INTRODUCTION
Emerging cases of bone tissue damage due to bone tumors, trauma, and an increasingly aging population have triggered advances in bone tissue engineering (BTE) (Li et al., 2019). Over the past few decades, synthetic bone grafts made of metal and ceramic have been clinically approved, but some drawbacks have been found, such as the need for secondary surgical removal after restoring tissue function and the low bioactivity (Elangomannan et al., 2017). Advanced BTE has developed into a rapidly growing field focusing on the application of various biodegradable scaffolds serving as temporary matrices to mimic the extracellular matrix of bone (Griffon et al., 2011).
Synthetic polymers are one of the promising scaffold materials due to their ability to control the geometry and strength of the scaffold (Kandelousi et al., 2019). One of them, polycaprolactone (PCL), an FDA-approved biomaterial-based scaffold, has been extensively studied for its outstanding mechanical properties, biodegradability, biocompatibility, and low toxicity (Nyberg et al., 2017). However, its hydrophobicity and lack of cell-specific ligands lead to an undesirable surface for cell attachment (Yao et al., 2014). Previous studies have identified that the addition of bioceramics such as hydroxyapatite [Ca10(PO4)6(OH)2, Hap] to synthetic polymers increased biocompatibility and affinity in bone tissue, facilitating cell proliferation, and osteoconduction. It works by ion release and deposition or mimics natural bone in vitro and in vivo (Cho et al., 2019; Elangomannan et al., 2017). The addition of natural biopolymers such as collagen, gelatin, or chitosan to scaffolds has also been proposed to overcome the lack of cell adhesion motifs (Asadpour et al., 2020; Bendtsen et al., 2017; Gao et al., 2017).
Bone scaffolds are designed to grow cells in a physiologically relevant three-dimensional (3D) space and form clinically functional tissues (Cho et al., 19). Among the various methods for the fabrication of scaffolds, electrospinning techniques produce nanoscale fibrous scaffolds with an attractive high surface area-to-volume ratio required for cell attachment and growth (Lu et al., 2013). However, such highly porous electrospun nanofibrous mats also present weaknesses, particularly in terms of fragility in handling, which remains a challenge. PCL, even though it has been blended with natural polymers, can still play a dominant role as a hydrophobic material, depending on the ratio of polymer and biopolymer used (Rajzer et al., 2014; Zafari et al., 2020). Poor relocation of cell suspensions in hydrophobic matrices along with low cell attachment is one of the challenging problems encountered during in vitro cell seeding. These problems are one of the barriers to providing reproducible bone scaffolds.
While many studies have attempted to develop PCL modifications (Cho et al., 2019; Hosseini et al., 2017; Rajzer et al., 2014), few have addressed the seeding methods that are prerequisites for the success of BTE (Impens et al., 2010; Kruyt et al., 2008). The seeding technique determines the initial number and spatial distribution of cells attached to the scaffold and will affect the specific proliferation, migration, and differentiation of the engineered implant, so it must be reliable and robust (Chen et al., 2011). The most common seeding techniques rely mainly on complex bioreactors or static steps (Fu et al., 2012), but few can achieve homogeneous distribution and efficient seeding. Several tools and strategies have been used for dynamic seeding but yielded contradictory results (Griffon et al., 2011; Lee et al., 2013). Although the list of seeding techniques tested for tissue engineering is extensive, the ideal technique for seeding cells on 3D matrices needs some improvement.
This study focuses on the feasibility of a facile seeding method on hydrophobic dominant nanofibrous mats as an alternative cell carrier for BTE. HAp-PCL with and without natural biopolymer addition was used as a scaffold model to evaluate the seeding method. Here, we propose a modified static seeding method using a support seeding construct to translocate cell suspension within the matrix for the early hour seeding period, together with a concentrated cell suspension in serum-deficient media to provide intimate cell presentation on nanofibrous mats. The seeding construct was also purposed for the safe handling of the nanofibrous mats during the reciprocating seeding process. This paper discusses some concerns in seeding methods to optimize cell-matrix interactions on hydrophobic matrices by emphasizing osteoblast preferential adhesion and growth patterns on different surface characteristics of biopolymer composites. Furthermore, applying this simple seeding method is expected to inspire those who are starting to work with the challenge of inducing bone morphogenesis in BTE scaffolds.
MATERIALS AND METHODS
Materials
Hydroxyapatite derived from common cockle (Cerastoderma edule) shells, Yogyakarta, at a Ca/P molar ratio of 1.68, was synthesized and characterized in the Department of Physics, Universitas Gadjah Mada, Yogyakarta, Indonesia. In brief, calcium carbonate (CaCO3) isolated from common cockle shells was reacted with diammonium hydrogen phosphate (NH4)2 HPO4 and alkalified with ammonium hydroxide (NH4OH) at above pH 9, 70°C for 1 hour. The reaction product was washed with distilled water and further calcined at 1,000°C for 6 hours.
Other materials used were as follows: PCL 45 kDa (Sigma-Aldrich, USA), fish collagen (CV Bio Chitosan, Indonesia), extra pure gelatin (Sumber Ilmiah Persada, Surabaya), chitosan with a deacetylation degree of more than 75% (Sigma-Aldrich, USA), acetone, chloroform (Merck, USA), antibiotic-antimycotic (Sigma-Aldrich), Dulbecco’s Modified Eagle Medium (DMEM), heat-inactivated fetal bovine serum (FBS), and PrestoBlueTM and the LIVE/DEAD Cell Viability Kit (Thermo Fisher Scientific, USA). The experiments utilized a cell line with osteoblastic properties (Saos-2), which was generously donated by the Gurdon Institute, University of Cambridge. All other chemicals were of reagent grade.
Preparation of PCL-HAp-based electrospun nanofibrous mats
Hydrophobic PCL-HAp nanofibrous mats with and without biopolymer reinforcement (collagen, gelatin, and chitosan) were fabricated in the Department of Physics, Airlangga University, Surabaya, using our previous method (Aminatun et al., 2021). Four composites were made, namely, PCL-HAp, PCL-HAp-collagen, PCL-HAp-gelatin, and PCL-HAp-chitosan, at a ratio of 15:1:1. In brief, the precursor solution was prepared by dissolving PCL 30% by weight in chloroform, 2 wt% of HAp, collagen, and gelatin in water, and 1 wt% of chitosan in acetic acid 2% (v/v). The PCL and biopolymer solutions were mixed, stirred, and formed into a single phase with the addition of acetone. Next, the HAp solution was mixed homogeneously to obtain a PCL-HAp-biopolymer solution. The nanofibrous mats were prepared by electrospinning of the precursor solution using a 26 kV, 0.5 l/hour flow rate with a flat aluminum foil collector arranged at a distance of 10 cm from the glass syringe tip.
