INTRODUCTION
Fungi, classified as kingdom fungi, are eukaryotic organisms whose functions are closely related to those of humans. Fungal infection, also known as mycosis, occurs when a fungus invades over an area of the human body and its immune system cannot handle it well (Kainz et al., 2020). Worldwide, around 150 million acute cases of fungal infections happen, resultant approximately 1.7 million deaths annually, which is much higher than malaria or tuberculosis (Bongomin et al., 2017). The mortality rate is alarmingly increasing, thus marking it as a global threat.
Although fungal infections are more common than bacterial infections, but it seems that the development of antifungal agents is far behind than that of antibacterial agents because of the slow growth of fungi in multicellular forms, hence making it more challenging to quantify than bacteria. Also, fungi are eukaryotes, and the maximum number of antifungal agents are found to be noxious to fungi and hosts as well, thereby making the evaluation of the biological activities of potent antifungal agents via in vitro or in vivo studies much more difficult (Dixon et al., 1996). Basic treatments of fungal infection still face many challenges, for example, drug resistance (Al-Wabli et al., 2018), alarming drug interactions, poor systemic availability, and low safety profiles (Sheng and Zhang, 2011). These challenges become a major hindrance, making the fungal infection very difficult to treat. Despite these drawbacks and difficulties, recent advancements in technology and well-defined literature backup support researchers in coping with these challenges by understanding the existing antifungal drugs, modifying them and synthesizing novel antifungal agents with improved safety profiles.
Antifungal medications are topically or systemically administered as pills, creams, powders, etc., and work either by killing fungi directly or preventing them from growing. Antifungal drugs include azoles, polyenes, allylamines, echinocandins, and others (https://www.healthline.com/health/fungal-infection/antifungal). Among them, azoles are the most commonly used anti-fungal drugs. Azole antifungals contain an azole ring and inhibit fungi from growing by slowing down the process. Azole antifungal drugs are further classified into three groups—(i) Imidazoles are possessing two atoms of nitrogen in one azole ring (e.g., miconazole, econazole, dapaconazole, and ketoconazole), (ii) Triazoles have three nitrogen atoms in an azole ring (e.g., fluconazole, fosfluconazole, and itraconazole), and (iii) Tetrazoles have four nitrogen atoms in one azole ring (e.g., quilseonazole, oteseseonazole, etc.) (Fig. 1) (Shafiei et al., 2020).
Triazole compounds are one of the most significant groups for medicinal as well as for organic chemistry. When combined with different molecules, the triazole moiety has demonstrated a variety of biological activities, including antimicrobial (Chen et al., 2000; Sherement et al., 2004), anticancer (Duran et al., 2002), antimalarial (Gujjar et al., 2009), analgesic (Hafez et al., 2008), anti-inflammatory (Banu et al., 1999), and anticonvulsant. (Guan et al., 2007) Triazoles possess specific structural and chemical features such as high aromatic stabilization, which provide them stability under different conditions.
Another important compound in the azole family is imidazole, a five-membered heterocyclic molecule with two nitrogen atoms, positioned at 1 and 3 places (Debus, 1858). As a very common scaffold, imidazole is present in many highly significant biomolecules such as essential amino acids, histamine, histidine, alkaloids (Kleeman et al., 1999; Verma et al., 2013) with extensive biological activities like antibacterial (Vijesh et al., 2011), analgesic (Uçucu et al., 2001), antifungal (Vita et al., 2012; Yang et al., 2012), anticancer (Yang et al., 2012); anti-HIV (Johns et al., 2009; Zhan et al., 2009), and antitubercular (Lu et al., 2012). Imidazole, a relatively simple molecule, is amenable to several structural modifications. Imidazole has several commendable properties like excellent tissue permeability and penetrability, good bioavailability, and comparatively less toxicity and adverse effects.
Etomidate, Cimetidine, Oxiconazole, Ketoconazole, Ornidazole, Metronidazole, Azomycin, Clonidine, etc. members of the imidazole family have been shown to have in vitro antifungal activity against a wide range of pathogenic fungi. As per a recent study, derivatives of imidazole, e.g., ketoconazole and miconazole, can cause the amassing of toxic peroxide compounds inside the cells of microbes (Gupta, 2001; Wright et al., 1992), where Ketoconazole, a water-soluble imidazole, can be used orally to treat both superficial as well as deep-seated fungal infections (Terrell et al., 1992).
A comprehensive literature review indicated that in medicinal chemistry, currently, piperazine, a nitrogen-containing heterocyclic, and its congeners are considered to be the most significant building blocks for drug synthesis (Alang et al., 2010; Al-Harthy et al., 2016; Al-Talib et al., 2016). Piperazine and its derivatives have attained a unique position as imperative pharmacophores in several therapeutic areas. Its multipurpose structure has always motivated researchers to use piperazine molecules as a scaffold to design novel drug molecules with numerous biological activities. The literature demonstrates the subsistence of several piperazine compounds along with triazoles and imidazole pharmacophores with varied pharmacological activities, including their incredible potential for analgesic and anti-inflammatory activities (Fromtling, 1988; Shao et al., 2007). These molecules contained a core structure of piperazine where the imidazole/triazole ring linked to a dihalophenyl ring via a chain of two carbons. Along with this, a hydroxyl group is present on the alpha carbon of the phenyl ring. These molecules possess three chiral carbons, two fixed chiral centers are present in the dioxolane ring and aryloxymethylene dioxolane-ring substituents, and triazolomethylene are cis to each other (Fig. 2). Literature supports modifications to the head and tail parts of the core structure of ketoconazole and itraconazole molecules to improve their biological activity and bioavailability (Liu et al., 2011; Pinal, 2004; Ruiz-Castillo and Buchwald, 2016).
Thus, to magnify the scope of piperazine derivatives as privileged therapeutic scaffolds and a considerable requirement for the discovery of novel chemical entities with potential biological activity and interest, we pursued our interest in developing new anti-fungal agents and strived for the synthesis and evaluation of biological activities of newly synthesized piperazine derivatives comprising imidazole and triazole moieties. The present paper reports the synthesis, characterization, and antimicrobial activity of novel piperazine analogs as prospective antifungal agents.
RESULTS AND DISCUSSION
Chemistry
Due to the varied antifungal properties of triazole and imidazole derivatives, we planned to synthesize new piperazine derivatives. By using the core structures of ketoconazole (KTZ) and ITZ as a platform, methodically, we altered the side chains to synthesize four effective systemic antifungal drug molecules comprising a broad spectrum of antifungal activity with less probability of developing resistance. All four novel derivatives were synthesized by coupling of Cis tosylate with synthesized piperazine derivatives comprising Imidazole/Triazole moieties to get an intermediate. This intermediate on further treatment with several phenyl derivatives produced the designed novel derivatives.
Biological activities of KTZ-1, KTZ-2, ITZ-1, and ITZ-2
The anti-oxidant activities and anti-microbial activities of all the synthesized novel azole piperazine congeners (KTZ-1, KTZ-2, ITZ-1, and ITZ-2) were evaluated using the 1, 1-diphenyl-2-picrylhydrazyl (DPPH) method and the Well Diffusion method against microbial strains of Candida albicans, Staphylococcus aureus Gram-positive bacteria, and Pseudomonas aeruginosa, Gram-negative bacteria, respectively.
