INTRODUCTION
Diabetes mellitus is a long-term metabolic disorder characterized by hyperglycemia which might be due to improper secretion of insulin (Okasha et al., 2017). The chronic hyperglycemic condition can impair the eyes, kidneys, brain system, and cardiovascular system, among several other body organs including peripheral nerves (Hashem et al., 2021). The previous report found that diabetic individuals with low anti-oxidant levels, glucose autooxidation, and excess glycosylated proteins promote tissue damage and microvascular complication which might responsible for peripheral neuropathy (Medakkel and Sheela, 2018; Zhong et al., 2018).
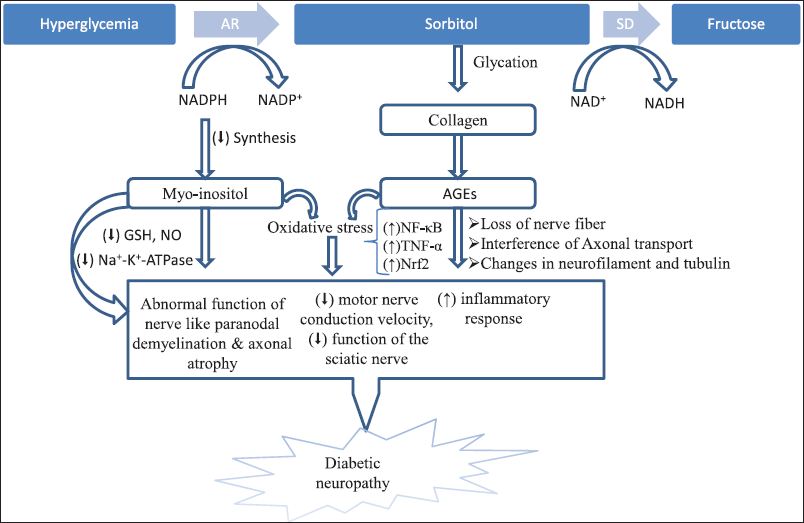
Peripheral neuropathy is the most common secondary complication of diabetic mellitus. It was found that when there is long-lasting hyperglycemia, the damage to the peripheral nerve is increased through different down-regulated cellular cascade processes. The most important metabolic cascade involved in the pathogenesis of diabetic-induced peripheral neuropathy are increased influx of polyol cascade, enhanced formation of glycation and advanced glycation end-products (AGE) cascade, increased generation of oxidative stress, activation of protein kinase-C (PKC) pathways, induction of pro-inflammatory processes, and formation of cellular and trophic factor (Fig. 1).
Polyol pathway
When the plasma glucose level becomes hyperglycemic, the activation of the aldose reductase (AR) enzyme is more prominent for increased glucose, and the increased flow of polyolcascade is mediated in nervous tissue. Increased influx of polyol resulted in intracellular hyperosmolarity as impermeable sorbitol accumulated in the cytoplasm, resulting in cell expansion and lysis according to previous research based on osmotic theory (Prabhakar and Sai laxmi, 2021). In addition, the polyol cascade increased the utilization of cofactor (reduced nicotinamide adenine dinucleotide phosphate),resulting in decreased formation of reduced glutathione and increasing the level of oxidized glutathione, decreasing the concentration of myo-inositol and nitric oxide (NO). The decreased synthesis of myo-inositol adversely affects the normal function (Na+-K+-ATPase activity, nodal remyelination, and conduction velocity) of nerves, suggesting that myo-inositol depletion could be a crucial factor in the genesis of diabetic neuropathy (Pathak et al., 2022). Sima et al. (1997) explored the role of myo-inositol in L-fucose-induced diabetic neuropathy in an experimental rat model. After supplementation of the L-fucose diet for 24 weeks to the rats, there were significant decreases in the level of myo-inositol and decreases in the Na+-K+-ATPase activity along with significant paranodal demyelination and axonal atrophy. The effect is similar to neuropathy of diabetes. However, the supplementation of a myo-inositol-enriched diet prevents the above-mentioned changes in nerves significantly, suggesting that myo-inositol deficiency could be an important pathological event in diabetic neuropathy (Sima et al., 1997). Further, the reduced level of glutathione and NO could increase the generation of reactive oxygen species (ROS), consequently producing cellular hemolysis and nerve cell damage. Kodani et al. (2000) illustrate the mechanism of action of NO in the involvement of diabetic neuropathy using streptozotocin (STZ)-induced male ddY mice. During the study, nociceptive thresholds and cyclic Guanosine monophosphate concentration were assessed after 8 weeks of STZ administration and the result indicates significant increases in nociceptive thresholds in diabetic animals than in the control group. However, injection of L-NG-nitro arginine methyl ester, which is a NO synthase inhibitor, exhibited a significant reduction of nociceptive thresholds in diabetic animals, concluding that NO has a potential role in the pathogenesis of diabetic neuropathy (Kodani et al., 2000).
