INTRODUCTION
Delivering drugs to the brain remained a challenge until recently when carbon nanotube (CNT) came up with a modification on the surface. These nanostructures in almost single dimension are delivering way out to several challenges in delivering drugs to the brain, crossing the tight barrier. Carbon-based nanostructures are becoming increasingly relevant in the field of neuroscience owing to their many exclusive chemical and physical properties. Recently, CNT based drug delivery has spawned great interest in medicine delivery and therapeutics, where significant modification of CNT helped in vaccine delivery systems (Bianco and Prato, 2003) as well as protein transporters (Kam et al., 2005) apart from being drug nano-carriers. Besides other nanomaterials, CNTs have been widely used in pharmaceutical and biomedical applications such as task-specific drug transport, cancer therapy and diagnosis, imaging, and or tissue engineering. While CNTs are used as drug delivery carriers, the nanotube aids in encapsulating drugs in its hollow tube-like structure. This is so since the drugs can be bound in their inner hollow area, whereas other molecules can be fabricated to the peripheral face to render them biocompatible while targeting the site (Martincic and Tobias, 2015).
Nevertheless, it is highly desirable that the drug transporter molecule must be non-immunogenic, non-toxic, have places for attachment of diagnostic or remedial agents, be electronically or spectroscopically accessible, as well as not exhibit long-term in vivo piling in vital organs. Since the remarkable breakthrough of CNTs by Iijima (1991), this particular structure in the nano range has exhibited valuable properties matching the above requirements for being ultra-lightweight, low deposition, highly flexible, high aspect ratio, inert with thermal and electrical conductivity, high penetrability, ultra-strong, and multiple attachable sites, ability to get surface modified, to name a few. These attributes along with many more have attracted scientists around the globe to seek its application in the healthcare and diagnostic arena. Approximately three decades of advances in carbon nanotechnology have divulged exciting perceptions and pioneering tactics in tissue regeneration and lately for nerve tissue healing (Dvir et al., 2011; Place et al., 2009). As a result, eventually, CNT is emerging as new promising aid for the management of neurological ailments like Parkinson’s disease, Alzheimer’s disease (AD), and ischemic stroke (Folch et al., 2016; Kakkar and Dahia, 2015; Komane et al., 2016). Effective application of CNTs in delivering drugs in vivo has been reported in many diseases and disorders like rheumatoid arthritis, osteoporosis, bone implants, and cancer (Malarkey and Parpura, 2007). Nonetheless, for the successful application of CNTs in neurological disorders, very limited preclinical studies have been performed (Fabbro et al., 2013). Investigations on fullerene derivatives have shown a promising role of this as neuroprotective agents (Dugan et al., 2001). For instance, reports for central nervous system (CNS) protection in rats against chronic alcohol were rendered by nanostructures of hydrated C60 fullerene (C60HyFn) (Dugan et al., 1997). Additionally, neurotransmitter metabolism has been suggested by the indirect participation of C60HyFn in the same report. There are several studies that have demonstrated that multiple synergistic mechanisms are offered by fullerene derivatives that can be employed for AD treatment (Kerna et al., 2020; Podolski et al., 2007; Tykhomyrov et al., 2008; Vorobyov et al., 2015). Because of their exceptional physical properties added to the recently discovered interface capacity with neuronal circuits and membranes, synapses, CNT-based techniques are deemed very appealing to enhance neuron healing after brain injury (Fabbro et al., 2013).
It is an established fact that aqueous solutions do not dissolve CNTs in their natural or unaltered state. Hence, the requirement of surface modification is an alternative to handle this kind of issue. Surface functionalization and chemical modification improve aqueous solubility, making them more useful for treating neurological diseases (Georgakilas et al., 2002). Because of its very high aspect ratio, studies have shown that the use of pure CNTs causes an inflammatory response along with a loss in cell viability. Furthermore, CNT aggregation caused by van der Waals interactions has a negative impact on cellular responses and causes pulmonary toxicity in vivo (Fisher et al., 2012; Liu et al., 2013). The above-mentioned limitations can be addressed through chemical modification. As a result, many of the neuro-therapeutic compounds never reach the market since they are unable to cross the blood–brain barrier (BBB) (Bicker et al., 2014). The growth configuration of neurons on as-grown functionalized multi-walled nanotubes has been investigated by Mattson et al. (2000) which came up with encouraging reports. Later, Pantarotto et al. (2004) in another study revealed that the internalization of nanotubes with fluorescent tagging had no obvious toxicity effects on cells; however, the uptake mechanism could not be identified. Paracellular transport across the BBB tight junctions can be improved via the functionalization of CNTs since these tight barriers allow certain particles only in the range of <20 nm (Chenthamara et al., 2019). CNTs offered a wide variety of unlike molecules that could be decorated onto the surface of nanotubes, as illustrated in Figure 1. Hence, a possibility of an easily customized way of ferrying molecules into cells in a smart way. Therapeutic and biomedical uses of CNTs in vitro and in vivo for brain cells-oriented disease treatments have seen modern advances so far (Xiang et al., 2020).
