INTRODUCTION
The endophytic fungi, among various microbial communities reported around the world, reside asymptomatically within plant tissues (Petrini, 1991). Endophytic fungi are the reservoir of novel bioactive metabolites capable of improving the plant growth and supporting survival of their host plants in the habitat (Chandra et al., 2021). These findings invoked considerable interest in scientists to search for possible agents for innovative medicinal and agricultural product development (Paramanantham et al., 2019). Consequently, the identification of bioactive substances with enormous potential has resulted from the separation and screening of naturally occurring substances from endophytes and their host plants. The co-existence of endophytic fungi in their host is a unique relationship which impacts the number and quality of metabolites derived from fungal endophytes (Khare et al., 2018).
Almost all studies to date on endophytic fungi of host plant origin focused on the importance of metabolites with antibacterial and antioxidant properties in human welfare (Dwibedi and Saxena, 2020). Following the development of drug resistance in clinical microbial pathogens, the exploration of natural resources of new antibacterial agents hostile to the resistant pathogens and treatment of diseases caused by them has been gaining much importance. Antioxidants are chemical substances that shield cells from harm caused by reactive oxygen species and free radicals that incites carcinogenesis, DNA damage, and degenerative disorders including Alzheimer’s disease (Adeleke and Babalola, 2021; Fadiji and Babalola, 2020). In this regard, the exploration of endophytic fungi with the ability to produce antioxidant compounds for the treatment of human disorders caused by reactive oxygen species has been taken up (Adeleke and Babalola, 2021; Ambele et al., 2019). Literature also indicated that metabolites secreted internally and externally by fungal endophytes may have therapeutic value in the management of a variety of microbial diseases in humans (Aharwal et al., 2021).
Cancer, a collective name for a variety of related diseases is caused by the uncontrolled division of cells following mutation and invades and destroys adjacent tissues and organs (Li et al., 2018). Cancer is treated by chemotherapy, radiation, immunotherapy, and suicide gene therapy (Ghobrial et al., 2005). Nonetheless, the multidrug-resistant feature of cancer cells and the adverse effect of medications still remain a significant impediment for a successful chemotherapy (Gottesman et al., 2002). Eventually, studies on the source of new compounds with pharmaceutical importance, drug discovery, and development have received much importance and attention (Newman and Cragg, 2007). The exploration of Taxomyces andreanae endophytic in Taxus brevifolia (Stierle et al., 1995) that produce the anticancer compound paclitaxel (taxol) resulted in further studies demonstrating a diverse range of chemical compounds with various biological activities produced by endophytic fungal communities resident in ethnomedicinal plants (Vieira et al., 2012). The fungal endophytes in comparison to their host plants are considered as a potential source for the mass production of biologically active chemicals (Sopalun et al., 2021).
The selection of the medicinal plants to isolate endophytic fungus is one of the routine methods to identify novel compounds. The ethnomedicinal plants are the fascinating source of fungal taxa that produce a variety of biologically active substances (de Carvalho et al., 2021). In this study, Jatropha heynei of Euphorbiaceae a rare and endemic medicinal herb growing in dry tropical regions of Karnataka, was chosen to investigate the diversity of fungal endophytes and to assess the capability of certain endophytic fungal taxa to produce metabolites with bactericidal, free radical scavenging, and cytotoxic properties. Such of the endophytic fungal extracts that contain chemical components with above biological activities were subjected to chemo-profiling. Further, the spectral compounds were analyzed for their functional groups.
METHODS AND MATERIALS
Selection of study region and plant species
Three locations in Surammanahalli village (13.2181° N, 75.6556° E) of Chitradurga district in Karnataka were selected for the collection of plant samples of J. heynei. The soil of the study site is red sandy loam soil and receives 593.5 mm of an average annual rainfall and the temperature of 32°C–36°C. The specimen plant sample was characterized based on the morphological characteristics (Gamble, 1934) as J. heynei N. P. Balakr, and the identity of the species was confirmed by Dr. K. G. Bhat, an eminent plant taxonomist of Karnataka. The herbarium specimen of the plant was prepared and deposited (Voucher No-KU/AB/05) in the Herbarium Center at Department of Applied Botany, Kuvempu University, Shankaraghatta.
Endophytic fungal isolation and identification
Jatropha heynei plants at the flowering stage during June–August 2020 were randomly selected from the study sites. The healthy plant samples like leaf, fruit, and root were collected in sterile polypropylene sheets and brought within 24 hours to the laboratory. The specimens were cleaned in running water, then subjected to surface disinfection successively with hydrogen peroxide (0.2%), ethyl alcohol (70%), and Sodium hypochlorite (0.3%) (Achar and Shivanna, 2013) and segmented (1 cm) in an aseptic environment and placed on potato dextrose agar (PDA), malt extract agar (MEA), czapek dox agar (CZA), or water agar media (WA) supplemented with or without chloramphenicol (100 mg l−1) and incubated in a regime of 12/12 hours of light and nUV light (350–400 nm) for 5–7 days at 23°C ± 2°C (Shivanna and Vasanthakumari, 2011). Simultaneously, the surface disinfected leaf samples were pressed on to the surface of PDA medium and incubated as above to ensure that the fungal isolates are not originating as the concomitant source of contamination but arising only from the inner regions of the plant tissues (Unterseher and Schnittler, 2009). The morphological characteristics of fruiting bodies and spores were used to identify the endophytes that were expressed on the incubated plant segments (Rama Rao and Manoharachary, 1990; Subramanian, 1983). Penicillium citrinum, an endophytic fungus with high incidence and in vitro antibacterial activity, was chosen for the molecular characterization. The internal transcribed spacer (ITS) DNA regions of fungal species were chosen for molecular identification by cetyltrimethylammonium bromide method (Aamir et al., 2015). Primers such as ITS1: 5?-TCC GTA GGT GAA CCT CGG-3? and ITS4: 5?-TCC TCC GCT TAT TGA TAT GC-3? were employed in order to amplify the ITS region. The reaction mixture for the polymerase chain reaction (PCR) amplification includes 10 μl of genomic DNA and 25 μl of PCR reaction mixture (Taq polymerase, 10× buffer, dNTPs, primers, and sterile water). The PCR protocol included 30 reactions of amplification, denaturation (95°C for 1 minute), annealing (50°C for 30 seconds), and extension (73°C for 1 minute) after a preliminary denaturation of 95°C for 5 minutes. The PCR products were purified and the amplification of PCR products was confirmed by the gel electrophoresis technique. The amplified DNA segments were sequenced by the Sanger’s technique. The phylogenetic tree was constructed using RAxML GUI software and by a maximum likelihood method of 1,000 bootstrap replications by the GTRGAMMA+I model as suggested by jModelTest (Darriba et al., 2012).
