INTRODUCTION
Hesperidin (C28H34O15) (Fig. 1) is a flavanone glycoside found in abundance in citrus fruits such as lemons, sweet oranges (Citrus sinensis), and grape fruits, as well as to a lesser level in vegetables such as tomatoes, mint, and other fresh herbs (Pandey and Khan, 2021; Sulaiman et al., 2020). Flavonoids are the most common type of dietary polyphenol. Its name comes from the Latin word “hesperidium,” which refers to the fruit of citrus trees. Recent studies have reported that hesperidin in experimental animals has a lower bioavailability and absorption due to the rutinoside moiety’s binding to the dietary flavonoid (Rekha et al., 2019). Hesperidin, on the other hand, has been identified as a promising phytocompound with antibacterial, anticancer, antioxidant, anti-inflammatory (Balakrishnan et al., 2021; Rao et al., 2018), antidiabetic, and cardiovascular protective properties. Hesperidin, in particular, has been shown to have chemotherapeutic and chemopreventive properties. A number of chromatographic methods, including high performance liquid chromatography (HPLC) and liquid chromatography mass spectrometry (LC/MS), have been used to quantify hesperidin in orange, lime peel, grapes, and plant extracts (Lee et al., 2017). Spectrophotometric, HPLC, and HPTLC techniques have all been used to estimate hesperidin in various species extracts and have been published in the literature. The procedures outlined have their own drawbacks, such as the fact that they take longer and demand the use of expensive and complex chemicals. There is no Food and drug administration (FDA)-approved reverse phase (RP)-HPLC method for evaluating hesperidin in its Ayurvedic formulation and nanotransferosome, according to the literature study. As a result, to estimate hesperidin, an RP-HPLC method must be designed and validated utilizing a suitable solvent system. The purpose of this research is to develop and design a new RP-HPLC technique for assessing hesperidin in plant extract, Ayurvedic formulation, and nanotransferosome. Many assays have been described for diosmin (Šatínský et al., 2013), hesperetin, rutin, and quercetin in combination with hesperidin, including HPLC, HPTLC (Lobo et al., 2010), gas chromatography-MS, and UV-Spectroscopy. There are currently no reports of a hesperidin RP-HPLC method. The goal of the current investigation was to design and establish a simple, delicate, exact, and reliable RP-HPLC technique for assessing hesperidin. Hesperidin is now offered in a range of pharmaceutical formulations as nutritional health supplements (powders and capsules). These dose formulations, however, fall short of delivering therapeutic benefits due to their limited bioavailability (Majumdar and Srirangam, 2009), low aqueous solubility, rapid decomposition at alkaline pH, and rapid elimination of the system (Li et al., 2008). Hesperidin stability during formulation and analysis is also an issue related to its sensitivity that can harm its therapeutic potential. Several nanocarrier techniques, like liposomes, solid lipid nanoparticles (Ferrari et al., 2019), nanoemulsions (Somasundaram et al., 2018), and nanoparticles have been studied to overcome these obstacles. The nanotransferosome (Yuan et al., 2022) is the most useful vesicular system among these nanocarriers. On the other hand, particle size has an impact on the effectiveness of drug delivery systems based on nanoformulation. In this regard, the current research focuses on the formulation of hesperidin-loaded nanotransferosome as well as the assessment of the utility of the created approach. The goal of this work was to provide a clear, simple, and accurate RP-HPLC method in compliance with the International Council for Harmonization guideline (ICH, Q2 [R1] (2005)) to assess hesperidin in commercial Ayurvedic formulation (Kumar and Devanna, 2013), plant extract, and hesperidin loaded nanotransferosome. As far as we are aware, there is no one approach that has been documented that can analyze the quantity of hesperidin in a range of systems, including Ayurvedic formulations, plant extracts, and lipid-based nanoformulations. The established strategy was applied to investigate the behavior of hesperidin’s degradation under diverse circumstances (acidic, basic, oxidation, and photochemical degradation). The stated approach can benefit the acceptable selection of experiments and its effects on design, formulation, and storage.
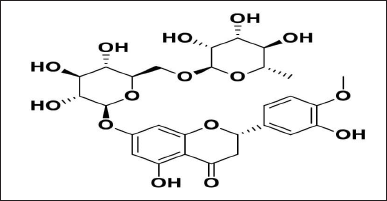 | Figure 1. Chemical structure of hesperidin. [Click here to view] |
MATERIALS AND METHODS
Chemicals and reagents
Shaanxi Yi An Biological Technology Co., Lt., China, and Himalaya wellness Bangalore provided the hesperidin as a gift sample. HPLC grade methanol and Ortho phosphoric acid used from Merck Limited (Mumbai, India), Marketed Ayurvedic formulation Madhiphala rasayana from well-established Ayurveda local dispensing store Belagavi.
Instrumentation
The Agilent 1220 Infinity II LC System is a high-performance liquid chromatography system by Agilent Technologies (Agilent technologies, Germany). The Sorvall MX 150 Ultracentrifuge from Thermo Scientific was utilized to extract unentrapped hesperidin from Ayurvedic formulation, as well as nanotransferosome.
