INTRODUCTION
Based on the traditional theory, adipose tissue is inert, and it regulates body temperature, stores fat, supplies energy, absorbs shock, and functions as an insulator. However, recent studies described it as an endocrine organ with multiple functions and active metabolism (Coelho et al., 2013). The factors influenced by adipose tissue secretion and metabolism are called adipokines which perform a substantial role in obesity, metabolic syndrome, atherosclerosis, and blood glucose regulation (Kim and Choi, 2020). Furthermore, they serve as growth factors, cytokines, acute phase reactants, other inflammatory mediators, adipose tissue-secreting hormones such as leptin and adiponectin, and biochemical messengers (Kang et al., 2016).
Vaspin, also known as SERPINA12 according to serpin nomenclature, is a unique insulin-sensitizing adipocytokine discovered by Hida et al. (2005) and was associated with the development of inflammation and metabolic syndrome. It was first discovered in the visceral adipose tissue (VAT) of Otsuka Long-Evans Tokushima fatty (OLETF) rat, an animal model with type 2 diabetes mellitus (T2DM) and truncal obesity.
Previous investigations of vaspin role in metabolic and vascular diseases produced conflicting and various results because the levels are always inconstant in both conditions. A study associated the increase of serum vaspin concentration in mice with central obesity and insulin resistance (IR) (Liu et al., 2018). However, vaspin has been presumed to be an insulin sensitizer with anti-inflammatory effects and might act as a compensatory process in response to IR (Wada, 2008). Furthermore, a study showed no significant vaspin role concerning insulin sensitivity in nondiabetic healthy humans (Von Loeffelholz et al., 2010).
A further study reported that VAT-derived adipokines such as vaspin have a local and endocrine function in developing early and late atherosclerosis in obesity by affecting vascular smooth muscle cells (VSMCs) of the endothelium (de Leal and Mafra, 2013). Meanwhile, another study stated that vaspin serum level is not related to carotid stenosis plaque severity, but its low level is associated with the risk of ischemic events in carotid stenosis patients (Aust et al., 2009).
Therefore, we conducted a systematic review and meta-analysis to determine the serum vaspin level alteration role in atherosclerosis and glucose tolerance disorders. We also conducted a critical appraisal of all selected articles to establish the serum vaspin role in atherosclerosis and glucose tolerance disorders. A common understanding and conclusion were eventually developed from the different assessed results.
Moreover, a brief additional review of the serum vaspin basic biochemical role and its genetic expression and polymorphism factor was also conducted. However, we did not consider and further analyze the relationship between genetic expression-polymorphism factor and serum vaspin levels in this study.
METHODS
A literature search was conducted using scientific databases such as PubMed, ScienceDirect, Scopus, and Google Scholar from September 2021 to January 2022. We performed this search based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart for the selection of studies (Page et al., 2021). Combinations of keywords associated with “vaspin” AND “glucose intolerance” including diabetes, hyperglycemia, and insulin plus either “atherosclerosis” involving coronary heart disease, ischemic heart disease, and stroke, “metabolic syndrome,” OR “obesity” were employed. The inclusion criteria included English language studies published between 2005 and 2022 with clinical or laboratory experimental and nonexperimental designs (cohort, case-control, and cross-sectional), as well as discussing vaspin serologically rather than in terms of genetic expression. Several in vitro and in vivo studies were also considered to provide insight into the potential mechanism of vaspin in atherosclerosis and glucose intolerance. Moreover, the citation-reference lists from the selected studies were also investigated to recognize related results.
The eligibility of the titles and abstracts was independently reviewed by two authors (RD and FM) based on the Cochrane Handbook for Systematic Reviews. Initially, 146 articles were obtained, including 92 derived from the keywords of “vaspin” AND “glucose intolerance” OR “atherosclerosis” and 54 from “vaspin” AND “metabolic syndrome” combination OR “obesity.” A total of 95 articles were excluded after manual investigation for unavailable full text and duplications. The articles were extracted using a data extraction template (Microsoft Office Excel 2016). We used the Cochrane risk of bias tool to assess the risk of bias in the included studies. This tool evaluates six potential issues: sequence generation, allocation concealment, incomplete data, blinding, selective outcome reporting, and other bias-related issues. Then, we screened again the eligibility of each article’s title and abstract, and eventually, 23 eligible articles were selected to be reviewed (Fig. 1). These articles were then exported and compiled by the Mendeley Citation Manager.
The selected articles used immunoassays product of Vaspin ELISA (Mediagnost Vaspin ELISA E106) by BioVendor R&D® (Asheville, NC). This Vaspin ELISA product uses a serum sample to quantify the vaspin concentration. It has a sensitivity of 4 pg/ml. Its reagents and material equipment provided included a microtiter plate, recombinant vaspin, dilution buffer, control sera, antibody conjugate, enzyme conjugate, washing buffer, substrate, stopping solution, and sealing tape for covering the microtiter plate. We also ensured that the selected articles used the standardized statistical analysis software of Statistical Package for the Social Sciences, at least the 22nd version.