Preparation of fungal extract
The endophytic fungus was cultured with occasional shaking at 26°C for 8–12 days in two 500 ml Erlenmeyer flasks consisting of potato dextrose broth (Gagana et al., 2020). The culture filtrate (CF) and mycelial parts were separated by using Whatman filter paper No. 1. In a separating funnel, ethyl acetate (Himedia, Mumbai, India) was used to extract the metabolites from the CF. The CF was condensed with rotary vacuum evaporators at room temperature and crude extract was re-extracted using the same solvent. The partially purified extract was pooled and kept at 4°C (Nischitha et al., 2020).
In vitro antibacterial assay
The antibacterial potential of the extract was tested by well diffusion technique (Nischitha et al., 2020) against five clinical bacterial pathogens procured from the Institute of Microbial Technology, Chandigarh. Bacterial strains are Gram-positive Staphylococcus aureus and Gram-negative Pseudomonas aeruginosa (MTCC-4734), Salmonella typhi (MTCC-734), Klebsiella pneumoniae (MTCC-7028), and Pseudomonas syringae (MTCC-1604). Antibiotics such as amoxicillin (Amozlin-250 Dr. Reddy’s Laboratories, Hyderabad, India), chloramphenicol (125 mg, Sanofi Aventis Pharma India), and ciprofloxacin (BIOCIP-TZ—Biochem, India) were served as standard controls, while dimethyl sulfoxide (DMSO; Himedia, Mumbai, India) was served as the negative control. The endophytic fungal extract (10 mg ml−1) was dissolved in DMSO and made into concentrations of 100%, 50%, and 25%. A sterilized cork-borer was used to create wells (0.5-mm diameter) in the solidified nutrient agar (Himedia, Mumbai, India) and 20 μl of the extract was poured in to every well and kept for incubation at 37°C for 24 hours. The antibacterial activity of extract and standards was evaluated by measuring the inhibition zone (ZI, mm).
Redox potential of extract by electrochemical assay
The oxidation potential was determined by the cyclic voltammetry technique (Arulpriya et al., 2010). The crude endophytic fungal extract (2 ml) was diluted in phosphate buffer (pH 7, 50 Mm of disodium hydrogen phosphate (65%, w/v) and 50 Mm sodium dihydrogen phosphate (35%, w/v). The cyclic voltammograms of the extract were produced using the Electrochemical Workstation CHI 660c (model potentiostat). The experiment used a carbon paste working electrode (3-mm diameter), a carbon paste electrode modified by methanol extract (CE), a counter electrode made of platinum wire, and a saturated calomel reference electrode. To determine the impact of various scan speeds on the anodic oxidation of the extract, the electrode was pre-treated with potassium chloride (1 Molar, 2 mg). The oxidation potential of extract was evaluated at a scan rate of 50 mVs−1 (Nischitha et al., 2020).
In vitro cytotoxic activity
The cytotoxic potential of P. citrinum metabolites was analyzed by using the 3-(4, 5-dimethylthiazole-2yl)-2, 5-diphenyl tetrazolium bromide (MTT) cell proliferation method (Mosmann, 1983) against MCF-7 human breast cancer cell line and A549 human adenocarcinoma cell line (NCCS, Pune, India). The working solution (1% v/v) was prepared by dissolving the extract in DMEM high glucose medium (Himedia, Mumbai, India). The cell suspension of 200 μl with the appropriate cell number (20,000 cells/well) and without test agent was taken in a 96-well microtiter plate and extract concentrations of 31.25, 62.5, 125, 250, or 500 μg ml−1 were then added in to well and kept for incubation at 37°C for 24 hours in 5% CO2 environment. Curcumin served as a standard, and DMSO served as a negative control. The incubated cell suspension was added with MTT reagent and 100 μl of solubilization solution (DMSO) was added. Finally, absorbance of cell suspension in the microtiter plate was read at 595 nm in ELISA plate reader reader (Agilent biotech, USA). The IC50 value was calculated by linear regression formula Y = mx + c
where, Y = 50, the viability graph was used to calculate M and C values.
Percentage of cell viability was calculated from the ELISA absorbance data by the following formula:
Chemical profiling of P. citrinum metabolites by orbitrap high resolution liquid chromatography mass spectroscopy (OHR-LCMS)
Based on the antibacterial, antioxidant and cytotoxic activities of P. citrinum crude extract, sample was subjected to OHR-LC-MS (Thermo Scientific Xcalibur, ver. 4.2.28.14). The analytic instrument consists of an outer electrode and a central electrode which function as analyzer and detector. Both positive and negative ionization modes were employed for direct infusion with the mass (m/z) which ranged from 50 to 8,000 amu. In this study, the data acquisition software Thermo Scientific Xcalibur (version 4.2.28.14) and the data processing software Compound Discoverer 2.1 SP1 was employed. The column material, Hypersil Gold 3 μm 100 × 2.1 MM (Thermo Scientific), was used in conjunction with a mobile phase of 0.1% formic acid in distilled water and methyl alcohol. The OHR-LCMS analysis was done at Sophisticated Analytical Instrument Facility (SAIF) in IIT Bombay, Mumbai, India. Search engines for open chemistry databases like Pub-Chem and Chem-spider were used to check the biological properties of detected compounds. Chem-Sketch software was used to draw the chemical structures.
Fourier transform infrared spectroscopic (FTIR) analysis
The FTIR observations in the range of 4,000–400 cm−1 were determined with FTIR accouterment (Bruker optic, Germany). The extract of 150 μl (0.5 mg ml−1) was mixed with methanol and then with newly prepared FTIR reagent (4.5 ml). The spectral data were obtained with resolution of 4 cm−1.
Statistical analysis
The data were analyzed to determine the colonization frequency (%) of endophytic fungal species, as well as the diversity indices like Simpson and Shannon evenness and species richness indices and antibacterial activity (Duncan’s multiple range test p < 0.05). All experiments were done with three trials. Results of data were shown as the mean and standard error. Phylogenetic tree was constructed using the RAxML GUI version 2.0.0.0 with maximum likelihood method.