 | Figure 1. Illustrate the possible mechanism of action involved during diabetic neuropathy. [Click here to view] |
Glycation and AGEs pathway
AGEs are heterogeneous molecules produced by reactive dicarbonyls during long-standing hyperglycemia. It is non-enzymatic glycation of plasma proteins via covalent adducts. Accumulation of AGEs in the peripheral nerves causes significant structural and functional modifications such as loss of nerve fiber, and alteration of neurofilament and tubulin. This alteration of nerve fibers leads to interference of axonal transport, which contributes to the genesis of atrophy and degeneration of nerve fibers, which play a vital role in the development of damage of nerve damage (Hotta et al., 2020). In addition, up-regulation of the receptor of AGEs (RAGE) leads to overexpression of endothelial cells of peri- and endoneurial blood vessels, which activates transcription factors of NF-κB and protein kinase (activator protein-1) to increase the expression of vascular cell adhesion molecule-1 and cytokines such as tumor necrosis factor and interleukin-6, suggesting that activation of RAGE by AGEs could be a crucial mechanism of pathologic processes of endoneurial vascular dysfunction and microangiopathy in the peripheral nerve. Toth et al. (2008) experimentally illustrate the relation between RAGE expression and diabetic neuropathy using STZ-induced diabetic mice. The result indicates significant up-regulation of RAGE mRNA and protein in epidermal axon and dorsal root ganglia (DRG) after 5 months of diabetic mice. The electrophysiological and quantitative morphometric test indicates structural abnormalities of the peripheral nerve. The study report also confirmed the activation of NF-κB and PKC in Schwann cells and peripheral nerves via RAGE expression. However, mice lacking RAGE have minimal activation of the peripheral nerve signal pathway (Toth et al., 2008). It is anticipated that the formation and accumulation of AGEs in the peripheral nerve involves the development of diabetic neuropathy, directly by affecting structural and functional proteins and indirectly by activating RAGE.
Oxidative stress
Hyperglycemia is an important factor in the mechanism of polyol pathway and AGEs generation. This cascade mechanism is involved in hyperglycemia-induced mitochondrial dysfunction which promotes oxidative stress and free radicals, in turn, stimulates nerve cellular injury and contributes to diabetic neuropathy. Oxidative stress in the peripheral nervous system can directly produce impairment in the synthesis of DNA or protein and modify intracellular signaling cascades such as redox-sensitive transcription factors and activation of protein kinase known as a mitogen-activated protein kinase (MAPK) in Schwann cells that contribute to the induction of inflammatory cytokines. The damaged protein in the nerve cell prevents metabolic functions like cell signaling and eventually leads to cell death. In addition, oxidative stress produced during hyperglycemia is also involved in the induction of endothelial abnormalities which leads to the development of micro- and macrovascular dysfunction. Furthermore, it was found that oxidative stress produced during persistent hyperglycemia modulates the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and NF-κB transcription factor. In healthy cells, the previous factor responsible for the maintenance of anti-oxidant defense and later involved with the production of the pro-inflammatory cytokine-maintained homeostasis condition in the cell. However, during increased oxidative stress, this cell homeostasis condition is perturbed, resulting in less generation of endogenous anti-oxidant defense, and consequently resulting in dysfunction of nerve microvascular structure and diabetic neuropathy (Kumar and Mittal, 2017; Yerra et al., 2013). Hence, targeting Nrf2 and NF-κB expression could be a beneficial approach in the management of diabetic neuropathy. Wang et al. (2020) illustrate the role of oxidative stress in diabetic peripheral neuropathy and also its alleviation with an anti-oxidant agent diphenyl diselenide using both in vitro and in vivo methods. During in vitro study, rat Schwann cells 96 were incubated with a higher concentration of glucose at 100 mM for about 24 hours, and then the test drug diphenyl diselenide was added with a concentration range of 1–50 uM to the cell lines for the evaluation of cytotoxicity and oxidative stress. The result indicates significant suppression of ROS and malondialdehyde (MDA) concentration in Schwann cells as well as overexpression of Nrf2 signaling and down-regulation of Keap1 expression in the sciatic nerve. Furthermore, the in vivo diabetic neuropathy was developed after the injection of STZ into rats. After administration of diphenyl diselenide (15 mg/kg) for 12 weeks, there was a significant improvement in motor nerve conduction velocity, mechanical hyperalgesia, the function of the sciatic nerve, and prevention of oxidative stress in the sciatic nerve. The result suggests that the anti-oxidant agent ameliorates the pathogenesis of diabetic-induced neuropathy induced by oxidative stress and could be considered a promising strategy for the management of neuropathy (Wang et al., 2020).
Protein kinase-C pathways
The involvement PKC in the pathophysiology of peripheral diabetic neuropathy has been well investigated (Mizukami et al., 2011). The condition of chronic hyperglycemia promotes the synthesis of 1,2-diacylglycerol which contributes to the activation of different isoforms of PKC such as PKC-α, -β1/2, and PKC-δ. These isoforms have been involved in the pathogenesis of neurovascular disorders by affecting blood flow and conduction velocity of the small vessel leading to diabetic neuropathy complications. Kim et al. (1991) illustrate the association of PKC in nerve tissue function in STZ-induced diabetic rats. The experiment reveals that diabetic rats have impaired Na+-K+-ATPase activity and alteration in PKC activity in the peripheral nerve which reduced nerve conduction as well as adversely affected nerve regeneration. This can be prevented by PKC agonists, suggesting the possible role of PKC in diabetic neuropathy (Kim et al., 1991).
Pro-inflammatory processes
The above illustration pointed out that chronic hyperglycemia gives rise to oxidative stress of the endoplasmic reticulum (ER) and mitochondria. The increased accumulation of ROS leads to the activation of inflammatory processes such as NF-kB, TNF-α, etc. (Cameron and Cotter, 2008). Kumar et al. (2012) illustrate the involvement of the NF-B inflammatory process in diabetic neuropathy using STZ-induced diabetic rats. BAY 11-7082 at two doses (1 and 3 mg/kg) and NF-kB inhibitor were administered to two groups of diabetic rats. The result of BAY 11-7082 treatment indicates a dose-dependent decrease in the level of inflammatory markers (IL-6 and TNF-α) and inducible enzymes (COX-2 and iNOS), decreased MDA level, and improvement in GSH level along with improvement of sensory responses and nerve function compared to the non-BAY 11-7082 treated group. The result suggests that down-regulation of NF-kB improved nerve function and prevents neuroinflammation by improving endogenous anti-oxidants and decreasing inflammatory cascades (Kumar et al., 2012). These inflammatory cascades show mild intraneural inflammation resulting in degeneration of peripheral nerve fiber, associated with diabetic neuropathy. Further, the infiltration of macrophages in the nerve fiber induced the generation of pro-inflammatory cytokines and chemokines which are also associated with dysfunction of peripheral nerve fiber and inflammation (Baum et al., 2021).
