INTRODUCTION
Actinomycetes are saprophytic bacteria that are Gram-positive and free-living. They are found in terrestrial and aquatic environments (Lacombe-Harvey et al., 2018). In their DNA, actinomycetes have a high guanine and cytosine content of over 55% (Devi et al., 2013). Actinomycetes include at least 350 genera (Takahashi and Nakashima, 2018). Although they are present in the soil at a density of 106–109 cells/g, more than 95% of actinomycetes isolated from the soil are Streptomycetes (Jakubiec-Krzesniak et al., 2018).
Actinomycetes are exceptionally rich, diverse, easily accessible, and the most potent sources for producing bioactive secondary metabolites with different biological activities (Janardhan et al., 2014; Sharma and Thakur, 2020). Economically and biotechnologically, they are considered the most important prokaryotes with significant biological activity (Dholakiya et al., 2017). They are widely distributed in soils and synthesize biologically active compounds with good economic value, including a lot of medical significance (Baltz, 2007). For example, it has been estimated that approximately two-thirds of the natural antibiotics, anticancer, anthelmintic, and antifungal compounds are obtained from actinomycetes (Jakubiec-Krzesniak et al., 2018). Geographical area, soil type, cultivation and organic matter content, soil temperature, pH, aeration, and moisture content play key roles in actinomycetes’ isolation from the soil (Hazarika and Thakur, 2020).
Dacrycarpus imbricatus is known as Jamuju in Indonesia. Its timber has been used in construction, furniture, firewood, and ornamental plant (Hau et al., 2017). Several chemical compounds with their bioactivities have been reported. For example, pimaric acid isolated from D. imbricatus has medium activity against human cancer cell lines KB and HepG2 (Hau et al., 2017). In addition, 20-hydroxyecdysone isolated from D. imbricatus bark has the activity to inhibit the proliferation of acute myeloid leukemia cells (Thuy et al. 2017).
Rhizosphere soil, the area around the plant roots, is inhabited by the microbial population, which is affected by compounds or root exudates released by the plant roots (McNear, 2013). The rhizosphere also acts as the center of interaction between microbes, plant roots, soil, and environments (Henneron et al., 2020). Therefore, the nearby plant affects actinomycetes from the rhizosphere soil. Furthermore, root exudates affect the type of microbes in the rhizosphere soil, and the root exudates are utilized as carbon and energy sources by microbes (Quian et al., 1997).
Currently, there is an increase in antibiotic resistance, which has become a global public health problem, with a prediction of causing 10 million deaths by 2050 and a global cost of 100 trillion USD (Trotter et al., 2019). In addition, antibiotic resistance may result in prolonged illness, disability, and even death (Chandra et al., 2020). Globally, infectious diseases are the second leading cause of death (Luzhetskyy et al., 2007). Therefore, the search for new antibiotic sources that can overcome resistance by improving binding complexity with the drug target and improving pharmacological properties (Hoffman, 2020) is necessary to be carried out.
On the other hand, normal cell metabolism produces free radicals continuously that harm our health. Because of their unpaired electrons, free radicals are extremely reactive and unstable. Free radicals destroy healthy tissue to become stable. Endogenous antioxidants produced within the cells might be inadequate. Therefore, exogenous antioxidants are required to eliminate the adverse effect of free radicals on DNA, proteins, lipids, and other biomolecules in the human body (Abu et al., 2017).
Dacrycarpus imbricatus has important ecological functions as a carbon source, supports the soil microbial community, improves soil nutrients, and conserves water (Rahadiantoro et al., 2013). Due to the high potential of actinomycetes in producing bioactive compounds and the relationship between microbes and the rhizospheres, a study on the potency of actinomycetes collected from the rhizosphere soil of D. imbricatus (Podocarpaceae) from Toba Samosir needs to be determined.
MATERIAL AND METHODS
Isolation and molecular identification of actinomycetes
The soil samples were taken from the rhizosphere soil of D. imbricatus in Toba Samosir, North Sumatra. At room temperature, soil samples were air-dried, mashed, and sifted to obtain uniform soil particles. Actinomycetes were isolated by sodium dodecyl sulfate-yeast extract (SDS-YE) (Hayakawa and Nonomura, 1989). About 9 ml of sterile aqua dest was used to dissolve 1 g of soil particles and homogenized using vortex for 10 minutes. Next, 500 µl of soil suspension was placed in a test tube containing SDS-YE and homogenized for 5 minutes. After homogenizing, tubes were incubated in a shaker water bath for 20 minutes at 40°C. After incubation was complete, the suspension was diluted to 106 unity. The diluted suspension (100 µl) was cultivated on humic acid-vitamin agar (HVA) growth media in a Petri dish. HVA medium was supplemented with nalidixic acid and chlortetracycline antibiotics (Wako Pure Chemical Industries) at 10 mg/l. This media was incubated at 30°C for 2 weeks. The emerging actinomycetes on HVA were purified by re-cultivating on yeast extract agar and incubated for 1–2 weeks. The pure isolates of actinomycetes were preserved in 10% glycerol at −80°C for further study. Actinomycetes isolate (1 × 1 cm2) from yeast extract agar was cultured in broth YIM 310 media (Hazarika and Thakur, 2020) and incubated in a shaker water bath for 2 weeks at 130 rpm, 22°C–24°C.