Chromatographic conditions
The analytical conditions were calibrated at room temperature using a Phenomenex Luna C18 Column (250 × 4.6 mm, 5 μm) with an Agilent 1220 infinity II LC System (Agilent technologies, Germany). Various combinations of methanol and 0.1% orthophosporic acid were tested on a C18 column in preliminary investigations in order to obtain a strong, isolated, and stable hesperidin peak. Methanol: 0.1% orthophosporic acid in a 50:50 (% v/v) ratio was shown to be the best combination. The mobile phase was filtered through a 0.45 mm membrane and ultrasonically degassed for 15 minutes (Chaudhary et al., 2013). With a run time of 7.0 minutes and a sample injection volume of 20 μl, the flow rate was maintained at 1.0 ml/minute. The system was equilibrated with the mobile phase for 1 hour prior to sample injections. Hesperidin absorbance was measured at 284 nm. All analysis tests were performed at room temperature.
Standard solution preparation
In the mobile phase, a stock solution was made (100 μg/ml). Dilutions from 0.5 to 16 μg/ml were used to develop the calibration curve (Kempwade et al., 2015; Kurangi et al., 2019).
Commercial orange peel and lime extracts samples
Precisely weighed 10 g and then mixed with 5 ml of methanol and 5 ml of dimethyl sulfoxide in 10 ml amber-colored volumetric flasks (Ahmed et al., 2021; Kurangi and Jalalpure, 2018; Rodrigues et al., 2022). The methanolic solution was vortex mixed and sonicated for 10 minutes to make sure that all of the hesperidin was extracted. For volume make-up, methanol was used. A 0.25 m membrane filter was used to filter the solution. After filtering, samples of orange and lime peel extract (filtrate) were used in the HPLC analysis (Jagwani et al, 2019; Kudatarkar et al.,2022; Kurangi and Jalalpure, 2020; Kurangi et al., 2021; Sharma et al.,2022).
Analysis of Madhiphala rasayana—a marketed Ayurvedic formulation
2 ml of the selected formulation was carefully measured and placed into a 10 ml amber-colored volumetric flask, where it was subjected to the identical method as the commercial formulation analysis and the Ayurveda formulation analysis. The HPLC analysis was used to quantify the parameter.
Preparation of hesperidin-loaded nanotransferosome
In the current investigation, a thin film hydration technique was used in the preparation process to develop the hesperidin-loaded nanotransferosome. Hesperidin was accurately weighed and dissolved in 1 ml of dimethyl sulfoxide. Phospholipon 90G and tween 80 were dissolved in a mixture of chloroform and methanol (2:1, v/v) and hesperidin. The solvent mixture was evaporated in a rotary evaporator under pressure at 60°C and 60 rpm, forming a thin layer on the flask walls. To ensure that almost all organic solvents were completely removed, the flask was placed under a vacuum for an entire night after the solvents must have completely evaporated (Asensio-Regalado et al., 2022).
The lipid film was hydrated to either phosphate buffer (pH 7.4) or by rotating at 60 rpm for an hour at 60°C. The resulting suspension was allowed to stand at room temperature for 1 hour to achieve complete hydration in order to produce large multilamellar vesicles (LMLVs). Using an ultrasonic sonicator, LMLV was probe sonicated for 2 minutes (nanotransferosome) at 4°C to create smaller vesicles. To get uniformly sized vesicles, the sonicated vesicles were extruded through 0.45 and 0.2 m sterile syringe filters (Minisart, Sartorius, AG, Germany), and they were then stored at 4°C for further research (Hsieh et al., 2021).
Method development
The development of method for analysis of hesperidin was done by using different mobile phase ratios, concentration, and pH values of ortho phosphoric acid (OPA) and flow rates. Whenever a robust baseline was obtained, the chromatographic variables were modified. Injecting a standard hesperidin solution assessed the chromatogram, and the study was carried out six times with the same method (Parab et al., 2021).
Approach evaluation
Linearity
The linear calibration curve is plotted from values of 0.5 to 16 μg/ml, according to studies on linearity. Using common calibration curves, concentrations versus peak area were calibrated. A chromatogram was created following a sample run of each standard solution in triplicate (Parab et al., 2022).
Precision and accuracy
Three different quality control methods were employed on the hesperidin samples to evaluate their precision and accuracy. These approaches used low, medium, and high levels of 2, 4, and 16 μg/ml. In order to assure intraday precision and accuracy, triplicates of standard solutions (50%, 100%, and 150%) were injected on the same day in triplicates of 50%, 100%, and 150% including over the course of 3 days. Analysis was performed to determine relative standard deviation (RSD) and percent error.
Limit of detection (LOD) and limit of quantification (LOQ)
The signal-to-noise level was set with a minimum of 3.3:1 for LOD and 10:1 for LOQ to estimate the LOD using a series of hesperidin stock solutions. Both variables restrict the amount of analyte that may be examined (Shetti and Jalalpure, 2021).
System suitability
The tailing factor, theoretical plates, peak area, and resolution values are one of the variables that are considered when conducting suitability tests to evaluate the chromatographic process.
