INTRODUCTION
The discovery of carbon nanotubes (CNTs) in the last decade of the 20th century has generated great interest in a wide range of engineering and industrial applications, including biomaterials and nanomedicine (Jha et al., 2020; Simon et al., 2019). Targeted delivery of drugs to specific organs and tissues and subsequent control of drug release from the carrier system are promising approaches of modern nanomedicine. Already today, nanoparticle surface engineering makes it possible to assemble such systems from molecular building blocks, which include CNTs, using feasible synthetic approaches (Cheng et al., 2018).
The CNTs structure is represented as a graphene honeycomb lattice layer rolled into a long cylinder with a hemispherical “cap” at each end, thought to be attractive to use as a “container” for targeted drug delivery systems. Such a CNT-based container has a significant internal volume for loading pharmaceutically active substances, a large surface area that allows conjugation with various biological molecules, and the ability to penetrate cell membranes (Jha et al., 2020; Raval et al., 2018).
The consideration of CNTs as a core component of drug delivery systems is related to their basic properties such as inertness and chemical stability, high aspect ratio, mechanical rigidity, and ability to encapsulate both hydrophobic and hydrophilic substances. The latter makes it possible to protect drugs from enzymatic degradation, i.e., to enhance the stability of the carried drug and to provide site-specific delivery (Niezabitowska et al., 2018). On the other hand, the high volume-to-surface ratio and huge surface area, which is considered to be an advantage, turn into the cause of xenobiotic toxicity of CNTs due to their tendency to aggregate with themselves and surrounding biomolecules (Li and Cao, 2018; Zhou et al., 2017).
Taking into account the extensive use of CNTs in a wide range of industries and the increasing human exposure to it, the safety and toxicity issues of CNTs have been and continue to be investigated. To date, a significant number of observations of the toxic effects of exposure to CNTs (most often pulmonary) have been accumulated. In many cases, it has been proven that exposure to CNTs was a pathogenic factor in the development of severe disease (Ema et al., 2016; Lanone et al., 2013). CNT toxicokinetics has been studied by many researchers using various cell, tissue, and animal models and sophisticated methodology for modeling human disease states to demonstrate the pathogenic effect of the accumulation of CNTs (Ong et al., 2016; Swidwinska-Gajewska and Czerczak, 2017).
Since the size and geometry of multiwalled CNTs (MWCNTs) are close to asbestos fibers, similar mechanisms of CNTs cytotoxicity have been proposed, including oxidative stress followed by inflammation. It has been shown that these toxic effects can be mitigated by reducing the effective length of asbestos-like long pristine MWCNTs (Ali-Boucetta et al., 2013). Oxidative stress increases the intracellular production of reactive oxygen species (ROS) and subsequently promotes the depletion of antioxidant reserves, which leads to the activation of the proinflammatory signaling cascade. This sequence of processes is considered the general mechanism of the reaction of organisms to various cellular damaging factors, including the effect of CNTs (Møller et al., 2014).
Understanding the development of CNT-induced immune responses, involving a number of cellular and humoral factors, is important for predicting the potential damage to certain tissues caused by inflammation, as well as for developing a therapeutic strategy for relevant human diseases (Dong, 2020).
In addition to activating proinflammatory signaling, the excessive production of ROS due to exposure to CNTs leads to damage of genetic material, oxidation of proteins and amino acids, and inactivation of enzymes. Together, these deleterious effects lead to apoptosis. It is not surprising that one of the promising therapeutic strategies in oncology is based on the apoptosis of tumor cells caused by the introduction of CNTs (Mohanta et al., 2019; Sheikhpour et al., 2020).
The results of numerous studies on the effects of CNTs on experimental animals have demonstrated that the manifestation of toxic effects is determined by a wide range of their physicochemical characteristics, which together form such general properties as biocompatibility, biodistribution, stability, and clearance of CNTs (Aoki and Saito, 2020; Galassi et al., 2020).
To a certain extent, these properties determining the interaction between CNTs and living organisms depend on the functionalization of the CNTs surface. The introduction of specific functional groups or chemical radicals for the formation of side chains by covalent bonding or attachment of various molecules through physical adsorption (noncovalent bonding) and electrostatic forces to CNTs makes it possible to increase their solubility and hydrophilicity, therefore making CNTs more biocompatible in order to improve their biodegradability and clearance. The functionalization of the CNTs surface is considered an effective tool for weakening the aggregation of lipophilic CNTs and the formation of agglomerates in the aqueous medium of living organisms to reduce their toxic effects (Ali et al., 2015; Huang, 2020). When assembling CNT-based systems for targeted drug delivery, surface functionalization makes it possible to load low-molecular-weight drugs, binding proteins, immunoglobulins, DNA fragments, or other parts of such supramolecular complexes (Caoduro et al., 2017; Kaufmann et al., 2017).
Extensive studies on the toxicity of CNTs that were carried out in hundreds of laboratories over the past 20 years have yielded a wealth of valuable data and allowed the development of several concepts of the mechanisms of CNTs’ pathogenic action. In general, it was shown that the toxicity of CNTs depends on structural and physicochemical factors, such as the method of CNTs’ synthesis, their type, shape, length, and diameter, surface characteristics and tendency to form agglomerates, the presence of certain functional groups, and impurities content. The aforementioned characteristics of CNTs can be accurately measured using sophisticated analytical and visualization techniques but cannot be reliably extrapolated to more complex properties such as biocompatibility or interaction with specific cell types. It is much more difficult to predict the reactivity of CNTs with animal organisms; therefore, generalizing conclusions about the toxicity of specific CNTs fabricated for a definite application may be elusive and require experimental research (Huang, 2020; Jha et al., 2020; Simon et al., 2019).
We faced the problem of the toxic effects of CNTs while participating in a project to develop a supramolecular nanocomplex for targeted delivery of doxorubicin to solid tumors. The starting point for our team was simulating a “container” consisting of MWCNTs, loading it with doxorubicin, and sequentially releasing this chemical entity in a controlled manner using infrared heating. The next step after obtaining a series of samples of the original MWCNTs with appropriate loading/release characteristics was to evaluate the toxic effects of the optimal samples, which were chosen as a potential vehicle for targeted delivery of doxorubicin for further work. An aqueous dispersion of the fabricated MWCNTs was chosen as an appropriate form of the targeted drug delivery system to allocate in the vascular system. Dispersions of CNTs in water are extensively used in biomedical research (Alshehri et al., 2016; Broz and Dixit, 2016; Kobayashi et al., 2017; Mohanta et al., 2019; Xu et al., 2016). To stabilize an aqueous dispersion of MWCNTs, two widely used and practically effective methods are applied: the addition of a nonionic surfactant and mechanical dispersion by sonication (Zaib and Ahmad, 2019). Taking into account the intravenous route of administration of the developed nanocomplex for targeted drug delivery and the intention to study its distribution through the circulatory system, a rat model was chosen for toxicological research.
The protocol of short-term toxicity assessment was chosen as the approach that allows obtaining complex data both on the general response of the animal organisms to xenobiotics and on the appearance of structural and functional changes in specific organs and tissues due to the administration of a potentially toxic agent. We assumed that relatively short (14 days) exposure would be sufficient to induce at least microstructural changes in the experimental animals. In addition, daily administration for 2 weeks is significantly more frequent than the recommended dosage regimen of doxorubicin (one injection per week or one injection every 3 weeks). As far as the purpose of the toxicological fragment of the mentioned project was to obtain a principal assessment of general toxic effects of intravascularly administered MWCNTs aqueous dispersion, which was chosen by optical properties, we did not investigate toxicokinetics during the postexposure period as it is recommended in Organization for Economic Co-operation and Development (OECD) guidelines.
MATERIALS AND METHODS
Test agent
Fabrication of MWCNTs
To study the short-term toxicity of the CNT-based vehicle for targeted delivery of doxorubicin, we used a highly purified fraction of multiwalled nanotubes, kindly provided by the Chu?ko Institute of Surface Chemistry of the National Academy of Sciences of Ukraine and LLC TM Spetsmash (Kyiv, Ukraine). A series of MWCNTs samples were synthesized by catalytic pyrolysis of unsaturated hydrocarbons at the pilot production set-up developed in the mentioned institutions. The chemical vapor deposition (CVD) method with iron oxide and nickel-based three-active-component catalysts was used to synthesize MWCNTs (Kartel et al., 2016; Kovalska and Sementsov, 2013; Sementsov and Kartel, 2019). The advantages of the CVD method are associated with low growth temperature, high efficiency, and high purity of the produced nanotubes (Nguyen et al., 2019).
The transmission electron microscopy (TEM) images of the MWCNTs produced by the catalytic CVD synthesis are shown in Figure 1. The JEOL JEM-100CX II (Japan), a tungsten-filament 100 kV TEMn with a 2.04 Å resolution, was used for observations. The multiwalled structure of nanotubes with a hollow internal channel is clearly visible in each image, especially at high magnification (lower fragments). In less magnified images (the upper fragments of Fig. 1), note the densely stained hollow dots located preferably at the tip position with a size approximately equal to the internal diameter of the bound nanotubes, which are the metal particles of the catalyst that provides the growth of MWCNTs.
Determination of the parameters of MWCNTs was conducted by a group of specialists from Chu?ko Institute of Surface Chemistry of the National Academy of Sciences of Ukraine and LLC TM Spetsmash (Kyiv, Ukraine). Dimensions and size distribution function of the MWCNTs fabricated in these institutions were measured employing photon correlation spectroscopy by means of the ZetaSizer-3 spectrometer (Malvern Instruments, UK) with a correlator 7032 and 25 MW helium-neon laser LH-111 (Newport Corp., USA) following standard operating procedures of the manufacturer lab (Sementsov and Kartel, 2019). The manufacturer provided the following parameters of nanotubes in the specification: the inner and outer diameters of nanotubes were 1–2 and 10–40 nm, respectively, the ash content was less than 0.4%, the specific surface area determined by the argon desorption method was 200–400 m2/g, the bulk density varied within 20–40 g/dm3, and the MWCNTs content in the product exceeded 95%.