Molecular identification of potential isolates and the phylogenetic analysis were carried out as follows. Actinomycetes were cultured on a yeast starch agar plate for 7 days. First, the mass of mycelia and spore were harvested and transferred into a 2 ml micro-tube, and the micro-tube was incubated overnight at −20°C. Then, 500 µl of distilled water was used to wash the mycelium and spores of actinomycetes (Putri and Sumerta, 2020). DNA extraction was carried out following Franco-Correa et al. (2010). Using a set of universal primers 27F and 1492R, polymerase chain reaction (PCR) amplification of 16S rDNA was performed. Initial denaturation was at 94°C for 1 minute, followed by 30 cycles of PCR amplification, denaturation at 95°C for 30 seconds, annealing at 50°C for 30 seconds, and then elongation at 72°C for 1 minute 30 seconds (Putri and Sumerta, 2020). The 16S rRNA gene fragment was sequenced at Macrogen®, Republic of Korea. Cromaspro program version 1.6 was used to analyze the raw sequencing data. The 16S rDNA sequence of the isolates was matched to those in the public database using the EzBioCloud tool (http://eztaxon-e.ezbiocloud.net) for phylogenetic analysis (Yoon et al., 2017). The isolates were aligned with the closest representative gene sequences by the Clustal W program. The construction of phylogenetic trees of the isolates was done by the neighbor-joining method with bootstrap analysis of 1,000 replicates performed in the MEGA version 6.
Extraction of bioactive metabolites
The method of bioactive metabolite extraction was following Praptiwi et al. (2019). Liquid media and biomass of actinomycetes were extracted three times with ethyl acetate as a solvent after 2 weeks of incubation. A separating funnel was used to separate the ethyl acetate extract, concentrated using an evaporator, and then stored at −4°C in a glass vial.
Chemical compounds analysis by thin-layer chromatography (TLC)
Analysis of chemical compounds using the TLC method was following Praptiwi et al. (2019). The TLC used silica plates (Merck GF254) to separate chemical compounds in actinomycetes extracts. Extracts (10 µl in a concentration of 10 mg/ml) were loaded on the normal phase of silica gel plates (Merck GF254). Silica plates were developed in a mobile phase containing dichloromethane: methanol (10:1). The chromatogram was observed by UV light at 254 and 366 nm and sprayed with cerium sulfate reagent and vanillin sulfate for the color reaction.
Detection of anti-bacterial activity by TLC-bioautography
Anti-bacterial activity by TLC-bioautography method was following Praptiwi et al. (2019). Anti-bacterial assay of 11 actinomycetes extracts was tested against Staphylococcus aureus and Escherichia coli by TLC-bioautography. The ethyl acetate extract of actinomycetes (10 µl at a concentration of 10 mg/ml) was loaded on silica plates. After drying the plates, they were dipped in the bacterial suspension. Plates were incubated at 37°C for 18 hours under humid conditions. Aqueous iodonitrotetrazolium (INT) chloride solution (4 mg/ml, Sigma) was used to spray the TLC plate after incubation. A clear zone against a purple background indicated an active anti-bacterial extract (Famuyide et al., 2019). The active extracts were further analyzed to determine the active anti-bacterial compounds in the extract by loading extract (10 µl at a concentration of 10 mg/ml) and were developed using the mobile phase of dichloromethane: methanol (10:1). The dried plates that have been developed were dipped in bacterial suspension followed by incubation under the humid condition at 37°C for 18 hours. Plates were sprayed with an INT solution after the incubation was completed. The formation of a clear zone with a purple background characterized the active anti-bacterial compounds. Positive control in this study used chloramphenicol, while ethyl acetate was used as a negative control.
Determination of minimum inhibitory concentration (MIC) by broth microdilution method
The MIC by broth microdilution method was following Praptiwi et al. (2019). The MIC value was performed by two-fold microdilution on 96 microwell plates in triplicate. All 12 wells in the first row were filled with 100 µl of Mueller Hinton broth (MHB) and 10 µl of extract (at the concentration of 10,240 ppm in dimethyl sulfoxide) and added with sterilized distilled water (90 µl). At the second to eighth row, 100 µl of MHB was added to every well. The well in row 1 was thoroughly mixed, took 100 µl from it, transferred to the well on the second row, and homogenized. Again, 100 µl of the mixture on the second row is transferred into the third row. The serial dilution is continued, and 100 µl is removed and discarded in the last row. After diluting, each well is filled with 100 µl bacterial suspension and incubated for 24 hours at 37°C. This assay was performed in the laminar airflow aseptically. After incubation, each well was added with 10 µl INT chloride (4 mg/ml). The lowest concentration of the well that had no change in color is determined as the MIC value.
