INTRODUCTION
The largest organ on the outside of the human body, the skin, is a barrier to shield interior organs from dehydration, microbial infection, and UV exposure. Besides these important functions, it is well known that a better skin appearance is also responsible for an individual’s awareness of looking more beautiful. Hence, various companies have attempted to pursue improving their innovative products in skincare cosmetics. The business sector in this area has been recognized as one of the most promising ones, with an annual revenue worth of billions of dollars (Kouassi et al., 2022). Currently, a phenomenon in healthy lifestyles contributes to shifting from synthetic skincare cosmetics to green ones, since synthetic materials reportedly have harmful side effects, such as low absorption ability and allergic reactions (irritation) (Morais et al., 2021). A previous study also indicated their negative influences on the environment (Amberg and Fogarassi, 2019). On the other hand, green technology offers several advantages, including safety, nontoxicity, having no adverse effects when used correctly, and biodegradability. These products are generally fabricated by incorporating bioactive compounds obtained from plants. Therefore, this trend motivates scientists worldwide to explore plant materials with appropriate properties, particularly those used in skincare cosmetics.
Euphorbiaceae is considered to be one of the largest families among flowering plants, with 218 genera and 5,735 species (Zixi et al., 2016). According to ethnobotanical studies, most plants in this family are frequently utilized in local medicine and skincare (Zahidin et al., 2017). Macaranga is a genus of Euphorbiaceae, which consists of more than 300 species, and it is widely distributed across tropical and subtropical countries in Africa, Asia, Australasia, and the Pacific (Huonga et al., 2019). Nevertheless, its distribution center has been recorded in tropical Asia (Syah and Ghisalberti, 2015). This fast-growing tree species, also known as the highest genus in the Euphorbiaceae, can be found in gaps between the forest canopy, disturbed forests, and open areas (Tanjung et al., 2018). Due to the change in light conditions after logging activities or forest fires, some Macaranga species are usually found with high dominance as pioneer species. The presence of Macaranga can be a reliable bioindicator of deforestation (Zapanta et al., 2019). Its rapid growth has been recorded to significantly influence the composition of the forest vegetation community during the secondary succession stage (Susanto et al., 2018). In Indonesia, its wide distribution has been found in Sumatera and on Kalimantan Island (Tanjung et al., 2018). Considering its ability to cover land quickly and attract wildlife, it is also becoming popular as a species to promote revegetation after coal mining in this country (Amirta and Candra, 2016). The leaves of several species from this genus are often utilized in traditional medicine and have the potential for use as antipyretics, antitussives, and anti-inflammatories (Pailee et al., 2015). Due to its good adaptation to various types of land, even with low available nutrient status, combined with its interesting properties, further domestication of Macaranga will allow the use of these plant species in various applications, including sustainable raw materials for additives in skincare production.
Nowadays, the practice of using direct herbal products for skincare purposes has been declining, since various modern skincare products containing bioactive ingredients with simpler usage have been introduced to the market (Milito et al., 2021). As a result, valuable information about crude extraction and isolated compounds, including those obtained from Macaranga, will need to be collected, as several authors have reported its remarkable bioactivity (Di et al., 2020; Hashim et al., 2022; Minarti et al., 2021). This review presents a comprehensive summary of the chemical and pharmacological studies on Macaranga spp. in terms of their suitability for use as skincare cosmetic products. Furthermore, the range of their potential application in large-scale industrial skincare products is also considered.
METHODOLOGY
The current review was conducted based on a number of publications, specifically, plant extracts and bioactive constituents of various Macaranga plants, which can be used as skincare cosmetic ingredients. Other potential applications of Macaranga’s bioactive constituents are also presented. There is no limitation on the year of publication. Exhaustive searches were performed on four electronic databases: PubMed, ScienceDirect, Scopus, and Google Scholar. Various keywords, such as Macaranga plant, Macaranga genus and species, chemical compounds of Macaranga, bioactivity of Macaranga, anti-inflammatory activity, antioxidant activity, antimicrobial activity, and antityrosinase activity, were employed to find appropriate data and articles.
Bioactive constituents of Macaranga plants
An earlier work summarized the chemical investigations of 25 species of Macaranga (Magadula, 2014). In this study, the phytochemical characterization has been updated to include 40 species. They are identified as M. adenantha, M. allorobinsonii, M. alnifolia, M. balansae, M. barteri, M. bicolour, M. conglomerata, M. conifera, M. constricta, M. deheiculata, M. denticulata, M. gigantea, M. gigantifolia, M. hemsleyana, M. heynei, M. hosei, M. hurifolia, M. hypoleuca, M. indica, M. javanica, M. kurzii, M. lowii, M. magna, M. mappa, M. monandra, M. peltata, M. pleiostemona, M. pruinosa, M. pustulata, M. recurvata, M. rhizinoides, M. rubiginosa, M. sampsonii, M. schweinfurthii, M. siamensis, M. sinensis, M. tanarius, M. trichocarpa, M. triloba, and M. vedeliana. This list reveals, however, that only a small percentage of species (less than 15%) have been further chemically investigated, despite the fact that there are more than 300 species of Macaranga available in the world.
Each plant species is distinctive regarding its bioactive constituents. Based on the previous study by Mai et al. (2020), it has been discovered that some Macaranga species are rich in phenolic compounds, particularly flavonoid and stilbene derivatives. Yang et al. (2015a, 2015b) reported the presence of two novel stilbenes, denticulatains A and B, and flavonoid derivatives, including denticulatain C, D, and E from M. denticulata fronds. C-methylated and isoprenylated chalcone derivatives, dentichalcones A, B, and C, were also discovered in related species. According to Pailee et al. (2015), macasiamenenes A-U, macasiamenin A, macasiamenone A, and macasiamenols A and B, were isolated from M. siamensis leaves and twigs. Furthermore, secondary metabolites in the class of tannins, terpenes, coumarins, steroids, and many other compounds were also detected. The most prevalent of these is scopoletin, a coumarins that is present in six different species of Macaranga: M. barteri, M. conglomerata, M. denticulata, M. kurzii, M. magna, and M. triloba. The number of compounds isolated from M. tanarius has been reported as the highest, with 98 compounds collected from its leaves, stem bark, and fruits. Mostly, the compounds in Macaranga are stilbenes and flavonoids, as shown in Figure 1. A list of all summarized constituents is presented in Table 1.
Application of Macaranga in cosmetics
Anti-inflammatory activity
Inflammation is the response of body to any kind of disturbance caused by irritation, injury, or bacteria. Anti-inflammatory products work by blocking the body response to this skin disorder by inhibiting the effects of certain enzymes that contribute to swelling and inflammation (Attiq et al., 2018). In terms of both skin appearance and health, dealing with inflammation is a major issue. Preventing the impact of skin inflammation is essential for a youthful appearance and avoiding the development of chronic or acute skin diseases (Hoang et al., 2021). In general, anti-inflammatory skincare products contain antioxidants, which help the body fight damaging free radicals. Antioxidants prevent collagen breakdown and skin DNA damage (Lin et al., 2018).
