INTRODUCTION
Herbal formulations are not uncommon in treating diseases as they have been utilized to treat different diseases in humans and animals for millennia. Despite screening conducted on several other plant species, research studies on plant-based bioactive compounds remain paramount as better and safer agents with a broad spectrum of bioactivity are greatly needed (Arifullah et al., 2014). There is a long history of the medicinal uses of the plant in Southeast Asian countries, with some being sold in different forms for health remedies (Liew et al., 2020). It is an appropriate time to develop novel bioactive compounds from natural sources such as higher plants (Manga et al., 2018). This is due to the antimicrobial, insecticidal, and antioxidant properties these plants possess; therefore, they are accepted for everyday use in both traditional and modern medicine (Arifullah et al, 2014). Herbs are essential in dealing with different health conditions; this could be attributed to their enormous bioactive compounds, thus attracting the interest of researchers to the field of drug discovery (Oliveira et al., 2020).
Melaleuca cajuputi Powell plant is a member of the Myrtaceae family, popularly called “Gelam,” “kayu putih,” “paperbark,” “Cajuput tree,” “Melaleuca leucadendron,” “Cajuput oil,” or “tea tree” (Sharif et al., 2019). The leaves are greyish-green and fragrant with a relaxing aroma. Its height ranges from 4 to 9 cm (Fig. 1). The flowers are whitish with a unique stamen with 16 cm long spikes, which are said to resemble bottlebrushes, and the encircled seeds are essential in folk medicine (Sharif et al., 2019). Melaleuca essential oil has a yellow-green color with a solid herbal aroma that is similar to eucalyptus essential oil (Wi?ska et al., 2019). The tea tree can be found naturally in tropical areas including Malaysia, Indonesia, Thailand, and Australia. Melaleuca forests grow well in estuaries and coastal swamps in the hot and humid tropics with a temperature range of 17°C–33°C. The Melaleuca-rich region receives an average of 1.3–1.7 mm of precipitation per year, with typical monsoon conditions (Lim and Midon, 2001).
 | Figure 1. Melaleuca cajuputi Powell in its natural habitat (source: author). [Click here to view] |
Several studies on the different parts of M. cajuputi Powell extracts have been reported previously (Al-Abd et al., 2015; Noor et al., 2020). However, the information obtained from these studies is extremely fragmented. Due to this, an in-depth literature review on M. cajuputi Powell will undoubtedly provide the necessary information which is crucial in understanding the knowledge gaps of this plant and stimulating future research opportunities. This scoping review focuses on the types of M. cajuputi Powell extracts examined and their phytoconstituents as well as the research findings on their biological activities.
METHODOLOGY
This review followed the methodological framework established by Arksey and O’Malley (2005). In addition, this study was processed according to the procedure of systematic database search, selection, and inclusion strategy as described by (Moher et al., 2009). This scoping review consisted of defining objectives, searching for relevant data, proper selection, gathering relevant information based on the study objectives, and summarizing the findings.
Relevant studies on the phytoconstituents and biological activities of M. cajuputi Powell published in the last two decades (2001 to 2022) were retrieved from Google, Science Direct, Google Scholar, Scopus, and PubMed. However, only research papers on M. cajuputi Powell written in English from January 2001 to early 2022 were reviewed. Theses, dissertations, and conference proceedings were included as well. Several key search terms were included such as “M. cajuputi Powell,” “Gelam extracts,” “tea tree,” “cajuput tree,” “M. leucadendron,” “antibacterial,” “antifungal,” “antimicrobial,” “antioxidant activities,” “insecticidal,” “toxicity effect,” “bioactive,” and “phytochemical compounds” by using the Boolean operators of AND/OR (Table 1).
Figure 2 illustrates the record selection criteria. Research articles from the various databases were first harmonized and sorted based on the titles to exclude all duplicates. Titles and abstracts were reviewed to exclude all records with inaccurate subjects or outcomes related to this review. Lastly, the full-text articles were screened further to exclude other irrelevant data.
Data recording
Relevant data regarding studies of M. cajuputi Powell were recorded. The information obtained consisted of various parts of the plant, such as leaves, flowers, stems, and essential oils (Fig. 3). The extraction procedures, bioactive compounds, type of biological activity, methods employed/outcomes, organisms tested, and references are shown in Table 2.
Collection, reporting, and summary of the review outcomes
All findings were recorded and summarized in Table 2. The research gaps were identified to establish a roadmap for future research to provide knowledge on novel drug discoveries from M. cajuputi Powell.
RESULT AND DISCUSSION
Medicinal plants are essential sources of unique bioactive compounds in creating new, effective, and safe medicines (3,311) research articles on the bioactivity of different parts of M. cajuputi Powell were retrieved via an electronic survey in interdisciplinary databases, with 325 duplicates removed from the list. As shown in Figure 2, The remaining 2,986 articles were then checked for title and abstract eligibility. In all, 2,892 irrelevant articles were excluded, leaving 94 possible relevant articles for full-text analysis, out of which only 34 papers were selected based on the review’s selection criteria and objectives.