The 23 articles were reviewed and analyzed by standard common questions for the critical appraisal (Al-Jundi and Sakka, 2017). The critical appraisal questions included the research objective or question, study design, selection issues, outcome factors, study factors, important potential confounders, statistical method used, statistical results, main conclusions about the research objective or question, and ethical issues considered. Data synthesis and statistical analysis were performed using RevMan version 5.4 for Windows. The authors conducted a subgroup analysis and meta-regression of heterogeneous papers and the relationship between serum vaspin levels and atherosclerotic-glucose tolerance diseases based on their respective study designs.
The study location was also considered due to population race effects on vaspin gene expression and polymorphism. However, this review did not analyze vaspin gene expression and polymorphism in detail but compared the data analysis components of every article. Finally, a common agreement and conclusion were reached concerning serum vaspin role in atherosclerosis and glucose tolerance disorders.
RESULTS AND DISCUSSION
We reviewed 23 studies investigating serum vaspin role in atherosclerosis and glucose tolerance disorders (Tables 1 and 2). In those tables, the authors also gave specific significant remarks to explain why the selected study was being listed. We also presented the risk of bias in the included studies in Figures 2 and 3. The forest plots of the meta-analysis showed atherosclerotic diseases versus normal healthy (MD −0.43; 95% CI: −1.35 to 0.50) (Fig. 4) and glucose tolerance disorders versus normal healthy (MD −0.07; 95% CI: −0.3 to 80.25) (Fig. 5). Those results indicated that higher vaspin levels in the disease group were preferred.
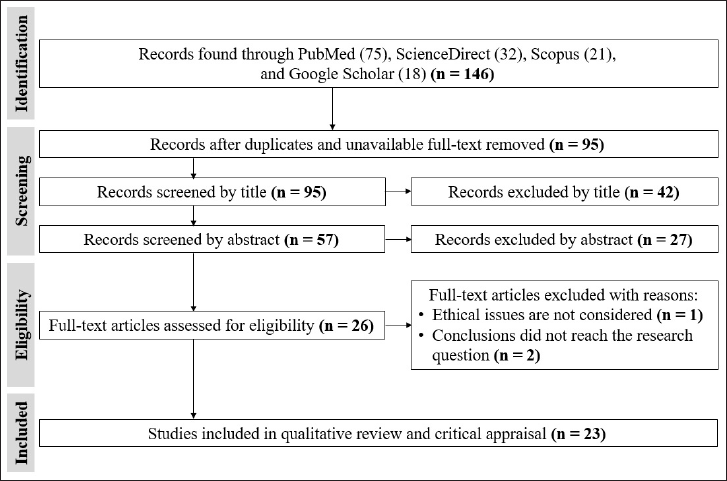 | Figure 1. Article selection during the literature search based on the PRISMA flow diagram (Page et al., 2021 ). [Click here to view] |
Mode of action and mechanism of vaspin
Vaspin is an adipokine with targets in kallikrein 7 (KLK) and KLK 14, which are involved in skin desquamation. This VAT-derived serine protease inhibitor has been identified to significantly increase in 30 weeks of OLETF (Hida et al., 2005). Once the circulatory or adipocyte vaspin concentration hits the peak in IR and obesity, the expression of this compound increases significantly. Furthermore, a study showed that KLK 7 co-expressed in rat islets suggests promising relevance of the vaspin-KLK7 mode of inhibition in early insulin secretion (Heiker et al., 2013) (Fig. 6). Meanwhile, the insulin sensitizers of pioglitazone normalize both vaspin serum levels and expression (Wada, 2008).
Vaspin is believed to act as an insulin sensitizer with a compensatory role in white adipose tissue because the favorable effect is involved in the gene expression of IR pathogenesis, including tumor necrosis factor-α (TNF-α), leptin, resistin, glucose transporter-4, and adiponectin (Li et al., 2008). It is considered that vaspin synthesis antagonizes the effect of uninvestigated proteases, which interrupt insulin action (Dwipayana et al., 2019). It could be compared to other proteins, such as neutrophil elastase and α1-antitrypsin (D’Souza et al., 2021; Motoyama et al., 2020).
Vaspin and atherosclerosis
In obesity-associated inflammation and cardiovascular diseases, several studies showed the establishment of vaspin anti-atherogenic and anti-inflammatory roles on smooth muscle and vascular endothelial cells. Evidence demonstrates that it protects endothelial cells from apoptosis and inflammation (Fig. 6). Vaspin inhibits TNF-α-induced expression of adhesion molecules in VSMCs and later reduces lymphocyte adhesion by decreasing reactive oxygen species (ROS), protein kinase C, and nuclear factor-κB (NF-κB) activation. Additionally, the blockade of ROS production attenuates and reduces cytoskeletal reorganization, platelet-derived growth factor (PDGF), and intercellular adhesion molecule 1 (ICAM1) expression (Li et al., 2013).
According to the critical appraisal in Table 1, seven studies, three cross-sectional, two cohorts, one case-control, and one interventional in vivo, suggested that vaspin has a protective and beneficial effect on atherosclerosis progression (Aust et al., 2009; Jung et al., 2011; Kastl et al., 2020; Li et al., 2012; Zhang et al., 2013, 2016, 2020). However, those three cross-sectional studies failed to indicate the cause-effect relationship of vaspin with atherosclerosis. A result showed confounding factors, including 52.5% of patients using angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and 66.3% receiving β-blockers (Zhang et al., 2016).
