INTRODUCTION
Cancer is a terrible disease that can start in any body organ from an abnormal genome function leading to the proliferation of cells at inappropriate times and locations. Cells acquire mutations that abolish the regulation of cell division and multiply continuously, forming tumors and invading new body parts (Weinberg, 2015). According to WHO, about 18.1 million cancer patients worldwide and about 10 million deaths in 2020 are due to cancer. Today, one of the six deaths is due to cancer, the second-largest killer in the world (https://www.who.int/news-room/fact-sheets/detail/cancer). Despite significant improvements in healthcare facilities, the cancer burden grows to cause tremendous financial strain to the human populace. At the same time, poor accessibility of quality medicines, high cost, off-the-target drug resistance, and the severity of the side effects remain significant challenges to successful cancer treatment. Exploring new anticancer compounds from plants and other biological sources could be a good choice. Plant-derived compounds are readily available, possess lesser side effects, and are sometimes more efficacious than the existing drugs (Atanasov et al., 2015).
Plant and plant-derived compounds serve as a promising source of medicine for several diseases (Basu et al., 2020; Fahad et al., 2021; Swargiary and Daimari, 2021). Since ancient times, searching for natural medicines from plants and organisms has remained an essential aspect of drug discovery. Today, about 60% of the approved anticancer drugs are directly or indirectly derived from plant sources (Fridlender et al., 2015). Several phytocompounds, such as paclitaxel, etoposide, camptothecin, vinblastine, vincristine, uvaribonin, 22-epicalamistrin, etc. are plant-derived natural products with considerable anticancer activity (Cragg et al., 2009; Pettit et al., 2008). Persicaria strigosa (R.Br.) Nakai belonging to the family Polygonaceae is a creeping herb that grows well in marshy places. Stems are greenish-brown simple or branched, and leaf blades are green above and prickly on nerves beneath. Geographically, P. strigosa is distributed mainly in Southeast Asian countries, including India. There is very little literature regarding the phytochemistry and bioactivities of P. strigosa. From a survey report, Deka and Devi (2015) opined that the leaves of P. strigosa can be used to control dysentery in cattle. The Bodo community of Assam uses the aerial part (mainly leaves) of P. strigosa as deworming medicine (Swargiary et al., 2019, 2020). The plant is also used to treat jaundice, typhoid, and common sickness. However, no scientific work has been carried out to investigate the bioactivity of the plant. Because of the rich ethnomedicinal values, this study explores the phytochemicals, antioxidants, and antiproliferative properties of P. strigosa.
MATERIALS AND METHODS
Identification of plant and preparation of solvent extracts
The sample plant was collected from the Tinali area of Kokrajhar town with the help of local people. The plant specimen was submitted to the Department of Botany, Bodoland University, and identified as P. strigosa Nakai. (BUBH2018021). Preparation of methanolic crude extract of P. strigosa leaves was carried out following the method described in our earlier publication (Swargiary et al., 2016). Dry, semi-solid P. strigosa methanolic extract (PSME) was kept at −20°C for further use. The PSME was processed for liquid–liquid solvent fractionation, and three solvent fractions were prepared in n-hexane (PSHE), diethyl ether (PSDE), and ethyl acetate Persicaria strigosa ethyl acetate (PSEE). The solid material obtained after solvent drying was kept at −20°C for further study.
Cytotoxicity study
Cell line and dose preparation
The antiproliferative activity of different solvent extracts was studied for 24, 48, and 72 hours treatment using Dalton’s lymphoma (DL) cell line. DL cells were cultured in RPMI-1640 medium supplemented with 10% FBS, gentamycin (20 mg/ml), streptomycin (100 mg/ml), and penicillin (100 IU) in a CO2 incubator at 37°C with 5% CO2; 80% confluent of exponentially growing cells were sub-cultured and used in the experiments. The different dosage (25, 50, 100, and 200 mg/ml) of plant extracts was freshly prepared in phosphate-buffered saline (PBS).
Cell proliferation and apoptosis assay
After the cells were treated with different concentrations of solvent extracts, cell proliferation was measured by 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay in DL (Mosmann, 1983; Verma and Prasad, 2013). In brief, the cells were treated with different doses (25, 50, 100, and 200 mg/ml) of plant extracts for 24, 48, and 72 hours, then 10 μl of the MTT reagent (5 mg/ml in PBS) was added into each well. Next, the treated plates were incubated for 4 hours under 5% CO2 and 95% air at 37°C after adding 100 μl of Dimethyl sulfoxide (DMSO) into each well and gently shaken. The plates were checked for complete solubilization of crystals, and then absorbance was recorded at 570 nm using Microplate Reader (Rapid Diagnostics; SKU, LISA-R, India). Furthermore, control and treated cells were stained with acridine orange and ethidium bromide (AO/Eb) for 5 minutes in a dark/cold room. The cells were observed under Fluorescence Microscope and photographed (Medlab Lx400 FLR Fluorescence Microscope). About 1,000 cells were counted, and the percentages of apoptotic cells were determined based on differential coloring patterns (red/green) of the nucleus.