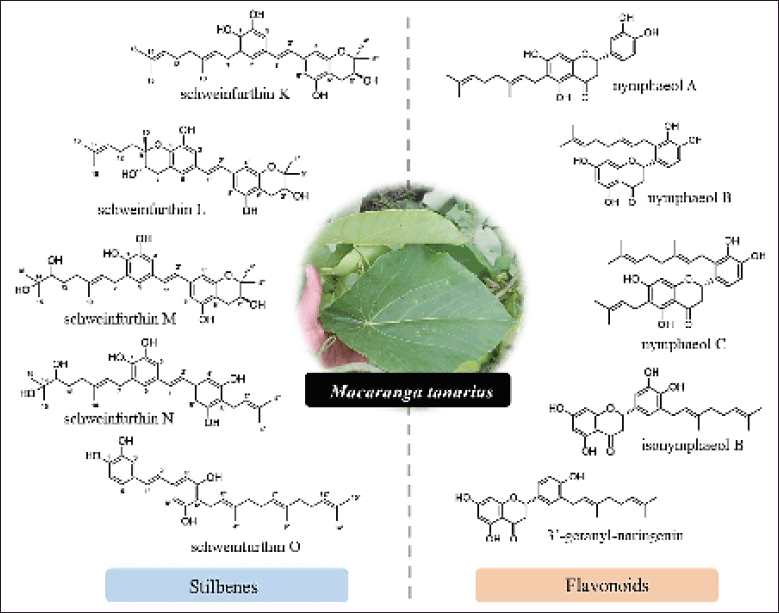 | Figure 1. Structure of several stilbenes and flavonoid derivatives isolated from Macaranga tanarius. [Click here to view] |
An earlier study found that dichloromethane extracts of M. siamensis leaves and twigs had a strong anti-inflammatory effect, with Macasiamenene F isolated from these extracts reducing tumor necrosis factor (TNF) alpha by 20% after 24 hours (Leláková et al., 2020). M. barteri methanol extract had a strong anti-inflammatory effect with potential compounds such as macabarterin, 3-O-methylellagic acid, 4-O-b-D-xylopyranoside, ellagic acid, 3-O-methylellagic acid, and gallic acid (Ngoumfo et al., 2008). Moreover, an assay on rats with carrageenan-induced edema showed that hydroalcoholic M. barteri bark extract reduced inflammation and skin hyperalgesia. This report also confirmed the traditional application of M. barteri’s bark to treat pain and inflammation, acting as an analgesic (Asante-Kwatia et al., 2019). M. tanarius demonstrated high inhibition of protein (albumin) denaturation with IC50 values of 0.26–1.02 mm with some bioactive compounds such as nymphaeol A, nymphaeol B, nymphaeol C, isonymphaeol B, and 3’-geranyl-naringenin (Shahinozzaman et al., 2021). Nymphaeol B of this species strongly inhibits acetylcholinesterase (Ache) at 50 µg/ml (Amir Rawa et al., 2022). Another study reported that M. hurifolia extract from Nigeria has an inflammatory effect (69.6%) at a dose of 300 mg/kg (Segun et al., 2019a).
Antioxidant activity
Natural antioxidants are used in the cosmetics industry for their capacity to reduce oxidative stress on the skin and protect products from oxidative deterioration (He et al., 2021; Hoang et al., 2021). Due to the natural aging process as well as extrinsic causes including UV radiation, air pollution, and pathogenic microorganisms, oxidative stress is the main factor accelerating skin aging (Farage et al., 2008; Rees, 2004). Antioxidant molecules prevent radical chain reactions, which inhibit reactive oxidants’ formation; as a result, they can also be used to cure cancer. In cosmetic products, including serums and creams, antioxidants can be employed to stabilize ingredients and prevent the rancidity of lipids (Leopoldini et al., 2011). Antioxidants inhibit lipid oxidation by reacting with lipids and peroxy radicals and converting them into more stable nonradical products (Lin et al., 2018; Petruk et al., 2018). Furthermore, antioxidants also help in overcoming inflammation.
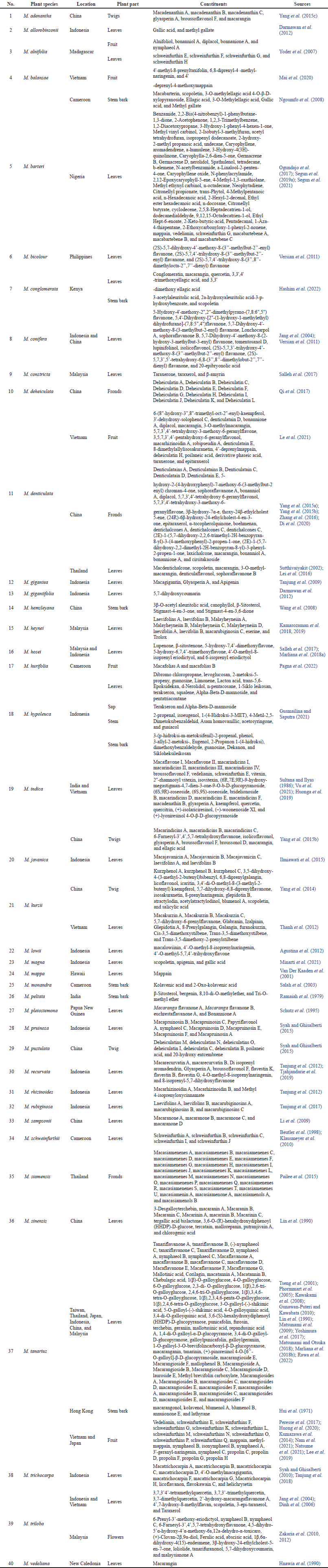 | Table 1. Current status of Bioactive Compounds Isolated from Macaranga species. [Click here to view] |
Ogundajo and Ashafa (2019) reported that the methanol extract of a popular traditional medicinal plant in west Africa, M. barteri, demonstrated a high antiradical effect against 1,1-diphenyl-2-picrylhydrazyl (DPPH) and nitric oxide (NO) with values of 0.47 and 1.68 mg/ml, respectively. M. tanarius fruit extracts exhibited strong antioxidant activity due to the presence of prenylflavonoids in the seed, pericarp, glandular trichome, and leaf (Kumazawa et al., 2014; Kumazawa et al., 2008). Chien et al. (2022) also reported that M. tanarius new and mature fruit extracts exhibited stronger free radical-scavenging activity and possessed lower IC50 than Taiwanese green propolis extract. Ethyl acetate extract of M. triloba leaves from Central Kalimantan, Indonesia, exhibited a strong ability to neutralize free radicals DPPH (Ardany et al., 2018). Kamarozaman et al. (2019) reported that new dihydrostilbenes isolated from M. heynei showed some antioxidant activity.