Modified seeding method
The nanofibrous mats were used as a model of a predominantly hydrophobic scaffold with a size of 10x10x3 mm. An illustration of the modified seeding method is shown in Figure 1. To support hydrophobic nanofibrous mats, a special construct was arranged, as shown in Figure 1A. It consisted of 15 × 15 cm flat carrier glass sandwiched between two cylindrical supports (1 cm diameter and 0.5 cm height). The lower cylinder, which can be a glass cylinder or an inert silicone ring, was used for safe handling and easy removal of nanofibrous mats, while the upper cylinder was intended to hold the hydrophobic matrix from floating on the culture medium.
 | Figure 1. An illustration of modified cell seeding method that utilizes carrier glass sandwiched between two cylindrical supports to prolong cell presentation on the hydrophobic surface and safe handling of nanofibrous mats during reciprocating seeding process. [Click here to view] |
The seeding apparatus and nanofibrous mats were sterilized by exposing each side of the component under a UV lamp (Analytic Jena, Germany) for 1 hour (Fig. 1B). The nanofibrous mats were placed on the carrier glass; then, the seedling construct was transferred into 35 mm sterile Petri dishes (Cellvis, USA). The matrix was moistened with 200 μl of DMEM (Gibco, Invitrogen) for 30 minutes through the top of the glass cylinder wall (Fig. 1C).
The experiments utilized a cell line with osteoblastic properties (Saos-2), which was generously donated by the Gurdon Institute, University of Cambridge. The Saos-2 cells were grown in 75 cm2 flasks incubated in 5% CO2 at 37°C with a relative humidity of 95% overnight until about 80% confluence. The cells were then centrifuged at 1,200 rpm after trypsinization, and the cells resuspended in a seeding medium consisting of a DMEM medium supplemented with 1% antibiotic-antimycotic (Sigma-Aldrich) and 2% FBS (Gibco, Invitrogen). The cell suspension (200 μl) containing 2 × 105 cells was seeded onto each nanofibrous mat arranged in the seeding construct. Subsequently, the constructs were incubated under static conditions at 37°C, 5% CO2, and relative humidity at 95% for 4 hours (Fig. 1D) with immediately returning the leaking medium every hour (Fig. 1E).
The seeded matrix assemblies were then transferred to 12-well plates (Fig. 1F), and 2 ml of fresh DMEM supplemented with 1% antibiotic-antimycotic and 10% FBS (complete medium) was added to each well and incubated at 37°C, 5% CO2, and relative humidity at 95% for 7 days (Fig. 1G). Samples were taken on days 1 and 7 for analysis of cell adhesion and proliferation analysis. Media replacement was done every 2 days. The conventional seeding method was used for comparison. In brief, sterilized nanofibrous mats were placed directly on each well on a 12-well plate and seeded with 2 × 105 cell suspensions in 2 ml of a DMEM complete medium. The seeded matrices were incubated at 37°C, 5% CO2, and relative humidity at 95% for 7 days.
Evaluation of cell adhesion and proliferation
The cellular responses of the Saos-2 osteoblast, namely, adhesion and proliferation on PCL-CAP nanofibrous mats with and without biopolymer modification, were determined using the PrestoBlueTM assay after 1 and 7 days of culture. This test is based on the resazurin reduction test by measuring the increase in mitochondrial activity with an increase in the number of cells represented as an increase in resorufin absorbance (pink) and a decrease in resazurin (blue) (Varçin et al., 2021). Both resazurin and resorufin are water-soluble and nontoxic (Sonnaert et al., 2015); therefore, the same sample was used for analysis on days 1 and 7. Briefly, the seeded nanofibrous mats arranged in the seeding constructs were transferred to new 12-well plates, rinsed with 500 μl of PBS twice. Then, 300 μl of the PrestoBlueTM mix solution (10% PrestoBlueTM 10× reagent diluted with 90% DMEM basal medium) was added and incubated at 37°C, 5% CO2 (relative humidity at 95%), for approximately 2 hours until the reagent changed from blue to pink. An aliquot of a 100 μl sample was taken and transferred to the 96-well plates in triplicate, and the optical density was measured at 570 and 600 nm wavelengths using the Multiskan GO Thermo Scientific Plate Reader (USA). The viability of 2 × 105 cells/ml of the Saos-2 cells grown on 12-well plates was analyzed on day 1 and used as a control. Cell viability was calculated using the PrestoBlue™ technical note: Processing Absorbance Data Obtained Using Viability Reagent shown in
where
A1 = absorbance of sample at 570 nm.
A2 = absorbance of sample at 600 nm.
M1 = absorbance of media only at 570 nm.
M2 = absorbance of media only at 600 nm.
C1 = absorbance of control cells at 570 nm.
C2 = absorbance of control cells at 600 nm.
The absorbance at 600 nm was used as a reference or normalization wavelength.
The proliferation and viability of cells in the nanofibrous mats were analyzed on day 7 in the culture using confocal microscopy. Briefly, the growth media were discharged, and the seeded mats were washed with phosphate-buffered saline (PBS, Sigma-Aldrich, USA) twice. Subsequently, 200 μl of the LIVE/DEAD Staining Reagent (LIVE/DEAD Cell Viability Kit, Thermo Fisher, USA) was incubated at room temperature for 10 minutes under a condition protected from light. The cells were observed using a confocal laser scanning microscope (CLSM, Olympus FV 1200) with alive and dead cells shown as green and red, respectively.
Evaluation of cell behaviour on natural biopolymer reinforced nanofibrous mats
The behavior of osteoblasts grown on different surfaces characteristic of nanofibrous mats was observed by a scanning electron microscope (SEM). In brief, the scaffolds were washed with PBS three times and gradually dehydrated with a gradient ethanol solution (30% and 70%), and each gradient was dehydrated for 15 minutes and dried. Finally, the cell-adhered nanofibrous mats’ sections were observed by SEM (Hitachi SU3500, Japan) after sputter-coating with gold (MC-1000, Hitachi™, Japan) to analyze the morphology and homogeneity.
Scaffold characteristics corresponding to cell adhesion properties were analyzed, including zeta potential, contact angle, and porosity. The change of electrical charge was measured using a zeta potential analyzer (Horiba SZ-100, Japan).
Contact angle measurement
To examine the wettability of the scaffold, the contact angles were analyzed by the principle of static contact angle measurement of a water droplet in an air atmosphere (Zhao and Jiang, 2018). 0.01 ml of distilled water was dropped on 1 cm × 1 cm scaffold composites, whose shape was expected to be flatter with time. The contact angles were measured by taking photos of the droplet after 10 seconds. Three points were chosen for each sample, and the average value was calculated as the result. Finally, the average contact angle with standard deviation (SD) was reported.
Determination of porosity of the nanofibrous mats
The porosity of the electrospun membranes was determined using the ethanol immersion method based on Song et al. (2017). In this method, dry weights of the scaffolds were recorded, and then the scaffolds were immersed in absolute ethanol for 1 hour. Wet weights were also recorded. Three specimens were evaluated for each sample, and the averages were reported. The porosity of each sample was determined through the following equation:
where P is porosity, Ws is swollen scaffold weight, Wd is dry scaffold weight, Dethanol is ethanol density, and Vscaffold is volume of the swollen scaffold.
Statistical analysis
Statistical tests used two-way analysis of variance (ANOVA) for proliferation analysis and one-way ANOVA for the other multiple comparisons with Tukey’s multiple comparison test or Student’s t-tests for comparison between two groups. p values greater than 0.05 were considered insignificant in all analyses.