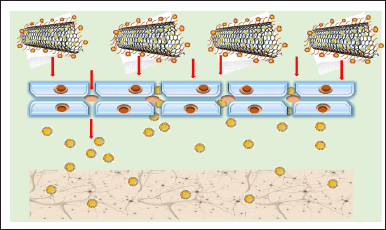 | Figure 1. Functionalized CNTs allow the passage of drugs via tight barriers to the brain cells. [Click here to view] |
Studies have revealed that if the size of a particle is less than 100 nm, it gets easily transferred through BBB (Costa et al., 2004). Thus, nanostructures that can be brain-targeted as a part of the therapeutic approach have led to improved brain delivery. CNTs are used as a nano-carrier in treating CNS disease for their distinct physical and chemical properties, which include large surface area, better solubility in physiological solvents for its nature to functionalize, unique carrying ability of drug molecules, and biocompatibility with the neural system (John et al., 2015). The permeability of antitumor medication molecules through the BBB is extremely poor, which has a detrimental influence on brain tumor therapy. A patent in this regard was filed by BRAINGUARD CO Ltd. wherein a thorough discussion and implementation of CNT could be made for prophylactic or treatment of any diseases of the brain (Jeong et al., 2011).
CNTS FOR GLIOBLASTOMA
Intrinsically, CNTs are hydrophobic in nature. As such this keeps them agglomerated and is one of the limitations in cellular uptake and or penetration. Surface functionalization of these nanostructures resolves this issue to a great extent. Conjugated CNT with cytosine–guanosine dinucleotide (CpG), short single-stranded synthetic DNA molecules) has established counteraction on the immunosuppressive setting when tested in intracranial GL261 gliomas in tumor-bearing mice. Hence, low doses of CpG-modified CNTs could boost immunity against tumors and also eliminate gliomas (malignant brain tumors) at low doses (Zhao et al., 2011). In another study, polyethylene glycosylated (PEGylated) multi-walled CNTs were surface modified with Angiopep-2 (a peptide that shows a high brain penetration capability) to overcome the BBB. Such surface-decorated CNTs were capable of distributing in brain cells and later get accumulated in the target tumor. The nature of CNTs offered exceptionally high surface area and significantly high loading capacity of doxorubicin, an anticancer drug. This functionalized CNT was able to deliver drugs to the cancer-affected cells of the brain after crossing the BBB (Ren et al., 2012).
Poor penetration and low permeability of delivery systems across the BBB and into the tumor tissue limits the therapeutic effect of glioma. In an attempt to overcome these two barriers, several proposals were made where Angiopep-conjugated drug-loaded CNTs could target glioma treatment. This assembly successfully targeted the glioma cells by traversing across BBB typically through lipoprotein receptor-related protein receptor-mediated trans-cytosis (Kafa et al., 2016).
In another study, –COOH functionalized single-walled carbon nanotube (SWCNT) had levodopa attached to the carrier system. This could successfully be delivered to PC12 cells (cell line obtained from a pheochromocytoma of the adrenal medulla of rats) and evaluate its effect on normal neuronal cells in vitro. This was a pH-dependent release of levodopa (LD) and -COOH modified LD attached SWCNT (SWCNT-COOH-LD) that affected the cell viability of PC12 cells negatively (Tan et al., 2015). Here, the surface decorated CNTs-based drug delivery could successfully be delivered to the nervous system.
CNTS FOR ALZHEIMER DISEASE
More than 10% of the world’s population over the age of 65 years is affected by Alzheimer disease (AD). Owing to the low accuracy and or expensive neuroimaging and neuropsychological investigations, early diagnosis of AD is still confusing. CNTs are becoming a promising nano-engineered technology for biomedical applications (Dugan et al., 1997; Vorobyov et al., 2015; Wang et al., 2014). Kim et al. (2020) reported that clinically precise and ultra-sensitive detection of several AD main biomarkers like Aβ40, Aβ42, p-tau181, and t-tau can be done using densely linearly associated CNTs in the blood plasma of human beings. Low coefficient of variation, close to <6%, femtomolar-level limit of detection, and a high degree of recovery of more than 93.0% were obtained in a study with meticulously packed and unidirectional CNT sensor arrangement that demonstrated high precision, sensitivity, and accuracy. Evidence for the treatment of AD was obtained by coating berberine-loaded MWCNTs (multi-walled CNTs) with phospholipids and polysorbate. The produced optimum formulation had a particle size of 186 nm exhibiting 68.6% absorption of the drug along with 96% release of the drug within a span of 16 hours (Kim et al., 2020). The study revealed that from day 18 to day 20, specially coated MWCNTs showed tremendous improvement in the memory performance of the subjects by reducing amyloid-induced AD. This was established further by standard biochemical markers in brain soft tissue. Neurite outgrowth for therapeutic application in nerve regeneration was promoted when Li et al. (2017) created a neural scaffold based on uniformly dispersed MWCN-hydrogel nanowires with tunable structural porosity. The newly fabricated MWCNTs with a noticeable electrical spur showed potential efficacy in promoting neurite extension for nerve regeneration, according to the findings of Guo et al. (2017).