Cellular and trophic factor
Neurotrophin depletion is a major contributor to diabetic neuropathy (Azoulay et al., 2020). The report found that diabetic mice model nerve growth factor release was inhibited in the skin, and nerve growth factor replacement improved peripheral diabetic neuropathic alterations of tiny fibers and autonomic disease (Schmidt et al., 2003). Literature has discovered the pathophysiology of neuropathy, chimeric cells comprised of local Schwann cells or neuronal cells, and migratory proinsulin-producing cells from the bone marrow in diabetic nerves (Busik et al., 2009).
Protective role of epicatechin in diabetic peripheral neuropathy
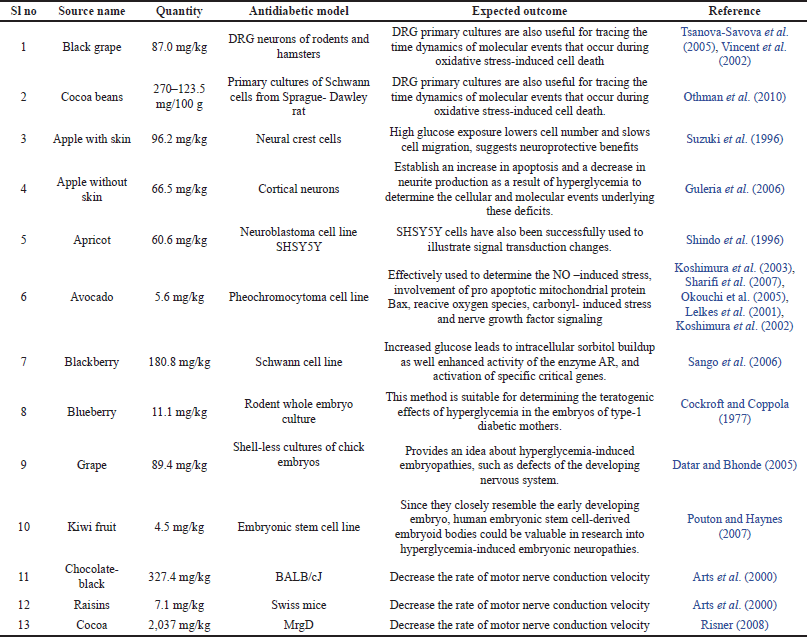
Epicatechin (flavon-3-ol monomer units) is a subclass of flavonoids known as flavanol monomers (Abdulkhaleq et al., 2017), and is a plant-based bioactive compound. The most common source of epicatechin is cocoa, green tea, black tea, peanuts, grapes, berries, apple, and dark chocolate (Manach et al., 2004). The chiral center on positions two and three of the plant-derived flavon-3-ol results in four diastereoisomers, namely (+)-epicatechin, (-)-epicatechin, (+)-catechin, and (-)-catechin (Bernatova, 2018). The chemical name of (-)-epicatechin is (2R, 3R). The 2,4-(dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol shows significant anti-inflammatory and anti-oxidant properties. The natural flavonoids, epicatechin, have been associated with a number of health benefits such as improving the function of the heart, brain, and muscles. Furthermore, epicatechin has been shown to have significant effects on the management of diabetes (Table 1) and its associated complications such as neuropathy, retinopathy, obesity, cardiovascular complications, and retinopathy (Basu and Basu, 2020). Among the different complications of diabetes, neuropathy is one of the insidious conditions in which nerve damage occurs throughout the body and adversely impacts the quality of life. The possible pathological involvement that modulates diabetic neuropathy includes decreased production of myo-inositol content in different autonomic nerves as well as increased oxidative stress, AGE, and PKC levels. Natural polyphenols have shown significant beneficial effects in the management of neuropathy. In this regard, an experimental finding indicates that the administration of catechin at the dose of 50 mg/kg body weight for the period of 28 days to STZ-induced diabetic rats exhibited a significant reduction of oxidative stress as well as neuronal damage in different nerves, suggesting flavonoids have a promising effect in the management of diabetic neuropathy (Addepalli and Suryavanshi, 2018). The anti-oxidant and anti-inflammatory properties of epicatechin are responsible for the reduction of damage to the pancreas, preventing diabetic-like conditions and improving the patient’s life span. Si et al. (2011) studied the effect of epicatechin on the diabetic db/db mouse model. The results indicated that administration of dietary epicatechin (0.1–8 mmol/l) for 15 weeks reduced the concentration of insulin-like growth factor-1, serum cholesterol level, blunted fat deposition, and degeneration of aortic vessels significantly. In addition, it improved hepatic anti-oxidant parameters like glutathione and superoxide dismutase (SOD) levels as well as the activity of AMP-activated protein kinase-α. The results suggest the longer, healthier lifespan could be due to the anti-oxidant properties of epicatechin, which could be a promising food-derived anti-aging bioactive compound (Si et al., 2011).
 | Table 1. Source of different epicatechin and their anti-diabetic properties. [Click here to view] |
The report found that the bioactive components of green tea play a significant role in the inhibition of AR, an enzyme that plays a vital role in the polyol pathway, one of the important pathological involvements of diabetic neuropathy. The experimental finding indicates strong inhibition of AR enzymes with IC50 = 79 μmol/l and 38 μmol/l for epicatechin and epicatechin gallate, respectively, suggesting flavonoids have a promising effect in the management of diabetic neuropathy (Murata et al., 1994). Another experiment evaluated the AR inhibitory activity using flavonoids. The result revealed that rich fractions obtained from Pedalium murex showed effective non-competitive inhibition of AR in STZ-induced diabetic rats. This suggests that flavonoids could be useful in associated complications of diabetic individuals (Patel, 2021).