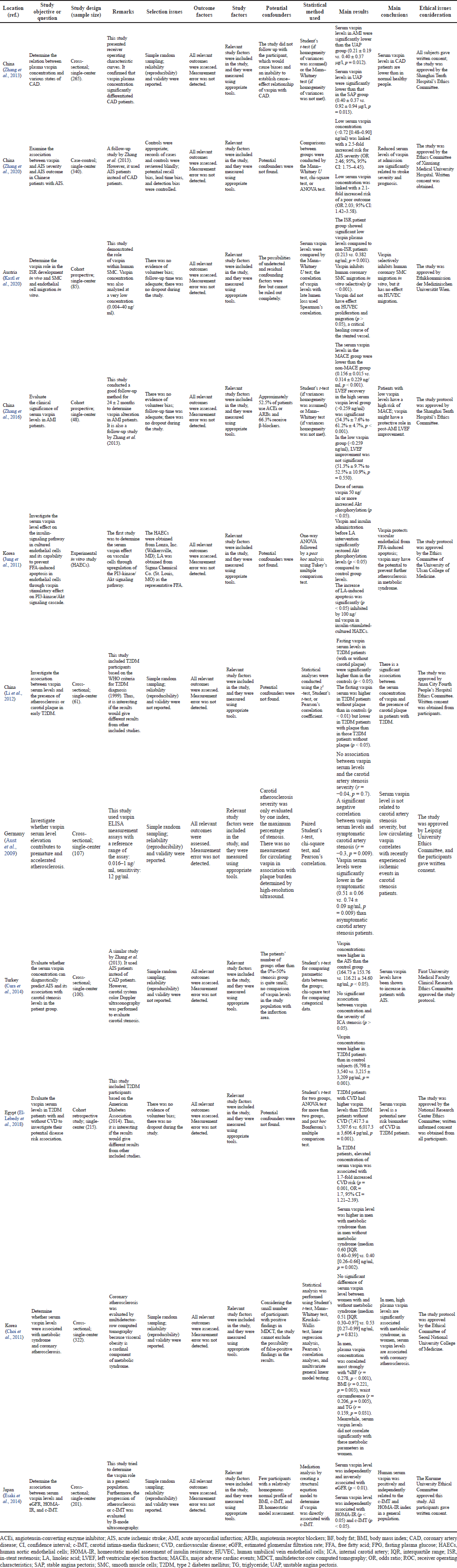 | Table 1. Critical appraisal from the review of the association between vaspin serum level and atherosclerosis. [Click here to view] |
Meanwhile, four studies, three cross-sectional and one cohort, demonstrated that serum vaspin elevation causes atherosclerosis (Choi et al., 2011; Cura et al., 2014; El-Lebedy et al., 2018; Esaki et al., 2014). Those three cross-sectional types stated no conclusive relationship between vaspin and atherosclerosis. One cross-sectional study had limitations due to small sample size with a relatively homogenous normal profile of body mass index (BMI), carotid intima-media thickness, and IR homeostatic model assessment (Esaki et al., 2014).
Vaspin and glucose tolerance disorders
Several studies showed a positive correlation of serum vaspin levels with waist fat mass, circumference, and BMI, while the higher levels in obese and T2DM patients are possibly the consequence reaction of IR (Jian et al., 2014). Another study explained this pathophysiology that the expression of insulin receptor substrate-2 messenger RNA (mRNA) was increased by administering 80–320 ng/ml vaspin. The VAT-derived serine protease inhibitor stimulated insulin secretion by the insulinoma cell line mediated through kinase Akt activation and the mTOR/p70S6K signaling pathway (Liu et al., 2017). It was also reported to increase β-cell activity by impeding the KLK 7 and KLK 14 inhibitory function (Heiker et al., 2013) (Fig. 6).
Pancreatic cell inflammation was inhibited by vaspin through NF-κB downregulation (Liu et al., 2017). It is an important fact that T2DM is associated with a chronic inflammatory process mediated by cytokines. Subsequently, high inflammatory cytokine in the pancreatic islets of T2DM patients causes pancreatic β-cell failure and activity impairment (Donath et al., 2009; Nordmann et al., 2017). Hence, high serum vaspin levels improve the β-cell function in patients with glucose tolerance disorders (Jian et al., 2014; Liu et al., 2017).
Based on the critical appraisal in Table 2, this current review indicated that 10 studies, 8 cross-sectional, 1 case-control, and 1 interventional human, demonstrated higher vaspin levels in glucose tolerance disorder patients (Dai et al., 2016; El-Mesallamy et al., 2011; Lal et al., 2018; Liu et al., 2020; Moradi et al., 2016; Yang et al., 2015, 2017a, 2017b; Ye et al., 2009; Youn et al., 2008). As stated in the previous review, all the cross-sectional types did not determine the cause–effect relationship between vaspin and T2DM. Furthermore, one interventional study showed that vaspin plays a role in compensating the insulin physiological and sensitivity function in IR conditions (Youn et al., 2008). The compound is believed to have no significant role in healthy normal subjects and physically active persons (Youn et al., 2008). A case-control study that evaluates the genotype and allele frequency in gestational diabetes mellitus (GDM) showed very little inconsistency in the relationship between vaspin levels and GDM compared with other existing reports. Conversely, the vaspin gene with a predominance of the AA genotype has been said to increase the risk of developing metabolic syndrome more than TT or TA (Lal et al., 2018).
