H. Bob Bazar Hospital in South Lampung. J Kedokteran dan Kesehatan: Publikasi Ilmiah Fakultas Kedokteran Univ Sriwijaya. 2022; 9(2). CrossRef
Lan KC, Wei KT, Lin PW, Lin CC, Won PL, Liu YF, Chen YJ, Cheng BH, Chu TM, Chen JF, Huang KE, Chang CE, Kang HY. Targeted activation of androgen receptor signaling in the periosteum improves bone fracture repair. Cell Death Dis, 2022; 13(2):1–3. CrossRef
Lee HR, Jeung EB, Cho MH, Kim TH, Leung PC, Choi KC. Molecular mechanism (s) of endocrine-disrupting chemicals and their potent oestrogenicity in diverse cells and tissues that express oestrogen receptors. J Cell Mol Med, 2013; 17(1):1. CrossRef
Lee HS, Lee TH, Lee DH, Yun BS, Lee KW, Kim JS, Goo YT, Kim JH. Evaluation of estrogen receptor agonistic activity of medicinal herbs using organization for economic cooperation and development transactivation assay with rat liver S9 fraction. J Med Food, 2021; 24(12):1285–92. CrossRef
Lee HS, Park T. Nuclear receptor and VEGF pathways for gene-blood lead interactions, on bone mineral density, in Korean smokers. PLoS One, 2018; 13(3):e0193323. CrossRef
Levine PM, Garabedian MJ, Kirshenbaum K. Targeting the androgen receptor with steroid conjugates. J Med Chem, 2014; 57(20):8224–37 CrossRef
Li Y, Jin D, Xie W, Wen L, Chen W, Xu J, Ding J, Ren D. PPAR-γ and Wnt regulate the differentiation of MSCs into adipocytes and osteoblasts respectively. Curr Stem Cell Res Ther, 2018; 13(3):185–92. CrossRef
Li X, Ning L, Ma J, Xie Z, Zhao X, Wang G, Wan X, Qiu P, Yao T, Wang H, Fan S, Wan S. The PPAR-γ antagonist T007 inhibits RANKL-induced osteoclastogenesis and counteracts OVX-induced bone loss in mice. Cell Commun Signal, 2019a; 17(1):136. CrossRef
Li R, Robinson M, Ding X, Geetha T, Al-Nakkash L, Broderick TL, Babu JR. Genistein: a focus on several neurodegenerative diseases. J Food Biochem, 2022a; 46(7):e14155. CrossRef
Li F, Song C, Zhang Y, Wu D. Structural overview and perspectives of the nuclear receptors, a major family as the direct targets for small-molecule drugs. Acta Biochim Biophys Sin (Shanghai), 2022b; 54(1):1–13.
Li XL, Sui L, Lin FH, Lian Y, Ai LZ, Zhang Y. Differential effects of genistein and 8-prenylgenistein on reproductive tissues in immature female mice. Pharm Biol, 2019b; 57(1):226–30. CrossRef
Li Y, Zhang R, Ren M, Liu H, Yang M. Experimental study on the effects of simvastatin in reversing the femoral metaphyseal defects induced by sodium valproate in normal and ovariectomized rats. Heliyon, 2022c; 8(9):e10480. CrossRef
Lin HF, Liao KF, Chang CM, Lin CL, Lai SW, Hsu CY. Correlation of the tamoxifen use with the increased risk of deep vein thrombosis and pulmonary embolism in elderly women with breast cancer: a case–control study. Medicine, 2018; 97(51):e12842. CrossRef
Liu C, Feng T, Zhu N, Liu P, Han X, Chen M, Wang X, Li N, Li Y, Xu Y, Si S. Identification of a novel selective agonist of PPARγ with no promotion of adipogenesis and less inhibition of osteoblastogenesis. Sci Rep, 2015; 5(1):1–3. CrossRef
Lombardi G, Delvin E. Micro-RNA: a future approach to personalized diagnosis of bone diseases. Calcif Tissue Int, 2022; 19:1–7. CrossRef
Ma X, Wang D, Zhao W, Xu L. Deciphering the roles of PPARγ in adipocytes via dynamic change of transcription complex. Front Endocrinol, 2018; 9:473. CrossRef
Macías I, Alcorta-Sevillano N, Infante A, Rodríguez CI. Cutting edge endogenous promoting and exogenous driven strategies for bone regeneration. Int J Mol Sci, 2021; 22(14):7724. CrossRef
