INTRODUCTION
Allergies are immune dysfunctions that affect quality of life and lead to death if not treated properly (Guillamón et al., 2008). The World Allergy Organization estimated that the prevalence of allergies in the entire world population ranges from 10% to 40% (Pawankar et al., 2013). Based on ISAAC’s study, the prevalence of allergic rhinitis among 13–14 years old is from 1.4% to 39.7% (Nurhutami et al., 2020). Food allergy is a condition caused by an IgE reaction to food ingredients (chemical compounds) and is a concerning type of allergy with the highest prevalence observed in infancy and early childhood (Chapman et al., 2006). One of the symptoms that arise from food allergies is allergic rhinitis that can cause nasal obstruction, causing a decrease in quality of life (Zachreini et al., 2015). IgE antibodies are usually synthesized in small amounts to optimize defense against microbes and other helminths by stimulating the release of inflammatory mediators by mastocytes (Zakaria et al., 1992a). An allergic reaction mediated by IgE is a health problem in developing and developed countries (Lestari Eka et al., 2016). In allergic reactions, IgE synthesis is unregulated and can be detected in large amounts in the blood. Not all dietary proteins trigger IgE synthesis. However, specific proteins will trigger their specific IgE antibodies, which can be used to determine the specific allergen-inducing protein (Zakaria et al., 1992b). IgE recognition of its protein allergens is the basis of most allergy analyses and determinations that can identify a single protein allergen in various foods (Wijaya et al., 2014), and it is the principle of the skin prick test (SPT) for allergy diagnosis (Astuti et al., 2018).
The winged bean is a local Indonesian plant that can be used as a raw material that is rich in protein sources (Wahyuni, 2015). The winged bean has a high protein content ranging from 33.83% to 38.31%, so it can be used as an alternative source of vegetable protein (Amoe et al., 2006). Protein contents significantly affect growth (Rachmawati and Samidjan, 2016).
The winged bean belongs to the family Fabaceae. The winged bean is a nonconventional legume crop that is similar to soybean in lipid and protein contents and one of the protein sources that are palatable but highly degradable in the rumen (Adiwimarta et al., 2018). According to Faeste et al. (2010), the Fabaceae family has potential as an allergen. Jimenez Lopez et al. (2015) and Guillamon et al. (2010) reported that a Fabaceae family plant, i.e., Lupinus angustifolius L., can cause allergies.
This study aimed to isolate the winged bean’s allergen protein and to characterize the allergen protein isolate from the SDS-PAGE, ELISA, and immunoblotting assays. The risk of allergy symptoms has not been assessed in Indonesia (Priyantini et al., 2020). To avoid allergies, people tend to avoid foods that are suspected of causing them. Therefore, an allergy diagnosis test is needed. Allergy tests that are carried out to find out the type of food that causes a person to be allergic are the skin prick test or skin prick test.
The SPT was the method for diagnosing IgE-mediated allergic diseases. The SPT is an easy, inexpensive, and relatively safe in vivo examination procedure (Sari et al., 2014). The main ingredient used for the SPT reagent in this study was an allergen protein extract from the winged bean (Psophocarpus tetragonolobus L.).
MATERIALS AND METHODS
Isolation of winged bean seed protein
The winged bean (P. tetragonolobus L.) was treated with two treatments of fresh and boiled samples (Setiawan et al., 2019; Wijaya et al., 2014; Wu et al., 2009). The winged beans were obtained from plantations in the Ciawi area, Bogor Regency, Indonesia. The winged bean seeds used are seeds that were harvested when the plants were 8 weeks old and soaked in water for 18 hours. The skin of the fresh winged bean (FWB) that had been soaked was peeled using an abrasive peeler, dried in a drying oven at 50°C, and pulverized. The flouring was carried out using a pin disc mill with a sieve size of 60 meshes. In the boiling process, the winged bean seeds were boiled for 30 minutes, and the skin of the winged bean was also peeled using an abrasive peeler, dried in a drying oven at 50°C, and mashed. The flours of FWBs and boiled wing beans, each as much as 100 g, were extracted using n-hexane (1:5) for 2 hours at room temperature and centrifuged at 4,000 rpm at 40°C for 15 minutes. The precipitate was dried in a drying oven at 50°C for 2 hours. The sample was mixed with distilled water (ratio 1:10 w/v), 1 N NaOH was added to raise the pH to 8–8.5, and the mixture was stirred at 40°C for 90 minutes and centrifuged at 4,500 rpm for 30 minutes at 4°C. The pH of the obtained supernatant was lowered to 3.45 using 1 N HCl; then, it was centrifuged at 4,500 rpm and 4°C for 20 minutes. The sample was dried with a freeze drier and analyzed for protein by the Bradford method.