A positive association of serum vaspin level with IR risk has been reported in all adolescents at the pubertal stage and their counterparts with a high percentage of body fat (%BF). Higher serum vaspin level (?: ≥0.87, ?: ≥1.50 μg/ml) was linked with a reduced IR risk independently of BMI, %BF, and pubertal stage (Pala et al., 2019). Those results did not conclude whether serum vaspin level affects IR directly because the insulin metabolism adjustment is possibly affected by regular physical activity. Insulin has a propensity to be the factor affecting serum vaspin levels. Nevertheless, the aforementioned study proves that the association between higher serum vaspin levels and lower risk for IR tends to be mediated by physical activity in adolescents. However, there were actual result biases, including 180 adolescents who did not complete the physical activity protocol (Pala et al., 2019). One interventional human study also produced unusual results, which suggested that the longer intervention duration further elucidates the nonsignificant difference between the intervention and control saline groups (Von Loeffelholz et al., 2010).
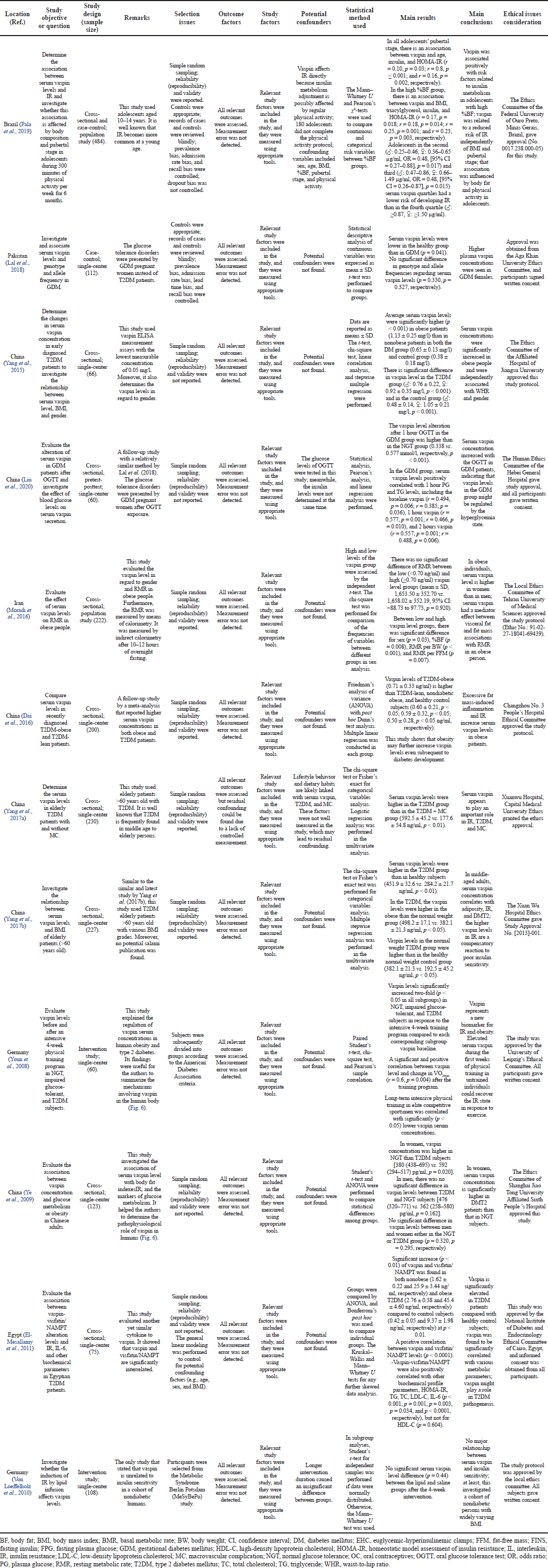 | Table 2. Critical appraisal from the review of the association between vaspin serum level and glucose tolerance disorders. [Click here to view] |
Vaspin and gender difference
The majority of the studies showed that serum vaspin concentrations were significantly higher in females than in males. Vaspin levels elevated with pubertal stage and age in females, but they were constant in males (Körner et al., 2011). The mechanism between serum vaspin levels and gender differences remains unsettled. Circulating estrogen concentration has been said to elevate serum leptin levels. This tends to also elucidate the elevated vaspin levels in females (Yang et al., 2015). A study reported GRP78 mRNA expression and vaspin levels in porcine oocyte and ovarian follicles (Kurowska et al., 2019). Those vaspin genes and serum depend on the ovarian follicle cycle while serum vaspin increases from the early to the late luteal phase (Kurowska et al., 2020).
A cross-sectional case-control study showed significantly high vaspin levels in females with polycystic ovary syndrome (PCOS). These individuals had stimulated vaspin mRNA expression and its protein product in omental adipose tissue (Dogan et al., 2020). Consequently, PCOS is highly associated with peripheral IR, hyperinsulinemia, and obesity (Kazemi Jaliseh et al., 2017).
