INTRODUCTION
Peperomia pellucida (L.) Kunth is a herbaceous plant that belongs to the Piperaceae family. In many countries, it has long been used traditionally to treat a wide range of diseases, including diabetes, muscle pain, aches, common cold, conjunctivitis, abscesses, boils and skin wounds, fever, headache, proteinuria, and convulsions, and it is also used as a diuretic and to lower blood cholesterol level, as well as its use in the Ayurvedic system of medicines. The plant is commonly found in several Asian and South American countries, is easy to find in yards and humid areas, and usually grows wild (Bojo et al., 1994; Majumder, 2011; Susilawati et al., 2017).
Many biological activity studies have been successfully conducted to reveal the potential of P. pellucida in treating diseases, including antihypertensive, anti-inflammatory, antipyretic, analgesic, antibacterial, antiamoeba, antioxidant, gastroprotective, and other pharmacological activities (Ahmad et al., 2019; Fakayode et al., 2021; Idris et al., 2016; Ng et al., 2021; Rojas-Martínez et al., 2013; Uwaya et al., 2021). The results are also supported by the fact that this plant contains phytochemical compounds responsible for pharmacological activities. For instance, pellucidin A, isolated from P. pellucida, inhibits the angiotensin-converting enzyme (ACE), and patuloside A exhibits antibacterial activity. The herbs are also believed to possess polyphenolics, flavonoids, fatty acids, volatile oils, and other bioactive constituents (Ahmad et al., 2019; Heinrich et al., 1998; Khan et al., 2010; Usman and Ismaeel, 2020).
Peperomia pellucida has great potential as it has been used empirically to treat diseases and is scientifically proven to possess biological activities related to its utilization in traditional medicine. However, currently, there is still limited data available about the use of this herb as a raw material for commercial herbal medicinal products. The plant is even considered a weed by some local farmers.
Recently, scientists have studied the development of the P. pellucida extraction method. These studies generally focus on increasing the yield obtained from the extraction process with a green chemistry approach. The results suggest that applying the suitable extraction method combined with optimal extraction parameters can produce more yields than those conventional techniques (Ahmad et al., 2017a, 2017c; Gomes et al., 2022; Hashim et al., 2020; Mun’im et al., 2017). Thus, it is possible to be applied to industrial-scale production. This current review aims to provide up-to-date information regarding the progress of research on P. pellucida herbs in terms of their phytochemical, pharmacological, and toxicological properties, as well as developments in the extraction technique. In addition, it also discussed the economic prospects of this plant.
MATERIALS AND METHOD
A literature search was conducted between mid-2021 and mid-2022 to find all published papers on P. pellucida L. Kunth In electronic databases such as Google Scholar, Directory of Open Access Journals, PubMed, and ScienceDirect. All the data included in the review were articles written in English.
DISCUSSION
Plant description and origins
Taxonomy
Kingdom: Plants
Subkingdom: Tracheobionta – vascular plants
Superdivision: Spermatophyta – seed plants
Division class: Magnoliophyta – flowering plants
Class: Magnoliopsida – Dicotyledons
Subclass: Magnoliidae
Order: Piperales
Family: Piperaceae
Genus: Peperomia
Species: Peperomia pellucida (L.) Kunth
Morphology
The P. pellucida herb (Fig. 1) is a plant that usually grows wild in damp clusters. This plant is easy to find in gardens and yards and on roadsides and edges of ditches and other watery places. This herb flowers throughout the year and is an annular herb; the leaves are bare and light green on the upper surface and green and white on the lower surface, thinly fleshy, ovoid or oval, with an area of +1.5–4 (−5) cm, width 1–3.3 cm, with fibrous roots, pale green translucent stems, erect or ascending, with a height of +15–45 cm (Abere and Okpalaonyagu, 2015; Majumder, 2012; Rahman et al., 2014).
Habitat
This plant is widespread in South America and many Asian countries and grows at about 400 m above sea level as a weed along roadsides, in moist soil and in shady places around houses that are usually in clusters, and in plantations (mainly oil palm plantations in Indonesia). Most of these plants are in the tropics (Majumder, 2011; Rahman et al., 2014). The herb of P. pellucida is widely distributed in many American and South Asian countries (Arrigoni-Blank et al., 2004; Ho et al., 2022).