It is a well-known fact that autophagy is a manner by which a cell in its cytoplasm disrupts and destroys aged, broken, or atypical proteins and other substances. The metabolized products are then reprocessed for vital cell functions, specifically, during times of stress or starvation. It was for the first time that Xue et al. (2014) could establish that autophagy is noticeably weakened in primary glia from CRND8 mice and set that autophagy dysfunction and autophagic substrate clearance are upturned by SWNT with the possibility of improving autophagy in AD and suggested an innovative approach to reinstating normal autophagy activity when the lysosomal function is diminished. Another report in Alzheimer’s News Today by Forest Ray in 2020 claimed that blood tests for Alzheimer’s could be possible with a CNT sensor. This report revealed that surface-modified nanotubes were able to detect microscopic concentrations of Alzheimer’s main protein biomarkers in blood plasma, thereby discriminating Alzheimer’s patients from a healthy population with usual accuracy of 88.6%. Another similar work done by Kim et al. (2020) reported in Nature Communications, could identify multiple AD core biomarkers, t-tau/Aβ42, p-tau181/Aβ42, and Aβ42/Aβ40 in blood plasma quantitatively for AD patients. In this study, densely arrayed CNT sensors could selectively segregate Alzheimer’s patients from normal individuals with a usual sensitivity of around 90.0% and a selectivity of 90.0%. Some time back, Zhang et al. (2017) functionalized CNT fibers with tunable defects to act as micro-sensor for the quantitative detection of ascorbic acid levels in the brain of AD-induced rats. In this study, the measurement of oxidation of ascorbic acid served as an indirect way to establish high sensitivity and high selectivity for possible causes of interference in the brain, in studying the brain activity of AD-induced rats (Zhang et al., 2017). Interestingly, a thorough study by Yang et al. (2010) demonstrated that CNTs can pass through tight BBB for acetylcholine delivery into the brain cells of mice for AD treatment. While a safe dose of CNTs (single-walled) was 12 mg/kg, a mere 5 mg/kg Acetylcholine-loaded SWCNTs enhanced the memory and learning proficiency of the AD-induced mouse model (Yang et al., 2010). This study affirmed that Acetylcholine could be transported into the neuron of mice via CNT as a carrier.
CNTS TO TREAT PARKINSON’S DISORDER
Neurodegenerative illnesses of the CNS, such as Parkinson’s disease in addition to AD, have received a lot of attention in recent years as a major cause of morbidity across the world. Pathognomonic indications of Parkinson’s disease are the result of the death of dopaminergic cells in the substantia nigra, and hence the disparity between the cholinergic and dopaminergic systems (Stephenson et al., 2018). Chiefly, bradykinesia, tremor, stiffness, and postural instability are the four cardinal motor symptoms of Parkinson’s disease. It is a fact that conventional drugs for handling Parkinson’s disease have considerably curtailed the intensity of such symptoms thereby improving the quality of life of patients. Additionally, other medications like amantadine, biperiden and dopamine replacement therapy have been the origin of the treatment for a long. These drugs can help to reduce the progression of Parkinson’s disease, but stop it (Barka et al., 2013). Ceasing the administration of the right medication, instead, will result in a return of signs with augmented intensity. More diversified symptoms like cognitive impairment, psychiatric problems, and autonomic dysfunction occur as the illness advances, suggesting the presence of a more widespread underlying pathophysiology, demanding the expansion of state-of-the-art therapies (Dawson et al., 2018). Additionally, superparamagnetic iron oxide nanoparticles, gold nanoparticles, quantum dots (QDs), nanotube derivatives, and grapheme (More et al., 2015) have been identified as agents that can impact the amyloid formation process in various ways. Very recently, Alimohammadi et al. (2021) doped nanotubes with phosphorus-forming P-CNT that could prevent the α-synuclein amyloid formation (the chief cause of Parkinson’s disease). Remarkably, this study established that phosphorus-doped CNT prevented α-synuclein amyloid formation effectively hinting that this could be a possible treatment for Parkinson’s disease (Alimuhammadi et al., 2021).
CNTS AS IMAGING TOOL AND DRUG DELIVERY VEHICLES IN THE CENTRAL NERVOUS SYSTEM FOR DETECTION OF ISCHEMIC STROKE
CNT is coming up as a handy tool in tissue imaging for locating sick sites and delivering drugs to the site of action (He and Dai, 2004; Kostarelos et al., 2009). CNTs let imaging of the whole thick tissue and they improve visibility (Heller et al., 2006). CNTs are easily detectable due to their bulky resonance and increased Raman scattering characteristics (Heller et al., 2005). Position emission tomography, computer tomography, and magnetic resonance imaging are three imaging techniques that have helped researchers better understand how neural circuits work. Imaging is an important technique for studying both, biochemical and physiological activity in the CNS, particularly in the spinal cord and brain. Advances in technology have enhanced our acceptance of the effects of the cellular injury on the CNS. In modern days, the accuracy of neurological therapies has improved, as has the degree of invasiveness to the CNS (Nunes et al., 2012). Electroencephalography and magnetoencephalography are two modern CNS imaging methods. Conductors are put on the skull bone to measure electrical impulses emanating from the brain in these approaches. The skull is surgically opened to get access to the brain so that electrodes may be inserted to measure brain impulses directly. This procedure is quite intrusive, necessitating the use of a noninvasive alternative. Small interfering RNA (siRNA)-modified CNT was injected cortically into an endothelin-1-induced stroke rat model in a study. It was observed that the perceptive ability of stroke in rats increased markedly (Khuloud, 2015).
When combined with fluorescein probe-functionalized CNTs, established medicines like methotrexate have been shown to increase visibility in the body during its functioning. Figure 2 illustrates a schematic representation of the fact that when CNTs are further modified, they can reach the remote cells in the brain crossing the tight barrier, and thus this device can further be modified to be used as an imaging tool for determining the location of an ischemic stroke and treating it when tagged with suitable biomarkers. Because SWCNTs have better photostability than QDs and fluorophores, they can accomplish a longer excitation duration at a higher laser intensity (Vidu et al., 2014). This permits the perceptibility of the impervious tissue in the range of 700–1,400 nm. Regardless of certain merits, this technique renders a few demerits as well, such as limited sensitivity, difficulty to penetrate the BBB, and a shorter t1/2 (half-life) after intravenous delivery (Nunes et al., 2012). To aid in visualizing the sick spot within a tissue, miniaturized video cameras can be encapsulated in CNTs as well and delivered orally (Beg et al., 2011). In a limited 1.3–1.4 m zone, Hong and the team pioneered that non-invasive brain imaging could be made possible with CNT alignment (named the NIR-IIa region). In an epifluorescence imaging mode, this method permits penetration through the intact scalp and skull, resolving cerebral vasculatures with a previously unreachable spatial resolution of sub-10 micrometers at a depth of >2 mm beyond the surface of the scalp skin. Furthermore, high temporal resolution (200 ms/frame) dynamic NIR-IIa cerebrovascular imaging was employed to indicate significantly decreased blood flow (Hong et al., 2014). Thus, CNT has the potential to be employed as a non-invasive imaging technique in the treatment of neurological diseases.