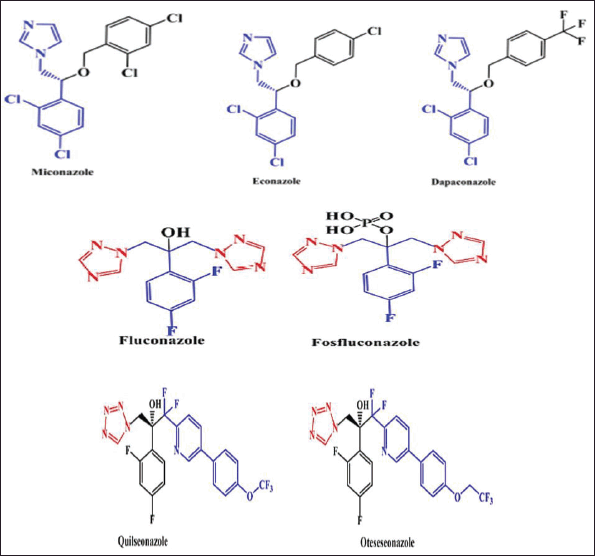 | Figure 1. Chemical structures of a few azole-based anti-fungal drugs which are in clinical use. [Click here to view] |
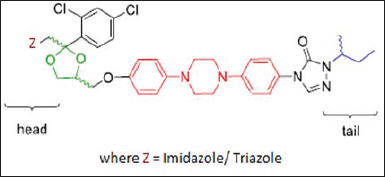 | Figure 2. Core structure of piperazine with imidazole/triazole moiety. [Click here to view] |
Antioxidant activity
When DPPH (2, 2-diphenyl-1-picrylhydrazyl, purple colored) free-radical interacts with odd electrons, absorption occurs at 517 nm. Free-radical scavengers react to DPPH to form DPPH-H, which exhibits lower absorbance due to the smaller number of hydrogens. As the number of electrons increases, DPPH gets decolorized (yellow hue).
Figure 3 reflects the relation between the absorbance and sample concentrations of KTZ-1, KTZ-2, ITZ-1, ITZ-2, and gallic acid (control). It can be observed from Figure 4 that the antioxidant activity of gallic acid as a control showed a linear relationship between concentration and percentage inhibition when absorbance is at 517 nm. The antioxidant activity of four synthesized novel molecules by the DPPH assay also shows that the antioxidant effect is proportional to the concentration. Comparative inhibition percentage of each synthesized derivative at different concentrations and gallic acid (control) are shown in Figure 5. In comparison to the IC50 of gallic acid (0.282 μg/ml), the IC50 of ITZ-1 and ITZ-2 is 0.070 and 0.224 μg/ml, indicating that these two molecules have very good antioxidant activity. For KTZ-1 and KTZ- 2, IC50 values are 1.259 and 1.438 μg/ml, respectively; their antioxidant activity is relatively moderate. This demonstrated that synthesized molecules possessed antioxidant potential.
Antimicrobial activity
Values of observed antimicrobial activities of control and all synthesized derivatives are collated in Figures 5 and 6. Antimicrobial results showed that all synthesized compounds, excluding ITZ-2, have significant antimicrobial activity against the P. aeruginosa strain (Gram-negative bacteria) and C. albicans fungi.
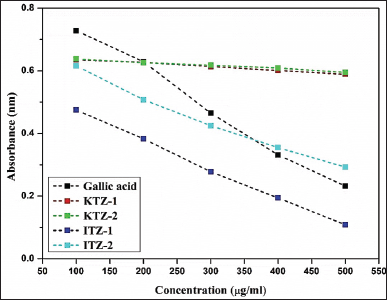 | Figure 3. Absorbance vs sample concentrations of KTZ-1, KTZ-2, ITZ-1, ITZ-2, and gallic acid (control). [Click here to view] |
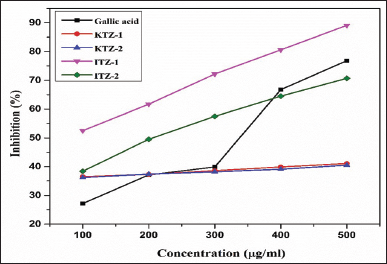 | Figure 4. Inhibition percentage vs sample concentrations of KTZ-1, KTZ-2, ITZ-1, ITZ-2, and Gallic acid (control). [Click here to view] |
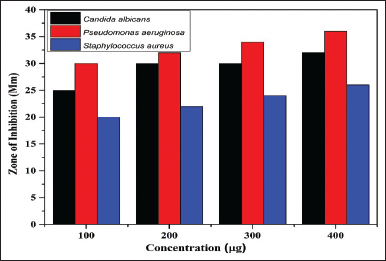 | Figure 5. ZOI of Tetracycline (control group) against P. aeruginosa strain, S. aureus strain and C. albicans fungi. [Click here to view] |
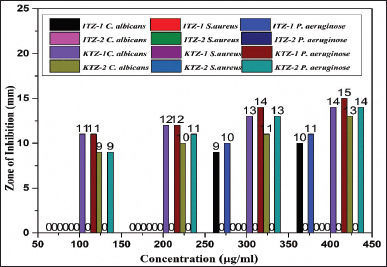 | Figure 6. ZOI of ITZ-1, ITZ-2, KTZ-1 and KTZ-2 against P. aeruginosa strain, S. aureus strain, and C. albicans fungi. [Click here to view] |
Antimicrobial results demonstrates that ITZ-1, KTZ-1, and KTZ-2 had the maximum zone of inhibition against P. aeruginosa strain (11 ± 1.2 mm), (15 ± 1.1 mm) and (14 ± 1.4 mm), respectively, at 400 μg/ml.
It is evident that for the P. aeruginosa strain and C. albicans fungi, KTZ-1, and KTZ-2 inhibit pathogen progression even at low concentrations (100–400 μg/ml). However, in the case of ITZ-1 maximum zone of inhibition was observed at higher concentration only, there is no significant antimicrobial activity at 100 to 200 μg/ml concentrations. No zone of inhibition (ZOI) was observed when all the three strains were exposed to ITZ-2 (100 to 400 μg/ml). Hence, apart from ITZ-2, ITZ-1, KTZ-1, and KTZ-2 are competently subduing the growth of pathogens thus displaying good antimicrobial activity against the two pathogen microbial strains (Fig. 6). It is noteworthy that none of the synthesized molecules were active against S. aureus (Gram-positive bacteria). Antimicrobial results demonstrate that piperazine derivatives with imidazole derivatives are more susceptible to antimicrobial activity than triazole piperazine derivatives. The Gram-positive organism, S. aureus strain, showed strong resistance to all synthesized piperazine derivatives. Among all four synthesized molecules, KTZ-1 was found to be the most active one.
CONCLUSION
In this study, four new piperazine derivatives with triazole and imidazole moieties have been synthesized and characterized for the first time. Furthermore, they were evaluated for antimicrobial and antioxidant studies. All synthesized compounds except ITZ-2, have shown significant antibacterial activity for the P. aeruginosa strain (MTCC 2453) and antifungal activity against Candida albicans (MTCC 3958) fungi. Staphylococcus aureus strain (MTCC 96) has shown strong resistance to all synthesized piperazine derivatives. ITZ- 1, KTZ-1, and KTZ-2 showed significant activity against P. aeruginosa strains and Candida albicans fungi. Among all these synthesized molecules, KTZ-1 exhibited excellent and promising antifungal and antibacterial activities, whereas ITZ-1 showed the weakest effect. The order of antimicrobial activity for the synthesized piperazine derivatives against P. aeruginosa strain is as follows: Imidazole ≥ Triazole. An antioxidant study shows that the IC50 of ITZ-1 and ITZ-2 is 0.70 and 0.224 μg/ml, respectively, indicating their strong antioxidant activity. For KTZ-1 and KTZ-2 it is moderate. This demonstrated that synthesized molecules possessed antioxidant potential. Therefore, ITZ-1, KTZ-1, and KTZ-2 may be further used for the synthesis of new antimicrobial and antioxidant drug molecules. Observed ZOI values and drug resistance of S. aureus (Gram-positive bacteria) against all synthesized novel piperazine derivatives in the present investigation will be helpful to use as a reference for auxiliary studies and further evaluation is worthwhile. However, the in vivo activity of these synthesized drugs should be assessed in clinical trials.
EXPERIMENTAL MATERIAL AND METHOD
All required chemicals and reagents were procured from Sigma-Aldrich Chemical Company (Sigma-Aldrich Chemical Company, St. Louis, MO) and used without any further purification. To perform the thin-layer chromatography (TLC), F254 plates (silica gel coated with fluorescent indicator) were purchased from Fluka Company and column chromatography was carried out on Silica gel 60 (0.063–0.2 mm). Gallenkamp melting point apparatus was used to determine the Melting points and were uncorrected. By using KBr pellets IR spectra were measured on a Thermo Nicolet model 470 Fourier-transform spectrophotometer. Nuclear magnetic resonance (NMR) (1H) was recorded on a Varian 400 MHz spectrophotometer. Chemical shifts were expressed in parts per million with tetramethylsilane as an internal standard.