Preparation of MWCNTs dispersion
MWCNTs in the form of a stable water dispersion were injected into the lateral tail vein of the experimental rats. To prepare the aqueous dispersion, the surfactant Tween 80 was used for the noncovalent functionalization of MWCNTs. The choice of Tween 80 (a hydrophilic nonionic detergent) among other nanotube solubilization methods was based on its widespread use as an ingredient in injectable medications for the solubilization of large molecules such as proteins. Tween 80 is often used for the dispersion and solubilization of nonfunctionalized CNTs (Fatemi and Foroutan, 2016). The following procedure was applied for the extemporal preparation of the dispersion: 2.4 mg of purified MWCNTs (exact weight) was taken into a 100 ml volumetric flask. An isotonic saline solution containing 0.25% of Tween 80 was added up to 100 ml. The flask was kept in a sonic bath for 10 minutes. The resulting dispersion was viewed in the transmitted light. Perceptible solid particles should not be present.
Monitoring based on UV-visible spectrophotometry was applied to assess the quality and stability of aqueous MWCNTs dispersions. This method, which provides fast and accurate measurements, has been chosen as a powerful nondestructive characterization tool for CNTs often used to measure dispersion, purity, and functionalization (Njuguna et al., 2015). The optical density and absorbance of a set of experimental compositions with a gradually increasing content of MWCNTs were measured following Chapter 2.2.25 of the European Pharmacopoeia (10th edition) at wavelengths of maximum absorption of 425 nm. The results of optical density measurements for nine compositions and calculated values of specific optical absorbance are presented in Table 1. Proportional elevation of MWCNTs content in the dispersion led to a commensurate rise in the optical density, whereas the values of the specific optical absorbance were practically the same. It is well known that specific optical absorbance for stable dispersive systems is a constant value since changes in optical density due to changes in the content of colloid particles do not lead to changes in the value of specific absorbance (A 1%, 1 cm). The specific optical absorbance will change over time due to the condensation processes for unstable dispersive systems (Hohl et al., 2016).
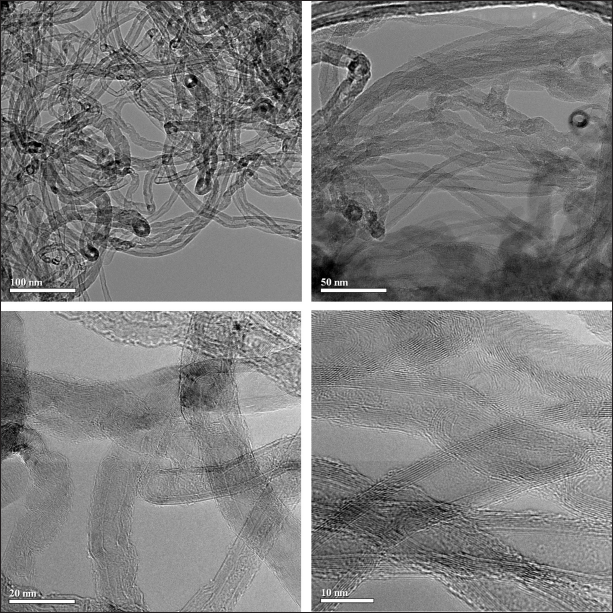 | Figure 1. TEM images of the MWCNTs produced by CVD method with nickel-based three-component catalyst (Al-Ni-Mo). [Click here to view] |
To characterize the stability of a surfactant-solubilized and sonicated aqueous dispersion of MWCNTs, the specific optical absorbance (A 1%, 1 cm) of the freshly prepared dispersion with a certain content of nanotubes was measured 1 hour after preparation and 48 hours after storage in a refrigerator at +4°C. Forty-eight-hour stability was considered enough for MWCNT-based drug delivery vehicles elaborated for extemporal preparation and administration. Paired comparison of the mean values of specific optical absorbance of nine freshly prepared and 48 hours stored aqueous MWCNTs dispersions revealed noticeable differences in light absorption only for Composition 1, containing the minimal amount of nanotubes—0.0032 mg/ml (Table 1). After 48 hours of storage in a refrigerator, the mean optical density of Composition 1 proved to be 0.0179 higher than the initial value; a related increase in specific optical absorbance constituted 8.49%. Although these differences did not reach the statistical significance level (p < 0.05), the tendency toward the minor rise of optical density and specific optical absorbance due to the low-temperature storage of MWCNTs dispersions was most pronounced in the diluted composition. This tendency got gradually less pronounced but was still seen in up to Composition 7, containing 0.028 mg/ml of nanotubes, but changed oppositely for aqueous MWCNTs dispersions with higher nanotubes content of 0.032 and 0.040 mg/ml (Compositions 8 and 9). The mechanism of such behavior of a surfactant-solubilized and sonicated aqueous dispersion of MWCNTs is not clear; it can be speculated that the reason is related to the achievement of the thermodynamic equilibrium of a multicomponent dispersion system in response to a change in temperature and the direction of the colloid system response is dependent on the nanotubes content and ratio of Tween 80 and MWCNTs content (? = ?Tw80 / ?CNTs, as shown in Table 1). Considering the inherent variability of living organisms, postulated at least 5%, the small statistically insignificant shifts in optical density and specific optical absorbance of aqueous MWCNTs dispersions that occurred due to 48 hours storage at +4°C may be neglected. From a practical point of view, the results of paired comparison of the mean values of optical density and specific optical absorbance of a series of MWCNTs compositions before and after 48 hours storage in a refrigerator showed no signs of colloid particles condensation and acceptable stability of all investigated dispersions, excluding diluted Composition 1. Of the nine compositions studied, Composition 6, containing 0.024 mg/ml of MWCNTs dissolved in a 0.25% Tween 80 saline solution, was chosen to evaluate the short-term toxicity in rats, considering minimal changes in spectrophotometric parameters after storage at a low temperature and consequently better colloidal stability of the dispersed MWCNTs in an aqueous solution (Table 1).
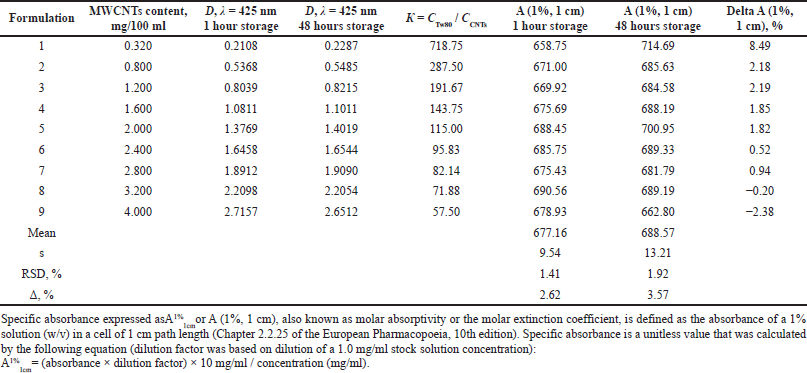 | Table 1. Optical density and specific optical absorbance of a series of experimental compositions of aqueous dispersions of MWCNTs with a gradual increase in the content of nanotubes. [Click here to view] |
A graphical representation of the relationship between the optical absorption of Composition 6 and wavelength is shown in Figure 2. The visual absorption curve did not demonstrate pronounced maxima or minima, which corresponds to the literature data on the UV-Vis spectra of different MWCNTs suspensions with various surfactant solutions (Njuguna et al., 2015).
Animals
The experiment was carried out in accordance with the recommendations of Chronic Toxicity Studies (OECD Guidelines for the Testing of Chemicals, 2009). Outbred Wistar rats were used for the study. The rats were kept under uniform conditions; standard chow and water were provided ad libitum.
Rats were maintained in autoclaved polycarbonate cages (EHRET, Germany), five animals of the same sex in each cage. The environmental temperature was maintained at 22°C–24°C interval and relative humidity at the level of the rat cages varied within 55%–60%. A regular 12/12-hour light/dark cycle was kept in the animals’ room. Standard granulated rodent chow (Phoenix LLC, Kyiv, Ukraine) was used for animal feeding. The quality of drinking water met the state standards of potable water. Dietary and water intakes were recorded daily.
Experiments were performed using 40 adult rats of both sexes (20 males and 20 females weighing 180–200 g) purchased from the Veterinary Clinics of the Institute of Pharmacology and Toxicology (Kyiv, Ukraine). After transportation, animals were allowed to acclimate to the conditions and diet in the lab’s vivarium for 2 weeks. Housing and management of the animals, as well as all experimental procedures, were performed in accordance with the Guide for Care and Use of Laboratory Animals (NRC, 1996) and Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes.
Animal groups and administration of chemical compounds
After 14 days of acclimatization, the rats were weighed and randomly assigned into two groups. Ten male and 10 female rats of the first (control) group received the dispersant: an isotonic saline solution (0.9%) prepared in a 0.25% Tween 80 solution. Animals of the second (dose) group (10 males and 10 females) received the aqueous dispersion of MWCNTs prepared as described above (see Paragraph 2.1). The dispersant solution and the aqueous dispersion of MWCNTs were prepared every second day of the experiment using the pyrogen-free sterile saline solution for injection (YURiA-PHARM LLC, Ukraine).
Each solution was injected into the tail vein at the same volume of 1.0 ml per animal (MWCNTs dispersion to the rats of the dose group and the dispersant solution to the parallel control) for 14 consecutive days in the morning (from 8:00 to 10:00). Before administration, both solutions were kept in a water bath at 38°C for 15 minutes. The single dose of purified MWCNTs that was administered daily to each animal of the dose group was 0.024 mg, and the cumulative dose for 14 days reached 0.336 mg/rat. The dose range was selected referring to the published data on acute effects developed after intravenous administration of a single 0.3 mg/rat (Hosseinpour et al., 2016) and multiple low doses (0.25, 0.5, and 0.75 mg/kg) of MWCNTs (Adedara et al., 2018). Low MWCNTs doses were chosen due to our aspiration to analyze the deposition and accumulation of nanotubes following repeated administration, which corresponds to doxorubicin chemotherapy protocols.