Recently, stereotactic surgery is emerging as an invasive process to transfer medicines and other physiologically relevant substances into the brain within therapeutic settings. This approach provides the path for direct access to a specific brain region of interest (Jacob and Hanein, 2008; Jacobs et al., 2014). Delivery of nanodrug for neuroprotection in chronic neurological illnesses, such as ischemic stroke, is of high significance following this mode of drug administration. This indicated that the use of nanotechnology to deliver medications over the BBB is an upcoming possibility (Barker and TRANSEURO Consortium, 2019; Sharma et al., 2013). The ability of nano-technological developments for neurotrophin delivery systems to trigger neurotrophin signaling for neuro-protection and neuro-regeneration brings new hope to the medical fraternity (Tan et al., 2012). Established evidences reveal that CNTs can carry neurotrophins to their sites of action, which are critical for the formation and function of neurons (Bardi et al., 2013; Lin et al., 2009).
A decade afore, a CNT drug delivery system was employed (Iverson et al., 2013) to improve CpG oligo-deoxynucleotide (short single-stranded synthetic DNA molecules that contain a cytosine triphosphate deoxynucleotide) immunotherapy in the treatment of glioblastoma. Polyethylene glycol (PEG) was used to functionalize SWCNTs, which was then conjugated with a CpG oligonucleotide. Toll-like receptors (TLR) family members are intracellular receptors that identify carbohydrates, lipids, peptides produced by microbes, and nucleic acid structures. SWCNTs were conjugated with CpG. The study delineated that there was an augmentation of the acceptance of CpG in intracranial gliomas, in vivo. CpG inspired TLR in the glial cells to impede tumor development in glioma models (Zhau et al., 2011).
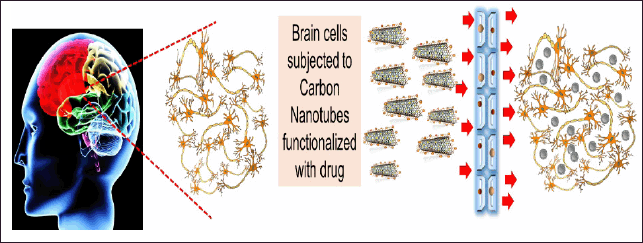 | Figure 2. Brain cells when exposed to surface modified CNTs, they can deliver the drug molecules, imaging elements or antibodies at the respective sites in the brain, crossing the tight junction. [Click here to view] |
CNT-based neurotherapy might be particularly beneficial in the treatment of a variety of neurological disorders, as well as ischemic stroke. SWNCNT decorated with amine groups through amidation reaction improves neuronal ischemia damage tolerance. Neurons are preserved from damage and their functions are restored without the use of therapeutics when amine-modified SWCNTs are used (Lee et al., 2011). In a mouse model with induced endothelin-1 stroke, Al-Jamal and coworkers established the efficiency of amine-MWCNTs in delivering small interfering RNA (siRNA) that reduced apoptosis at the wounded site and encouraged retrieval of functioning motor neurons (Al-Jamal et al., 2011).
CNT-BASED TUMOR-TARGETED DELIVERY SYSTEMS
Several studies have stated that surface-modified CNTs can navigate through cell membranes passively whereas, many such movements are done actively like phagocytosis or endocytosis (Cauduro et al., 2017; Li et al., 2017). Functionalization of CNTs with suitable ligands apart from single or multiple active agent(s) and or biomarkers enables these carriers to locate and get hooked to tumors or cancerous cells effectively (Kaur et al., 2017; Yang et al., 2017). (Lee et al 2011), established that the high superficial energy of the positively charged SWNTs provided a favorable environment. This, in other words, is a source for neuronal regeneration, wherein the neuroprotective effect was realized due to a reduction in apoptosis, inflammation, and glia activation (Lee et al., 2011). In another recent report, improved cell penetration in addition to cellular uptake of CNTs than free mangiferin allowed 55% apoptosis for conjugated mangiferin on a CNT-PEG platform when tested against 21% for plain mangiferin against the U-87 cells (Harsha et al., 2019).
The BBB is extremely selective, allowing only a few compounds in the bloodstream to enter the CNS (Guo et al., 2017; Lee et al., 2017). The existence of the BBB makes it difficult to transfer medications to the brain for the management of tumors and other neurological illnesses, such as stroke. It hinders therapeutic molecule distribution into the CNS, resulting in fewer than 1% of the supplied medicine being delivered to the CNS by intravenous injection (Bjartmarz and Rehncrona, 2017; Zhang et al., 2017). This careful selection of chemicals that enter the brain is critical for maintaining CNS homeostasis. It also protects the CNS against external invaders such as poisons, viruses, bacteria, and other undesired substances (Barka et al., 2019; Dawson et al., 2018; Ji et al., 2010; Zhang et al., 2017). In the future, neuroprotection might be accomplished in chronic neurological illnesses by using nano-drug delivery.