Antimicrobial activity
In cosmetic goods, antimicrobial compounds are used to both inhibit the growth of unfavorable microorganisms in skincare products and increase the shelf life of products (Nowak et al., 2021). The skin is the largest organ in the body, and it is exposed to the environment, making it an ideal location for bacteria, viruses, or fungi to grow. In order to protect consumers and stabilize shelf life, especially against potentially harmful bacteria, the formulation of cosmetic products (water based vs. oil based) has considered multifunctional antimicrobial ingredients (Hoang et al., 2021). This is in line with rising consumer awareness and demands for clean beauty and avoiding artificial ingredients, especially preservatives. It is realized that the use of conventional antimicrobial agents, which are usually loaded with preservatives, has disturbed the balance of skin microflora, so the skin is vulnerable to exposure to harmful microorganisms (Nowak et al., 2021).
Methanolic extract of M. barteri leaves grown in Nigeria has been reported to inhibit Pseudomonas aeruginosa, Enterococci faecalis, and Cryptococcus neoformans (>80% inhibition (Ogbole et al., 2018). Purayil et al. (2019) used a combination solvent, chloroform and water (1:10), to extract from the leaves of M. peltata, which resulted in a clearance zone of the well diffusion method against Staphylococcus aureus. Panda et al. (2017) tested acetone, water, and ethanol solvents to extract from M. peltata leaves, which had antimicrobial activity against Escherichia coli, S. aureus, and Candida albicans. As described in another report, both the leaves and stem bark of M. peltata also had positive activity against four bacterial strains: E. coli, P. aeruginosa, Bacillus subtilis, and S. aureus (Verma et al., 2009).
Bradacs et al. (2010) reported that the inner bark extract of Macaranga dioica, commonly used as traditional medicine in the South Pacific Archipelago of Vanuatu, exhibited moderate activity against C. albicans. Ogundajo et al. (2017) extracted hexane, ethyl acetate, and methanol from M. barteri leaves. They found that methanolic extract had the best antibacterial activity against S. aureus, Bacillus pumilus, Streptococcus faecalis, Listeria sp., P. aeruginosa, Plesiomonas shigelloides, Aeromonas hydrophila, Shigella sonnei, Salmonella typhi, Salmonella typhimurium, E. coli, Proteus vulgaris, Proteus vulgaris, Enterobacter faecalis, and Klebsiella pneumoniae. The authors also discovered that a hexane extract of the plant was effective against fungi such as Candida neoformans, Trichophyton mucoides, and Candida albicans. Lee et al. (2019) isolated five propolins from M. tanarius. Among them, propolin D showed the highest inhibition of biofilm formation by strains of S. aureus, Staphylococcus epidermidis, and C. albicans, with MICs of 10–50 µg/ml. Isolated prenylated kaempferol and conglomeratin, from M. conglomerata, reportedly showed significant permeation of P. aeruginosa (MIC = 7.8 mg/ml) and moderate activity against S. aureus, E. coli, and Klebsiella pneumoniae (MIC = 62.5 mg/ml) (Hashim et al., 2022).
Tyrosinase inhibitory effect
Antimelanogenesis activity is one of the most important criteria for determining the suitability of plant compounds for use in skincare agents. It was previously reported by Arung et al. (2019) that they isolated glyasperin A, a prenylated flavonoid isolated from M. pruinosa that greatly inhibited melanin in B16 melanoma. Another report from Mazlan et al. (2013) demonstrated that they had extracted M. denticulata, M. pruinosa, and M. gigantea using methanol to assess their tyrosinase inhibitory activity. Among them, methanolic extract of M. denticulata bark showed the highest inhibition (68.7%). Methanol extract from the leaves and stem bark of M. hurifolia has been tested to show their application in tyrosinase inhibition, in which they found appropriate activity with values of 159.42 [mg Kojic acid equivalent (KAE)/g] and 160.95 (mg KAE/g), respectively (Sadeer et al., 2019). Lim et al. (2009) screened the bioactivity of methanolic extracts of M. gigantea, M. pruinosa, M. tanarius, and M. triloba, which resulted in the best tyrosinase inhibition activity obtained by M. pruinosa among all the Macaranga tested. KAE values and quercetin equivalent were 6.8 and 20.7 mg/g, respectively.
Limitations of natural ingredient in cosmetics
Macaranga extract contains antioxidant, anti-inflammatory, and antimicrobial compounds. Some of these compounds have potential as topical cosmetic preparations. However, it is acknowledged that not all of these natural ingredients are safe because they may be associated with carcinogenic, mutagenic, and reprotoxic chemicals (Hoang et al., 2021). As a result, their application as skincare products that protect the integrity of cosmetics and the skin at the same time must be considered wisely. Furthermore, despite their promising effects, validation with clinical results is required. Due to a lack of clinical data and the limited relevance of data, some of the practical significance of the effects mentioned has not been adequately proven. However, these data provide interesting points for dermatologists to consider in the application of natural ingredients in relation to topical therapies (Hoang et al., 2021).
Although uncommon, skin contact with cosmetics containing plant extracts can result in allergic responses, contact dermatitis, erythema multiforme, and xanthomatous reactions (Hoang et al., 2021). It is possible, due to the lack of separation techniques, that many plant extracts have not been investigated for their compounds (Zorzi et al., 2016). Antioxidants may also have a variety of adverse effects, including acute toxicity, skin and eye irritation, skin sensitization, and photosensitization (Hoang et al., 2021; Mujtaba et al., 2021). In light of this, the application of natural ingredients requires that they are applied in safe concentrations; for instance, the application of essential oils is only recommended at 0.1–0.8%, or diluting with carrier oils (Guzmán and Lucia, 2021; Vostinaru et al., 2020). The use of some natural extracts, such as polyphenols, in cosmetics is constrained due to their low stability and sensitivity to heat and light (Hoang et al., 2021). Due to this condition, some cosmetic products that contain furocoumarins, compounds that are also present in Macaranga extract, should only be applied at night. Sun exposure has phototoxicity effects, causing skin damage.