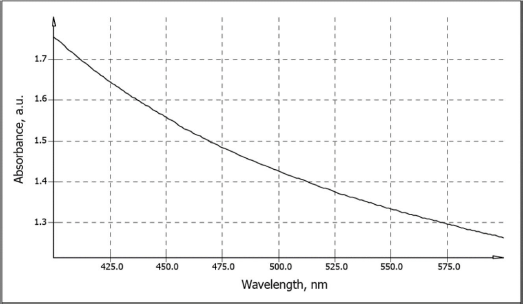 | Figure 2. Optical absorption of experimental Composition 6 (0.024 mg/ml MWCNTs in 0.25% Tween 80 saline solution) used for intravenous administration in rats to study short-term toxicity. [Click here to view] |
Clinical observations and lab tests
The rats were observed twice daily to detect uncharacteristic behavior, signs of pain, distress, experimental animals’ discomfort, and other toxicity manifestations. By visual inspection, the state of the skin, mucosa, eyes, respiratory frequencies, nasal secretions, salivation, tremors, convulsions, changes in activity levels, posture, gait, and sensory responses to stimuli was checked. Food and water consumption was measured daily, and the weights of the rats were assessed once a week. The next day after the last intravenous administration of the test solutions (15th day of the experiment), venous blood samples were taken from the tail veins of the control and experimental rats for hematological and coagulation tests. Blood samples for biochemical analysis were collected just after carbon dioxide euthanasia, followed by decapitation; 1 ml of blood was collected at the decapitation site. To perform hematological analysis, a semiautomatic analyzer Alcon 871 (AL Systems, Germany) was used to measure the number of erythrocytes, leukocytes, and platelets, the concentration of hemoglobin, hematocrit, the average volume of red blood cells, the average volume of platelets, the average hemoglobin content, and the average concentration of hemoglobin in the erythrocyte. The leukocyte formula was calculated in blood smears stained according to Pappenheim. To assess the state of coagulation hemostasis, prothrombin time (PT) and activated partial thromboplastin time (APTT) were measured using a Helena C-2 semiautomatic coagulometer (Helena Biosciences Europe, UK). For clinical chemistry, an Ra50 semiautomatic biochemical analyzer (Bayer-Siemens, Germany) was used to determine the following parameters: activity of alanine aminotransferase and aspartate aminotransferase (Reitman-Frankel method), alkaline phosphatase activity (kinetic method), total protein concentration (biuret method), glucose (glucose oxidase method), concentration of urea (glutamate dehydrogenase urease method), total cholesterol concentration (enzymatic colorimetric method), triglycerides (Trinder enzymatic method), concentration of creatinine (Jaffe method), total bilirubin (Jendrassik and Gróf method), and albumin (colorimetric method). The concentration of the globulins was calculated by subtraction from the total protein amounts the concentration of the albumin. These data were used to calculate the albumin/globulin ratio (A/G). The aforementioned determinations were carried out using kits from Biosystems S.A. (Spain) and Erba Lachema s.r.o. (Czech Republic).
Final evaluation and histopathological examination
The final evaluation of the rats was carried out on the 15th day of the experiment. Just before euthanasia, samples of venous blood were taken from the tail veins of the control and experimental rats for hematological and biochemical studies. The animals were sacrificed in a CO2 gas chamber and then subjected to a total autopsy; the pathological findings were recorded. The brain, lungs, heart, liver, kidneys, spleen, adrenals, thymus, and testes with epididymis in the males and ovaries in the females were weighed, and their relative weights were calculated (i.e., the % of body weight). Tissues and organs were examined histologically. Samples for the histopathological examinations were taken from the heart, liver, pancreas, stomach, esophagus, intestine, kidneys, adrenals, brain (three parts and membranes), spleen, thymus, thyroid, salivary glands and lymph nodes, trachea, aorta, testicles, ovaries, and uterus. Parts of the organs were fixed in a 10% solution of buffered formalin, dehydrated with alcohol, and embedded in paraffin. Sections 5 mcm thick were cut from paraffin blocks of the embedded organs using a sledge microtome. The sections were stained with hematoxylin-eosin, sealed in celloidin, and then observed under an optical microscope equipped with a micrograph system. The microscopic features of the organs of the experimental rats were compared with those of the control group.
Statistical analysis
All numerical data (body weight gain, relative organ weights, food and water consumption, and hematological and clinical chemistry values) are expressed as mean ± SEM. Comparisons between groups were performed using a one-way analysis of variance (ANOVA). P value of < 0.05 was considered significant.
RESULTS
Clinical signs, consumption of food and water
The administration of the aqueous dispersion of MWCNTs did not cause mortality in any rats. No clinical signs of adverse effects associated with toxicity were observed in the rats that were exposed to repeated intravenous MWCNTs administration during 14 consecutive days. Exposure to MWCNTs did not have a noticeable effect on the general condition and behavior patterns of the experimental rats. Physical observations indicated no signs of changes in the skin, fur, and visible mucous membranes.
The rats receiving the aqueous MWCNTs dispersion responded normally to external stimuli. There were no changes in posture, muscle tone, motor behavior, and coordination of movements. Tremors, fasciculation, convulsions, and seizures were not noticed either. Ophthalmic sensitivity to light was normal. Salivation conformed to the physiological norm. Animals did not develop diarrhea. The consistency and color of stool and urine in the rats of the experimental and control groups did not differ. Food and water consumption corresponded to the age norms established for rats; no statistically significant differences (p < 0.05) were found between the experimental and control rats.
Body and organ weights
The average body weight gains of the male and female rats subjected to repeated intravenous administration of the aqueous dispersion of MWCNTs for 14 days and the animals of the control group are shown in Figure 3. Compared to the control rats, exposure to MWCNTs did not lead to changes in the dynamics of body weight gain in the experimental rats. Differences in the mean body weight values between the male and female rats that received MWCNTs intravenously and the parallel control were minor and did not reach the statistically significant level (p < 0.05).
The mean values of relative weights of the brain, lungs, heart, liver, kidneys, spleen, adrenals, thymus, and testes with epididymis in the males and ovaries in the females of the experimental and control rats are presented in Table 2. Statistically significant differences between the relative weights of all studied organs were not found except for the liver. Intravenous administration of the aqueous MWCNTs dispersion for 14 days led to a noticeable increase in the value of this index in both the male and female rats of the dose group as compared with the parallel control. In the dose group, the mean values of relative liver weight were 33.1% higher in the male and 21.0% in the female rats than the control rats. A statistically significant (p < 0.05) increase in relative liver weight in the experimental rats of both sexes was considered a sensitive indicator of possible liver hypertrophy due to the toxic effect of repeated intravenous administration of the aqueous MWCNTs dispersion (Table 2).
Also of note is the marked increase in mean relative lung weight in the male rats of the dose group; the indicator was higher by 23.4% than the male control rats. Although the difference did not reach statistical significance (p > 0.05) due to the high variability of individual parameters, this finding deserves attention, given the specific anatomical position of the pulmonary blood vessels in the circulatory system, as the first vast capillary bed network which receives intravenously administered materials or chemical compounds (Table 2). This observation correlates with the data of a macroscopic examination of the lungs during the macropathology procedure performed after the end of the administration of the aqueous dispersion of MWCNTs. Visual examination revealed a light gray shade of the surface of the lungs with scarcely spread black specks and spots. Surprisingly, in the female rats of the dose group, the macroscopic picture of the lungs was the same as in the males, while the mean value of the relative lung weight proved to be 11.5% lower than the parallel control (p > 0.05); the difference did not reach statistical significance (Table 2).
Similarly directed changes in the mean values of the relative weights of the heart and adrenal glands in the male and female rats of the dose group should be mentioned as well. The relative heart weights were 11.5% and 1.2% lower and relative adrenals weights were 8.8% and 11.1% lower in the male and female rats of the dose group, respectively, than the control rats (Table 2).
Considering the cumulative dose of MWCNTs administered intravenously during the 14-day experiment, which equaled 0.336 mg/rat with a body weight of about 200 g, direct aggregation or accumulation of nanotubes in any of the organs studied could not lead to the growth and fall of relative organs weight. A comparative histopathological study was performed to evaluate the reasons for these findings.
Hematological parameters
Venous blood samples for hematological and clinical chemistry testing were taken from the tail veins of the control rats treated with the dispersant (0.25% Tween 80 saline solution) and experimental rats subjected to intravenous administration of the aqueous MWCNTs dispersion for 14 consecutive days at the next day after the last injection. The mean values of the basic hematological parameters of the experimental and control rats, as well as the results of their comparative analysis, are presented in Table 3.
Red blood cell indices
Fourteen-day exposure to MWCNTs did not lead to noticeable changes in key cellular indexes of erythrocytes like mean cell volume (MCV) characterizing the average size of erythrocytes and the average quantity [mean corpuscular hemoglobin (MCH)] and concentration [mean corpuscular hemoglobin concentration (MCHC)] of hemoglobin present in a single red blood cell. Comparison of red blood cell characteristics in the control and experimental groups rats revealed minimal elevation of mean values of all these indexes as in the male and female rats subjected to intravenous injections of the MWCNTs dispersion. Although the elevation of the mentioned erythrocytes’ indexes in the dose group relative to the control varied from 0.7% to 2.5% and was statistically insignificant (p > 0.05), the similar direction of changes in the male and female rats should be mentioned (Table 3). The absence of noticeable changes in the mean volume of erythrocytes and the mean hemoglobin content in cells indicates the lack or minimal effect of MWCNTs intravascular exposure on erythrocyte hemopoiesis.