In both the CNS and the PNS, neurotrophins are required for the growth and activity of neurons. CNTs can be used to transport them into the brain. The usage of CNTs as a delivery tool for handling CNS pathology is established on structural features such as improved solubility in biological solvents due to surface modification, large surface area, capability to get easily adapted with drug molecules, and biocompatibility with neural systems (He and Dai, 2004). Soligo et al. (2021) confirmed that modification of MWCNTs leading to electro-conductivity could employ neuroprotectivity via variation of a prime neurotrophic agent and improve neurodegeneration-related gliosis.
Due to the extreme conditions laid by the BBB while reaching the brain cells, there are hardly a few FDA-approved CNT-mediated therapeutic agents for serious brain-related diseases like Parkinson’s Disease and AD (Pardridge, 2020). Interestingly, there are almost 10 smart drugs like Durvalumab, Ponatinib, Epitinib succinate, Bavituximab, Temozolomide with procarbazine and cilengitide, and many more that are approved by USFDA and are under various stages of clinical trials. Although there are multiple challenges like the adaptation of the formulation by large companies for commercial production, manufacturing cost, no consistency of data regarding toxicity tests, intra- and inter-batch variation, variation in in vivo–in vitro co-relationship and many more common men is eagerly waiting to adapt these extremely sophisticated and smart drugs once they are in the market (Kumar et al., 2021).
PHOTO THERMAL BRAIN TUMOR THERAPY
CNTs bear a unique property to absorb near-infrared (NIR) radiation. This property was utilized by Santos et al. (2014) to convert it to heat, thereby leading to photo-thermal-related brain tumor treatment. The team delivered a report and convinced many that a permutation of intra-tumoral SWNT insertion and NIR radiation in athymic glioblastoma-bearing mice not only diminished tumor growth relative to SWNTs but also suppressed tumor recurrence for up to 80 days (Santos et al., 2014). The anticancer activity of polyvinylpyrrolidone-coated (PVP-G) and SWCNTs were studied by Markovic et al. (2011). The study revealed that PVP-G had better photo-thermic sensitivity and imparted apoptotic and necrotic death in vitro by means of caspase activation/DNA fragmentation and cell membrane damage via partial thromboplastin time test (PTT) in U251 glioma cells (Markovic et al., 2011).
TOXICITY
It is a well-known fact that BBB offers restricted access to any particle that seems foreign material to get access to the brain. While finding an alternative to passing through this tight junction, contact with CNTs interrupts this delicate equilibrium resulting in cytotoxicity (Costa et al., 2004). So far, there have been no reports that consider brain toxicity to CNT being administered smartly. Interestingly, there are indications that approved CNTs are biodegraded inside human brain cells by human (myeloperoxidase) MPO and hydrogen peroxide (H2O2) present therein. In a study related to this fact, Kagan et al. (2010) confirmed via Raman spectroscopy that MPO stimulated SWNT to decompose into simpler substances via a pathway that supports the production of spontaneous hypochlorite that oxidizes parts of the CNT wall structure. A serious observation of this study was that PEG used as coating material (to amplify in vivo bioavailability) did not impede this process (Bhattacharya et al., 2014; Kagan et al., 2010).
CONCLUSION AND PROSPECTS
So far, there is enough evidence that CNTs have prospective use as medication delivery vehicles to targeted brain sites. Although functionalized CNTs bring a ray of hope in the smart delivery and access to the brain crossing the BBB, there is at present no conformity about the related animal models that could be utilized to consider the short- and long-term impact of CNT exposure to biological cells and tissues. Hence, it becomes vital that distinct procedures and guidelines can be provided by the right authority, so that the results and their understanding as well as analysis remain unaltered by dissimilarity in testing methods.
AUTHOR’S CONTRIBUTION
Rajkumar Ghosh and Jagabandhu Bag drafted the manuscript. Aparna Datta conceptualized and finally gave approval to the work. The figures were also contributed by her. Arup Pramanick contributed to the necessary corrections. Isa Hassan Abubakar made the final revision of the manuscript. The final draft was checked and agreed upon by all the authors.
FINANCIAL SUPPORT
This research received no external funding.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Alimohammadi E, Nikzad A, Khedri M, Rezaian M, Jahromi AM, Rezaei N, Maleki R. Potential treatment of Parkinson’s disease using new-generation carbon nanotubes: a biomolecular in silico study. Nanomedicine (Lond), 2021; 16(3):189–204. CrossRef
Al-Jamal KT, Gherardini L, Bardi G, Nunes A, Guo C, Bussy C, Herrero MA, Bianco A, Prato M, Kostarelos K, Pizzorusso T. Functional motor recovery from brain ischemic insult by carbon nanotube-mediated siRNA silencing. Proc Natl Acad Sci, 2011; 108(27):10952–7. CrossRef