Natural antioxidants are susceptible to deterioration, and their bioavailability is limited by low absorption. The use of active phytomolecules with the application of nanotechnology in cosmetics has drawn a lot of attention since it can boost absorption by the skin (Salvioni et al., 2021). Various types of nanoemulsions, nanoparticles, liposomes, niosomes, and dendrimers have influenced the formulation of cosmetic products (Musthaba et al., 2009). According to test results, the application of nano formulations can minimize hematological toxic effects, boost bioavailability, and lessen other side effects, such as alopecia, nausea, vomiting, diarrhea, exhaustion, and skin rashes (Hoang et al., 2021; Saklani and Kutty, 2008; Salvioni et al., 2021; Zorzi et al., 2016). Recently, major concerns regarding its safety have been raised, and much more exploration is needed to determine its efficacy in delivering active ingredients into the skin. New regulations established by the European Union have passed amendments in its (new regulations) cosmetics directory for safer nanocosmetics to enter the market, safeguarding the beauty and health of consumers (Hoang et al., 2021; Musthaba et al., 2009). Natural antioxidants are more expensive than synthetic ones in cosmetics, despite the fact that these molecules are safer than synthetic antioxidants.
Other potential uses of Macaranga plants
Hendra et al. (2017) reported that the extract of M. tanarius exhibited positive antihyperlipidemic and hepatoprotective effects. Some active compounds isolated from ethyl acetate extract of M. tanarius leaves, i.e., mallotinic acid, corilagin, chebulagic acid, macatannins A and B, can be developed for diabetes treatment agents (Gunawan-Puteri and Kawabata, 2010). Another Macaranga species, M. hurifolia extract from Nigeria, showed potential for anti-inflammatory and antidiabetic effects (Segun et al., 2019b). Ehile et al. (2018) reported that the aqueous extract of M. barteri exhibited a gastric antiulcer effect at doses ranging from 62.5 to 500 mg/kg b.w. This extract has been reported to be safe for oral medicine due to major toxic anthropometric and hematological effects not being present in tested rats, despite the fact that biochemical and histological studies are needed to ensure safe use of the extract (Ehile et al., 2018). Furthermore, as reported earlier, Macaranga spp. contains rich prenylated flavonoids (Shahinozzaman et al., 2021). These exhibited several potential effects: antivirus activity (Feng et al., 2010), antiallergic activity (Quan et al., 2008), and larvicidal activity against the fourth-instar larvae of Aedes albopictus and Culex pipiens quinquefasciatus (Niu et al., 2010). Muhaimin et al. (2019) reported that ethanolic extract of M. gigantea exhibited antiplasmodial activity and can be developed as an alternative agent for controlling malaria. The study also confirmed that M. denticulata leaf extract has antinociceptive activity. At doses of 200 and 400 mg/kg, it demonstrated dose-dependent and statistically significant antinociceptive activity in acetic acid and formalin tests. Furthermore, among six major M. denticulata compounds, Macaranga had the best fitness score of 5.81 with the COX-1 enzyme (Hasanat et al., 2017).
CONCLUSIONS
A comprehensive review of the potential of Macaranga plants as skincare cosmetics ingredients has been conducted. Macaranga plants contain bioactive compounds which have anti-inflammatory, antioxidant, antibacterial, and tyrosinase inhibitory activity. Therefore, Macaranga plants have the potential to be used as skincare cosmetic ingredients.
AUTHORS’ CONTRIBUTIONS
Conceptualization is done by E.T.A. and M.T.H.; methodology is carried out by E.T.A., M.T.H., and A.S.P.; investigation is done by E.T.A., I.W.K., H.K., R.A., and E.R.; writing original draft preparation is conducted by M.T.H., I.W.K., H.K., R.A., E.R., Y.Y., W.S., S.P., R.R., D.T., M.I., H.A.A., W.F., A.A., C.R.K., N.I.W.A., Y.K., and E.T.A.; writing review and editing is done by E.T.A., W.F., D.T., and H.A.A.; visualization is carried out by M.T.H., D.T., W.D., H.A.A., and E.T.A.; project administration is done by E.T.A. and W.F.; funding acquisition is done by E.T.A. All authors have read and agreed to the published version of the manuscript.
FUNDING
This research received funding from the Deputy of Research and Innovation, National Research and Innovation Agency (BRIN) for “Pusat Kolaboratif Riset Kosmetik Berteknologi Nano Berbasis Bio-massa” in the Fiscal Year 2022 (grant number: 398/II/FR/3/2022).
CONFLICTS OF INTEREST
The authors have no relevant financial or nonfinancial interests to disclose.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this review article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliations.
REFERENCES
Agustina W, Juliawaty L, Hakim E, Syah Y. Flavonoids from Macaranga lowii. ITB J Sci, 2012; 44. CrossRef
Amberg N, Fogarassy C. Green consumer behavior in the cosmetics market. Resources, 2019; 8:137. CrossRef
Amir Rawa MS, Nurul Azman NA, Mohamad S, Nogawa T, Wahab HA. In vitro and in silico anti-acetylcholinesterase activity from Macaranga tanarius and syzygium jambos. Molecules, 2022; 27(9):2648. CrossRef
Amirta R, Candra KP. Comparative characterization of Macaranga species collected from secondary forests in East Kalimantan for biorefinery of unutilized fast growing wood. Biodivers J Biol Divers, 2016; 17:116–23. CrossRef
Ardany SD, Mulia DS, Rosawanti P. Antioxidant activity of ethyl acetate fraction of Macaranga triloba leaves from central Kalimantan. Asian J Pharm Clin Res, 2018; 11(3):39–42. CrossRef
Arung ET, Sinamabela JR, Rosamah E, Kusuma IW, Kuspradini H, Alam AE, Amen Y, Tanaka H, Satria D, Shimizu K, Ishikawa H. Antioxidant and antimelanogenesis activities of glyasperin a from Macaranga pruinosa leaves. Nat Prod Commun, 2019; 14(7):1934578X19867192. CrossRef
Asante-Kwatia E, Jibira Y, Mensah A, Osei-Sarfoh D. Macaranga barteri stem bark extract exerts anti-inflammatory and anti-hyperalgesia activity in murine models. Discover Phytomed, 2019; 6(3):130–7. CrossRef
Attiq A, Jalil J, Husain K, Ahmad W. Raging the war against inflammation with natural products. Front Pharmacol, 2018; 9:976. CrossRef
Beutler JA, Shoemaker RH, Johnson T, Boyd MR. Cytotoxic geranyl stilbenes from Macaranga schweinfurthii. J Nat Prod, 1998; 61(12):1509–12. CrossRef
Bradacs G, Maes L, Heilmann J. In vitro cytotoxic, antiprotozoal and antimicrobial activities of medicinal plants from Vanuatu. Phytother Res, 2010; 24(6):800–9. CrossRef
Chien YH, Yu YH, Ye SR, Chen YW. Antibacterial and antioxidant activity of the fruit of Macaranga tanarius, the plant origin of Taiwanese green propolis. Antioxidants, 2022; 11(7):1242. CrossRef
Darmawan A, Kosela S, Kardono LBS, Syah Y. Scopoletin a coumarin derivative compound isolated from Macaranga gigantifolia. Merr J Appl Pharm Sci, 2012; 2:17–25.