Many of the consequences of diabetes mellitus, including diabetic neuropathy, are linked to the buildup of AGEs. AGE inhibitors were developed to combat diabetes complications and age-related disorders. According to Vasan et al. (1996), the AGE-breakers are a new class of reagents that involve the breaking down of AGE cross-links between tissue proteins that have already formed (Vasan et al., 1996). The use of botanical components, such as anti-AGE agents, has gained popularity as natural compounds have been demonstrated to be reasonably safer for human intake than synthetic substances (Lee et al., 2007). One of the phytoconstituents, epicatechin, has shown effective prevention of oxidative stress in pancreatic beta cells (Martín et al., 2014). Epicatechin is an anti-oxidant that protects lipids from oxidation and scavenges oxygen radicals (Ngawhirunpat et al., 2010). Peng et al. (2008) studied the role of epicatechin in the inhibition of the production of AGE. The results found that phytoconstituents such as epicatechin, catechin, and procyanidin B2 obtained from aqueous cinnamon bark exhibited significant inhibition of AGE production in the bovine serum albumin-glucose assay. The anti-oxidant and insulin-enhancing properties of bioactive compounds could be responsible for the entrapment of methylglyoxal and highly reactive carbonyl species, resulting in inhibition of AGE production and might be used as potential agents to alleviate diabetic complications (Peng et al., 2008). Another article demonstrated the inhibition of accumulation of AGE of (-)-epicatechin using both in vitro glycated human serum albumin and in vivo rat models. Experimental findings indicate that the intraperitoneal administration of epicatechin at the dose of 50 and 100 mg/kg for 15 days reduced AGE in both models in a dose-dependent manner, which is confirmed using immunohistochemical and western blotting tests. This suggests that epicatechin could be applied in diabetic individuals for the management of neuropathy by depleting the level of AGE (Kim et al., 2015). Similarly, the presence of anti-oxidant polyphenols such as (-)-epicatechin 3-O-gallate and (-)-epigallocatechin 3-O-gallate in green tea has exhibited a significant reduction in AGE production. Experimental findings indicate that administration of green tea extract at the dose of 300 mg/kg body weight to STZ-induced diabetic rats for the period of 1 month showed inhibition of AGE formation. These findings conclude that epicatechin might be used as a promising agent in the management of diabetic neuropathy and other complications (Wu et al., 2011). In normoglycemic rats injected with exogenous AGEs, the effects of epicatechin on retinal vascular impairment were also investigated; in vitro results revealed that epicatechin eliminates preformed antigenic AGEs (Kim et al., 2015). From these findings, we can conclude that by inhibiting the formation of AGE, which is responsible for the pathogenesis of neuropathy, epicatechin may pose a potential therapeutic target to overcome both microvascular and macrovascular diabetic complications.
The chronic hyperglycemic condition of diabetes causes oxidative stress which might damage the nerve tissue of the autonomic nervous system leading to peripheral neuropathy. Agents having potential anti-oxidant properties, for example, natural polyphenols like epicatechin and catechin, obtained from different diet sources could be utilized in the management of hyperglycemia-induced oxidative stress in neuropathy. The report found that administration of polyphenol (catechin) at the dose of 50 mg/kg for 28 days improved the oxidative stress parameters such as MDA, reduced glutathione, SOD, and catalase in STZ-induced diabetic rat vagus nerves significantly in addition to the reduction of random plasma blood glucose level. Further, the treatment of polyphenol reduces multifocal proliferation of Schwann cells and fragmentation of myelin and axon cells which was confirmed using histopathology examinations of the vagus nerve. The finding suggests that polyphenol could have the ability to prevent neuronal degeneration in the peripheral nervous system by reducing oxidative stress in hyperglycemic animal models (Addepalli and Suryavanshi, 2018). Another research article cites the effect of polyphenols in the management of diabetic neuropathy by reducing oxidative stress using the in vitro Alamar Blue assay. Incubation of (-)-epigallocatechin-3-gallate (EGCG) in the concentration of 50 μM in oxidative-stress-damaged cell lines of rat hippocampal neurons for 24 hours showed better cellular resistance to glucose oxidase by stimulating the protein expression of heme oxidase-1 enzymes via the Nrf2 signaling pathway. These results suggest the cytoprotective effect of EGCG is due to the reduction of oxidative stress in damaged cells and could be a key agent in the management of diabetic neuropathy (Romeo et al., 2009). Similarly, the reduction of oxidative stress activity of EGCG was evaluated in an in vivo STZ-induced male Wistar rat diabetic model. Oral administration of EGCG (40 mg/kg) for 7 weeks to diabetic rats shows a significant reduction of diabetic-induced serum nitrite and MDA content and increases the level of erythrocyte SOD in serum. These results suggest the attenuation of the oxidative stress effect of EGCG could be utilized as a therapeutic agent in the treatment of diabetic hyperalgesia or neuropathy (Baluchnejadmojarad and Roghani, 2012).