Determination of anti-mycobacterial activity by resazurin reduction assay against Mycobacterium smegmatis
Anti-mycobacterial activity by resazurin reduction assay against M. smegmatis was following Fathoni et al. (2022). Resazurin reduction assay was carried out only on potential extracts (A18-TE1-1 and A18-TE1-8). Mycobacterium smegmatis was cultivated in Middlebrook (7H9) broth media and incubated overnight on a shaker incubator. After incubation, M. smegmatis was diluted 1,000× using Middlebrook media. Each well on a 96-well microplate was filled with 2 µl of extract (2.5 mg/ml in 50% DMSO), followed by filling with 48 µl of diluted M. smegmatis cells. The positive control well contained 2 µl of 50% DMSO and 48 µl of diluted M. smegmatis cells. The negative control well contained 2 µl of 50% dimethyl sulfoxide (DMSO) and 48 µl of 7H9 media. After overnight incubation, each well was added with 20 µl of resazurin mixed with tween 80 (1:1) and incubated overnight. The absorbance was carried out using Varioscan LUX and determined at 530 nm with the emission at 590 nm.
TLC-bioautography for DPPH-free radicals scavenging activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH)-free radical scavenging activity by TLC-bioautography was following Praptiwi et al. (2019). Screening of radical scavenging activity on DPPH was performed by the TLC-bioautographic method on TLC silica plates. About 10 µl of extract (containing 10 mg/ml) were loaded on silica plates. After loading, the plates were sprayed with 0.2% DPPH methanolic solution. The plates were incubated at room temperature for 30 minutes. After incubation, the extracts producing yellowish-white color against a purple background were considered DPPH-free radical scavengers. The extracts with DPPH-free radical scavenging activity were further analyzed by separating the compounds on TLC plates. The mixture of dichloromethane and methanol (10: 1, v/v) was used as the mobile phase. After the TLC plate dried, the plates were sprayed with DPPH solution in methanol. Observations were made 30 minutes after spraying. Yellowish-white band formation against a purple background was considered the active compound with DPPH-free radical scavenging activity.
Determination of IC50 value for DPPH-free radicals scavenging activity
The determination of the IC50 value for free radical scavenging activity was following Praptiwi et al. (2019). The determination of IC50 for DPPH-free radicals scavenging activity was performed by serial microdilution on 96 microwell plates in triplicate. Each well was filled with 100 µl methanol p.a. Wells in the first row of the microwell plate were added with 5 µl of extract and 95 µl methanol and homogenized. Next, 100 µl of the mixture from the first row was removed, transferred into the second row, and homogenized. Again, 100 µl of the mixture from the second row was removed and transferred into the third row. Finally, in the last row, 100 µl of the mixture was discarded. After serial dilution, 100 µl of DPPH (61.5 µl/ml) was added to each well, and the final concentration of DPPH was 30.75 µg/ml. The microwell plate was incubated for 90 minutes at room temperature in the dark. A Vario Scan Flash microplate reader (Thermo Scientific) at a wavelength of 517 nm was used to determine the absorbance of the samples. Inhibitory concentration was calculated using the following equation:
The sample concentration inhibits 50% of free radicals DPPH as the IC50 value. The linear regression curve was determined from the graph of the inhibitory percentage against extract concentration (Guchu et al., 2020).
RESULTS AND DISCUSSION
Isolation and molecular identification of actinomycetes from D. imbricatus
Eleven actinomycetes isolates were successfully isolated and identified from the rhizosphere soil of D. imbricatus (Table 1). Several isolates also have been identified at the genus or species level, and the potential isolates were identified further using molecular identification and phylogenetic tree reconstruction. The result in Table 1 showed that the rhizosphere soil of D. imbricatus was inhabited by several genera, even several species of actinomycetes.
Anti-bacterial activity of actinomycetes
Pathogenic microorganisms’ emergence and antibiotic resistance pose a serious global (Aslam et al., 2018). Therefore, it is necessary to search for a new source of anti-bacterial compounds, such as actinomycetes, that may overcome resistance problems. Eleven actinomycete isolates were cultured on HVA medium from the rhizosphere soil of D. imbricatus in Toba Samosir and determined their anti-bacterial activity. TLC-bioautography carried out the anti-bacterial screening of actinomycetes extracts from Toba Samosir. TLC-bioautography combines planar chromatographic separation and in situ detection of biological activity (Zang et al., 2020). It enables rapid detection of antimicrobial compounds due to the response of the active compound as a clear zone around the compound, as well as the Rf value of the compound on the TLC plate after INT spraying. In addition, the Dot-Blot test was carried out to detect the anti-bacterial activity of the unseparated compounds in the extract.