Robustness
By performing a simple adjustment to the optimal value, the robustness of the approach was examined. The parameters like flow rate ( ±0.1 ml/min), wavelength ( ±2 nm), and data were used to estimate the hesperidin peak areas and the % RSD of robustness testing (Chimagave et al., 2020; Joshi et al, 2018).
Specificity
By analyzing the chromatograms from the standard hesperidin solution and phosphate buffer at the retention time of hesperidin, the specificity study chances of interferences from phosphate buffer and hesperidin based nano transferosome were investigated.
Force degradation studies
The devised HPLC method stability-indicating property was tested according to the ICH criteria. Hesperidin was subjected to acidic, basic, oxidative, and photochemical conditions to study its forced degradation (Anwer et al., 2014; Bhalerao et al., 2019; Kudatarkar et al; 2021; Peram et al., 2019; Patil and Mall, 2012; Tavade et al., 2022).
RESULTS
Conditions for sample preparation and chromatographic analysis
The composition of the mobile phase was modified to carry out various studies. Finally, a methanol solution comprising 50:50 v/v and 0.1% orthophosphoric acid resulted in the best separation. The injection volume of 20 μl and the flow rate of 1 ml/minutes were constant throughout this study.
Analytical method validation suitability of system
Table 1 provides an overview of the system suitability results. The theoretical plate average was higher than 6,000, however, the tailing factor was less than 2. The maximum retention time was 5.45 minutes. Figure 2 shows that the tailing factor was less than 2, and the average theoretical plate was > 6,000. A more efficient process has higher theoretical plates and a lower tailing number. The system was also well-equipped, showing percent RSD values of less than 1% for every parameter including retention time, peak area, theoretical plates, and tailing factor.
Linearity and range
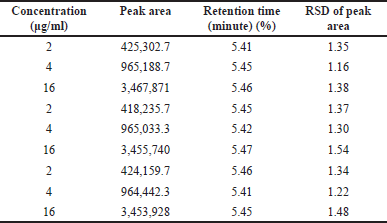
Table 2 and Figure 3 present the linearity and regression results. The hesperidin peaks were not affected by any of the nanotransferosome ingredients or stress treatment derivatives. The concentration of hesperidin (×) was plotted against the peak area to produce a calibration curve. It was shown that there was linearity in the concentration range of 0.5–16 μg/ml. (R2 = 0.999; equation y = 216,488 ×–21,910). Hesperidin was detected with an accuracy and recovery of 100.85 to 101.43 using a C18 column with methanol to 0.1% orthophosphoric acid as the mobile phase.
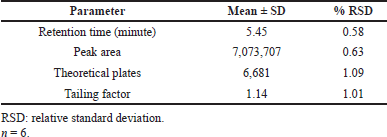 | Table 1. Results of system suitability studies of quality control samples of hesperidin. [Click here to view] |
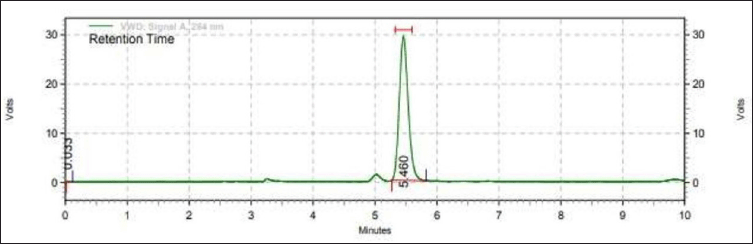 | Figure 2. Chromatogram of standard hesperidin solution (10 μgml). [Click here to view] |
 | Table 2. Results of linearity and regression analysis of hesperidin. [Click here to view] |
LOD and LOQ
According to studies, hesperidin LOD and LOQ are 0.008 and 0.024 μg/ml, respectively.
Accuracy and precision
The findings are shown in Tables 3, 4, and 5. The intra-day precision of hesperidin was 1.16% to 1.54%, and its accuracy was 0.74% to 1.72%. The intraday precision was 1.04%, and the interday precision was 1.72%. The best recovery of three injected samples was 100.85%–103.69%, demonstrating that the approach is more accurate.
Robustness and ruggedness
Table 6 displays the findings of the robustness analysis. Mobile phase, flow rate, wavelength, and column type still have no influence on resolution in terms of the same retention time and minimum RSD values.
Stability-indicating study
Table 7 displays the outcomes of the experiments performed to identify how acid, base, oxidation, and photolysis affected the standard hesperidin solution. Figures 4 and 5 illustrate the chromatograms of the standard stressed hesperidin solution after two hours under different stress levels. In the present investigation, the percentage of hesperidin degradation varies from 9.99% for oxidative degradation to 53% for base degradation. In this study, samples were subjected to photochemical stress, which caused 10.84%. Additionally, 18.5%, 9.99%, and 53.14% of the samples were affected by acid, oxidation and bases analysis (Jagwani et al., 2019).