Bardi G, Nunes A, Gherardini L, Bates K, Al-Jamal KT, Gaillard C, Prato M, Bianco A, Pizzorusso T, Kostarelos K. Functionalized carbon nanotubes in the brain: cellular internalization and neuroinflammatory responses. PLoS One, 2013; 8(11):e80964. CrossRef
Barker RA, TRANSEURO consortium. Designing stem-cell-based dopamine cell replacement trials for Parkinson’s disease. Nat Med, 2019; 25(1):1045–53. CrossRef
Beg S, Rizwan M, Sheikh AM, Hasnain MS, Anwer K, Kohli K. Advancement in carbon nanotubes: basics, biomedical applications and toxicity. J Pharm Pharmacol, 2011; 63(2):141–63. CrossRef
Ben-Jacob E, Hanein Y. Carbon nanotube microelectrodes for neuronal interfacing. J Mater Chem, 2008; 18(43):5181. CrossRef
Bhattacharya K, Sacchetti C, El-Sayed R, Fornara A, Kotchey GP, Gaugler JA, Star A, Bottini M, Fadeel B. Enzymatic ‘stripping’ and degradation of PEGylated carbonnanotubes. Nanoscale, 2014; 6(24):14686–90. CrossRef
Bianco A, Prato M. Can carbon nanotubes be considered useful tools for biological applications? Adv Mater Lett, 2003; 15(1):1765–8. CrossRef
Bicker J, Alves G, Fortuna A, Falcão A. Blood–brain barrier models and their relevance for a successful development of CNS drug delivery systems: a review. Eur J Pharm Biopharm, 2014; 87(3):409–32. CrossRef
Bjartmarz H, Rehncrona S. Comparison of accuracy and precision between frame-based and frameless stereotactic navigation for deep brain stimulation electrode implantation. Stereotact Funct Neurosurg, 2007; 85(5):235–42. CrossRef
Caoduro C, Hervouet E, Girard-Thernier C, Gharbi T, Boulahdour H, Delage-Mourroux R, Pudlo M. Carbon nanotubes as gene carriers: focus on internalization pathways related to functionalization and properties. ActaBiomater, 2017; 49(1):36–44. CrossRef
Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin FH, Qoronfleh MW. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res, 2019; 23;20. CrossRef
Costa PM, Bourgognon M, Wang JT, Al-Jamal KT. Functionalized carbon nanotubes: From intracellular uptake and cell-related toxicity to systemic brain delivery. J Control Release, 2004; 241(1):200–219. CrossRef
Dawson TM, Golde TE, Lagier-Tourenne C. Animal models of neurodegenerative diseases. Nat Neurosci, 2018; 21(10):1370–9. CrossRef
Dugan LL, Lovett EG, Quick KL, Lotharius J, Lin TT, O’Malley KL. Fullerene-based antioxidants and neurodegenerative disorders. Parkinson Relat Disord, 2001; 7(1):243–6. CrossRef
Dugan LL, Turetsky DM, Du C, Lobner D, Wheeler M, Almli CR, Shen CK, Luh TY, Choi DW, Lin TS. Carboxyfullerenes as neuroprotective agents. Proc Natl Acad Sci USA, 1997; 94(1):9434–9. CrossRef
Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol, 2011; 6(1):13–22. CrossRef
Fabbro A, Prato M, Ballerini L. Carbon nanotubes in neuroregeneration and repair. Adv Drug Deliv Rev, 2013; 65(1):2034–44. CrossRef
Fisher C, Rider AE, Han ZJ, Kumar S, Levchenko I, Ostrikov K. Application and nanotoxicity of carbon nanotubes and graphene in biomedicine. J Nanomater, 2012; 2012(1):315185. CrossRef
Folch J, Petrov D, Ettcheto M, Abad S, Sánchez-López E, García ML, Olloquequi J, Beas-Zarate C, Auladell C, Camins A. Current research therapeutic strategies for Alzheimer’s disease treatment. Neural Plasti, 2016; 8501693. CrossRef
Georgakilas V, Kordatos K, Prato M, Guldi DM, Holzinger M, Hirsch A. Organic functionalization of carbon nanotubes. J Am Chem Soc, 2002; 124(5):760–1. CrossRef
Guo Q, Shen XT, Li YY, Xu SQ. Carbon nanotubes-based drug delivery to cancer and brain. J Huazhong Univ Sci Technolog Med Sci, 2017; 37(5):635–41. CrossRef
Harsha P, Thotakura N, Kumar M, Sharma S, Mittal A, Khurana RK, Singh B, Negi P, Raza K. A novel PEGylatedcarbon nanotube conjugated mangiferin: an explorative nanomedicine for brain cancer cells. J Drug DelivSciTechnol, 2019; 53(1):101186. CrossRef
He P, Dai L. Aligned carbon nanotube-DNA electrochemical sensors. Chemcomm, 2004; 10(3):348–9. CrossRef
Heller DA, Baik S, Eurell TE, Strano MS. Single walled carbon nanotube spectroscopy in live cells: towards long-term labels and optical sensors. Adv Mater Lett, 2005; 17(23):2793–9. CrossRef
Heller DA, Jeng ES, Yeung TK, Martinez BM, Moll AE, Gastala JB, Strano MS. Optical detection of DNA conformational polymorphism on single-walled carbon nanotubes. Science, 2006; 311(5760):508–11. CrossRef
Hong G, Diao S, Chang J, Antaris AL, Chen C, Zhang B, Zhao S, Atochin DN, Huang PL, Andreasson KI, Kuo CJ, Dai H. Through-skull fluorescence imaging of the brain in a new near-infrared window. Nat Photonics, 2014; 8(9):723–30. CrossRef
Iijima S. Helical microtubules of graphitic carbon. Nature, 1991; 354(1):56–8. CrossRef
Iverson NM, Barone PW, Shandell M, Trudel LJ, Sen S, Sen F, Ivanov V, Atolia E, Farias E, McNicholas TP, Reuel N, Parry NMA, Wogan GN, Strano MS. In vivo biosensing via tissue-localizable near-infrared-fluorescent single walled carbon nanotubes. Nat Nanotechnol, 2013; 8(11):873–80. CrossRef
Jacobs CB, Ivanov IN, Nguyen MD, Zestos AG, Venton BJ. High temporal resolution measurements of dopamine with carbon nanotube yarn microelectrodes. Anal Chem, 2014; 86(12):5721–7. CrossRef
Jeong Y, Lee HJ, Lee DY, Noh YH, Do Hee KI, Kim OH, Park JA, Jiwon LE, inventors. BRAINGUARD CO Ltd. Use of carbon nanotubes for preventing or treating brain disease. European Patent EP2594289A4. 2011-07-14.