RESULTS AND DISCUSSION
Isolation and identification of endophytic fungal community
The isolation of fungal endophytes in J. heynei was carried out during July 2019–May 2020 (rainy, winter, and summer seasons), at three different locations and by four isolation methods such as PDA, MEA, CZA, and Water agar methods. The assemblages of endophytic fungal species varied depending on the season which played vital role in the colonization of fungi, as suggested by season-wise isolation. The endophytic fungal colonization frequency was more in the winter season when compared to the rainy and summer seasons. The occurrence of the endophytic fungal assemblages in plant parts varied based on the nutritional supplement; fungal incidence was more in PDA followed by Water agar, MEA, and CZA media (Table 1). Thirty three endophytic fungal species were recovered from 5,400 segments of J. heynei and fungal species were characterized based on their morphological features. Cladosporium cladosporioides, Aspergillus, and Penicillium species were dominant during rainy season while C. cladosporioides, Colletotrichum truncatum, and Fusarium sp. were dominant in the winter season. Aspergillus flavus, C. cladosporioides, P. citrinum, and Fusarium sp. were predominantly found in the summer season and in all parts of J. heynei. The fungal emergence was more in the leaf than in the root and fruit regions. Based on the preliminary study of antibacterial activity in certain fungal species, an isolate of P. citrinum (Fig. 1) with good antibacterial activity was used for molecular identification. Based on the ITS regions of the rDNA, the fungal isolate identified as P. citrinum (sequence deposited to NCBI GeneBank, Accession. No. OP049986) and based on the maximum likelihood method P. citrinum grouped with the most closely related reference species and formed separate clades in the phylogenetic tree (Fig. 2). The diversity indices of Shannon and Simpson and evenness indices of the endophytic fungi were high in the leaf than in the root and fruit (Table 2). Amongst the regions of the plant, the leaf followed by root harbored both anamorphic as well as teleomorphic stages of ascomycetes. Similar findings were recorded in previous research work (Gagana et al., 2020).
The qualitative mycochemical analysis of metabolites produced by P. citrinum detected the presence of alkaloids, glycosides, sterols, phenols, tannins, and triterpenoids. The secondary metabolites in fungal endophytes produced by distinct metabolic pathways (Tan and Zou, 2001) have been reported for the antimicrobial, antioxidant, and other biological activities (Chandrappa et al., 2013).
Antibacterial potential of fungal metabolites in vitro
The antibacterial activity of P. citrinum extract against selected bacterial strains is detailed in Table 3. Penicillium citrinum extract caused more antibacterial activity against Gram-negative strain P. syringae (17.65 ± 1 mm) and Gram-positive strain –S. aureus (16.32 ± 0.5 mm) than the rest of the strains. The activity of extract against the above strains was lesser than that of ciprofloxacin and choramphenicol standards. However, the activity was almost equivalent to that of amoxicillin control. Further, the extract was less in effectiveness against other test bacterial strains. The colony suppression of bacterial pathogens could be attributed to the inhibition of DNA or protein synthesis or damage caused to the integrity of cell membranes, ultimately leading to cell death (George et al., 2019). The inhibitory effect of endophytic fungal extract against clinical bacterial pathogens could be caused by the presence of active bactericidal compounds or synergistic effect of other bio-active chemicals present in the extract.
Antioxidant activity of fungal metabolites by cyclic voltammetry
Since the redox potential and antioxidant properties have a strong link, the reducing ability of extract was assessed by cyclic voltammetry. The CF generated positive oxidative peak at Ipa, 0.14 V (Fig. 3) when anodic current potential was present. The compounds with strong scavenging ability are known to oxidize at low potential (Gagana et al., 2020).The high oxidation potential suggested that CF extract exhibited considerable antioxidant activity. At pH 7, the anodic peak was generated between 0.1 and 0.2 V. The shift in anodic peak potential toward a positive value indicated the presence of oxygen-free radicals that can be scavenged in the fungal extract (Keffous et al., 2016). The presence of flavonoids and phenolic substances is linked to the good antioxidant property of ethyl acetate extract (Sochor et al., 2013). The antioxidant compounds have always been demonstrated for their antibacterial, antiinflammatory, antiviral, and anticancer activities (Cai et al., 2004; Prasher and Sharma, 2021).
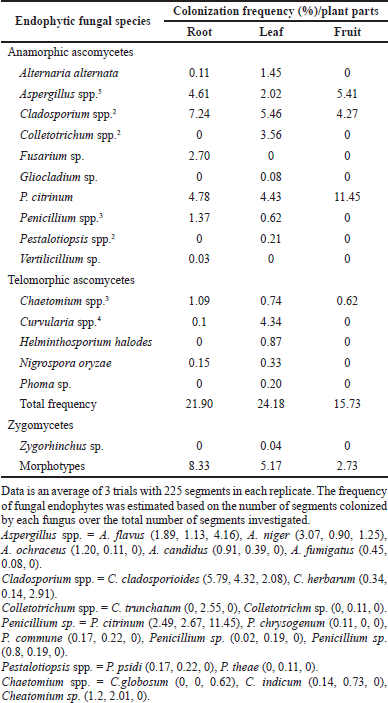 | Table 1. Colonization frequency (%) of endophytic fungi in J. heynei during rainy and winter seasons of 2019–2020. [Click here to view] |
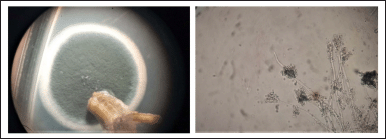 | Figure 1. Photograph showing colony and spore structure of P. citrinum. [Click here to view] |
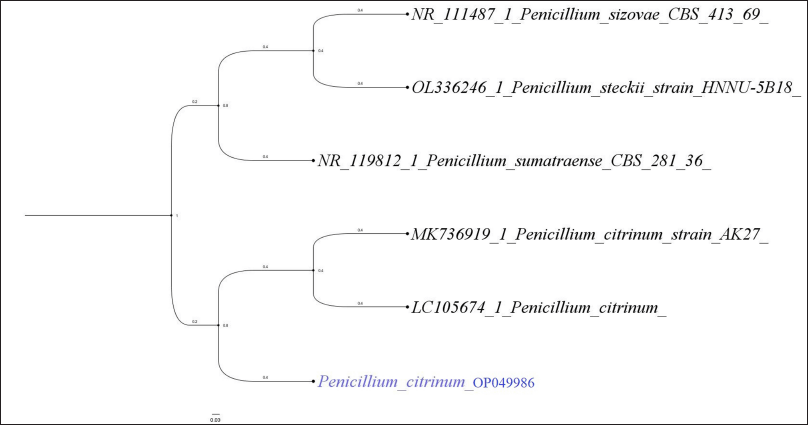 | Figure 2. Phylogenetic analysis of rDNA ITS sequences of P. citrinum (GenBank Accession No. OP049986) isolated from J. heynei. [Click here to view] |
 | Table 2. Species abundance, diversity, and evenness of endophytic fungal populations in root, leaf, and fruit regions of J. heynei. [Click here to view] |
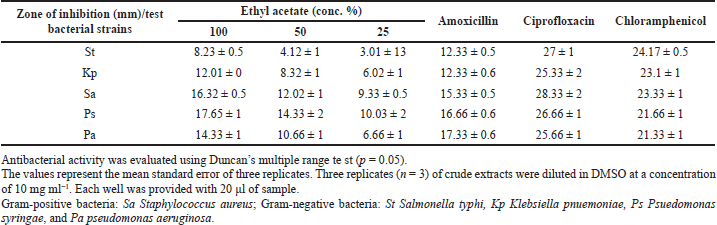 | Table 3. In vitro antibacterial activity of metabolites of P. citrinum in J. heynei. [Click here to view] |
The primary drawback of spectrophotometric assays was identified as interference from biomolecules which absorbs the same wavelength while evaluating the antioxidant activity (Amamra et al., 2018). In this study, the electrochemical measurement demonstrated its uniqueness in determining antioxidant activity by enabling the detection of the substantial potential for oxidation of certain chemicals at shifting pH and in various reaction settings. This implied that the cyclic-voltammetric technique could be used in the place of spectrophotometric assay of antioxidant compounds (Nischitha et al., 2020).
