INTRODUCTION
Naturally occurring substances have been used as another option, instead of conventional treatments, for several ailments because of their respectable effectiveness and reduced toxicity (Ijaz et al., 2017). Biologically active compounds from natural resources have received exceptional interest among scientists working on different diseases (Krishnakumar et al., 2013). To date, several modern pharmaceutical products derived from plants or plant-based medicine have been utilized in treating various conditions (Antony et al., 2017; Gajalakshmi et al., 2012; Himaja and Neelufar Shama, 2015; Sanusi et al., 2017). Due to their extraordinary curative powers and fewer side effects, traditional medicines continue to be used as alternatives or supplemental therapies despite the increasing global acceptance of contemporary drugs (Divya et al., 2017; Monton et al., 2014).
One of the biggest genera of woody bamboos, with over 100 species, Bambusa Schreber is a member of the subtribe Bambusinae J. S. Presl, with its subfamily being known as Bambusoideae Kunth ex Ascherson and Graebner (Ohrnberger, 1999). Bambusa spp. can be identified by its dominant primary branch with several secondary branches, thick-walled culms, and erect triangular blades of the culm sheaths (Dransfield, 1992; Dransfield and Widjaja, 1995). They possess unique sheathing structures, a spreading architecture, petiolate foliage, a highly effective rhizome network, hollowed woody stems, and tree-like characteristics from a physiological perspective (Banik, 2015). This genus is located between southern Asia and the Pacific Rim in the tropics and subtropics, and many species are arboreal in Africa, India, China, and Brazil, with widespread utility and economic value (Bahru and Ding, 2021; Pereira and Beraldo, 2007; Tewari et al., 2019; Wu et al., 2009). Due to its several applications, this plant also plays a significant financial and socioeconomic part in the lives of the locals in some Asian nations including China, India, Japan, and the countries of Southeast Asia, especially in rural regions (Cho et al., 2011; Khairi et al., 2020; Ruiz-Sanchez et al., 2019). They are present in about 1,500 consumer products (Li and Kobayashi, 2004), that are used in a variety of contexts, including musical instruments, food profiles, and building supplies (Cho et al., 2011) to the production of paper pulp, fencing, basketry (Pearson et al., 1994), water pipes, utensils (Liu et al., 2008), bridges (Xiao et al., 2010), and low-rise housing (Chung and Yu, 2002).
Bamboos have drawn interest from all around the world and are essential to the drug and food industry sectors due to their nutritional and medicinal capabilities (Nirmala et al., 2018). Thus, Bambusa spp. harbor many pharmaceutical compounds, such as steroids, terpenoids, tannins, flavonoids, polyphenols, alkaloids, glycosides, phytosterols, ginsenosides, and fatty acids (Carey et al., 2009; Jawaid et al., 2015; Lodhi et al., 2016; Saducos, 2021; Thamizharasan et al., 2015; Yakubu and Bukoye, 2009; Zihad et al., 2018). For centuries, Bambusa spp. have been used in folk medicines for the treatment and prevention of various diseases (Esakkimuthu et al., 2016; Luo et al., 2020; Prabhu et al., 2021). Pharmacological properties of Bambusa spp. include anti-inflammatory (Carey et al., 2009; Lodhi et al., 2016; Muniappan and Sundararaj, 2003; Vanitha et al., 2016), antifungal (Abiala et al., 2015; Saducos, 2021; Tyagi et al., 2018), antibacterial (Badwaik et al., 2014; Jayarambabu et al., 2021), antimalarial (Esmaeili et al., 2015; Komlaga et al., 2016), antioxidant (Alok et al., 2017; Karnjanapratum et al., 2019; Kong et al., 2020; Sandhiya et al., 2013), anticancer (Jayarambabu et al., 2021; Kalaiarasi et al., 2015), antidiabetic (Dey et al., 2018; Goyal et al., 2017), abortifacient (Yakubu and Bukoye, 2009; Yakubu et al., 2009), and cytotoxicity (Komlaga et al., 2016; Valdés et al., 2010) activities. Resultantly, the bioactive compounds of Bambusa spp. can serve as a fundamental idea for drug development.
In order to promote future investigation into the widespread use and therapeutic application of this medicinal plant, this review presents the existing information on plant ethnobotany, phytochemistry, and the pharmacological activities of Bambusa spp.
MATERIALS AND METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009) were used to assist this research on the ethnobotanical, phytochemical, and pharmacological aspects of the genus Bambusa and scoping study methodology (Arksey and O’Malley, 2005) as a systematic approach. The methodological framework for the scoping review consisted of five stages: (1) establishing the research questions; (2) locating relevant studies; (3) study selection; (4) charting the data; (5) compiling, summarizing, and reporting the results. Figure 1 illustrates the PRISMA flow diagram for article selection.
Stage 1: establishing the research questions
The research questions were: (1) What are the beneficial health effects of ethnobotanical and pharmacological properties of Bambusa species? (2) What type of phytochemicals can be found in Bambusa species?
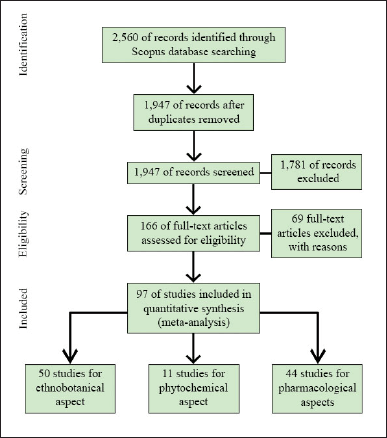 | Figure 1. PRISMA flow diagram for article selection process. [Click here to view] |
Stage 2: locating relevant studies
The source of data was the Scopus database. Articles published between 2003 and 2021 were considered for the search process. The literature search was conducted using specific keywords, comprising “Bambusa/Poaceae ethnobotany” or “Bambusa/Poaceae traditional knowledge” and “Bambusa/Poaceae phytochemistry” and “Bambusa/Poaceae pharmacology” or “Bambusa/Poaceae medicine” or “Bambusa/Poaceae biological activities”. A total of 2,560 total articles were retrieved from the literature search.
Stage 3: study selection
Studies were considered in regards to inclusion in this study if they satisfied the criteria which follow: (1) emphasizing all species names from the genus Bambusa; (2) reporting single or multiple data of the ethnobotany, phytochemistry, and pharmacology in Bambusa species; (3) showing bioactive compound with its figure and Chemical Abstracts Service Registry Number (CAS RN) that can be found in Bambusa species; (4) highlighting the beneficial health effects of the ethnobotany in Bambusa species; and (5) evaluating the mechanism of each pharmacological property in Bambusa species.
Stage 4: charting the data
The data were presented according to the following: (1) species, local names, diseases or ailments, parts used, preparation and administration, and references for ethnobotanical aspect; (2) compound names, CAS RN, species, pharmacological activities, and references for phytochemical aspect; and (3) pharmacological properties, species, material basis, methods, results, and references for pharmacological aspects.
Stage 5: compiling, summarizing, and reporting the results
The findings of the genus Bambusa were presented systematically in the form of tables for ethnobotanical and pharmacological aspects, whereas both tables and figures were utilized for the phytochemical aspect.
ETHNOBOTANICAL ASPECT
Bambusa spp. (Fig. 2) have been used to cure a variety of diseases in folk medicine. For instance, the people of Subhartipuram in India prescribed the leaves of Bambusa vulgaris for the treatment of rheumatism (Singh et al., 2020). Additionally, B. vulgaris is prescribed to cure malaria, heart problems, and clean-out dilation. Kani tribals consume the seeds of B. arundinacea as a paste to cure rheumatism (Ayyanar and Ignacimuthu, 2011). The paste of B. bambos seeds is used to treat rheumatism (Silambarasan and Ayyanar, 2015). Traditional healers in Kanyakumari apply a paste made from the entire plant, turmeric, and Areca catechu to cure bruising and reduce swelling (Sukumaran et al., 2014). The people of the Rakhain tribe used the leaves and roots of B. multiplex to cure fever and skin itches in Bangladesh (Hanif et al., 2009), whereas the root of B. arundinacea is used in treating joint pains (Sharkar et al., 2013).
Bambusa arundinacea and B. vulgaris are the two most utilized species in traditional medicine. Hence, the medical applications of Bambusa spp. are detailed in Table 1.