Epicatechin has been considered a promising anti-inflammatory agent and shows strong action in the reduction of pro-inflammatory processes such as NF-κB, iNOS, and c-JUN N-terminal kinase (JNK) which might be responsible for the progression of diabetic neuropathy. In this context, myristate 13-acetate was used to induce NF-κB in Jurkat T cells 4.6-fold more. In vitro results show that pretreatment with epicatechin (20 nM) reduced the expression of NF-κBup to 78% via preventing the NF-κBto gene, suggesting the modulating properties of epicatechin could affect the immune function properties of diabetic neuropathy individuals (Mackenzie et al., 2004). Recent discoveries demonstrate that ER stress plays a significant role in the development of diabetic neuropathy and points to a potential new treatment target. Grape seeds proanthocyanidins (GSP) and their derivatives such as epicatechin and catechin obtained from grape seeds have been reported to mitigate pro-inflammatory markers which are involved in neuropathic pain in murine models. In vivo results have shown that oral administration of GSP at the dose of 250 or 500 mg/kg body weight to STZ-induced diabetic rats exhibited significant improvement in the level of nerve conduction velocity and morphology of myelin sheath along with increases in ER and mitochondria, suggesting GSP treatment could prevent neuropathy by mitigating Ca2+ content in sciatic nerves (Ding et al., 2014). Similarly, flavonoids have shown considerable action in the alleviation of neurodegenerative disease by protecting the nervous system, which might be helpful in diabetic individuals. The proposed mechanism of the neuroprotective effect of epicatechin is the significant inhibition of Ca2+-dependent JNK signal and caspase-3-like protease expression in neurons induced by oxidized low-density lipoprotein, which suggests epicatechin has strong neuroprotective properties (Schroeter et al., 2001).
The report found that epicatechin and catechin obtained from the diet have significant actions in the protection of central neurons and preventing neuroinflammation. The result shows that 1-month administration of epicatechin (50 μg) produces a significant reduction of pro-inflammatory cytokines. TNF-α and IL-6 suggest flavonoids as promising neuroprotective agents and could be considered a potential therapeutic approach in the management of diabetic neuropathy (Noll et al., 2013). Al-Gayyar et al. (2011) investigated the function of pro nerve growth factor in neuroglial activation and the neuroprotective benefits of epicatechin, a specific inhibitor of tyrosine nitration in an experimental rat model of diabetes at a dose of 100 mg/kg body weight and found that epicatechin can successively restore neuronal survival (Al-Gayyar et al., 2011). Quiñonez-Bastidas et al. (2013) suggested that in diabetic rats, daily or every other day pre-treatment with epicatechin (0.03–30 mg/kg, i.p.) for 2 weeks reduced formalin-induced nociception (Quinonez-Bastidas et al., 2013). Recently, in chronic constriction injury-induced neuropathic pain models, researchers looked at the antinociceptive efficacy and underlying mechanism of Camellia japonica leaf extract having epicatechin and rutin, and found C. japonica leaf extract and its active components might have antinociceptive benefits against CCI4-induced neuropathic pain, which may be mediated through DRG MAPK activation and spinal microglial activation (Lim et al., 2022). Furthermore, epicatechin activates the NO-cyclic GMP-K+ channel, 5-HT1A/1B/1D/5A serotonin receptors, and opioid receptors to produce antinociception (Quiñonez-Bastidas et al., 2018). Similarly, (-)-epicatechin has demonstrated significant anti-diabetic activity by altering the relative expression of the GLUT 3 and TNF- proteins in an in vivo high fat, STZ-induced diabetic model, suggesting that it may alleviate neuropathy (Gonzalez, 2014). Lopes et al. (2012) studied the mechanism of antinociceptive action of epicatechin isolated from the hydroalcoholic fraction of Combretum leprosum using Swiss albino mice model. Initially, the nociception was induced in the paw of animals by using 20 μmol of glutamate. The use of epicatechin (50 mg/kg p.o.) to the nociceptive mice, after pretreatment with naloxone (2 mg/kg s.c.), glibenclamide (mg/kg s.c.), ketanserin (0.3 mg/kg s.c.), yohimbine (0.15 mg/kg s.c.), pindolol (1 mg/kg s.c.), and atropine (0.1 mg/kg s.c.) exhibited reverse antinociceptive effect. The result indicates the participation of opioid receptors and potassium channels sensitive to ATP, as well as serotoninergic (receptors 5HT1A and 5HT2A), adrenergic (α2 receptor), and cholinergic receptors in the alleviation of pain receptors and might be useful in the management of diabetic neuropathy (Lopes et al., 2012). The above findings suggest that epicatechin having anti-oxidant activity can be employed to ameliorate neuropathic pain by modifying the complementary biochemical processes. The stimulation of essential components in the Nrf2 pathway, NF-kB pathway, NO pathway, PI3K/AKT system, and the particular regulation of mitochondrial activity, endothelial function, muscle growth, and inhibitors are all important for epicatechin’s biological actions.
In addition to pre-clinical data on epicatechin in hyperglycemia, there are limited data on blood glucose control in pre-diabetic human patients. In this regard, a double-blinded, placebo-controlled, randomized, Phase I trial was conducted by using (+)-epicatechin with a dose of 30 mg/day for 7 days, and the result shows glycemic control in treated volunteers when compared to the placebo group (ClinicalTrials.gov, 2016). Another clinical trial was conducted for the testing of safety and effectiveness of synthetic prepared (+) epicatechin capsules at the dose of 25 mg thrice a day for 4 months in patients having Friedreich’s Ataxia, a neurological disorder. The result showed enhanced mitochondrial biogenesis, resulting in neuroprotection (ClinicalTrials.gov, 2016).
CONCLUSION
We found that the plant-derived flavonoid, epicatechin, has a promising effect on the prevention and management of diabetic neuropathy by modulating different pathological processes. The presence of anti-oxidant properties of epicatechin prevents the polyol pathway and AGEs, improves autonomic nerve function, and prevents neuronal damage caused by diabetic-induced free radicals. Furthermore, the significant anti-inflammatory activity of epicatechin helps in the prevention of neuroinflammation and protects the central as well as peripheral neurons. The effect of epicatechin in improving various neuropathic pain conditions, including mechanisms with preclinical information, suggests that it has a significant role in the control of neuropathic pain. As a result, it can be concluded that epicatechin has a great deal of potential for the development of innovative neuropathic pain treatments. Nevertheless, more research is required.