Protein isolate profile analysis with SDS-PAGE
Winged bean protein isolates were characterized by SDS-PAGE using acrylamide gel (Wijaya et al., 2014). A whole 40 μl sample was transferred into a 0.5 ml microtube, and 10 μl of sample buffer was added. The tube was then heated in boiling water at 100°C for 5 minutes. The resulting gel was transferred to a closed container that already contained a dye of Coomassie Brilliant Blue G-250.
Serum preparation of allergy patients
A total of 10 food allergy patients had their serum taken by medical personnel from the Indrajana Allergy Clinic in Jakarta (Research Code of Ethics No. 168/IT3.KEPMSM-IPB/SK/2019) (Astuti et al., 2016; Wijaya et al., 2014). The blood was placed in a tube containing no EDTA and was centrifuged at 2,500 rpm (1,250 g) for 20 minutes. The serum was stored at −20°C.
Total serum IgE analysis by ELISA method
100 μl of serum which had undergone a 1:10 dilution (in a 0.05 M carbonate-bicarbonate buffer with pH 9.6) was tied to a microtiter plate and was incubated overnight at 4°C (Wijaya et al., 2014). As a negative control, normal serum was used. The remaining samples were discarded, and the microtiter plate was washed five times using PBST (250 μl/well). Then, 200 μl of 5% skim milk in PBST was added to the microtiter plate and was incubated at 37°C for 1 hour. After washing five times using PBST (250 μl/well), HRP-conjugated monoclonal mouse anti-human IgE 1:6,000 antibody was added to PBST at 100 μl/well and was incubated at 37°C for 1 hour. Then, the microtiter plate was washed using PBST (250 μl/well) 10 times, and then 100 μl/well of a TMB substrate was added. Positive results were indicated by the appearance of a blue color. After 5 minutes, the reaction was stopped using 2 M H2SO4 of 100 μl/well, and the solution turned bright yellow. Optical density (OD) was measured using an ELISA reader at a wavelength of 450 nm.
Serum specific IgE analysis by ELISA method
The protein sample (10 μg/ml) was dissolved in a carbonate-bicarbonate buffer (0.05 M, pH 9.8), and then it was incubated overnight at 4°C. The plates were then washed five times using PBST of 250 μl/well, blocked with 5% skim milk in PBST of 200 μl/well, and incubated at 37°C for 1 hour. Then, the microtiter plate was washed using PBST (250 μl/well) five times. The serum of allergy patients that had been diluted 1:10 in PBST was added, as much as 100 μl/well, and then incubated at 37°C for 1 hour. After incubation, the plate was washed using PBST (250 μl/well) five times; then, 100 μl/well of HRP-conjugated monoclonal mouse anti-human IgE 1:6,000 antibody in PBST was added and then incubated at 37°C for 1 hour. The plate was washed using PBST (250 μl/well) 10 times, and 100 μl/well of a TMB substrate was added. Positive results were indicated by the appearance of a blue color. After 5 minutes, the reaction was stopped using 2 M H2SO4 of 100 μl/well, and the solution turned bright yellow. OD was measured using an ELISA reader at a wavelength of 450 nm.