GC-MS analysis of phytocompounds
The phytocompounds present in the best bioactive fraction, PSEE were further investigated by GC-MS system (Perkin Elmer (USA), make GC-MS instrument, Model: Clarus 680 GC & amp; Clarus 600C MS comprising a liquid auto-sampler). The software used in the system was TurboMass Ver. 5.4.2. The capillary column used was ‘Elite-5MS’, having dimensions, length of 60 m, ID- 0.25 mm, and film thickness of 0.25 μm, and the stationary phase was 5% diphenyl 95% dimethylpolysiloxane. Helium (99.99%) was used as carrier gas at a flow rate of 1 ml/minute. An injection volume of 2 μl was employed in split-less mode. Injector and ion-source temperatures were kept at 280°C and 180°C, respectively. The oven temperature was programmed at 60°C (1 minute) with an increasing rate of 7°C/minute up to 200°C (hold for 3 minutes), and then again increased at the rate of 10°C/minute up to 300°C (hold for 5 minutes). The total run time was ~39 minutes. The solvent delay was kept for 8 minutes. MS Protocol Mass Spectra were taken in Electron Impact positive (EI+) mode at 70 eV. A solvent delay of 8 minutes was there for MS scan. Mass range, i.e., m/z range is 50–600 amu.
Identification of peaks
Interpretation of the peaks of the GC-MS chromatogram was carried out by library search of the mass spectrum using the National Institute Standard and Technology-2008 database.
Molecular docking
To further elucidate the antiproliferative activity of the plant, molecular docking was carried out with phytocompounds of P. strigosa with six anti-apoptotic proteins, BCL-2 (PDB: 1YSW), BCL-XL (4QVX), MCL-1 (5KU9), BCL-W (2Y6W), BCL-B (4B4S), and BCL-A1/Bfl-1 (5UUK). The 3D structures of proteins and compounds were retrieved from PDB (https://www.rcsb.org/) and PubChem databases (https://pubchem.ncbi.nlm.nih.gov/). Docking was performed in AutoDock vina (Trott and Olson, 2010). The grid parameters were set as x, y, and z size-coordinate and grid box center-coordinates: 14.496, −3.140, 1.690, and 46, 82, 58 for BCL-2, −15.653, 19.048, 0.789, and 30, 48, 40 for BCL-W, −6.783, 9.665, 2.169 and 38, 38, 30 for BCL-B, 0.308, −21.586, 11.404 and 74, 54, 52 for BCL-XL, −2.209, -10.161, 24.412 and 54, 42, 52 for MCL-1, and −2.219, 20.505, −4.937, and 36, 44, 44 for BCL-A1/Bfl-1 proteins, respectively. Docking was performed in triplicate (n = 3), and the best docking output was visualized in Discovery studio software.
Analysis of drug-likeness and absorption, distribution, metabolism, excretion, and toxicity (ADMET) profile
The phytocompounds of P. strigosa were studied for their drug-likeness properties using SwissADME (Daina et al. 2017) and PubChem database. The drug-likeness property of compounds was evaluated based on Lipinski’s rule (Lipinski, 2004). Similarly, in-silico ADMET properties of identified compounds were studied using the ADMETlab server (Dong et al., 2018).
Statistical analysis
All the statistical calculations were carried out in Microsoft excel. A one-way ANOVA correlation study was carried out using OriginPro software. Data were expressed as mean ± SD, n = 3 at p ≤ 0.05 probability level.
RESULTS
Antiproliferative and apoptotic-inducing study
The antiproliferative property of different solvent extracts of P. strigosa is shown in Figure 1. In vitro bioassay showed dose-dependent mortality of DL cells in all the solvent fractions. PSEE showed the most potent cytotoxicity, while PSHE showed the weakest activity. At 25−200 mg/ml, the percentage (%) mortality of cells ranged from 9 ± 19% to 38 ± 4%, 15 ± 2% to 46 ± 4%, and 21 ± 2% to 59 ± 6% at 24, 48, and 72 hours treatment, respectively. The mortality of cells at 72 hours treatment was found to be highest in PSEE, followed by PSDE, PSME, and PSHE. The LD50 of plant extracts for cell death at 72 hours treatment was 74.99, 140.93, 308.06, and 396.87 mg/ml, respectively, for PSEE, PSDE, PSME, and PSHE. Statistical analysis showed a significant difference (p ≤ 0.05) in the mortality of cells at high treatment doses of all the solvent extracts. However, at a lower dose (25 mg/ml), PSDE and PSEE showed no significant difference. Similarly, PSHE and PSME did not show significant differences in terms of mortality of the cells. Like antiproliferative assay, the fluorescence microscopy study also revealed a similar pattern of apoptosis-inducing potential in medicinal plants. Figure 2 shows the fluorescent microscopy images of cells treated with 200 mg/ml plant extracts after 72 hours of treatment. Of the four plant extracts, PSEE shows the best apoptosis-inducing property. The study observed membrane blebbing, chromatin condensation, and apoptotic bodies in plant extracts treated DL cells. In contrast, the control DL cells showed green nuclei with intact cell and nuclear membrane structures.