ACKNOWLEDGMENTS
The authors are thankful to the management of Centurion University and Management and Technology; they are also grateful to the authorities of the Sikhsa ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, India, for providing the required facilities to complete this assignment.
AUTHOR CONTRIBUTIONS
The authors confirm their contribution to the paper as follows: study conception and design: JD and GP; data collection: JD, BK; draft manuscript preparation: GG, GD, BK. All authors reviewed the results and approved the final version of the manuscript.
CONFLICT OF INTEREST
There are no conflicts of interest declared by the authors.
FUNDING
The authors do not have any funding sources.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdulkhaleq LA, Assi MA, Noor MH, Abdullah R, Saad MZ, Taufiq-Yap YH. Therapeutic uses of epicatechin in diabetes and cancer. Vet World, 2017; 10(8):869. CrossRef
Addepalli V, Suryavanshi SV. Catechin attenuates diabetic autonomic neuropathy in streptozotocin induced diabetic rats. Biomed Pharmacother, 2018; 1(108):1517–23. CrossRef
Arts IC, van de Putte B, Hollman PC. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. J Agric Food Chem, 2000; 48(5):1746–51. CrossRef
Al-Gayyar MMH, Matragoon S, Pillai BA, Ali TK, Abdelsaid MA, El-Remessy AB. Epicatechin blocks pro-nerve growth factor (proNGF)-mediated retinal neurodegeneration via inhibition of p75 neurotrophin receptor proNGF expression in a rat model of diabetes. Diabetologia, 2011; 54(3):669–80. CrossRef
Azoulay D, Abed S, Sfadi A, Sheleg O, Shaoul E, Shehadeh M, Kaykov E, Nodelman M, Bashkin A. Low brain-derived neurotrophic factor protein levels and single-nucleotide polymorphism Val66Met are associated with peripheral neuropathy in type II diabetic patients. Acta Diabetol, 2020; 57(7):891–8. CrossRef
Baluchnejadmojarad T, Roghani M. Chronic oral epigallocatechin-gallate alleviates streptozotocin-induced diabetic neuropathic hyperalgesia in rat: involvement of oxidative stress. Iran J Pharm Res, 2012; 11(4):1243.
Basu P, Basu A. In vitro and in vivo effects of flavonoids on peripheral neuropathic pain. Molecules, 2020; 25(5):1171. CrossRef
Baum P, Toyka KV, Blüher M, Kosacka J, Nowicki M. Inflammatory mechanisms in the pathophysiology of diabetic peripheral neuropathy (DN)-new aspects. Int J Mol Sci, 2021; 22(19):10835. CrossRef
Bernatova I. Biological activities of (−)-epicatechin and (−)-epicatechin-containing foods: focus on cardiovascular and neuropsychological health. Biotechnol Adv, 2018; 36(3):666–81. CrossRef
Busik JV, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, Caballero S, Player D, Nakagawa T, Afzal A, Kielczewski J, Sochacki A, Hasty S, Calzi SL, Kim S, Duclas SK, Segal MS, Guberski DL, Esselman WJ, Boulton ME, Grant MB. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med, 2009; 206(13):2897–906. CrossRef
Cameron NE, Cotter MA. Pro-inflammatory mechanisms in diabetic neuropathy: focus on the nuclear factor kappa B pathway. Curr Drug Targets, 2008; 9(1):60–7. CrossRef
Cockroft DL, Coppola PT. Teratogenic effects of excess glucose on head-fold rat embryos in culture. Teratology, 1977; 16(2):141–6. CrossRef
Datar S, Bhonde RR. Shell-less chick embryo culture as an alternative in vitro model to investigate glucose-induced malformations in mammalian embryos. Rev Diabet Stud, 2005; 2(4):221. CrossRef
Ding Y, Dai X, Zhang Z, Jiang Y, Ma X, Cai X, Li Y. Proanthocyanidins protect against early diabetic peripheral neuropathy by modulating endoplasmic reticulum stress. J Nut Biochem, 2014; 25(7):765–72. CrossRef
Early Phase Pre-Clinical and Initial Clinical Research on Epicatechin (Part 2) - Full Text View - ClinicalTrials.gov, 2016
Gonzalez N. Effects of epicatechin on glucose transporters in cardiac tissue and peripheral nerves in type 2 diabetes (1086.5). FASEB J, 2014; 28:1086–5. CrossRef
Guleria RS, Pan J, DiPette D, Singh US. Hyperglycemia inhibits retinoic acid–induced activation of Rac1, prevents differentiation of cortical neurons, and causes oxidative stress in a rat model of diabetic pregnancy. Diabetes, 2006; 55(12):3326–34. CrossRef
Hashem MM, Esmael A, Nassar AK, El-Sherif M. The relationship between exacerbated diabetic peripheral neuropathy and metformin treatment in type 2 diabetes mellitus. Sci Rep, 2021; 11(1):1–9. CrossRef
Hotta N, Kawamura T, Umemura T. Does the breakdown of the detoxification system for aldehydes as a result of aldose reductase upregulation lead to alcohol–induced liver injury in humans and mice? J Diabetes Investig, 2020; 11(6):1426. CrossRef
Kim J, Kim CS, Moon MK, Kim JS. Epicatechin breaks preformed glycated serum albumin and reverses the retinal accumulation of advanced glycation end products. Eur J Pharm, 2015; 748:108–14. CrossRef