Synthesis of KTZ-1 and KTZ-2
A detailed synthetic scheme of KTZ-1 and KTZ-2 with key intermediates is shown in scheme 1. For that purpose, first, key intermediates KTZ-a and KTZ-b were synthesized as per the standard procedure (Heeres et al., 1984).
Synthesis of 1-(4-(4-((2-((1H-imidazol-1-yl) methyl-2-(2, 4-dichlorophenyl)-1, 3-dioxolan- 4-yl) methoxy) phenyl) piperazin-1-yl) ethenone-(KTZ-a)
For the synthesis of KTZ-a, in the suspension of NaH (50% dispersion) in 10 ml of dimethylformamide (DMF), 0.484 g of 1-(4-(4) hydroxyphenylpiperazin-1-yethanone was added and the solution was stirred for 70 minutes. Afterward, 1.0 g of (2 ((IH-imidazol-1-yl)methyl)-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl)methyl 4 methylbenzene sulfonate (Cis Tosylate) was added and stirring was continued for 5 hours at 80°C. Progress of the reaction was monitored by TLC using a solvent system of ethyl acetate: methanol (9:1). After completion of the reaction, the mixture was cooled and extracted with Dichloromethane (CH2Cl2). The obtained organic layer was dried up and evaporated to afford a solid residue of KTZ-a.
Synthesis of 1-(4-(4-((2-((1H-imidazol-1-yl) methyl)-2-(2, 4-dichlorophenyl)-1, 3- dioxolan-4 yl) methoxy) phenylpiperazine-(KTZ-b)
A solution of 1-(4-(4-((2-((1H-imidazol-1-yl) methyl)-2-(2,4-dichlorophenyl)-1,3-dioxolan-4 yl) methoxy phenylpiperazin-1-yl) ethanone, containing 1.0 g of KTZ-a in 10 ml of MeOH and 0.1 g of NaOH pellets, was refluxed with overnight stirring at 60°C. Progress of reaction was monitored by TLC using a solvent system of ethyl acetate: methanol in 9:1 ratio. After completion of the reaction, the reaction mixture was cooled and subsequently extracted with chloroform. The organic layer was dried and evaporated in vacuo to obtain KTZ-b.
Synthesis of 1-(4-((2-((1H-imidazol-1-yl) methyl)-2-(2, 4-dichlorophenyl)-1, 3-dioxolan-4 ylmethoxy) phenyl)-4-3 methyl-benzylpiperazine-(KTZ-1)
A solution of 1-(4-((2-((1H-imidazol-1-yl) methyl)-2-(2, 4-dichlorophenyl)-1, 3-dioxolan-4 yl) methoxy phenyl piperazin is prepared by taking 0.5 g of KTZ-b in 10 ml of DMF. On adding 0.3 g of potassium carbonate and 0.5 g of 1-(bromo-methyl)-3-methylbenzene, mixture was refluxed with overnight stirring at 145°C. Reaction progress was mentioned by TLC using the solvent system ethyl acetate:methanol in 9:1 ratio. After the completion of the reaction, the reaction mixture, water was added. The mixture was subsequently extracted with chloroform (CHCl3). The organic layer was dried using MgSO4 and evaporated in a vacuum to obtain a chocolate-colored product (KTZ-1).
1HNMR: δ-7.63 (1H, J = 8), δ-7.48 (s,1H, J = 12), δ-7.47 (1H, J = 9), δ-7.44 (1H, J = 9), δ-7.28 (1H, J = 7), δ-7.24 (1H, J = 8), δ-7.14 (1H, J = 16), δ-4.50 (1H, J = 14), δ-4.43 (1H, J = 30), δ-4.23 (2H, J = 20), δ-4.23 (1H, J = 20), δ-3.73 (2H, J = 8), δ-3.56–3.33 (2H), 2.51 (s, 3H). IR (KBr, cm−1): 3,871.2 and 3,673.8 cm−1 (N-H stretching of imidazole and piperazine), 2,916.8 cm−1 (C-H stretching of C-CH3), 1,632 cm−1 (C=C aromatic). 1,225.6 cm−1 (C-O stretching of the ether linkage), 1,102.9 and 1,025.7 cm−1 (C-O stretching), 817.6 cm−1 (C-Cl linkage) (Kujawski et al., 2017). Further confirmation was done by finding the mass by liquid chromatography-mass spectrometry (LC-MS) and comparing it with the calculated one. Calculated LC/MS (m/z): 593.4 while from the graph, it is 591.14 g/mol.
Synthesis of phenyl 4-(4-((2-((1H-imidazol-1-yl) methyl)-2-(2, 4-dichlorophenyl)-1, 3- dioxolan-4-yl) methoxy) phenyl) piperazine-1-Carboxylate -(KTZ-2)
A 0.5 g solution of 1-(4-((1H-imidazol-1-yl) methyl)-2-(2,4-dichlorophenyl)-1,3-dioxolan-4 yl) methoxy) phenyl) piperazine (De acetylation ketoconazole) in 10 ml of DMF, 0.202 g of triethylamine (TEA), and 0.18 g of Phenyl Chloroformate was refluxed with overnight stirring at 145°C. Reaction progress was monitored and a cream-colored product (KTZ-2) was obtained.
1HNMR: δ-7.74 (1H, J = 22), δ-7.63 (1H, J = 8), δ-7.47 (1H, J = 9), δ-7.44 (1H, J = 9), δ-7.30 (1H, J = 7), δ- 7.28 (1H, J = 7), δ-7.24 (1H, J = 8), δ-7.14 (1H, J = 16), δ-4.50 (1H, J = 14), δ-4.43 (1H, J = 30), δ- 4.23 (2H, J = 20), δ-4.23 (1H, J =20), δ-3.8 (1H, J = 7), δ-3.73 (2H, J = 8), δ-3.56–3.33 (2H). IR (KBr, cm−1): 3,871.2 and 3,673.8 cm−1 (N-H stretching of imidazole and piperazine), 2,916.8 cm−1 (N-H stretching), 1,716 cm−1 (C=O-O ester linkage), 1,632 cm−1 (C=C aromatic), 1,225.6 cm−1 (C-O stretching of ether linkage), 1,102.9 cm−1, and 1,025.7 cm−1 (C-O stretching), 817.6 cm−1 (C-Cl linkage) (Gao et al., 2005). For the KTZ-2 molecule, calculated LC/MS (m/z) is 609.5 whereas graphical LC/MS (m/z) is 608.49 g/mol.
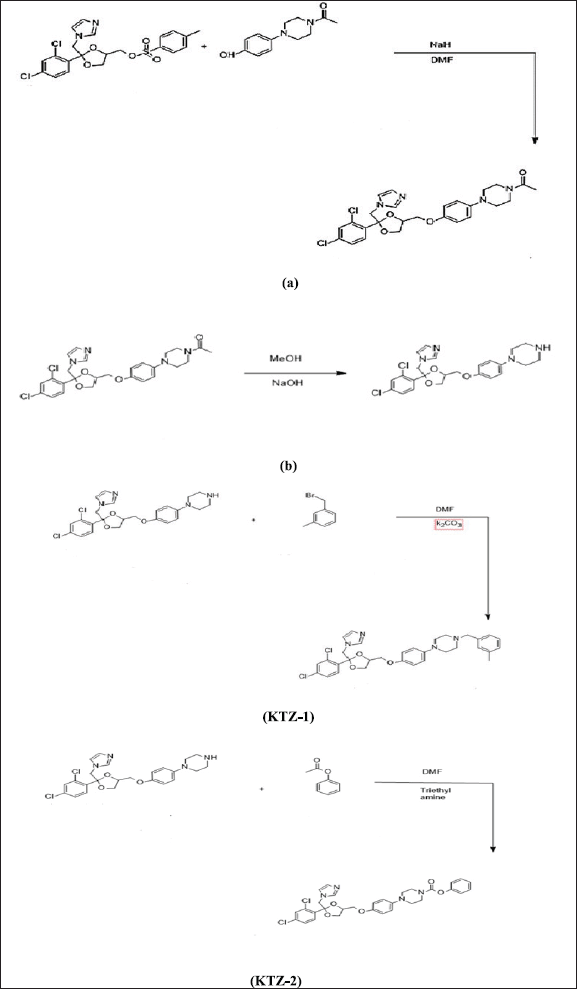 | Scheme 1. Synthesis of KTZ-a, KTZ-b, KTZ-1 and KTZ-2. [Click here to view] |
SYNTHESIS OF ITZ-1 AND ITZ-2
Furthermore, the key intermediate ITZ-(a–e) was synthesized as stated in the literature (Heeres et al., 1984). By esterification and acylation of ITZ-e, final molecules, ITZ-1 and ITZ-2 were synthesized. Synthesis of ITZ-1 and ITZ-2 is shown in Scheme 2.