RESULTS AND DISCUSSION
Modified seeding method
The distribution of cells in the early stages plays an important role in the regeneration of defective tissues supported by temporary scaffolds (Abbasi et al., 2020). It is of utmost importance to anticipate the hydrophobic characteristic of synthetic polymer scaffolds against cell seeding barriers. It was evident that when cells in the culture medium were seeded on top of the porous hydrophobic scaffold or injected into their interior for seeding, most of the pores remained empty because the scaffold did not absorb the culture medium, resulting in heterogeneous cell seeding (Oh et al., 2013). Since the advanced use of PCL in BTE accounted for its hydrophobic nature resulting in slow degradation and marked increase in mechanical strength, efforts are needed to improve the functionality of the scaffold in providing adequate cells with a homogeneous spatial distribution to support bone growth. Previous research proposed mineralized PCL composite scaffolds, but the contact angle was still very high (over 130°), eliminating attempts to increase the wettability of the matrix (Gorodzha et al., 2017). Greater attention should be paid to seeding methods for developing functional scaffolds; therefore, several concerns have been observed during this study to minimize the physicochemical barriers of HAp-PCL nanofibrous mats.
First, a high-density cell suspension in a small volume of seeding medium was applied to immerse the entire nanofibrous mat to allow effective cellular contact with the surrounding nanofibers for adhesion. Our preliminary study revealed that a volume of 200 μl containing 2 × 105 cells was sufficient to submerge a 10 × 10 × 3 mm matrix. Having too much volume that reduces cell contact with the matrix or too small a volume that causes an uneven distribution of cells throughout the scaffold should be avoided. This attempt was in accordance with Yassin et al. (2015), who found that higher seeding density resulted in higher cell adhesion to poly(l-lactide-co-ε-caprolactone) and promoted better new bone formation in animal studies. Other similar evidence was reported by Grayson et al. (2008), who applied a high seeding density to a 3D-printed porous PCL functionalized with various minerals that induced bone regeneration, in addition to the porous structure of the scaffold.
Second, a static seeding method was applied to prolong the contact time of cell suspensions in the matrix and to reduce the tendency of media leaking from the hydrophobic matrix. We noticed that HAp-PCL mats did not exhibit media adsorption (wettability) until 10 minutes when the cell suspension was dropped on the surface before leaking into the well, indicating their hydrophobic characteristics. The advantageous characteristics of dynamic cell seeding in providing homogeneous cell distribution and nutritional support (Grayson et al., 2008) were considered counterproductive for seeding cells of the hydrophobic matrix due to the limited chance for cell anchorage to matrix surfaces in constant fluid perfusion. From our observations, the cell suspension seeded by the conventional method took only a few seconds before falling into the well, thus giving no time for the cells to adhere to the surface of the scaffold. The modified seeding method was emphasized to draw back the cells that fall from the scaffold.
Accommodating these two purposes, a simple seeding apparatus was implemented consisting of a glass carrier sandwiched between two cylindrical glasses for physical support during seeding. The nanofibrous mats were vulnerable during the reciprocating seeding procedure; therefore, flat glass was used to translocate the cell suspension and support the matrix, whereas the two glass cylinders allowed the sliding glass to be lifted and avoid the matrix floating in the culture medium. The low cell adhesion capacity of untreated glass (Lerman et al., 2018) with optical clarity for easy observation of the seeding process was considered for this material of choice. Spare sliding glass may be needed to make it easier for the researchers to flip through the nanofibrous mats. The assembly does not need to be glued, so the glass can be easily lifted and removed from the culture plate.
Third, instead of using 10% FBS supplementation, the Saos-2 cells were resuspended in seeding media supplemented with reduced FBS aiming to avoid the inherent adsorption competition between albumin and cells for hydrophobic surfaces. This attempt was based on previous findings, such as an increase in the number of cells adhering to the scaffold by 90% or more in the absence of FBS during the initial seeding step (Carré et al., 2010). Our preliminary study applying the modified seeding method on HAp-PCL using DMEM supplemented with 10% FBS resulted in a very low percentage of cell viability (unpublished data). However, a low concentration of 2% FBS was used to supplement cell adhesion substances, such as fibronectin present in the serum (Carré et al., 2010). After 4 hours of seeding in which cells were assumed to have adhered to the matrix, 2 ml of a complete medium supplemented with 10% FBS was refilled onto the cultured cells and further incubated for up to 7 days of culture.
Cell adhesion performance of nanofibrous mats following the conventional and modified seeding methods
The performance of HAp-PCL nanofibrous mats in supporting cell adhesion after cell seeding using unmodified (conventional, CD) and modified (MD) methods was analyzed using the PrestoBlueTM assay, as shown in Figure 2. This analysis was based on a positive correlation between metabolic activity and the number of attached cells (Sonnaert et al., 2015). As shown in Figure 2A and B, the modified seeding method (MD1) on HAp-PCL nanofibrous mats and biopolymer composites resulted in significantly higher cell viability indicating higher cell adhesion than the conventional method (CD1). The results of the one-way ANOVA analysis showed that the CD1 data were in the lower group compared to MD1, namely, in groups E, F, and G, with no significant difference between HAp-PCL nanofibrous composites with and without biopolymer supplementation. The encouraging results were shown by the modified seeding method wherein nanofibers supplemented with collagen, gelatin, and chitosan gave higher cell viability in groups A, B, C, and D with varying degrees of increased cell viability than HAp-PCL without a biopolymer that was in groups E and F.
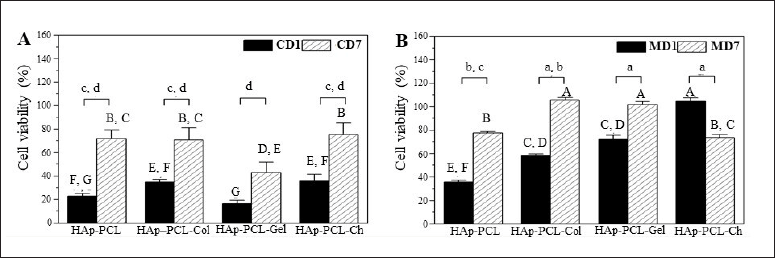 | Figure 2. The viability of osteoblast cells attached to the nanofibrous mats analyzed by PrestoBlueTM after seeding using (A) conventional and (B) modified methods determined at day 1 (CD1 and MD1) and day 7 (CD7 and MD7) of culture. Statistical analysis: significant differences in data were analyzed by ANOVA with Tukey’s pairwise post hoc test at p<0.05 for means that do not share a letter; (i) A to G (capital letters) for cell adhesion analysis by one-way ANOVA with F = 90.70, p = 0.000; (ii) a to d (small letters) for cell proliferation analysis by two-way ANOVA with F = 53.02, p = 0.000 for day factor and F = 13.51, p = 0.000 for nanofibrous formula factor. [Click here to view] |
Cells attached to the HAp-PCL nanofibers showed relatively low viability on day 1 after seeding by conventional (22.72 ± 2.32%) with a slight increase in modified (35.49 ± 1.94%) methods. This feature is likely due to osteoblasts not adapting to the new environment in the matrix as mentioned by Song et al. (2017) even in a hydrophilic collagen scaffold. Previous studies have also demonstrated low adhesion of mineralized PCL scaffolds (Elangomannan et al., 2017; Gorodzha et al., 2017). One explanation is due to the hydrophobicity of PCL, which did not show an improvement in wettability upon the addition of silicate containing hydroxyapatite, which was otherwise beneficial in increasing the hydrophilicity and cell adhesion of another hydrophobic polymer (Gorodzha et al., 2017). Efforts to improve osteoblast adhesion to mineralized PCL scaffolds have been carried out previously by adding pores on nanofibers with a carbon nanotube (Elangomannan et al., 2017) or a porous mini-deposition produced matrix (Jiang et al., 2012). A recent report has also emphasized the adhesion preference of osteoblasts on porous charged PCL fibers prepared by controlled electrical electrospinning with increased hydrophilicity implied by a reduced contact angle as well as increased surface roughness on porous fibers (Metwally et al., 2020). Here, we found that cell adhesion was augmented on biopolymer-reinforced nanofibrous mats after modified seeding with viability reaching 50% to 100% at day 1 after seeding, suggesting a beneficial effect of chemical surface modification favoring cell-matrix interactions through either nonspecific or specific integrin-ligand binding (Chen et al., 2021; Metwally et al., 2020). These results indicated that the modified seeding method provided better cell adhesion with success in applying the simultaneous effect of prolonged cell presentation on the hydrophobic surface of the matrix at a high cell density (2 × 105 cells in 200 μl media or 106 cells/ml) while minimizing albumin competition in low-serum media.