Preparation and characterization of nanotransferosome
The thin-film hydration method was successfully used to prepare the hesperidin-loaded nanotransferosome, which reveals results like, particle size as 160 nm and the blank nanotransferosome showed 182 nm. According to Table 8, the polydispersity index for drug-free and drug-based nano-transferosome was 0.22 and 0.27, correspondingly. These observations highlighted that the polydispersity index (PDI) value is less than 0.3 and suggested narrow homogeneous distribution of particle size dispersion. On the chromatogram of the nanotransferosome, as shown in Figure 5 and Table 8, hesperidin displays a visible, recognizable peak. The average particle size of hesperidin-based nanotransferosome was in the range from 177 ± 4.1 to 270.2 nm. The PDI ranges from 0.205 ± 0.02 to 0.220 ± 0.050 and being highly capable of being formulated into nutraceuticals. Additional research, hesperidin-loaded polymer-based nanoparticles (PLGAs) showed a particle size of 204.4 nm, PDI of 0.253. Hesperidin-based PLGA nanoparticles may be used as a good source of anti-cancer agent (Balakrishnan et al., 2021; Chimagave et al., 2022; Patil et al., 2022).
Comparison with previously published HPLC methods
On the basis of mobile phase ratios, mobile phase flow rate, wavelength, column, stability study, limits, and application of the HPLC methods, a comparison of previously published methods and the recently created HPLC method was carried out. The comparison details were shown in Table 9. There is currently no single HPLC method that can be used for a variety of analyses, such as the estimation of hesperidin in nanoformulation, plant extract, and Ayurvedic-marketed products like Madhiphala rasayana, as well as the evaluation of hesperdin’s degradation behavior using the same parameters of developed HPLC method. When compared to previously developed methods and other hesperidin-related published literature, the current method, which uses methanol as the mobile phase and 0.1% OPA (50:50 v/v), 1 ml/minute flow rate, and 284 nm as the detection wavelength, is shown to be more accurate, affordable, and stable. In accordance with ICH rules that were within allowable bounds, the established procedure was validated. The developed approach shows a precise and simple assessment of hesperidin in nanotransferosomes, plant extracts, and Ayurvedic-marketed products, demonstrating the method is sensitive and reliable. Hesperidin appeared to be relatively resistant to acidic, alkaline, and high heat conditions, according to the results of a study on stress degradation. However, piperine was vulnerable to breakdown under conditions of oxidative and photolytic stress. This easy, stability-indicating RP-HPLC approach is therefore useful for further routine quality control analysis. Thus, this technique might be applied to regularly estimate hesperidin in vitro and in vivo.