Di Q, Zhu H, Pu D, Zhao X, Li X, Ma X, Xiao W, Chen W. The natural compound Cirsitakaoside enhances antiviral innate responses against vesicular stomatitis virus in vitro and in vivo. Int Immunopharmacol, 2020; 86:106783. CrossRef
Dinh V, Zhang HP, Duc NM, Tuu NV, Qin GW. A new geranyl flavanone from Macaranga triloba. J Asian Nat Prod Res, 2006; 8(1–2):155–8. CrossRef
Ehile E, Goze N, Kouakou KL, Yapo A, Ehile E. Acute toxicity and gastric anti-ulcer activity of an aqueous extract of the leaves of Macaranga barteri Mll. Arg (Euphorbiaceae) on rat models. J Med Plants Res, 2018; 12:96105. CrossRef
Farage MA, Miller KW, Elsner P, Maibach HI. Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmetic Sci, 2008; 30(2):87–95. CrossRef
Feng T, Wang RR, Cai XH, Zheng YT, Luo XD. Anti-human immunodeficiency virus-1 constituents of the bark of Poncirus trifoliata. Chem Pharm Bull, 2010; 58(7):971–5. CrossRef
Gunawan-Puteri MDPT, Kawabata J. Novel α-glucosidase inhibitors from Macaranga tanarius leaves. Food Chem, 2010; 123(2):384–9. CrossRef
Gusmailina R, Saputra N. Identification on chemical organic compounds of Macaranga hypoleuca by using Py-GCMS. IOP Conf Ser Earth Environ Sci, 2021; 914:012065. CrossRef
Guzmán E, Lucia A. Essential oils and their individual components in cosmetic products. Cosmetics, 2021; 8(4):114. CrossRef
Hasanat A, Chowdhury TA, Kabir MSH, Chowdhury MS, Chy MNU, Barua J, Chakrabarty N, Paul A. Antinociceptive activity of Macaranga denticulata Muell. Arg. (Family: Euphorbiaceae): in vivo and in silico studies. Medicines (Basel), 2017; 4(4):88. CrossRef
Hashim I, Onyari JM, Omosa LK, Maru SM, Nchiozem-Ngnitedem VA, Karpoormath R. Conglomeratin: a new antibacterial flavonol derivative from Macaranga conglomerata Brenan (Euphorbiaceae). Nat Prod Res, 2022; 36:1–9. CrossRef
He H, Li A, Li S, Tang J, Li L, Xiong L. Natural components in sunscreens: topical formulations with sun protection factor (SPF). Biomed Pharmacother, 2021; 134:111161. CrossRef
Hendra P, Jamil O, Maharani D, Suhadi M, Putri C, Fenty, Julianus J. Antihyperlipidemic and hepatoprotective studies on leaves of Macaranga tanarius. Asian J Pharmaceutical Clin Res, 2017; 10:239–41. CrossRef
Hnawia E, Thoison O, Guéritte-Voegelein F, Bourret D, Sévenet T. A geranyl substituted flavonol from Macaranga vedeliana. Phytochemistry, 1990; 29(7):2367–8. CrossRef
Hoang HT, Moon JY, Lee, YC. Natural antioxidants from plant extracts in skincare cosmetics: recent applications, challenges and perspectives. Cosmetics, 2021; 8(4):106. CrossRef
Hui WH, Ng KK, Fukamiya N, Koreeda M, Nakanishi K. Isolation and structure of macarangonol, a diterpene ketol from Macaranga tanarius. Phytochemistry, 1971; 10(7):1617–20. CrossRef
Huong DTM, Linh NT, Van TTT, Litaudon M, Roussi F, Van Nam V, Van Cuong P. Stilbenes from Macaranga tanarius (Euphorbiaceae) growing in Vietnam. Vietnam J Chem, 2020; 58(3):338–42. CrossRef
Huonga DTM, Vu LTN, Anh L, Cuc NT, Nhiem NX, Tai BH, Van Kiem P, Litaudon M, Dang Thach T, Van Minh C, Pham VC. Cytotoxic prenylated flavonoids from the leaves of Macaranga indica. Phytochemy Lett, 2019; 34:39–42. CrossRef
Ilmiawati A, Hakim EH, Syah YM. Prenylated 9,10-dihydrophenanthrenes from Macaranga javanica. Zeitschrift für Naturforschung B, 2015; 70(9):659–63. CrossRef
Jang DS, Cuendet M, Pawlus AD, Kardono LB, Kawanishi K, Farnsworth NR, Fong HH, Pezzuto JM, Kinghorn AD. Potential cancer chemopreventive constituents of the leaves of Macaranga triloba. Phytochemistry, 2004; 65(3):345–50. CrossRef
Kamarozaman A, Ahmat N, Rahman NF, Yen KH. Prenylated dihydrostilbenes from Macaranga heynei (Euphorbiaceae). Malaysian J Anal Sci, 2018; 22:258–63. CrossRef
Kamarozaman AS, Ahmat N, Isa SNM, Hafiz ZZ, Adenan MI, Yusof MIM, Azmin NFN, Latip J. New dihydrostilbenes from Macaranga heynei IM. Johnson, biological activities and structure-activity relationship. Phytochem Lett, 2019; 30:174–80. CrossRef
Kawakami S, Harinantenaina L, Matsunami K, Otsuka H, Shinzato T, Takeda Y. Macaflavanones A-G, prenylated flavanones from the leaves of Macaranga tanarius. J Nat Prod, 2008; 71(11):1872–6. CrossRef
Keeyari Purayil S, Chew A, Paulraj P, Pattammadath S, Mohamed J, Selvarani J, P R, Ravibalan T, Petchi I, Samrot A. Evaluation of antioxidant and antimicrobial activity of some plants collected from Malaysia. J Pure Appl Microbiol, 2019; 13:2363–73. CrossRef
Klausmeyer P, Van QN, Jato J, McCloud TG, Beutler JA. Schweinfurthins I and J from Macaranga schweinfurthii. J Nat Prod, 2010; 73(3):479–481. CrossRef
Kouassi MC, Grisel M, Gore E. Multifunctional active ingredient-based delivery systems for skincare formulations: a review. Colloids Surf B Biointerfaces, 2022:112676. CrossRef
Kumazawa S, Murase M, Momose N, Fukumoto S. Analysis of antioxidant prenylflavonoids in different parts of Macaranga tanarius, the plant origin of Okinawan propolis. Asian Pac J Trop Med, 2014; 7(1):16–20. CrossRef
Kumazawa S, Nakamura J, Murase M, Miyagawa M, Ahn MR, Fukumoto S. Plant origin of Okinawan propolis: honeybee behavior observation and phytochemical analysis. Naturwissenschaften, 2008; 95(8):781–6. CrossRef