Immunoblotting
The unstained electrophoresis gel was transferred to a nitrocellulose membrane (0.45 m) (Wijaya et al., 2014). Blotting was performed at 90 V for 90 minutes and the membrane was soaked or fixed with 50% methanol for 2 minutes and then blocked with 5% skimmed milk in PBST at room temperature for 1 hour. The membranes were washed using PBST three times, added with serum from allergy patients (1:10) in PBST, and incubated at room temperature for 2 hours. Washing was performed again using PBST three times, and then HRP-conjugated monoclonal mouse anti-human IgE antibody (1:3,000 dilution in PBST) was added and incubated for 1 hour while shaking. The membrane was washed again using PBST three times, and a DAB substrate was added. The positive detection result of the allergen protein complex with serum was indicated by the formation of a brown band on the nitrocellulose membrane.
Skin prick test
The SPT was performed by an allergologist at the Allergy and Asthma Clinic, DR. Indrajana, Jakarta (Jeong et al., 2013; Maleki et al., 2010; Wijaya et al., 2014; Wijaya et al., 2015). The research subjects for this SPT were 10 people with allergies. The skin prick test was performed on the volar part of the forearm, which was previously disinfected with alcohol and marked with a ballpoint pen. As a positive control, histamine 1 μg/μl was used, and as a negative control, a 50% glycerol-saline solution was used. The result was declared 0 if the size of the bumps was the same as the negative control (no bumps formed). The result was declared +1 if the size of the bumps was 25%–50% larger than the negative control (<3 mm). The result was +2 if the size of the bumps was 50%–75% larger than the negative control (3–5 mm), +3 if the size of the bumps was the same as the positive control (5–7 mm), +4 if the size of the bumps was 25%–50% larger than the positive control, and >+4 if the size of the bumps was more than 50% of the positive control.
RESULTS AND DISCUSSION
Isolation of winged bean seed protein
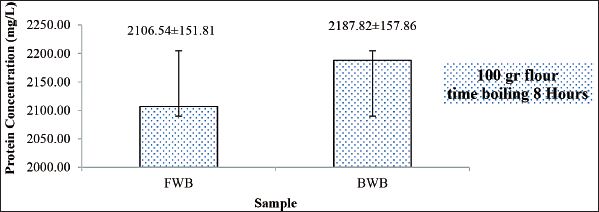
The concentration of the boiled winged bean (BWB) protein extract was 2,187 mg/l, while the total concentration of the FWB protein extract was 2,106 mg/l (Fig. 1). The protein concentration of BWBs was higher than that of FWBs. This could be due to the boiling process.
The heating process causes changes in protein solubility so that it affects the amount and type of protein that can be extracted in the protein isolation process (Nirbaya et al., 2020; Nirbaya et al., 2021). The treatment also caused the winged bean’s allergenicity to change. According to Vissers et al. (2011), the thermal process of a protein can cause changes in protein structure that can cause changes in its allergenicity. Protein allergenicity can be decreased or increased by treatment because it can change the structure of the allergen so that the allergen is not recognized by antibodies (Suseno et al., 2016; Zakaria et al., 1992a).
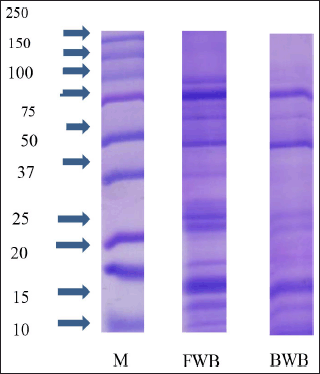
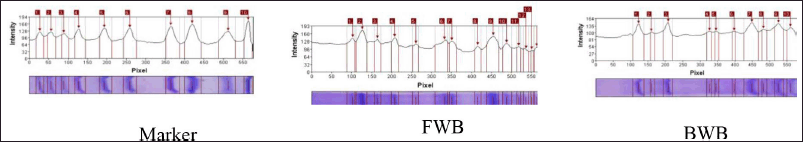
The SDS-PAGE profile of winged bean extracts is presented in Figure 2. In SDS-PAGE, protein bands were formed consisting of many proteins with different band thicknesses indicating the high and low concentrations of the proteins in a sample (Ma et al. 2009). The area of the protein band thickness was calculated using the GelAnalyzer 2010a software.