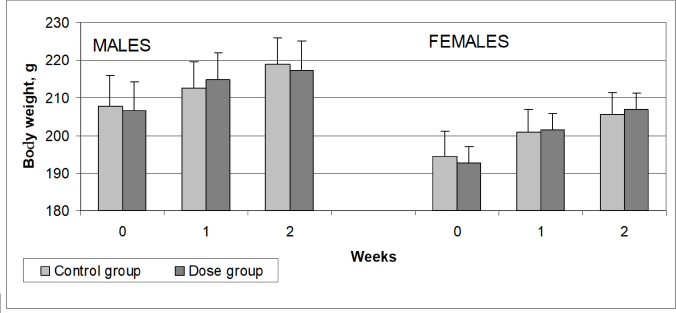 | Figure 3. The mean body weight of rats that received aqueous dispersion of MWCNTs intravenously for 14 days and animals of the control group that received carrier solution. Values are expressed as mean ± SEM (10 males and 10 females in each group). [Click here to view] |
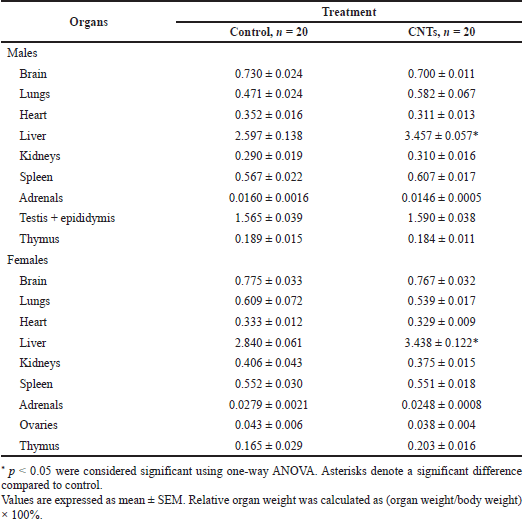 | Table 2. Relative organ weights of rats receiving repeated doses of CNTs for 14 days in comparison with control rats receiving intravenous injections of the dispersant solution. [Click here to view] |
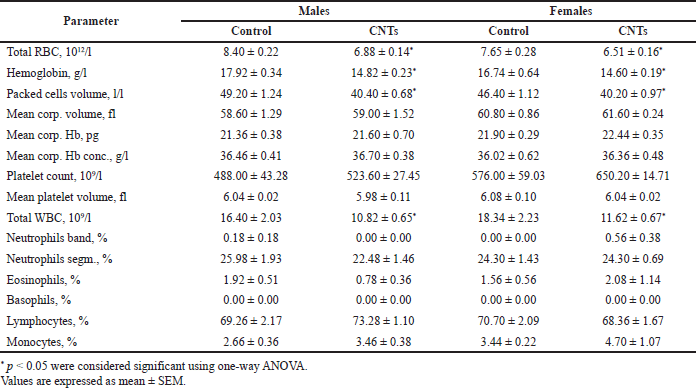 | Table 3. The effects of 14-days CNTs administration on hematological parameters of experimental rats in comparison with the control animals receiving the dispersant solution. [Click here to view] |
In contrast to minimal changes in the mean values of the cellular indexes of red blood cells, the parameters related to erythrocytes count (RBC, Hct, and hemoglobin content) changed considerably. The pattern of changes in these hematological parameters in the male and female rats of the dose group was quite similar. Compared with the parallel control, the total number of erythrocytes decreased in males by 18.0% and in females by 14.8%, the hemoglobin content in the blood decreased by 17.3% and 12.8% in males and females, respectively, and the hematocrit decreased in males by 17.9% and in females by 13.4%; all these differences reached the level of statistical significance (p < 0.05). Comparable percentage parallel decreases in RBC, Hct, and Hb by 18.1%, 17.9%, and 17.3% in the male and 14.9%, 13.4%, and 12.8% in the female rats, respectively, can be explained by the loss of red blood cells in the result of MWCNTs intravascular administration (Table 3).
Reduction of the total red blood cell count probably can occur due to the direct effect of MWCNTs on erythrocytes in the vascular bed, which leads to damage and removal of less resistant cells. Evaluation of this assumption’s correctness is beyond this study’s scope; it is only a possible explanation for a parallel decrease in the total number of erythrocytes, blood hemoglobin, and hematocrit without noticeable changes in the parameters characterizing the cellular properties of erythrocytes (Table 3).
White blood cell indices
A statistically significant decrease in the total white blood cell count after 14 days intravenous administration of the aqueous dispersion of MWCNTs was characteristic of the rats of the dose group. Compared with the control, the number of leukocytes in the male rats decreased by 34.0% (p < 0.05) and in the female rats by 36.6% (p <0.05). The mean values of total leukocyte count dropped equally in the male and female rats of the dose group, but the leukogram changes were different. Compared with the control, the male rats of the dose group demonstrated a relative decrease in the number of granulocytes (neutrophilic by 13.5% and eosinophilic by 59.4%), as well as an increase in the number of lymphocytes by 5.8% and monocytes by 5.8%. In contrast, no such effects were observed in the female rats, except for an increase in the number of monocytes by 36.6%. Despite significant shifts in the leukogram pattern, these differences in the number of leukocyte subpopulations were not statistically significant (p > 0.05) due to the high variability of individual values (Table 3).
In general, the reaction of the leukocyte component of the blood to repeated intravenous injections of the MWCNTs dispersion can be characterized as moderate leukopenia due to a reduction in the blood neutrophil count and monocytosis. Neutropenia and agranulocytosis are well-known toxic effects of xenobiotics, including a wide range of medications like anticancer drugs, antibiotics, psychotropic agents, etc. Mechanisms include direct toxic effects upon the bone marrow and the formation of antibodies against hematopoietic precursors or involve peripheral destruction of cells. Given the role of monocytes as effectors of pathogen ingestion, antigen-presenting, and cytokine-secreting cells, monocytosis can be explained as a response to inflammatory processes initiated in tissues interacting with MWCNTs.
Thrombocytes
In the rats of the dose group, a slight increase in the content of platelets in the peripheral blood was found; the rise in platelet counts in the males was 7.3% (p > 0.05) and in the females 12.9% (p > 0.05) compared with the control and was not associated with changes in the volume of platelets (Table 3). The absence of changes in the mean volume of platelets in the rats of the dose group indicates the absence of an effect of 14-day exposure to MWCNTs on the processes of platelet formation in the bone marrow since young platelets are characterized by a large volume.
Summing up the review of hematological parameters, we note the uncertainty in interpreting the results obtained as hematological toxicity or as an unspecific functional response to exogenic material introduced inside the organisms of the experimental rats. From one side, we saw statistically significant but light or moderate shifts in hematological parameters, characterizing the cellular component of the blood, which did not exceed the physiological range and represented part of a systemic response to the exogenic impact. Alternatively, such shifts in hematological parameters can be considered the initial stage of the inflammatory process with severe consequences. To be sure about hematological toxicity, at the next stage of the project, we plan to evaluate dependence on the dose of MWCNTs and recovery dynamics after the end of the exposure.
Hemostasis and biochemical analysis
This section presents the results of the laboratory evaluation of blood samples collected the next day after the end of the 14-day intravenous administration of the aqueous dispersion of MWCNTs to the rats of the dose group and the dispersant (0.25% Tween 80 saline solution) to the rats of the control group. Blood samples were taken immediately after carbon dioxide euthanasia, followed by decapitation; 1 ml of blood was collected at the decapitation site.
Coagulation testing
The mean values of PT in the male and female rats of the control group were 13.40 ± 0.92 and 12.71 ± 1.12 seconds, and those of APTT were 43.82 ± 2.34 and 40.76 ± 2.44 seconds, respectively. In the rats of the dose group, the mean values of these indicators were as follows: PT in males 11.68 ± 1.18 seconds and females 14.37 ± 1.46 seconds and APTT in males 58.14 ± 3.07 seconds and females 51.32 ± 3.43 seconds. The mean values of PT and APTT proved to be slightly different in the rats treated with the aqueous dispersion of MWCNTs and control rats subjected to intravascular administration of the vehicle solution; the differences did not reach statistical significance (p > 0.05). These results provide evidence that key indicators characterizing the integrity and performance of the extrinsic (PT) and intrinsic (APTT) pathways of blood coagulation systems did not differ significantly in the MWCNT-treated rats from the parallel control, except the tendency toward a slight decrease in PT in the male rats and some elevation of APTT as in the males and females of the dose group. The APTT elevation tendency deserves attention as an indirect sign of possible hepatic injury and related impairments in blood coagulation factors production.
Blood biochemistry testing
The basic biochemical parameters of the experimental rats receiving the aqueous dispersion of MWCNTs and control rats receiving the dispersant solution are presented in Table 4. The mean values of total protein content in the plasma of the male and female rats of the dose group were lower than in the control animals: in males by 7.1% (p > 0.05) and in females by 9.9% (p > 0.05). The fraction analysis revealed that the albumin fraction changed slightly; in contrast, the globulin fraction dropped by 13.1% in the males (p < 0.05) and by 19.2% in the females (p > 0.05); the differences did not reach the level of statistical significance due to high variability of individual values in the female rats of the dose group. Accordingly, the value of the albumin-globulin ratio was 11%–12% higher than in the parallel control; the differences compared to control rats were statistically significant in the males (p < 0.05) but insignificant in the females (p > 0.05) due to outliers in primary data (Table 4). Of all the possible reasons for the decrease in the globulin fraction, inflammation and activation of the immune system seem to be the most likely.
Fourteen-day intravascular exposure to MWCNTs dispersion led to unidirectional changes in the main indicators of the functional state of the liver, such as alanine transaminase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (AP); the activity of all these enzymes in the blood serum of the experimental rats increased to varying degrees in relation to the parallel control (Table 4). In the male rats of the dose group, the ALT, AST, and ALP activities proved to be higher by 53.6% (p < 0.05), 6.9% (p > 0.05), and 33.3% (p > 0.05) than the male rats of the control group. Compared with the control group, in the female rats subjected to repeated intravenous injections of the MWCNTs dispersion, the activities of ALT, AST, and ALP were also increased by 22.9% (p > 0.05), 34.9% (p < 0.05), and 21.3 % (p > 0.05), respectively (Table 4).