PHYTOCHEMICAL ASPECT
The phytochemical analyses of ethanol extract from B. vulgaris yielded positive results for carbohydrates, flavonoids, glycosides, proteins, tannins, and terpenoids. The extraction of aqueous and ethyl acetate fractions was found to be 1.82% w/w and 1.13% w/w, respectively (Lodhi et al., 2016). The phytochemical analysis of B. arundinacea leaves revealed the presence of amino acids, carbohydrates, flavonoids, proteins, and steroids in the crude extract (Jawaid et al., 2015). Furthermore, the presence of phytoconstituents such as alkaloids, flavonols, and phenolics in B. arundinacea was attributed to the laxative properties found in the shoot (Zihad et al., 2018). Bambusa blumeana (known as Kawayang Tinik) extracts of whole plants contain phytochemicals such as alkaloids, flavonoids, phenolics, sterols, and tannins. Hence, more research is crucial to discover the compounds responsible for the antifungal properties (Saducos, 2021). This study of B. arundinacea seed extracts showed the presence of flavonoids, phenolics, quinines, steroids, and tannins (Thamizharasan et al., 2015). Additionally, early phytochemical testing of a fluid extract of B. vulgaris foliage revealed the presence of alkaloids, anthraquinones, flavonoids, glycosides, phenolics, saponins, and tannins. However, terpenes, steroids, and chalcones were not identified. Alkaloids made up the majority of the B. vulgaris leaves’ aqueous extract, whereas flavonoids made up the least amount of the phytochemicals (Yakubu and Bukoye, 2009). The early phytochemical screening of B. vulgaris methanol extract revealed the presence of alkaloids, carbohydrates, flavonoids, glycosides, and proteins (Carey et al., 2009).
 | Figure 2. Example of Bambusa spp. [Click here to view] |
The phytochemistry of Bambusa spp. including B. balcooa, B. nutans, and B. textilis has been investigated in detail and classified into certain chemical compounds (Table 2). In Figures 3–14, the structural properties of these compounds (1-49) are also depicted (Liu et al., 2016; Pande et al., 2018; Sarkar et al., 2020; Soumya et al., 2014).
PHARMACOLOGICAL ASPECTS
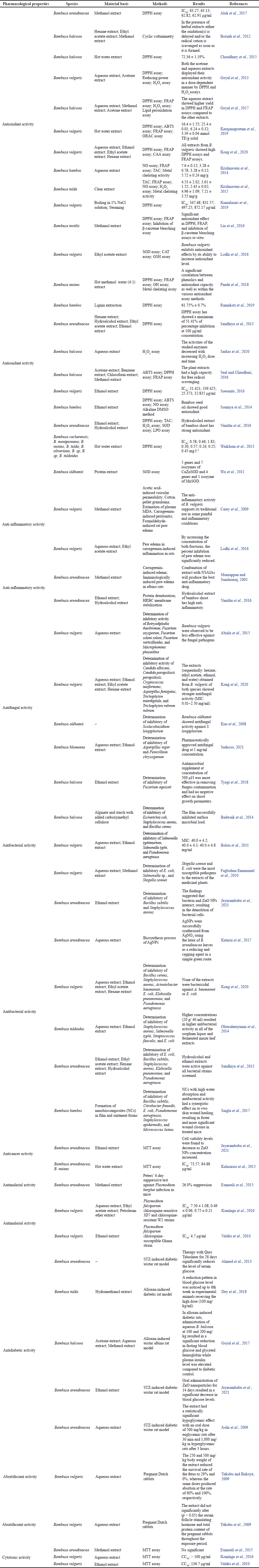
Table 3 shows a variety of pharmaceutical activities of Bambusa spp. that exhibit clinically relevant biological activities.
Antioxidant activity
The antioxidant activity of Bambusa spp. has been extensively investigated. The methanolic extract of B. arundinacea as one of the ingredients in the Ayurvedic formula of Drakshavaleha (DKV) had a maximum DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of 68.24% at 100 μg/ml. Furthermore, at 100 μg/ml, the maximum DPPH radical scavenging activity of marketed DKV formulations (DKV-1, DKV-2, and DKV-3) was 67.90%, 68.35%, and 68.40%, respectively (Alok et al., 2017). The ethyl acetate and hexane extracts of fresh B. balcooa did not show instant radical scavenging capacity as the second oxidation wave was delayed (Boruah et al., 2012). DPPH radical scavenging activity was 8.57%, 14.31%, and 17.85% for biscuits merged with 5%, 10%, and 15% of B. balcooa levels, respectively. Meanwhile, the value for the control was 3.50%, depicting improved functional and nutraceutical properties for biscuit preparation and other food products (Choudhury et al., 2015). By absorbing hydrogen peroxide and DPPH radicals, both acetone and the aqueous extracts of B. vulgaris demonstrated their antioxidant properties in a manner dependent on dosage (Goyal et al., 2013).
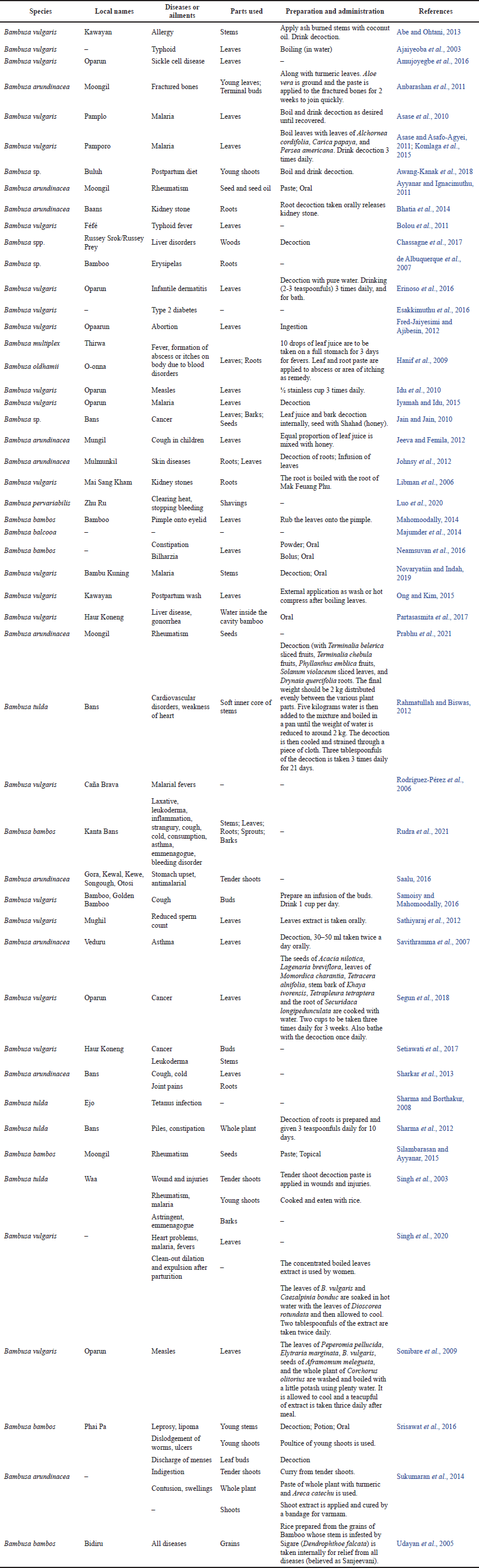 | Table 1. List of Bambusa spp. used as medicinal plant worldwide. [Click here to view] |
The maximum scavenging of DPPH radicals was seen in the methanolic extract from B. balcooa, which was followed by the aqueous extract. However, the B. balcooa extract in acetonic form had the least amount of action. The maximum ferric reducing antioxidant power (FRAP) result was seen in the aqueous extract of B. balcooa (0.217), followed by the acetonic extract (0.027), and the methanolic extract (0.079) when compared to ascorbic acid (0.041) as a reference at a concentration of 200 μg/ml. Therefore, it can be inferred that aqueous extract contains in vitro antioxidant effect (Goyal et al., 2017). The fiber hydrolysate from B. vulgaris shoots displayed ABTS [2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonate)] radical scavenging activities, DPPH, FRAP, and oxygen radical absorbance capacity. Bambusa vulgaris can thus be successfully synthesized via enzymatic hydrolysis with antioxidant activity and is appropriate for application in fortified fiber products (Karnjanapratum et al., 2019).