 | Figure 1. Peperomia pellucida (L) Kunth in natural habitat. [Click here to view] |
Empirical uses
Ethnobotanical studies on the P. pellucida herb show that every part of this plant has long been used as a medicinal plant. Empirically, the herb is used by Indonesians to treat headaches accompanied by fever, and the leaves extract is used to treat stomach pain, gout, and hypertension (Heyne, 1988). In Bolivia, all parts of this plant are used to stop bleeding. The herbaceous root of P. pellucida is used to treat fever and wounds. In northeastern Brazil, this herb is used for blood pressure and lowering cholesterol levels, and this plant is used in religious activities (de Albuquerque et al., 2007). In Guyana and the Amazon, it acts as a cough medicine, emollient, and diuretic and can be used to treat proteinuria (Nwokocha et al., 2012). In the Philippines, the decoction of this plant is used to reduce uric acid levels and kidney disorders. In various areas of Bangladesh, the leaves of this plant are used by local people as a treatment for mental illness. In South America, the fresh juice of the stems and leaves of this plant can be used as a treatment for inflammation of the eyes (Majumder, 2011).
Phytochemistry
Peperomia pellucida plants have been known to have various types of chemical constituents, including amino acids, protein carbohydrates, minerals consisting of sodium, calcium, and iron (Ooi et al., 2012), tannins, saponins, phenols, steroids, terpenoids, amino acids, alkaloids (Abere et al., 2012; Abere and Okpalaonyagu, 2015; de Albuquerque et al., 2007; Awe et al., 2013; Gini and Jothi, 2013; Gomes et al., 2022; Majumder and Majumder, 2013; Mengome et al., 2010; Pappachen and Chacko, 2013), essential oils (Manalo et al., 1983; Usman and Ismaeel, 2020; Verma et al., 2015), and fatty acid (linoleic acid and α-linoleic acid) (Heinrich et al., 1998). Some studies have succeeded in isolating and identifying the chemical compounds contained in the P. pellucida herb, and these studies have been carried out since 1983, as presented in Table 1.
Pharmacology activity
A comprehensive study of pharmacological activities of P. pellucida has been discussed by Kartika et al. (2016). The review describes P. pellucida herbs (in the form of pure compound isolates, fractions, or extracts) for their pharmacological activities such as cytotoxic, lipase inhibitory, fibrinolytic, and thrombolytic, hypotensive, gastroprotective, depressant, burn healing, analgesic, antioxidant, antipyretic, anti-inflammatory, antiosteoporotic, antidiarrheal, antisickling, antimicrobial, antihyperuricemic, and antihyperglycemic. However, in this subsection, we discuss some of the updated potential activities in detail.
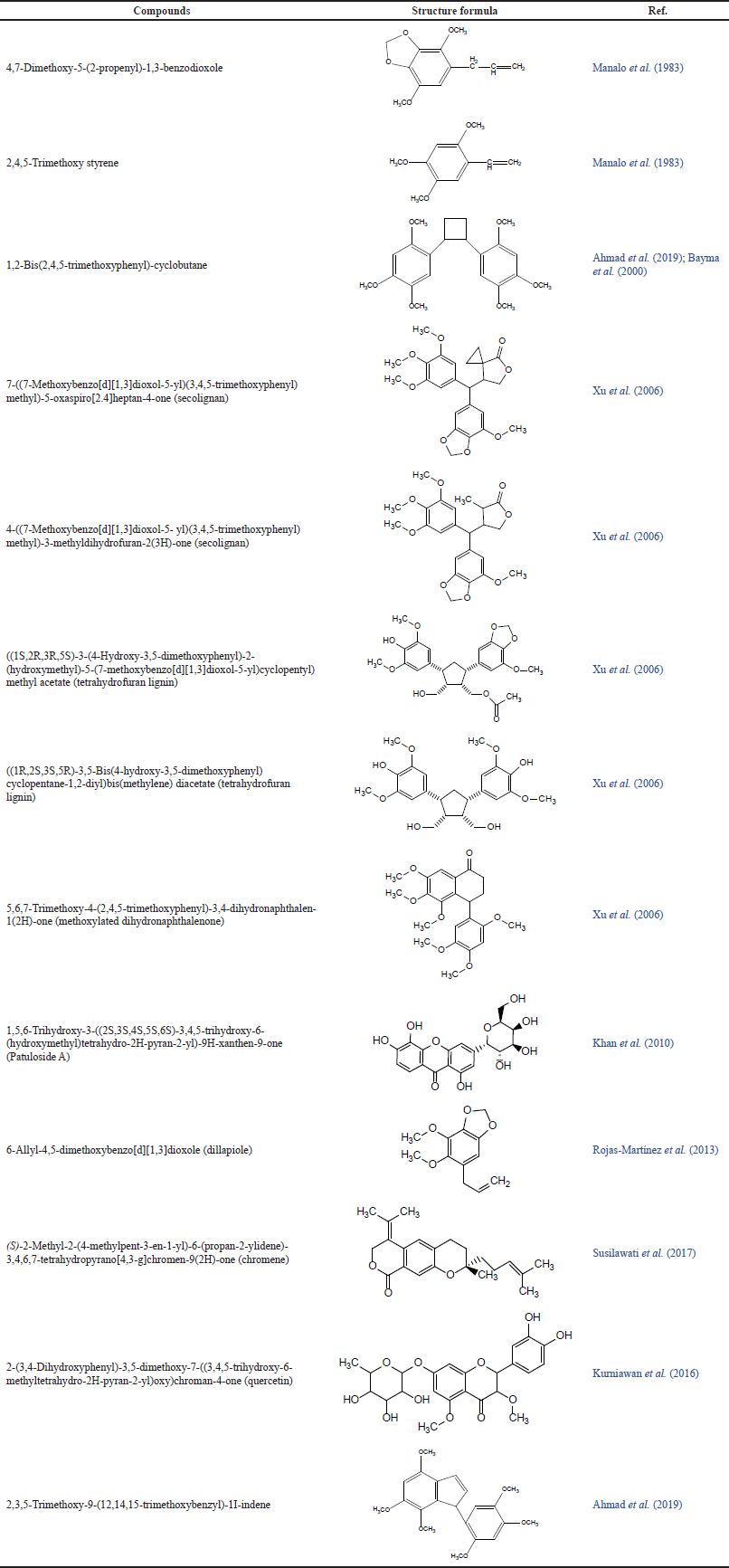 | Table 1. Isolated bioactive compounds from P. pellucida. [Click here to view] |