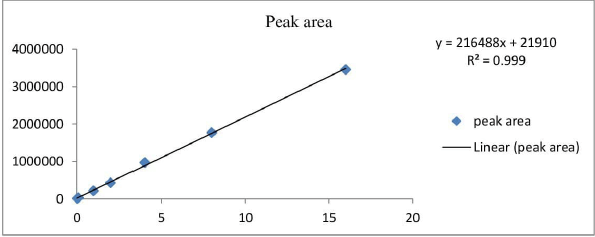 | Figure 3. Calibration curve for hesperidin 284 nm. [Click here to view] |
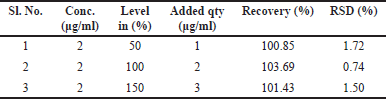 | Table 3. Results of accuracy study of hesperidin. [Click here to view] |
 | Table 4. Results of intra-day precision of hesperidin solution. [Click here to view] |
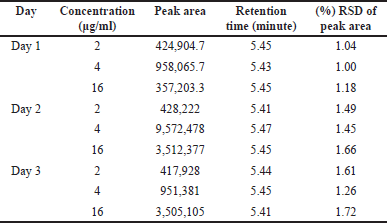 | Table 5. Results of interday precision data of hesperidin samples. [Click here to view] |
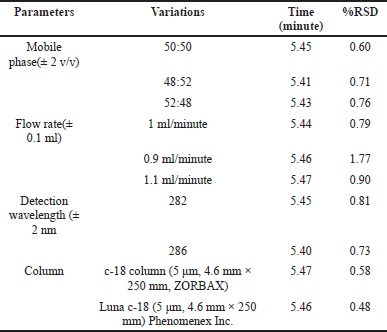 | Table 6. Analysis of robustness and ruggedness using hesperidin solution. [Click here to view] |
 | Table 7. Results of the stress degradation studies of hesperidin solution at 2 hours. [Click here to view] |
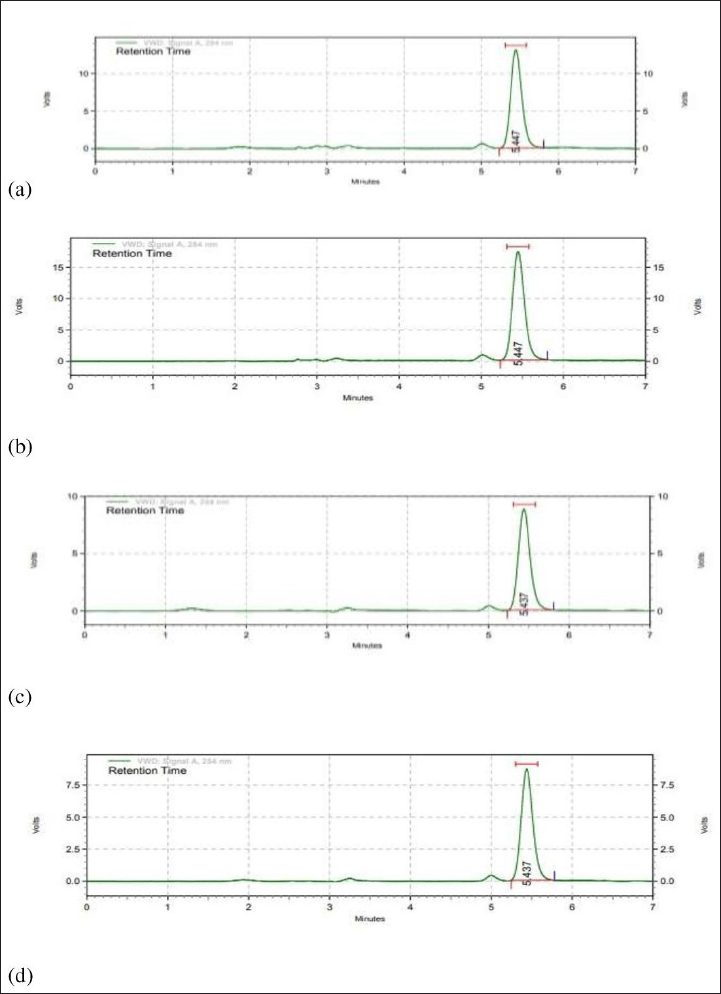 | Figure 4. Chromatograms of stressed hesperidin samples (10 μgml) under a) acid hydrolysis (0.1 N HCI), b) base hydrolysis (0.1 N NaOH), c) oxidative degradation (3% H2O2), and d) photolytic. [Click here to view] |
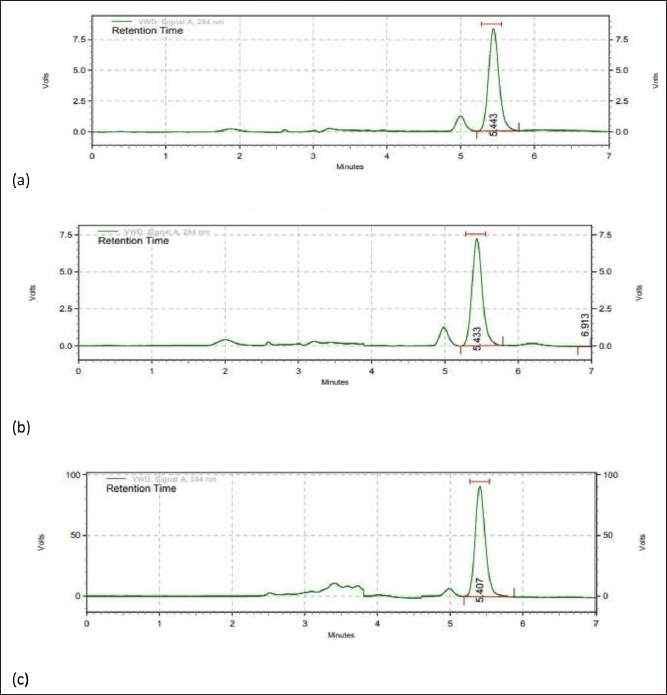 | Figure 5. RP-HPLC chromatogram of a) plant extract (orange peel), b) hesperidin-loaded nano transferosome, and c) marketed Ayurveda formulation. [Click here to view] |
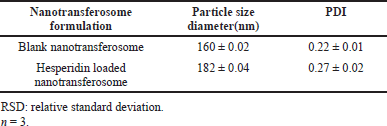 | Table 8. Results of characterization of hesperidin loaded nanotransferosome. [Click here to view] |
DISCUSSION
The presence of higher theoretical plates with a lower tailing number indicates the system’s efficiency. The percent RSD values were also less than 1% for all parameters, including retention interval, peak area, theoretical plates, and tailing number, indicating that the system was more efficient. Hesperidin peaks were not affected by any of the nano transferosome components or stress treatment. A calibration curve was generated by plotting the quantity of hesperidin (×) against the peak area. For the concentration range of 0.5–16 μg/ml, linearity of r = 0.999 was obtained using the equation y = 216,488 ×–21,910. Hesperidin was found using a C18 column with methanol and 0.1% orthophosphoric acid as the mobile phase. The best recovery of three injected samples was 100.85%–103.69%, demonstrating that the approach is more accurate. The method’s RSD values for intra- and interday precision were less than 2%. In terms of similar retention time and lower RSD values, parameters such as mobile phase, flow rate, detection wavelength, and column type had no effect on resolution. Triplicate analysis of the samples revealed that the established procedure was more robust than those previously described. Hesperidin exhibited a degree of degradation ranging from 9.99% for oxidative degradation to 53% for base damage in the current investigation. The developed approach accurately predicted the degradation of hesperidin.