Evidence of cell proliferation on HAp-PCL composite nanofibrous mats
Viability data analysis using two-way ANOVA showed that cells attached to HAp-PCL and natural biopolymer composites increased significantly after the 7 days of the culture with a significant difference in both seeding methods (Fig. 2). This is because the cells have adapted to the nanofibers, mainly due to the presence of HAp which provides surface roughness that mimics the natural environment for osteoblasts to adhere and grow (Elangomannan et al., 2017; Li et al., 2019). The conventional seeding method resulted in an increase in cell viability from 16% to35% on day 1 to 43%–76% with a high SD in the range of 7.5%–10.5%. This is a consequence of the poor cell contact on the hydrophobic surface resulting in a high variation of cell attachment and subsequent proliferation.
A substantial attenuation of cell adhesion was shown by the modified seeding method (p = 0.000). Increasing the presentation time of cells on the surface of the hydrophobic matrix by applying the seeding apparatus shown in Figure 1 had a positive impact on the uniformity of cell adhesion. Furthermore, a high cell density in low-serum media provided adequate cell adhesion substances while minimizing serum albumin competition (Carré et al., 2010; Kruyt et al., 2008). Extracellular matrix is important in promoting intercellular communication, migration, and proliferation. Cell proliferation on day 7 was magnified 1.5–2 times from the first day with a narrow variation in the SD range of 1%–3.3% (Fig. 2B).
Evidence of cell proliferation and viability was also revealed by fluorescence labeled microscopy using the LIVE/DEAD Staining Reagent at 7 days after seeding (Fig. 3). Most of the nanofibrous mats were populated by green fluorescence indicating live cells with a certain level of red fluorescence dead cells after both conventional (Fig. 3A) and modified (Fig. 3B) seeding methods. Distinct cellularity was found in nanofibers after the two seeding methods proved the superior effect of modified seeding in providing a favorable environment for cell adhesion. It is advantageous to facilitate the activity of adhering cells to adapt the mineralized PCL environment to further signal-transduce, migrate, and proliferate populating the matrix (Grayson et al., 2008; Nyberg et al., 2017). It can be concluded that the modified seeding method provided significant cell proliferation with low variability even in hydrophobic HAp-PCL composites.
Insights into diverse cell behaviors on HAp-PCL nanofibrous mats reinforced with natural biopolymers
The PCL nanofibrous mats studied here were reinforced with HAp and natural biopolymers, namely, collagen, gelatin, and chitosan, at a weight ratio of 15:1:1. PCL mineralization with HAp was proposed to increase surface roughness at the submicron scale to mimic the natural bone matrix environment for osteoblast morphogenesis by providing functional areas for cell elongation and alignment. This HAp-PCL environment allows elongation of cellular filopodia and communal migration of attached osteoblasts to surrounding adhesion areas (Metwally et al., 2020; Refaaq et al., 2020). Moreover, mineralization is also important for stimulating DNA and collagen synthesis that simultaneously enhance cell viability and bone matrix differentiation and growth (Abdal-hay et al., 2020).
Sufficient cell numbers attached to the matrix are another determinant factor for initiating cellular contact and communication to form transient cell junctions. Cells communicating together lose their initial cell-matrix adhesion, which induces cell polarization away from the contact adhesion. The cells then generate new cell-matrix adhesions and migrate collectively to the surrounding areas (Bischoff et al., 2021). These events will trigger cellular communication, migration, proliferation, and differentiation of osteoblasts. Taking this into account, natural biopolymers were added to the nanofibrous formula in which collagen and gelatin were intended to provide ligands for integrin-mediated cell adhesion, while chitosan mediated nonspecific electrostatic binding via positively charged aminoglycosides (Bischoff et al., 2021; Davidenko et al., 2016; Monteiro et al., 2011; Wu et al., 2014).
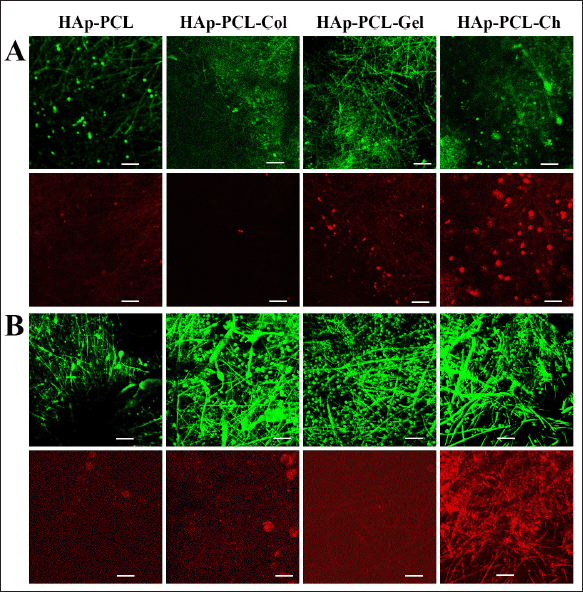 | Figure 3. Confocal images of LIVE/DEAD-stained cells grown on nanofibrous mats after 7 days of culture, which were seeded by (A) conventional and (B) modified methods. Green fluorescence indicates living cells attached and proliferated on the matrix, while red fluorescence indicates dead cells. The scale bars are 20 μm. [Click here to view] |
In addition, the surface charge of the scaffold is another essential regulator of cellular activity (Metwally et al., 2020). The addition of ionized groups on the surface increases the zeta potential of the biomaterial, increasing its interaction with the electrolyte components of the medium. In the case of hydrophobic synthetic polymers, this interaction will promote surface biodegradation to allow cell penetration into the matrix. Furthermore, the surface potential induces adhesion proteins in serum or cell membranes to adsorb and orient to the matrix, triggering cellular interactions and activity (Metwally et al., 2020). The increase in surface potential is also beneficial in avoiding unexpected albumin deposition from serum by inhibiting buried hydrophobic amino acids from binding to the hydrophobic surface (Carré et al., 2010). Therefore, PCL reinforced with HAp and natural biopolymers can reduce the hydrophobic properties of PCL and otherwise provide simultaneous physical and chemical cues to initiate cellular activity of osteoblasts to adhere and grow across the scaffold to elicit effective new bone formation.