GC-MS study
The presence of volatile phytocompounds present in PSEE was analyzed by GC-MS. Figure 3 shows the GC-MS chromatogram of the identified compounds. Peak structure with retention time (RT) 19.218 and RT-28.272 showed the highest percentage height, followed by RT-21.064, RT-17.552, and RT-22.264 compared to other structures. The names of the probable phytocompounds with the RT and m/z data have been provided in Table 1. The most abundant phytocompounds were identified to be carbonic acid, tridecyl vinyl ester [C3] and tetradecanoic acid,10,13-dimethyl-, methyl ester [C9], followed by 3N-hexylthiane-S,S-dioxide [C5] and trans-2-methyl-4-n-butylthiane-S, S-dioxide [C2].
Molecular docking study
Table 2 shows the binding affinities of six anti-apoptotic proteins with the phytocompounds. Among the six proteins, BCL-XL showed the best binding affinity with the phytocompounds (average, −5.40 kcal/mol), followed by BCL-2 (average, −5.21 kcal/mol), BCL-A1/Bfl-1 (average, −4.93 kcal/mol), and BCL-B (average, −4.47 kcal/mol). BCL-W showed the weakest binding affinity (average, −2.14 kcal/mol) with phytocompounds. Of the 12 compounds, C12 showed the strongest binding affinity of −8.57 kcal/mol with BCL-XL. Similarly, C7, C5, C5, C6, and C6 showed the best affinities with BCL-2, BCL-W, BCL-B, MCL-1, and BCL-A1/Bfl-1, respectively (Table 3). Overall, C12, C6, C7, and C2 showed the best binding affinity with all the proteins compared to other compounds, while C10 showed the weakest affinity.
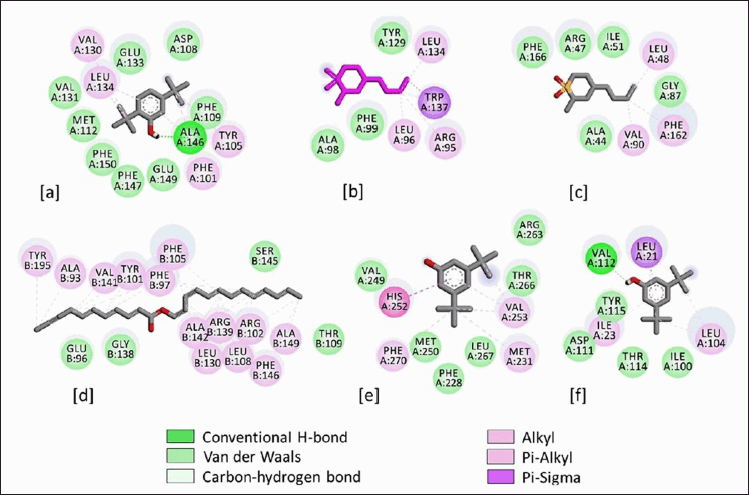
The binding interactions of phytocompounds with the anti-apoptotic proteins are shown in Figures 4 and 5. The 2D display of the binding interactions revealed 13 amino acid residues of BCL-2 interacting with C7. A maximum of 17 residues was observed between BCL-XL and C12. Similarly, C2 interacted with 7 and 8 residues of BCL-W and BCL-B, while C6 involved 10 and 8 amino acid residues with MCL-1 and BCL-A1/Bfl-1. Van der Waal’s interactions and alkyl bonds were the most prominent binding energies between the proteins and phytocompounds. However, in the case of the best docking complex, BCL-XL & C12, alkyl bonds were the most predominant interactions (Figure 4d). Alternatively, two docking complexes, BCL-2 & C7 and BCL-A1/Bfl-1 & C6 showed one hydrogen bond between proteins and phytocompounds.