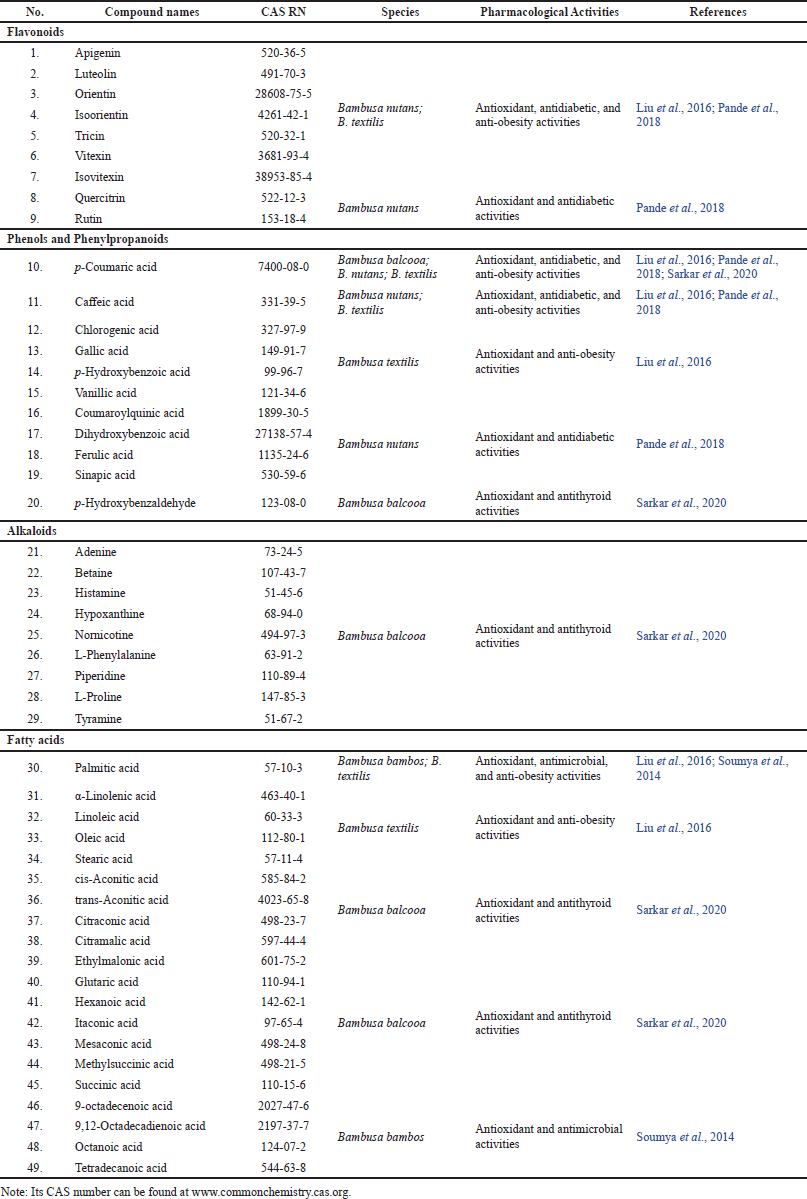 | Table 2. Phytoconstituents isolated from Bambusa spp. [Click here to view] |
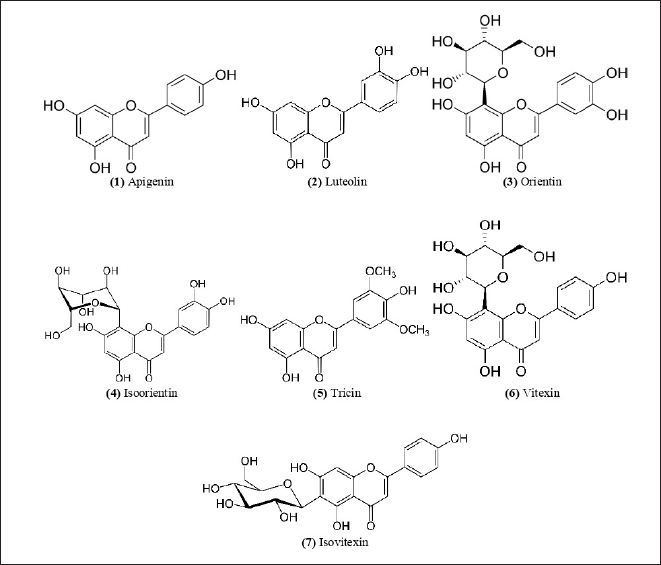 | Figure 3. Chemical structures (1-7) isolated from B. nutans and B. textilis. [Click here to view] |
Anti-inflammatory activity
All experimental models, including acetic acid-induced vascular permeability, carrageenan-induced peritonitis, cotton pellet granuloma, formaldehyde-induced paw edema, and estimation of plasma malondialdehyde (MDA), were significantly and dose-dependently inhibited (p < 0.01) by B. vulgaris (100 mg/kg, 200 mg/kg, and 400 mg/kg, p.o.). The results indicated that B. vulgaris significantly contains anti-inflammatory properties, which are reflected in the use of this traditional plant for treating painful and inflammatory conditions (Carey et al., 2009). The inhibitory activities of paw edema were significantly decreased by increasing the amounts of ethyl acetate and aqueous fractions from B. vulgaris, which contains anti-inflammatory properties (Lodhi et al., 2016). When compared to the usual medications recognized as ulcerogenic, the methanolic extract of B. arundinacea leaves against immunologically generated paw edema and carrageenan-induced edema was shown to be better (Muniappan and Sundararaj, 2003). Additionally, by stabilizing the membrane of human red blood cells and using the protein denaturation technique, the hydroalcoholic extract of B. arundinacea shows potent anti-inflammatory properties (Vanitha et al., 2016).
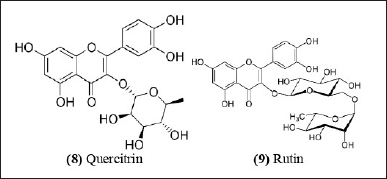 | Figure 4. Chemical structures (8-9) isolated from B. nutans. [Click here to view] |
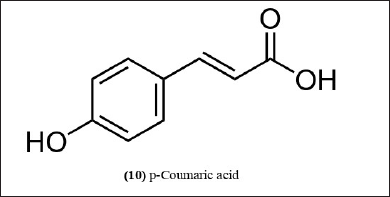 | Figure 5. Chemical structure (10) isolated from B. balcooa, B. nutans, and B. textilis. [Click here to view] |
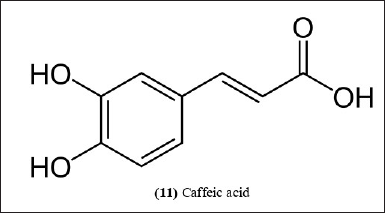 | Figure 6. Chemical structure (11) isolated from B. nutans and B. textilis. [Click here to view] |
Antibacterial activity
At doses ranging from 2.5 mg/ml to > 80 mg/ml, both aqueous and ethanolic extracts of B. vulgaris demonstrated bactericidal action against at least one of the test pathogens, as analyzed by Minimum Bactericidal Concentration and Minimum Inhibitory Concentration (MIC) (Bolou et al., 2011). In contrast to the ethanolic leaf extract, which lacked antibacterial activity against Salmonella typhi and Streptococcus faecalis, fresh leaf extracts of B. tuldoides from sorghum liquors and fermented maize at a concentration of 20 g/100 ml demonstrated antibacterial activity against all of the bacteria tested. Higher concentrations (20 g/40 ml) of both fresh and dried extracts of B. tuldoides demonstrated greater antibacterial activity than sorghum liquors and fermented maize (Oluwahenyinmi et al., 2014). For increasing concentrations (25, 50, 100, and 150 μl) of ethanolic extract of B. arundinacea, the inhibition zone for Staphylococcus aureus was 14, 16, 17, and 19 mm, whereas that of Bacillus subtilis was 12, 14, 15, and 17 mm, respectively (Jayarambabu et al., 2021). Following the use of the agar well diffusion method, B. vulgaris extracts may include compounds with antibacterial activities that can be incorporated into novel antimicrobial medicines (Sandhiya et al., 2013).