Ethnomedicinal uses of M. cajuputi Powell
Medicinal plants have been reported and investigated for natural bioproducts to control diseases and lessen reliance on conventional antibiotics worldwide (Isah et al., 2020). According to historical records, herbal therapy and Ayurvedic medicine have been utilized in China for over 5,000 years (Idris et al., 2019). Moreover, humans also have been relying heavily on natural products such as plant-based food and treatment for thousands of years (Idris et al., 2019). Herbal decoctions can be used in oral administration, topical application, or steaming for disease treatment, depending on the formulation and plant parts. Tablespoons, cups, and bottles are used to measure the dosage of these herbal formulations (Bunalema et al., 2014).
The leaves of Gelam are edible and are used to treat digestive problems, cough, common cold, and stomach pain (Daud et al., 2015). Meanwhile, herbal preparation made from M. cajuputi Powell leaves and stems is used to treat joint soreness, abdominal discomfort, and muscle ache (Wolter et al., 2002). Furthermore, tea tree leaves exhibit antimicrobial, antioxidant, anodyne, insect repellent, and anti-inflammatory properties and are utilized in traditional medicine to treat dyspepsia, burns, pain, and influenza (Ko et al., 2009).
Similarly, M. cajuputi Powell is an excellent producer of therapeutic essential oil that contains many phytochemicals (Hai et al., 2019). Steam or hydrodistillation extracts the Cajuput oil from the plant’s leaves or other parts. It is utilized to heal wounds, body itch, coughs, stomach pains, asthma, and rashes in folk medicine, particularly in Southeast Asia (Toan et al., 2020). Additionally, the oil has a synergistic effect when combined with other plants in decoction to treat rheumatoid arthritis (Toan et al., 2020). Externally, the utilization of M. cajuputi oil includes relieving neuralgia and rheumatism (typically in the form of ointments and salves), as well as toothache, cancer, worms (particularly roundworms), and infections of the genitourinary system (Silva et al., 2007).
 | Table 1. Keywords and search phrases used for literature search. [Click here to view] |
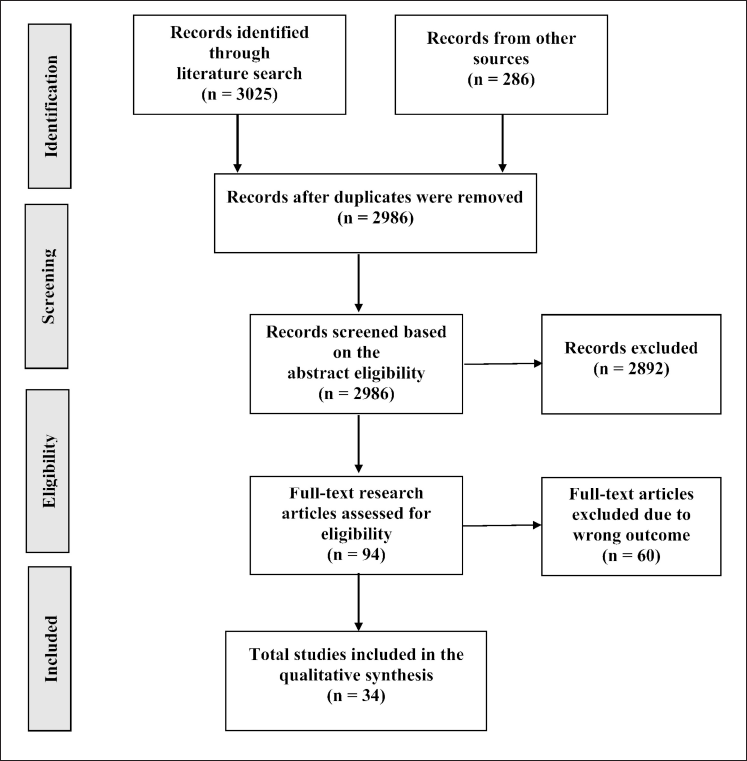 | Figure 2. Record identification and selection protocol based on PRISMA guidelines (PRISMA, 2009 ). [Click here to view] |
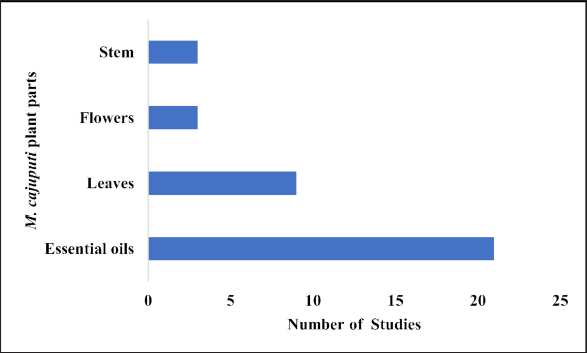 | Figure 3. Bioactivity studies on the different parts of M. cajuputi Powell. [Click here to view] |
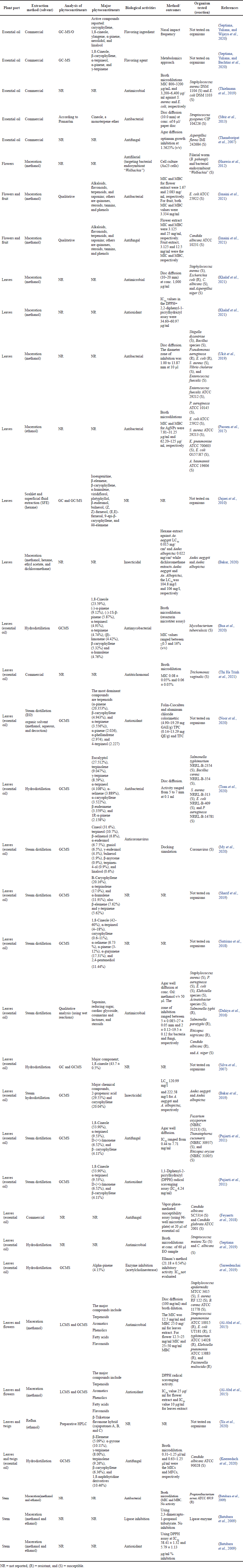 | Table 2. Biological activities and phytochemical constituents of M. cajuputi Powell. [Click here to view] |
Solvent selection and methods for extracting phytochemicals from M. cajuputi Powell extracts