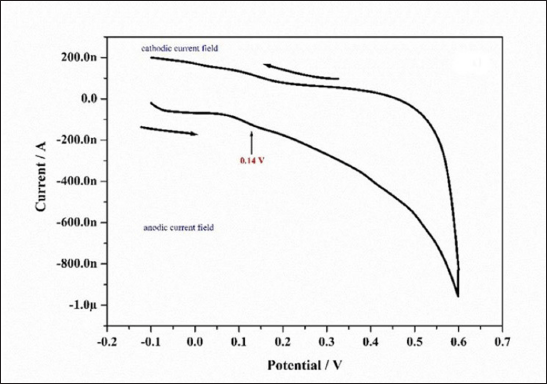 | Figure 3. Voltammogram of the ethyl acetate extract of P. citrinum culture filtrate. The measurement was performed at a scan rate of 50 mVs−1 with a 3-mm diameter carbon paste electrode as the working electrode, saturated calomel as the reference electrode, and a platinum wire as the auxiliary electrode at pH 7.0. [Click here to view] |
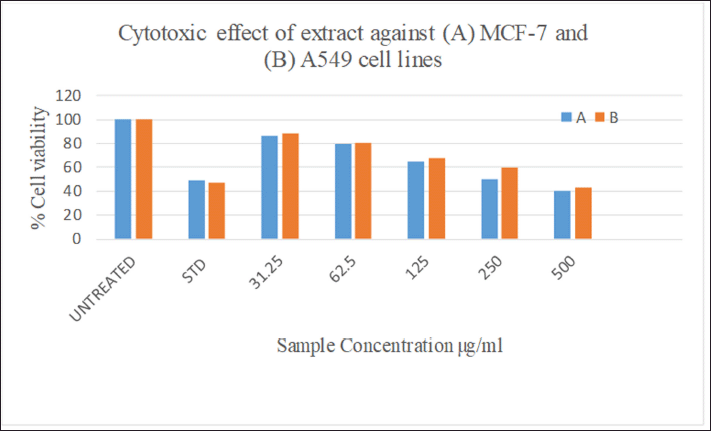 | Figure 4. Cytotoxic effect of P. citrinum extract in vitro against (A) MCF-7 and (B) A549 cancer cell lines. [Click here to view] |
In vitro cytotoxic activity of fungal metabolites
The MTT colorimetric assay is frequently used to assess the cytotoxicity in vitro and gauge the rate at which the enzyme mitochondrial dehydrogenase converts MTT into formazan. A visible spectrophotometer measures the quantity of blue formazan, and it is positively correlated to the number of cells living because of the reduction reaction which can occur when the enzyme mitochondrial reductase was produced (Chapdelaine, 2001). The effect of the endophytic fungal extract against human cancer cell lines A-549 and MCF-7 is shown in Figures 4 and 7. The extract exhibited moderate cytotoxicity to both A549 and MCF-7 cell lines at the concentration of 500 μg after the period of 24 hours with IC50 values of 280.7 and 283.0 μg ml−1, respectively. The standard curcumin showed 48% cell viability (IC50 of 52.41 μg ml−1) at concentration of 10 μM. The cytotoxic effect of fungal extract could be attributed to the involvement of anticancer and antiinflammatory metabolites in the extract (Ghasemzadeh et al., 2010).
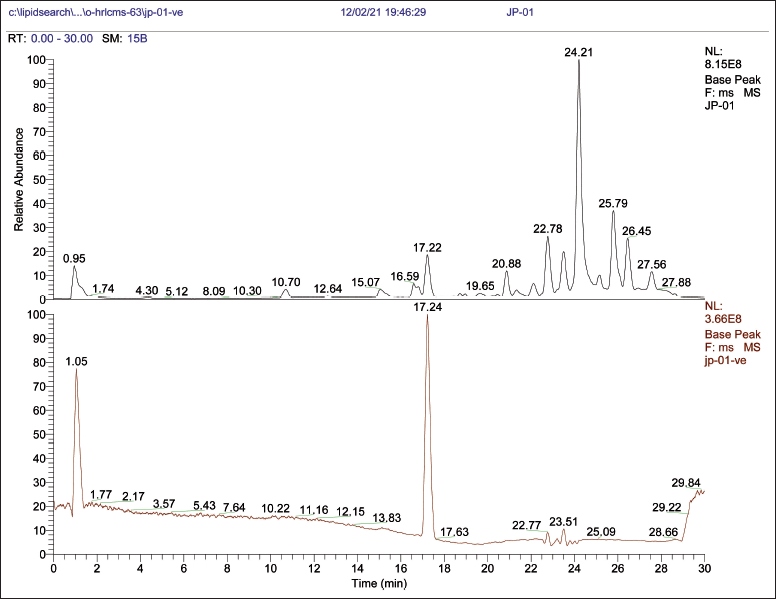 | Figure 5. OHR-LCMS chromatogram of metabolites of P. citrinum. [Click here to view] |
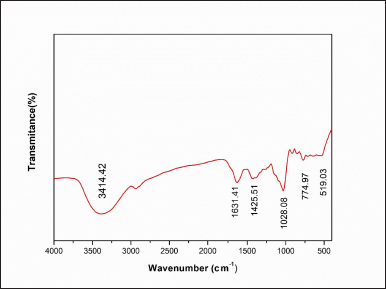 | Figure 6. FTIR chromatogram of ethyl acetate extract of P. citrinum. [Click here to view] |
The observation of the presence of antibacterial, antioxidant, and cytotoxic activities in the fungal extract prompted the authors for further investigation of extract for metabolite profiling using OHR-LCMS method.