Antifungal activity
The extracts (sequentially ethanol, ethyl acetate, hexane, and water) acquired from B. vulgaris boiled shoots exhibited a higher antifungal effect (MIC: 0.01–2.50 mg/ml) compared to antibacterial effect (MIC: 0.31–2.50 mg/ml) (Kong et al., 2020). Class III chitinase cDNA (BoChi3-1) was cloned from suspension-cultured bamboo (B. oldhamii) cells and then produced in yeast by using a cDNA library (Pichia pastoris X-33). Both recombinant BoChi3-1 isoforms inhibited Scolecobasidium longiphorum growth (Kuo et al., 2008). At a concentration of 1 mg/ml, all Kawayang Tinik (B. blumeana) extracts showed a statistically equivalent zone of inhibition (ZOI) against Aspergillus niger, whereas ethanolic leaf and root extracts produced a wider ZOI against Penicillium chrysogenum than other B. blumeana extracts. In addition, antifungal assay findings demonstrated that B. blumeana extracts have a comparable antifungal effect to fluconazole, an FDA-approved antifungal agent at 1 mg/ml concentration (Saducos, 2021).
Antimalarial activity
Plasmodium berghei was evaluated for in vivo antimalarial efficacy against B. arundinacea methanolic extract, and it demonstrated 26.0%, showing excellent antimalarial potential (Esmaeili et al., 2015). The organic solvent fractions of B. vulgaris exhibited antimalarial activity with ethyl acetate and petroleum ether fractions, displaying an IC50 below 1 μg/ml against the Plasmodium falciparum 3D7 strain (Komlaga et al., 2016). Bambusa vulgaris hydroalcoholic extract also demonstrated the most specific and potent antimalarial property (IC50 = 4.7 μg/ml, SI = 28.9) (Valdés et al., 2010).
Antidiabetic activity
Treatment of streptozotocin-induced diabetic rats with Qurs Tabasheer (including B. arundinacea) for 28 days significantly reduced serum glucose, cholesterol, fructose-1-6-biphosphatase, glucose-6-phosphatase, and triglycerides, whereas the magnitude of hexokinase and high-density lipoprotein cholesterol was amplified (Ahmed et al., 2013). Compared to diabetic animals, the animals given B. tulda leaves showed a gain in body weight. Hence, hydromethanolic extract proved that B. tulda leaves possess antidiabetic activity. Although the effect was significant, B. tulda leaves could be utilized in managing diabetic levels (Dey et al., 2018). While plasma insulin levels were raised relative to diabetic control, the aqueous extract of B. balcooa at 100 mg/kg and 200 mg/kg effectively reduced fasting blood glucose and glycated hemoglobin in alloxan-induced diabetic rats. Both dosages of glibenclamide (standard antidiabetic agent) were efficient against diabetic rats (Goyal et al., 2017). The hypoglycemic effect of B. arundinacea aqueous extract was statistically significant and equivalent to that of glibenclamide at 0.9 mg/kg in euglycemic rats at 30 minutes and 1,000 mg/kg in hyperglycemic rats at 3 hours with an oral dose of 500 mg/kg (Joshi et al., 2009).
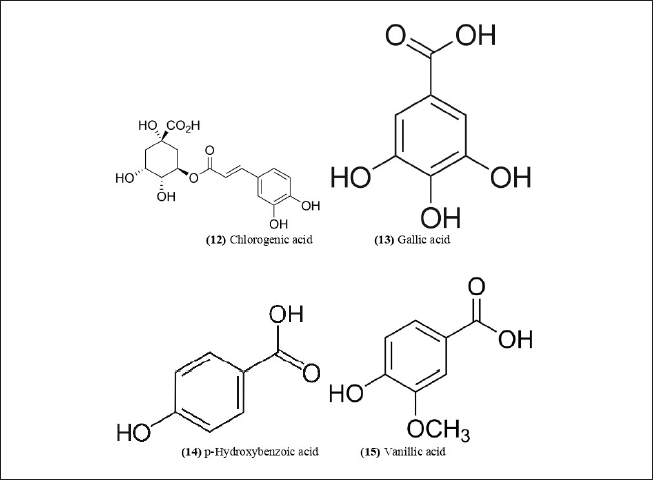 | Figure 7. Chemical structures (12-15) isolated from B. textilis. [Click here to view] |
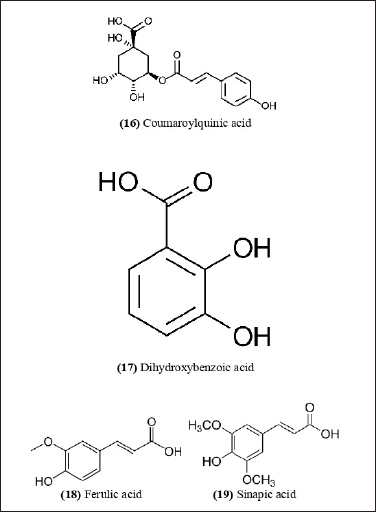 | Figure 8. Chemical structures (16-19) isolated from B. nutans. [Click here to view] |
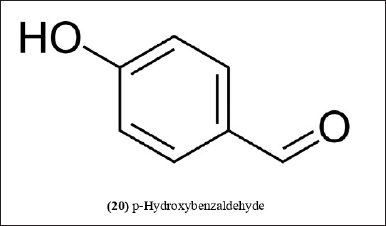 | Figure 9. Chemical structure (20) isolated from B. balcooa. [Click here to view] |
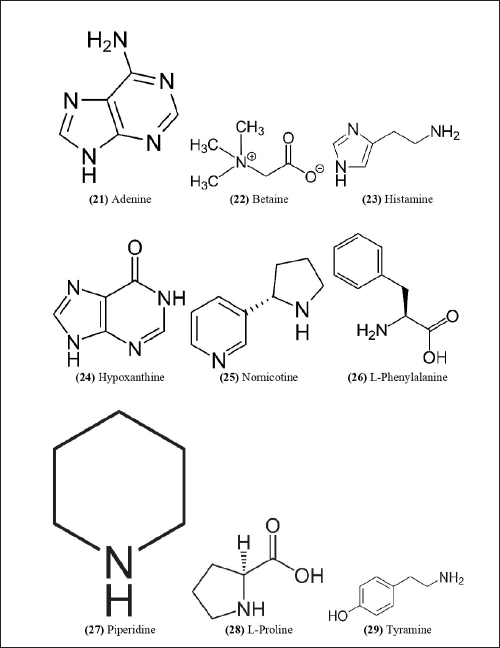 | Figure 10. Chemical structures (21-29) isolated from B. balcooa. [Click here to view] |
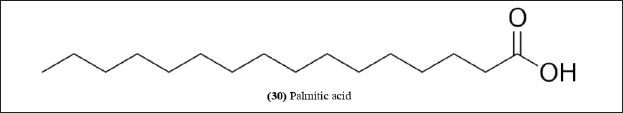 | Figure 11. Chemical structure (30) isolated from B. bambos and B. textilis. [Click here to view] |
Anticancer activity
Bambusa arundinacea-derived zinc oxide nanoparticles (ZnO NPs) demonstrated anticancer activity against MCF-7 cell lines. These results indicate that biosynthesized ZnO NPs may have therapeutic properties (Jayarambabu et al., 2021). Kalaiarasi et al. (2015) examined the antitumor effectiveness of in vitro-grown B. arundinacea (Ba) and B. nutans (Bn) leaf samples for the formation of metallic silver nanoparticles (AgNPs) from silver ions against human prostate cancer cell lines (PC-3). BaAgNPs and BnAgNPs had IC50 values for PC3 cancer cells of 73.57 μg/ml and 84.88 μg/ml, respectively, while Vero cells had IC50 values of 93.58 μg/ml and 96.41 μg/ml, respectively. For BaAgNPs and BnAgNPs, the percentages of apoptotic bodies as measured by acridine orange/ethidium bromide staining were 76% and 62%, respectively. The observations clearly suggest that synthesized BaAgNPs may have anticancer activity against human PC-3 cell lines in contrast to BnAgNPs.
