INTRODUCTION
The aging process is defined as the accumulation of various progressive changes in skin cells and tissues, divided into intrinsic and extrinsic aging (Kammeyer and Luiten, 2015). Intrinsic aging is a variety of changes in stem cells and their progeny that include the expression of microRNA, telomeres, apoptotic proteins, and gene expression influenced by genetics, hormones, and free radicals, in contrast to extrinsic aging which is influenced by lifestyle and the environment, especially exposure to UV rays (Kammeyer and Luiten, 2015; Xiong et al., 2021).
The human skin is composed of an extracellular matrix (ECM) with collagen as one of its constituent proteins (Rani and Ritter, 2016). Collagen that plays a role in skin tissue is type I and type III collagen (Poljšak et al., 2012; Wong et al., 2016). Type I collagen is the main component of Extracellular Matrix (ECM) which functions as a form of structural integrity and mechanical resistance of the skin (Stamov and Pompe, 2012). Degradation of type I collagen by matrix metalloproteinases (MMP) can result in loss of the skin’s mechanical strength during the aging process, considering that the amount of type I collagen can reach 90% of the total collagen in the skin (Cole et al., 2018). MMP-1 can degrade type I and III fibrillar collagen through domain recognition such as hemopexin. Decreased distribution and mechanical strength of fibroblasts as precursors of collagen induce MMP-1 expression through the transcription factor c-JUN/AP-1, the cyclooxygenase 2-prostaglandin pathway, and oxidative stress during the aging process. Oxidative stress induces MMP and elastase release from neutrophils which triggers the degradation of collagen and elastic fibers, leading to significant damage to MES and increasing skin susceptibility. The damage causes structural changes, such as cis/trans-conversion in fats, loss of enzyme or membrane function, and DNA damage. Type III collagen is an ECM protein synthesized by cells as preprocollagen. Type III collagen is composed of 5%–20% of the total collagen in the body. Type III collagen is the largest structural component found in hollow organs, such as the blood vessels. Type III collagen also plays a role in blood clotting and intermolecular interactions for wound healing. In the skin, type III collagen makes up 50% of fetal skin but only about 20% of adult skin. Type III collagen is able to facilitate skin expansion and contraction; besides that, it also plays an important role in type I collagen fibrillogenesis (Kuivaniemi and Tromp, 2019; Nielsen and Kasdal, 2016). Elastin is an extracellular protein that functions in the elasticity of tissues, such as the skin. Elastin is a major ECM fiber that plays a role in maintaining cutaneous tissue homeostasis because it plays the role of elasticity as it has the ability to recover after continuous stretching (Wang et al., 2021). Overall, elastin and type I and II collagen comprise an ECM with functions in stretching and restoring tissues, including the skin, to their original condition; the three ECMs can be used as skin parameters.
Exosomes defined as bilayer extracellular nanovesicles measuring 40–100 nm are derived from the endocytic membrane and contain biomolecules such as proteins, lipids, RNA, and DNA (Yang et al., 2021). Exosomes were first discovered in 1983 and have contributed to various aspects of physiology disease, including cell-to-cell communication, tissue homeostasis, cell differentiation, and organ development and remodeling. The name “exosomes” for these extracellular vesicles (EVs) was coined by Rose Johnstone, referring to membrane fragments isolated from biological fluids. The term “exosome complex” has also been used as an intracellular particle involved in RNA modification (Harding et al., 2013; Xiong et al., 2021).
Exosomes are known as small spheroidal membrane blebs which are released from reticulocytes and peripheral blood platelets. In the case of reticulocytes, exosome formation has been proposed as a mechanism for releasing excess cell membrane and unnecessary surface receptors (e.g., transferrin receptor) during cell maturation into erythrocytes (Zhang et al., 2022). More than a decade after the discovery of exosomes in reticulocytes, Raposo, G. (1996) demonstrated that the MHC-II-enriched exosomes in β-lymphocytes fused with the plasma membrane and these exosomes were able to present peptide-MHC-II complexes to activate T-cell responses. Soon after, it was shown that dendritic cells produce exosomes. The ability of exosomes to stimulate T-cell responses sparked an interest in the potential of exosomes in the field of immunology, which also shows that exosomes carry low immunogenicity with high hemocompatibility and stability in body fluids to fuse with recipient cells (Harding et al., 2013; Raposo and Stoorvogel, 2013; Yang et al., 2021).