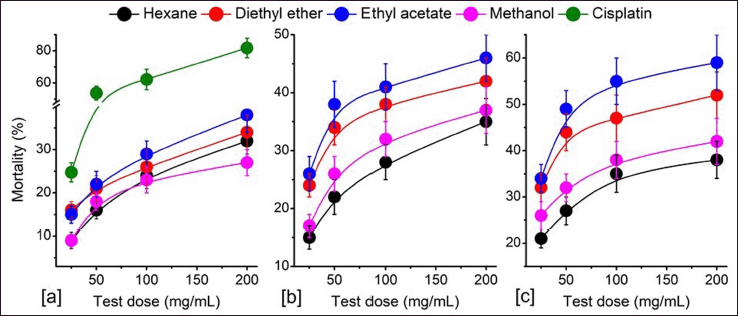 | Figure 1. Cytotoxicity study of the solvent fractions of P. strigosa against DL cells. (a) 24 hours, (b) 48 hours, and (c) 72 hours treatment. Values are expressed as mean ± SD, n = 3. [Click here to view] |
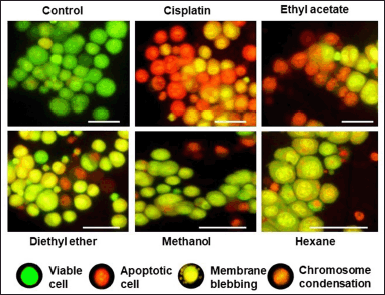 | Figure 2. Morphological features of apoptotic and viable cells observed under a fluorescence microscope after 72 hours treatment with different solvent fractions of P. strigosa. The ethyl acetate extract shows higher apoptotic cells compared to other plant extracts. Scale bar 10 μm. [Click here to view] |
Drug-likeness and ADMET study
The drug-likeness properties of the top five compounds identified from P. strigosa are presented in Table 3. All the compounds showed drug-likeness properties as per the Lipinski rule. According to the rule, compounds with <500 Da molecular weight, <5 lipophilicity, <5 H-bond donors, and <10 H-bond acceptors fulfill the drug-likeness property. A compound is found to have drug-likeness properties if the compound does not violate two or more rules. The in-silico study revealed that all five compounds possess drug-likeness properties. A major drug-likeness property is the compounds’ molecular weight and surface area. All the top five compounds were smaller than 500 Da, and the topological polar surface area (TPSA) values were less than 100 °C². Similarly, the LD50 values showed a low toxicity profile suggesting good drug-likeness profiles.
Figure 6 shows the ADMET profiles of the top five best-binding phytocompounds. Physiochemically, all the compounds showed poor aqueous solubility but high lipophilicity. C12 showed the best lipid permeability property. Similarly, the phytocompounds were predicted to have high absorption through intestinal cells. C12 showed moderate values in bioavailability, while the other four compounds have high bioavailability. All the phytocompounds showed high blood-brain barrier properties, indicating less seepage of the compounds from the blood circulation. In addition, all five compounds showed low metabolism profiles suggesting their suitable drug-likeness property. There is a very low probability that the phytocompounds would act as substrates or inhibitors to the cytochromes-P450 enzyme superfamily. In addition to other ADMET properties, the compounds also had low toxicity and carcinogenic effect.
DISCUSSION
Growth and development are essential characteristics of life. An organism grows and becomes an adult because of the new cells’ continuous multiplication and replacement of the dead cells. All living cells possess a sophisticated and controlled mechanism of cell division. Aged or damaged cells are eliminated from the body by a standard cellular mechanism called apoptosis, also known as programmed cell death. Avoidance of normal cellular death leading to the unregulated growth and division of cells and invading other body parts is the hallmark of cancer disease (Wong, 2011). Dysregulation of apoptosis-related genes and their product is a recurring event in cancer that inhibits cell death induced by many cellular stimuli (Ashkenazi et al., 2017). Anti-apoptotic proteins belonging to the BCL-2 family protein (such as BCL-2, BCL-XL, MCL-1, BCL-W, BCL-B, and BCL-A1/Bfl-1) suppress normal apoptosis. Therefore, it represents an exciting chemotherapeutic target in cancer treatment (Yip and Reed, 2008). In this study, the ethyl acetate fraction of P. strigosa showed the most potent antiproliferative and apoptosis-inducing activities. The antiproliferative property of the plant may be due to the phytochemicals present in the plant. Cisplatin, the reference chemical, showed a significant difference (p ≤ 0.05) compared to plant extracts. GC-MS study identified 12 probable phytocompounds from the ethyl acetate extract of P. strigosa. Again, molecular docking was carried out with P. strigosa phytocompounds and six anti-apoptotic proteins. The study showed a strong binding affinity of C2, C6, C7, and C12 phytocompounds with the anti-apoptotic proteins.