Table 2 showed that five extracts (nos. 2, 3, 8, 9, and 10) were active against E. coli, and seven were active against S. aureus (1, 4, 6, 8, 9, 10, and 11). The anti-bacterial activity was visualized by spraying the TLC plate with an INT solution. The white area formation against a purple background indicated the growth inhibition of bacteria (Praptiwi et al., 2019). The purple background resulted from converting tetrazolium salt by the dehydrogenase enzymes in living microorganisms into a purple formazan (Villegas-Mendoza et al., 2019). The formazan production per cell increases following an increase in growth rate (Cretu and Morlock, 2014). In the presence of anti-bacterial compounds, INT reduction to the color formazan did not occur, as indicated by the white area formation (Aslam et al., 2018).
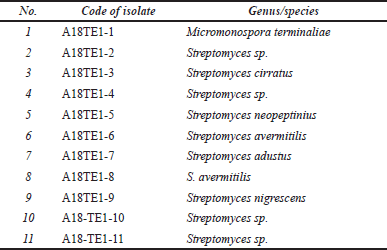 | Table 1. Identified actinomycetes isolate from rhizosphere soil of D. imbricatus. [Click here to view] |
The MIC value in Table 3 showed that E. coli was less sensitive than S. aureus toward actinomycetes extracts. The MIC values of actinomycetes extract against S. aureus ranged from 8 to >256 µg/ml, while the MIC value against E. coli was >256 µg/ml. The differences in Gram-negative and Gram-positive bacteria cell wall structures might influence the sensitivity of bioactive compounds in the extract of actinomycetes. The outer membranes of Gram-negative cell walls were arranged phospholipidic, containing a thick layer of lipopolysaccharide components as a barrier that protects the cell wall and inner membrane (Shaku et al., 2020; Vaara, 1992). Gram-negative bacteria have a synergistic action of outer membrane permeability, efflux pump activities, and enzymatic degradation that efficiently reduce the intracellular concentrations of small molecules (Vergalli et al., 2020). Gram-positive bacteria’s cell walls have a multilayer peptidoglycan structure without an outer cell membrane (Shaku et al., 2020). As a result, the hydrophobic anti-bacterial component can damage Gram-positive bacterial cell walls (Vaara, 1992). Cell membranes of Gram-positive bacteria become more permeable and cause bacterial death.
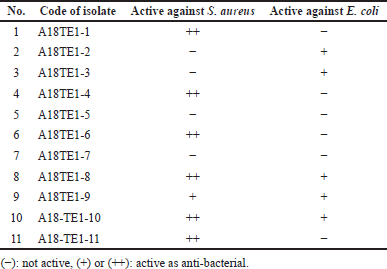 | Table 2. Anti-bacterial activity of 11 actinomycetes extracts from Toba Samosir against S. aureus and E. coli by Dot-Blot method. [Click here to view] |
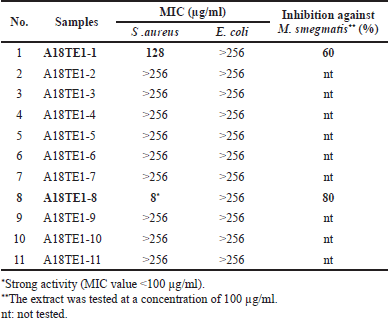 | Table 3. The MIC of actinomycetes extracts from D. imbricatus rhizosphere soil in Toba Samosir, North Sumatra, against S. aureus and E. coli and the inhibition percentage against M. smegmatis. [Click here to view] |
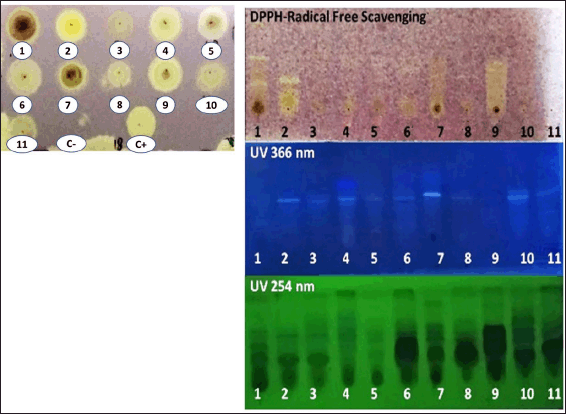 | Figure 1. Dot-Blot assay for DPPH-free radical scavenging activity of 11 actinomycetes extracts (A18TE1-1–A18TE1-11) (left). The plates were eluted in a dichloromethane: methanol (10:1) solvent system (right). A 0.2% DPPH methanolic solution was sprayed onto the plate. Catechin was used as a positive control (C+). A yellowishwhite spot or band on a purple background is considered a DPPH-free radical scavenger. [Click here to view] |
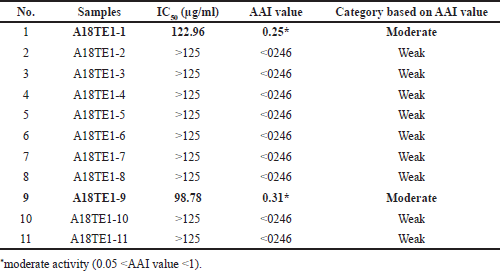 | Table 4. The value of IC50 and Antioxidant Activity Index (AAI) of actinomycetes extracts from D. imbricatus rhizosphere soil in Toba Samosir, North Sumatra. [Click here to view] |
The results of the resazurin reduction assay on two extracts (A18-TE1-1 and A18TE1-8) showed that A18-TE1-1 had the inhibition of 60% while A18-TE1-8 had the inhibition of 80% on the tested concentration of 100 µg/ml. Based on this result, both extracts will be addressed for further isolation and purification of the active compound.