Synthesis of 1-(4-methoxy phenyl) piperazine-(ITZ-a)
In a 500 ml round bottom flask fitted with a stirrer and reflux condenser, 20 ml of isopropyl alcohol (IPA) was taken. Then, 2 g of each potassium carbonate and Bis (2-Chloro Ethyl) Amine hydrochloride were added. After stirring for 10 minutes, 2 g of P-Anisidine was added and refluxed at 85°C overnight. The reaction was supervised by checking TLC. Once the reaction was completed, the mixture was allowed to attain the room temperature, further followed by filtration and distillation of the mother liquor. Solid 1-(4-methoxy phenyl) piperazine was obtained by precipitation using hexane.
Synthesis of 1-(4-methoxy phenyl)-4-(4-nitrophenyl)-(ITZ-b)
In a dried and clean round bottom flask fitted with a reflux condenser, 1 g of 1-(4-methoxy phenyl) piperazine (ITZ-a), 10 ml of DMF, and 0.56 g of potassium carbonate were added. Stirring was carried out at room temperature for 15 minutes. After the addition of 0.86 g of 1- chloro-4-nitro benzene, the reaction mixture was refluxed at 125°C for 8 hours and the reaction was monitored by checking TLC. Once the reaction was completed, the reaction mixture was quenched with distilled water and extracted using Dichloromethane (DCM). In the DCM layer, sodium sulfate was added to remove any traces of water. Complete distillation of the organic layer gives the solid ITZ-b.
Synthesis of 4-[4-(4-Nitrophenyl)-1-piperazinyl] phenol-(ITZ-c)
In a 50 ml two-neck round bottom flask (fitted with a reflux condenser), 30 ml of HBr (63%) was taken. In this, 5 g of 1-(4-methoxy phenyl)-4-(4-nitrophenyl) (ITZb) was added portion-wise over a period of 10 minutes. The reaction mixture was refluxed for 16 hours at 150°C and it was monitored by checking TLC. After the completion of the reaction, the reaction mixture was quenched with water. A solid product was made into an amorphous powder using pestle and mortar.
Synthesis of ((2S, 4S)-2-((1H-1, 2, 4-triazol-1-yl) methyl)-2-(2, 4-dichlorophenyl)-1, 3- dioxolan-4-yl) methyl 4- methylbenzenesulfonate (Cis Tosylate) to 4-[4-(4-Nitrophenyl)- 1-piperazinyl] phenol-(ITZ-d)
In a 50 ml two-neck round bottom flask containing 10 ml of DMF, 1 g of ITZ-c was added. The solution was stirred while the reaction mixture was heated for 1 hours at 500°C. To dissolve it in the DMF solution, 1 g of (2S, 4S) -2-((1H-1,2,4-triazol-1-yl) methyl)-2-(2,4- dichlorophenyl)-1,3-dioxolan-4-yl) methyl 4-methylbenzenesulfonate (Cis Tosylate) was added slowly. The reaction was kept at 950–1,000°C overnight. After cooling to room temperature, the mixture was quenched with sodium chloride (aqueous solution) and extracted with ethyl acetate for three times. The organic layer was dried over Na2SO4 and distilled to get a solid ITZ-d.
Reduction of the nitro group of ITZ-d to amine group to get ITZ-e
In a two-neck round bottom flask, 2.5 g of ITZ-d and 10 ml of methanol were taken and for 30 minutes the reaction mixture was stirred at room temperature. Then zinc powder and ammonium formate in the 1:1 ratio were added. In the beginning, the color of the reaction mixture was yellow, but after 3 hours the yellowish color changed to a brown color. The reaction was constantly monitored by TLC. Once, the reaction was completed, the mixture is filtered using methanol. The collected filtrate was distilled to get a solid product. Further filtration and distillation were carried out to get a solid, pure product (ITZ-e). The obtained ITZ-e was further subjected to acylation and esterification to get two new derivatives of itraconazole—ITZ-1 and ITZ-2.
Acylation reaction to obtain ITZ-1
In a two neck round bottom flask, ITZ-e and DCM were taken in a 1:20 ratio after the dissolution of ITZ-e, TEA and benzoyl chloride (1:1) were added and the reaction was allowed to proceed for 5 hours at room temperature. Reaction was supervised by checking TLC. After the quenching, separation, and distillation, ITZ-1 was obtained as a cream-colored amorphous powder.
1H NMR: δ-3.6 (2H, J = 5, piperizine ring), δ-3.7 (2H, J = 3, 1,3 dioxalan), δ-3.8 (s J = 6, R-OCH3), δ-4.2 (s, Oxygenated bridge), δ-5.2 (s, amide linkage), 6.8 to 8.2 (J = 2 to 9 aromatic rings), δ-8.1 (s, 1,2,4 triazole), δ-7.8 (J = 7, benzene attached with chlorine), δ-6.8 (J = 9, Benzene attached to Nitrogen)]. IR (KBr, cm−1): 1,700 cm−1 (-NH-C=O amide group), 1,600–1,800 cm−1 (-C=O stretching carbonyl group), 1,584, 1,552 (– C-C aromatic), 1,450, 1,380, 1,333 cm−1 - N=N and C-N triazole, 1,229 cm−1—alkyl aryl ether, 824, 731, 701 cm−1 (– C-Cl aromatic) (Singh et al., 2000). Confirmation of the synthesized molecule was done by performing LC-MS. The LCMS result revealed that the calculated LC/MS (m/z) is 685.9 while from the LC/MS graph, it is found to be 686.53 g/mol.
Esterification reaction to obtain ITZ-2
Similar to the above procedure, in a two-neck round bottom flask, ITZ-e and DCM were taken in a 1:25 ratio. After the dissolution of ITZ-e, 3-4 drops of each TEA and Phenyl chloroformate were added. The reaction was continued for 3 hours at room temperature. Reaction was constantly monitored by checking TLC. After the quenching, separation, and distillation, ITZ-2 was obtained as a light-yellow colored powder.
1H NMR: δ-3.6 (2H, J = 5, piperizine ring), δ-3.7 (2H, J = 3, 1,3 dioxalan), δ-3.8 (s J = 6, R-OCH3), δ-4.2 (s, Oxygenated bridge), δ-5.2 (s, ester-amide linkage), 6.8 to 8.2 (J = 2 to 9 aromatic rings), δ-8.1 (s, 1,2,4 triazole), δ-7.8 (J = 7, benzene attached with chlorine), δ-6.8 (J = 9, Benzene attached to Nitrogen. IR (KBr, cm−1): 1,725 cm−1 (O-C=O aromatic ester, 1,584, 1,552 (– C-C aromatic), 1,585 cm−1 1,450, 1,380, 1,333—N=N and C-N triazole, 1,274, 1,185 (– C-H aromatic), 1,229 cm−1—alkyl aryl ether, 810, 736, and 685 cm−1 (C-Cl stretching) (Madgulkar et al., 2008). Calculated LC/MS (m/z) is 701.59 and, graphically, the LC/MS value is found to be 702.66 g/mol(Supplementary Figures S1–12). The physical properties of all synthesized piperazine derivatives are summarized in Table 1.