Several studies have reported the antiproliferative and inhibitory properties of anti-apoptotic proteins of several medicinal plants and phytocompounds (Erdogan et al., 2020; Kamaruddin et al., 2019; Swargiary et al., 2021). Phytocompounds such as betulinic acid (Betula alba), dentatin (Clausena excavata), etc. were found to downregulate the expression of the anti-apoptotic BCL-2 gene family (Arbab et al., 2012; Rzeski et al., 2006). The identified compounds have also been reported from many other plants. The essential oil, Trans-2-Methyl-4-n-butylthiane-S,S-dioxide has also been reported in the ethyl acetate fraction of Croton macrostachyus (Gabrehiwot et al., 2018). Tricosanoic acid, an important fatty acid, has been reported from plants with rich bioactivities, such as Cuminum cyminum and Coffea robusta (Dong et al., 2015; Shukla et al., 2018). The bioactive compound Trans-2-Methyl-4-n-butylthiane-S,S-dioxide isolated from the ethyl acetate extract from Streptomyces parvulus VITJS11 have been reported in treating microbial infections and indicated their broad spectrum of activity with beneficial virtues for therapeutic use (Naine et al., 2014). 3,5-Bis(1,1-dimethylethyl) phenol, a bioactive compound isolated from the leaves of Ageratum houstonianum showed strong binding affinity with human polo-like kinase-1 enzyme suggesting the anticancer potential of the compound (Rizvi et al., 2014). Again, another study found that the bioactive compound 3,5-bis (1,1-dimethylethyl) -phenol extracted from Moringa oleifera had the potential as a new anthelmintic drug compound (Novian, 2019). Similarly, the presence of hexacosyl acetate and many other phytocompounds has been attributed to the α-amylase inhibitory activity of Croton bonplandianum (Keerthana et al., 2013).
Molecular docking is an important in-silico tool to study drug–protein interactions that can help design therapeutic protein inhibitors. Van der Waals interaction was the major interaction between the phytocompounds and proteins. Similarly, anonaine, a benzylisoquinoline alkaloid isolated from Annona muricata, showed a high binding affinity with BCL-2, BCL-W, and MCL-1 proteins (Rosdi et al., 2018). Similarly, ginsenosides from Panax ginseng showed a strong binding affinity with BCL-2, BCL-XL, and MCL-1 (Sathishkumar et al., 2012). Adewole and Ishola (2019) screened 47 phytocompounds from Morinda lucida, of which two triterpenes (ursolic and oleanolic acid) and four phytosterols (cycloartenol, campesterol, stigmasterol, and β-sitosterol) were found to have a strong binding affinity to BCL-2 proteins. Phenolics and flavonoids are bioactive compounds having numerous biological properties. Our study also revealed high phenolic and flavonoid content in the ethyl acetate fraction of P. strigosa compared to other extracts (data not published). Of the five flavonoids, biochanin-A, myricetin, apigenin, galangin, and fisetin, BCL-2 and BCL-XL showed the strongest binding interaction with fisetin (Abd Ghani et al., 2020). In this study, Undec-10-ynoic acid, Tridec-2-yn-1-yl ester showed the most promising inhibitory activity among the phytocompounds. 3,5-bis(1,1-dimethylethyl) phenol and 2,5-Bis(1,1-Dimethylethyl) phenol as well as Trans-2-methyl-4-n-butylthiane-S,S-dioxide showed potential apoptosis-inducing property.
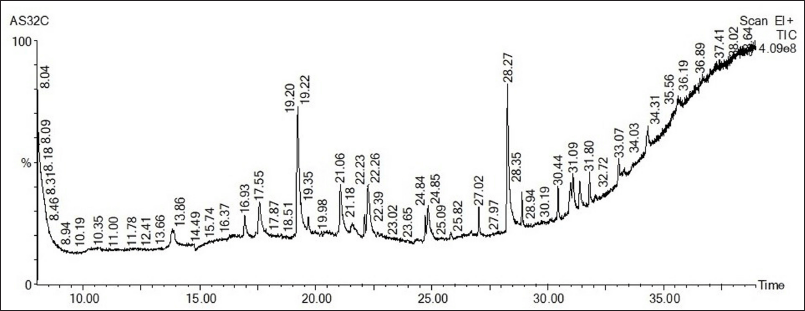 | Figure 3. GC-MS chromatogram of ethyl acetate extracts of P. strigosa leaves. [Click here to view] |