Accumulated studies state that exosomes derived from different cells showed a positive effect in wound healing application (Yang et al., 2021). Specific molecular mechanisms of different exosomes may be related to the origin of exosomes (Zhao et al., 2020). Exosomes have been reported to have functional properties similar to mesenchymal stem cells (MSCs) without significant side effects; therefore, exosomes-based therapy has the potential to be safer than direct cell use and offers a promising alternative as a tissue regenerative modality (Li et al., 2019, 2020). Recent studies have shown that various types of endogenic exosomes are important regulators in shaping the physiological and pathological development of the skin while exogenous exosomes, such as stem cell exosomes, may serve as novel treatment options to repair, regenerate, and rejuvenate skin tissue (Xiong et al., 2021). In addition, the cell survival and regenerative effects of exosomes are dose-dependent on the use of exosomes (Yang et al., 2021).
Exosomes can be isolated from the mesenchymal stem. The predominant localization of exosomes in the perinuclear area suggests that exosomes can enter human umbilical vein endothelial cells (HUVECs) and regulate their biological behavior. HUVECs are primary cells isolated from the umbilical cord. HUVECs are commonly used as a model to investigate endothelial function related to hypoxia, oxidative stress, inflammation, angiogenesis, and immune system response, in which endothelial cell activation can be stimulated by cytokines, such as Tumor Necrosis Factor-Alpha (TNF-α) and Interferon Gamma (IFN-γ) (Cao et al., 2017). Systemically, exosomes have an anti-inflammatory and antiapoptotic effect (Zhang et al., 2022). HUVECs can internalize exosomes and significantly enhance proliferation, migration, and angiogenesis both in vitro and in vivo (Li et al., 2020; Wang et al., 2019). In vivo models show accelerated reepithelialization, increased collagen maturation, and improved angiogenesis (Li et al., 2020). Meanwhile, the in vitro model shows that human induced pluripotent stem cells (iPSCs)-MSC-exosomes can increase the proliferation and migration of both human fibroblasts and HUVECs while increasing the secretion of collagen and elastin fibroblasts (Zhang et al., 2015).
Compared to the control group, Masson’s trichrome staining and western blot of HUVEC exosomes showed increased collagen deposition and collagen I protein expression (Zhao et al., 2020). More significant collagen deposition was observed in wounds in mice in the exosome group than in the control group. Recent studies have shown that various types of endogenic exosomes are essential regulators in the physiological and pathological development of the skin. Meanwhile, exogenous exosomes, such as stem cell exosomes, can be a new treatment option to repair, regenerate, and rejuvenate skin tissue (Xiong et al., 2021). Therefore, this study aimed to determine the effect of HUVEC exosomes on type I collagen deposition in the skin of intrinsic aging Wistar rats.
MATERIALS AND METHODS
This study uses an experimental laboratory design with a posttest-only control group and was conducted at the Setia Budi Laboratory, Surakarta; Dermama Biotech Laboratory, Surakarta; and Anatomical Pathology Laboratory, Faculty of Medicine, Sebelas Maret University, Surakarta, in the period December 2021–January 2022.
Exosomes
Exosomes were obtained from HUVEC secretomes isolated according to the standard. The HUVEC secretome was transferred into the tube, and the total exosome isolation reagent in vitro was added. The tube content was subsequently mixed with vortex, followed by incubation (2°C–8°C for one night) and centrifugation (10,000× g, 2°C –8°C for 1 hour). The supernatant was discarded so that the exosomes on the bottom pellet of the tube could be obtained. Using the affinity method, resuspension pellets with Phosphate Buffer Saline (PBS) and exosomes were ready for purification. Exosomes were isolated (2°C–8°C for 1 week). Each Eppendorf contained 1 ml of liquid containing 100 g of exosomes dissolved in PBS. The dose in this study was 1 l.