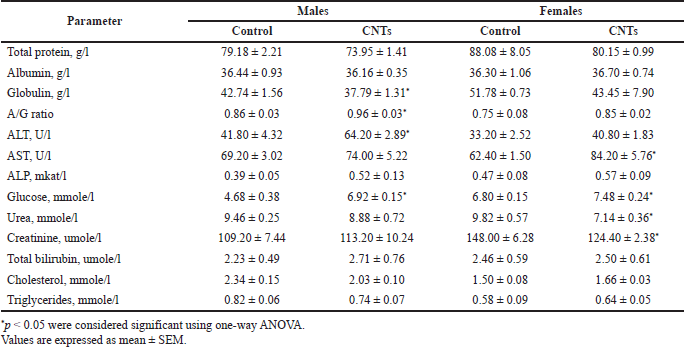 | Table 4. The effects of 14-days CNTs administration on clinical biochemistry parameters of experimental rats in comparison with the control animals receiving the dispersant solution. [Click here to view] |
Parallel and proportional elevation of cytosolic and mitochondrial enzymes housed in high concentrations in the liver provides evidence of possible hepatocellular injury followed by the release of these enzymes into circulation. On the other hand, drug-induced liver damage is associated with a significant (three to five times) increase in the activity of aminotransferases; therefore, a slight increase in the activity of ALT, AST, and ALP can be regarded as a temporary functional change. As liver damage is suspected, it is important to evaluate glycemic status, serum albumin concentration, bilirubin levels, cholesterol, and triglycerides concentrations as indicators of liver function in carbohydrate and lipid metabolism, plasma protein synthesis, and hemoglobin (heme) catabolism. There were no marked differences between the experimental and control rats in serum albumin and bilirubin levels as well as in cholesterol and triglycerides concentrations. In contrast, blood glucose concentration was considerably higher in the dose group rats than in the control rats. The mean values of glucose concentrations in the blood serum of the male and female rats subjected to intravascular exposure to MWCNTs were 47.9% (p < 0.05) and 10.0% (p < 0.05) higher than the control rats receiving the vehicle solution (Table 4).
The study of renal function (the level of urea and creatinine in the blood serum) in the experimental and control rats revealed contradictory and somewhat unexpected changes. The mean values of creatinine content, a commonly used marker of glomerular function, proved to be quite the same in the male rats receiving the MWCNTs dispersion and vehicle solution; in contrast, in the females of the dose group, this indicator was 15.9% (p < 0.05) lower than in the parallel control (Table 4). The same paradoxical effect was noticed in the female rats of the dose group for blood urea content, which was decreased relative to control rats by 23.7% (p < 0.05).
Thus, the changes in the biochemical indicators characterizing the state of the main functional systems of the organisms of the rats subjected to repeated intravenous injections of an aqueous dispersion of MWCNTs for 14 days did not exhibit severe metabolic impairments or pronounced deviations in the internal organs functioning.
The main findings of this section can be listed as follows: (1) a slight decrease in the content of total protein (by 6.6% in males and by 9.0% in female rats) due to a decrease in the globulin fraction and the associated decrease in the albumin-globulin ratio; (2) parallel and proportionately elevated activity of transaminases (ALT and AST) and alkaline phosphatase against the background of unaltered levels of serum albumin, bilirubin, cholesterol, and triglycerides concentrations; and (3) a marked increase in blood glucose content in the male rats of the dose group (Table 4). The expressiveness of changes in the biochemical parameters was weak or moderate and did not exceed the physiological ranges, which allowed considering them as functional shifts, but following the worst-case rule, they should be regarded as signs of liver injury caused by 14 days repeated intravenous injections of the aqueous dispersion of MWCNTs. An increase in the fraction of globulins (consisting mainly of gamma globulins) may also indicate the activation of the immune system.
Macropathology and histopathology
Gross examination of the vital organs of the treated animals revealed no abnormalities in the size, texture, color, and surface properties when compared with the organs of the control group rats. The serous membranes were thin, wet, smooth, and shiny. However, the lungs of some animals of the dose group had a light gray shade. On close examination, many small black specks were found spread across the entire surface of the lungs. In isolated cases, larger black spots were visible on the surface of the lungs.
Hematoxylin-eosin-stained microscopic preparations of the studied organs obtained from the rats exposed to MWCNTs were examined for signs of toxic damage to the tissue microstructure in comparison with slides of the corresponding organs obtained from the control rats. A comparative histological examination showed a normal structure and the absence of noticeable pathological lesions in most of the internal organs of the rats of the dose group, but in some organs such changes were revealed. Repeated intravenous administration of the aqueous MWCNTs dispersion led to more or less pronounced alterations in the histological structure of the lungs, heart, liver, spleen, salivary glands and adjacent lymph nodes, and thymus. In almost every animal in the dose group, these pathological changes were accompanied by a pronounced response of macrophages and corresponding activation of the immune system.
Macrophage infiltration was found in the spleen tissue, in the lymph node sinuses, in the thymus, and in the peribronchial and perivascular spaces of the lung tissue, where lymphatic vessels and clefts are usually present. The histological picture of macrophage infiltration of the tissues of the mentioned organs of the rats subjected to 14 days repeated intravenous injections of the aqueous dispersion of MWCNTs is shown in Figure 4. In most cases, macrophages were characterized by large hyperchromic nuclei without nucleoli and a thin rim of cytoplasm. In some cases, the cytoplasm of these cells (often found in the tissue of the spleen and sinuses of the lymph nodes) was wide and eosinophilic and contained one large nucleus and several nuclei (Fig. 4). In addition to the large size of the macrophages, their clustering and preferential localization in the perivascular spaces attract attention. Given the key role of macrophages in the innate immune system in recognizing and removing pathogens and exogenous materials, the grouping of macrophages may indicate the sites where nanotubes initially pass out of the capillaries into the surrounding tissues.
In addition to macrophage infiltration, histological examination of the lung and liver tissues of the rats in the dose group revealed a granulomatous reaction with the formation of granulomatous nodules of various sizes and the presence of giant cells with a wide cytoplasmic rim and several nuclei (Fig. 5). Granulomatous nodules also contained small lymphocytes, which varied in number. Intracellular accumulations of a brown-black pigment (possibly hemosiderin) were found in the cytoplasm of cells in the lung parenchyma, as well as in the lumen of the capillaries and interalveolar septa (Fig. 5).
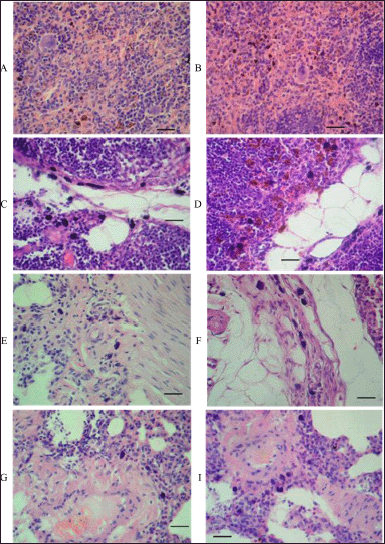 | Figure 4. Representative images showing macrophage infiltration in tissues of different organs of rats subjected to 14 days intravenous injections of aqueous MWCNTs dispersion. A and B: higher-power microscopy of two splenic tissue specimens revealed macrophage infiltration. Clusters of large macrophages in the splenic red pulp with eosinophilic cytoplasm and single nucleus (sometimes with fragmented nucleus or several separated nuclei). C and D: higher-power microscopy of two lymph node specimens showed macrophage infiltration. Groups of large macrophages in the sinuses of the submandibular lymph nodes, some of them with a large homogeneous basophilic nucleus, indistinguishable nucleoli, and a very narrow rim of cytoplasm, and others also with a large nucleus, but a wide edge of the cytoplasm stained with eosin. E, F, G, and I: a set of four lung tissue specimens examined under higher-power microscopy showed macrophage infiltration. The accumulation of large macrophages in the peribronchial and perivascular spaces of the lung tissue with dustlike inclusions of a brown-black pigment in the cytoplasm. Scale bar, 50 μm. [Click here to view] |
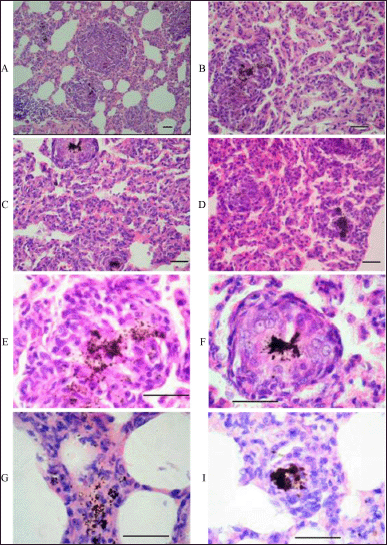 | Figure 5. Histopathologic findings in the lung tissue of rats subjected to intravenous administration of the aqueous dispersion of MWCNTs for 14 days. A: scanning power microscopy showing a general view of nodular aggregations of activated macrophages with brown-black deposits in a lung tissue specimen. B: higher-power, more detailed image of a small granuloma in the lung tissue with a significant accumulation of brown-black material in the center. C and D: higher-power microscopy of two lung tissue specimens revealed chronic inflammation with focal accumulations of macrophages around brown-black deposits in the lung tissue. E: high-magnification images of a granulomatous nodule in lung tissue. In the center of the granuloma, there is a large accumulation of macrophages containing a brown-black pigment in the cytoplasm. F: high-magnification image of the granuloma shown at the top of fragment C. G: accumulation of small lymphocytes in granulomatous nodule. I: depositions of the brown-black pigment in the center of the granulomas and thickened interalveolar septa. Scale bar, 50 μm. [Click here to view] |
Another lesion of the histological structure of the lungs in the rats of the dose group was interstitial (focal) pneumonia with accumulations of xanthoma cells in some alveoli, which can also be associated with the activation of the immune system (Fig. 6).
Several other alterations were found in the histological structure of the studied internal organs. Microscopic examination of liver tissue specimens revealed granulomas of various sizes in some hepatic lobules of almost all the rats subjected to intravenous injections of the aqueous dispersion of MWCNTs for 14 days. Unlike the lungs, the granulomatous nodules in the liver tissue did not contain deposits of the pigment (Fig. 7). The absence of brown-black deposits in granulomatous nodules in the liver tissue of the rats exposed to short-term intravenous exposure to MWCNTs may indicate their possible destruction and elimination by liver parenchyma cells. Liver granulomas mainly consisted of small lymphocytes, sometimes with the presence of macrophages—large cells with abundant foamy cytoplasm and light nuclei in the form of watch glasses (Fig. 7).
Abnormalities in the myocardium microstructure were found in some animals in the dose group. Histopathological changes were manifested by irregular staining of cardiomyocytes located in groups, the disappearance of the pattern of cross-striation, the presence of cells with homogenized cytoplasm, and the absence of nuclei in some of them (Fig. 8). In general, such lesions are characteristic of severe parenchymal dystrophy, which probably developed against the background of hypoxia.