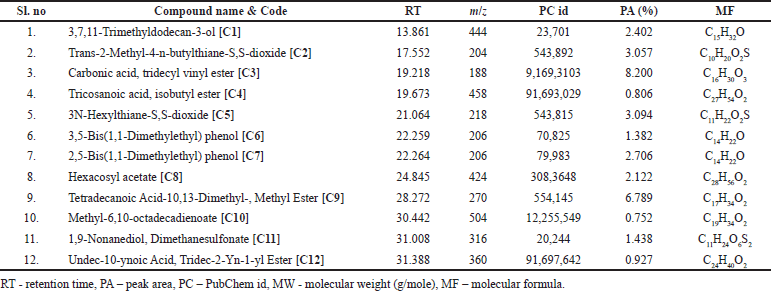 | Table 1. GC-MS properties of phytocompounds identified from ethyl acetate extract of P. strigosa leaves. [Click here to view] |
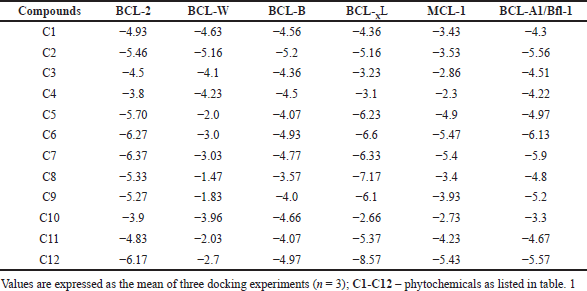 | Table 2. Binding affinity (−kcal/mol) of anti-apoptotic proteins with 12 phytocompounds from P. strigose. [Click here to view] |
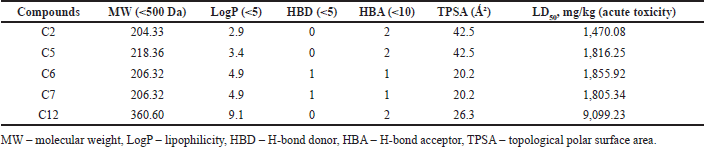 | Table 3. In-silico drug-likeness properties of top five phytocompounds of P. strigosa. [Click here to view] |
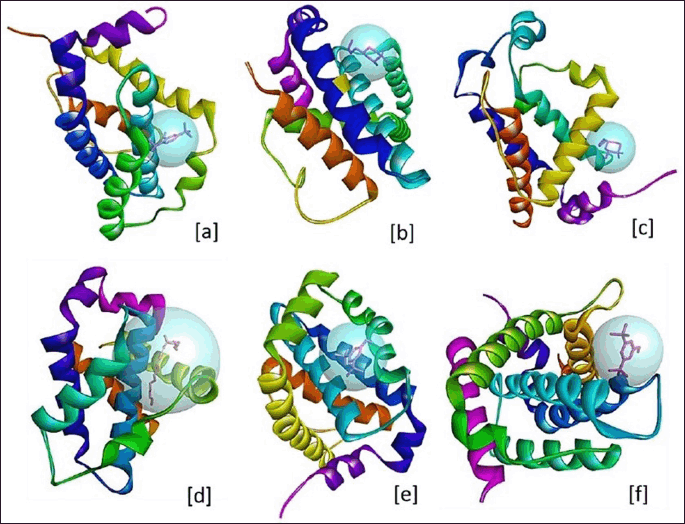
 | Figure 4. 3D structure of ligand binding site and sphere view of anti-apoptotic proteins. (a) BCL-2 and C7, (b) BCL-W and C2, (c) BCL-B and C2, (d) BCL-XL and C12, (e) MCL-1 and C6, and (f) BCL-A1/Bfl-1 and C6. [Click here to view] |
Studying the drug-likeness and ADMET properties is essential in the present-day drug discovery pipeline. Lipinski’s rule predicts that an oral drug is effective if it follows all four rules. According to the rule, a molecule is not considered orally active if it violates two or more of the four rules (Guan et al., 2019). In addition, a candidate drug must also satisfy other properties such as less toxicity, high cell membrane permeability, and easy excretion from the body (DiMasi et al., 2003). Such in-silico studies may be helpful because pharmaceutical companies spend millions of dollars to advance a new drug through clinical trials, and failure in the later stages results in significant economic losses (Sertkaya et al., 2016). Undesirable pharmacokinetic properties and high toxicity are the leading causes of such failure at the clinical trial stage. Therefore, a good balance of potency and ADMET profile offers a helpful guideline for further optimization and drug discovery (Jia et al., 2020). In this study, all five best phytocompounds showed suitable drug-likeness properties with considerable ADMET properties. The high lipophilicity of phytocompounds suggests higher permeability through cell membranes. Similarly, small molecular mass and smaller surface area allow the compounds to pass through the membrane. ADMET study of the P. strigosa compounds showed low toxicity properties, suggesting the potentiality of a lead therapeutic compound. Compounds having LD50 values in the range of 1−50, 51−500, and 501−5,000 mg/kg body weight are considered high, moderate, and low toxicity properties (Lei et al., 2016). The LD50 of all five compounds showed more than 600 mg/kg suggesting a low risk of toxicity in the host body.
 | Figure 5. 2D binding interactions of Persicaria strigosa phytocompounds with the anti-apoptotic proteins. (a) BCL-2 and C7, (b) BCL-W and C2, (c) BCL-B and C2, (d) BCL-XL and C12, (e) MCL-1 and C6, and (f) BCL-A1/Bfl-1 and C6. [Click here to view] |
 | Figure 6. Heat map of ADMET properties of P. strigosa phytocompounds (best five). [Click here to view] |
CONCLUSION
This study investigated the phytochemistry, antiproliferative, and apoptosis-inducing activity of different solvent extracts of P. strigosa. The plant extracts revealed promising apoptosis-inducing properties, which may be attributed to the secondary metabolites present in the ethyl acetate extract of the plant. The bioactivity could be due to the downregulation of the BCL-2 family gene or inhibition of anti-apoptotic proteins by the phytocompounds. However, further study must be conducted to establish the exact molecular mode of action.