Kim J, Rushovich EH, Thomas TP, Ueda T, Agranoff BW, Greene DA. Diminished specific activity of cytosolic protein kinase C in sciatic nerve of streptozotocin-induced diabetic rats and its correction by dietary myo-inositol. Diabetes, 1991; 40(11):1545–54. CrossRef
Kodani T, Tanaka N, Chougahara M, Nitanda A, Kohno Y, Koike A, Shiomi H, Nakamura A. Pathophysiological role of nitric oxide in streptozotocin-induced diabetic neuropathy. Japanese J Pharmacol, 2000; 82:157. CrossRef
Koshimura K, Tanaka J, Murakami Y, Kato Y. Involvement of nitric oxide in glucose toxicity on differentiated PC12 cells: prevention of glucose toxicity by tetrahydrobiopterin, a cofactor for nitric oxide synthase. Neurosci Res, 2002; 43(1):31–8. CrossRef
Koshimura K, Tanaka J, Murakami Y, Kato Y. Effect of high concentration of glucose on dopamine release from pheochromocytoma-12 cells. Metabolism, 2003; 52(7):922–6. CrossRef
Kumar A, Mittal R. Nrf2: a potential therapeutic target for diabetic neuropathy. Inflammopharmacology, 2017; 25(4):393–402. CrossRef
Kumar A, Negi G, Sharma SS. Suppression of NF-κB and NF-κB regulated oxidative stress and neuroinflammation by BAY 11-7082 (IκB phosphorylation inhibitor) in experimental diabetic neuropathy. Biochimie, 2012; 94(5):1158–65. CrossRef
Lee HS, Jung SH, Yun BS, Lee KW. Isolation of chebulic acid from Terminaliachebula Retz and its antioxidant effect in isolated rat hepatocytes. Arch Toxicol, 2007; 81(3):211–8. CrossRef
Lelkes E, Unsworth BR, Lelkes PI. Reactive oxygen species, apoptosis and alte1red NGF-induced signaling in PC12 pheochromocytoma cells cultured in elevated glucose: an in vitro cellular model for diabetic neuropathy. Neurotox Res, 2001; 3(2):189–203. CrossRef
Lim EY, Lee C, Kim YT. The antinociceptive potential of Camellia japonica leaf extract,(−)-epicatechin, and rutin against chronic constriction injury-induced neuropathic pain in rats. Antioxidants, 2022; 11(2):410. CrossRef
Lopes LDS, Marques RB, Fernandes HB, Pereira SDS, Ayres MC, Chaves MH, Almeida FRC. Mechanisms of the antinociceptive action of (−) epicatechin obtained from the hydroalcoholic fraction of Combretumleprosum mart & eic in rodents. J Biomed Sci, 2012; 19(1):1–6. CrossRef
Mackenzie GG, Carrasquedo F, Delfino JM, Keen CL, Fraga CG, Oteiza PI. Epicatechin, catechin, and dimericprocyanidins inhibit PMA–induced NF–κB activation at multiple steps in Jurkat T cells. FASEB J, 2004; 18(1):167–9. CrossRef
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nut, 2004; 79(5):727–47. CrossRef
Martín MÁ, Fernández–Millán E, Ramos S, Bravo L, Goya L. Cocoa flavonoid epicatechin protects pancreatic beta cell viability and function against oxidative stress. Mol Nut Food Res, 2014; 58(3):447–56. CrossRef
Medakkel AA, Sheela P. Vibration perception threshold values and clinical symptoms of diabetic peripheral neuropathy. J Clin Diagn Res, 2018; 1:12(5). CrossRef
Mizukami H, Ogasawara S, Yamagishi SI, Takahashi K, Yagihashi S. Methylcobalamin effects on diabetic neuropathy and nerve protein kinase C in rats. Eur J Clin Invest, 2011; 41(4):442–50. CrossRef
Murata M, Irie J, Homma S. Aldose reductase inhibitors from green tea. LWT-Food Sci Technol, 1994; 27(5):401–5. CrossRef
Ngawhirunpat T, Opanasopi P, Sukma M, Sittisombut C, Kat A, Adachi I. Antioxidant, free radical-scavenging activity and cytotoxicity of different solvent extracts and their phenolic constituents from the fruit hull of mangosteen (Garciniamangostana). Pharm Biol, 2010; 48(1):55–62. CrossRef
Noll C, Lameth J, Paul JL, Janel, N. Effect of catechin/epicatechin dietary intake on endothelial dysfunction biomarkers and proinflammatory cytokines in aorta of hyperhomocysteinemic mice. Eur J Nut, 2013; 52(3):1243–50. CrossRef
Okasha AH, Gaballah HH, Zakaria SS, Elmeligy SM. Role of autophagy and oxidative stress in experimental diabetes in rats. Tanta Med J, 2017; 45(3):122. CrossRef
Okouchi M, Okayama N, Aw TY. Hyperglycemia potentiates carbonyl stress-induced apoptosis in naive PC-12 cells: relationship to cellular redox and activator protease factor-1 expression. Curr Neurovasc Res, 2005; 2(5):375–86. CrossRef
Othman A, Jalil AM, Weng KK, Ismail A, Ghani NA, Adenan I. Epicatechin content and antioxidant capacity of cocoa beans from four different countries. Afr J Biotechnol, 2010; 9(7):1052–9. CrossRef
Patel DK. Anti-diabetic activity of flavonoid rich fraction of Pedalium murex in the medicine: biological importance of aldose reductase in polyol pathway for prevention of microvascular complications. Bone Rep, 2021; 14:100932. CrossRef
Pouton CW, Haynes JM.Embryonic stem cells as a source of models for drug discovery. Nat Rev Drug Discov, 2007; 6(8):605–16. CrossRef