Plant extraction can be performed with different solvent systems ranging from nonpolar, intermediate, and highly polar to separate and purify bioactive compounds (Ukit et al., 2019). The resulting filtrate is tested for bioactivity screening at different concentrations. The most common solvent systems used to extract the bioactive compounds from M. cajuputi Powell were methanol, ethanol, hexane, ethyl acetate, dichloromethane, and water (Table 3). Methanol, in particular, was highlighted several times in previous studies due to its excellent properties in dissolving polar and nonpolar bioactive compounds from plant material (Ukit et al., 2019). Bioactive compounds can be separated and isolated by using different chromatographic techniques. Subsequently, various spectrometric approaches can be employed to identify the plant’s chemical structure and functional groups of extracted bioactive components (Xu et al., 2020).
Phytochemical composition of M. cajuputi Powell
The bioactive compounds reported in M. cajuputi Powell extracts include monoterpenes, sesquiterpenes, flavonoids, and phenolic compounds (Table 2). Figure 4 depicts the chemical structures of the main bioactive molecules. Monoterpenes are the most common chemical compounds, with structures comprised two isoprene units and organic functional groups involving hydrocarbons (pinene, terpinene, p-cimene, and terpinene), alcohols (linalool, cineol, and 4-terpineol), and ethers like 1,8-cineol (Jajaei et al., 2010). Sesquiterpenes are unsaturated chemicals that are comprised of hydrocarbons (caryophyllene), oxygenated sesquiterpenes (nerolidol), and alcohols (bisabolol and nerolidol). Other organic compounds such as steroids, quinones, saponins, tannins, and alkaloids were also reported in M. cajuputi Powell extracts (Dahiya et al., 2016; Isnaini et al., 2021).
 | Table 3. Solvent’s system used in M. cajuputi Powell extraction process from 2001 to 2021. [Click here to view] |
Plants usually produce primary metabolites such as simple sugars, amino acids, polypeptides, and lactic acid, which are found virtually in all plants as part of their regular metabolic activity. They also produce secondary metabolites such as antibiotics, poisons, organic acids, and pigments for defense purposes (Angelo, 2015). As most of the products are used for self-defense, these metabolites and pigments could contain bioactive molecules, including inulin, quinine, morphine, and codeine (Angelo, 2015). Some of these bioactive compounds reported in M. cajuputi Powell extracts could interfere with the cell membrane integrity and distort cell wall structure, leading to cell death. For example, terpenoids could divide lipid membranes and cause irreversible leakage of cellular components, leading to cell destruction (Sharif et al., 2019). Another bioactive compound is 4-terpineol, an isomer of terpineol that blocks respiration and interferes with cellular metabolism (Sharif et al., 2019).
Antimicrobial activity of M. cajuputi Powell
The antimicrobial efficacy of M. cajuputi Powell extracts from leaves, flowers, stem bark, and essential oil against bacteria, fungi, protozoa, and viruses was evaluated in vitro using agar well diffusion, disc diffusion, and broth microdilution. Melaleuca cajuputi Powell crude extracts showed antimicrobial activity at 0.007–25.00 mg/ml and 0.062–50.0 mg/ml as minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)values, respectively. The diameter zone of inhibition ranged from 1.00 to 27.00 mm (Table 2).