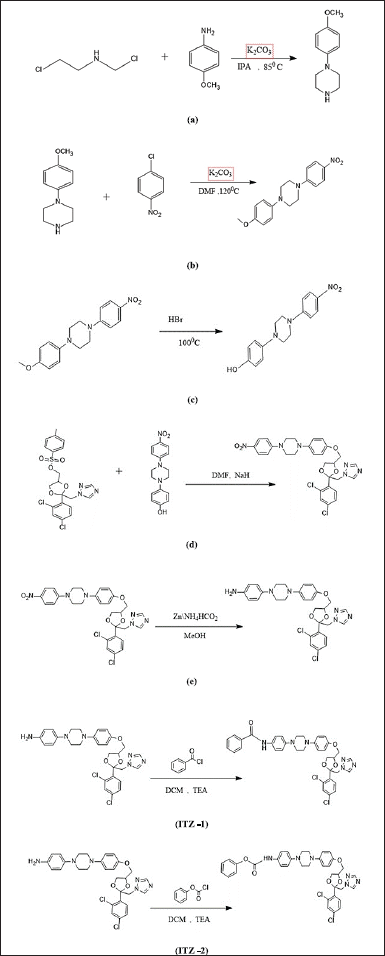 | Scheme 2. Synthesis of ITZ-(a-e), ITZ -1 and ITZ -2. [Click here to view] |
ANTIOXIDANT ACTIVITY
DPPH assay
The free radical scavenging capacity of all four synthesized molecules was estimated using the stable DPPH radical (Benzie and Strain, 1999; Katalinic et al., 2006). Different concentrations (100–500 μg/ml) of the synthesized molecules were taken in separate test tubes. By using methanol, the volume of each test tube was made up to 0.1. 3 ml of DPPH solution (whose absorbance was pre-set to 1) was added in all the test tubes containing sample and methanol. Further all the test tubes incubated in dark conditions for 15 min. After incubation, by keeping methanol as a blank, the absorbance was read at 517 nm spectrophotometrically. Percentage inhibition was calculated using the formula:
Percentage inhibition = [(Control Ab – Sample Ab)]/ [Control Ab] * 100
ANTI-MICROBIAL ACTIVITY
Bacteria, fungi, and culture media.
In the present work, C. albicans, S. aureus (Gram positive), and P. aeruginosa (Gram negative) were used to investigate the antifungal and antibacterial activity of all four synthesized compounds. Tetracycline is used as a control against these organisms (Magaldi et al., 2004; Valgas et al., 2007).
The well diffusion method
The samples ITZ-1, ITZ-2, KTZ-1, and KTZ-2 were tested in three replicates for their ZOI measurement against organisms (C. albicans, S. aureus, and P. aeruginosa).
Culture preparation
Culture and media preparation for fungus
30 ml of Dextrose Broth (PDB: Potato-200 g, Dextrose-20 g, and Distilled water-1,000 ml) was prepared in Erlenmeyer flasks by boiling 6 g of potato in 30 ml of distilled water and filtering. After adding 0.6 g of dextrose to the above filtrate, the final volume was made up to 30 ml using distilled water. 15 minutes in an autoclave at 121°C. Candida albicans (MTCC 3958) was inoculated and incubation was done at 25°C for 72 hours.
Bacterial culture media preparation
In two Erlenmeyer flasks, LB broth (Sodium chloride 10 g, Tryptone 10 g, Yeast extract 6 g, 1,000 ml Distilled water) was prepared by adding Tryptone 0.3 g, Sodium chloride 0.3 g, Yeast extract 0.18 g, and distilled water 30 ml and autoclaved at 121°C for 15 minutes. Pseudomonas aeruginosa (MTCC 2453) and S. aureus (MTCC 96) strains were inoculated in 30 ml sterilized LB broth flasks and incubation was done at 37°C for 24 hours.
Sample preparation
10 mg of samples (ITZ-1, ITZ-2, KTZ-1, and KTZ-2) were dissolved in 1 ml of dimethyl sulfoxide (DMSO), respectively, to prepare different aliquots of samples of 100, 200, 300, and 400 μg/ml concentrations.
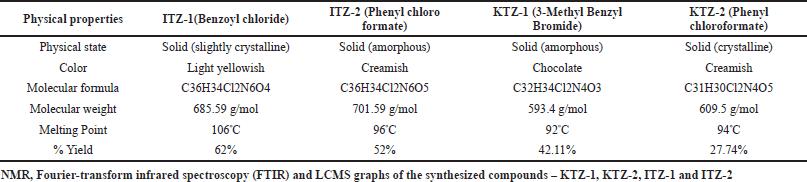 | Table 1. Summarized physical properties of novel ITZ-1, ITZ-2, KTZ-1 and KTZ-2 molecules. [Click here to view] |
Mediation preparation for ZOI
For fungal plate—Potato Dextrose Agar (PDA: Potato-200 g, Dextrose-20 g, Agar-20 g, distilled water-1,000 ml). 30 g of potatoes were boiled in 100 ml of distilled water and filtered and by using distilled water final volume was made up to 150 ml. 3 g of Dextrose and 3 g of Agar were added and further autoclaved for 15 minutes at 121°C.
For bacterial plate-Luria Bertani (LB) agar media (Tryptone 10 g, Sodium chloride 10 g, Yeast extract 6 g, Agar 20 g, Distilled water 1,000 ml), 400 ml was prepared in Erlenmeyer flasks by adding Tryptone 4 g, Sodium chloride 4 g, Yeast extract 2.4 g, Agar 8 g, Distilled water 400 ml and further autoclaved at 121°C for 15 minutes.
Plating to measure ZOI against all three organisms
About 25 ml of the media (LB agar and PDA) was poured on the sterilized petri- plates and then it was allowed to solidify. A 200 μl inoculum (S. aureus, P. aeruginosa and C. albicans) was poured on agar plates. By using a plate spreader it was spread thoroughly. Five wells of 0.6 cm were made in each plate by using the borer. 50 μl of prepared sample containing 100, 200, 300, and 400 μg were loaded into the respective wells, and as a control blank, 50 μl of DMSO was loaded in the middle well. Further all plates were kept for the incubation. Incubation period for the bacterial plates was 24 hours at 37°C and for fungal plates it was 72 hours at 25°C. Afterwards, the zone of inhibition was measured in Supplementary Figure S13a–e.
CONFLICT OF INTEREST
There are no conflicts to declare.
ACKNOWLEDGMENTS
The authors would like to thank M.S. Ramaiah University of Applied Sciences, Bangalore, India for providing research resources, chemicals, equipment, and labs to carry out this research work. This work was partially funded by the Karnataka Science and Technology Academy (KSTA), Department of Science and Technology, Government of Karnataka, India.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Alang G, Kaur R, Kaur G, Singh A, Singla P. Synthesis and antibacterial activity of some new benzothiazole derivatives. Acta Pharm Sci, 2010; 52(2):213–8.
Al-Harthy T, Zoghaib WM, Pflüger M, Schöpel M, Önder K, Reitsammer M, Hundsberger H, Stoll R, Abdel-Jalil R. Design, synthesis, and cytotoxicity of 5-fluoro-2-methyl-6-(4- aryl-piperazin-1-yl) benzoxazoles. Molecules, 2016; 21(10):1290. CrossRef
Al-Talib M, Al-Soud YA, Abussaud M, Khshashneh S. Synthesis and biological evaluation of new benzothiazoles as antimicrobial agents. Arabian J Chem, 2016; 9:926–30. CrossRef
Al-Wabli RI, Al-Ghamdi AR, Primsa IP, Ghabbour HA, Al-Agamy MH, Joe IH, Attia MI. (2 E)-2-[1-(1,3-Benzodioxol-5-yl)-3-(1 H -imidazol-1-yl) propylidene]- N -(4-methoxyphenyl) hydrazinecarboxamide: Synthesis, crystal structure, vibrational analysis, DFT computations, molecular docking and antifungal activity. J Mol Struct, 2018; 1166:121–30. Available at: https://www.healthline.com/health/fungal-infection/antifungal (Accessed 16 May 2022). CrossRef
Banu KM, Dinaker A, Ananthnarayan C. Synthesis, characterization of antimicrobial studies and pharmacological screening of some substituted 1,2,3-triazoles. Indian J Pharm Sci, 1999; 61(4):202–5.