Pathak R, Sachan N, Chandra P. Mechanistic approach towards diabetic neuropathy screening techniques and future challenges: a review. Biomed Pharm, 2022; 150:113025. CrossRef
Peng X, Cheng KW, Ma J, Chen B, Ho CT, Lo C, Chen F, Wang M. Cinnamon barkproanthocyanidins as reactive carbonyl scavengers to prevent the formation of advanced glycation endproducts. J Agric Food Chem, 2008; 56(6):1907–11. CrossRef
Prabhakar O, Sai laxmi M. Inflammatory biomarkers as a part of diagnosis in diabetic peripheral neuropathy. J Diabetes Metab Disord, 2021; 20(1):869. CrossRef
Quiñonez-Bastidas GN, Cervantes-Durán C, Rocha-González HI, Murbartián J, Granados-Soto V. Analysis of the mechanisms underlying the antinociceptive effect of epicatechin in diabetic rats. Life Sci, 2013; 93(17):637–45. CrossRef
Quiñonez-Bastidas GN, Pineda-Farias JB, Flores-Murrieta FJ, Rodríguez-Silverio J, Reyes-García JG, Godínez-Chaparro B, Granados-Soto V, Rocha-González HI. Antinociceptive effect of (−)-epicatechin in inflammatory and neuropathic pain in rats. Behav Pharmacol, 2018; 29(2):270–9. CrossRef
Risner CH. Simultaneous determination of theobromine, (+)-catechin, caffeine, and (−)-epicatechin in standard reference material baking chocolate 2384, cocoa, cocoa beans, and cocoa butter. J Chromatogr Sci, 2008; 46(10):892–9. CrossRef
Romeo L, Intrieri M, D’Agata V, Mangano NG, Oriani G, Ontario ML, Scapagnini G. The major green tea polyphenol,(-)-epigallocatechin-3-gallate, induces hemeoxygenase in rat neurons and acts as an effective neuroprotective agent against oxidative stress. J Am Coll Nut, 2009; 28(sup4):492S–9S. CrossRef
Sango K, Suzuki T, Yanagisawa H, Takaku S, Hirooka H, Tamura M, Watabe K. High glucose-induced activation of the polyol pathway and changes of gene expression profiles in immortalized adult mouse Schwann cells IMS32. J Neurochem, 2006; 98(2):446–58. CrossRef
Schmidt RE, Dorsey DA, Beaudet LN, Parvin CA, Escandon E. Effect of NGF and neurotrophin-3 treatment on experimental diabetic autonomic neuropathy. J Neuropathol Exp Neurol, 2001; 60(3):263–73. CrossRef
Schroeter H, Bahia P, Spencer JPE, Sheppard O, Rattray M, Cadenas E, Rice-Evans C, Williams RJ. (–) Epicatechin stimulates ERK–dependent cyclic AMP response element activity and up–regulates GluR2 in cortical neurons. J Neurochem, 2007; 101(6):1596–606. CrossRef
Sharifi AM, Mousavi SH, Farhadi M, Larijani B. Study of high glucose-induced apoptosis in PC12 cells: role of bax protein. J Pharm Sci, 2007; 104(3):258–62. CrossRef
Shindo H, Thomas TP, Larkin DD, Karihaloo AK, Inada H, Onaya T, Stevens MJ, Greene DA. Modulation of basal nitric oxide-dependent cyclic-GMP production by ambient glucose, myo-inositol, and protein kinase C in SH-SY5Y human neuroblastoma cells. J Clin Investig, 1996; 97(3):736–45. CrossRef
Si H, Fu Z, Babu PVA, Zhen W, LeRoith T, Meaney MP, Voelker KA, Jia Z, Grange RW, Liu D. Dietary epicatechin promotes survival of obese diabetic mice and Drosophila melanogaster. J Nut, 2011; 141(6):1095–100. CrossRef
Sima AA, Dunlap JA, Davidson EP, Wiese TJ, Lightle RL, Greene DA, Yorek MA. Supplemental myo-inositol prevents L-fucose–induced diabetic neuropathy. Diabetes, 1997; 46(2):301–6. CrossRef
Suzuki N, Svensson K, Eriksson UJ. High glucose concentration inhibits migration of rat cranial neural crest cells in vitro. Diabetologia, 1996; 39(4):401–11. CrossRef
Tsanova-Savova S, Ribarova F, Gerova M. (+)-Catechin and (−)-epicatechin in Bulgarian fruits. J Food Compost Anal, 2005; 18(7):691–8. CrossRef
Toth C, Rong LL, Yang C, Martinez J, Song F, Ramji N, Brussee V, Liu W, Durand J, Nguyen MD, Schmidt AM, Zochodne DW. Receptor for advanced glycation end products (RAGEs) and experimental diabetic neuropathy. Diabetes, 2008; 57(4):1002–17. CrossRef
Vasan S, Zhang X, Zhang X, Kapurniotu A, Bernhagen J, Teichberg S, Basgen J, Wagle D, Shih D, Terlecky I, Bucala R, Cerami A, Egan J, Ulrich P. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature, 1996; 382(6588):275–8. CrossRef
Vincent AM, Brownlee M, Russell JW. Oxidative stress and programmed cell death in diabetic neuropathy. Ann N Y Acad Sci, 2002; 959(1):368–83. CrossRef
Wang X, Huan Y, Li C, Cao H, Sun S, Lei L, Liu Q, Liu S, Ji W, Liu H, Huang K, Zhou J, Shen Z. Diphenyldiselenide alleviates diabetic peripheral neuropathy in rats with streptozotocin-induced diabetes by modulating oxidative stress. Biochem Pharmacol, 2020; 182:114221. CrossRef
Wu CH, Huang SM, Lin JA, Yen GC. Inhibition of advanced glycation endproduct formation by foodstuffs. Food Funct, 2011; 2(5):224–34. CrossRef
Yerra VG, Negi G, Sharma SS, Kumar A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol, 2013; 1(1):394–7. CrossRef
Zhong JM, Lu YC, Zhang J. Dexmedetomidine reduces diabetic neuropathy pain in rats through the Wnt 10a/β-catenin signaling pathway. Biomed Res Int, 2018; 2018:9043628. CrossRef