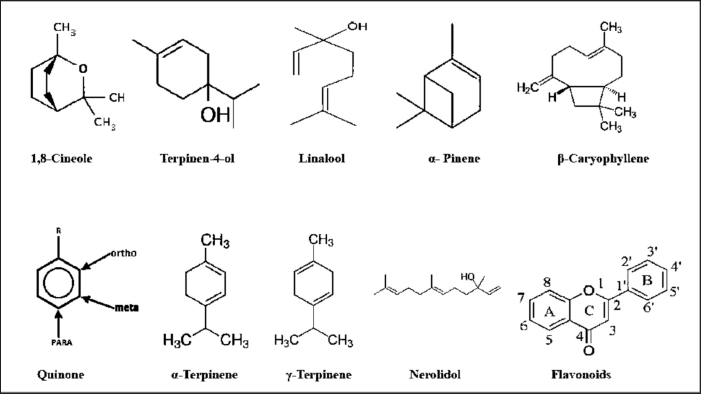 | Figure 4. Major bioactive compounds reported in M. cajuputi Powell extracts and their chemical structures. [Click here to view] |
The report on the antimicrobial properties showed that M. cajuputi Powell extracts were effective against fungal species, bacteria, viruses, and protozoa at varying concentrations (Bua et al., 2020; Shivappa et al., 2015; Thanaboripat et al., 2007; Trinh et al., 2021).
The essential oil at a concentration of 0.714% (w/w) was reported to show antibacterial activity against pathogenic bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) (Wahab et al., 2022). As determined in silico and verified by flow cytometry and transmission electronic microscopy, M. leucadendra essential oil enhances cell membrane permeability and disruption as well as cell wall lysis of bacteria by inhibiting enzymes (peptidoglycan glycosyltransferase) (Bautista-Silva et al., 2020). According to Chaudhari et al. (2022), M. cajuputi essential oil interferes with the integrity of the plasma membrane of Aspergillus flavus cells by interfering with the biosynthesis of ergosterol.
When compared to other parts of the plant, Cajuput oil was far more effective against all the microorganisms tested, including the virulent coronavirus (My et al., 2020). Furthermore, the plant was reported to have an antifilarial effect against Brugia pahangi by inhibiting the bacterial endosymbiont “Wolbachia” (Al-Abd et al., 2016). Figure 5 depicts the number of microorganisms used in the previous studies.
Antioxidant properties of M. cajuputi Powell
Melaleuca cajuputi Powell possesses significant antioxidant activity. Different parts of the plant have several antioxidant compounds, such as α-terpineol, D-(+)-limonene, and β-caryophyllene, which possess excellent antioxidant activities (Table 2).
Several physiological processes in the human and animal body produce free radicals, and they are involved in cellular biochemical functions such as gene regulation, transcription, translation, and signaling (Pérez-Rosés et al., 2016). However, it is important to highlight that there must be a balance between free radicals and antioxidants for normal physiological functioning. Oxidative stress can arise when free radicals outstrip the body’s ability to manage them, leading to diabetes, inflammation, cancer, and cardiovascular disease (Abubakar and Loh, 2016). Therefore, natural antioxidant compounds from a natural source, such as high plants, are essential for the body’s normal physiological function (Dandashire et al., 2019).
Toxicological studies
There are ongoing debates on the safety of herbal medicine in modern medicine and disease treatment, but scientists have continued to be fascinated with herbal medicine. Therefore, it is essential to provide knowledge of previous reports regarding the toxicity profile of M. cajuputi Powell to support its usage in disease treatment.
Melaleuca cajuputi Powell essential oil causes termite mortality at LC50 = 4.60% (Roszaini et al., 2013). Furthermore, M. cajuputi Powell essential oil caused contact toxicity on Sitophilus zeamais and Tribolium castaneum at LC50 values of 178.23 and 213.17 μl L−1, respectively (Ko et al., 2009). Similarly, the methanolic extract of M. cajuputi Powell stems induced a toxicity effect on Camponotus sp. at LT50 = 84.3% (Visheentha et al., 2018). On the other hand, the methanolic leaves extract at doses between 50 and 200 mg/kg showed no toxic effect on Sprague-Dawley rats (Daud et al., 2018). However, in a brine shrimp lethality test, the extract induced a mild toxicity effect at LC50 427 μg/ml (Noor et al., 2020). The aqueous and decoction extracts of M. cajuputi Powell had no toxicity effect at LC50 1062 and 2477 μg/ml, respectively (Noor et al., 2020). According to the Meyer toxicity index, the plant extract is considered toxic when the LC50 is less than 1,000 μg/ml, and it is nontoxic when the LC50 is greater than 1,000 μg/ml (Noor et al., 2020). Clarkson on the other hand defined the toxicity as nontoxic if the LC50 is greater than 1,000 μg/ml, lowly toxic if the LC50 is between 500 and 1,000 μg/ml, highly toxic if the LC50 is between 100 and 500 μg/ml, and extremely toxic if the LC50 is between 0 and 100 μg/ml (Hamidi et al., 2014).