ACKNOWLEDGMENTS
The authors would like to thank SERB, Govt. of India, for providing financial assistance in a research project to A.S. (EEQ/2017/000071). The authors also thank Dr. Sanjib Baruah, Assistant Professor in the Department of Botany, for the scientific validation of the plant. The authors acknowledge the GC-MS facilities of Biotech Park, IIT, Guwahati, Assam.
AUTHOR CONTRIBUTIONS
A.S. designed the study, performed statistical calculations, and wrote the manuscript. A.K.V. conducted the antiproliferative and apoptosis study. M.K.R. is involved in literature collection, manuscript drafting, and docking studies. H.B. was involved in the antioxidant study and statistical calculations. M.D. was involved in data acquisition, and J.K.D. was involved in statistical calculation and manuscript drafting. All the authors read the final manuscript and approved it for publication.
FINANCIAL SUPPORT
The present study does not have any external funding from any source.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
No ethical approval is required for the current study.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abd Ghani MF, Othman R, Nordin N. Molecular docking study of naturally derived flavonoids with anti-apoptotic BCL-2 and BCL-XL proteins toward ovarian cancer treatment. J Pharm Bioall Sci, 2020; 12:S676−80.
Adewole K, Ishola A. Phytosterols and triterpenes from Morinda lucida Benth (Rubiaceae) as potential inhibitors of anti-apoptotic BCL-XL, BCL-2, and MCL-1: an in-silico study. J Recept Signal Transduct Res J, 2019; 39(1):87−97.
Arbab IA, Looi CY, Abdul AB, Cheah FK, Wong WF, Sukari MA, Abdullah R, Mohan S, Syam S, Arya A, Taha MME, Muharram B, Mustafa MR, Abdelwahab SI. Dentatin induces apoptosis in prostate cancer cells via bcl-2, bcl-xl, survivin downregulation, caspase-9,-3/7 activation, and nf-κb inhibition. Evid Based Complement Alternat Med, 2012:1−15, 2012.
Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov, 2017; 16(4):273−84.
Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH, Rollinger JM, Schuster D, Breuss JM, Bochkov V, Mihovilovic MD, Kopp B, Bauer R, Dirsch VM, Stuppner H. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv, 2015; 33(8):1582−614.
Basu P, Meza E, Bergel M, Maier C. Estrogenic, antiestrogenic and antiproliferative activities of Euphorbia bicolor (Euphorbiaceae) latex extracts and its phytochemicals. Nutrients, 2020; 12(1):59.
Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anticancer agents. Chem Rev, 2009; 109(7):3012−43.
Daina A, Michielin O, Zoeteb V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep, 2017;7:42717.
Deka N, Devi N. Aquatic angiosperm of BTC area, Assam, with reference to their traditional uses. Asian J Plant Sci Res, 2015; 5(5):9−13.
DiMasi JA, Hansen RW, Grabowsk HG. The price of innovation: new estimates of drug development costs. J Health Econ, 2003; 22:151−85.
Dong J, Wang N, Yao Z, Zhang L, Cheng Y, Ouyang D, Lu A, Cao D. ADMETlab: a platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J Cheminformatics, 2018;10:29.
Dong W, Tan L, Zhao J, Hu R, Lu M. Characterization of fatty acid, amino acid and volatile compound compositions and bioactive components of seven coffee (Coffea robusta) cultivars grown in Hainan Province, China. Molecules, 2015; 20(9):16687−708.
Erdogan MK, Geçibesler IH, Behcet L. Chemical constituents, antioxidant, antiproliferative and apoptotic effects of a new endemic Boraginaceae species: Paracaryum bingoelianum. Results Chem, 2020; 2:100032.
Fahad FI, Barua N, Islam MS, Sayem SAJ, Barua K, Uddin MJ, Chy MNU, Adnan M, Islam MN, Sayeed MA, Emran TB, Simal-Gandara J, Pagano E, Capasso R. Investigation of the pharmacological properties of Lepidagathis hyaline Nees through experimental approaches. Life, 2021; 11(3):180.
Fridlender M, Kapulnik Y, Koltai H. Plant-derived substances with anticancer activity: from folklore to practice. Front Plant Sci, 2015; 6:799.
Gabrehiwot H, Zelelew D, Gebremariam A. Chemical analysis and medicinal activities of volatile components from the seeds of Croton Macrostachyus. Int J Sci Basic Appl, 2018; 37(2):316−30.
Guan L, Yang H, Cai Y, Sun L, Di P, Li W, Liu G, Tang Y. ADMET-score – a comprehensive scoring function for evaluation of chemical drug-likeness. Med Chem Comm, 2019; 10(1):148−57.
Jia CY, Li JY, Hao GF, Yang GF. A drug-likeness toolbox facilitates ADMET study in drug discovery. Drug Discov Today, 2020; 25(1):248−58.
Kamaruddin MF, Hossain MZ, Mohamed AA, Mohd BM. The antiproliferative and apoptotic effects of capsaicin on an oral squamous cancer cell line of Asian Origin, ORL-48. Medicina, 2019; 55:322.