Determination of the activity of DPPH-free radicals scavenging
The DPPH-free radical scavenging activity of actinomycetes extracts from Toba Samosir was evaluated by TLC-bioautography (Dot-Blot assay and developed TLC) on DPPH. Since DPPH is a stable free radical that embraces an electron or hydrogen radical, it is commonly used and approved for testing natural products’ antiradical activity (Pattusamy and Changa, 2017). In addition, bioautography is a fast, simple, and inexpensive method for screening biological and chemical extracts (Praptiwi et al., 2018). In the Dot-Blot assay, the individual activity of the extract was visible.
A yellowish-white spot suggested that almost all extracts have DPPH-free radical scavenging activity, as shown in Figure 1and Table 4. The yellowish-white spot on the purple background is due to free radicals DPPH, with the purple color reduced by natural antioxidants or reducing compounds to pale yellow hydrazine (Nurul et al., 2013). The capacity of the extract as a DPPH-free radical scavenger could be indicated by the yellowish-white color intensity (Praptiwi et al., 2018). A TLC plate was developed in dichloromethane: methanol solvent system to further analyze active extracts (10:1).
Figure 1 also showed the presence of yellowish-white bands against a purple background on the TLC plate and several chemical compounds with DPPH-free radicals scavenging activity, but it varied amongst extracts. The yellowish-white band indicated reducing compounds that can donate electrons and result in the disappearance of its violet color (AlNeyadi et al., 2020).
The DPPH method was also used to assess the quantitative DPPH-free radical scavenging activity of actinomycetes extracts and catechin as a positive control. According to Scherer and Godoy (2009), a free radical scavenger on DPPH can be classified based on the AAI values as follows: weak (low) < 0.05 < moderate < 1.00 < strong (significant) < 2.00 < very strong activity as an antioxidant agent. Among the 11 extracts used in this study, 2 extracts (A18TE1-1a and A18TE1-9) displayed moderate DPPH-free radical scavenging activity. In the previous study, a bioactive metabolite derivative of anthracene-9,10-dione isolated from actinomycetes from mangrove soil has antioxidant activity near values to the standard ascorbic acid (Janardhan et al., 2014). Another research also showed that some extracts of actinomycetes from rhizospheric soil have strong anti-bacterial activity against S. aureus and E. coli, and one extract had strong antioxidant activity (Praptiwi et al., 2019).