Animals
The experimental animals in this study were male Wistar rats aged 18 months with a body weight of 300–350 g. Rats were obtained from the Laboratory of Setia Budi University, Surakarta. Rats undergoing intrinsic aging are clinically characterized by cataracts in the eyes with a redder color and larger body size than young rats, yellowish-white fur, and brown spots on the rat’s tail. After 7 days of adaptation, three groups of rats were placed under standard environmental conditions (18°C–26°C, 12 hours of light and dark) and fed (BW 1) and provided with standard drinking water ad libitum. All treatments in this study abode by the research ethics of Declaration of Helsinki items 11 and 12 and had obtained permission from the Ethics Committee of Public General Hospital Dr. Moewardi, Surakarta.
HUVEC exosomes treatment
A sample for the preliminary test to calculate collagen deposition was taken from rat skin samples that had 1 × 1 cm excision biopsied. Then, it was continued by giving treatment to 30 samples divided into 3 groups (n = 10) randomly into a control group, HUVEC 1% exosome group, and 1.5% HUVEC exosome group. Exosomes were given by a subcutaneous injection into the back area once a week. After 28 days, an excisional biopsy measuring 1 × 1 cm was performed at the injection site. Termination of experimental animals was performed with euthanasia using chloroform.
The exosome delivery system is concerned with the biological effects brought about by exosomes. The biological effects of exosomes can only be produced through internalization by target cells via the endocytic pathway; therefore, prolonging the half-life of exosomes until they can reach target cells and reach the target dose is necessary. Previous studies have shown that exosome delivery by intravenous, intraperitoneal, and subcutaneous injection is more capable of prolonging the half-life of exosomes than topical application. Topical application of exosomes, for example, to the skin or eyes, allows rapid clearance of vesicles by secretions, skin sweat or tears, and exposure to external elements (Riau et al., 2019). Although direct injection of exosomes can also be cleared by the blood circulation quickly, the subcutaneous tissue tends to have fewer blood vessels, so it is expected that exosome clearance is slower and the half-life of exosomes is increased, and subcutaneous injection is simpler than other injections (Kim et al., 2017). The choice of Exo-HUVEC application via subcutaneous injection compared to skin dermabrasion was made because the parameters of skin repair and rejuvenation in this study were collagen, especially type I collagen. Collagen is located in the reticular dermis, and skin dermabrasion application is recommended within safe limits, namely from the superficial area to the mid-reticular-dermis, so subcutaneous injection is better because it is able to reach the deep dermis, which contains collagen. Dermabrasion exceeding the recommended area will disrupt collagen infrastructure, wounds, and depigmentation; besides that, dermabrasion can cause complications in the form of infection, milia, or persistent erythema which tends to be difficult to control in experimental rats (Bedford and Daveluy, 2021). To minimize wound trauma caused by the subcutaneous injection, several things were carried out, such as 1) fixing the experimental animals properly so that there were no unwanted movements that could interfere with the injection process; 2) marking the injection area; 3) performing the injection by a standard certified laboratory technician; and 4) carrying out the experimental animal cages individually, to avoid stress and fights between the experimental animals to reduce the risk of infection which can interfere with the wound healing process.
Histopathological preparation
Skin samples were colored using Masson’s trichrome staining: (1) fixation: the tissue was put in a 10% formalin solution (pH 7.0 for 18–24 hours) and then soaked in distilled water (1 hour); (2) dehydration: the tissue was placed in a graded concentration of alcohol and subsequently in an alcohol-xylol solution (1 hour), followed by a pure xylol solution (2 × 2 hours); (3) impregnation: the tissue was cut and placed in liquid paraffin (2 × 24 hours); (4) deparaffinization: the tissue was prepared in an oven (10 minutes); and (5) Masson’s trichrome staining: the tissue was given a 0.5% neutral red solution (5 minutes), then washed with running water (5 minutes) and rinsed with distilled water, and then given a solution of acid fuchsin (5 minutes) and rinsed with distilled water once again (5 minutes).
Collagen deposition calculation
Collagen deposition was observed with a microscope with 40× magnification in five fields of view using Optilab. An anatomical pathologist performed the interpretation of the results with repeated readings. Quantitative data were obtained using the ImageJ software (%) in the blue area of observation.
Statistical analysis
Data analysis was conducted using IBM Statistical Package for the Social Sciences version 21. The normality test was performed using the Shapiro–Wilk test. If a normal data distribution was obtained (p > 0.05), one-way analysis of variance was used to assess collagen deposition, followed by the post-hoc Least Significance Different (LSD) test. Supposing the data distribution is not normal (p < 0.05), the Kruskal–Wallis test was used to assess the increase in collagen deposition, followed by the Mann–Whitney test. The group difference was significant if the p value <0.05.