The results of the GelAnalyzer 2010a software showed that the FWB had 13 protein bands with molecular weights of 89, 68, 48, 35, 27, 24, and 23 kDa and 6 bands with a molecular weight of 22 kDa. Meanwhile, the BWB had 10 protein bands with molecular weights of 73, 51, 36, 24, and 23 kDa and 5 bands of 22 kDa (Fig. 3).
The treated winged bean sample (BWB) had fewer protein bands than the sample without a treatment (FWB). The greatest intensity in FWB was the protein with a BM of 68 kDa at 13.7%, while the greatest intensity in BWB was the protein with a BM of 22 kDa at 19.5%. Based on the WHO/IUIS Allergen Nomenclature database, winged beans have no allergen data.
Analysis of Total IgE and Specific IgE
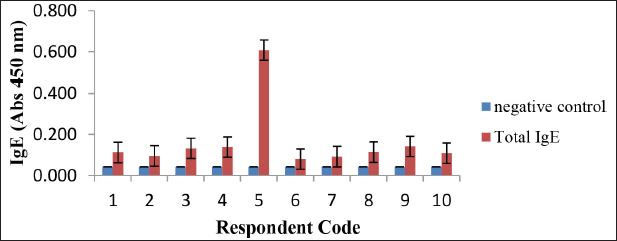
The results of the total IgE analysis of 10 positive food allergy respondents are described in Figure 4. All the results of the total IgE analysis of the respondents showed that all respondents were allergy patients with different levels of allergies. The greater total IgE of a person indicates the higher level of allergy (Zakaria et al., 1998). Figure 4 shows that the respondent who had the highest level of allergy was respondent 5 and the respondent who had the lowest level of allergy was respondent 6. IgE antibodies in serum that can bind to protein isolates are specific IgE antibodies to isolate proteins as allergens. A high specific IgE value indicates a strong binding of IgE in allergic subjects with specific allergen proteins (Chalid et al., 2019).
The analysis of specific IgE extracted from the winged bean in the 10 respondents was performed using the ELISA method because it is a fairly good method to show specific IgE reactivity with serum IgE at low concentrations (Astuti et al., 2015).
 | Figure 1. FWB and BWB proteins. [Click here to view] |
The results of the specific IgE analysis from the winged bean sample showed that, from 10 respondents, only 9 respondents were positive for allergies to fresh and BWBs, i.e., respondents 2, 3, 4, 5, 6, 7, 8, 9, and 10 (Fig. 5).
Total IgE results were not positively correlated with specific IgE results (Astuti et al., 2018). Total IgE only shows if a person is suffering from allergies, but it cannot show the type of allergen. This was indicated in respondent 1, who had positive total IgE, but the specific IgE was negative. It is possible that respondent 1 had an allergy to a type of food other than winged beans.
The immunoblotting results of 10 allergy-positive respondents, whose serum was taken, were also analyzed using the GelAnalyzer 2010a software. The results showed that the FWB protein could bind to specific IgE antibodies in respondents 1, 5, 7, and 9. Meanwhile, the BWB protein could bind to specific IgE antibodies in respondents 7 and 8. The allergen protein of the FWB, which could bind to specific IgE in respondent 1, was a protein band with a BM of 24 kDa. In respondent 5, it was a protein band with a BM of 219 kDa. In respondent 7, the positive IgE was bound to protein bands in FWB samples at 29 and 23 kDa. In respondent 9, positive IgE was bound to the protein band in the FWB samples at 17 kDa (Fig. 6).
 | Figure 2. Profile of SDS-PAGE of FWB and BWB extracts. [Click here to view] |
In the BWB samples, the allergen protein was bound to specific IgE only in respondents 7 and 8. The allergen protein of the BWB that was bound to specific IgE in respondent 7 was in the protein band with BM of 3 and 24 kDa. Meanwhile, in respondent 8, the allergen protein of the BWB was bound to specific IgE in the protein band with BM of 31 and 23 kDa.