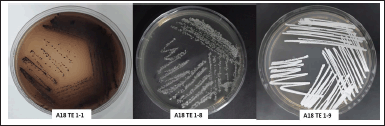 | Figure 2. Tree of potential isolates M. terminaliae A18TE 1(1), S. avermitilis A18TE 1(8), and S. nigrescens A18TE 1(9) from D. imbricatus rhizosphere soil in Toba Samosir. [Click here to view] |
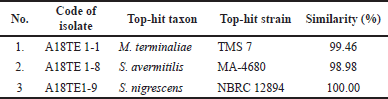 | Table 5. Identification of three potential actinomycetes based on 16S rRNA gene similarity. [Click here to view] |
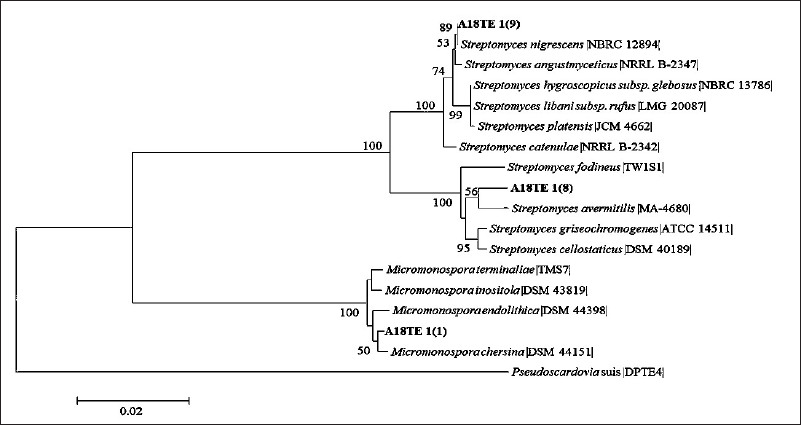 | Figure 3. Phylogenetic tree of isolates A18TE 1(1), A18TE 1(8), and A18TE 1(9) from D. imbricatus rhizosphere soil in Toba Samosir and their closely related type strains based on the 16S rDNA sequences in the 1,000 bootstrap replicates. The bar represents 0.02 substitutions per nucleotide position. Pseudocordavia suis DPTE4 was used as an out-group. [Click here to view] |
Molecular identification of three potential isolates
Three potential isolates (A18TE1-1, A18TE1-8, and A18TE1-9) (Fig. 2) were identified based on 16S rDNA sequencing analysis and compared with strain type from the EzBioCloud database. The potential strains were obtained based on a similarity search including Streptomyces (two strains) and Micronospora (one strain). In addition, three strains belonged to the species Micromonaspora chersina (A18TE 1-1) with a similarity of 99.48%, S. avermitilis (A18TE 1-8) with a similarity of 98.98%, and S. nigrescens (A18TE 1-9) with a similarity of 100% (Table 5). In addition, the phylogenetic tree analysis based on the neighbor-joining tree was performed for molecular identification (Fig. 3).
Actinomycetes isolates such as Micromonaspora spp. and Streptomyces spp. that are isolated from rhizospheric soil of plants Barringtonia racemosa, Albizia odoratissima, Spondias pinnata, and Azadirachta indica have anti-bacterial and antifungal activity (Malisorn et al., 2020). Most of the bioactive metabolites derived from the genus of Micromonospora have shown excellent antimicrobial and anticancer activities,such as aminoglycosides and ansamycins (Hifnawy et al., 2020). The isolates of Streptomyces spp. showed significant antimicrobial activity and was found to possess an antioxidant potential for DPPH and 2,2′-Azinobis- (3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS) radical scavenging activity (Siddharth et al., 2020). Streptomyces sp. MUM212 demonstrated potent antioxidant activity as a DPPH-free radical scavenger (Tan et al., 2017). Comparatively, S. avermitilis has been a potential species for producing avermectin as an anthelmintic (Kitani et al., 2011). In addition, S. avermitilis can produce avenolide, a hormone that controls antibiotic production (Kitani et al., 2011). Due to this ability, isolate A18TE1-8, identified as S. avermitilis, has good anti-bacterial activity against S. aureus and M. smegmatis.
CONCLUSION
This study showed that two actinomycetes extracts, i.e., S. avermitilis A18TE-8 and M. terminaliae A18TE1-1, had moderate-strong anti-bacterial activity against S. aureus and the growth inhibition percentage against M. smegmatis was moderate (60%–80%). Two extracts of actinomycetes, i.e., S. avermitilis A18TE -8 and S. nigrescens A18TE1-9, had moderate antioxidant activity (AAI value > 0.05). All extracts are weak against E. coli. This study concluded that actinomycetes collected from the rhizosphere soil of D. imbricatus in Toba Samosir could be used as a source of anti-bacterial and antioxidants.
ACKNOWLEDGMENTS
The authors acknowledge the financial support from the DIPA of the Indonesian Institute of Sciences. The authors also thank Yunita for her technical assistance. The authors declare that they have no conflicts of interest. All the authors equally contributed as main contributors.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abu F, Taib CNM, Moklas MAM, Akhir SM. Antioxidant properties of crude extract, partition extract, and fermented medium of Dendrobium sabin flower. Evid Based Complement Altern Med, 2017; 2017(2907219):1–9.
AlNeyadi SS, Amer N, Thomas TG, Ajeil RA, Breitener P, Munawar N. Synthesis, characterization, and antioxidant activity of some 2-methoxyphenols derivatives. Heterocycl Comm, 2020; 26(1):112–22.
Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, Nisar MA, Alvi RF, Aslam MA, Qamar MU, Salamat M, Baloch Z. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist, 2018; 11:1645.
Baltz RH. Antimicrobials from actinomycetes: back to the future. Actinomycetes are the source of most clinically relevant antibiotics in use today and may continue to be so. Microbe, 2007; 2(3):125–31.