RESULTS AND DISCUSSION
This study was a posttest-only study. The average collagen deposition is shown in Figure 1. The lowest average collagen deposition (%) at 40× magnification was in the control group, and the highest was in the group receiving HUVEC 1.5%. This study used the Kruskal–Wallis test since the p-value of the Shapiro–Wilk normality test was p < 0.05. The results of the Kruskal–Wallis test on the research variables showed a significant difference between the three groups (p < 0.05). The Kruskal–Wallis test indicated a significant difference between at least two research groups to find out which group to which group had a significant difference, and then statistical analysis was continued using the Mann–Whitney test. The Mann–Whitney test also indicated which one is the most effective treatment in increasing collagen deposition levels in the skin of intrinsic aging Wistar strain rats. The Mann–Whitney test obtained p < 0.05 for each group against the other. The results showed a significant difference based on the Mann–Whitney test between the three groups. The results of the Mann–Whitney test can be interpreted as the 1% and 1.5% HUVEC groups were equally effective in increasing collagen deposition compared to the control group in the skin of intrinsic aging Wistar rats. Collagen deposition preparations are shown in Figure 2.
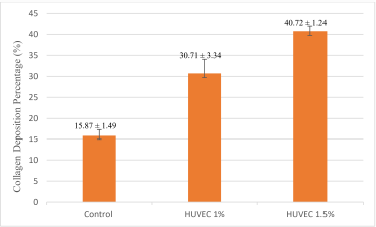 | Figure 1. Means ± SD collagen deposition percentage (%) [Number of mice per group (N) = 10]. [Click here to view] |
The previous statement explained that the use of HUVEC has a function similar to stem cells, with one of the sources being the umbilical cord (UC). Stem cells are useful in cell-based therapy considering that stem cells have varying potencies, are easy to isolate from the original tissue, have simpler ethical problems than embryonic stem cells (ESC), and have a lower risk of iPSCs teratoma formation. Moreover, they are beneficial for many therapeutic applications due to their ability to migrate to chemotactically damaged areas. Therefore, researchers can utilize stem cells in various tissues with different origins, including skin tissue (Jo et al., 2021).
The skin is composed of dermal ECM, with the primary components of type I and III collagen (Kammeyer and Luiten, 2015; Wong et al., 2016). With increasing age, the skin will experience a decrease in collagen and its elasticity ability, marked by an increase in oxidative stress and MMP, which will cause matrix degradation The aging process is also caused by reactive oxygen species (ROS). ROS is an activator of transcription factors that can increase the expression of MMP-1. Reaction with reactive molecule species (RMS) can convert biological molecules into free radicals and trigger a chain reaction that leads to a series of changes in the skin. RMS also triggers metabolic and catabolic processes in cellular homeostasis, growth and development, repair, immune defense, and apoptosis. Collagen fragments inhibit novel collagen synthesis in fibrocytes. Tissue degeneration and decreased potential for keratinocyte regeneration and differentiation contribute to the skin’s aging process (Wölfle et al., 2014).
In addition to the aging aspect, the skin is also the most often injured tissue. The skin has a basement membrane area that connects the basal keratinocytes with the collagen fibers on the surface of the dermis. This relationship has the primary function of adhesion between the two layers so that the basement membrane functions as a provider of oxygen and nutrients from the vascular dermis to the epidermis, considering that the epidermis is an avascular tissue (Jo et al., 2021).
Wounds on the skin have the following overlapping stages of healing: 1) hemostasis stage, in the form of formation of coagulation; 2) inflammatory stage, in the form of mononuclear cell infiltration; 3) proliferative stage, in the form of epithelialization, fibroplasia, and angiogenesis; and 4) maturation stage, in the form of increased collagen deposition and the formation of cicatricial tissue. MSCs can enhance the skin healing process and reduce scar tissue formation due to their ability to migrate to the injured area, inhibit inflammation, and promote the proliferation and differentiation of fibroblasts, epidermis, and endothelium. Recent studies have also stated that MSC derivatives, including exosomes, can treat wounds on various types of tissues, including skin tissue in the regeneration process of wound healing and rejuvenation (Jo et al., 2021).