Chemical profiling of fungal metabolites by OHR-LCMS
OHR-LCMS is one of the most recent and effective current techniques for identifying chemicals contained in biological samples (Wu et al., 2012). In this study, the chemical composition of crude extract was evaluated by using OHR-LCMS. The method involves the separation as well as identification of Chemical constituents in accordance with their retention period, data base metabolite class, difference (library), experimental m/z, MS/MS fragments, and suggested compounds. The positive and negative ionization modalities of MS data were reported. The ethyl acetate extract of P. citrinum detected 21 bio-active compounds by the positive ionization mode (+ESI) and also negative ionization mode (−APCI) (Fig. 5). The major chemicals with bio-active properties detected by positive mode are 8-hydroxyquinoline (1), trigonelline (2), 3-isobutylhexahydropyrrolo[1,2-a]pyrazine-1,4-dione (3), sulfamethazine (4), psoralidin (5), spectinomycin (6), kanosamine (7), 4-aminobenzoic acid (8), 2,3-dimethoxy-5-(6,7,8-trimethoxychroman-3-yl)cyclohexa-2,5-diene-1,4-dione (9), 2,4,6-trihydroxy-5-((1S)-1-((1aR,4R,4aR,7S,7bR)-4-hydroxy-1,1,4,7-tetramethyldecahydro-1H- cyclopropa[e]azulen-7-yl)-3-methylbutyl)isophthalaldehyde (10), phenethylamine (11), (2S,3R,4S,5S,6R)-2-[3-hydroxy-5-[2-(4-methoxyphenyl)ethenyl]phenoxy]-6-(hydroxymethyl)oxane-3,4,5-triol (12), 6,6’-oxybis(4-methylbenzene-1,2-diol (13), anthranillic acid (14), (S)-5-hydroxy-1,7-diphenylheptan-3-one (15), cyclosporine (16), 3-(3,4,5-trimethoxyphenyl)propanoic acid (17), artemisinin (18), and (E)-N-(4-acetamidobutyl)-3-(4-hydroxy-3-methoxyphenyl)acrylamide (19). Those compounds detected by both positive and negative ionization modes include nicotinic acid (+/−) (20) and 3’-adenosine monophosphate (+/−) (21). Most compounds detected in the negative ionization mode are carbohydrates (glucose, mannose, xylose, and malic acid) and citric acid as well as small peptides (Table 4 and Fig. 8).
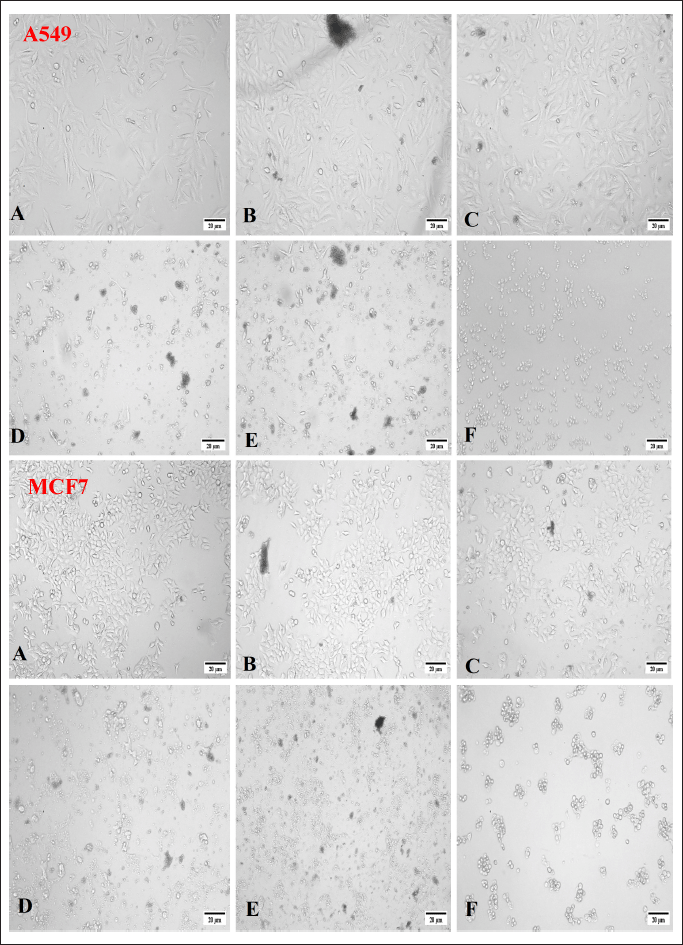 | Figure 7. Morphology showing the cytotoxic effect of P. citrinum CF extract against A549 and MCF-7 cancer cell lines at different concentrations. A = 31.25 μg, B = 62.5 μg, C = 125 μg, D = 250 μg, E = 500 μg, F = 10 μM of standard. [Click here to view] |
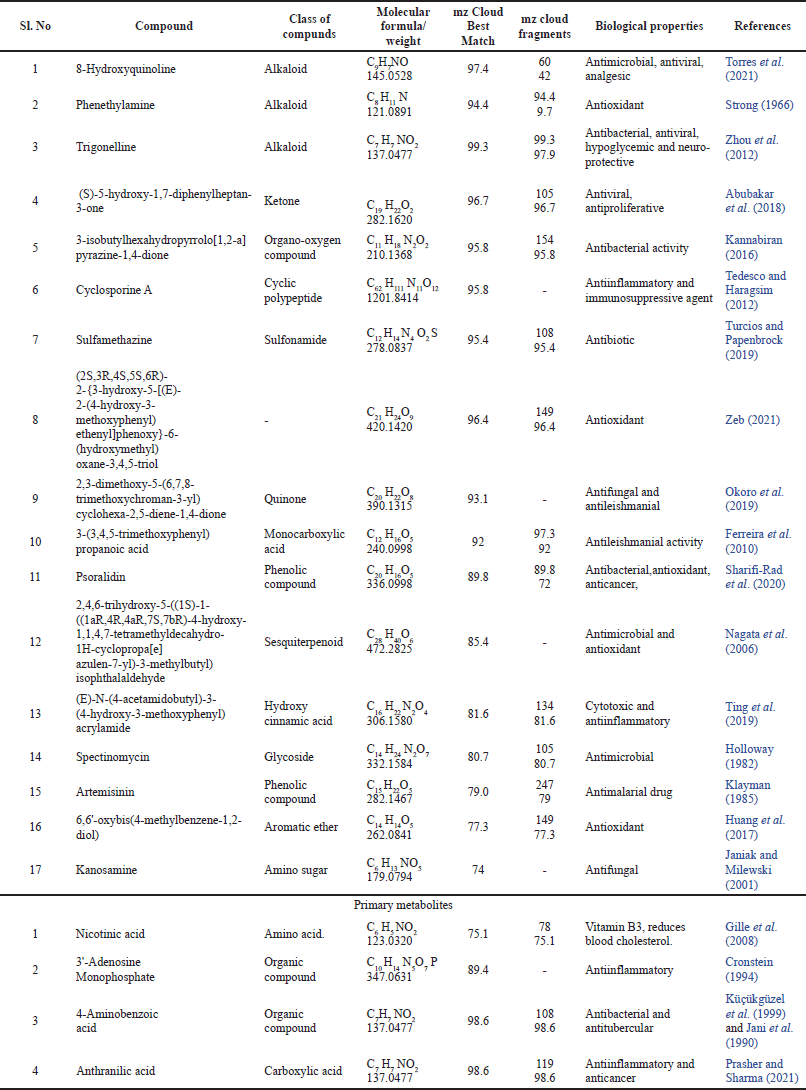 | Table 4. Metabolite profiling of P. citrinum by OHR-LCMS analysis. [Click here to view] |
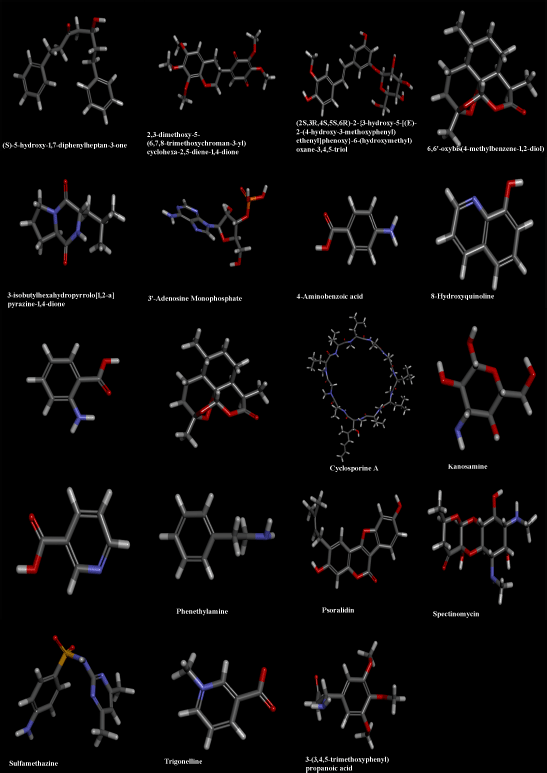 | Figure 8. Structures of certain important compounds identified in ethyl acetate extract of P. citrinum endophytic in J. heynei. [Click here to view] |
Among the above, compounds numbered 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 are previously documented for antimicrobial properties (Holloway, 1982; Janiak and Milewski, 2001; Kannabiran, 2016; Nagata et al., 2006; Okoro et al., 2019; Sharifi-Rad et al., 2020; Torres et al., 2021; Turcios and Papenbrock, 2019; Zhou et al., 2012). Compounds 11, 12, and 13 are reported for antioxidant activities (Huang et al., 2017; Strong, 1996; Zeb, 2021), while compounds 5 and 10 are both antimicrobial and antioxidant (Nagata et al., 2006; Sharifi-Rad et al., 