We measured the zeta potential of the composite nanofibers, as shown in Table 1. The HAp-PCL nanofibers in the water had a negative surface charge with a zeta potential of −22.5 ± 0.3 mV. This is the total potential HAp, the acid solvent used in the precursor preparation, and the charge potential applied in the electrospinning process (Metwally et al., 2020). In the HAp-PCL-Col nanofibers, the addition of collagen with an isoelectric point of pH 9.3 (Metwally et al., 2020) likely formed a positive charge at the neutral pH of the culture medium and thus caused a significant increase of the zeta potential to −0.5 ± 0.1 mV. A similar change in near-neutral charge was found in the chitosan-reinforced nanofibrous mats (HAp-PCL-Ch) due to a contribution from the positively charged deacetylated amine groups. As the chance of obtaining protein aggregation increases at the near isoelectric point, higher cell adhesion to the collagen and chitosan composites may occur. In contrast to the three matrices, a significant change towards a more negative charge was found in HAp-PCL-Gel. A wide variation of surface charges of these formulas is likely to influence osteoblast adhesion and proliferation in culture.
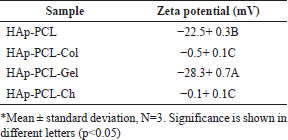 | Table 1. Zeta potential of nanofibrous mats. [Click here to view] |
The observation of cell adhesion and proliferation is in good agreement with contact angle measurement, as shown in Figure 4. The incorporation of HAp in PCL did not significantly change the contact angle, while the blending of HAp-PCL and the biopolymer led to a decrease in the contact angle. The contact angle of PCL-HAp decreased significantly from 71.59 + 2.20° to 63.55 + 2.22°, 65.39 + 2.33°, and 57.15 + 1.87° by reinforcement with collagen, gelatin, and chitosan, respectively. Generally, the surface wettability of the scaffold is increased by the introduction of the polar functional groups to the surfaces of polymers (Kim et al., 2022). The HAp-PCL-Ch composite showed maximum wettability amongst all samples, suggesting the high contribution of positively charged deacetylated amine groups to composite wettability.
The supporting components in these HAp-PCL composites may explain the success of the proposed modified seeding method in prolonging cell exposure and presentation to hydrophobic PCL nanofibrous mats, thereby enhancing the aforementioned cell adhesion and proliferation. The cell morphology after seeding using the modified method was revealed by SEM imaging shown in Figure 5C and D, whereas the poor cell adhesion resulting from the conventional method is shown in Figure 5A and B. The cells on the composite seeded with the modified method were uniformly distributed (Fig. 5C and D), whereas the cells on the composite were unevenly distributed and almost neglected (Fig. 5A and B). This was consistent with the results of the cell viability assay (Fig. 2). The fluorescence micrographs and the SEM micrographs both suggested the composite seeded with the modified method is more conducive to the adhesion and proliferation of osteoblasts.
Cells attached to HAp-PCL and HAp-PCL-Col showed a mixed diffuse and nonspreading spheroid morphology indicating nonadapted cells in the early stages of seeding (Fig. 5). Higher cell counts were found in HAp-PCL-Col indicating the benefit of the various adhesion motifs present in collagen binding more cells (Chen et al., 2021; Davidenko et al., 2016). Meanwhile, overlapping cell alignment with HAp-PCL-Gel nanofibrous mats implies the occurrence of integrin-mediated cell binding due to the presence of free RGD in gelatin. This allows adequate extension of the filopodia and binding of the more charged gelatin (Azarudeen et al., 2020). The inhibitory effect of cell binding on negatively charged HAp-PCL-Gel after seeding by the modified method was not found; this is due to the beneficial effect of extended cell presentation that can stimulate RGD-free motifs in gelatin to bind the cell surface integrin of the osteoblast. This in turn increased the hydrophilicity of the nanofibers allowing distribution of cells in the matrix, thereby increasing cell viability on day 1 and further growth on day 7 (Fig. 5D).
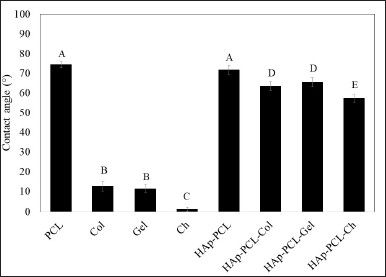 | Figure 4. The contact angle with its respective sessile drop pictures of PCL, biopolymers (collagen = Col, gelatin = Gel, and chitosan = Ch), and polymer composites of hydroxyapatite-polycaprolactone (HAp-PCL) blended with biopolymer (HAp-PCL-Col, HAp-PCL-Gel, and HAp-PCL-Ch). *Mean ± SD, N = 3. Significance is shown in different letters (p < 0.05). [Click here to view] |
Although collagen provided slightly lower cell adhesion than gelatin on day 1, it substantially increased osteoblast proliferation from 58.39±1.24% to 105.84 ± 1.87% during the 7 days of the culture (Fig. 2B). This could be explained by the presence of a subset of hydrophilic and hydrophobic ligands naturally found in this polymer, which are recognized well by osteoblasts (Chen et al., 2021; Davidenko et al., 2016; Subramanian et al., 2015; Wu et al., 2014). These ligands provide integrin-mediated cell adhesion and proliferation, resulting in profound cell growth with predominantly spreading cells (Fig. 5D).
On the other hand, conventional seeding methods could not support the adhesion performance of the HAp-PCL composites. This is mostly caused by short-term contact of cell suspensions in nanofibrous with low concentrations of natural polymers in a ratio of 1 to 15 wt. PCL was not sufficient to present cells to ligands. Furthermore, the presence of albumin competitors to the hydrophobic matrix precludes the presentation of cells to the hydrophobic PCL and therefore inhibits cell adhesion. This was particularly the case found in HAp-PCL-Gel with a negative charge, which was not a preferential condition for adhesion of negatively charged mammalian cells (Verma et al., 2007) and thus exhibited very low adhesion and proliferation during the 7 days of the culture (Fig. 2A). The higher degree of swelling of gelatin in the culture medium which tends to reduce the surface area for cell adhesion (Verma et al., 2007) may also explain this occurrence. This is also supported by the porosity measurement using ethanol with water impurities prone to cause gelatin swelling, which resulted in the porosity of HAp-PCL-Gel being significantly lower than other composites (Fig. 6).
Distinct cell behavior was found on chitosan-reinforced nanofibrous mats (HAp-PCL-Ch) at day 7 in the culture, as revealed by the SEM images (Fig. 5B). Cells mostly formed clusters inhabited by spherical cells, which showed poor filopodia formation. Strong cell membrane interactions with positively charged aminoglycosides of chitosan are likely to occur, leading to inhibition of cell-matrix rebounding needed for transient cell junction formation at cell contact points (Bischoff et al., 2021; Refaaq et al., 2020), therefore limiting cell migration and proliferation. This phenomenon was supported by the decreased cell viability compared to day 1 (Fig. 2B) and the remarkable increase in cell death found on LIVE/DEAD fluorescence labeled images (Fig. 3B). However, the morphology of these cells did not show cell membrane lyse as reported by Qi et al. (2005) in cells treated with a high concentration of chitosan nanoparticles. Gomes et al. (2015) also observed a contradictory result in the reduction viability, but an increased cell number was observed from confocal microscopy of fibroblasts grown in chitosan (500 kDa) nanofibrous mats for 7 days. This supports our conclusion that the contradictory viability and cell count are due to the high cell binding strength of the chitosan nanofibers and are not indicated as cell necrosis. Regardless of the contradictory results found at day 7, chitosan aminoglycoside that neutralized the negative charge of HAp-PCL (Table 1) is likely to enhance the adsorption tendency of adhesion proteins on near uncharged hydrophobic PC (Metwally et al., 2020), thereby showing the highest cell adhesion at day 1 (Fig. 2B). However, like other composites, the adhesion performance of HAp-PCL-Ch was inhibited after conventional seeding implying high adsorption competition with serum albumin in a 10% FBS supplementary medium (Fig. 2A).