The results of immunoblotting analyzed with the GelAnalyzer 2010a software were different from those in SDS-PAGE. The protein band in SDS-PAGE was more abundant than the protein band in immunoblotting. This is because not all proteins in the sample can bind to specific IgE in the serum. This means that not all protein fractions in the extract can produce IgE if ingested and not all winged bean protein fractions can cause allergies. Therefore, for use as a SPT reagent, it is better to use whole extracts and it is not necessary to separate the winged bean protein to produce SPT reagents (Wijaya et al., 2014). For the manufacture of SPT reagents, the use of whole protein extracts without protein fractionation is much safer because with whole extracts the presence of all protein fractions is guaranteed to be in the whole extract.
Skin prick test
In Table 1, the total IgE results show that 10 respondents were allergy sufferers. Specific IgE results from FWBs showed 9 people had positive results and only 1 person had negative specific IgE results. The results of the SPT showed that respondent 1 was negative for winged bean-specific IgE but showed positive results for the SPT in the FWB (+1) and BWB (+1) samples. Respondents 4, 5, and 8 had negative results on the SPT for all samples, both FWB and BWB, even though the specific IgE results were positive. Respondents 6, 7, 8, 9, and 10 showed varying results between specific IgE and SPT results. Respondents 6, 9, and 10 had a positive specific IgE, but the SPT results only showed a positive result (+1) for FWB, while they showed a negative result for BWB. In respondent 7, the SPT with a positive result was only the BWB (+1).
The SPT results showed the appearance of red bumps on the skin (Fig. 7). This occurred because of the cross-link between the rice eel allergens used and the IgE bound to the mastocyte cells of allergy sufferers. This further caused the release of histamine and other mediators from the mastocyte cells, which resulted in vasodilation and increased blood vessel permeability, resulting in redness of the skin and bumps (Wijaya et al., 2015). The results of total IgE and specific IgE of all subjects showed a positive reaction. However, the results of the SPT showed varying results. This is presumably due to the protein content of the different samples. However, overall, all subjects showed positive SPT results.
 | Figure 3. SDS-PAGE protein marker (M), FWB protein, and BWB after analysis using GelAnalyzer 2010a software. [Click here to view] |
 | Figure 4. Analysis of total IgE in the serum of 10 respondents with allergies. [Click here to view] |
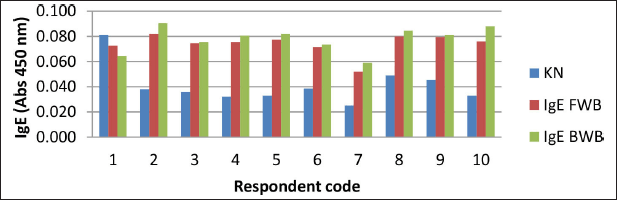 | Figure 5. Specific IgE antibodies on samples of FWB and BWB in the serum of 10 allergy patients. [Click here to view] |
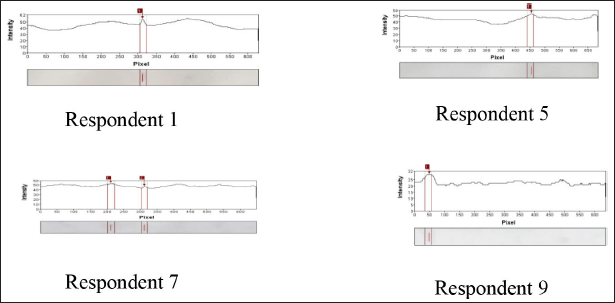 | Figure 6. Immunoblotting results of FWB protein on the serum from respondents 1, 5, 7, and 9. [Click here to view] |
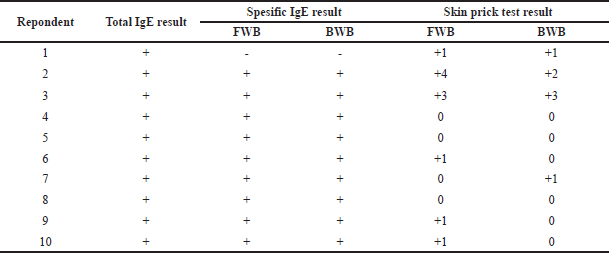 | Table 1. Results of total IgE, specific IgE, and skin prick test of winged beans. [Click here to view] |