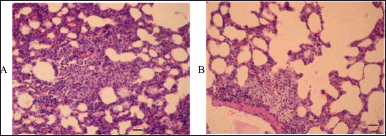 | Figure 6. Nonspecific interstitial pneumonia in rats subjected to 14 days intravenous injections of aqueous MWCNTs dispersion. A and B: scanning power microscopy of two lung tissue specimens revealed focal pneumonia with clusters of xanthoma cells in some alveoli. Scale bar, 50 μm. [Click here to view] |
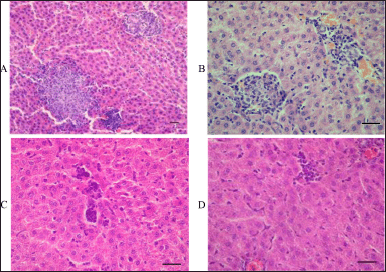 | Figure 7. Histopathologic findings in the liver tissue of rats subjected to intravenous administration of the aqueous dispersion of MWCNTs for 14 days. A: a general view of intralobular granulomas of various sizes revealed by scanning power microscopy. B: a more detailed image of small granulomas in the liver tissue shown by higher-power microscopy. C and D: higher-power microscopy of two specimens of liver tissue revealed granulomas of various sizes consisting of small lymphocytes and few macrophages. Scale bar, 50 μm. [Click here to view] |
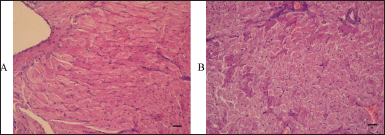 | Figure 8. Histopathological signs of parenchymal myocardial dystrophy in two rats of the dose group subjected to 14 days intravenous administration of the aqueous dispersion of MWCNTs. A and B: scanning power microscopy of two specimens of myocardial tissue revealed irregular staining of cardiomyocytes located in groups, the disappearance of cross-striation, the presence of cells with homogenized cytoplasm, and the absence of nuclei. [Click here to view] |
DISCUSSION
The widening application of CNTs in modern industries is generating interest in toxicological studies with a focus on airway absorption (Khalid et al., 2016; Kobayashi et al., 2017), while the biomedical and pharmaceutical applications of CNTs as a vehicle of targeted drug delivery require toxicological studies using the intravenous route of administration. The latter was the principal goal of this work.
Taking part in the project to create an MWCNT-based supramolecular nanocomplex for targeted delivery of doxorubicin to solid tumors, we investigated short-term (14 days) toxicity and assessed the safety of an MWCNT-based drug delivery vehicle in experiments on adult rats. The agent tested was high-purity MWCNTs obtained by catalytic pyrolysis of unsaturated hydrocarbons, which were administered intravenously in the form of an aqueous dispersion. The route of administration was selected to be the same as planned for the ultimate nanocomplex.
We found that multiple injections of the aqueous dispersion of MWCNTs did not have a noticeable influence on the status and behavior of the experimental rats.
There were no statistically significant differences in body weight dynamics and food and water consumption. No clinical signs and symptoms of intoxication were registered, including abnormal body weight changes.
The results of hematological, hemostasis, and biochemical laboratory testing of blood samples taken from the experimental rats that received intravascular injections of MWCNTs and the control rats that received the carrier solution revealed a complex of pathological changes in the cellular composition and plasma components.
Fourteen-day exposure to MWCNTs did not lead to noticeable changes in key cellular indexes of erythrocytes (MCV, MCH, and MCHC); in contrast, parameters related to erythrocytes count (RBC, Hct, and hemoglobin content) changed considerably. Compared with the parallel control, the mean value of the total number of erythrocytes in the dose group decreased by 18.0% in the male and by 14.8% in the female rats, with comparable decreases in the Hct and Hb indicators. A parallel decrease in RBC, Hct, and Hb against the background of stable cellular erythrocyte indices (MCV, MCH, and MCHC) made it possible to exclude the toxic effect of the 14 days intravascular exposure to MWCNTs to erythropoiesis but to put forward a hypothesis about the direct effect of MWCNTs on erythrocytes in the vascular bed, which led to the damage and removal of less resistant cells.
The reaction of the leukocyte blood component to repeated intravenous injections of the MWCNTs dispersion can be characterized as moderate leukopenia due to a decrease in the blood neutrophil count (neutropenia and agranulocytosis) and monocytosis. Compared with the control, the number of leukocytes in the male rats of the dose group decreased by 34.0% and in the female rats by 36.6%; the number of monocytes in the female rats increased by 36.6%. Neutropenia and agranulocytosis are well-known toxic effects of xenobiotics, whereas monocytosis can be explained as a response to inflammatory processes initiated in tissues interacting with MWCNTs.
Comparison of the biochemical indicators characterizing the state of the main functional systems of the organisms of the experimental and control rats did not exhibit severe metabolic impairments or pronounced deviations in the internal organs functioning. The main findings of the biochemical blood testing were a slight decrease in the content of total protein due to a decrease in the globulin fraction; parallel and proportionately elevated activity of transaminases (ALT and AST) and alkaline phosphatase against the background of unaltered levels of serum albumin, bilirubin, cholesterol, and triglycerides concentrations; and a marked increase in blood glucose content in the male rats of the dose group. The expressiveness of these changes was weak or moderate, but in combination, they were indicative of signs of liver injury caused by 14-day intravascular exposure to MWCNTs. An increase in the fraction of globulins (consisting mainly of gamma globulins) may also indicate the activation of the immune system.
A comparative microscopic examination of the internal organs’ microstructure in the rats of the dose group that received multiple intravenous injections of the MWCNTs dispersion and the control animals that received the dispersant solution revealed certain pathological changes in the histological structure of the lungs, heart, liver, spleen, salivary glands and adjacent lymph nodes, and thymus in the experimental animals.
Pronounced macrophage activation (cells were characterized by large hyperchromatic nuclei without nucleoli) was found in the lung, liver, and blood-forming organs (spleen, lymphatic nodes, and thymus). In almost all the animals treated with MWCNTs, accumulation of macrophages in the peribronchial and perivascular tissue of the lungs was observed. The probable reason for the observed macrophage infiltration was the MWCNTs agglomerations in the intercellular spaces.
Other characteristic histopathological lesions were granulomas of various sizes and granulomatous reactions found in the liver and lung tissues of the rats exposed to 14 days MWCNTs administration. Both the liver and lung tissue granulomatous nodules contained small lymphocytes, which occupied the peripheral part of the granulomas predominantly. In contrast to the liver, lung parenchyma cells, the capillary lumen, and interalveolar septa contained accumulations of a brown-black substance or pigment (possibly hemosiderin).
Considering that brown iron-containing pigment hemosiderin is derived from the disintegration of extravasated erythrocytes, our hypothesis about the direct impact of MWCNTs on erythrocytes in the vascular bed, leading to damage and removal of less resistant cells, was indirectly confirmed. To confirm the nature of this brown-black pigment as hemosiderin, special iron staining was required, which was beyond the scope of the present study.
It has been established that the severity of nonspecific macrophage infiltration of the organs under study correlates well with the intensity of the organs’ blood supply, which is the highest in the lungs and heart, and less in the liver, spleen, and kidneys.. The total amount of MWCNTs that can potentially be accumulated in the tissue of a specific organ depends on the anatomical position of the organ in the circulatory system, the level of perfusion, and the microstructure of the capillaries. The higher severity of macrophage infiltration in the lung tissue confirms the assumption of a relationship between the quantities of MWCNTs agglomerated in the intercellular spaces and the degree of infiltration by macrophages. A possible reason for the observed infiltration of macrophages was the agglomeration of intravenously administered MWCNTs in the intercellular spaces after crossing the barrier of the capillary endothelium.
Unlike individual small-size nanotubes, which have a weak immunostimulant effect, CNTs agglomerates can strongly activate the macrophage system of the body (Li and Cao, 2018; Yang and Zhang, 2019). The fact that macrophage infiltration was highly expressed in the lungs and heart tissues compared to other organs can be explained by the anatomical structure of the mammalian circulatory system. After injection of the aqueous MWCNTs dispersion into the tail vein of the experimental rats, a portion of blood carrying the “cloud” of dispersed nanotubes reaches the right atrium via the vessels of the inferior vena cava and then is expelled by the right ventricle via the pulmonary artery to the arterioles and capillary bed of the lungs. After that, a portion of blood, enriched with nanotubes, reaches the left heart, is ejected into the coronary arteries, and ultimately enters systemic circulation. Consequently, peak concentrations of MWCNTs are reached in the capillaries of the lungs and heart, as well as in the perivascular parenchyma of these organs, which leads to greater accumulation and agglomeration of nanotubes than in other organs. In fact, an extensive capillary bed of the lungs turns out to be the primary barrier that adsorbs the maximal amount of MWCNTs.
These observations are in concert with published toxicological data on different CNTs types (Ema et al., 2016; Lanone et al., 2013; Khalid et al., 2016; Mohanta et al., 2019; Ong et al., 2016; Zhou et al., 2017). It is quite natural that histological manifestation of CNTs toxicity proved to be similar in cases of respiratory and intravenous routes of administration, appearing in the form of pulmonary inflammation, injury, and fibrosis. The similarity of microstructural disorders suggests the similarity of the mechanisms of pathogenesis leading to the initiation and progression of inflammation and the development of associated long-term consequences. In the case of respiratory administration, the starting point of this process is the penetration of CNTs into the pulmonary interstitial sites (formation of depositions in the lung tissue) with subsequent entry into the systemic circulation. It was shown that the deposition of CNTs in the interstitial compartment is accompanied by their translocation into blood and lymph vessels with further distribution by these transport systems throughout the body (Qin et al., 2017; Xu et al., 2016).