Serum vaspin level and its genetic expression
Serum vaspin is regulated and encoded by the SERPINA12 gene consisting of 1,245 nucleotides on the 14q32.1 human chromosome (Hida et al., 2005). This review did not consider vaspin genetic expression aspects; hence, the vaspin expression and serum concentration are indirectly influenced by the gene and single nucleotide polymorphisms (SNPs) role (Fig. 6). Moreover, each type of vaspin gene SNP is strongly influenced by population race.
In the Japanese population, the SNPs restriction site (rs) 77060950 indicated high serum levels by modulating vaspin protein transcription (Teshigawara et al., 2012). Another study on the serum vaspin concentration and T2DM in the Iranian population showed that rs2236242 allele A is protective for T2DM and it indirectly causes a low vaspin level compared to T. According to Hosseini et al. (2021), rs2236242 SNPs have no association with IR. Therefore, the population race and its most suitable SNP type need to be considered in every vaspin serological study. Therefore, the population race and its most suitable SNP type need to be considered in every vaspin serological study.
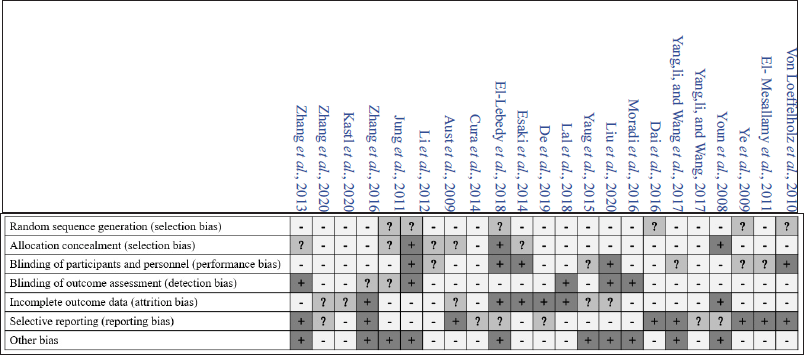 | Figure 2. Risk of bias assessment by the authors’ judgments for each reviewed study. [Click here to view] |
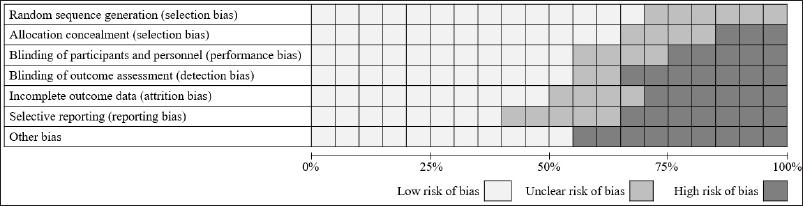 | Figure 3. Risk of bias graph by the authors’ judgments for each reviewed study. [Click here to view] |
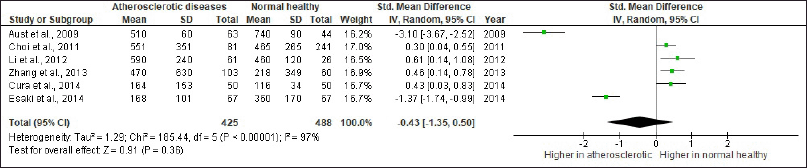 | Figure 4. Forest plot of serum vaspin levels (ng/ml) comparison between atherosclerotic diseases and normal healthy. CI, confidence interval and SD, standard deviation. [Click here to view] |
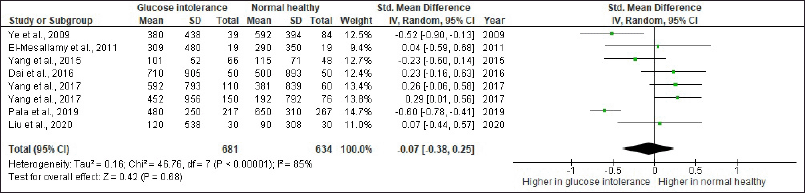 | Figure 5. Forest plot of serum vaspin levels (ng/ml) comparison between glucose tolerance disorders and normal healthy. CI, confidence interval and SD, standard deviation. [Click here to view] |
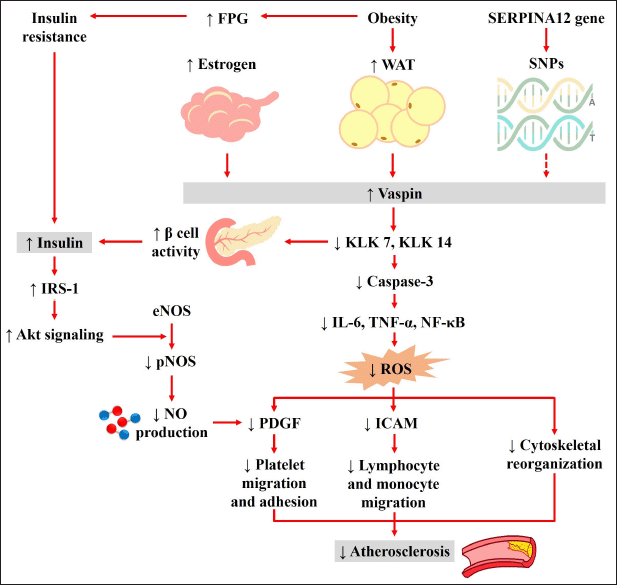 | Figure 6. Anti-inflammatory, anti-atherosclerotic, and IR compensatory molecular pathways that are affected by serum vaspin. Vaspin showed a beneficial role in endothelial cells; meanwhile, vaspin increases insulin levels as a compensatory mechanism due to IR. eNOS, endothelial nitric oxide synthase; FPG, fasting plasma glucose; ICAM1, intracellular adhesion molecule 1; IL, interleukin; IRS-1, insulin receptor substrate 1; KLK, kallikrein; NF-κB, nuclear factor-κB; NO, nitric oxide; PDGF, platelet-derived growth factor; pNOS, phosphorylated endothelial nitric oxide synthase; ROS, reactive oxygen species; SNPs, single nucleotide polymorphisms; TNF-α, tumor necrosis factor-alpha; and WAT, white adipose tissue. [Click here to view] |
CONCLUSION
High serum vaspin levels represent insulin compensatory adipokines during IR conditions in T2DM or obesity. Consequently, the levels of this compound significantly increase in patients with glucose intolerance. In vascular studies, a high serum vaspin level demonstrates a favorable role and beneficial effect in preventing atherosclerosis in either VSMCs or endothelial cells. The main limitation of this review was a failure to consider the effect of population SNP types on serum vaspin levels. Hence, a further review needs to include a detailed discussion of vaspin gene expression, population race, and specific SNP types.