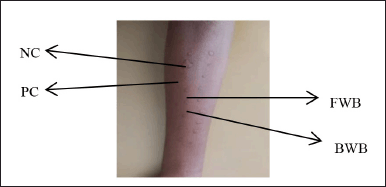 | Figure 7. Skin prick test results for fresh and boiled winged bean allergens. Description: NC = negative control, PC = positive control, FWB = fresh winged bean, and BWB = boiled winged bean. [Click here to view] |
CONCLUSIONS
The SDS-PAGE profile in this study showed that the FWB had 11 protein bands with molecular weights of 89, 67, 47, 34, 24, and 23 kDa and 5 bands of 22 kDa. Meanwhile, the boiled winged bean (BWB) contained 9 protein bands with molecular weights of 73, 35, 24, and 23 kDa with 2 bands and 4 bands of 22 kDa. The protein extract was tested for protein allergenicity using the ELISA method, and it showed the presence of allergenicity. The winged bean protein extract had the ability to bind IgE in the subject’s serum, so it can be used for making SPT allergens. The SPT results showed that there was an allergen protein in the fresh and boiled winged bean samples that can be used for the SPT reagent.
The reagent SPT from FWB and BWB complies with the Standardization of Allergen Extracts European Pharmacopoeia Monograph of Allergen Products and has been trialed in humans. For application as a reagent, it is sufficient to use whole protein extracts and there is no need to use protein fractions because as seen in the results of the immunoblotting and SPT not all protein fractions from the protein extracts reacted positively and specifically to the IgE sera of the allergic subjects. The cost of this SPT is relatively low, so people do not mind doing allergy tests so they can easily find out which protein is right for them. It is hoped that people, especially children, do not have to worry about consuming various proteins. The need for protein is very important considering that children, especially those who are still growing, need adequate amounts of it to prevent protein deficiency due to a condition of lack of nutritional intake for a long time, which results in growth disorders in children, namely, high body height, lower than their age.
In addition, a SPT also needs to be carried out considering the consequences that arise from allergic reactions similar to reactions due to infection with microbes, such as diarrhea, abdominal pain, and vomiting. This is very important because it can prevent the inappropriate use of antibiotics due to frequent misdiagnosis, especially in children.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This research was approved by Indrajana Allergy Clinic in Jakarta (Research Code of Ethics No. 168/IT3.KEPMSM-IPB/SK/2019).
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Adiwimarta K, Budisatria IGS, Rusman R, Adiwinarti R. Effects of total mixed rations containing treated or untreated soybean meal on the energy utilization of kacang goats. Pak J Nutr, 2018; 17(11):563–7.
Amoe IA, Adebayo, Oyeleye. Chemical evaluation of winged beans (Psophocarpus tetragonolobus), Pitanga Cherries (Eugenia uniflora) and Orchid Fruit (Orchid fruit myristica). Afr J Food Agr Nutr Dev, 2006; 6(2):1–12.
Astuti RM, Palupi NS, Zakaria FR. Allergic reactivity of bambara groundnut (Vigna subterranea) proteins. Food Agric Immunol, 2016; 27(4):535–46.