Le TNV, Truong BN, Le TP, Litaudon M, Tran DT, Chau VM, Mai HDT, Pham VC. Cytotoxic phenolic compounds isolated from the fruits of Macaranga denticulata. Nat Prod Res, 2021; 35(11): 861–1868. CrossRef
Lee JH, Kim YG, Khadke SK, Yamano A, Woo JT, Lee, J. Antimicrobial and antibiofilm activities of prenylated flavanones from Macaranga tanarius. Phytomedicine, 2019; 63:153033. CrossRef
Lei C, Zhang LB, Yang J, Gao LX, Li JY, Li J, Hou AJ. Macdentichalcone, a unique polycyclic dimeric chalcone from Macaranga denticulata. Tetrahedron Letters, 2016; 57(49):5475–8. CrossRef
Leláková V, Béraud-Dufour S, Hošek J, Šmejkal K, Prachyawarakorn V, Pailee P, Widmann C, Václavík J, Coppola T, Mazella J, Blondeau N, Heurteaux C. Therapeutic potential of prenylated stilbenoid macasiamenene F through its anti-inflammatory and cytoprotective effects on LPS-challenged monocytes and microglia. J Ethnopharmacol, 2020; 263:113147. CrossRef
Leopoldini M, Russo N, Toscano M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem, 2011; 125(2):288–306. CrossRef
Li X, Xu L, Wu P, Xie H, Huang Z, Ye W, Wei X. Prenylflavonols from the leaves of Macaranga sampsonii. Chem Pharm Bull (Tokyo), 2009; 57(5):495–8. CrossRef
Lim TY, Lim YY, Yule CM. Evaluation of antioxidant, antibacterial and anti-tyrosinase activities of four Macaranga species. Food Chem, 2009; 114(2):594–99. CrossRef
Lin JH, Ishimatsu M, Tanaka T, Nonaka GI, Nishioka I. Tannins and related compounds. XCVI, Structures of macaranins and macarinins, new hydrolyzable tannins possessing macaranoyl and tergalloyl ester groups, from the leaves of Macaranga sinensis (BAILL.) MUELL. ARG. Chem Pharm Bull, 1990; 38:1844–51. CrossRef
Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci, 2018; 19(1):70. CrossRef
Magadula J. Phytochemistry and pharmacology of the genus Macaranga: a review. J Med Pl Res, 2014; 8(12):489–503. CrossRef
Mai HDT, Toan TP, Huu GT, Le TN, Oanh VTK, Hang NTM, Thu HT, Chau VM, Litaudon M, Pham VC. New flavonoid and stilbene derivatives from the fruits of Macaranga balansae. Nat Prod Res, 2020; 34(19):2772–8. CrossRef
Marliana E, Astuti W, Kosala K, Hairani R, Tjahjandarie T, Tanjung M. Chemical composition and anticancer activity of Macaranga hosei Leaves. Asian J Chem, 2018a; 30:795–8. CrossRef
Marliana E, Hairani R, Tjahjandarie TS, Tanjung M. Antiplasmodial activity of flavonoids from Macaranga tanarius leaves. IOP Conf Ser Earth Environ Sci, 2018b; 144:012011. CrossRef
Matsunami K, Otsuka H. Okinawan subtropical plants as a promising resource for novel chemical treasury. Chem Pharm Bull (Tokyo), 2018; 66(5):519–26. CrossRef
Matsunami K, Otsuka H, Kondo K, Shinzato T, Kawahata M, Yamaguchi K, Takeda Y. Absolute configuration of (+)-pinoresinol 4-O-[6″-O-galloyl]-β-d-glucopyranoside, macarangiosides E, and F isolated from the leaves of Macaranga tanarius. Phytochemistry, 2009; 70(10):1277–85. CrossRef
Mazlan NA, Mediani A, Abas F, Ahmad S, Shaari K, Khamis S, Lajis NH. Antioxidant, antityrosinase, anticholinesterase, and nitric oxide inhibition activities of three malaysian Macaranga species. ScieWorld J, 2013; 2013:312741. CrossRef
Milito A, Castellano I, Damiani E. From Sea to Skin: is there a future for natural photoprotectants? Mar Drugs, 2021; 19(7):379. CrossRef
Minarti Cahyana AH, Darmawan A. Isolation, characterization and antidiabetic activity of phenolic compounds isolated from Macaranga magna turrill. Rasayan J Chem, 2021; 14(4):2420–7. CrossRef
Morais T, Cotas J, Pacheco D, Pereira L. Seaweeds compounds: an ecosustainable source of cosmetic ingredients? Cosmetics, 2021; 8. CrossRef
Muhaimin M, Yusnaidar Y, Wilda S, Madyawati L, Riski Dwimalida P, Andita U, Anis Yohana C, Andreas Yoga A, Josephine Elizabeth S. Antiplasmodial activity of ethanolic extract of Macaranga Gigantea leaf and its major constituent. Pharmacognosy J, 2019;11(6) CrossRef
Mujtaba SF, Masih AP, Alqasmi I, Alsulimani A, Khan FH, Haque S. Oxidative-stress-induced cellular toxicity and glycoxidation of biomolecules by cosmetic products under sunlight exposure. Antioxidants, 2021; 10(7):1008. CrossRef
Musthaba S, Ahmad S, Ahuja A, Ali J, Baboota S. Nano approaches to enhance pharmacokinetic and pharmacodynamic activity of plant origin drugs. Curr Nanoscie, 2009; 5(3):344–52. CrossRef
Nam SH, Yamano A, Kim JA, Lim J, Baek SH, Kim JE, Kwon TG, Saito Y, Teruya T, Choi SY, Kim YK, Bae YC, Shin HI, Woo JT, Park EK. Prenylflavonoids isolated from Macaranga tanarius stimulate odontoblast differentiation of human dental pulp stem cells and tooth root formation via the mitogen-activated protein kinase and protein kinase B pathways. Int Endod J, 2021; 54(7):1142–54. CrossRef
Natsume N, Yonezawa T, Saito Y Woo JT, Teruya T. Prenylflavonoids from fruit of Macaranga tanarius promote glucose uptake via AMPK activation in L6 myotubes. J Nat Med, 2021; 75(4):813–23. CrossRef
Ngoumfo RM, Ngounou GE, Tchamadeu CV, Qadi MI, Mbazoa CD, Begum A, Ngninzeko FN, Lontsi D, Choudhary MI. Inhibitory effect of macabarterin, a polyoxygenated ellagitannin from Macaranga barteri, on human neutrophil respiratory burst activity. J Nat Prod, 2008; 71(11):1906–10. CrossRef
Niu HM, Zeng DQ, Long CL, Peng YH, Wang YH, Luo JF, Wang HS, Shi YN, Tang, GH, Zhao FW. Clerodane diterpenoids and prenylated flavonoids from Dodonaea viscosa. J Asian Nat Prod Res, 2010; 12(1):7–14. CrossRef