MSCs in the healing process have the role of regeneration and rejuvenation. The regeneration roles include anti-inflammatory, modulator immune system, increase in keratinocytes and fibroblasts, angiogenesis, and nerve regeneration. Meanwhile, the role of rejuvenation includes modulating the immune system, increasing collagen and elastin synthesis, decreasing ROS, and inhibiting MMP activation and hair regeneration. For example, in burn healing, UC-MSCs have been shown to enhance the healing process through their immunosuppressive effect (Jo et al., 2021).
UC-MSCs are well known for their ability to proliferate rapidly and immunomodulating capacity and are easy to isolate compared to other mesenchymal cells in antiaging aspects. In addition, in their antiaging role, UC-MSCs have various growth factors, including the epidermal growth factor, basic fibroblast growth factor, transforming growth factor-β (TGF-β), platelet-derived growth factor, hepatocyte growth factor, collagen type I, and growth differentiation factor-11. UC-MSCs have also been applied in the form of a cream that showed that 4 weeks of daily use could increase skin density by up to 2.46% and reduce eye wrinkles. Another application using UC-MSCs derivatives in the form of EVs can trigger fibroblast proliferation in vitro and increase collagen, elastin, and fibronectin and inhibit MMP-1 and MMP-3. Theoretically, the results obtained in this study showed appropriate results, where the administration of exosomes at a dose of either 1% or 1.5% was able to increase type I collagen as an indicator of skin tissue rejuvenation of Wistar strain rats experiencing intrinsic aging (Jo et al., 2021).
Other supporting research in the form of in vivo studies by Li et al. (2020) using experimental rat animals showed that administration of HUVEC exosomes was able to regulate protein expression. It could also be interpreted that exosomes could increase the ability of proliferation, migration, and angiogenesis, as well as increase the expression of related proteins. More significant collagen deposition was observed in rat wounds in the exosome-treated group than in the control (Li et al., 2020). The study by Zhao et al. (2020) also reported that exosomes derived from HUVECs could promote proliferative activity and migration of keratinocytes and fibroblasts, which are two essential effector cells for skin regeneration. Other in vivo results show accelerated reepithelialization, increased collagen maturity, and improved angiogenesis. This study that performed a histological evaluation by Masson’s trichrome staining and western blot showed that HUVEC exosomes could increase collagen deposition and expression of collagen I protein compared with the control group (Zhao et al., 2020).
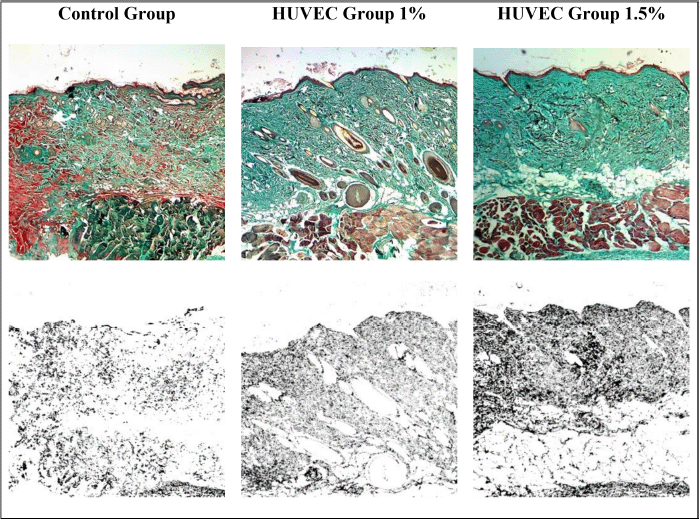 | Figure 2. Trichrome Masson staining on Wistar rat skin preparations in 100× magnification, collagen is indicated by green color (top row). The results of the quantitative analysis using ImageJ software, collagen is indicated by black color (bottom row). Control group with 16.4% (bottom left); 1% HUVEC exosome treatment group with 29.7% (bottom center); and HUVEC 1.5% exosome treatment group with 40.3% (bottom right). [Click here to view] |
Previous supporting research conducted by Kang et al. (2017) showed that the exosome treatment group had increased collagen synthesis and decreased MMP-1 production. MMP-1 lowering is due to the exposure of skin fibroblasts to exosomes that trigger an increase in collagen and elastin production (Yang et al., 2021). The antiaging properties of exosomes derived from ESCs, and it was found that mmu-miR-291a-3p containing these exosomes was able to reverse the aging process in dermal fibroblasts mechanically via the TGF-β pathway (Bae et al., 2019).