Chandra S, Prithvi PPR, Srija K, Jauhari S, Grover A. Antimicrobial resistance: call for rational antibiotics practice in India. J Fam Med Prim Care, 2020; 9(5):2192–9.
Cretu GC, Morlock GE. Analysis of anthocyanins in powdered berry extracts by planar chromatography linked with bioassay and mass spectrometry. Food Chem, 2014; 146:104–12.
Devi SC, Kumari A, Jain N, Jemimah NS, Mohanasrinivasan V. Screening of actinomycetes isolated from soil samples for anti-bacterial and antioxidant activity. Int J Pharm Pharm Sci, 2013; 5(4):483–9.
Dholakiya RN, Kumar R, Mishra A, Mody KH, Jha B. Anti-bacterial and antioxidant activities of novel Actinobacteria strain isolated from Gulf of Khambhat, Gujarat. Front Microbiol, 2017; 8:2420.
Fathoni A, Hudiyono S, Cahyana AH, Nurkanto A, Agusta A. (+)-epoxydone: a major secondary metabolite as antibacterial agent from Phomopsis sp. TcBt1Bo-6 isolated from stem of Brotowali plant (Tinospora crispa). Rasayan J Chem, 2022; 15(4):2210–7.
Famuyide IM, Aro AO, Fasina FO. Anti-bacterial and antibiofilm activity of acetone leaf extracts of nine under-investigated south African Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complement Altern Med, 2019; 19:141.
Franco-Correa M, Quintana A, Duque C, Suarez C, Rodríguez MX, Barea JM. Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Appl Soil Ecol, 2010; 45(3):209–17.
Guchu BM, Machocho AK, Mwihia SK, Ngugi P. In vitro antioxidant activities of methanolic extracts of Caesalpinia volkensii Harms., Vernonia lasiopus O. Hoffm., and Acacia hockii De Wild. Evid Based Complement Altern Med, 2020; 2020(3586268):1–10.
Hau DV, Nguyen TT, Nguyen THA, Quan TD, Thien DD, Nhung LTH, Tinh BX, Nguyen LT, Loc TV, Sung TV, Thuy TT. Terpenoids from Dacrycarpus imbricatus. Vietnam J Chem, 2017; 55(6):734–7.
Hayakawa M, Nonomura H. A new method for the intensive isolation of actinomycetes from soil. Actinomycetologica, 1989; 3(2):95–104.
Hazarika SN, Thakur D. Chapter 21—Actinobacteria. In: Amaresan N, Kumar MS, Annapurna K, Kumar K, Sankaranarayanan A (Eds.). Beneficial microbes in agro-ecology, Academic Press, London, UK, pp 443–76, 2020.
Henneron L, Kardol P, Wardle DA, Cros C, Fontaine S. Rhizosphere control of soil nitrogen cycling: a key component of plant economic strategies. New Phytol, 2020; 228:1269–82.
Hifnawy MS, Fouda MM, Sayed AM, Mohammed R, Hassan HM, AbouZid SF, Rate ME, Keller A, Adamek M, Ziemert N, Abdelmohsen UR. The genus Micromonospora as a model microorganism for bioactive natural product discovery. RSC Adv, 2020; 10:20939–59.
Hoffman PS. Anti-bacterial discovery: 21st century challenges. Antibiotics, 2020; 9(5):213.
Jakubiec-Krzesniak K, Rajnisz-Mateusiak A, Guspiel A, Ziemska J, Solecka J. Secondary metabolites of actinomycetes and their antibacterial, antifungal and antiviral properties. Pol J Microbiol, 2018; 67(3):259–72.
Janardhan A, Kumar AP, Buddola VB, Saigopal DVR, Narashima G. Production of bioactive compounds by actinomycetes and their antioxidant properties. Biotechnol Res Int, 2014; 2014:217030.
Kitani S, Miyamoto KT, Takamatsu S, Herawati E, Iguchi H, Nishitomi K, Uchida M, Nagamitsu T, Omura S, Ikeda H, Nihira T. Avenolide, a Streptomyces hormone controlling antibiotic production in Streptomyces avermitilis. Proc Natl Acad Sci USA, 2011; 108(39):16410–5.
Lacombe-Harvey ME, Brzezinski R, Beaulieu C. Chitinolytic functions in actinobacteria: ecology, enzymes, and evolution. Appl Microbiol Biotechnol, 2018; 102:7219–30.
Luzhetskyy A, Pelzer S, Bechthold A. The future of natural products as a source of new antibiotic. Curr Opin Investig Drugs, 2007; 8(8):608–13.
Malisorn K, Embaen S, Sribun A, Saeng-in P, Phongsopitanun W, Tanasupawat S. Identification and antimicrobial activities of Streptomyces, Micromonospora, and Kitasatospora strains from rhizosphere soils. J Appl Pharm Sci, 2020: 10(2):123–8.