The complex cascade of immune mechanisms with the recruitment of various immune cells, chemical mediators, and proinflammatory agents initiated in the lung tissue upon respiratory administration of CNTs was studied to a certain extent. Interaction of CNTs with alveolar type II epithelial cells leads to ROS generation during CNTs phagocytosis. The intensity of the inflammatory process correlates with the generation of ROS, and therefore, the severity of the toxic lesions depends on the reactive surface of the CNTs (Dong, 2020; Khalid et al., 2016; Møller et al., 2014). ROS system activation disrupts the balance between oxidative and antioxidative systems due to the depletion of glutathione, which leads to the activation of nuclear transcription factors (NF-kB) and elevates the production of proinflammatory cytokines (Dong and Ma, 2019; Liu et al., 2017; Qin et al., 2017). IL-8 is released from epithelial cells leading to the recruitment and activation of phagocytes, neutrophil influx, and inflammation (Dong, 2020; Vlaanderen et al., 2017). The sequence of events leading to inflammation in the case of the intravenous route of CNTs administration is principally the same, being more direct and immediately involving the reticuloendothelial (mononuclear phagocyte) system in highly perfused organs such as the liver, the heart, the spleen, and the kidney (Alshehri et al., 2016; Broz and Dixit, 2016; Kobayashi et al., 2017; Mohanta et al., 2019).
The ability of CNTs to penetrate cell and tissue barriers is determined by a complex of their specific properties, the main ones of which are size, shape (less studied), general chemical activity (surface modification by chemical functional groups), free surface energy, electrical charge, and hydrophilic–lipophilic balance. A number of studies on the disposition of various types of single- and MWCNTs have demonstrated that an understanding of the differences in the distribution and recirculation patterns, aggregation and accumulation, retention and clearance of CNTs after parenteral administration can be achieved by comparing the aforementioned physicochemical properties with the methods and conditions for the synthesis of CNTs, as well as subsequent transformations into the finished product (Jha et al., 2020; Swidwinska-Gajewska and Czerczak, 2017).
Peculiarities of disposition and distribution in the body’s fluid compartments are considered important factors for CNT-based drug delivery systems of direct relevance to tissue- and organ-specific targeting. Several pharmacokinetic and in vivo biodistribution studies were carried out during the last decade to analyze the effects of different types of CNTs surface functionalization on their disposition and clearance using various methods for tracking CNTs’ pathways in test organisms like radioisotope labeling, photoluminescence, semiconducting measurements, and Raman and isotope-ratio mass spectrometry (Zhou et al., 2017; Aoki and Saito et al., 2020).
Methods of radiolabeling nanotube tracking were used more often for short-term analysis of CNTs disposition due to comparable technical simplicity and sufficient temporal and spatial resolution. For long-term studies, alternative methods for tracking CNTs made it possible to obtain poorly reproducible results due to the spontaneous release of radioactive labels from CNTs. Since the application of other methodologies did not provide more accurate and detailed data on the biodistribution and the fate of CNTs in living organisms after parenteral administration, the results of such studies were, to a certain extent, contradictory, which causes further efforts to emerge (Raval et al., 2018; Qin et al., 2017; Zhang et al., 2017).
Nanotubes injected intravenously are exposed to blood plasma containing a huge variety of complex biomolecules, first of all, proteins, which bind competitively with the surface of CNTs. Adsorption of proteins, lipids, and other biomolecules changes the parent chemical modification of CNTs surface, electric charges distribution, and water displacement and actually forms a certain multilayer shell of an inner strongly bound immobile layer of biomolecules with high affinity to CNTs surface (hard corona) and outer weakly bound mobile layer (soft corona). It is assumed that a hard corona, being a more stable structure, determines the biochemical interaction between CNTs and cells (Baimanov et al., 2019; Czarnecka et al., 2020; Liu et al., 2020).
Every aspect of the interaction between a living organism and CNTs can be analyzed in terms of their biological activity, which is the ability to influence the structures and processes of an organism, and reasonable modification of CNTs properties to balance desirable and undesirable biological effects. Critical properties that determine CNTs’ biological activity (and potential toxicity) are the following: (i) surface area/mass ratio, which determines a contact zone with the cellular membrane, as well as the capacity of CNTs to adsorb and carry biomolecules; (ii) retention time as the integral index characterizing the ability of CNTs to persist in the organism and indicating the duration of exposition which, in turn, depends on other specific properties like biocompatibility, solubility, tendency for aggregation and accumulation in tissues, and the possibility to be biotransformed and excreted; and (iii) contaminants, first of all, metal impurities, the presence of which is directly correlated with cytotoxicity of CNTs (Aoki and Saito et al., 2020; Jha et al., 2020; Khalid et al., 2016; Kobayashi et al., 2017; Raval et al., 2018).
Designing and fabricating CNT-based structures for therapeutic applications with a well-balanced combination of rather opposite properties is a challenging task akin to dance with Mephisto, i.e., managing to achieve sufficient drug loading/release characteristics and reduce the inherent cytotoxicity of CNTs by manipulating their size, structure, and surface functionalization. Controversial or even conflicting results of the toxicological studies of CNT-based drug delivery systems, as well as other carbon nanostructures developed for biomedical applications, reflect the current state of the issue and are a logical consequence of the still existing uncertainty in design criteria of size, shape, aggregation tendency, types, positions of functional groups, etc. Poor results of risk-to-benefit assessments of CNT-based systems for targeted drug delivery, uncertain outcomes of efficacy and toxicology studies, and obscure data on pharmacokinetics, biotransformation, clearance, and long-term fate discourage their use for solving clinical problems. Undoubtedly, positive numeral results of experiments with carbon nanomaterials as carriers for anticancer drug delivery carried out on cell cultures or animal models that have demonstrated improved anticancer activity compared to that of the free drugs are very promising and cause further efforts toward the development of smart carbon nanomaterial-based nanocarriers to emerge.
CONCLUSION
The short-term (14 days) toxicity of the original (self-synthesized) purified MWCNTs (without related impurities and heavy metals) with a noncovalently functionalized surface designed as a carrier of doxorubicin was studied in experiments on adult rats. Repeated intravenous administration of an aqueous MWCNTs dispersion did not exert a noticeable toxic effect on the organisms of the experimental rats or their general status, physiological functions, and behavior. However, a complex of biochemical and, to a greater extent, multiple histological findings demonstrated noticeable toxic effects associated with MWCNTs aggregation in the capillaries and perivascular parenchyma of highly perfused organs, especially in the lungs, which are the primary barrier that adsorbed the maximum amount of MWCNTs. The similar pictures of inflammatory histological changes in these organs indicated the same pathogenetic mechanisms involved and the formation of similar immune responses. The results modified our team’s strategy in further developing a nanocomplex for anticancer drug delivery with a focus on smaller-sized nanomaterials like carbon nanohorns and graphene nanoplatelets, improving hydrophobicity and surface chemistry and comparative in vitro cytotoxicity tests with long-term toxicity experiments in animals.
ACKNOWLEDGMENTS
This work was possible through a collaboration with Chuiko Institute of Surface Chemistry, National Academy of Sciences of Ukraine, Kyiv, Ukraine. The authors would like to express deep gratitude to the scientific staff of Chuiko Institute of Surface Chemistry.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
FUNDING
There is no funding to report.
ETHICAL APPROVAL
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Bioethics Committee of the National University of Pharmacy (Protocol No. 2 of 4 November 2019, Bioethics Expertise No. 2/19/07).
AUTHORS’ CONTRIBUTION
KIE, KSM, and MTK worked on concept and design; KOM, GYuI, and KIE worked on the experimental part; KIE and BND contributed to data analysis and interpretation; GYuI and KIE conducted the statistical analysis; KIE and KSM wrote the article; KSM supervised and administered the study. All authors have read and agreed to the published version of the manuscript.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Adedara I, Anao O, Forcados G, Awogbindin I, Agbowo A, Ola-Davies O, Patlolla A, Tchounwou P, Farombi E. Low doses of multi-walled carbon nanotubes elicit hepatotoxicity in rats with markers of oxidative stress and induction of pro-inflammatory cytokines. Biochem Biophys Res Commun, 2018; 503(4):3167–73; doi:10.1016/j.bbrc.2018.08.112 CrossRef