ACKNOWLEDGMENTS
The authors are grateful to the endocrinology and cardiovascular medicine colleagues at Sebelas Maret University for evaluating this review and providing necessary feedback.
AUTHOR CONTRIBUTIONS
All authors read and approved the final manuscript. RD, SRT, BP, and DI: Conceptualization, literature search, critical appraisal, discussion, software, and logistics support. FM: Literature search, critical appraisal, data extraction, statistical analyses, writing-original initial draft, and final manuscript. DKM, VW, SNO, SUB, PB, YH, BLH, HRP, EAJH, IR, TT, and RAAT: Reviewing, assessment of the risk of bias, supervision, and validation.
FINANCIAL SUPPORT
No funding or grant was received for this review.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Al-Jundi A, Sakka S. Critical appraisal of clinical research. J Clin Diagn Res, 2017; 11(5):JE01. CrossRef
Aust G, Richter O, Rohm S, Kerner C, Hauss J, Klöting N, Ruschke K, Kovacs P, Youn BS, Blüher M. Vaspin serum concentrations in patients with carotid stenosis. Atherosclerosis, 2009; 204(1):262–6. CrossRef
Choi SH, Kwak SH, Lee Y, Moon MK, Lim S, Park YJ, Jang HC, Kim MS. Plasma vaspin concentrations are elevated in metabolic syndrome in men and are correlated with coronary atherosclerosis in women. Clin Endocrinol (Oxf), 2011; 75(5):628–35. CrossRef
Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci, 2013; 9(2):191. CrossRef
Cura HS, Özdemir HH, Demir CF, Bulut S, Ilhan N, Inci MF. Investigation of vaspin level in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis, 2014; 23(3):453–6. CrossRef
D’Souza RF, Masson SWC, Woodhead JST, James SL, MacRae C, Hedges CP, Merry TL. α1-Antitrypsin A treatment attenuates neutrophil elastase accumulation and enhances insulin sensitivity in adipose tissue of mice fed a high-fat diet. Am J Physiol Endocrinol Metab, 2021; 321(4):E560–70. CrossRef
Dai R, Dong Z, Qian Y, Han Y. Obese type 2 diabetes mellitus patients have higher serum vaspin concentrations. J Diabetes, 2016; 8(3):445–7. CrossRef
de Leal VO, Mafra D. Adipokines in obesity. Clin Chim Acta, 2013; 419:87–94. CrossRef
Dogan K, Helvacioglu C, Baghaki S, Ekin M. Comparison of body mass index and metabolic parameters with serum vaspin levels in women with polycystic ovary syndrome. Diabetes Metab Syndr, 2020; 14(2):137–9. CrossRef
Donath MY, Böni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda), 2009; 24(6):325–31. CrossRef
Dwipayana IMP, Semadi IMS, Gotera W, Saraswati MR, Suastika K. Vaspin in developing obesity (Vande-Ob); the correlation of waist circumference and visceral fat percentage with vaspin levels in patients with type ii diabetes mellitus. Open Access Maced J Med Sci, 2019; 7(1):50. CrossRef
El-Lebedy DH, Ibrahim AA, Ashmawy IO. Novel adipokines vaspin and irisin as risk biomarkers for cardiovascular diseases in type 2 diabetes mellitus. Diabetes Metab Syndr Clin Res Rev, 2018; 12(5):643–8. CrossRef
El-Mesallamy HO, Kassem DH, El-Demerdash E, Amin AI. Vaspin and visfatin/Nampt are interesting interrelated adipokines playing a role in the pathogenesis of type 2 diabetes mellitus. Metabolism, 2011; 60(1):63–70. CrossRef