Benzie IFF and Strain JJ. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol, 1999; 299:15–27. CrossRef
Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi, 2017; 3(4):57. CrossRef
Chen MD, Lu SJ, Yuag GP, Yang SY, Du XL. Synthesis and antimicrobial activity of some heterocyclic beta-enamino ester derivatives with 1, 2, 3-triazole. Heterocyclic Comm, 2000; 6(5):421–6. CrossRef
Debus H. Ueber die Einwirkung des Ammoniaks auf Glyoxal. Annalen der Chemie und Pharmacie, 1858; 107(2):199–208. CrossRef
Dixon DM, Walsh TJ. Antifungal Agents. In: Baron S (ed.). Medical microbiology. University of Texas Medical Branch at Galveston, Galveston, TX, 1996. Available via https://www.ncbi.nlm.nih.gov/books/NBK8263/
Duran A, Dogan HN, Rollas H. Synthesis and preliminary anticancer activity of new 1,4-Dihydro-3-(3-hydroxy-2-naphthyl)-4-substituted-5H-1,2,4-triazoline-5-thiones. Farmaco, 2002; 57(7):559–64. CrossRef
Fromtling RA. Overview of medically important antifungal azole derivatives. Clin Microbiol Rev, 1988; 1(2):187–219. CrossRef
Gao ZH, GU JY, Wang XM, Li ZG, Bai XD. FTIR and XPS study of the reaction of phenyl isocyanate and cellulose with different moisture contents. Pigment Resin Technol, 2005; 34(5):282–9. CrossRef
Guan LP, Jin QH, Tian GR, Chai KY, Quan ZS. Synthesis of some quinoline-2(1H)- one and 1, 2, 4-triazolo [4, 3-a] quinoline derivatives as potent anticonvulsants. J Pharm Sci, 2007; 10(3):254–62.
Gujjar R, Marwaha A, White J, White KL, Creason S, Shackleford DM, Baldwin J, Charman WN, Buckner FS, Charman S, Rathod PK, Phillips MA. Identification of a metabolically stable triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with antimalarial activity in mice. J Med Chem, 2009; 52(7):1864–72. CrossRef
Gupta KL. Fungal infections and the kidney. Indian J Nephrol, 2001; 11:147–54. Hafez HN, Abbas HA, El-Gazzar AR. Synthesis and evaluation of analgesic, anti-inflammatory and ulcerogenic activities of some triazolo- and 2-pyrazolyl-pyrido [2, 3-d]- pyrimidines. Acta Pharm, 2008; 58(4):359–78. CrossRef
Heeres, J., Backx, L. and Van Cutsem, J., Antimycotic azoles. 7. Synthesis and antifungal properties of a series of novel triazol-3-ones. J Med Chem, 1984; 27(7):894–900. CrossRef
Johns BA, Weatherhead JG, Allen SH, Thompson JB, Garvey EP, Foster SA. The use of oxadiazole and triazole substituted naphthyridines as HIV-1 integrase inhibitors Part 1: Establishing the pharmacophore. Bioorg. Med Chem Lett, 2009; 19(6):1802–6. CrossRef
Kainz K, Bauer MA, Madeo F, Carmona GD. Fungal infections in humans: the silent crisis. Microb Cell, 2020; 7(6):143–5. CrossRef
Katalinic V, Milos M, Kulisic T, Jukic M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem, 2006; 94(4):550–7. CrossRef
Kleeman A, Engel J, Kutscher B and Reichert D. 1999. Pharmaceutical Substances: Syntheses, Patents, Applications of the Most Relevant APIs. Thieme Medical, New York.
Kujawski J, Czaja K, Jod?owska-Siewert E, Dettlaff K, ?wawiak J, Kujawski R, Ratajczak T, Bernard MK. Structural and spectroscopic properties of itraconazole and ketoconazole–experimental and theoretical studies. J Mol Struct, 2017; 1146:259–66. CrossRef
Liu Y, Liu Z, Cao X, Liu X, He H and Yang Y. Design and synthesis of pyridine- substituted itraconazole analogues with improved antifungal activities, water solubility and bioavailability. Bioorg Med Chem Lett, 2011; 21(16):4779−83. CrossRef
Lu X, Liu X, Wan B, Franzblau SG, Chen L, Zhou C, You Q. Synthesis and evaluation of anti-tubercular and antibacterial activities of new 4-(2,6-dichlorobenzyloxy) phenyl thiazole, oxazole and imidazole derivatives. Part 2. Eur J Med Chem, 2012; 49:164–71. CrossRef
Madgulkar A, Kadam S, Pokharkar V. Studies on formulation development of mucoadhesive sustained release itraconazole tablet using response surface methodology. AAPS Pharm SciTech, 2008; 9(3):998–1005. CrossRef
Magaldi S, Mata-Essayag S, Hartung de Capriles C, Hartung de Capriles C, Perez C, Colella MT, Olaizola C, Ontiveros Y. Well diffusion for antifungal susceptibility testing. Int J Infect Dis, 2004; 8(1):39–45. CrossRef
Manfredini S, Vicentini CB, Manfrini M, Bianchi N, Rustigliano C, Mischiati C, Gambari R. Pyrazolo-triazoles as light DNA cleaving agents. Bioorg Med Chem Lett, 2000; 8(9):2343–6. CrossRef
Pinal R. Effect of molecular symmetry on melting temperature and solubility. Org Biomol Chem, 2004; 2:2692−9. CrossRef
Ruiz-Castillo P, Buchwald SL. Applications of Palladium-Catalyzed C–N Cross- Coupling Reactions. Chem Rev, 2016; 116(19):12564−649. CrossRef
Shafiei M, Peyton L, Hashemzadeh M, Foroumadi A. History of the development of antifungal azoles: a review on structures, SAR, and mechanism of action. Bioorg Chem, 2020; 104:104240. CrossRef
Shao P, Huanga L, Hsueh P. Recent advances and challenges in the treatment of invasive fungal infections. Int J Antimicrob Agents, 2007; 30(6):487–95. CrossRef
Sheng C, Zhang W. New lead structures in antifungal drug discovery. Curr Med Chem, 2011; 18(5):733–66. CrossRef
Sherement EA, Tomanov RI, Trukhin EV, Berestovitskaya VM. Synthesis of 4-aryl-5- nitro-1, 2, 3-triazoles. Russ J Org Chem, 2004; 40:594–5. CrossRef
Singh RB, Gupta DC, Bajpai UC, Saxena M and Saxena AK. Fourier transform infrared spectra and normal mode analysis of 1- [(3-methyl phenyl) piperazin-1-yl]-3-[thio (4- acetamido-phenyl] propane: a potent 5-HT2 and D2 receptor ligand. Spectrochim Acta Part A: Mol Biomol Spectrosc, 2000; 56A(7):1267–75. CrossRef
Terrell CL, Hughes CE. Antifungal agents used for deep-seated mycotic infections. Mayo Clinic Proc, 1992; 67(1):69–91.