Nowak K, Jab?o?ska E, Ratajczak-Wrona W. Controversy around parabens: alternative strategies for preservative use in cosmetics and personal care products. Environment Res, 2021; 198:110488. CrossRef
Ogbole OO, Segun PA, Fasinu PS. Antimicrobial and antiprotozoal activities of twenty-four Nigerian medicinal plant extracts. S Afr J Botany, 2018; 117:240–6. CrossRef
Ogundajo A, Okeleye B, Ashafa AO. Chemical constituents, in vitro antimicrobial and cytotoxic potentials of the extracts from Macaranga barteri Mull-Arg. Asian Pac J Trop Biomed, 2017; 7(7):654–9. CrossRef
Ogundajo AL, Tom Ashafa AO. Chemical profiling, antioxidant and carbohydrate-metabolising enzymes inhibitory potential of fractions from the leaves of Macaranga bateri Mull-Arg. Trans R Soc S Afr, 2019; 74(1):27–37. CrossRef
Pagna JIM, Awazi T, Mbarga PE, Mbekou IMK, Mkounga P, Fotie J, Frese M, Fabrice FB, Lenta BN, Sewald N, Nkengfack EA. Antibacterial flavonoids from the fruits of Macaranga hurifolia. J Asian Nat Prod Res, 2022: 1–1. CrossRef
Pailee P, Sangpetsiripan S, Mahidol C, Ruchirawat S, Prachyawarakorn V. Cytotoxic and cancer chemopreventive properties of prenylated stilbenoids from Macaranga siamensis. Tetrahedron, 2015; 71(34):5562–71. CrossRef
Panda SK, Padhi L, Leyssen P, Liu M, Neyts J, Luyten W. Antimicrobial, anthelmintic, and antiviral activity of plants traditionally used for treating infectious disease in the similipal biosphere reserve, Odisha, India. Front Pharmacol, 2017; 8:658. CrossRef
Péresse T, Jézéquel G, Allard PM, Pham VC, Huong DTM, Blanchard F, Bignon J, Lévaique H, Wolfender JL, Litaudon M, Roussi F. Cytotoxic prenylated stilbenes isolated from Macaranga tanarius. J Nat Prod, 2017; 80(10):2684–91. CrossRef
Petruk G, Del Giudice R, Rigano MM, Monti DM. Antioxidants from plants protect against skin photoaging. Oxid Med Cell Longev, 2018; 2018:1454936. CrossRef
Phommart S, Sutthivaiyakit P, Chimnoi N, Ruchirawat S, Sutthivaiyakit, S. Constituents of the leaves of Macaranga tanarius. J Nat Prod, 2005; 68(6):927–30. CrossRef
Qi WY, Shen Y, Wu Y, Leng Y, Gao K, Yue JM. Deheiculatins A-L, 20-oxygenated cembranoids from Macaranga deheiculata. Phytochemistry, 2017; 136:101–7. CrossRef
Quan W, Lee H, Noh C, Um ABH, Oak MH, Kim KM. Anti-allergic prenylated flavonoids from the roots of sophora flavescens. Planta Med, 2008; 74:168–70. CrossRef
Ramaiah PA, Row LR, Reddy DS, Anjaneyulu ASR, Ward RS, Pelter A. Isolation and characterisation of bergenin derivatives from Macaranga peltata. J Chem Soc Perkin Trans 1, 1979; (0):2313–6. CrossRef
Rees J. The genetics of sun sensitivity in humans. Am. J. Hum. Genet., 2004; 75(5):739–51. CrossRef
Sadeer, N, Llorent-Martínez E, Bene K, Mahomoodally F, Mollica A, Ibrahime S, Stefanucci A, Ruiz Riaguas A, Fernández de Cordova ML, Zengin G. Chemical profiling, antioxidant, enzyme inhibitory and molecular modelling studies on the leaves and stem bark extracts of three African medicinal plants. J Pharm Biomed Anal, 2019; 174:19–33 CrossRef
Saklani A, Kutty SK. Plant-derived compounds in clinical trials. Drug Discov Today, 2008; 13(3-4):161–71. CrossRef
Salah MA, Bedir E, Toyang NJ, Khan IA, Harries MD, Wedge DE. Antifungal clerodane diterpenes from Macaranga monandra (L) Muell. et Arg. (Euphorbiaceae). J Agric Food Chem, 2003; 51(26):7607–10. CrossRef
Salleh WMNHW, Razak N, Farediah A. Phytochemicals and biological activities of Macaranga hosei and Macaranga constricta (Euphorbiaceae). Marmara Pharm J, 2017; 21:881–8. CrossRef
Salvioni L, Morelli L, Ochoa E, Labra M, Fiandra L, Palugan L, Prosperi D, Colombo M. The emerging role of nanotechnology in skincare. Adv Colloid Interface Sci, 2021; 293:102437.
Schütz BA, Wright AD, Rali T, Sticher O. Prenylated flavanones from leaves of Macaranga pleiostemona. Phytochemistry, 1995; 40(4):1273–7. CrossRef
Segun P, Gbadebo M, Adebowale M, Olufolabo K, Fred-Jaiyesimi A. Investigation of the anti-inflammatory and hypoglycaemic effects of Macaranga hurifolia beille (eurphorbiaceae) extract on wistar albino rats. ACTA Pharm Sci, 2019a; 57:93. CrossRef
Segun PA, Ogbole OO, Akinleye TE, Faleye TOC, Adeniji AJ. In vitro anti-enteroviral activity of stilbenoids isolated from the leaves of Macaranga barteri. Nat Prod Res, 2021; 35(11):1909–13. CrossRef
Segun PA, Ogbole OO, Ismail FMD, Nahar L, Evans AR, Ajaiyeoba EO, Sarker SD. Bioassay-guided isolation and structure elucidation of cytotoxic stilbenes and flavonols from the leaves of Macaranga barteri. Fitoterapia, 2019b; 134:151–7. CrossRef
Shahinozzaman M, Obanda DN, Tawata S. Chemical composition and pharmacological properties of Macaranga-type Pacific propolis: a review. Phytother Res, 2021; 35(1):07–22. CrossRef
Sultana S, Ilyas M. Chromenoflavones from Macaranga indica. Phytochemistry, 1986; 25(4):953–4. CrossRef
Susanto D, Kusuma R, Amirta R. Nutrient distribution on soil and abovegroundbiomass of Macaranga gigantea five years after planting. Asian J Forest, 2018; 2:12–9. CrossRef
Sutthivaiyakit S, Unganont S, Sutthivaiyakit P, Suksamrarn A. Diterpenylated and prenylated flavonoids from Macaranga denticulata. Tetrahedron, 2002; 58(18):3619–22. CrossRef