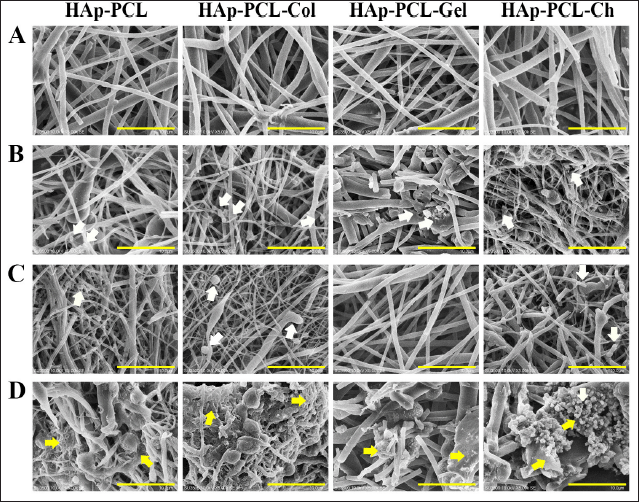 | Figure 5. SEM images of cells attached to nanofibrous mats on day 1 and day 7 of culture after seeding using the conventional method (A and B) and modified method (C and D), respectively. Arrows indicate attached cells: white arrows are nonspreading cells, and yellow arrows are spreading cells. The scale bars are 100 μm. [Click here to view] |
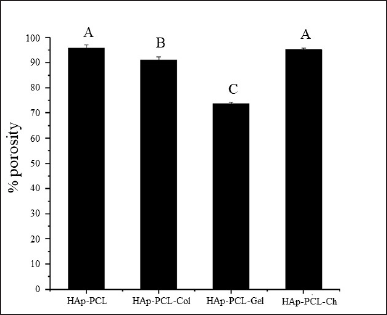 | Figure 6. The porosity of nanofibrous mats: HAp-PCL, collagen (HAp-PCL-Col), gelatin (HAp-PCL-Gel), and chitosan (HAp-PCL-Ch). *Mean ± SD, N = 3. Significance is shown in different letters (p < 0.05) [Click here to view] |
It is noteworthy to consider the molecular weight (MW) and deacetylation degree (DD) of chitosan in designing tissue engineering scaffolds. The higher chitosan chains (>10 to 200 kDa) at high DD (>85%) present more primary amine groups to bind to the zwitterionic lipid head groups of the cell membrane, which tends to reduce cell viability (Huang et al., 2004). In contrast, small molecule chitosan studied by de Oliveira Pedro et al. (2020) at 3.8 kDa and 97% DD has maintained the integrity of mammalian cells, although this size otherwise has formed strong electrostatic bonds to bacterial membranes that effectively destroy the microbes. The high molecular weight of chitosan also presents several obstacles in the preparation of the scaffolds, such as the acidic pH required to solubilize polymers with protonated amino groups with a pKa of 6.4, which can compromise the stability of bioactive materials. The high viscosity of a large chitosan solution is another limitation for some scaffold preparations, such as electrospinning (Nezarati et al., 2013). Many attempts to improve the cell adhesion performance of chitosan-based scaffolds have been studied, such as increasing the density of the RGD ligand (Hansson et al., 2012; Wu et al., 2014) and mixing with another ligands-rich biopolymer, i.e., collagen (Verma et al., 2007). Although chitosan exhibited compromised cell viability within the 7 days culture in vitro, it displayed better physical support for new tissue growth in vivo than PCL and gelatin (Gomes et al., 2015).
CONCLUSION
We succeeded in overcoming the limitations of conventional cell seeding applications on hydrophobic PCL-HAp nanofibrous mats due to poor cell-matrix contact, along with high albumin adsorption tendencies on hydrophobic surfaces. The reduction of the hydrophobic surface was carried out by applying a modified seeding method using a seeding construct with a static seeding culture to prolong the presentation of cells on the matrix. The construct consists of a glass carrier sandwiched between two cylindrical supports to extend the translocation of the cell suspension within the matrix. Furthermore, the use of a high cell density in 2% serum media increased the exposure of the cells to the surface charges provided by the addition of natural biopolymers to the PCL-HAp nanofibrous mats, thereby increasing the surface hydrophilicity and cell-matrix interactions. These seeding conditions allow expansion of the osteoblast filopodia on the HAp-mimicked surface roughness bone environment, allowing cell adhesion, migration, and proliferation to provide an adequate cell population for initiating new bone formation. The experimental results showed that PCL-HAp blended with collagen had good cell adhesion on day 1 and increased proliferation on day 7. This could be due to the high positive charge, thereby minimizing the movement of cells to the surrounding area, which in turn resulted in contact inhibition. Therefore, modification with PCL-HAp-Gel and PCL-HAp-Col gave the best performance for potential use in bone tissue engineering. Therefore, HAp-PCL mixed with collagen (PCL-HAp-Col) and gelatin (PCL-HAp-Gel) gave the best performance for potential use in bone tissue engineering. Therefore, this facile seeding method is a potential tool to support the performance of bone scaffolds using hydrophobic synthetic polymers to provide temporary physical support in long bone morphogenesis.