Another study by Fiqri et al. (2021) showed that the administration of exosomes from Wharton’s jelly at doses of 0.75 and 1.5 mcg increased type I collagen deposition in rat skin induced with UVB rays to 48.41% and 58%, respectively, compared to the control group, whose value was 23.72%. Therefore, both this study and previous similar studies gave appropriate results, where the administration of HUVEC exosomes was able to increase collagen expression in the skin tissue of intrinsic aging Wistar rats.
As for limitations, this study only examined collagen deposition in the excision part of skin tissue and did not examine other skin parts, so the systemic effect of increased collagen deposition could not be determined.
CONCLUSION
HUVEC exosomes can increase type I collagen deposition significantly in intrinsic aging Wistar rats. Therefore, they can be considered a therapeutic option for skin rejuvenation in future studies.
CONFLICT OF INTERESTS
The authors report no financial or any other conflicts of interest in this work.
FUNDING
There is no funding to report.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Bae YU, Son Y, Kim CH, Kim KS, Hyun SH, Woo HG, Jee BA, Choi JH, Sung HK, Choi HC, Park SY, Bae JH, Doh KO, Kim JR. Embryonic stem cell-derived mmu-miR-291a-3p inhibits cellular senescence in human dermal fibroblasts through the TGF-β receptor 2 pathway. J Gerontonol Series A Biol Sci Med Sci, 2019; 74(9):1359–67; doi:10.1093/gerona/gly208. CrossRef
Bedford L, Daveluy S. Skin resurfacing dermabrasio. StatPearls Publishing, Treasure Island, FL, 2021.
Cao Y, Gong Y, Liu L, Zhou Y, Fang X, Zhang C, Li Y, Li J. The use of human umbilical vein endothelial cells (HUVECs) as an in vitro model to assess the toxicity of nanoparticles to endothelium: a review. J Appl Toxicol, 2017; 37(12):1359–69; doi:10.1002/jat.3470. CrossRef
Cole MA, Quan T, Voorhees JJ, Fisher GJ. Extracellular matrix regulation of fibroblast function: redefining our perspective on skin aging. J Cell Commun Signal, 2018; 12(1):35–43; doi:10.1007/s12079-018-0459-1. CrossRef
Fiqri A, Widhiati S, Mawardi P, Novan AS, Arrochman F, Julianto I, Margiana R. A histophatological review of conditioned medium exosomes from wharton’s jelly derived mesenchymal stem cells administration to skin collagen deposition of aged wistar rats. Ann Rom Soc Cell Biol, 2021; 25(2):1377–983. Available via https://www.annalsofrscb.ro/index.php/journal/article/view/1094
Harding C, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol, 2013; 200(4):367–71; doi:10.1083/jcb.201212113. CrossRef
Jo H, Brito S, Kwak BM, Park S, Lee MG, Bin BH. Applications of mesenchymal stem cells in skin regeneration and rejuvenation. Int J Mol Sci, 2021; 22(5):1–18; doi:10.3390/ijms22052410. CrossRef
Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev, 2015; 21:16–9; doi:10.1016/j.arr.2015.01.001. CrossRef
Kang HH, Kim IK, Lee HI, Joo H, Lim JU, Lee J, Lee SH, Moon HS. Chronic intermittent hypoxia induces liver fibrosis in mice with diet-induced obesity via TLR4/MyD88/MAPK/NF-kB signaling pathways. Biochem Biophys Res Commun, 2017; 490(2):349–55; doi:10.1016/j.bbrc.2017.06.047. CrossRef
Kim H, Park H, Lee SJ. Effective method for drug injection into subcutaneous tissue. Sci Rep, 2017; 7:9613; doi:10.1038/s41598-017-10110-w. CrossRef
Kuivaniemi H, Tromp G. Type III collagen (COL3A1): gene and protein structure, tissue distribution, and associated diseases. Gene, 2019; 707:151–71; doi:10.1016/j.gene.2019.05.003. CrossRef
Li M, Wang T, Tian H, Wei G, Zhao L, Shi Y. Macrophage-derived exosomes accelerate wound healing macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif Cells Nanomed Biotechnol, 2019; 47(1):3793–803; doi:10.1080/21691401.2019.1669617. CrossRef
Li X, Wang Y, Shi L, Li B, Li J, Wei Z, Lv H, Wu L, Zhang H, Yang B, Xu X, Juang J. Magnetic targeting enhances the cutaneous wound healing effects of human mesenchymal stem cell-derived iron oxide exosomes. J Nanobiotechnol, 2020; 18(1):1–14; doi:10.1186/s12951-020-00670-x. CrossRef
Nielsen MJ, Karsdal MA. Type III collagen. In: Karsdal MA (ed.). Biochemistry of collagens, laminins, and elastin: structure, function and biomarkers, Elsevier, Denmark and Southern Danish University, Denmark, pp 21–30, 2016; doi:10.1016/B978-0-12-809847-9.00003-9. CrossRef
Poljšak B, Dahmane RG, Godic A. Intrinsic skin aging: the role of oxidative stress. Acta Dermatovenerol Alp Panon Adriat, 2012; 2(2):33–6; doi:10.2478/V10162-012-0009-0.