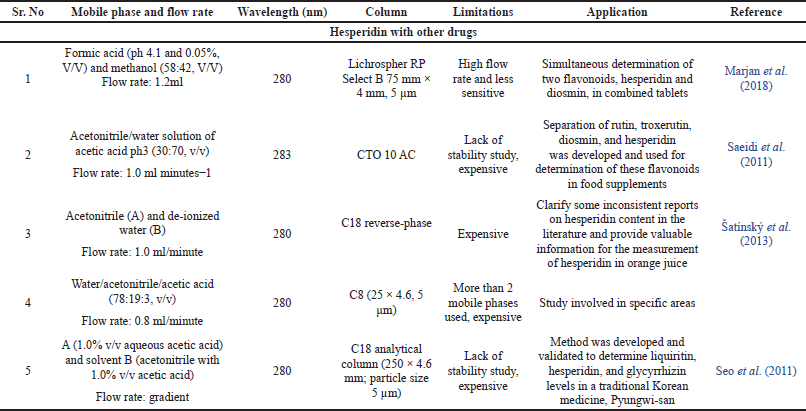 | Table 9. Comparison between previously published HPLC methods. [Click here to view] |
CONCLUSION
The present approach was effectively established and validated, using the same mobile phase to estimate hesperidin in plant extract, Ayurvedic formulation, and hesperidin-loaded nanotransferosome, as well as to determine degradation behavior. The established RP-HPLC method was reliable, accurate, and easy, and it met the FDA’s criterion for hesperidin quantitative determination and its validation. Hesperidin may withstand acidic, oxidative, and photolytic environments, but is more likely to get damaged in base degradation conditions, according to forced degradation experiments. The results of the degradation experiments are used to determine the best conditions for formulation, development, and storage. The lack of excipient peaks in hesperidin analysis in plant extract, Ayurvedic formulation, and nanotransferosome further proved that the established method may be used for routine hesperidin analysis in various dosage forms.
ACKNOWLEDGMENTS
We appreciate the gifts of pure hesperidin drug from Shaanxi Yi a Biologicals in China and Himalaya Wellness Company, Bengaluru, and also the (LIPOID 90G) Phospholipid provided as a gift sample for the study by Lipoid GmbH in Ludwigshafen, Germany. This work was financially supported by the KLE Academy of Higher Education and Research in Belagavi, Karnataka, India.
LIST OF ABBREVIATIONS
ICH: International Council for Harmonisation; LOD: Limit of detection; LOQ: Limit of quantification; RP-HPLC: Reverse phase high performance liquid chromatography; RSD: Relative standard deviation.
CONFLICT OF INTEREST
The authors declare no conflict of interests for this manuscript.
ETHICAL APPROVAL
This study does not involve any animals or human subjects.
AUTHOR’S CONTRIBUTION
All the authors have equally contributed to the method development and its validation as well as design and characterization of hesperidin loaded nanoformulation. Supriya S. Chimagave contributed in the conceptualization and development & validation of RP-HPLC method for Hesperidin. Dr. Sunil S. Jalalpure guided for this project and given a technical support. Akshay K. Patil involved in design of nanotransferosome. Bhaskar K. Kurangi guided for the HPLC validation work and manuscript writing.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ahmed HA, Aboul-Enein AM, Abou-Elella F, Salem SH, Aly HF, Nassrallah A, Salama Z. Nano-formulations of hesperidin and essential oil extracted from sweet orange peel: chemical properties and biological activities. Egypt J Chem, 2021l 1;64(9):5373–85. CrossRef
Asensio-Regalado C, Alonso-Salces RM, Gallo B, Berrueta LA, Era B, Pintus F, Caddeo C. Tempranillo grape extract in transfersomes: a nanoproduct with antioxidant activity. Nanomaterials, 2022; 23;12(5):746. CrossRef
Anwer MK, Jamil S, Ansari MJ, Al-Shdefat R, Abdel-Kader MS. A new improved stability-indicating RP-HPLC method for determination of diosmin and hesperidin in combination. Int. J Biol Sci, 2014; 3(6):41–6.
Balakrishnan K, Casimeer SC, Ghidan AY, Ghethan FY, Venkatachalam K, Singaravelu A. Bioformulated hesperidin-loaded PLGA nanoparticles counteract the mitochondrial-mediated intrinsic apoptotic pathway in cancer cells. J InorgOrganometPolym Mater, 2021; 31(1):33143. CrossRef
Bhalerao A. Rasika, Mehta R. Divya, Raka K. Neha, Markad M. Akansha, Pawar R. Aishwarya, Undre S. Pratiksha, Damle C. Mrinalini, stability indicating HPTLC method for hesperidin, Int J Pharm Biol, 2019; 9(3):832–7.