Mahmoud AM, Martin IK, Schlicht MJ, Nonn L, Bosland MC. Differential effects of the isoflavone genistein on androgen receptor expression and cell proliferation comparing prostate cancer cells with mutant and wild type androgen receptor. Cancer Res, 2011; 71(8_Supplement):1864. CrossRef
Makar S, Saha T, Swetha R, Gutti G, Kumar A, Singh SK. Rational approaches of drug design for the development of selective estrogen receptor modulators (SERMs), implicated in breast cancer. Bioorg Chem, 2020; 94:103380. CrossRef
Maldonado-Rojas W, Salinas-Torres J, Olivero-Verbel J. Identification of potential human protein targets for soybean isoflavones. J Braz Chem Soc, 2021; 32:767–76. CrossRef
Manayi A. Soybeans and phytoestrogen rich foods (Genistein, Daidzein) against cancer. In: Nutraceuticals and cancer signaling, Springer, Cham, Switzerland, pp 419–49, 2021. CrossRef
Marciano DP, Kuruvilla DS, Boregowda SV, Asteian A, Hughes TS, Garcia-Ordonez R, Corzo CA, Khan TM, Novick SJ, Park H, Kojetin DJ. Pharmacological repression of PPARγ promotes osteogenesis. Nat Commun, 2015; 6(1):1–7. CrossRef
Martinkovich S, Shah D, Planey SL, Arnott JA. Selective estrogen receptor modulators: tissue specificity and clinical utility. Clin Interv Aging, 2014; 9:1437. CrossRef
Mayo B, Vázquez L, Flórez AB. Equol: a bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients, 2019; 11(9):2231. CrossRef
Mbachu OC, Howell C, Simmler C, Malca Garcia GR, Skowron KJ, Dong H, Ellis SG, Hitzman RT, Hajirahimkhan A, Chen SN, Nikolic D, Moore TW, Vollmer G, Pauli GF, Bolton JL, Dietz BM. SAR study on estrogen receptor α/β activity of (iso)flavonoids: importance of prenylation, C-ring (un)saturation, and hydroxyl substituents. J Agric Food Chem, 2020; 68(39):10651–63. CrossRef
Mehedintu C, Carp-Veliscu A, Edu A, Plotogea M, Petca A, Andreescu CV, Secara D, Dumitrascu M, Rotaru AM. Non-hormonal management for menopause. Rom J Med Pract, 2021; 16(6):82. CrossRef
Melville KM, Kelly NH, Khan SA, Schimenti JC, Ross FP, Main RP, van der Meulen MC. Female mice lacking estrogen receptor-alpha in osteoblasts have compromised bone mass and strength. J Bone Miner Res, 2014; 29(2):370–9. CrossRef
Melville KM, Kelly NH, Surita G, Buchalter DB, Schimenti JC, Main RP, Ross FP, van der Meulen MC. Effects of deletion of ERα in osteoblast-lineage cells on bone mass and adaptation to mechanical loading differ in female and male mice. J Bone Miner Res, 2015; 30(8):1468–80. CrossRef
Ming LG, Ge BF, Wang MG, Chen KM. Comparison between 8-prenylnarigenin and narigenin concerning their activities on promotion of rat bone marrow stromal cells’ osteogenic differentiation in vitro. Cell Prolif, 2012; 45:508–15. CrossRef
Morito K, Aomori T, Hirose T, Kinjo J, Hasegawa J, Ogawa S, Inoue S, Muramatsu M, Masamune Y. Interaction of phytoestrogens with estrogen receptors α and β (II). Biol Pharm Bull, 2002; 25(1):48–52. CrossRef
Murphy C, Deplazes E, Cranfield CG, Garcia A. The role of structure and biophysical properties in the pleiotropic effects of statins. Int J Mol Sci, 2020; 21(22):8745. CrossRef
Muruganandan S, Ionescu AM, Sinal CJ. At the crossroads of the adipocyte and osteoclast differentiation programs: future therapeutic perspectives. Int J Mol Sci, 2020; 21(7):2277. CrossRef
Mutha RE, Tatiya AU, Surana SJ. Flavonoids as natural phenolic compounds and their role in therapeutics: an overview. Future J Pharm Sci, 2021; 7(1):1–3. CrossRef
Nagai N, Ogata F, Otake H, Nakazawa Y, Kawasaki N. Design of a transdermal formulation containing raloxifene nanoparticles for osteoporosis treatment. Int J Nanomedicine, 2018; 13:5215. CrossRef