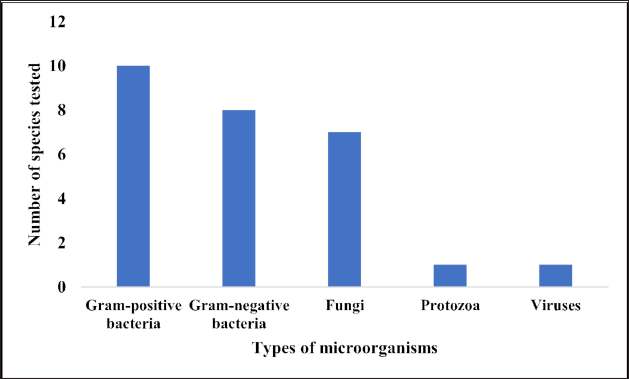 | Figure 5. Types of microorganisms susceptible to M. cajuputi Powell extracts as reported in the various literatures (n = 27). [Click here to view] |
CONCLUSION
The extracts from M. cajuputi Powell showed a wide range of in vitro and in vivo biological effects. The reported phytoconstituents such as phenolic, aromatic, flavonoid, and alcohol groups could be responsible for the biological activities in the plant. The antimicrobial, antioxidant, and toxicity profiles of M. cajuputi Powell signified that the plant could be an excellent source of particular pharmacological active compounds. Further studies on bioassay-guided fractionation to obtain the pure compounds of M. cajuputi Powell extracts and the investigation of their antimicrobial mechanisms are necessary to unveil the plant’s full potential as a good source of novel pharmaceutical compounds for disease remedy.
AUTHOR’S CONTRIBUTIONS
Assoc. Prof. Dr. Mohd Dasuki Sul’ain, Dr. Wan-Nor-Amilah Wan Abdul Wahab, and Isah Musa contributed to the concept and design. Dr. Wan-Nor-Amilah Wan Abdul Wahab and Isah Musa contributed to data acquisition and interpretation. Isah Musa drafted the manuscript. Prof. Dr. Wan Rosli Wan Ishak, Assoc. Prof. Dr. Hasmah Abdullahi, Dr. Wan-Nor-Amilah Wan Abdul Wahab, and Rasmaizatul Akma Rosdi critically reviewed the manuscript.
CONFLICT OF INTEREST
The authors have no known conflicts of interest.
FUNDING
The authors are highly grateful to the School of Health Sciences, Universiti Sains Malaysia (USM), for their financial support under RUI 1001.PPSK.8012209 grant.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abubakar IB, Loh HS. A review on ethnobotany, pharmacology and phytochemistry of Tabernaemontana corymbosa. J pharm pharmacol, 2016; 68(4):423–32; doi:10.1111/JPHP.12523 CrossRef
Al-Abd MN, Nor MZ, Mansor M, Azhar F, Hasan MS, Kassim M. Antioxidant, antibacterial activity, and phytochemical characterization of Melaleuca cajuputi extract. BMC Complement Altern Med, 2015; 15(1):1–13; doi:10.1186/s12906-015-0914-y CrossRef
Al-Abd NM, Nor ZM, Mansor M, Hasan MS, Kassim M. Antifilarial and antibiotic activities of methanolic extracts of Melaleuca cajuputi flowers. Korean J Parasitol, 2016; 54(3):273–80; doi:10.3347/KJP.2016.54.3.273 CrossRef
Angelo RU. Efficacy of Acacia nilotica extracts towards microbicidal activity against pathogens. Int J Curr Microbiol Appl Sci, 2015; 4(10):33–42.
Arifullah M, Vikram P, Chiruvella KK, Shaik MM, Husna I, Ripain BA. A review on malaysian plants used for screening of antimicrobial activity. Annu Res Rev Biol, 2014; 4(13):2088–132; doi:10.9734/arrb/2014/8258 CrossRef
Azlinda Abu B. Bioactivity evaluation of Melaleuca cajuputi (Myrtales: Myrtaceae) crude extracts against Aedes mosquito. Pertanika J Trop Agric Sci, 2020; 43(3):303–13.