Esaki E, Adachi H, Hirai Y, Yamagishi S Ichi, Kakuma T, Enomoto M, Fukami A, Kumagai E, Ohbu K, Obuchi A, Yoshimura A, Nakamura S, Nohara Y, Fujiyama T, Fukumoto Y, Imaizumi T. Serum vaspin levels are positively associated with carotid atherosclerosis in a general population. Atherosclerosis, 2014; 233(1):248–52. CrossRef
Heiker JT, Klöting N, Kovacs P, Kuettner EB, Sträter N, Schultz S, Kern M, Stumvoll M, Blüher M, Beck-Sickinger AG. Vaspin inhibits kallikrein 7 by serpin mechanism. Cell Mol Life Sci, 2013; 70(14):2569–83. CrossRef
Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, Hashimoto I, Okada T, Yasuhara A, Nakatsuka A, Shikata K, Hourai S, Futami J, Watanabe E, Matsuki Y, Hiramatsu R, Akagi S, Makino H, Kanwar YS. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci, 2005; 102(30):10610–5. CrossRef
Hosseini M, Nezhadali M, Hedayati M. Association of vaspin rs2236242 gene polymorphism with serum vaspin level, insulin resistance and diabetes in an Iranian diabetic/pre-diabetic population. J Med Biochem, 2021; 40(1):33. CrossRef
Jian W, Peng W, Xiao S, Li H, Jin J, Qin L, Dong Y, Su Q. Role of serum vaspin in progression of type 2 diabetes: a 2-year cohort study. PLoS One, 2014; 9(4):e94763. CrossRef
Jung CH, Lee WJ, Hwang JY, Seol SM, Kim YM, Lee YL, Park JY. Vaspin protects vascular endothelial cells against free fatty acid-induced apoptosis through a phosphatidylinositol 3-kinase/Akt pathway. Biochem Biophys Res Commun, 2011; 413(2):264–9. CrossRef
Kang YE, Kim JM, Joung KH, Lee JH, You BR, Choi MJ, Ryu MJ, Ko YB, Lee MA, Lee J, Ku BJ, Shong M, Lee KH, Kim HJ. The roles of adipokines, proinflammatory cytokines, and adipose tissue macrophages in obesity-associated insulin resistance in modest obesity and early metabolic dysfunction. PLoS One, 2016; 11(4):e0154003. CrossRef
Kastl SP, Katsaros KM, Krychtiuk KA, Jägersberger G, Kaun C, Huber K, Wojta J, Speidl WS. The adipokine vaspin is associated with decreased coronary in-stent restenosis in vivo and inhibits migration of human coronary smooth muscle cells in vitro. PLoS One, 2020; 15(5):1–13. CrossRef
Kazemi Jaliseh H, Ramezani Tehrani F, Behboudi-Gandevani S, Hosseinpanah F, Khalili D, Cheraghi L, Azizi F. Polycystic ovary syndrome is a risk factor for diabetes and prediabetes in middle-aged but not elderly women: a long-term population-based follow-up study. Fertil Steril, 2017; 108(6):1078–84. CrossRef
Kim JA, Choi KM. Newly discovered adipokines: pathophysiological link between obesity and cardiometabolic disorders. Front Physiol, 2020; 11:568800. CrossRef
Körner A, Neef M, Friebe D, Erbs S, Kratzsch J, Dittrich K, Blüher S, Kapellen TM, Kovacs P, Stumvoll M, Blüher M, Kiess W. Vaspin is related to gender, puberty and deteriorating insulin sensitivity in children. Int J Obes (Lond), 2011; 35(4):578–86. CrossRef
Kurowska P, Mlyczynska E, Barbe A, Staub C, Gregoraszczuk E, Dupont J, Rak A. Vaspin in the pig ovarian follicles: expression and regulation by different hormones. Reproduction, 2019; 158(2):135–46. CrossRef
Kurowska P, Mlyczy?ska E, Dawid M, Grzesiak M, Dupont J, Rak A. The role of vaspin in porcine corpus luteum. J Endocrinol, 2020; 247(3):283–94. CrossRef
Lal KK, Jarwar R, Farhat S, Fatima SS. Association of vaspin levels and its snp rs2236242 with gestational diabetes at a tertiary care setting. J Pak Med Assoc, 2018; 68(11):1736–40.