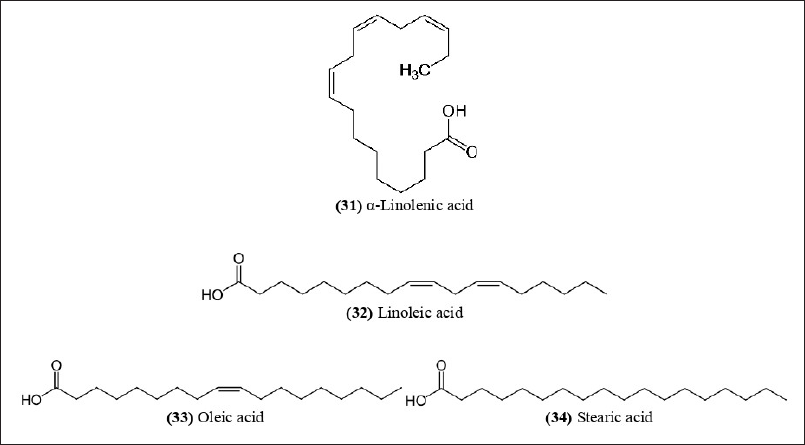 | Figure 12. Chemical structures (31-34) isolated from B. textilis. [Click here to view] |
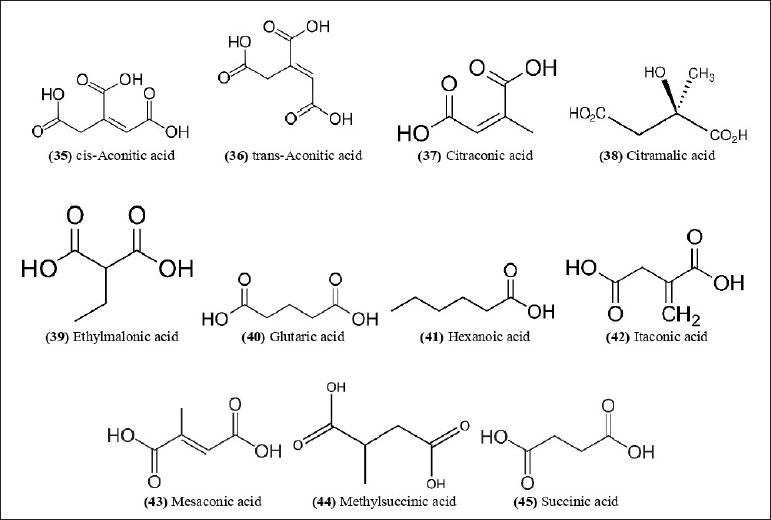 | Figure 13. Chemical structures (35-45) isolated from B. balcooa. [Click here to view] |
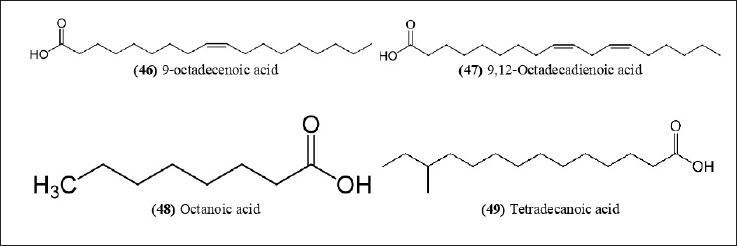 | Figure 14. Chemical structures (46-49) isolated from B. bambos. [Click here to view] |
 | Table 3. Pharmacological properties of Bambusa spp. [Click here to view] |
Abortifacient activity
Aqueous extracts of B. vulgaris leaves were tested for their toxicity at 250 mg/kg and 500 mg/kg body weight in a pregnant Dutch rabbit test. The extract increased kidney gamma-glutamyl transferase (GGT) at a dose of 250 mg/kg. Contrarily, kidney GGT activity decreased at 500 mg/kg while the 250 mg/kg dose did not affect the liver and serum GGT. Furthermore, the extract increased the levels of total and conjugated bilirubin, bicarbonate ions, creatinine, potassium, sodium, and uric acid in the blood. The alteration of biochemical markers by the aqueous extract of B. vulgaris leaves suggests a negative impact on the excretory, secretory, synthetic, and reabsorptive functions of the liver and kidney in animals (Yakubu et al., 2009). Yakubu and Bukoye (2009) also showed B. vulgaris aqueous extract to be an abortifacient. The dosage of the extract at 250 mg/kg and 500 mg/kg decreased the fetus survival rate to 29% and 0%, correspondingly, while the same levels resulted in 60% and 100% more abortions, accordingly. The implantation index and preimplantation loss were evaluated in relation to the control. Both dosages resulted in an increase in the post-implantation loss and resorption index. The extract also decreased serum progesterone, luteinizing hormone, and follicle-stimulating hormone levels. These results verified the abortifacient properties of the aqueous extract of B. vulgaris leaves.
Cytotoxic effect
Methanolic extract of B. arundinacea was screened for cytotoxicity effect on Madin-Darby bovine kidney cells and showed no IC50 (Esmaeili et al., 2015). Nevertheless, the aqueous extract of B. vulgaris showed CC50 > 100 μg/ml when tested for cytotoxicity against human umbilical vein endothelial cells, supporting its classical usage in Ghana as a malaria therapy (Komlaga et al., 2016). A hydroalcoholic extract of B. vulgaris over human cell line MRC-5 to examine cytotoxicity showed CC50 of 136.7 μg/ml, thereby reflecting that the extract could possess cytotoxicity activity (Valdés et al., 2010).
SUMMARY AND OUTLOOK
This review has elucidated the significance of Bambusa spp. as a medicinal plant with diverse pharmacological spectra. Bambusa spp. contain a variety of phytochemical constituents that are responsible for their diverse ethnomedicinal and pharmacological properties. Developing novel clinical therapeutics from Bambusa spp. requires additional investigation of the current data. Given that this review compiled vital information on various aspects of this medicinal plant, it provides an excellent opportunity for future research.
ACKNOWLEDGMENTS
Sincere appreciation is extended to Sabah Parks and Sabah Biodiversity Centre for giving access permission [JKM/MBS.1000-2/2 JLD.12 (89)].
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The authors would like to thank Universiti Malaysia Sabah for the financial support provided by the Postgraduate Research Grant (UMSGreat) [GUG0507-2/2020] throughout the research period.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abe R, Ohtani K. An ethnobotanical study of medicinal plants and traditional therapies on Batan Island, the Philippines. J Ethnopharmacol, 2013; 145(2):554–65.
Abiala MA, Ayandeko FM, Odebode AC. Antifungal effects of selected botanicals on fungal pathogens of watermelon fruit. Arch Phytopathol Plant Prot, 2015; 48(7):569–77.
Ahmed D, Sharma M, Mukerjee A, Ramteke PW, Kumar V. Improved glycemic control, pancreas protective and hepatoprotective effect by traditional poly-herbal formulation “Qurs Tabasheer” in streptozotocin induced diabetic rats. BMC Complement Altern Med, 2013; 13:10.
Ajaiyeoba EO, Oladepo O, Fawole OI, Bolaji OM, Akinboye DO, Ogundahunsi OAT, Falade CO, Gbotosho GO, Itiola OA, Happi TC, Ebong OO, Ononiwu IM, Osowole OS, Oduola OO, Ashidi JS, Oduola AMJ. Cultural categorization of febrile illnesses in correlation with herbal remedies used for treatment in Southwestern Nigeria. J Ethnopharmacol, 2003; 85(2–3):179–85.
Alok S, Kumar MD, Karunakar S. Evaluation of antioxidant potential of Drakshavaleha: a polyherbal ayurvedic formulation. Asian J Pharm, 2017; 11(4):331–5.
Amujoyegbe OO, Idu M, Agbedahunsi JM, Erhabor JO. Ethnomedicinal survey of medicinal plants used in the management of sickle cell disorder in Southern Nigeria. J Ethnopharmacol, 2016; 185:347–60.
Anbarashan M, Parthasarthy N, Padmavathy A. Ethno-floristic survey in sacred groves, Pudukottai district, Tamil Nadu- India. J Med Plant Res, 2011; 5(3):439–43.
Antony E, Sathiavelu M, Arunachalam S. Synthesis of silver nanoparticles from the medicinal plant Bauhinia acuminata and Biophytum sensitivum–a comparative study of its biological activities with plant extract. Int J Appl Pharm, 2017; 9(1):22–9.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol, 2005; 8(1):19–32.
Asase A, Akwetey GA, Achel DG. Ethnopharmacological use of herbal remedies for the treatment of malaria in the Dangme West District of Ghana. J Ethnopharmacol, 2010; 129(3):367–76.
Asase A, Asafo-Agyei T. Plants used for treatment of malaria in communities around the Bobiri forest reserve in Ghana. J Herbs Spices Med Plants, 2011; 17(2):85–106.
Awang-Kanak F, Abu Bakar MF, Mohamed M, Norazlimi NA. Utilization of natural resources: preliminary study on ethnopharmacological application of “ulam” or traditional vegetables among Sama-Bajau of Kampung Menunggui, Kota Belud, Sabah. AIP Conf Proc, 2018; 2016:020029.
Ayyanar M, Ignacimuthu S. Ethnobotanical survey of medicinal plants commonly used by Kani tribals in Tirunelveli hills of Western Ghats, India. J Ethnopharmacol, 2011; 134(3):851–64.