Ali MA, Solanki PR, Srivastava S, Singh S, Agrawal VV, John R, Malhotra BD. Protein functionalized carbon nanotubes-based smart lab-on-a-chip. ACS Appl Mater Inter, 2015; 7(10):5837–46; doi:10.1021/am509002h CrossRef
Ali-Boucetta H, Nunes A, Sainz R, Herrero MA, Tian B, Prato M, Bianco A, Kostarelos K. Asbestos-like pathogenicity of long carbon nanotubes alleviated by chemical functionalization. Angew Chem Int Ed Engl, 2013; 52(8):2274–8; doi:10.1002/anie.201207664 CrossRef
Alshehri R, Ilyas AM, Hasan A, Arnaout A, Ahmed F, Memic A. Carbon nanotubes in biomedical applications: factors, mechanisms, and remedies of toxicity. J Med Chem, 2016; 59(18):8149–67; doi:10.1021/acs.jmedchem.5b01770 CrossRef
Aoki K, Saito N. Biocompatibility and carcinogenicity of carbon nanotubes as biomaterials. Nanomaterials, 2020; 10(2):264; doi:10.3390/nano10020264 CrossRef
Baimanov D, Cai R, Chen C. Understandihe Chemical Nature of Nanoparticle-Protein Interactions. Bioconjug Chem, 2019; 30:1923–37; doi:10.1021/acs.bioconjchem.9b00348 CrossRef
Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol, 2016; 16(7):407–20; doi:10.1038/nri.2016.58 CrossRef
Caoduro C, Hervouet E, Girard-Thernier C, Gharbi T, Boulahdour H, Delage-Mourroux R, Pudlo M. Carbon nanotubes as gene carriers: focus on internalization pathways related to functionalization and properties. Acta Biomaterialia, 2017; 49:36–44; doi:10.1016/j.actbio.2016.11.013 CrossRef
Cheng Y, Xu P, Feng Q, Yang X, Liu S, Xu C, Huang L, Mengwei C, Feng L. Near infrared light triggered cucurbit[7]uril-stabilized gold nanostars as a supramolecular nanoplatform for combination treatment of cancer. Bioconjug Chem, 2018; 29(8):2855–66; doi:10.1021/acs.bioconjchem.8b00438 CrossRef
Czarnecka J, Wi´sniewski M, Forbot N, Bolibok P, Terzyk AP, Roszek K. Cytotoxic or not? Disclosing the toxic nature of carbonaceous nanomaterials through nano–bio interactions. Materials, 2020; 13:2060; doi:10.3390/ma13092060 CrossRef
Dong J. Signaling pathways implicated in carbon nanotube-induced lung inflammation. Front Immunol, 2020; 11:552613; doi:10.3389/fimmu.2020.552613 CrossRef
Dong J, Ma Q. In vivo activation and pro-fibrotic function of NF-κB in fibroblastic cells during pulmonary inflammation and fibrosis induced by carbon nanotubes. Front Pharmacol, 2019; 2(10):1140; doi:10.3389/fphar.2019.01140 CrossRef
Ema M, Hougaard KS, Kishimoto A, Honda K. Reproductive and developmental toxicity of carbon-based nanomaterials: a literature review. Nanotoxicology, 2016; 10(4):391–412; doi:10.3109/17435390.2015.1073811 CrossRef
Fatemi S, Foroutan M. Recent developments concerning the dispersion of carbon nanotubes in surfactant/polymer systems by MD simulation. J Nanostruct Chem, 2016; 6:29–40; doi:10.1007/s40097-015-0175-9 CrossRef
Galassi TV, Antman-Passig M, Yaari Z, Jessurun J, Schwartz RE, Heller DA. Long-term in vivo biocompatibility of single-walled carbon nanotubes. PLoS One, 2020; 15(5):e0226791; doi:10.1371/journal.pone.0226791 CrossRef
Hohl L, Schulz J, Paul N, Kraume M. Analysis of physical properties, dispersion conditions and drop size distributions in complex liquid/liquid systems. Chem Eng Res Design, 2016; 108:210–6; doi:10.1016/j.cherd.2016.01.010 CrossRef
Hosseinpour M, Azimirad V, Alimohammadi M, Shahabi P, Sadighi M, Nejad G. The cardiac effects of carbon nanotubes in rat. Bioimpacts, 2016; 6(2):79–84; doi:10.15171/bi.2016.11 CrossRef
Huang B. Carbon nanotubes and their polymeric composites: the applications in tissue engineering. Biomanuf Rev, 2020; 5:3; doi:10.1007/s40898-020-00009-x CrossRef
Jha R, Singh A, Sharma PK, Fuloria NK. Smart carbon nanotubes for drug delivery system: a comprehensive study. J Drug Deliv Sci Technol, 2020; 58:101811; doi:10.1016/j.jddst.2020.101811 CrossRef
Kartel N, Sementsov Y, Mahno S, Trachevskiy V, Wang B. Polymer composites filled with multiwall carbon nanotubes. Univ J Mater Sci, 2016; 4(2):23–31; doi:10.13189/ujms.2016.040202 CrossRef
Kaufmann A, Hampel, S, Rieger C, Kunhardt D, Schendel D, Füssel S, Schwenzer B, Erdmann K. Systematic evaluation of oligodeoxynucleotide binding and hybridization to modified multi-walled carbon nanotubes. J Nanobiotechnol, 2017; 15(1):53; doi:10.1186/s12951-017-0288-z CrossRef
Khalid P, Hussain MA, Suman VB, Arun AB. Toxicology of carbon nanotubes – a review. Int J Appl Eng Res, 2016; 11(1):148–57.
Kobayashi N, Izumi H, Morimoto Y. Review of toxicity studies of carbon nanotubes. J Occup Health, 2017; 59(5):394–407; doi:10.1539/joh.17-0089-RA CrossRef
Kovalska E, Sementsov YI. Carbon nanotubes deagglomeration in aqueous solutions. In: Fesenko O, Yatsenko L, Brodin M (eds.). Nanomaterials imaging techniques, surface studies, and applications, 1st edition, Springer, New York, NY, vol. 146, pp 61–72, 2013; doi:10.1007/978-1-4614-7675-7_5 CrossRef
Lanone S, Andujar P, Kermanizadeh A, Boczkowski J. Determinants of carbon nanotube toxicity. Adv Drug Deliv Rev, 2013; 65(15):2063–9; doi:10.1016/j.addr.2013.07.019 CrossRef
Li Y, Cao J. The impact of multi-walled carbon nanotubes (MWCNTs) on macrophages: contribution of MWCNTs characteristics. Sci China Life Sci, 2018; 61:1333–51; doi:10.1007/s11427-017-9242-3 CrossRef
Liu N, Tang M, Ding J. The interaction between nanoparticles-protein corona complex and cells and its toxic effect on cells. Chemosphere, 2020; 245:125624; doi:10.1016/j.chemosphere.2019.125624 CrossRef
Liu T, Zhang L, Joo D, Sun SC. NF-kappa B signaling in inflammation. Signal Transduct Target Ther, 2017; 2:17023; doi:10.1038/sigtrans.2017.23 CrossRef
Mohanta D, Patnaik S, Sood S, Das N. Carbon nanotubes: evaluation of toxicity at biointerfaces. J Pharma Anal, 2019; 9:293–300; doi:10.1016/j.jpha.2019.04.003 CrossRef
Møller P, Christophersen DV, Jensen DM, Kermanizadeh A, Roursgaard M, Jacobsen NR, Hemmingsen JG, Danielsen PH, Yi C, Jantzen K, Klingberg H, Hersoug LG, Loft S. Role of oxidative stress in carbon nanotube-generated health effects. Arch Toxicol, 2014; 88(11):1939–64; doi:10.1007/s00204-014-1356-x CrossRef
Nguyen D, Quyen DQK, Tran NT, Dang XT, Bui THD. Carbon nanotubes: synthesis via chemical vapour deposition without hydrogen, surface modification, and application. J Chem, 2019; 2019(10):1–14; doi:10.1155/2019/4260153 CrossRef
Niezabitowska E, Smith J, Prestly MR, Akhtar R, von Aulock FW, Lavallée Y, Ali-Boucetta H, McDonald TO. Facile production of nanocomposites of carbon nanotubes and polycaprolactone with high aspect ratios with potential applications in drug delivery. RSC Adv, 2018; 8:16444–54; doi:10.1039/C7RA13553J CrossRef
Njuguna J, Vanli OA, Liang, R. A review of spectral methods for dispersion characterization of carbon nanotubes in aqueous suspensions. J Spectr, 2015; 2015:463156; doi:10.1155/2015/463156 CrossRef
Ong LC, Chung FFL, Tan YF, Leong CO. Toxicity of single-walled carbon nanotubes. Arch. Toxicol, 2016; 90:103–18; doi:10.1007/s00204-014-1376-6 CrossRef
Qin Y, Li S, Zhao G, Fu X, Xie X, Huang Y, Cheng X, Wei J, Liu H, Lai Z. Long-term intravenous administration of carboxylated single-walled carbon nanotubes induces persistent accumulation in the lungs and pulmonary fibrosis via the nuclear factor-kappa B pathway. Int J Nanomedicine, 2017; 23(12):1515; doi:10.2147/IJN.S133229 CrossRef
Raval JP, Joshi P, Chejara DR. Carbon nanotube for targeted drug delivery. In: Inamuddin A, Asiri AM, Mahommad A (eds.). Applications of nanocomposite materials in drug delivery, 1st edition, Woodhead Publishing, pp 203–16, 2018; doi:10.1016/b978-0-12-813741-3.00009-1 CrossRef
Sementsov YuI, Kartel N?. The influence of small concentrations of carbon nanotubes on the structuralization in matrices of different nature. Chem Phys Technol Surf, 2019; 10(2):174–89; doi:10.15407/hftp10.02.174 CrossRef
Sheikhpour M, Naghinejad M, Kasaeian A, Lohrasbi A, Shahraeini SS, Zomorodbakhsh S. The applications of carbon nanotubes in the diagnosis and treatment of lung cancer: a critical review. Int J Nanomed, 2020; 15:7063–78; doi:10.2147/ijn.s263238 CrossRef
Simon J, Flahaut E, Golzio M. Overview of carbon nanotubes for biomedical applications. Materials (Basel), 2019; 12(4):624; doi:10.3390/ma12040624 CrossRef
Swidwinska-Gajewska AM, Czerczak S. Carbon nanotubes - characteristic of the substance, biological effects and occupational exposure levels. Med Pr, 2017; 68:259–76; doi:10.13075/mp.5893.00504 CrossRef
Vlaanderen J, Pronk A, Rothman N, Hildesheim A, Silverman D, Hosgood HD, Spaan S, Kuijpers E, Godderis L, Hoet P, Lan Q, Vermeulen R. A cross-sectional study of changes in markers of immunological effects and lung health due to exposure to multi-walled carbon nanotubes. Nanotoxicology, 2017; 11(3):395–404; doi:10.1080/17435390.2017.1308031 CrossRef
Xu YY, Ge J, Zhang MH, Sun WJ, Zhang J, Yu PL, Zheng YF, Yang J, Zhu XQ. Intravenous administration of multiwalled carbon nanotubes aggravates high-fat diet-induced nonalcoholic steatohepatitis in sprague dawley rats. Int J Toxicol, 2016; 35(6):634–43; doi:10.1177/1091581816653363 CrossRef
Yang M, Zhang M. Biodegradation of carbon nanotubes by macrophages. Front Mater, 2019; 6:225; doi:10.3389/fmats.2019.00225 CrossRef
Zaib Q, Ahmad F. Optimization of carbon nanotube dispersions in water using response surface methodology. ACS Omega, 2019; 4(1):849–59; doi:10.1021/acsomega.8b02965 CrossRef
Zhang T, Tang M, Zhang S, Hu Y, Li H, Zhang T, Xue Y, Pu Y. Systemic and immunotoxicity of pristine and PEGylated multi-walled carbon nanotubes in an intravenous 28 days repeated dose toxicity study. Int J Nanomed, 2017; 27(12):1539–54; doi:10.2147/IJN.S123345 CrossRef
Zhou L, Forman HJ, Ge Y, Lunec J. Multi-walled carbon nanotubes: a cytotoxicity study in relation to functionalization, dose and dispersion. Toxicol Vitro, 2017; 42:292–8; doi:10.1016/j.tiv.2017.04.027 CrossRef