Nicks KM, Fujita K, Fraser D, McGregor U, Drake MT, McGee-Lawrence ME, Westendorf JJ, Monroe DG, Khosla S. Deletion of estrogen receptor beta in osteoprogenitor cells increases trabecular but not cortical bone mass in female mice. J Bone Miner Res, 2016; 31(3):606–14. CrossRef
Nishide Y, Tadaishi M, Kobori M, Tousen Y, Kato M, Inada M, Miyaura C, Ishimi Y. Possible role of S-equol on bone loss via amelioration of inflammatory indices in ovariectomized mice. J Clin Biochem Nutr, 2013; 53(1):41–8. CrossRef
Notini AJ, McManus JF, Moore A, Bouxsein M, Jimenez M, Chiu WS, Glatt V, Kream BE, Handelsman DJ, Morris HA, Zajac JD, Davey RA. Osteoblast deletion of exon 3 of the androgen receptor gene results in trabecular bone loss in adult male mice. J Bone Miner Res, 2007; 22(3):347–56. CrossRef
Palaniappan M, Nguyen L, Grimm SL, Xi Y, Xia Z, Li W, Coarfa C. The genomic landscape of estrogen receptor α binding sites in mouse mammary gland. PLoS One, 2019; 14(8):e0220311. CrossRef
Palomer X, Barroso E, Pizarro-Delgado J, Peña L, Botteri G, Zarei M, Aguilar D, Montori-Grau M, Vázquez-Carrera M. PPARβ/δ: a key therapeutic target in metabolic disorders. Int J Mol Sci, 2018; 19(3):913. CrossRef
Papageorgiou L, Shalzi L, Pierouli K, Papakonstantinou E, Manias S, Dragoumani K, Nicolaides NC, Giannakakis A, Bacopoulou F, Chrousos GP, Eliopoulos E. An updated evolutionary study of the nuclear receptor protein family. World Acad Sci J, 2021; 3(6):1–8. CrossRef
Park K, Ju WC, Yeo JH, Kim JY, Seo HS, Uchida Y, Cho Y. Increased OPG/RANKL ratio in the conditioned medium of soybean-treated osteoblasts suppresses RANKL-induced osteoclast differentiation. Int J Mol Med, 2014; 33(1):178–84. CrossRef
Pawlowski JW, Martin BR, McCabe GP, McCabe L, Jackson GS, Peacock M, Barnes S, Weaver CM. Impact of equol-producing capacity and soy-isoflavone profiles of supplements on bone calcium retention in postmenopausal women: a randomized crossover trial. Am J Clin Nutr, 2015; 102(3):695–703. CrossRef
Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. J Steroid Biochem Mol, 2014; 142:142–54. CrossRef
Puranik NV, Srivastava P, Bhatt G, John Mary DJ, Limaye AM, Sivaraman J. Determination and analysis of agonist and antagonist potential of naturally occurring flavonoids for estrogen receptor (ERα) by various parameters and molecular modelling approach. Sci Rep, 2019; 9(1):1. CrossRef
Rana K, Davey RA, Zajac JD. Human androgen deficiency: insights gained from androgen receptor knockout mouse models. Asian J Androl, 2014; 16(2):169–77. CrossRef
Ranhotra, HS. The estrogen-related receptors in metabolism and cancer: newer insights. J Recept Signal Transduct Res, 2018; 38(2):95–100. CrossRef
Rocha RF, Rodrigues T, Menegatti AC, Bernardes GJ, Terenzi H. The antidiabetic drug lobeglitazone has the potential to inhibit PTP1B activity. Bioorg Chem, 2020; 100:103927. CrossRef
Rosati L, Falvo S, Chieffi Baccari G, Santillo A, Di Fiore MM. The aromatase–estrogen system in the testes of non-mammalian vertebrates. Animals, 2021; 11(6):1763. CrossRef
Russell PK, Clarke MV, Skinner JP, Pang TP, Zajac JD, Davey RA. Identification of gene pathways altered by deletion of the androgen receptor specifically in mineralizing osteoblasts and osteocytes in mice. J Mol Endocrinol, 2012; 49(1):1. CrossRef
Sathyapalan T, Aye M, Rigby AS, Thatcher NJ, Dargham SR, Kilpatrick ES, Atkin SL. Soy isoflavones improve cardiovascular disease risk markers in women during the early menopause. Nutr Metab Cardiovasc Dis, 2018; 28(7):691–7. CrossRef