Bakar AA, Ahmad H, Sulaiman S, Omar B, Ali RM. Evaluation of in vitro bioactivity of Melaleuca cajuputi powell essential oil against Aedes aegypti (L.) and Aedes albopictus (Skuse). Sains Malays, 2019; 48(9):1919–26; doi:10.17576/jsm-2019-4809-13 CrossRef
Batubara I, Mitsunaga T. Ohashi H. Screening antiacne potency of Indonesian medicinal plants: antibacterial, lipase inhibition, and antioxidant activities. J Wood Sci. 2009; 55(3):230–5. Available via https://jwoodscience.springeropen.com/articles/10.1007/s10086-008-1021-1 (Accessed 5 August 2021). CrossRef
Bautista-Silva JP, Seibert JB, Amparo TR, Rodrigues IV, Teixeira LFM, Souza GHB, dos Santos ODH. Melaleuca leucadendra essential oil promotes loss of cell membrane and wall integrity and inhibits bacterial growth: an in silico and in vitro approach. curr microbio, 2020; 77(9):2181–91; doi:10.1007/s00284-020-02024-0 CrossRef
Bua A, Molicotti P, Donadu MG, Usai D, Le LS, Tran TTT, Ngo VQT, Marchetti M, Usai M, Cappuccinelli P, Zanetti S. ‘In vitro’ activity of Melaleuca cajuputi against mycobacterial species. Nat Prod Res, 2020; 34(10):1494–7; doi:10.1080/14786419.2018.1509335 CrossRef
Bunalema L, Obakiro S, Tabuti JRS, Waako P. Knowledge on plants used traditionally in the treatment of tuberculosis in Uganda. J Ethnopharmacol, 2014; 151(2):999–1004; doi:10.1016/j.jep.2013.12.020 CrossRef
Chaudhari AK, Singh VK, Das S, Kujur A, Deepika, Dubey NK. Unveiling the cellular and molecular mode of action of Melaleuca cajuputi Powell. essential oil against aflatoxigenic strains of Aspergillus flavus isolated from stored maize samples. Food Control, 2022; 138(April):109000; doi:10.1016/j.foodcont.2022.109000 CrossRef
Dahiya DP, Bharat CS, Praveen D. Evaluation of in vitro antimicrobial potential and phytochemical analysis of spruce, cajeput and jamrosa essential oil against clinical isolates. Int J Green Pharm, 2016; 10(1):1–6; doi:10.22377/IJGP.V10I1.602
Dandashire BS, Magashi AM, Abdulkadir B, Abbas, MA, Goni MD, Yakubu A. Toxicological studies and bioactivity-guided identification of antimicrobially active compounds from crude aqueous stem bark extract of Boswellia dalzielii. J Adv Vet Anim Res, 2019; 6(2):183; doi:10.5455/javar.2019.f330 CrossRef
Daud D, Alias NLR, Ali MTM, Tawang A. Physical signs of illness, liver functions and reproductive parameters of female rats supplemented with Melaleuca cajuputi methanolic extract. J Appl Pharm Sci, 2018; 8(04):139–42; doi:10.7324/JAPS.2018.8420 CrossRef
Daud D, Gan NNMS, Ali MTM, Tawang A. The effect of Melaleuca cajuputi methanolic leaves extract on body growth, puberty and sperm quality of juvenile male rats. BioTechnol Indian J Trade Sci Inc, 2015. Available via https://www.tsijournals.com/abstract/the-effect-of-melaleuca-cajuputi-methanolic-leaves-extract-on-body-growth-puberty-and-sperm-quality-of-juvenile-male-rat-2168.html (Accessed 1 July 2021).
Feyaerts AF, Mathé L, Luyten W, Van Dijck P. Comparison between the vapor-phase-mediated anti-candida activity of conventional and organic essential oils. Nat Volat Essential Oils, 2018; 5(1):1–6.
Hai HNT, Rimbawanto A, Prastyono, Kartikawati NK, Wu H. Genetic improvement for essential oil yield and quality in Melaleuca cajuputi. Ind Crops Prod, 2019; 137(June):681–6; doi:10.1016/j.indcrop.2019.05.061 CrossRef
Hamidi MR, Jovanova B, Panovska TK. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Macedonian Pharm Bull, 2014; 60(01):9–18; doi:10.33320/maced.pharm.bull.2014.60.01.002 CrossRef
Hnawia E, Brophy JJ, Craven LA, Lebouvier N, Cabalion P, Nour M. An examination of the leaf essential oils of the endemic Melaleuca (Myrtaceae) species of new Caledonia. 2012; 24(3):273–8; doi:10.1080/10412905.2012.676776. Available via http://dx.doi.org/10.1080/10412905.2012.676776 CrossRef
Idris OA, Wintola OA, Afolaya AJ. Comparison of the proximate composition, vitamins (Ascorbic acid, α-Tocopherol and retinol), anti-nutrients (phytate and oxalate) and the GC-MS analysis of the essential oil of the root and leaf of Rumex crispus l. Plants, 2019; 8(3): doi:10.3390/plants8030051 CrossRef
Isah M, Manga SS, Muhammad A, Tondi ZY, Sa’adu M. The in vitro antibacterial effects of Ficus sycomorus stem bark extracts on Salmonella typhi and Escherichia coli. Equity J Sci Technol, 2020; 7(1):108–13. Available via www.equijost.com CrossRef
Isnaini I, Biworo A, Khatimah H, Gufron KM, Puteri SR. Antibacterial and antifungal activity of Galam (Melaleuca cajuputi subsp. Cumingiana (Turcz.) Barlow) extract against E. coli bacteria and C. albicans fungi. J Agromedicine Med Sci, 2021; 7(2):79–83; doi:10.19184/AMS.V7I2.23467 CrossRef
Jajaei SM, Daud WRW, Markom M, Zakaria Z, Lo Presti M, Costa R, Mondello L, Santi L. Extraction of Melaleuca cajuputi using supercritic fluid extraction and solvent extraction. J Essential Oil Res, 2010; 22(3):205–10; doi:10.1080/10412905.2010.9700304 CrossRef
Keereedach P, Hrimpeng K, Boonbumrung K. Antifungal activity of Thai cajuput oil and its effect on efflux-pump gene expression in fluconazole-resistant Candida albicans clinical isolates. Int J Microbiol, 2020, 1–10; doi:10.1155/2020/5989206 CrossRef
Khalaf MO, Abdel-Aziz MS, El-Hagrassi AM, Osman AF, Ghareeb MA. Biochemical aspect, antimicrobial and antioxidant activities of Melaleuca and Syzygium Species (Myrtaceae) grown in Egypt. J Phys Conf Ser, 2021; 1879(2):022062; doi:10.1088/1742-6596/1879/2/022062 CrossRef
Ko K, Juntarajumnong W, Chandrapatya A. Repellency, fumigant and contact toxicities of Melaleuca cajuputi Powell against Sitophilus zeamais Motschulsky and Tribolium castaneum herbst. J Agric Sci, 2009; 42(1):27–33. Available via www.thaiagj.org (Accessed 5 August 2021).