McNear Jr DH. The rhizosphere—roots, soil and everything in between. Nat Sci Educ, 2013; 4(3):1.
Nurul MAA, Shafik H, Maria AP, Maria GG. Solvent effect on antioxidant activity and total phenolic content of Betula alba and Convolvulus arvens. Int J Biotechnol Bioeng, 2013; 7(5):351–6.
Pattusamy N, Changa M. Antioxidant activity of 3-arylidene-4-piperidones in the 1,1-diphenyl-2-picrylhydrazyl scavenging assay. J Taibah Univ Sci, 2017; 11:40–5.
Praptiwi P, Raunsai M, Wulansari D, Fathoni A, Agusta, A. Anti-bacterial and antioxidant activities of endophytic fungi extracts of medicinal plants from Central Sulawesi. J App Pharm Sci, 2018; 8(8):69–74.
Praptiwi P, Fathoni A, Putri AL, Wulansari D, Agusta A. Assessment of actinomycetes isolated from soils on Simeuleu Island as antibacterial and antioxidant, AIP Conf Proc, 2019; 2120:080011.
Putri AL, Sumerta IN. Selective isolation of Dactylosporangium and Micromonaspora from the soil of karst cave of Simuelue Island and their anti-bacterial potency. Ber Bio, 2020; 19(3A):257–68.
Quian JH, Doran JW, Walters DT. Maize plant contributions to root zone available carbon and microbial transformation of nitrogen. Soil Biol Biochem, 1997; 29:1451–62.
Rahadiantoro A, Hakim L, Aruningtyas EL. Genetic variation of Dacrycarpus imbricatus in Bromo Tengger Semeru National Park (BTS-NP), East Java. J Trop Life Sci, 2013; 3(2):127–31.
Shaku M, Ealand C, Matlhabe O, Lala R, Kana BD. Chapter Two—Peptidoglycan biosynthesis and remodeling revisited. In: Gadd GM, Sariaslani S (Eds.). Advances in applied microbiology, Academic Press, London, UK, vol. 112, pp 67–103, 2020.
Scherer R, Godoy HT. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem, 2009; 112(3):654–8.
Sharma P, Thakur D. Antimicrobial biosynthetic potential and diversity of culturable soil actinobacteria from forest ecosystems of Northeast India. Sci Rep, 2020; 10:4104.
Siddharth S, Vittal RR, Wink J, Steinert M. Diversity and bioactive potential of actinobacteria from unexplored regions of Western Ghats, India. Microorganisms, 2020; 8(2):225.
Takahashi Y, Nakashima T. Actinomycetes, an inexhaustible source of naturally occurring antibiotics. Antibiotics, 2018; 7(3):74.
Tan LTH, Chan KG, Khan TM, Bukhari SI, Saokaew S, Duangjai A, Pusparajah P, Lee LH, Goh BH. Streptomyces sp. MUM212 as a source of antioxidants with radical scavenging and metal chelating properties. Front Pharmacol, 2017; 8:276.
Thuy TT, Tam NT, Anh NTH, Hau DV, Phong DT, Thang LQ, Adorisio S, Sung TV, Delfino DV. 20-Hydroxyecdysone from Dacrycarpus imbricatus bark inhibits the proliferation of acute myeloid leukemia cells. Asian Pac J Trop Med, 2017; 10(2):157–9.
Trotter AJ, Aydin A, Strinden MJ, O’Grady J. Recent and emerging technologies for the rapid diagnosis of infection and antimicrobial resistance. Curr Opin Microbiol, 2019; 51:39–45. https://doi.org/10.1016/j.mib.2019.03.001
Vaara M. Agents that increase the permeability of the outer membrane. Microbial Rev, 1992; 56(30):395–411.
Vergalli J, Bodrenko IV, Masi M, Lucile M, Acosta-Gutiérrez S, Naismith JH, Davin-Regli A, Ceccarelli M, van den Berg B, Winterhalter M, Pagès JM. Porins and small-molecule translocation across the outer membrane of Gram-negative bacteria. Nat Rev Microbiol, 2020; 18:164–76.
Villegas-Mendoza J, Cajal-Medrano R, Maske H. The chemical transformation of the cellular toxin INT (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(phenyl) tetrazolium chloride) as an indicator of prior respiratory activity in aquatic bacteria. Int J Mol Sci, 2019; 20(3):782.
Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J. Introducing EzBioCloud: a taxonomically united database of 16S rRNA and whole genome assemblies. Int J Syst Evol Microbiol, 2017; 67:1613–7.
Zang Y, Cheng Z, Wu T. TLC Bioautography on screening of bioactive natural products: an update review. Curr Anal Chem, 2020; 16(5):545–56.