Ji S, Liu C, Zhang B, Yang F, Xu J, Long J, Jin C, Fu D, Ni Q, Yu X. Carbon nanotubes in cancer diagnosis and therapy. Biochim Biophys Acta Rev Cancer, 2010; 1806(1):29–35. CrossRef
John AA, Subramanian AP, Vellayappan MV, A. Balaji, Mohandas H, Jaganathan SK. Carbon nanotubes and graphene as emerging candidates in neuroregeneration and neurodrug delivery. Int J Nanomedicine, 2015; 10(312):4267–77. CrossRef
Kafa H, Wang JT, Rubio N, Klippstein R, Costa PM, Hassan HA, Sosabowski JK, Bansal SS, Preston JE, Abbott NJ, Al-Jamal KT. Translocation of LRP1 targeted carbon nanotubes of different diameters across the blood-brain barrier in vitro and in vivo. J Control Release, 2016; 225(1):217–29. CrossRef
Kagan VE, Konduru NV, Feng W, Allen BL, Conroy J, Volkov Y, Vlasova II, Belikova NA, Yanamala N, Kapralov A, Tyurina YY, Shi J, Kisin ER, Murray AR, Franks J, Stolz D, Gou P, Klein-Seetharaman J, Fadeel B, Star A, Shvedova AA. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat Nanotechnol, 2010; 5(5):354–9. CrossRef
Kakkar AK, Dahiya N. Management of Parkinson’s disease: current and future pharmacotherapy. Eur J Pharmacol, 2015; 750(1):74–81. CrossRef
Kam NWS, Liu Z, Dai H. Functionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. J Am Chem Soc, 2005; 127(36):12492–3. CrossRef
Kaur S, Mehra NK, Jain K, Jain NK. Development and evaluation of targeting ligand-anchored CNTs as prospective targeted drug delivery system. Artif Cells Nanomed Biotechnol, 2017; 45(2):242–50. CrossRef
Kerna NA, Flores JV, Pruitt KD, Nwokorie U, Holets H. The application of fullerene derivatives in human nutrition: brain health, immunity, longevity, quality of life, skin tone, sports performance, vitality, and weight Loss. EC Nutrition, 2020; 15(1):01–06.
Khuloud A. Carbon nanotubes deliver in medicine Am Sci, 2015; 103(1):122–8. CrossRef
Kim K, Kim MJ, Kim DW, Kim SY, Park S, Park CB. Clinically accurate diagnosis of Alzheimer’s disease via multiplexed sensing of core biomarkers in human plasma. Nat Commun, 2020; 11(1):119. CrossRef
Komane PP, Choonara YE, du Toit LC, Kumar P, Kondiah PDP, Modi G, Pillay V. Diagnosis and treatment of neurological and ischemic disorders employing carbon nanotube technology. J Nanomat, 2016; 9417874:19. CrossRef
Kostarelos K, Bianco A, Prato M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat Nanotechnol, 2009; 4(10):627–33. CrossRef
Kumar LA, Pattnaik G, Satapathy BS, Swapna S, Mohanty D. Targeting to brain tumor: nanocarrier-based drug delivery platforms, opportunities, and challenges. J Pharm Bioallied Sci, 2021; 13(2):172–7. CrossRef
Lee HJ, Park J, Yoon OJ, Kim HW, Lee DY, Kim DH, Lee WB, Lee NE, Bonventre JV, Kim SS. Amine-modified single-walled carbon nanotubes protect neurons from injury in a rat stroke model. Nat Nanotechnol, 2011; 6(2):121–5. CrossRef
Lee SJ, Zhu W, Nowicki M, Lee G, Heo DN, Kim J, Zuo Y, Zhang LG. 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration. J Neural Eng, 2018; 15(1):016018. CrossRef
Li S, Gu X, Yi S. The regulatory effects of transforming growth factor-β on nerve regeneration. Cell Transplant, 2017; 26(1):381–94. Li Z, de Barros ALB, Soares DCF, Moss SN, Alisaraie L. Functionalized single-walled carbon nanotubes: cellular uptake, biodistribution and applications in drug delivery. Int J Pharm, 2017; 524(1–2):41–54. CrossRef
Lin CM, Lee YT, Yeh SR, Fang W. Flexible carbon nanotubes electrode for neural recording. Biosens Bioelectron, 2009; 24(9):2791–7. CrossRef
Liu Y, Zhao Y, Sun B, Chen C. Understanding the toxicity of carbon nanotubes. Acc Chem Res, 2013; 46(3):702–13. CrossRef
Malarkey EB, Parpura V. Applications of carbon nanotubes in neurobiology. Neurodegener Dis, 2007; 4(1):292–9. CrossRef
Markovic ZM, Harhaji-Trajkovic LM, Todorovic-Markovic BM, Kepi? DP, Arsikin KM, Jovanovi? SP, Pantovic AC, Drami?anin MD, Trajkovic VS. In vitro comparison of the photothermal anticancer activity of graphene nanoparticles and carbon nanotubes. Biomaterials, 2011; 32(4):1121–9. CrossRef
Martincic M, Tobias G. Filled carbon nanotubes in biomedical imaging and drug delivery. Expert Opin Drug Deliv, 2015; 12(4):563–81. CrossRef
Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J Mol Neurosci, 2000; 14(3):175–82. CrossRef
More SV, Choi DK. Promising cannabinoid-based therapies for Parkinson’s disease: motor symptoms to neuroprotection. Mol Neurodegener, 2015; 10(17):1–26. CrossRef