Syah YM, Ghisalberti EL. Flavanone derivatives from Macaranga tanarius. Biochemical Systematics and Ecology, 2015; 62:151–4. CrossRef
Syah YM, Ghisalberti EL. Phenolic derivatives with an irregular sesquiterpenyl side chain from Macaranga pruinosa. Nat Prod Commun, 2010; 5(2):219–2. CrossRef
Tanjung M, Hakim EH, Elfahmi Latip J, Syah YM. Dihydroflavonol and flavonol derivatives from Macaranga recurvata. Nat Prod Commun, 2012; 7(10):1309–10. CrossRef
Tanjung M, Hakim EH, Mujahidin D, Hanafi M, Syah YM. Macagigantin, a farnesylated flavonol from Macaranga gigantea. J Asian Nat Prod Res, 2009; 11(11):929–32. CrossRef
Tanjung M, Hakim EH, Syah YM. Prenylated dihydrostilbenes from Macaranga rubiginosa. Chem Nat Compd, 2017; 53(2):215–8. CrossRef
Tanjung M, Juliawaty LD, Hakim EH, Syah YM. Flavonoid and stilbene derivatives from Macaranga trichocarpa. Fitoterapia, 2018; 126:74–7. CrossRef
Tjahjandarie T, Tanjung M, Saputri R, Nadar P, Aldin M, EvaMarliana, Permadi A. Flavestin K. An isoprenylated stilbene from the leaves of Macaranga recurvata gage. Nat Prod Sci, 2019; 25:244. CrossRef
Trinh Thi Thanh V, Doan Thi Mai H, Pham VC, Litaudon M, Dumontet V, Guéritte F, Nguyen VH, Chau VM. Acetylcholinesterase inhibitors from the leaves of Macaranga kurzii. J Nat Prod, 2012; 75(11):2012–5. CrossRef
Tseng MH, Chou CH, Chen YM, Kuo YH. Allelopathic prenylflavanones from the fallen leaves of Macaranga tanarius. J Nat Prod, 2001; 64(6):827–8. CrossRef
Van Der Kaaden JE, Hemscheidt TK, Mooberry SL. Mappain, a new cytotoxic prenylated stilbene from Macaranga mappa. J Nat Prod, 2001; 64(1):103–5. CrossRef
Verma M, Raj V, Hariharapura R, Rao J, Udupa N. Screening of plant Macaranga peltata for its antioxidant, antimicrobial and cytotoxicity activity. Int Conference Biomed Pharm Eng Singapore, 2009. CrossRef
Versiani M, Ratnayake R, Henrich C, Bates S, McMahon J, Gustafson K. flavonoids from eight tropical plant species that inhibit the multidrug resistance transporter ABCG2. J Nat Prod, 2011; 74:262–6. CrossRef
Vostinaru O, Heghes S, Filip L. Essential oils—bioactive compounds. New perspectives and applications. IntechOpen, Paris, France, 2020.
Vu LTN, Anh LT, Cuc NT, Nhiem NX, Tai BH, Van Kiem P, Litaudon M, Thach TD, Van Minh C, Mai HDT, Van Cuong P. Prenylated flavonoids and other constituents from Macaranga indica. Nat Prod Res, 2021; 35(13):2123–0. CrossRef
Wang TS, Liu BJ, Hua SY, Li TL, Chen GY. Study on the chemical constituents of liposoluble steroidal and triterpenoid compounds from the stem and bark of Macaranga hemsleyana. Zhong Yao Cai, 2008; 31(3):372–4.
Yang DS, Li ZL, Wang X, Yan H, Yang YP, Luo HR, Liu KC, Xiao WL, Li XL. Denticulatains A and B: unique stilbene-diterpene heterodimers from Macaranga denticulata. RSC Advances, 2015a; 5(18):13886–90. CrossRef
Yang DS, Peng WB, Yang YP, Liu KC Li, X.-L, Xiao WL. Cytotoxic prenylated flavonoids from Macaranga indica. Fitoterapia, 2015b; 103. CrossRef
Yang DS, Wang SM, Peng WB, Yang YP, Liu KC, Li XL, Xiao WL. Minor prenylated flavonoids from the twigs of Macaranga adenantha and their cytotoxic activity. Nat Prod Bioprospect, 2015c; 5(2):105–9. CrossRef
Yang DS, Wei JG, Peng WB, Wang SM, Sun C, Yang YP, Liu KC, Li XL. Cytotoxic prenylated bibenzyls and flavonoids from Macaranga kurzii. Fitoterapia, 2014; 99:261–6. CrossRef
Yoder BJ, Cao S, Norris A, Miller JS, Ratovoson F, Razafitsalama J, Andriantsiferana R, Rasamison VE, Kingston DG. Antiproliferative prenylated stilbenes and flavonoids from Macaranga alnifolia from the Madagascar rainforest. J Nat Prod, 2007; 70(3):342–6. CrossRef
Yoshimura K, Hosoya T, Fujinami M, Ohta T, Kumazawa S. Nymphaeol-C a prenylflavonoid from Macaranga tanarius, suppresses the expression of fibroblast growth factor 18. Phytomedicine, 2017; 36:238–42. CrossRef
Zahidin NS, Saidin S, Zulkifli RM, Muhamad II, Ya’akob H, Nur H. A review of Acalypha indica L. (Euphorbiaceae) as traditional medicinal plant and its therapeutic potential. J Ethnopharmacol, 2017; 207:146–73. CrossRef
Zakaria I, Ahmat N, Ahmad R, Mohd Jaafar F, Ghani N, Ghani S, Khamis. Flavanones from the Flower of Macaranga triloba. World Appl Sci J, 2010; 9:1003–7.
Zakaria I, Ahmat N, Jaafar FM, Widyawaruyanti A. Flavonoids with antiplasmodial and cytotoxic activities of Macaranga triloba. Fitoterapia, 2012; 83(5):968–72. CrossRef
Zhang LB, Lei C, Gao LX, Li JY, Li J, Hou AJ. Isoprenylated flavonoids with PTP1B inhibition from Macaranga denticulata. Nat Prod Bioprosp, 2016; 6(1):25–30. CrossRef
Zapanta BR, Achondo MJMM, Raganas AFM, Fritzie A, Delima AGD, Mantiquilla, JA, Salvaña FRP. Species richness of trees in disturbed habitats within a protected area and its implications for conservation: the case of Mt. Apo natural park, Mindanao Island, Philippines. Biodivers J Biol Divers, 2019; 20: 2081–91. CrossRef
Zixi W, Bainian S, Jin P, Deng P, Chen J, Sun F. A new species of Macaranga from the middle miocene of Fujian, China and its significance. Hist Biol, 2016; 29: 1–2. CrossRef
Zorzi GK, Caregnato F, Moreira JCF, Teixeira HF, Carvalho ELS. Antioxidant effect of nanoemulsions containing extract of Achyrocline satureioides (Lam) D.C.—Asteraceae. AAPS Pharm Sci Tech, 2016; 17(4):844–50. CrossRef