ACKNOWLEDGMENTS
This research was funded by the Directorate of Research and Community Engagement of the Bandung Institute of Technology through the Indonesian Collaborative Research Program (Riset Kolaborasi Indonesia, RKI). The authors would like to thank the Gurdon Institute, Cambridge University, for generously donating Saos-2 cells and Ms. Sri Anendya Lestari for assisting in the culture of these cells.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abbasi N, Hamlet S, Love RM, Nguyen NT. Porous scaffolds for bone regeneration. J Sci Adv Mater Devices, 2020; 5(1):1–9; doi: 10.1016/j.jsamd.2020.01.007 CrossRef
Abdal-hay A, Raveendran NT, Fournier B, Ivanovski S. Fabrication of biocompatible and bioabsorbable polycaprolactone/magnesium hydroxide 3D printed scaffolds: Degradation and in vitro osteoblasts interactions. Compos B: Eng, 2020; 197:108158; doi: 10.1016/j.compositesb.2020.108158 CrossRef
Aminatun A, Suciati T, Sari YW, Sari M, Alamsyah KA, Purnamasari W, Yusuf Y. Biopolymer-based polycaprolactone-hydroxyapatite scaffolds for bone tissue engineering. Int J Polym Mater Polym Biomater, 2021; 1–10; doi: 10.1080/00914037.2021.2018315 CrossRef
Asadpour S, Kargozar S, Moradi L, Ai A, Nosrati H, Ai J. Natural biomacromolecule based composite scaffolds from silk fibroin, gelatin and chitosan toward tissue engineering applications. Int J Biol Macromol, 2020; 154:1285–94; doi: 10.1016/j.ijbiomac.2019.11.003 CrossRef
.Azarudeen RS, Hassan MN, Yassina MA, Thirumarimurugan M, Muthukumarasamy N, Velauthapillai D, Mustafa K. 3D printable polycaprolactone-gelatin blends characterized for in vitro osteogenic potency. React Funct Polym, 2020; 146:104445; doi: 10.1016/j.reactfunctpolym.2019.104445 CrossRef
Bendtsen ST, Wei M. In vitro evaluation of 3D bioprinted tri-polymer network scaffolds for bone tissue regeneration. J Biomed Mater Res A, 2017; 105(12):3262–72; doi: 10.1002/jbm.a.36184 CrossRef
Bischoff MC, Lieb, S., Renkawitz-Pohl R, Bogdan S. Filopodia-based contact stimulation of cell migration drives tissue morphogenesis. Nat Comm, 2021; 12(1):1–18; doi: 10.1038/s41467-020-20362-2 CrossRef
Carré A, Lacarrière V. How substrate properties control cell adhesion. A physical-chemical approach. J Adhes Sci Technol, 2010; 24(5):815–30; doi: 10.1163/016942409X12598231567862 CrossRef
Chen Q, Zhang D, Zhang W, Zhang H, Zou J, Chen M, Li J, Yuan Y, Liu R. Dual mechanism β-amino acid polymers promoting cell adhesion. Nat Commun, 2021;12(1):1–13; doi:10.1038/s41467-020-20858-x CrossRef
Chen Y, Bloemen V, Impens S, Moesen M, Luyten FP, Schrooten J. Characterization and optimization of cell seeding in scaffolds by factorial design: quality by design approach for skeletal tissue engineering. Tissue Eng Part C Methods, 2011; 17(12):1211–21; doi: 10.1089/ten.tec.2011.0092 CrossRef
Cho YS, Quan M, Lee SH, Hong MW, Kim YY, Cho YS. Assessment of osteogenesis for 3D-printed polycaprolactone/ hydroxyapatite composite scaffold with enhanced exposure of hydroxyapatite using rat calvarial defect model. Compos Sci Technol, 2019; 184:107844; doi: 10.1016/j.compscitech.2019.107844 CrossRef
Davidenko N, Schuster CF, Bax DV, Farndale RW, Hamaia S, Best SM, Cameron RE. Evaluation of cell binding to collagen and gelatin: a study of the effect of 2D and 3D architecture and surface chemistry. J Mater Sci Mater Med, 2016; 27(10):1–14; doi: 10.1007/s10856-016-5763-9 CrossRef
de Oliveira Pedro R, Pereira AR, Oliveira ON, Miranda PB. Interaction of chitosan derivatives with cell membrane models in a biologically relevant medium. Colloids Surf B Biointerfaces, 2020; 192:111048; doi: 10.1016/j.colsurfb.2020.111048 CrossRef
Elangomannan S, Louis K, Dharmaraj BM, Kandasamy VS, Soundarapandian K, Gopi D. Carbon nanofiber polycaprolactone/mineralized hydroxyapatite nanofibrous scaffolds for potential orthopedic applications. ACS Appl Mater Interfaces, 2017; 9(7):6342–55; doi: 10.1021/acsami.6b13058 CrossRef
Fu WJ, Xu YD, Wang ZX, Li G, Shi JG, Cui FZ, Zhang Y, Zhang X. New ureteral scaffold constructed with composite poly(L-lactic acid)–collagen and urothelial cells by new centrifugal seeding system. J Biomed Mater Res A, 2012; 100(7):1725–33; doi: 10.1002/jbm.a.34134 CrossRef
Gao C, Peng S, Feng P, Shuai C. Bone biomaterials and interactions with stem cells. Bone Res, 2017; 5:17059; doi: 10.1038/boneres.2017.59 CrossRef
Gomes SR, Rodrigues G, Martins GG, Roberto MA, Mafra M, Henriques CMR, Silva JC. In vitro and in vivo evaluation of electrospun nanofibers of PCL, chitosan and gelatin: a comparative study. Mater Sci Eng C Mater Biol Appl, 2015; 46:348–58; doi: 10.1016/j.msec.2014.10.051 CrossRef
Gorodzha SN, Muslimov AR, Syromotina DS, Timin AS, Tcvetkov NY, Lepik KV, Petrova AV, Surmeneva MA, Gorin DA, Sukhorukov GB, Surmenev RA . A comparison study between electrospun polycaprolactone and piezoelectric poly (3-hydroxybutyrate-co-3-hydroxyvalerate) scaffolds for bone tissue engineering. Colloids Surf B Biointerfaces, 2017; 160:48–59; doi: 10.1016/j.colsurfb.2017.09.004 CrossRef
Grayson WL, Bhumiratana S, Cannizzaro C, Chao PHG, Lennon DP, Caplan AI, Vunjak-Novakovic G. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng Part A, 2008; 14(11):1809–20; doi: 10.1089/ten.tea.2007.0255 CrossRef
Griffon DJ, Abulencia JP, Ragetly GR, Fredericks LP, Chaieb S. A comparative study of seeding techniques and three-dimensional matrices for mesenchymal cell attachment. J Tissue Eng Regen Med, 2011; 5(3):169–79; doi: 10.1002/term.302 CrossRef
Hansson A, Hashom N, Falson F, Rousselle P, Jordan O, Borchard G. In vitro evaluation of an RGD-functionalized chitosan derivative for enhanced cell adhesion. Carbohydr Polym, 2012; 90(4):1494–500; doi: 10.1016/j.carbpol.2012.07.020 CrossRef
Hosseini Y, Emadi R, Kharaziha M. Surface modification of PCL-diopside fibrous membrane via gelatin immobilization for bone tissue engineering. Mater Chem Phys, 2017; 194:356–66; doi: 10.1016/j.matchemphys.2017.03.051 CrossRef
Huang M, Khor E, Lim LY. Uptake and cytotoxicity of chitosan molecules and nanoparticles: effects of molecular weight and degree of deacetylation. Pharm Res, 2004; 21(2):344–53; doi: 10.1023/b:pham.0000016249.52831.a5 CrossRef
Impens S, Chen Y, Mullens S, Luyten F, Schrooten J. Controlled cell-seeding methodologies: a first step toward clinically relevant bone tissue engineering strategies. Tissue Eng Part C Methods, 2010; 16(6):1575–83; doi: 10.1089/ten.TEC.2010.0069 CrossRef