Antihypertension
Peperomia pellucida herb has activity as an antihypertensive, mainly as an ACE inhibitor, based on several studies that have been reported (Ahmad et al., 2019; Kurniawan et al., 2016; Saputri et al., 2021, 2015). An antihypertensive activity assay has been reported by Saputri et al. (2015), where the ethyl acetate fraction of the P. pellucida herb extract has activity as an ACE inhibitor (in vitro method) with an IC50 value of 7.17 μg/ml, and an in vivo assay method has also been carried out which shows that at the dose of 50 mg/kg body weight (BW) it has an ACE inhibitory effect similar to that of captopril. These results align with the research conducted by Nwokocha et al. (2012). The extract of the P. pellucida herb exhibited dose-dependent antihypertensive, vasodilating, and bradycardic effects by targeting nitric oxide-dependent signaling pathways in mice. ACE inhibitory compounds from the P. pellucida herb have been isolated and identified, including quercetin (Kurniawan et al., 2016), pellucidin A, and 2,3,5-trimethoxy-9-(12,14,15-trimethoxybenzyl)-1H-indene (Ahmad et al., 2019). In addition, an in silico molecular docking study has also been carried out on several other phenylpropanoid compounds against the ACE receptor, showing that most of these compounds have strong binding energy interactions with the receptor (Ahmad et al., 2019).
Anti-inflammatory, antipyretic, and analgesic
The aqueous extract of the P. pellucida herb was tested as an anti-inflammatory (carrageenan and arachidonic acid induction method) and analgesic (acetic acid induction and heat induction using hot plates) in rats and mice orally, with anti-inflammatory activity at doses of 200 and 400 mg/kg, respectively, based on the effect of prostaglandin synthesis (Arrigoni-Blank et al., 2004). In addition, anti-inflammatory activity has also been demonstrated by Ng et al. (2021), who showed that the fermentation and drying processes affected the effectiveness of the anti-inflammatory activity of the P. pellucida herb extracts, where the anti-inflammatory potential of P. pellucida increased significantly with the drying process compared to the results of the fermentation process. Furthermore, the fresh and dried leaf extracts of P. pellucida exhibited various antioxidant and anti-inflammatory potentials comparable to those obtained in the standard (Fakayode et al., 2021).
The methanol extract of the P. pellucida herb was administered orally at a dose range of 70–210 mg/kg and showed significant analgesic activity in mice induced with acetic acid (Aziba et al., 2001; Sheikh et al., 2013). The analgesic activity dose with the highest activity at a dose of 400 mg/kg is induced with acetic acid, and a dose of 100 mg/kg is the best dose in the heat induction method using a hot plate (Arrigoni-Blank et al., 2004).