2020). Certain compounds numbered 14, 15, 16, 19, and 21 are related to antiinflammatory and anticancer activities (Abubakar et al., 2018; Cronstein, 1994; Prasher and Sharma, 2021; Tedesco and Haragsim, 2012; Ting et al., 2019). Compounds with multiple pharmacological activities include 8-hydroxyquinoline (1) (Torres et al., 2021), trigonelline (2) (Zhou et al., 2012), and psoralidin (5) (Sharifi-Rad et al., 2020). Apart from the above, Compound 3-(3, 4, 5-trimethoxyphenyl) propanoic acid (17) is antileishmanial (Ferreira et al., 2010), while artemisinin (18) produced from this endophytic fungi is a sesquiterpene lactone consist of peroxide bridge responsible for their antimalarial properties (Klayman, 1985). Therapies based on a combination of artemisinin are now considered as standard treatment world-wide against Plasmodium falciparum and other species of Plasmodium and it is usually extracted from the Chinese traditional medicinal plant Artemisia annua (Arsenault et al., 2008). Nicotinic acid (20) in the extract is vitamin B3 which reduces the blood cholesterol level (Gille et al., 2008) (Table 4).
The FTIR spectrum of P. citrinum extract (Fig. 6) confirmed the presence of functional substituents of antibacterial and antioxidant chemicals as evaluated by the OHR-LCMS. A broad stretching peak at 3,414.42 cm−1 was assigned to the stretching vibration of amide groups (N–H), indicating the presence of alkaloids, while the band at 2,800.42 cm−1 was attributed to carboxyl acid’s (C=O) presence and (O–H) groups that indicated the presence of polyphenols and flavonoids; a sharp peak at 1,634.16 cm−1 (C=C) alkenes, and 1,425.51 cm−1, aromatic compounds (C=C), and 1,028.08–519.03 cm−1 (C–H) groups. Functional groups like carboxylic acids, anhydrides, alcohols, phenols, amines, amides, and esters showed that the fungal extract contained biologically active metabolites (Nischitha and Shivanna, 2021). This observation further supported the antioxidant activity due to phenols and flavonoids as shown by cyclic voltammetry in this study.
CONCLUSION
This study documented that variation in the occurrence of endophytic fungal assemblages in different plant parts depends on the season and isolation methods. Among the large number of endophytic fungi isolated, P. citrinum was the dominant endophyte. This study indicated that P. citrinum produced compounds with antibacterial, antioxidant, and cytotoxic potentials. This finding is supported by the metabolite profiling of fungal metabolites by OHR-LCMS and further determination of functional groups by FTIR spectroscopy. In this study, several compounds known to literature have been documented that expressed antimicrobial and antioxidant as well as other pharmacological activities. This study also documented the presence of several unknown compounds that might be of future pharmaceutical importance. Further research is needed before these bioactive chemicals can be used to benefit humans.
ACKNOWLEDGMENTS
We would like to acknowledge Ministry of Tribal Affairs, UGC New Delhi for financial help to carryout the research work and we are also grateful to SAIF, IIT Bombay for providing OHR-LCMS, and FTIR analyses.