Nunes A, Al-Jamal K, Nakajima T, Hariz M, Kostarelos K. Application of carbon nanotubes in neurology: clinical perspectives and toxicological risks. Arch Toxicol, 2012; 86(7):1009–20. CrossRef
Nunes A, Al-Jamal KT, Kostarelos K. Therapeutics, imaging and toxicity of nanomaterials in the central nervous system. J Control Release, 2012; 161(2):290–306. CrossRef
Pardridge WM. Blood-brain barrier and delivery of protein and gene therapeutics to brain. Front Aging Neurosci, 2020; 11(373):1–27. CrossRef
Pantarotto D, Briand JP, Prato M, Bianco A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem Commun (Camb), 2004; 1(1):16–7. CrossRef
Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater, 2009; 8(1):457–70. CrossRef
Podolski IY, Podlubnaya ZA, Kosenko EA, Mugantseva EA, Makarova EG, Marsagishvili LG, Shpagina MD, Kaminsky YG, Andrievsky GV, Klochkov VK. Effects of hydrated forms of C60 fullerene on amyloid 1-peptide fibrillization in vitro and performance of the cognitive task. J NanosciNanotechnol, 2007; 7(4–5):1479–85. CrossRef
Ren J, Shen S, Wang D, Xi Z, Guo L, Pang Z, Qian Y, Sun X, Jiang X. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials, 2012; 33(11):3324–33. CrossRef
Santos T, Fang X, Chen MT, Wang W, Ferreira R, Jhaveri N, Gundersen M, Zhou C, Pagnini P, Hofman FM, Chen TC. Sequential administration of carbonnanotubes and near-infrared radiation for the treatment of gliomas. Front Oncol, 2014; 4(1):180. CrossRef
Sharma HS, Muresanu DF, Sharma A. Novel therapeutic strategies using nanodrug delivery, stem cells and combination therapy for CNS trauma and neurodegenerative disorders. Expert Rev Neurother, 2013; 13(10):1085–8. CrossRef
Soligo M, Felsani FM, Ros TD, Bosi S, Pellizzoni E, Bruni S, Isopi J, Marcaccio M, Mannia L, Fiorito S. Distribution in the brain and possible neuroprotective effects of intranasally delivered multi-walled carbon nanotubes. Nanoscale Adv, 2021; 3(1):418–31. CrossRef
Stephenson J, Nutma E, van der Valk P, Amor S. Inflammation in CNS neurodegenerative diseases. Immunology, 2018;154(2):204–19. CrossRef
Tan J, Wang Y, Yip X, Glynn F, Shepherd RK, Caruso F. Nanoporous peptide particles for encapsulating and releasing neurotrophic factors in an animal model of neurodegeneration. Adv Mater, 2012; 24(25):3362–6. CrossRef
Tan JM, Foo JB, Fakurazi S, Hussein MZ. Release behaviour and toxicity evaluation of levodopa from carboxylated single-walled carbon nanotubes. Beilstein J Nanotechnol, 2015; 6(1):243–53. CrossRef
Tykhomyrov AA, Nedzvetsky VS, Klochkov VK, Andrievsky GV. Nanostructures of hydrated C60 fullerene (C60HyFn) protect rat brain against alcohol impact and attenuate behavioral impairments of alcoholized animals. Toxicology, 2008; 246(1):158–65. CrossRef
Vidu R, Rahman M, Mahmoudi M, Enachescu M, Poteca TD, Opris I, Nanostructures: a platform for brain repair and augmentation. Front Syst Neurosci, 2014; 8(91)1–24. CrossRef
Vorobyov V, Kaptsov V, Gordon R, Makarova E., Podolski I, Sengpiel F. Neuroprotective effects of hydrated fullerene C60: cortical and hippocampal EEG interplay in an amyloid-infused rat model of Alzheimer’s disease. J Alzheimer’s Dis, 2015; 45(1):217–33. CrossRef
Wang W, Zhu Y, Liao S, Li J. Carbon nanotubes reinforced composites for biomedical applications. Biomed Res Int, 2014; 2014(1):1–15. CrossRef
Xiang C, Zhang Y, Guo W, Liang XJ. Biomimetic carbon nanotubes for neurological disease therapeutics as inherent medication. Acta Pharm Sin B, 2020; 10(2):239–48. CrossRef
Xue X, Wang LR, Sato Y, Jiang Y, Berg M, Yang DS, Nixon RA, Liang XJ. Single-walled carbon nanotubes alleviate autophagic/lysosomal defects in primary glia from a mouse model of Alzheimer’s disease. Nano Lett, 2014; 14(9):5110–7. CrossRef
Yang T, Wu Z, Wang P, Mu T, Qin H, Zhu Z, Wang J, Sui L. A large-inner-diameter multi-walled carbon nanotube-based dual-drug delivery system with pH-sensitive release properties. J Mater Sci Mater Med, 2017; 28(7):110. CrossRef
Yang Z, Zhang Y, Yang Y, Sun L, Han D, Li H, Wang C. Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease. Nanomed J, 2010; 6(3):427–41. CrossRef
Zhang L, Liu F, Sun X, Wei G, Tian Y, Liu Z, Huang R, Yu Y, Peng H. Engineering carbon nanotube fiber for real-time quantification of ascorbic acid levels in a live rat model of Alzheimer’s disease. Anal Chem, 2017; 89(3):1831–7. CrossRef
Zhao D, Alizadeh D, Zhang L, Liu W, Farrukh O, Manuel E, Diamond DJ, Badie B. Carbon nanotubes enhance CpG uptake and potentiate antiglioma immunity. Clin Cancer Res, 2011; 17(4):771–82. CrossRef