Badwaik LS, Borah PK, Deka SC. Antimicrobial and enzymatic antibrowning film used as coating for bamboo shoot quality improvement. Carbohydr Polym, 2014; 103(1):213–20.
Bahru T, Ding Y. A review on bamboo resource in the African region: a call for special focus and action. Int J For Res, 2021; 2021:8835673.
Banik RL. Morphology and growth. In: Liese W, Köhl M (eds.). Bamboo: the plant and its uses, Springer, Cham, Switzerland, pp 43–90, 2015.
Bhatia H, Sharma YP, Manhas RK, Kumar K. Ethnomedicinal plants used by the villagers of district Udhampur, J&K, India. J Ethnopharmacol, 2014; 151(2):1005–18.
Bolou GEK, Bagré I, Ouattara K, Djaman AJ. Evaluation of the antibacterial activity of 14 medicinal plants in Côte d’Ivoire. Trop J Pharm Res, 2011; 10(3):335–40.
Boruah MP, Mahanta D, Handique JG. Comparative evaluation of antioxidant activities of some fresh and preserved herbal products of North East India by cyclic voltammetry. Indian J Nat Prod Resour, 2012; 3(1):40–7.
Carey WM, Dasi JMB, Rao NV, Gottumukkala KM. Anti-inflammatory activity of methanolic extract of Bambusa vulgaris leaves. Int J Green Pharm, 2009; 3(3):234–8.
Chassagne F, Deharo E, Punley H, Bourdy G. Treatment and management of liver diseases by Khmer traditional healers practicing in Phnom Penh area, Cambodia. J Ethnopharmacol, 2017; 202:38–53.
Cho E, Um Y, Yoo SK, Lee H, Kim HB, Koh S, Shin HC, Lee Y. An expressed sequence tag analysis for the fast-growing shoots of Bambusa edulis Munro. J Plant Biol, 2011; 54(6):402–8.
Choudhury M, Badwaik LS, Borah PK, Sit N, Deka SC. Influence of bamboo shoot powder fortification on physico-chemical, textural and organoleptic characteristics of biscuits. J Food Sci Technol, 2015; 52(10):6742–8.
Chung KF, Yu WK. Mechanical properties of structural bamboo for bamboo scaffoldings. Eng Struct, 2002; 24(4):429–42.
de Albuquerque UP, Monteiro JM, Ramos MA, de Amorim ELC. Medicinal and magic plants from a public market in northeastern Brazil. J Ethnopharmacol, 2007; 110(1):76–91.
Dey SK, Middha SK, Usha T, Brahma BK, Goyal AK. Antidiabetic activity of giant grass Bambusa tulda. Bangladesh J Pharmacol, 2018; 13(2):134–6.
Divya BJ, Suman B, Venkataswamy M, Thyagaraju K. A study on phytochemicals, functional groups and mineral composition of Allium sativum (garlic) cloves. Int J Curr Pharm Res, 2017; 9(3):42–5.
Dransfield S. The bamboos of Sabah. Forestry Department, Sabah, Malaysia, 1992.
Dransfield S, Widjaja EA. Plant resources of South-East Asia. No. 7: Bamboos. Backhuys Publishers, Leiden, The Netherlands, 1995.
Erinoso SM, Fawibe OO, Oyelakin AS, Ajiboye AA, Agboola DA. Herbal recipes used for the traditional management of infantile dermatitis in Odeda, Southwestern Nigeria. Afr J Tradit Complement Altern Med, 2016; 13(3):33–43.
Esakkimuthu S, Mutheeswaran S, Arvinth S, Paulraj MG, Pandikumar P, Ignacimuthu S. Quantitative ethnomedicinal survey of medicinal plants given for cardiometabolic diseases by the non-institutionally trained Siddha practitioners of Tiruvallur district, Tamil Nadu, India. J Ethnopharmacol, 2016; 186:329–42.
Esmaeili S, Ghiaee A, Naghibi F, Mosaddegh M. Antiplasmodial activity and cytotoxicity of plants used in traditional medicine of Iran for the treatment of fever. Iran J Pharm Res, 2015; 14(S):103–7.
Fagbohun Emmanuel D, David Oluwole M, Adeyeye Emmanuel I, Oyedele O. Chemical composition and antibacterial activities of some selected traditional medicinal plants used in the treatment of gastrointestinal infections in Nigeria. Int J Pharm Sci Rev Res, 2010; 5(3):192–7.
Fred-Jaiyesimi AA, Ajibesin KK. Ethnobotanical survey of toxic plants and plant parts in Ogun State, Nigeria. Int J Green Pharm, 2012; 6(3):174–9.
Gajalakshmi S, Vijayalakshmi S, Devi Rajeswari V. Phytochemical and pharmacological properties of Annona muricata: a review. Int J Pharm Pharm Sci, 2012; 4(2):3–6.
Goyal AK, Middha SK, Sen A. Bambusa vulgaris Schrad. ex J. C. Wendl. var. vittata Riviere & C. Riviere leaves attenuate oxidative stress- an in vitro biochemical assay. Indian J Nat Prod Resour, 2013; 4(4):436–40.
Goyal AK, Middha SK, Usha T, Sen A. Analysis of toxic, antidiabetic and antioxidant potential of Bambusa balcooa Roxb. leaf extracts in alloxan-induced diabetic rats. 3 Biotech, 2017; 7(2):120.
Hanif A, Hossan MS, Mia MMK, Islam MJ, Jahan R, Rahmatullah M. Ethnobotanical survey of the Rakhain tribe inhabiting the Chittagong hill tracts region of Bangladesh. Am J Sustain Agric, 2009; 3(2):172–80.
Himaja N, Neelufar Shama S. Herbal wealth for hepatotoxicity: a review. Asian J Pharm Clin Res, 2015; 8(1):3–9.
Idu M, Erhabor JO, Efijuemue HM. Documentation on medicinal plants sold in markets in Abeokuta, Nigeria. Trop J Pharm Res, 2010; 9(2):110–8.
Ijaz H, Tulain UR, Qureshi J, Danish Z, Musayab S, Akhtar MF, Saleem A, Khan KAUR, Zaman M, Waheed I, Khan I, Abdel-Daim M. Nigella sativa (Prophetic Medicine): a review. Pak J Pharm Sci, 2017; 30(1):229–34.
Iyamah PC, Idu M. Ethnomedicinal survey of plants used in the treatment of malaria in Southern Nigeria. J Ethnopharmacol, 2015; 173:287–302.
Jain R, Jain SK. Traditional medicinal plants as anticancer agents from Chhattishgarh, India: an overview. Int J Phytomedicine, 2010; 2(3):186–96.
Jawaid T, Awasthi A, Kamal M. Estrogenic activity of a hydro-alcoholic extract of Bambusa arundinacea leaves on female wistar rats. J Adv Pharm Technol Res, 2015; 6(1):19–24.
Jayarambabu N, Venkatappa Rao T, Rakesh Kumar R, Akshaykranth A, Shanker K, Suresh V. Anti-hyperglycemic, pathogenic and anticancer activities of Bambusa arundinacea mediated Zinc Oxide nanoparticles. Mater Today Commun, 2021; 26:101688.
Jeeva S, Femila V. Ethnobotanical investigation of Nadars in Atoor village, Kanyakumari district, Tamilnadu, India. Asian Pac J Trop Biomed, 2012; 2(2S):S593–600.
Johnsy G, Davidson Sargunam S, Kaviyarasan V. Indigenous knowledge of medicinal plants used for the treatment of skin diseases by the Kaani tribal of Kanyakumari district. Int J Pharm Pharm Sci, 2012; 4(1):309–13.
Joshi RK, Patil PA, Mujawar MHK, Kumar D, Kholkute SD. Hypoglycemic activity of Bambusa arundinacea leaf aqueous extract in euglycemic and hyperglycemic wistar rats. Pharmacologyonline, 2009; 3:789–95.
Kalaiarasi K, Prasannaraj G, Sahi SV, Venkatachalam P. Phytofabrication of biomolecule-coated metallic silver nanoparticles using leaf extracts of in vitro-raised bamboo species and its anticancer activity against human PC3 cell lines. Turkish J Biol, 2015; 39(2):223–32.
Karnjanapratum S, Kaewthong P, Takeungwongtrakul S, Sae-Leaw T, Hong JH, Nalinanon S. Production of fiber hydrolysate from bamboo shoot with antioxidative properties by enzymatic hydrolysis. Curr Appl Sci Technol, 2019; 19(3):225–34.