Chaudhary H, Kohli K, Kumar V. Nano-transfersomes as a novel carrier for transdermal delivery. Int J Pharm, 2013; 454(1):36780. CrossRef
Chimagave SS, Jalalpure SS, Kurangi BK. Preparation and development of polyherbal formulation of medicinal plants for antiarthritic activity. Indian J Health Sci Biomed Res (KLEU), 2020; 13(2):120. CrossRef
Chimagave S. Supriya, Jalalpure S. Sunil, Patil K. Akshay, Kurangi KB. Development and validation of stability indicating uv-spectrophotometric method for the estimation of hesperidin in bulk drugs, plant extract, Ayurveda formulation and nanoformulation. Indian J Pharm Educ Res, 2022; 56(3):865–72. CrossRef
Ferrari PC, Correia MK, Somer A, Ribeiro MA, Astrath NG, Sato F, Novatski A. Hesperidin-loaded solid lipid nanoparticles: development and physicochemical properties evaluation. J Nanosci Nanotechno, 2019; 19(8):4747–57. CrossRef
Hsieh WC, Fang CW, Suhail M, Vu QL, Chuang CH, Wu PC. Improved skin permeability and whitening effect of catechin-loaded transfersomes through topical delivery. Int J Pharm, 2021; 607:121030. CrossRef
ICH. Validation of analytical procedure: Methodology International Conference on Harmonization. ICH harmonised tripartite guideline - validation of analytical procedures: text and methodology Q2(R1). ICH, Geneva, Switzerland, 2005.
Jagwani S, Jalalpure S, Dhamecha D, Hua GS, Jadhav K. A stability indicating reversed phase HPLC method for estimation of trans-resveratrol in oral capsules and nanoliposomes. Anal Chem Lett, 2019; 9(5):711–26. CrossRef
Joshi SA, Jalalpure SS, Kempwade AA, Peram MR. Development and validation of HPLC method to determine colchicine in pharmaceutical formulations and its application for analysis of solid lipid nanoparticles. Curr Pharm Anal, 2018; 14(1):76–83. CrossRef
Kempwade A, Taranalli A, Jadhav K. Development and validation of UV Spectrophotometric method to study stress degradation behaviour of Rizatriptan Benzoate. Spectros Spect Anal 2015; 35(1):137–40.
Kumar S, Devanna N. Spectroscopic determination of hesperidine in ayurvedic formulation madiphalRasayana. Der Pharmacia Sinica, 2013; 4(3):22–29.
Kurangi BK, Jalalpure SS. Review of selected herbal phytoconstituents for potential melanoma treatment. Indian J Health Sci Biomed Res (KLEU), 2018; 11(1):3. CrossRef
Kurangi B, Jalalpure S, Jagwani S. A validated stability-indicating HPLC method for simultaneous estimation of resveratrol and piperine in cubosome and human plasma. J Chromatog. B, 2019; 1122:39–48. CrossRef
Kurangi B, Jalalpure S. A validated stability-indicating RP-HPLC method for piperine estimation in black pepper, marketed formulation and nanoparticles. Indian J Pharm Educ Res, 2020; 54(3):S677–86. CrossRef
Kurangi B, Jalalpure S, Jagwani S. Formulation and evaluation of resveratrol loaded cubosomal nanoformulation for topical delivery. Curr Drug Deliv, 2021; 18(5):607–1. CrossRef
Kudatarkar N, Jalalpure S, Balekundri A, Kurangi B. Analytical method development and validation for estimation of chrysin in chrysin loaded phytosomes using high performance thin layer chromatography. J Liq Chromatogr Relat Technol, 2022; 6:1–6.
Kudatarkar N, Jalalpure S, Patil VS, Kurangi B. System biology and chemoinformatics approaches to decode the molecular mechanisms of Chrysin against colon cancer. J Appl Pharm Sci, 2021; 11(09):057–65.
Lee JT, Pao LH, Hsieh CD, Huang PW, Hu OY. Development and validation of an LC-MS/MS method for simultaneous quantification of hesperidin and hesperetin in rat plasma for pharmacokinetic studies. Anal Methods, 2017; 9(22):3329–37. CrossRef
Li YM, Li XM, Li GM, Du WC, Zhang J, Li WX, Xu J, Hu M, Zhu Z. In vivo pharmacokinetics of hesperidin are affected by treatment with glucosidase-like BglA protein isolated from yeasts. J Agric Food Chem, 2008; 56(14):5550–7. CrossRef
Lobo R, Richa A, Prabhu KS, Shirwaikar AA, Mamtha B, Nipun D, Vijay S, Annie S. Development and validation of a stability-indicating HPTLC method for analysis of hesperidin in pharmaceutical dosage form. J Pharm Res, 2010; 3(7):1648–51.
Majumdar S, Srirangam R. Solubility, stability, physicochemical characteristics and in vitro ocular tissue permeability of hesperidin: a natural bioflavonoid. Pharm Res, 2009,;26(5):1217–25. CrossRef
Pandey P, Khan F. A mechanistic review of the anticancer potential of hesperidin, a natural flavonoid from citrus fruits. Nutr Res, 2021; 92:21–31. CrossRef
Parab Gaonkar V, Hullatti K. Quality assessment and RP-HPLC method development for estimation of curcuminoids in Curcuma longa: a quality by design approach. J Liq Chromatogr Relat Technol, 2021; 44(1-2):95–102. CrossRef
Parab Gaonkar V, Mannur VK, Hullatti K. Quality assessment and analytical quality by design-based RP-HPLC method development for quantification of Piperine in Piper nigrum L. Future J Pharm Sci, 2022; 8(1):1–9. CrossRef
Patil K. Akshay, Gaonkar P. Vishakha, Chimagave SS, Hullatti K. Pharmacognostical and biological evaluation of Mayurshikha (Actiniopteries dichotoma Bedd): an ayurvedic medicinal plant. Int J Ayurvedic Med, 2022; 13(2):338–44. CrossRef
Patil K, Mall A. Hepatoprotective activity of Menthaarvensis Linn. leaves against CCl4 induced liver damage in rats. Asian Pac J Trop, 2012; 2:S223–6. CrossRef
Peram MR, Jalalpure SS, Joshi SA. A simple RP-HPLC method for the determination of curcumin in commercial turmeric herbal products. Indian J Nat Prod Resour, 2019; 33(1):30–4.