Sebo ZL, Rodeheffer MS. Testosterone metabolites differentially regulate obesogenesis and fat distribution. Mol Metab, 2021; 44:101141. CrossRef
Sims NA, Clément-Lacroix P, Minet D, Fraslon-Vanhulle C, Gaillard-Kelly M, Resche-Rigon M, Baron R. A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor–deficient mice. J Clin Investig, 2003; 111:1319–27. CrossRef
Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, Resche-Rigon M, Gaillard-Kelly M, Baron R. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-β in bone remodeling in females but not in males. Bone, 2002; 30(1):18–25. CrossRef
Sinnesael M, Claessens F, Laurent M, Dubois V, Boonen S, Deboel L, Vanderschueren D. Androgen receptor (AR) in osteocytes is important for the maintenance of male skeletal integrity: evidence from targeted AR disruption in mouse osteocytes. J Bone Miner Res, 2012; 12:2535–43. CrossRef
Sinnesael M, Jardi F, Deboel L, Laurent MR, Dubois V, Zajac JD, Davey RA, Carmeliet G, Claessens F, Vanderschueren D. The androgen receptor has no direct antiresorptive actions in mouse osteoclasts. Mol Cell Endocrinol, 2015; 411:198–206. CrossRef
Sivo?ová MK, Kaplán P, Tatarková Z, Lichardusová L, Dušenka R, Jure?eková J. Androgen receptor and soy isoflavones in prostate cancer. Mol Clin Oncol, 2019; 10(2):191–204.
Slana? O, Hronová K, Bartošová O, Šíma M. Recent advances in the personalized treatment of estrogen receptor-positive breast cancer with tamoxifen: a focus on pharmacogenomics. Expert Opin Drug Metab Toxicol, 2021; 17(3):307–21. CrossRef
Solomon ZJ, Mirabal JR, Mazur DJ, Kohn TP, Lipshultz LI, Pastuszak AW. Selective androgen receptor modulators: current knowledge and clinical applications. Sex Med Rev, 2019; 7(1):84–94. CrossRef
Stanis?awska IJ, Figat R, Kiss AK, Bobrowska-Korczak B. Essential elements and isoflavonoids in the prevention of prostate cancer. Nutrients, 2022; 14(6):1225. CrossRef
Suetsugi M, Su L, Karlsberg K, Yuan YC, Chen S. Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol Cancer Res, 2003; 1(13):981–91.
Tanida T. Molecular dynamics of estrogen-related receptors and their regulatory proteins: roles in transcriptional control for endocrine and metabolic signaling. Anat Sci Int, 2022; 97:15–29. CrossRef
Terkawi MA, Matsumae G, Shimizu T, Takahashi D, Kadoya K, Iwasaki N. Interplay between inflammation and pathological bone resorption: insights into recent mechanisms and pathways in related diseases for future perspectives. Int J Mol Sci, 2022; 23(3):1786. CrossRef
Tomlinson B, Chan P, Lam CW. An overview of alogliptin+ pioglitazone for the treatment of type 2 diabetes. Expert Opin Pharmacother, 2022; 23(1):29–42. CrossRef
Tousen Y, Ishiwata H, Ishimi Y, Ikegami S. Equol, a metabolite of daidzein, is more efficient than daidzein for bone formation in growing female rats. Phytother Res, 2015; 29(9):1349–54. CrossRef
Wang X, Ha D, Yoshitake R, Chan YS, Sadava D, Chen S. Exploring the biological activity and mechanism of xenoestrogens and phytoestrogens in cancers: emerging methods and concepts. Int J Mol Sci, 2021; 22(16):8798. CrossRef
Wang L, Nanayakkara G, Yang Q, Tan H, Drummer C, Sun Y, Shao Y, Fu H, Cueto R, Shan H, Bottiglieri T. A comprehensive data mining study shows that most nuclear receptors act as newly proposed homeostasis-associated molecular pattern receptors. J Hematol Oncol, 2017; 10(1):1–41. CrossRef