Kataria B, Shyam V, Kaushik B, Vasoya J, Joseph J, Savaliya C, Kumar S, Parikh SP, Thakar CM, Pandya DD, Ravalia AB, Markna JH, Shah NA. Green synthesis of silver nanoparticle using Bambusa arundinacea leaves. AIP Conf Proc, 2017; 1837:040043.
Khairi NM, Yong WTL, Kulip J, Rodrigues KF. A glance at molecular identification of bamboo (Poaceae: Bambusoideae). Borneo Int J Biotechnol, 2020; 1:19–33.
Komlaga G, Agyare C, Dickson RA, Mensah MLK, Annan K, Loiseau PM, Champy P. Medicinal plants and finished marketed herbal products used in the treatment of malaria in the Ashanti region, Ghana. J Ethnopharmacol, 2015; 172:333–46.
Komlaga G, Cojean S, Dickson RA, Beniddir MA, Suyyagh-Albouz S, Mensah MLK, Agyare C, Champy P, Loiseau PM. Antiplasmodial activity of selected medicinal plants used to treat malaria in Ghana. Parasitol Res, 2016; 115(8):3185–95.
Kong CK, Tan YN, Chye FY, Sit NW. Nutritional composition and biological activities of the edible shoots of Bambusa vulgaris and Gigantochloa ligulata. Food Biosci, 2020; 36:100650.
Krishnakumar S, Dooslin Mercy Bai V, Rajan RA. Evaluation of bioactive metabolites from halophilic microalgae Dunaliella salina by GC – MS analysis. Int J Pharm Pharm Sci, 2013; 5(4):296–303.
Krishnaveni M, Eswari V, Silpavathi G, Silambarasan V, Senthil Kumar R, Sabari M. Free radical scavenging activity (in vitro) of plants collected near cement industry. Int J Curr Pharm Rev Res, 2015; 6(2):134–41.
Krishnaveni M, Kalimuthu R, Ponraj K, Lavanya K, Magesh P, Jasbin Shyni G. Antioxidant activities of plants studied in Yercaud road sides, Salem, Tamilnadu India. Int J Pharm Sci Rev Res, 2014; 27(1):61–5.
Kumalasari R, Iwansyah AC, Ratnawati L, Fitrianti I, Darmajana DA. Effect of pre-treatment on nutrient, antinutrient, and antioxidant properties of dried shoots from some edible Indonesian bamboo species. Afr J Food Agric Nutr Dev, 2019; 19(4):14932–49.
Kuo CJ, Liao YC, Yang JH, Huang LC, Chang CT, Sung HY. Cloning and characterization of an antifungal class III chitinase from suspension-cultured bamboo (Bambusa oldhamii) cells. J Agric Food Chem, 2008; 56(23):11507–14.
Li Z, Kobayashi M. Plantation future of bamboo in China. J For Res, 2004; 15(3):233–42.
Libman A, Bouamanivong S, Southavong B, Sydara K, Soejarto DD. Medicinal plants: an important asset to health care in a region of Central Laos. J Ethnopharmacol, 2006; 106(3):303–11.
Liu MH, Ko CH, Ma N, Tan PW, Fu WM, He JY. Chemical profiles, antioxidant and anti-obesity effects of extract of Bambusa textilis McClure leaves. J Funct Foods, 2016; 22:533–46.
Liu NT, Wu FH, Tsay HS, Chang WC, Lin CS. Establishment of a cDNA library from Bambusa edulis Munro in vitro-grown shoots. Plant Cell Tissue Organ Cult, 2008; 959(1):21–7.
Lodhi S, Jain AP, Rai G, Yadav AK. Preliminary investigation for wound healing and anti-inflammatory effects of Bambusa vulgaris leaves in rats. J Ayurveda Integr Med, 2016; 7(1):14–22.
Luo B, Ahmed S, Long C. Bamboos for weaving and relevant traditional knowledge in Sansui, Southwest China. J Ethnobiol Ethnomed, 2020; 16:63.
Mahomoodally MF. A quantitative ethnobotanical study of common herbal remedies used against 13 human ailments categories in Mauritius. Afr J Tradit Complement Altern Med, 2014; 11(6):1–32.
Majumder J, Bhattacharjee PP, Datta BK, Agarwala BK. Ethno-medicinal plants used by Bengali communities in Tripura, northeast India. J For Res, 2014; 25(3):713–6.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med, 2009; 151(4):264–9.
Monton C, Suksaeree J, Pathompak P. Can makjong (Scaphium macropodum) powder formed gel in effervescent blend? Int J Pharm Pharm Sci, 2014; 6(6):610–2.
Muniappan M, Sundararaj T. Antiinflammatory and antiulcer activities of Bambusa arundinacea. J Ethnopharmacol, 2003; 88(2–3):161–7.
Neamsuvan O, Phumchareon T, Bunphan W, Kaosaeng W. Plant materials for gastrointestinal diseases used in Chawang district, Nakhon Si Thammarat province, Thailand. J Ethnopharmacol, 2016; 194:179–87.
Nirmala C, Bisht MS, Bajwa HK, Santosh O. Bamboo: a rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends Food Sci Technol, 2018; 77:91–9.
Novaryatiin S, Indah I. The medicinal plants used in Anjir Pulang Pisau, Central Kalimantan-Indonesia. Pharmacogn J, 2019; 11(6):1572–9.
Ohrnberger D. The bamboos of the world: annotated nomenclature and literature of the species and the higher and lower taxa. Elsevier, Amsterdam, The Netherlands, 1999.
Oluwahenyinmi GARA, Christianah COM, Olugbenga OV, Joy AF. Effect of Bambusa tuldoides cv. ventricosa leaf extracted with fermented steep liquors of maize and sorghum on some pathogenic organisms. Malays J Microbiol, 2014; 10(4):280–9.
Ong HG, Kim YD. Herbal therapies and social-health policies: Indigenous Ati Negrito women’s dilemma and reproductive healthcare transitions in the Philippines. Evid Based Complement Altern Med, 2015; 2015:491209.
Pande H, Kumar B, Varshney VK. HPLC-ESI-QTOF-MS analysis of phenolic compounds, antioxidant capacity and α-glucosidase inhibitory effect of Bambusa nutans leaves. Indian J Chem, 2018; 57B(7):988–96.
Partasasmita R, An’Amillah A, Iskandar J, Mutaqin AZ, Annisa, Ratningsih N. Karangwangi people’s local knowledge of bamboo and its role: implications for management of cultural keystone species. Biodiversitas, 2017; 18(1):275–82.
Pearson AK, Pearson OP, Gomez IA. Biology of the bamboo Chusquea culeou (Poaceae: Bambusoideae) in southern Argentina. Vegetatio, 1994; 111(2):93–126.
Pereira MAR, Beraldo AL. 2007. Bambu de corpo e alma. Canal 6 Projetos Editoriais, Bauru, Brazil.
Prabhu S, Vijayakumar S, Morvin Yabesh JE, Prakashbabu R, Murugan R. An ethnobotanical study of medicinal plants used in Pachamalai hills of Tamil Nadu, India. J Herb Med, 2021; 25:100400.
Rahmatullah M, Biswas KR. Traditional medicinal practices of a Sardar healer of the Sardar (Dhangor) community of Bangladesh. J Altern Complement Med, 2012; 18(1):10–9.
Ramakoti B, Dhanagopal H, Deepa K, Rajesh M, Ramaswamy S, Tamilarasan K. Solvent fractionation of organosolv lignin to improve lignin homogeneity: structural characterization. Bioresour Technol Reports, 2019; 7:100293.
Rodríguez-Pérez M, Martínez JM, Rivero LR, Álvarez HMH, Valdez AFC, Rodríguez DA, Lizama RS, Payrol JA. Evaluación de la actividad antimalárica de algunas plantas utilizadas en la medicina tradicional cubana. Rev Ciencias Farm Basica Apl, 2006; 27(3):197–205.
Rudra S, Islam KN, Rahman MM, Uddin SB. Medicinal plant diversity and their therapeutic uses in selected village common forests in Chittagong Hill Tracts, Bangladesh. J Herbs Spices Med Plants, 2021; 27(1):83–107.
Ruiz-Sanchez E, Sosa V, Ortiz-Rodriguez AE, Davidse G. Historical biogeography of the herbaceous bamboo tribe Olyreae (Bambusoideae: Poaceae). Folia Geobot, 2019; 54(3–4):177–89.