LIST OF ABBREVIATIONS
CF: Culture filtrate, DMSO: Dimethyl sulfoxide, ITS: Internal transcribed spacer, PCR: Polymerase chain reaction, OHR-LCMS: Orbitrap high resolution-liquid chromatography mass spectroscopy, FTIR: Fourier transform infrared spectroscopy.
AUTHOR CONTRIBUTIONS
A.G.B. carried out the research, conducted the experimental work and wrote the first draught. M.B.S. reviewed and edited the manuscript before it was finalized. The final manuscript was read and approved by all authors.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study does not use animals used as human test subjects in in vivo experiments.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aamir S, Sutar S, Singh SK, Baghela A. A rapid and efficient method of fungal genomic DNA extraction, suitable for PCR based molecular methods. Plant Pathol Quar, 2015; 5(2):74–81. CrossRef
Abubakar IB, Malami I, Yahaya Y, Sule SM. A review on the ethnomedicinal uses, phytochemistry and pharmacology of Alpinia officinarum Hance. J Ethnopharmacol, 2018; 224:5–62. CrossRef
Achar KS, Shivanna MB. Colletotrichum leaf spot disease in Naravelia zeylanica and its distribution in Bhadra Wildlife Sanctuary, India. Indian Phytopathol, 2013; 66(2):125–31.
Adeleke BS, Babalola OO. Pharmacological potential of fungal endophytes associated with medicinal plants: a review. J Fungi, 2021; 7(2):147. CrossRef
Aharwal RP, Kumar S, Sandhu SS. Endophytic mycoflora: antibacterial secondary metabolites and their therapeutic potential. Curr Pharmacol Rep, 2021; 7(4):150–70. CrossRef
Amamra S, Cartea ME, Belhaddad OE, Soengas P, Baghiani A, Kaabi I, Arrar L. Determination of total phenolics contents, antioxidant capacity of Thymus vulgaris extracts using electrochemical and spectrophotometric methods. Int J Electrochem Sci, 2018; 13(8):7882–93. CrossRef
Ambele CF, Bisseleua HD, Akutse KS, Babalola OO, Humbert P, Patel A, Vidal S, Djuideu CT, Ekesi S. Testing a co-formulation of CO2 releasing material with an entomopathogenic fungus for the management of subterranean termite pests. Mycol Prog, 2019; 18:1201–11. CrossRef
Arsenault PR, Wobbe KK, Weathers PJ. Recent advances in artemisinin production through heterologous expression. Curr Med Chem, 2008; 15(27):2886–96. CrossRef
Arulpriya P, Lalitha P, Hemalatha S. Cyclic voltammetric assessment of the antioxidant activity of petroleum ether extract of Samanea saman (Jacq). Adv Appl Sci Res, 2010; 1:24–35.
Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci, 2004; 74(17):2157–84. CrossRef
Chandra H, Kumari P, Prasad R, Gupta SC, Yadav S. Antioxidant and antimicrobial activity displayed by a fungal endophyte Alternaria alternata isolated from Picrorhiza kurroa from Garhwal Himalayas, India. Biocataly Agric Biotechnol, 2021; 33:101955. CrossRef
Chandrappa CP, Anitha R, Jyothi P, Rajalakshmi K, Seema Mahammadi H, Govindappa M, Sharanappa P. Phytochemical analysis and antibacterial activity of endophytes of Embelia tsjeriam cottam Linn. Int J Pharma Bio Sci, 2013; 3:201–3.
Chapdelaine JM. MTT reduction-a tetrazolium-based colorimetric assay for cell survival and proliferation. Pharm Res Int, 2001:1–6.
Cronstein BN. Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol, 1994; 76(1):5–13. CrossRef
Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods, 2012; 9(8):772. CrossRef
de Carvalho CR, Maia MQ, Sobral M, Pereira GMD, da Silva K, Vital MJS, Rosa LH. Diversity and antimicrobial activity of culturable endophytic fungi associated with the neotropical ethnomedicinal plants Copaifera langsdorffii and Copaifera pubiflora. S Afr J Bot, 2021; 142:305–15. CrossRef
Dwibedi V, Saxena S. In vitro anti-oxidant, anti-fungal and anti-staphylococcal activity of resveratrol-producing endophytic fungi. In: Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, vol. 90, no. 1, pp 207–19, 2020. CrossRef
Fadiji AE, Babalola OO. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front Bioeng Biotechnol, 2020; 8:467. CrossRef
Ferreira MGP, Kayano AM, Silva-Jardim I, Silva TOD, Zuliani, JP, Facundo VA, Stábeli RG. Antileishmanial activity of 3-(3, 4, 5-trimethoxyphenyl) propanoic acid purified from Amazonian Piper tuberculatum Jacq. Piperaceae, fruits. Rev Bras Farmacogn, 2010; 20:1003–6. CrossRef
Gagana SL, Kumaraswamy BE, Shivanna MB. Diversity, antibacterial and antioxidant activities of the fungal endophytes associated with Schleichera oleosa (Lour.) Merr S Afr J Bot, 2020; 134:369–81. CrossRef
Gamble JS. Flora of the Presidency of Madras, vol. III. Botanical Survey of India, 1934.
George, TK, Joy A, Divya K, Jisha MS. In vitro and in silico docking studies of antibacterial compounds derived from endophytic Penicillium setosum. Microb Pathog, 2019; 131:87–97. CrossRef
Ghasemzadeh A, Jaafar HZE, Rahmat A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia Young Ginger (Zingiber officinale Roscoe). Molecules, 2010; 15:4324–33. CrossRef
Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin, 2005; 55(3):178–94. CrossRef
Gille A, Bodor ET, Ahmed K, Offermanns S. Nicotinic acid: pharmacological effects and mechanisms of action. Annu Rev Pharmacol Toxicol, 2008; 48:79–106. CrossRef
Gottesman MM, Fojo T, Bates E. Multidrug resistance in cancer: role of ATP–dependent transporters. Nat Rev Cancer, 2002; 2(1):48–58. CrossRef
Holloway WJ. Spectinomycin. J Urol, 1982; 128(4):876. CrossRef
Huang Z, Nong X, Ren Z, Wang J, Zhang X, Qi S. Anti-HSV-1, antioxidant and antifouling phenolic compounds from the deep-sea-derived fungus Aspergillus versicolor SCSIO 41502. Bioorgan Med Chem Lett, 2017; 27(4):787–91. CrossRef
Janiak AM, Milewski S. Mechanism of antifungal action of kanosamine. Med Mycol, 2001; 39(5):401–8. CrossRef
Kannabiran K. Bioactivity-guided extraction and identification of antibacterial compound from marine actinomycetes strains isolated from costal soil samples of Rameswaram and Dhanushkodi, Tamil Nadu, India. Asian J Pharm, 2016; 10(04).