Piponski M, Stoimenova TB, Topkoska M, Stefov S, Piponska M, Serafimovska GT. Development and validation of a fast and simple rp-hplc method for the determination of diosmin and hesperidin in combined tablet dosage form. Maced J Chem Chem Eng, 2018; 37(2):127–34. CrossRef
Rao K, Aziz S, Roome T, Razzak A, Sikandar B, Jamali KS, Imran M, Jabri T, Shah MR. Gum acacia stabilized silver nanoparticles based nano-cargo for enhanced anti-arthritic potentials of hesperidin in adjuvant induced arthritic rats. Artif Cells Nanomed Biotechnol, 2018; 46(sup1):597–607. CrossRef
Rekha SS, Pradeepkiran JA, Bhaskar M. Bioflavonoid hesperidin possesses the anti-hyperglycemic and hypolipidemic property in STZ induced diabetic myocardial infarction (DMI) in male Wister rats. J Nutr Intermed Metab, 2019; 15:58–64. CrossRef
Rodrigues K, Nadaf S, Rarokar N, Gurav N, Jagtap P, Mali P, Ayyanar M, Kalaskar M, Gurav S. QBD approach for the development of hesperetin loaded colloidal nanosponges for sustained delivery: in-vitro, ex-vivo, and in-vivo assessment. OpenNano, 2022; 7:100045. CrossRef
Šatínský D, Jägerová K, Havlíková L, Solich P. A new and fast HPLC method for determination of rutin, troxerutin, diosmin and hesperidin in food supplements using fused-core column technology. Food Anal Methods, 2013; 6(5):1353–60. CrossRef
Saeidi I, Hadjmohammadi MR, Peyrovi M, Iranshahi M, Barfi B, Babaei AB, Dust AM. HPLC determination of hesperidin, diosmin and eriocitrin in Iranian lime juice using polyamide as an adsorbent for solid phase extraction. J Pharm Biomed Anal J, 2011; 56(2):419–22. CrossRef
Seo CS, Lee JA, Jung D, Lee HY, Lee JK, Ha H, Lee MY, Shin HK. Simultaneous determination of liquiritin, hesperidin, and glycyrrhizin by HPLC-photodiode array detection and the anti-inflammatory effect of Pyungwi-san. Arch Pharm Res, 2011; 34(2):203–10. CrossRef
Sharma RK, Jalalpure SS, Chouhan MK, Deshpande S, Acharya R, Hegde S. Decipher the inhibitory potential of phytocompounds from Leptadeniareticulata on dopamine D2 receptor to enhance prolactin secretion. Drug Res, 2022; 72(04):189–96. CrossRef
Shetti P, Jalalpure SS. A single robust stability-indicating RP-HPLC analytical tool for apigenin quantification in bulk powder and in nanoliposomes: a novel approach. Future J Pharm Sci, 2021; 7(1):1–9. CrossRef
Somasundaram I, Sumathi S, Bhuvaneshwari SP, Shafiq KM, Shanmugarajan TS. Formulation and evaluation of hesperidin-loaded chitosan nanosuspension for brain targeting. Drug Invent, 2018; 10(3):279–84.
Sulaiman GM, Waheeb HM, Jabir MS, Khazaal SH, Dewir YH, Naidoo Y. Hesperidin loaded on gold nanoparticles as a drug delivery system for a successful biocompatible, anti-cancer, anti-inflammatory and phagocytosis inducer model. Sci Rep, 2020; 10(1):1–6. CrossRef
Tavade S, Patil K, Kurangi B, Suryawanshi S. Development and validation of UV-spectrophotometric method for estimation of Berberine Hydrochloride in Tree Turmeric, marketed formulation & PLGA nanoparticles. Indian J Pharm Educ Res, 2022; 22(3):1–8. CrossRef
Yuan M, Niu J, Xiao Q, Ya H, Zhang Y, Fan Y, Li L, Li X. Hyaluronan-modified transfersomes based hydrogel for enhanced transdermal delivery of indomethacin. Drug Deliv, 2022; 29(1):1232–42. CrossRef
Zhang L, Ling W, Yan Z, Liang Y, Guo C, Ouyang Z, Wang X, Kumaravel K, Ye Q, Zhong B, Zhang J. Effects of storage conditions and heat treatment on the hesperidin concentration in Newhall navel orange (Citrus sinensis Osbeck cv. Newhall) juice. J Food Compost Anal, 2020; 85:103338. CrossRef