Uçucu U, Karaburun NG, I?ikdag I. Synthesis and analgesic activity of some 1-benzyl- 2-substituted-4, 5-diphenyl-1H-imidazole derivatives. Farmaco, 2001; 56(4):285–90. CrossRef
Valgas C, Souza SM, Smania EFA, Smania Jr A. Screening methods to determine antibacterial activity of natural products. Braz J Microbiol, 2007; 38(2):369–80. CrossRef
Verma A, Joshi S, Singh D. Imidazole: having versatile biological activities. J Chem, 2013; 2013:1–12. CrossRef
Vijesh AM, Isloor AM, Telkar S, Peethambar SK, Rai S, Isloor N. Synthesis, characterization and antimicrobial studies of some new pyrazole incorporated imidazole derivatives. Eur J Med Chem, 2011; 46(8):3531–6. CrossRef
Vita LD, Scipione S, Tortorella S, Mellini P, Di Rienzo B, Simonetti G, D’Auria FD, Panella S, Cirilli R, Di Santo R, Palamara AT. Synthesis and antifungal activity of a new series of 2-(1H-imidazol-1-yl)- 1-phenylethanol derivatives. Eur J Med Chem, 2012, 49:334–42. CrossRef
Wright SW, Harris RR, Collins RJ, Corbett RL, Green AM, Wadman EA, Batt DG. Novel l-(pyridylphenyl)-l-phenyl-2-imidazolyl ethanols with topical anti-inflammatory activity. J Med Chem, 1992, 35(17):3148–55. CrossRef
Yang WC, Li J, Chen Q, Yang GF. Novel synthetic methods for N-cyano-1H- imidazole-4-carboxamides and their fungicidal activity. Bioorg Med Chem Lett, 2012a; 22(3):1455–8. CrossRef
Yang XD, Wan WC, Deng XY, Li Y, Yang LJ, Li L, Zhang HB. Design, synthesis and cytotoxic activities of novel hybrid compounds between 2-phenylbenzofuran and imidazole, Bioorg. Med Chem Lett, 2012b; 22(8):2726–9. CrossRef
Zhan P, Liu X, Zhu J, Fang Z, Li Z, Pannecouque C, Clercq ED . Synthesis and biological evaluation of imidazole thioacetanilides as novel non-nucleoside HIV-1 reverse transcriptase inhibitors. Bio Med Chem, 2009; 17(16):5775–81. CrossRef
SUPPLEMENTARY MATERIAL