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ, 2009; 339:1–27; doi:10.1136/BMJ.B2700 CrossRef
Liew KY, Hafiz MF, Chong YJ, Harith HH, Israf DA, Tham CL. A review of Malaysian herbal plants and their active constituents with potential therapeutic applications in sepsis. Evid-based Complement Altern Med, 2020, 1–20; doi:10.1155/2020/8257817 CrossRef
Lim SC, Midon MS. Timber of Gelam (Melaleuca cajuputi Powell). Available via https://info.frim.gov.my/infocenter/booksonline/ttb/TTBNO23.PDF (Accessed 24 June 2021).
Manga SS, Isah M, Danlam, BM. Antbacterial property of ethanolic extract from the leaves of Acacia nilotica WILD. on Staphylococcus aureus and Pseudomonas aeruginosa. Ujmr, 2018; 3(1):115–120.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med, 2009; 6(7):e1000097; doi:10.1371/JOURNAL.PMED.1000097 CrossRef
My TTA, Loan HTP, Hai NTT, Hieu DLT, Hoa PTT, Thuy DBTP, Quang PDT, Triet DNT, Anh DTTV, Dieu DNTX, Trung DNT, Hue DNV, Tat DPV, Tung DVT, Nhung DNTA. Evaluation of the inhibitory activities of COVID-19 of Melaleuca cajuputi oil using docking simulation. ChemistrySelect, 2020; (21):6312–20; doi:10.1002/slct.202000822 CrossRef
Noor AAM, Yusuf SM, Wahab WNAWA, Adam MFIC, Sul’ain MD. Evaluation on composition, antioxidant and toxicity of Melaleuca cajuputi leaves. Adv Trad Med, 2020; (September):1–7; doi:10.1007/s13596-020-00479-x
Oliveira TR, Teixeira AL, Barbosa JP, de Feiria SNB, Boni GC, Maia FC, Aniba PC, Wijesinghe GK, Höfling JF. Melaleuca spp. essential oil and its medical applicability. A brief review. Braz J Nat Sci, 2020; 3(1):249; doi:10.31415/bjns.v3i1.89 CrossRef
Paosen S, Saising J, Septama AW, Voravuthikunchai SP. Green synthesis of silver nanoparticles using plants from Myrtaceae family and characterization of their antibacterial activity. Mater Lett,2017; 209:201–6; doi:10.1016/j.matlet.2017.07.102 CrossRef
Pérez-Rosés R, Risco E, Vila R, Peñalver P, Cañigueral S. Biological and nonbiological antioxidant activity of some essential oils. J Agric Food Chem, 2016; 64(23):4716–24; doi:10.1021/acs.jafc.6b00986 CrossRef
Pujiarti R, Ohtani Y, Widowati TB, Kasmudjo K. Utillization of Melaluca leucadendron essential oil. J Wood Sci, 2011; 2(2):94–9.
Roszaini K, Azah MAN, Mailina J, Zaini S, Faridz ZM. Toxicity and antitermite activity of the essential oils from Cinnamomum camphora, Cymbopogon nardus, Melaleuca cajuputi and Dipterocarpus sp. against Coptotermes curvignathus. Wood Sci Technol, 2013; 47(6):1273–84; doi:10.1007/s00226-013-0576-1 CrossRef
Septiana S, Bachtiar BM, Yuliana ND, Wijaya CH. Cajuputs candy impairs Candida albicans and Streptococcus mutans mixed biofilm formation in vitro. F1000Research, 2019; (5):1–25; doi:10.12688/F1000RESEARCH.20700.2 CrossRef
Sareedenchai V, Petrachaianan T, Chaiyasirisuwan S, Athikomkulchai S. Screening of acetylcholinesterase inhibitory activity in essential oil from Myrtaceae. Thai J Pharm Sci, 2019; 43(1):63–8. Available via http://www.tjps.pharm.chula.ac.th/ojs/index.php/tjps/article/view/779 (Accessed 7 August 2021).