Saalu LC. Nigerian folklore medicinal plants with potential antifertility activity in males: a scientific appraisal. Res J Med Plant, 2016; 10(3):201–27.
Saducos AG. Antimycotic potential of Kawayang tinik against pathogenic fungal species. Plant Sci Today, 2021; 8(2):403–9.
Samoisy AK, Mahomoodally F. Ethnopharmacological appraisal of culturally important medicinal plants and polyherbal formulas used against communicable diseases in Rodrigues Island. J Ethnopharmacol, 2016; 194:803–18.
Sandhiya S, Subhasree N, Shivakumar S, Agrawal A, Dubey GP. In vitro antioxidant and antimicrobial potential of Bambusa arundinacea (Retz.) Willd. Int J Pharm Pharm Sci, 2013; 5(2):359–62.
Sanusi SB, Abu Bakar MF, Mohamed M, Sabran SF, Mainasara MM. Southeast Asian medicinal plants as a potential source of antituberculosis agent. Evid Based Complement Altern Med, 2017; 2017:7185649.
Sarkar D, Chandra AK, Chakraborty A, Ghosh S, Chattopadhyay S, Singh LH, Ray I. Effects of bamboo shoots (Bambusa balcooa) on thyroid hormone synthesizing regulatory elements at cellular and molecular levels in thyrocytes. J Ethnopharmacol, 2020; 250:112463.
Sathiyaraj K, Sivaraj A, Thirumalai T, Senthilkumar B. Ethnobotanical study of antifertility medicinal plants used by the local people in Kathiyavadi village, Vellore district, Tamilnadu, India. Asian Pac J Trop Biomed, 2012; 2(3S):S1285–8.
Savithramma N, Sulochana C, Rao KN. Ethnobotanical survey of plants used to treat asthma in Andhra Pradesh, India. J Ethnopharmacol, 2007; 113(1):54–61.
Seal T, Chaudhuri K. Effect of solvent extraction system on the antioxidant activities of some selected wild edible plants used by the ethnic people of Arunachal Pradesh. Int J Curr Pharm Rev Res, 2016; 7(3):180–5.
Segun PA, Ogbole OO, Ajaiyeoba EO. Medicinal plants used in the management of cancer among the Ijebus of Southwestern Nigeria. J Herb Med, 2018; 14:68–75.
Setiawati T, Mutaqin AZ, Irawan B, An’Amillah A, Iskandar J. Species diversity and utilization of bamboo to support life’s the community of Karangwangi village, Cidaun sub-district of Cianjur, Indonesia. Biodiversitas, 2017; 18(1):58–64.
Sharkar P, Rahman MM, Haque Masum GZ, Nayeem MA, Hossen MM, Azad AK. Ethnomedicinal importance of the plants in villages in Kushtia Sador and Mirpur Upozila, Bangladesh. J Herbs Spices Med Plants, 2013; 19(4):401–17.
Sharma TP, Borthakur SK. Ethnobotanical observations on bamboos among Adi tribes in Arunachal Pradesh. Indian J Tradit Knowl, 2008; 7(4):594–7.
Sharma UK, Pegu S, Hazarika D, Das A. Medico-religious plants used by the Hajong community of Assam, India. J Ethnopharmacol, 2012; 143(3):787–800.
Silambarasan R, Ayyanar M. An ethnobotanical study of medicinal plants in Palamalai region of Eastern Ghats, India. J Ethnopharmacol, 2015;172:162–78.
Singh HB, Kumar B, Singh RS. Bamboo resources of Manipur: an overview for management and conservation. J Bamboo Ratt, 2003; 2(1):43–55.
Singh RAJ, Upadhyay SK, Rani A, Kumar P, Kumar A. Ethnobotanical study of Subhartipuram, Meerut, Uttar Pradesh, India. II. Diversity and pharmacological significance of shrubs and climbers. Int J Pharm Res, 2020; 12(2):383–93.
Singla R, Soni S, Kulurkar PM, Kumari A, Mahesh S, Patial V, Padwad YS, Yadav SK. In situ functionalized nanobiocomposites dressings of bamboo cellulose nanocrystals and silver nanoparticles for accelerated wound healing. Carbohydr Polym, 2017; 155:152–62.
Soesanto E. Antioxidant activity of extracts from Bambusa vulgaris and Gigantochloa apus Kurz bamboo shoots. Pak J Nutr, 2016; 15(6):580–4.
Sonibare MA, Moody JO, Adesanya EO. Use of medicinal plants for the treatment of measles in Nigeria. J Ethnopharmacol, 2009; 122(2):268–72.
Soumya V, Muzib YI, Venkatesh P. GC-MS characterization, in vitro antioxidant and antimicrobial activity of newly isolated oil from edible wild bamboo rice (Bambusa bambos). J Biol Act Prod Nat, 2014; 4(3):209–15.
Srisawat T, Suvarnasingh A, Maneenoon K. Traditional medicinal plants notably used to treat skin disorders nearby Khao Luang mountain hills region, Nakhon Si Thammarat, Southern Thailand. J Herbs Spices Med Plants, 2016; 22(1):35–56.
Sukumaran S, Brintha TSS, Subitha P, Sheebha YA, Jeeva S. Usage of medicinal plants by two cultural communities of Kanyakumari district, Tamilnadu, South India. J Chem Pharm Res, 2014; 6(8):67–79.
Tewari S, Negi H, Kaushal R. Status of bamboo in India. Int J Econ Plants, 2019; 6(1):30–9.
Thamizharasan S, Umamaheswari S, Hari R, Ulagaratchagan V. Quantitative phytochemical analysis of Bambusa arundinacea seeds. Int J Pharmacogn Phytochem Res, 2015; 7(5):980–3.
Tyagi B, Tewari S, Dubey A. Biochemical characterization of fungus isolated during in vitro propagation of Bambusa balcooa. Pharmacogn Mag, 2018; 13(S4):S775–9.
Udayan PS, George S, Tushar KV, Balachandran I. Some common medicinal plants used by the Nayaka community, Savandurga forest of Magadi taluk, Bangalore rural district, Karnataka, India. J Nat Remedies, 2005; 5(1):35–40.
Valdés AFC, Martínez JM, Lizama RS, Gaitén YG, Rodríguez DA, Payrol JA. In vitro antimalarial activity and cytotoxicity of some selected Cuban medicinal plants. Rev Inst Med Trop Sao Paulo, 2010; 52(4):197–201.
Vanitha B, Rajesh Kumar R, Duraiswamy B, Gnanavel G, Neelamma G. Estimation of total phenol and total flavonoid content with antioxidant and anti-inflammatory activity of Bambusa arundinacea (bamboo shoot) grown in Nilgiris. Der Pharma Chem, 2016; 8(20):307–16.
Waikhom SD, Louis B, Sharma CK, Kumari P, Somkuwar BG, Singh MW, Talukdar NC. Grappling the high altitude for safe edible bamboo shoots with rich nutritional attributes and escaping cyanogenic toxicity. Biomed Res Int, 2013; 2013:289285.
Wu TH, Liao MH, Kuo WY, Huang CH, Hsieh HL, Jinn TL. Characterization of copper/zinc and manganese superoxide dismutase in green bamboo (Bambusa oldhamii): cloning, expression and regulation. Plant Physiol Biochem, 2011; 49(2):195–200.
Wu YQ, Peng WX, Qing Y, Tian WL, Li XG. Study on bioenergy recovery of chemical components of Bambusa blumeana by Py-GC/MS. IEEE, 2009; 5:9–11.
Xiao Y, Zhou Q, Shan B. Design and construction of modern bamboo bridges. J Bridg Eng, 2010; 15:533–41.
Yakubu MT, Bukoye BB. Abortifacient potentials of the aqueous extract of Bambusa vulgaris leaves in pregnant Dutch rabbits. Contraception, 2009; 80(3):308–13.
Yakubu MT, Bukoye BB, Oladiji AT, Akanji MA. Toxicological implications of aqueous extract of Bambusa vulgaris leaves in pregnant Dutch rabbits. Hum Exp Toxicol, 2009; 28(9):591–8.
Zihad SMNK, Saha S, Rony MS, Banu H, Uddin SJ, Shilpi JA, Grice ID. Assessment of the laxative activity of an ethanolic extract of Bambusa arundinacea (Retz.) Willd. shoot. J Ethnopharmacol, 2018; 214:8–12.