Wang H, Wang J. Estrogen-related receptor alpha interacts cooperatively with peroxisome proliferator-activated receptor-gamma coactivator-1alpha to regulate osteocalcin gene expression. Cell Biol Int, 2013; 37(11):1259–65. CrossRef
Wang J, Xu J, Wang B, Shu FR, Chen K, Mi MT. Equol promotes rat osteoblast proliferation and differentiation through activating estrogen receptor. Genet Mol Res, 2014; 13(3):5055–63. CrossRef
Wei W, Schwaid AG, Wang X, Wang X, Chen S, Chu Q, Saghatelian A, Wan Y. Ligand activation of ERRα by cholesterol mediates statin and bisphosphonate effects. Cell Metab, 2016; 23(3):479–91. CrossRef
Wei W, Wan Y. Thiazolidinediones on PPARγ: the roles in bone remodeling. PPAR Res, 2011; 2011:867180. CrossRef
Weivoda MM, Chew CK, Monroe DG, Farr JN, Atkinson EJ, Geske JR, Eckhardt B, Thicke B, Ruan M, Tweed AJ, McCready LK. Identification of osteoclast-osteoblast coupling factors in humans reveals links between bone and energy metabolism. Nat Commun, 2020; 11(1):1–3. CrossRef
Wu GJ, Chen JT, Cherng YG, Chang CC, Liu SH, Chen RM. Genistein improves bone healing via triggering estrogen receptor alpha-mediated expressions of osteogenesis-associated genes and consequent maturation of osteoblasts. J Agric Food Chem, 2020; 68(39):10639–50. CrossRef
Wu J, Henning P, Sjögren K, Koskela A, Tuukkanen J, Movérare-Skrtic S, Ohlsson C. The androgen receptor is required for maintenance of bone mass in adult male mice. Mol Cell Endocrinol, 2019; 479:159–69. CrossRef
Xie Y, Tian Y, Zhang Y, Zhang Z, Chen R, Li M, Tang J, Bian J, Li Z, Xu X. Overview of the development of selective androgen receptor modulators (SARMs) as pharmacological treatment for osteoporosis (1998–2021). Eur J Med Chem, 2022; 12:114119. CrossRef
Yang Y, Liu G, Zhang Y, Xu G, Yi X, Liang J, Zhao C, Liang J, Ma C, Ye Y, Yu M, Qu X. Association between bone mineral density, bone turnover markers, and serum cholesterol levels in type 2 diabetes. Front Endocrinol (Lausanne), 2018; 9:646. CrossRef
Yang D, Wan Y. Molecular determinants for the polarization of macrophage and osteoclast. Semin Immunopathol, 2019; 41(5):551–63. CrossRef
Zhang L, Liu Q, Zeng X, Gao W, Niu Y, Ma X, Xie H, Zhou X, Yu W, Xu G. Association of dyslipidaemia with osteoporosis in postmenopausal women. J Int Med Res, 2021; 49(3):300060521999555. CrossRef
Zhang Y, Zhou LP, Li XL, Zhao YJ, Ho MX, Qiu ZC, Zhao DF, Mok DK, Shi Q, Wang YJ, Wong MS. 8-Prenylgenistein, a prenylated genistein derivative, exerted tissue selective osteoprotective effects in ovariectomized mice. Oncotarget, 2018; 9(36):24221–36. CrossRef
Zheng J, Brion MJ, Kemp JP, Warrington NM, Borges MC, Hemani G, Richardson TG, Rasheed H, Qiao Z, Haycock P, Ala-Korpela M, Davey Smith G, Tobias JH, Evans DM. The effect of plasma lipids and lipid-lowering interventions on bone mineral density: a mendelian randomization study. J Bone Miner Res, 2020; 35(7):1224–35. CrossRef
Zheng X, Lee SK, Chun OK. Soy isoflavones and osteoporotic bone loss: a review with an emphasis on modulation of bone remodeling. J Med Food, 2016; 19(1):1–4. CrossRef
Zhou T, Gai Z, Gao X, Li L. The potential mechanism of exercise combined with natural extracts to prevent and treat postmenopausal osteoporosis. J Healthc Eng, 2021; 2021:2852661. CrossRef
Zou W, Rohatgi N, Chen TH, Schilling J, Abu-Amer Y, Teitelbaum SL. PPAR-γ regulates pharmacological but not physiological or pathological osteoclast formation. Nat Med, 2016; 22(11):1203–5. CrossRef
Zuo H, Wan Y. Nuclear receptors in skeletal homeostasis. Curr Top Dev Biol, 2017; 125:71–107. CrossRef