Astuti RM, Palupi NS, Zakaria FR. Quality performance of protein allergen isolates for allergy diagnostic test (Case: Indonesian soybeans (Glycine max) and peanuts (Arachis hypogaea). Int Food Res J, 2018; 25(1):217–26.
Chalid SY, Syah D, Giriwono PE, Zakaria FR. Profil Dan Sensitivitas Protein Alergen Ikan Tongkol (Thunnus albacares) Sebagai Reagen Skin Prick Test (SPT). Jurnal Kimia Valensi, 2019; 5(1):44–55; doi: 10.15408/jkv.v5i1.9678
Chapman J, Bernstein L, Lee R, Oppenheimer J. Food allergy: a practice parameter. Ann Allergy Asthma Immunol, 2006; 96:S1–68.
Faeste CK, Christians U, Egaas E, Jonscher RK. Characterization of potential allergens in fenugreek (Trigonella foenum-graecum) using patient sera and MS-based proteomic analysis. J Proteomics, 2010; 73(7):1321–33; doi:10.1016/j.jprot.2010.02.011
Guillamón E, Rodríguez J, Burbano C, Muzquiz M, Pedrosa MM, Cabanillas B, Crespo JF, Sancho AI, Mills EN, Cuadrado C. Characterization of lupin major allergens (Lupinus albus L.). Mol Nutr Food Res, 2010; 54(11):1668–76.
Guillamón E, Rodríguez J, Burbano C, Muzquiz M, Pedrosa MM, Cabanillas B, Haahtela T, von Hertzen L, Mkel M, Hannuksela M. Finnish allergy programme 2008–2018—time to act and change the course. Allergy, 2008; 63:634–45; doi: 10.1111/j.1398-9995.2008.01712.x
Jeong KY, Choi SY, Han IS, Lee JH, Lee JS, Hong CS, Park JW. The effects of storage conditions on the stability of house dust mite extracts. Allergy Asthma Immunol, 2013; 5(6):397–401; doi:10.4168/Aair.2013.5.6.397
Jimenez-Lopez JC, Cabello EL, Melser Su, Foley RC, Singh KB, Juan AD. Lupin allergy: uncovering structural features and epitopes of β-conglutin proteins in Lupinus angustifolius L. with a focus on cross-allergenic reactivity to peanut and other legumes. Springer International Publishing, Cham, Switzerland, pp 96–107, 2015.
Lestari Eka Y, Santoso YI. Effect of vitamin D supplementation on the number of lung tissue eosinophils in allergic patients experimental study in ovalbumin-induced BALB/C mice. Diponegoro Med J, 2016; 5(4):761–71.
Ma J, Pavase TR, Li ZX, Lin H. Optimisation of an extraction technique of fish allergens suitable for detection and diagnosis. Czeh J Food Sci, 2009; 35(1):24–31; doi: 10.17221/578/2015-CJFS
Maleki SJ, Casillas AM, Kaza U, Wilson BA, Nesbit JB, Reimoneqnue C, Cheng H, Bahna SL. Differences among heat-treated, raw, and commercial peanut extracts by skin testing and immunoblotting. Ann Allergy Asthma Immunol, 2010; 105(6):451–7; doi:10.1016/j.anai.2010.09.025
Nirbaya A, Zakaria FR, Wijaya H. Stability of bioactive compound and fatty acid composition after thermal processing of winged bean (Psophocarpus tetragonolobus). Int J Adv Sci Technol, 2020; 29(7):1602–10.
Nirbaya A, Zakaria FR, Wijaya H, Erniati. Immunomodulatory potentials of winged bean (Psophocarpus tetragonolobus) bioactive compounds on human lymphocyte proliferation. Int J Adv Sci Eng Inform Technol, 2021; 11(3):849–55; doi:10.18517/ijaseit.11.3.11527
Nurhutami AD, Supriharti, Marliyawati D, Kusuma Dewi AM. Risk factors for allergic rhinitis in children aged 13-14 years in Semarang. Diponegoro Med J, 2020; 9(2):154–60.