Keerthana G, Kalaivani MK, Sumathy A. In-vitro α-amylase inhibitory and antioxidant activities of ethanolic leaf extract of Croton bonplandianum. Asian J Pharm Clin Res, 2013; 6(4):32−6.
Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discovery Today, 2004;4:337-41.
Lei T, Li Y, Song Y, Li D, Sun H, Hou T. ADMET evaluation in drug discovery: 15. Accurate prediction of rat oral acute toxicity using relevance vector machine and consensus modeling. J Cheminformatics, 2016; 8:6.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods, 1983; 65(1–2):55−63.
Naine SJ, Devi CS, Mohanasrinivasan V, Vaishnavi B. Antimicrobial, antioxidant and cytotoxic activity of marine Streptomyces parvulus VITJS11 crude extract. Braz Arch Biol Technol, 2014; 58:198–207.
Novian DR. Anthelmintic potential of Moringa Oleifera as inhibitor mitochondrial rhodoquinol-fumarate reductase from Ascaris suum using the docking method. J Farm Sains Praktis, 2019;5(2):106–14.
Pettit GR, Mukku VJ, Craqq G, Herald DL, Knight JC, Herald CL, Chapuis JC. Antineoplastic agents. 558. Ampelocissus sp. cancer cell growth inhibitory constituents. J Nat Prod, 2008; 71(1):130−3.
Rizvi SD, Shakil S, Zeeshan M, Khan MS, Shaikh S, Biswas D, Ahmad A, Kamal MA. An enzoinformatics study targeting polo-like kinases-1 enzyme: comparative assessment of anticancer potential of compounds isolated from leaves of Ageratum houstonianum. Phcog Mag, 2014; 10(S1):14−21.
Rosdi MMN, Arif MS, Bakar AMH, Razali SA, Zulkifli RM, Ya’akob H. Molecular docking studies of bioactive compounds from Annona muricata Linn. as potential inhibitors for Bcl-2, Bcl-w and Mcl-1 anti-apoptotic proteins. Apoptosis, 2018; 23:27–40.
Rzeski W, Stepulak A, Szyma?ski M, Sifringer M, Kaczor J, Wejksza K, Zdzisi?ska B, Kandefer-Szersze? M. Betulinic acid decreases expression of Bcl-2 and cyclin D1, inhibits proliferation, migration and induces apoptosis in cancer cells. Naunyn Schmied Arch Pharmacol, 2006; 374:11–20.
Sathishkumar N, Sathiyamoorthy S, Ramya M, Yang D, Nyeong LH, Yang D. Molecular docking studies of anti-apoptotic BCL-2, BCL-XL, and MCL-1 proteins with ginsenosides from Panax ginseng. J Enzyme Inhib Med Chem, 2012; 27(5):685−92.
Sertkaya A, Wong HH, Jessup A, Beleche T. Key cost drivers of pharmaceutical clinical trials in the United States. Clin Trials, 2016; 13(2):117−26.
Shukla N, Rahul SN, Sharma J, Tiwari S. Evaluation of volatile compounds and fatty acid methyl ester (Fame) through gas chromatography in cumin seeds (Cuminum cyminum). J Pharmacog Phytochem, 2018; 7(4):1125−9.
Swargiary A, Daimari A, Daimari M, Basumatary N, Narzary E. Phytochemicals, antioxidant and anthelmintic activity of selected traditional wild edible plants of lower Assam. Indian J Pharmacol, 2016; 48(4):418−23.
Swargiary A, Daimari M. GC–MS analysis of phytocompounds and antihyperglycemic property of Hydrocotyle sibthorpioides Lam. SN Appl Sci, 2021; 3(1):1−11.
Swargiary A, Daimari M, Roy MK. Survey and documentation of anthelmintic plants used in traditional medicine system of tribal communities of Udalguri district of Assam, India. J Appl Pharm Sci, 2020; 10(1):46−54.
Swargiary A, Roy MK, Daimari M. Survey and documentation of putative anthelmintic plants used in ethnomedicinal systems of tribal communities of Baksa district of Assam. Med Plant, 2019; 11(4):368−79
Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem, 2010; 31(2):455−61.
Verma AK, Prasad SB. Changes in glutathione, oxidative stress and mitochondrial membrane potential in apoptosis involving the anticancer activity of cantharidin isolated from redheaded blister beetles, Epicauta hirticornis. Anticancer Agent ME, 2013; 13:1096.
Weinberg RA. Cancer: principles and overview. In: Plopper G, Sharp D, Sikorski E (eds.). Lewin’s cell, Jones & Bartlett Learning, LLC, Burlington, MA, pp 751−2, 2015.
Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res, 2011; 30(87):1−14.
Yip K, Reed J. Bcl-2 family proteins and cancer. Oncogene, 2008; 27:6398–406.