Li H, Peng W, Zhuang J, Lu Y, Jian W, Wei Y, Li W, Xu Y. Vaspin attenuates high glucose-induced vascular smooth muscle cells proliferation and chemokinesis by inhibiting the MAPK, PI3K/Akt, and NF-κB signaling pathways. Atherosclerosis, 2013; 228(1):61–8. CrossRef
Li Q, Chen R, Moriya J, Yamakawa J, Sumino H, Kanda T, Takahashi T. A novel adipocytokine, visceral adipose tissue-derived serine protease inhibitor (vaspin), and obesity. J Int Med Res, 2008; 36(4):625–9. CrossRef
Li Z, Ma C, Li L, Pan X, Chen L. Vaspin serum concentration in patients with type 2 diabetes and carotid plaque. J Int Med Res, 2012; 40(5):1670–6. CrossRef
Liu S, Li X, Wu Y, Duan R, Zhang J, Du F, Zhang Q, Li Y, Li N. Effects of vaspin on pancreatic β cell secretion via PI3K/Akt and NF-κB signaling pathways. PLoS One, 2017; 12(12):e0189722. CrossRef
Liu S, Duan R, Wu Y, Du F, Zhang J, Li X, Guo S, Wang M, Zhang Q, Li Y, Li N. Effects of vaspin on insulin resistance in rats and underlying mechanisms. Sci Rep, 2018; 8(1):13542. CrossRef
Liu Y, Gong M, Liu S, Pan Y, Huo Y. Effects of blood glucose on vaspin secretion in patients with gestational diabetes mellitus. Gynecol Endocrinol, 2020; 0(0):1–4.
Moradi S, Mirzaei K, Abdurahman AA, Keshavarz SA, Hossein-Nezhad A. Mediatory effect of circulating vaspin on resting metabolic rate in obese individuals. Eur J Nutr, 2016; 55(3):1297–305. CrossRef
Motoyama S, Yamada H, Yamamoto K, Wakana N, Terada K, Kikai M, Wada N, Saburi M, Sugimoto T, Kubota H, Miyawaki D, Kami D, Ogata T, Ibi M, Yabe-Nishimura C, Matoba S. Social stress increases vulnerability to high-fat diet-induced insulin resistance by enhancing neutrophil elastase activity in adipose tissue. Cells, 2020; 9(4):996. CrossRef
Nordmann TM, Dror E, Schulze F, Traub S, Berishvili E, Barbieux C, Böni-Schnetzler M, Donath MY. The role of inflammation in β-cell dedifferentiation. Sci Rep, 2017; 7(1):6285. CrossRef
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ, 2021; 372:n71. CrossRef
Pala D, Carlos-Cândido AP, Leandro-da-Cruz L, Oliveira-Barbosa P, Teixeira-Silva C, Carolina A, Pinheiro-Volp, Lins-Machado-Coelho GL, Nascimento-de-Freitas R. Vaspin association with insulin resistance is related to physical activity and body fat in Brazilian adolescents—a cross-sectional study. Nutr Hosp, 2019; 34(1):15–8.
Teshigawara S, Wada J, Hida K, Nakatsuka A, Eguchi J, Murakami K, Kanzaki M, Inoue K, Terami T, Katayama A, Iseda I, Matsushita Y, Miyatake N, McDonald JF, Hotta K, Makino H. Serum vaspin concentrations are closely related to insulin resistance, and rs77060950 at SERPINA12 genetically defines distinct group with higher serum levels in Japanese population. J Clin Endocrinol Metab, 2012; 97(7):E1202–7. CrossRef
Von Loeffelholz C, Möhlig M, Arafat AM, Isken F, Spranger J, Mai K, Randeva HS, Pfeiffer AFH, Weickert MO. Circulating vaspin is unrelated to insulin sensitivity in a cohort of nondiabetic humans. Eur J Endocrinol, 2010; 162(3):507–13. CrossRef
Wada J. Vaspin: a novel serpin with insulin-sensitizing effects. Expert Opin Investig Drugs, 2008; 17(3):327–33. CrossRef
Yang L, Chen SJ, Yuan GY, Wang D, Chen JJ. Changes and clinical significance of serum vaspin levels in patients with type 2 diabetes. Genet Mol Res, 2015; 14(3):11356–61. CrossRef
Yang W, Li Y, Tian T, Wang L, Lee P, Hua Q. Serum vaspin concentration in elderly patients with type 2 diabetes mellitus and macrovascular complications. BMC Endocr Disord, 2017a; 17(1):67. CrossRef
Yang W, Li Y, Tian T, Wang L. Serum vaspin concentration in elderly type 2 diabetes mellitus patients with differing body mass index: a cross-sectional study. Biomed Res Int, 2017b; 2017:4875026. CrossRef
Ye Y, Hou XH, Pan XP, Lu JX, Jia WP. Serum vaspin level in relation to postprandial plasma glucose concentration in subjects with diabetes. Chin Med J (Engl), 2009; 122(21):2530–3.
Youn BS, Klöting N, Kratzsch J, Lee N, Park JW, Song ES, Ruschke K, Oberbach A, Fasshauer M, Stumvoll M, Blüher M. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes, 2008; 57(2):372–7. CrossRef
Zhang B, Peng W, Li H, Lu Y, Zhuang J, Wang K, Su Y, Xu Y. Plasma vaspin concentrations are decreased in acute coronary syndrome, but unchanged in patients without coronary lesions. Clin Biochem, 2013; 46(15):1520–5. CrossRef
Zhang B, Peng W, Wang K, Li H, Xu Y. Vaspin as a prognostic marker in patients with acute myocardial infarction. Hear Lung Circ, 2016; 25(3):257–64. CrossRef
Zhang P, Wang G, Gui Y, Guo Z, Ren R, Sun Y, Song J. Serum vaspin as a predictor of severity and prognosis in acute ischemic stroke patients. Nutr Neurosci, 2020; 0(0):1–9.