Pawankar R, Holgate ST, Rosenwasser LJ (eds). Allergy frontiers: classification and pathomechanisms, Vol ke-2. Springer, Tokyo, Japan, 2013.
Priyantini S, Suprihati, Widyastiti NS, Soemantri. The low umbilical cord zinc levels lead to atopic allergic infants: a cohort study during 0-4 months of age. Bangladesh J Med Sci, 2020; 19(1):114–21.
Rachmawati D, Samidjan I. The dietary protein requirement in formulated feed for specific growth, digestibility and survival of spiral babylon (Babylonia spirata). Jurnal Teknologi, 2016; 78(4-2):39–43.
Sari WP, Bahtiar, Emiyarti. Studi preferensi habitat siput tutut (Bellamya javanica) di desa amonggedo kabupaten Konawe. Jurnal Manajemen Sumber Daya Perairan, 2016; 1(2):213–24.
Setiawan RD, Zakaria FR, Sitanggang AB, Prangdimurti E, Adawiyah DR, Erniati. Effect of harvesting time on the chemical properties of winged bean seed. Jurnal Teknol dan Industri Pangan, 2019; 30(2):133–42.
Suseno R, Palupi NS, Prangdimurti E. Alergenitas sistem glikasi isolat protein kedelai-fruktooligosakarida. Agritech, 2016; 36(4):450–8; doi: 10.22146/agritech.16770
Vissers YM, Blanc F, Skov PS, Johnson PE, Rigby NM, Nicaise LP, Bernard H, Wal JM, Weber BB, Jongejan LZ, Sze ZP, Koerts JR, Jansen AP, Savelkoul HFJ, Wichers HJ, Mackie AR, Mills CEN, Patient KA. Effect of heating and glycation on the allergenicity of 2S albumins (Ara h 2/6) from peanut. PLoS One, 2011; 6(8):1–9.
Wahyuni RR. Pengaruh hipoglikemik ekstrak protein kecipir (Psophocarpus tetragonolobus) pada tikus diabetik induksi alloksan. Jurnal Ilmiah Teknosains, 2015; 1(1):1–6.
Wijaya H, Zakaria FR, Syah D, Prangdimurti E. Extraction of squid (Photololigo Duvaucelii) myofibrillar and sarcoplasmic proteins to create a skin prick test reagent in the diagnosis of food allergy. J Pharmacy, 2014; 4(9):06–14.
Wijaya H, Zakaria FR, Syah D , Prangdimurti E. Extraction of crab (Scylla serrata) myofibrillar and sarcoplasmic proteins to create a skin prick test reagent for crab allergy diagnosis. Procedia Food Sci, 2015; 3:52–68.
Wu H, Wang Q, Ma T, Ren J. Comparative studies on the functional properties of various protein concentrate preparations of peanut protein. Food Res Int, 2009; 42:343–8.
Zachreini I, Suprihati A, Lubis MND, Aman AK. Role of histamine 1 receptor as risk factor for hypertrophic concha caused by allergic rhinitisimmunohistochemistrily. Int J PharmTech Res, 2015; 7(2):339–43.
Zakaria FR, Khatib A, Ispurwanto RA. Telaah sifat alergenisitas udang putih (Penaeus marguensis) untuk produksi isolat alergen. Bul Teknol dan Industri Pangan, 1998; 9:54–9.
Zakaria FR, Belleville P, Nabet P, Linden G. Allergenicity of bovine casein. I: Specific lymphocyte proliferation and histamine accumulation in the mastocyte as a result of casein feeding in mice. Food Agric Immunol, 1992a; 4(1):41–50; doi:10.1080/0954010920935475
Zakaria FR, Belleville F, Nabet P, Linden G. Allergenicity of bovine casein. II: Casein and its digestive enzyme hydrolysate-induced lymphocyte proliferation and mastocyte histamine accumulation in casein-free mice. Food Agric Immunol, 1992b; 4:51–62; doi:10.1080/09540109209354752