Septiana S, Yuliana ND, Bachtiar BM, Putri SP, Fukusaki E, Laviña WA, Wijaya CH. Metabolomics approach for determining potential metabolites correlated with sensory attributes of Melaleuca cajuputi essential oil, a promising flavor ingredient. J Biosci Bioeng, 2020; 129(5):581–7; doi:10.1016/j.jbiosc.2019.12.005 CrossRef
Septiana S, Yuliana ND, Wijaya CH, Bachtiar BM, Wijaya CH. Aroma-active compounds of Melaleuca cajuputi essential oil, a potent flavor on Cajuputs Candy. AIMS Agric Food, 2020; 5(2);292–306; doi:10.3934/agrfood.2020.2.292 CrossRef
Sfeir J, Lefrançois C, Baudoux D, Derbré S, Licznar P. In vitro antibacterial activity of essential oils against Streptococcus pyogenes. Evid Based Complement Altern Med, 2013; doi:10.1155/2013/269161 CrossRef
Sharif ZM, Kamal AF, Jalil NJ. Chemical composition of Melaleuca cajuputi Powell. Int J Eng Adv Technol, 2019; 9(1):3479–83; doi:10.35940/ijeat.A2668.109119 CrossRef
Shivappa RB, Christian LS, Noga EJ, Law JM, Lewbart GA. Laboratory evaluation of safety and efficacy for Melafix (Melaleuca cajuputi extract). J Exotic Pet Med, 2015; 24(2):188–92; doi:10.1053/j.jepm.2015.04.020 CrossRef
Silva CJ, Barbosa LCA, Maltha CRA, Pinheiro AL, Ismail FMD. Comparative study of the essential oils of seven Melaleuca (Myrtaceae) species grown in Brazil. Flavour Fragr J, 2007; 22(6):474–8; doi:10.1002/FFJ.1823 CrossRef
Sutrisno S, Retnosari R, Asmaningrum HP. Profile of the Indonesian essential oil from Melaleuca cajuputi. In Proceedings of the Seminar Nasional Kimia-National Seminar on Chemistry (SNK 2018), 14–9; doi:10.2991/snk-18.2018.3 CrossRef
Thanaboripat D, Suvathi Y, Srilohasin P, Sripakdee S, Patthanawanitchai O, Charoensettasilp S. Inhibitory effect of essential oils on the growth of Aspergillus flavus. Curr Appl Sci Technol, 2007; 7(1):1–7; Available via https://li01.tci-thaijo.org/index.php/cast/article/view/164767 (Accessed 2 September 2021).
Trinh NTH, Anh TNP, Rappelli P. Evaluation of the susceptibility of Trichomonas vaginalis isolates to Melaleuca cajuputi. Int J Multidiscip Res Publ M. cajuputi, 2021; 3(8):1–4.
Thielmann J, Muranyi P, Kazman P. Screening essential oils for their antimicrobial activities against the foodborne pathogenic bacteria Escherichia coli and Staphylococcus aureus. Heliyon, 2019; 5(6):e01860; doi:10.1016/j.heliyon.2019.e01860 CrossRef
Toan TQ, Thao LPP, Chien NQ, Van NTH, Phuong DL, Huong TT, Quan PM, Pham HHT, Bich HT, Ngan TTK, Thai TV, Nguyen TV, Bach LG, Long PQ, Inh CT. Determination of chemical composition and antimicrobial activity of Melaleuca cajuputi essential oil from Quang Tri Province, Vietnam. Asian J Chem, 2020; 32(9):2203–7; doi:10.14233/ajchem.2020.22458 CrossRef
Ukit U, Widiana A, Rahmawati E, Hasby RM. Antibacterial activities test of Cajuput leaf waste extract (Melaleuca cajuputi Powell) on pathogenic bacteria. J Phys Conf Ser, 2019; 1402(3); doi:10.1088/1742-6596/1402/3/033030 CrossRef
Visheentha M, Appalasamy S, Nivaarani A, Boon JG, Weeraya K, Charoen P. The Action of Gelam (Melaleuca cajuputi) stem crude extract as natural insecticide for Camponotus Sp. J Biodivers, Bioprospect Dev, 2018; 05(02):2–7; doi:10.4172/2376-0214.1000173 CrossRef
Wahab NZA, Ja’afar NSA, Ismail SB. Evaluation of antibacterial activity of essential oils of Melaleuca cajuputi Powell. J Pure Appl Microbiol, 2022; 16(1):549–56; doi:10.22207/JPAM.16.1.52 CrossRef
Wi?ska K, M?czka W, ?yczko J, Grabarczyk M, Czubaszek A, Szumny A. Essential oils as antimicrobial agents—myth or real alternative?. Molecules, 2019; 24(11):1–20; doi:10.3390/molecules24112130 CrossRef
Wolter F, Clausnitzer A, Akoglu B, Stein J. Piceatannol, a natural analog of resveratrol, inhibits progression through the s phase of the cell cycle in colorectal cancer cell lines. J Nutr, 2002; 132(2):298–302; doi:10.1093/jn/132.2.298 CrossRef
Xu WJ, Xie X, Wu L, Tian XM, Wang CC, Kong LY, Luo J. Cajuputones A–C, β-triketone flavanone hybrids from the branches and leaves of Melaleuca cajuputi. Chem Biodivers, 2020; 17(11):e2000706; doi:10.1002/cbdv.202000706 CrossRef