Jiang W, Shi J, Li W, Sun K. Morphology, wettability, and mechanical properties of polycaprolactone/hydroxyapatite composite scaffolds with interconnected pore structures fabricated by a mini-deposition system. Polym Eng Sci, 2012; 52(11):2396–402; doi: 10.1002/pen.23193 CrossRef
Kim HY, Kim BH, Kim MS. Amine plasma-polymerization of 3D polycaprolactone/β-tricalcium phosphate scaffold to improving osteogenic differentiation in vitro. Materials, 2022; 15(1):366; doi: 10.3390/ma15010366 CrossRef
Kandelousi PS, Rabiee SM, Jahanshahi M, Nasiri F. The effect of bioactive glass nanoparticles on polycaprolactone/chitosan scaffold: melting enthalpy and cell viability. J Bioact Compat Polym, 2019; 34:97–111; doi: 10.1177/0883911518819109 CrossRef
Kruyt M, De Bruijn J, Rouwkema J, van Blitterswijk C, Oner C, Verbout A, Dhert W. Analysis of the dynamics of bone formation, effect of cell seeding density, and potential of allogeneic cells in cell-based bone tissue engineering in goats. Tissue Eng Part A, 2008; 14(6):1081–8; doi: 10.1089/ten.tea.2007.0111 CrossRef
Lee H, Ahn S, Bonassar LJ, Chun W, Kim G. Cell-laden poly(?-caprolactone)/alginate hybrid scaffolds fabricated by an aerosol cross-linking process for obtaining homogeneous cell distribution: fabrication, seeding efficiency, and cell proliferation and distribution. Tissue Eng Part C Methods, 2013; 19(10):784–93; doi: 10.1089/ten.TEC.2012.0651 CrossRef
Lerman MJ, Lembong J, Muramoto S, Gillen G, Fisher JP. The evolution of polystyrene as a cell culture material. Tissue Eng B Rev, 2018; 24(5):359–72; doi: 10.1089/ten.TEB.2018.0056 CrossRef
Li Y, Liao C, Tjong SC. Synthetic biodegradable aliphatic polyester nanocomposites reinforced with nanohydroxyapatite and/or graphene oxide for bone tissue engineering applications. Nanomaterials, 2019;9(4):590; doi: 10.3390/nano9040590. CrossRef
Lu LLX, Zhang XF, Wang YY, Ortiz L, Mao X, Jiang ZL, Xiao ZD, Huang NP. Effects of hydroxyapatite-containing composite nanofibers on osteogenesis of mesenchymal stem cells in vitro and bone regeneration in vivo. ACS Appl Mater Interfaces, 2013; 5(2):319–30; doi: 10.1021/am302146w CrossRef
Metwally S, Ferraris S, Spriano S, Krysiak ZJ, Kaniuk ?, Marzec MM, Kim SK, Szewczyk PK, Gruszczy?ski A, Wytrwal-Sarna M, Karbowniczek JE, Bernasik A, Kar-Narayan S, Stachewicz U. Surface potential and roughness-controlled cell adhesion and collagen formation in electrospun PCL fibers for bone regeneration. Mater Des, 2020; 194:108915; doi: 10.1016/j.matdes.2020.108915 CrossRef
Monteiro GA, Fernandes AV, Sundararaghavan HG, Shreiber DI. Positively and negatively modulating cell adhesion to type I collagen via peptide grafting. Tissue Eng Part A, 2011; 17(13–14):1663–73; doi: 10.1089/ten.tea.2008.0346 CrossRef
Nezarati RM, Eifert MB, Cosgriff-hernandez E. Effects of humidity and solution viscosity on electrospun fiber morphology. Tissue Eng Part C: Methods, 2013; 19(10): 810–9; doi:10.1089/ten.tec.2012.0671 CrossRef
Nyberg E, Rindone A, Dorafshar A, Grayson WL. Comparison of 3d-printed poly-?-caprolactone scaffolds functionalized with tricalcium phosphate, hydroxyapatite, bio-oss, or decellularized bone matrix. Tissue Eng Part A, 2017; 23(11–12):503–14; doi: 10.1089/ten.TEA.2016.0418 CrossRef
Oh SH, Lee JH. Hydrophilization of synthetic biodegradable polymer scaffolds for improved cell/tissue compatibility. Biomed Mater, 2013; 8(1):014101; doi: 10.1088/1748-6041/8/1/014101 CrossRef
Qi LF, Xu ZR, Li Y, Jiang X, Han XY. In vitro effects of chitosan nanoparticles on proliferation of human gastric carcinoma cell line MGC803 cells. World J Gastroenterol, 2005; 11(33):5136; doi: 10.3748/wjg.v11.i33.5136 CrossRef
Rajzer I, Menaszek E, Kwiatkowski R, Planell JA, Castano O. Electrospun gelatin/poly(ε-caprolactone) fibrous scaffold modified with calcium phosphate for bone tissue engineering. Mater Sci Eng C Mater Biol Appl, 2014; 44:183–90; doi: 10.1016/j.msec.2014.08.017 CrossRef
Refaaq FM, Chen X, Pang SW. Effects of topographical guidance cues on osteoblast cell migration. Sci Rep, 2020; 10(1):1–11; doi: 10.1038/s41598-020-77103-0. CrossRef
Song X, Zhu C, Fan D, Mi Y, Li X, Fu RZ, Duan Z, Wang Y, Feng RR. A novel human-like collagen hydrogel scaffold with porous structure and sponge-like properties. Polymers, 2017; 9(12):638; doi: 10.3390/polym9120638 CrossRef
Sonnaert M, Papantoniou I, Luyten FP, Schrooten J. Quantitative validation of the presto blue metabolic assay for online monitoring of cell proliferation in a 3d perfusion bioreactor system. Tissue Eng Part C Methods, 2015; 21(6):519–29; doi: 10.1089/ten.TEC.2014.0255 CrossRef
Subramanian G, Bialorucki C, Yildirim-Ayan E. Nanofibrous yet injectable polycaprolactone-collagen bone tissue scaffold with osteoprogenitor cells and controlled release of bone morphogenetic protein-2. Mater Sci Eng C Mater Biol Appl, 2015; 51:16–27; doi: 10.1016/j.msec.2015.02.030 CrossRef
Varçin M, ?ener BB, Bayraç C. Adsorption of resazurin by poly(acrylic acid) hydrogels and evaluation of its use in reduction assay for quantification of cell viability. Dye Pigment, 2021; 186:109038; doi: 10.1016/j.dyepig.2020.109038 CrossRef
Verma V, Verma P, Kar S, Ray P, Ray AR. Fabrication of agar-gelatin hybrid scaffolds using a novel entrapment method for in vitro tissue engineering applications. Biotechnol Bioeng, 2007; 96(2):392-400; doi:10.1002/bit.21111 CrossRef
Wu TY, Zhou ZB, He ZW, Ren WP, Yu XW, Huang Y. Reinforcement of a new calcium phosphate cement with RGD-chitosan-fiber. J Biomed Mater Res A, 2014; 102(1):68–75; doi: 10.1002/jbm.a.34669 CrossRef
Yao Q, Nooeaid P, Detsch R, Roether JA, Dong Y, Goudouri OM, Schubert DW, Boccaccini AR. Bioglass®/chitosan–polycaprolactone bilayered composite scaffolds intended for osteochondral tissue engineering. J Biomed Mater Res A, 2014; 102(12):4510–8; doi: 10.1002/jbm.a.35125 CrossRef
Yassin MA, Leknes KN, Pedersen TO, Xing Z, Sun Y, Lie SA, Finne-Wistrand A, Mustafa K. Cell seeding density is a critical determinant for copolymer scaffolds-induced bone regeneration. J Biomed Mater Res A, 2015; 103(11):3649–58; doi: 10.1002/jbm.a.35505 CrossRef
Zafari M, Mansouri M, Omidghaemi S, Yazdani A, Pourmotabed S, Hasanpour Dehkordi A, Nosrati H, Validi M, Sharifi E. Physical and biological properties of blend-electrospun polycaprolactone/chitosan-based wound dressings loaded with N-decyl-N, N-dimethyl-1-decanaminium chloride: an in vitro and in vivo study. J Biomed Mater Res B Appl Biomater, 2020; 108(8):3084–98; doi: 10.1002/jbm.b.34636 CrossRef
Zhao T, Jiang L. Contact angle measurement of natural materials. Colloids Surf B Biointerfaces, 2018; 161:324–30; doi: 10.1016/j.colsurfb.2017.10.056 CrossRef