Keffous F, Belboukhari N, Sekkoum K, Djeradi H, Cheriti A, Aboul-Enein HY. Determination of the antioxidant activity of Limoniastrum feei aqueous extract by chemical and electrochemical methods. Cogent Chem, 2016; 2(1):1186141. CrossRef
Khare E, Mishra J, Arora NK. Multifaceted interactions between endophytes and plant: developments and prospects. Front Microbiol, 2018; 9:2732. CrossRef
Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science, 1985; 228(4703). CrossRef
Li SJ, Zhang X, Wang XH, Zhao CQ. Novel natural compounds from endophytic fungi with anticancer activity. Eur J Med Chem, 2018; 156:316–43. CrossRef
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods, 1983; 65(1–2):55–63. CrossRef
Nagata H, Inagaki Y, Yamamoto Y, Maeda K, Kataoka K, Osawa K, Shizukuishi S. Inhibitory effects of macrocarpals on the biological activity of Porphyromonas gingivalis and other periodontopathic bacteria. Oral Microbiol Immunol, 2006; 21(3):159–63. CrossRef
Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod, 2007; 70(3):461–77. CrossRef
Nischitha R, Shivanna MB. Metabolite fingerprinting, in vitro antimicrobial and antioxidant activities and in-silico docking in Alloteropsis cimicina and its endophytic fungus Penicillium pinophilum. Mol Biol Rep, 2021:1–17. CrossRef
Nischitha R, Vasanthkumari MM, Kumaraswamy BE, Shivanna MB. Antimicrobial and antioxidant activities and chemical profiling of Curvularia tsudae endophytic in Cynodon dactylon (L.) Pers. 3 Biotech, 2020; 10(7):1–2. CrossRef
Okoro EE, Ahmad MS, Osoniyi OR, Onajobi FD. Antifungal and antileishmanial activities of fractions and isolated isoflavanquinones from the roots of Abrus precatorius. Comp Clin Path, 2019:1–6. CrossRef
Paramanantham P, Pattnaik S, Siddhardha B. Natural products from endophytic fungi: synthesis and applications. In: Singh B (Ed.). Advances in endophytic fungal research, Springer, Cham, Switzerland, pp 83–103, 2019. CrossRef
Petrini O. Fungal endophytes of tree leaves. Microbial ecology of leaves. Springer, New York, NY, pp 179–97, 1991. CrossRef
Prasher P, Sharma M. Medicinal chemistry of anthranilic acid derivatives: a mini review. Drug Dev Res, 2021; 82(7):945–58. CrossRef
Rama Rao P, Manoharachary C. Soil fungi from Andhra Pradesh. Osmania University, Hyderabad, India, pp 159–64, 1990.
Sharifi-Rad J, Kamiloglu S, Yeskaliyeva B, Beyatli A, Alfred MA, Salehi B, Martorell M. Pharmacological activities of Psoralidin: a comprehensive review of the molecular mechanisms of action. Front Pharmacol, 2020; 11. CrossRef
Shivanna MB, Vasanthakumari MM. Temporal and spatial variability of rhizosphere and rhizoplane fungal communities in grasses of the subfamily Chloridoideae in the Lakkavalli region of the Western Ghats in India. Mycoshere, 2011; 2(3):255–71. CrossRef
Sochor J, Dobes J, Krystofova O, Ruttkay-Nedecky B, Babula P, Pohanka M, Kizek R. Electrochemistry as a tool for studying antioxidant properties. Int J Electrochem Sci, 2013; 8(6):8464–89.
Sopalun K, Laosripaiboon W, Wachirachaikarn A, Iamtham S. Biological potential and chemical composition of bioactive compounds from endophytic fungi associated with Thai mangrove plants. S Afr J Bot, 2021; 141:66–76. CrossRef
Stierle A, Strobel G, Stierle D, Grothaus P, Bignami G. The search for a taxol-producing microorganism among the endophytic fungi of the pacific yew, Taxus brevifoliaJ. Nat Prod, 1995; 58:1315–24. CrossRef
Strong FM. Toxicants occurring naturally in foods. Nutr Rev, 1966; 1354:94.
Subramanian CV. Hyphomycetes. Taxonomy and biology. Academic Press, New York, NY, 1983.
Tan RX, Zou WX. Endophytes: a rich source of functional metabolites. Nat Prod Rep, 2001; 18:448–59. CrossRef
Tedesco D, Haragsim L. Cyclosporine: a review. J Transplant, 2012; 2012:230386. CrossRef
Ting L, Yuan-peng H, Qiu-ping L, Guang-Xiong Z. Study on chemical constituents from root and stem of Lycium barbarum L. Nat Prod Res Dev, 2019; 31(9):1491.
Torres M, Ribeiro MA, Oliveira JAS, Meurer EC, Schwan-Estrada KR. Partial chemical characterization of the yeast extracts Lachancea thermotolerans CCMA 0763. Afr J Microbiol Res, 2021; 15(7):388–95. CrossRef
Turcios AE, Papenbrock J. Enzymatic degradation of the antibiotic sulfamethazine by using crude extracts of different halophytic plants. Int J Phytoremediation, 2019; 21(11):1104–11. CrossRef
Unterseher M, Schnittler M. Dilution-to-extinction cultivation of leaf-inhabiting endophytic fungi in beech (Fagus sylvatica L.)—different cultivation techniques influence fungal biodiversity assessment. Mycol Res, 2009; 113(5):645–54. CrossRef
Vieira ML, Hughes AF, Gil VB, Vaz AB, Alves TM, Zani CL, Rosa LH. Diversity and antimicrobial activities of the fungal endophyte community associated with the traditional Brazilian medicinal plant Solanum cernuum Vell. (Solanaceae). Can J Microbiol, 2012; 58(1):54–66. CrossRef
Wu L, Hao H, Wang G. LC/MS-based tools and strategies on qualitative and quantitative analysis of herbal components in complex matrixes. Curr Drug Metab, 2012; 13(9):1251–65. CrossRef
Zeb A. Chemistry of phenolic antioxidants. Phenolic antioxidants in foods: chemistry, biochemistry and analysis. Springer, Cham, Switzerland, pp 25–87, 2021. CrossRef
Zhou J, Chan L, Zhou S. Trigonelline: a plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr Med Chem, 2012; 19(21):3523–31. CrossRef