For all four synthesized molecules (KTZ-1, KTZ-2, ITZ-1, and ITZ-2), a separate file is attached containing their respective:
- 1HNMR,
- FTIR,
- LCMS graphs and
- ZOI (Zone of Inhibition) plate images against all three pathogens and control.
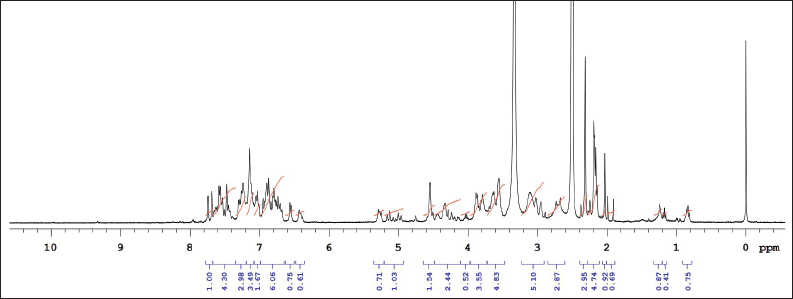 | Supplementary Figure S1. 1NMR data of KTZ-1 in DMSO. [Click here to view] |
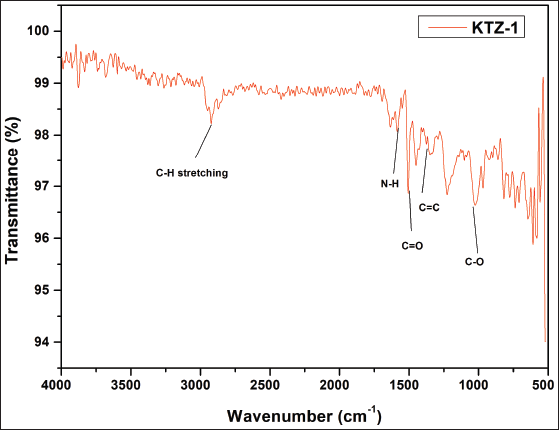 | Supplementary Figure S2. FTIR data of KTZ-1. [Click here to view] |
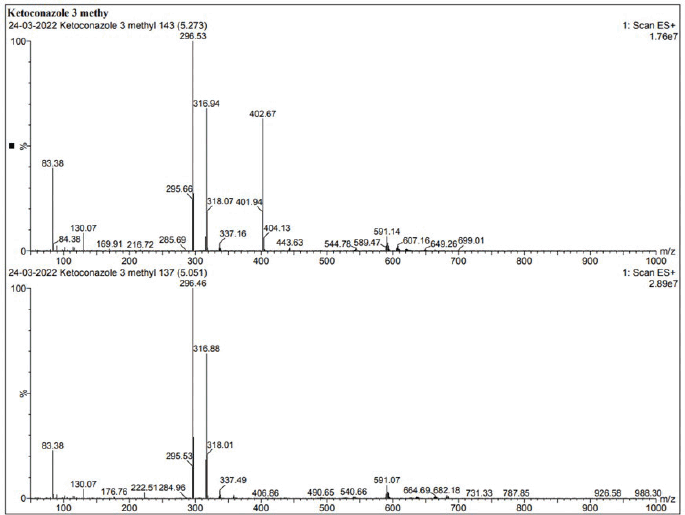 | Supplementary Figure S3. LCMS data of KTZ-1. [Click here to view] |
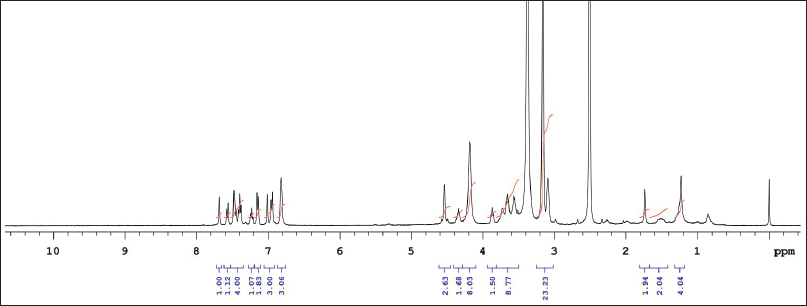 | Supplementary Figure S4. 1NMR data of KTZ-2 in DMSO. [Click here to view] |
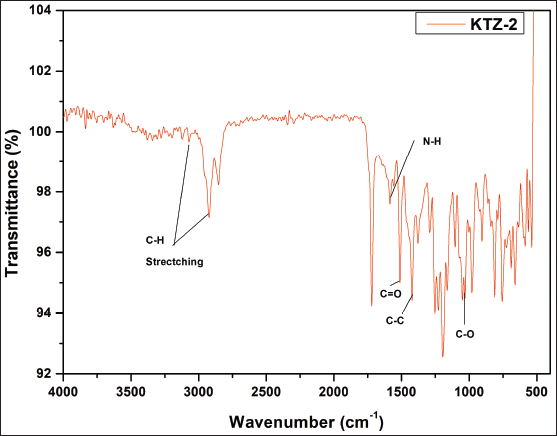 | Supplementary Figure S5. FTIR data of KTZ-2. [Click here to view] |
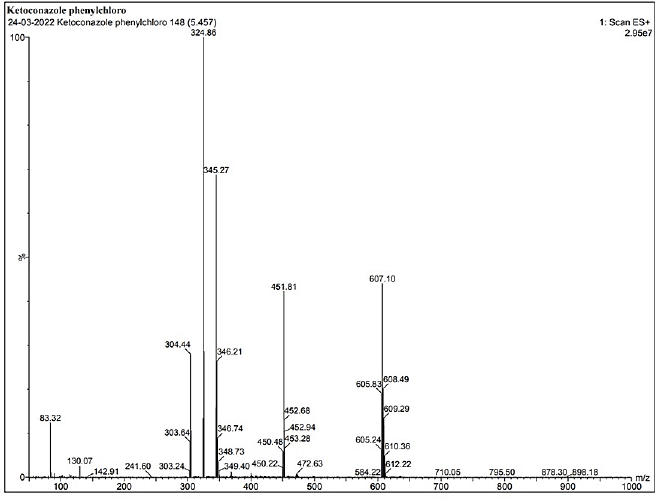 | Supplementary Figure S6. LCMS data of KTZ-2. [Click here to view] |
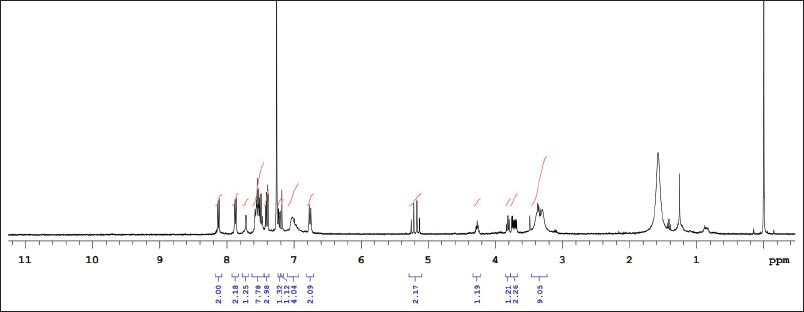 | Supplementary Figure S7. 1NMR data of ITZ-1 in CDCl3. [Click here to view] |
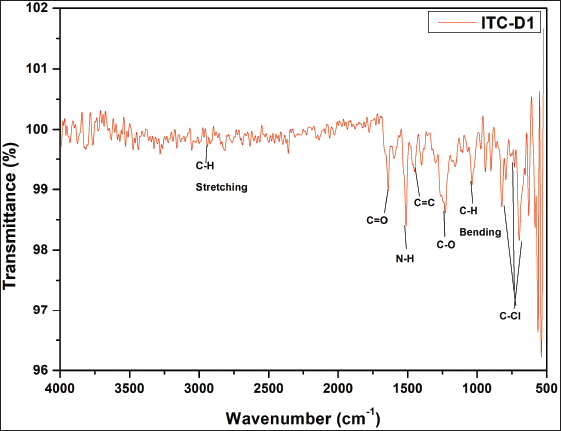 | Supplementary Figure S8. FTIR data of ITZ-1. [Click here to view] |
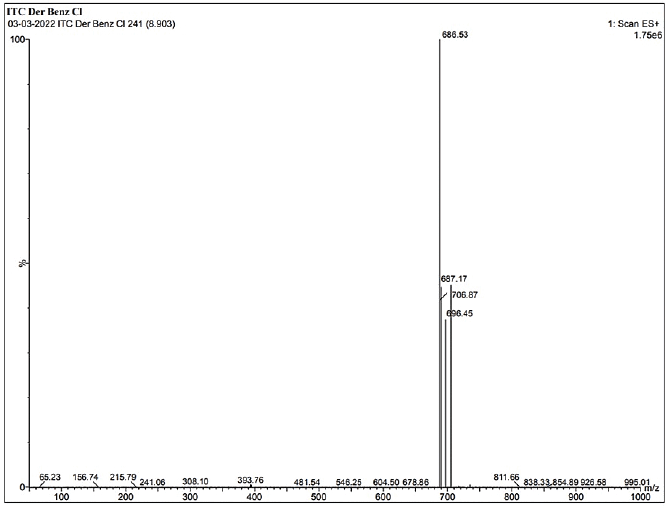 | Supplementary Figure S9. LCMS data of ITZ-1. [Click here to view] |
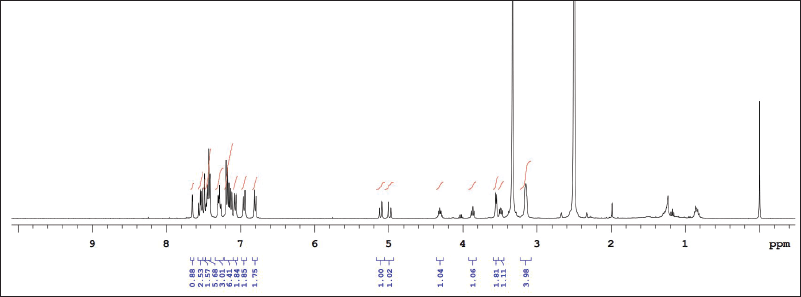 | Supplementary Figure S10. 1NMR data of ITZ-2 in DMSO. [Click here to view] |
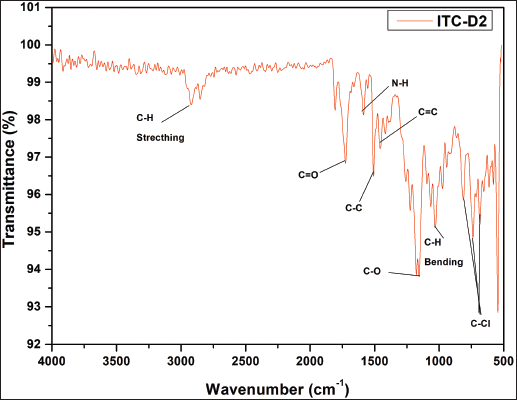 | Supplementary Figure S11. FTIR data of ITZ-2. [Click here to view] |
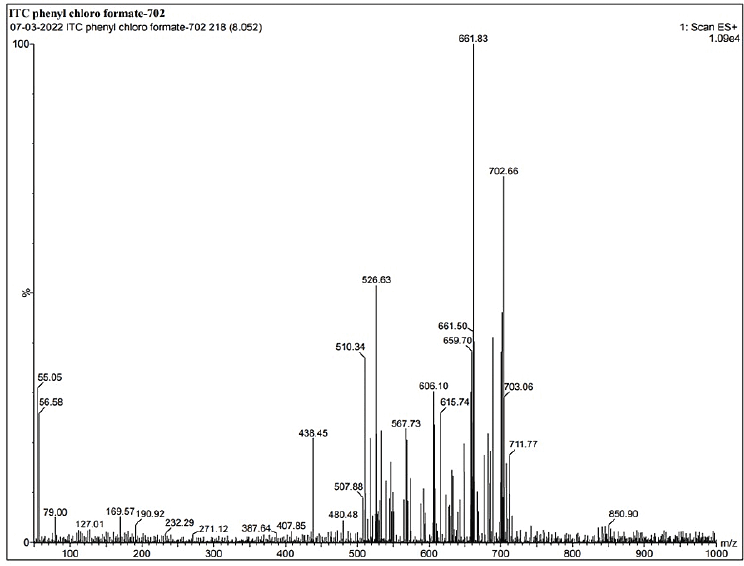 | Supplementary Figure S12. LCMS data of ITZ-2. [Click here to view] |
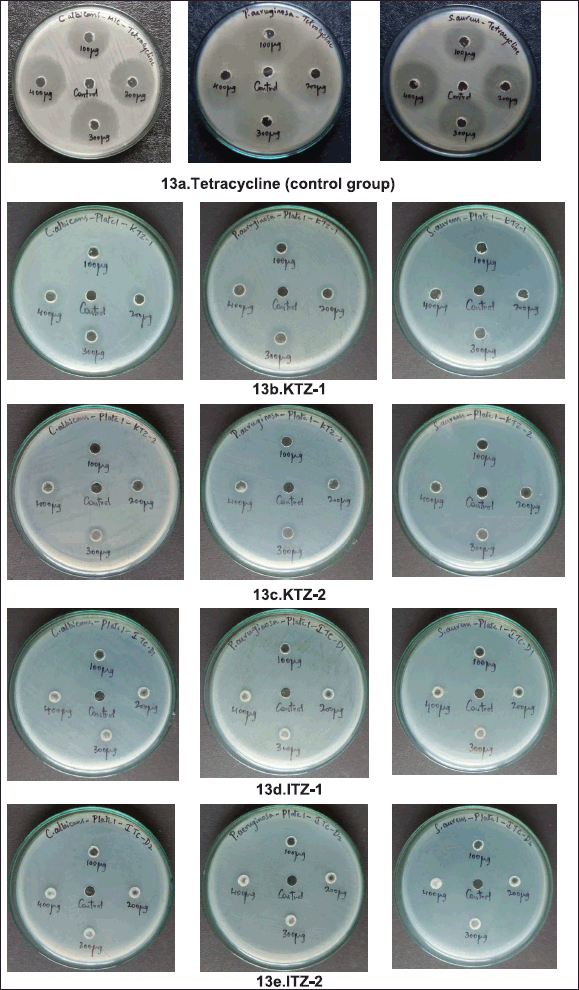 | Supplementary Figure S13. MIC plate images of (a) tetracycline (control group), (b) KTZ-1, (c) KTZ-2, (d) ITZ-1, and (e) ITZ-2 against pathogens. [Click here to view] |