Rani S, Ritter T. The exosome—a naturally secreted nanoparticle and its application to wound healing. Adv Mater, 2016; 28(27):5542–52; doi:10.1002/adma.201504009. CrossRef
Raposo, G. (1996). B lymphocytes secrete antigen-presenting vesicles. Journal of Experimental Medicine, 183(3), 1161–1172. doi:10.1084/jem.183.3.1161 CrossRef
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol, 2013; 200(4):373–83; doi:10.1083/jcb.201211138. CrossRef
Riau AK, Ong HS, Yam GHF, Mehta JS. Sustained delivery system for stem cell-derived exosomes. Front Pharmacol, 2019; 10:1368; doi:10.3389/fphar.2019.0136. CrossRef
Stamov D, Pompe T. Structure and function of ECM-inspired composite collagen type 1 scaffold. Soft Matter, 2012; 8(40):10200–12; doi:10.1039/c2sm26134k. CrossRef
Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W, Xu H, Lei B, Mao C. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostic, 2019; 9(1):65076; doi:10.7150/THNO.29766. CrossRef
Wang K, Meng X, Guo Z. Elastin structure, synthesis, regulatory mechanism and relationship with cardiovascular disease. Front Cell Dev Biol, 2021; 9(596702); doi:org/10.3389/fcell.2021.596702. CrossRef
Wölfle U, Seelinger G, Bauer G, Meinke MC, Lademann J, Schempp CM. Reactive molecule species and antioxidative mechanisms in normal skin and skin aging. Skin Pharmacol Physiol, 2014; 26(7):316–32; doi:10.1159/000360092. CrossRef
Wong R, Geyer S, Weninger W, Guimberteau JC, Wong JK. The dynamic anatomy and patterning of skin. Exp Dermatol, 2016; 25(2):92–8; doi:10.1111/exd.12832. CrossRef
Xiong M, Zhang Q, Hu W, Zhao C, Lv W, Yi Y, Wang Y, Tang H, Wu M, Wu Y. The novel mechanisms and applications of exosomes in dermatology and cutaneous medical aesthetics. Pharmacol Res, 2021; 166:105490; doi:10.1016/j.phrs.2021.105490. CrossRef
Yang GH, Lee YB, Kang D, Choi E, Nam Y, Lee KH, You HJ, Kang HJ, An SH, Jeon H. Overcome the barriers of the skin: exosome therapy. Biomater Res, 2021; 25(22); doi:10.1186/s40824-021-00224-8. CrossRef
Zhang C, Deng R, Zhang G, He X, Chen H, Chen B, Wan L, Kang X. Therapeutic effect of exosomes derived from stem cells in spincal cord injury: a systematic review based on animal studies. Front Neurol, 2022; 13(847444); doi:10.3389/fneur.2022.847444.
Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Xie Z, Zhang C, Wang Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med, 2015; 13:49; doi:10.1186/s12967-015-0417-0.
Zhao D, Yu Z, Li Y, Wang Y, Li Q, Han D. GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and faciliting skin regeneration. J Mol Histol, 2020; 51(3):251–63; doi:10.1007/s10735-020-09877-6. CrossRef