Antibacterial and antiamoeba
The n-butanol fraction of the P. pellucida herb extract has antibacterial activity (Khan and Omoloso, 2002), and there are also reported antibacterial tests on four Gram-positive bacteria (Bacillus megaterium, Bacillus subtilis, Streptococcus β-haemolyticus, and Staphylococcus aureus) and six Gram-negative bacteria (Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa, Shigella dysenteriae, Shigella sonnei, and Shigella flexneri), with minimal inhibitory concentration (MIC) values against all these bacteria in the concentration range from 8 to 64 g/ml, and antifungal tests on Aspergillus flavus and Candida albicans (Khan et al., 2010). Peperomia pellucida has broad-spectrum antimicrobial activity on Penicillium notatum, Aspergillus niger, Rhizopus stolon, Candida albicans, Salmonella typhi, Klebsiella pneumonae, Pseudomonas aeruginosa, Bacillus subtilis, Staphylococcus aureus, Escherichia coli (Oloyede et al., 2011), Vibrio sp., Flavobacterium sp., and Edwardsiella tarda (Wei et al., 2011). Idris et al. (2016) also proved the antibacterial activity of the n-hexane extract of P. pellucida herb with a MIC value of 25 μg/ml. Khan et al. (2010) succeeded in isolating and identifying the compound patuloside A, which has antibacterial activity, from this herb. Another study revealed that P. pellucida has an antiamoebic activity where the methanol fraction can damage the morphology and change the structure of Acanthamoeba cysts (IC50 = 29.28% ± 3.64%) detected using toluidine dye and observed using a light microscope (Sangsuwon et al., 2015).
Antidiabetic and antioxidant
The antidiabetic activity of the P. pellucida extract was observed in diabetic rats where doses of 10% and 20% w/w given for 6 days can reduce blood glucose levels (Hamzah et al., 2012). Sheikh et al. (2013) also proved the hypoglycemic effect of the ethyl acetate extract of the herbs at a dose of 300 mg/kg in vivo. Then, Susilawati et al. (2017) isolated a new active compound (8,9-dimethoxy ellagic acid) from the plant, which exhibited antidiabetic properties in experimental animals.
On the other hand, the antioxidant properties of the P. pellucida herbs have been widely studied (Adhitia et al., 2017; Mun’im et al., 2017; Oloyede et al., 2011; Uwaya et al., 2021; Wei et al., 2011; Yunarto et al., 2018). The methanol extract of the herbs possesses higher free radical scavenging activity than petroleum ether and chloroform extract (Uwaya et al., 2021; Wei et al., 2011). The high antioxidant activity of the plant at low concentrations indicates that it could be beneficial for treating ailments resulting from oxidative stress (Oloyede et al., 2011). Meanwhile, the ethanol extract has antioxidant activity with an IC50 value of 32.94 μg/ml (Yunarto et al., 2018). This result is relatively weaker than the methanol extract. However, there was an increase in the activity of the ethanol extract combined with the microwave-assisted extraction (MAE) method (Mun’im et al., 2017) and the effect of gamma irradiation (Yusuf et al., 2017).
Other pharmacology activities
Scientists also successfully revealed the potency of the P. pellucida herbs in other pharmacological activities, including gastroprotection, antimalaria, ultraviolet (UV) filter, and antihyperuricemia. Dillapiole, a chemical compound isolated from these herbs, showed gastroprotective activity in gastric ulcer experiment rats (Rojas-Martínez et al., 2013). The study of antimalaria properties on P. pellucida fractions (n-hexane, ethyl acetate, and methanol) suggested that all tested fractions were active against malaria culture with IC50 of 12.80, 2.90, and 10.74 μg/ml, respectively (Bialangi et al., 2016). Besides that, this plant also possesses UV protection activity (Ahmad, 2015). The ethanolic extract of P. pellucida demonstrated antihyperuricemia in rats and mice (Tarigan et al., 2012). In addition to the pharmacological activity of P. pellucida herbs, it has also been reported that the extracts are rich in carbohydrates, proteins, and total ash content (31.22%), whose composition consists of main elements including sodium, calcium, and iron. This indicates that the plant can benefit humans in terms of protein and energy supplements (Ooi et al., 2012). Moreover, the administration of the P. pellucida ethanol extract at a certain dose (200 mg/kg) can stimulate bone regeneration in the fractured part (Ngueguim et al., 2013).
Toxicity activity
In some countries, P. pellucida has been eaten as salads, cooked as greens, and brewed as tea by the local communities (Wakhidah et al., 2020). Moreover, the plant has historical data about its utilization as a herb to treat some diseases. This implies that this plant is safe to be consumed as food for humans. Several toxicological studies of this herb have been carried out to evaluate the safety of the extracts. An acute toxicity test on mice (Mus musculus) reported that the lethal dose 50% (LD50) of the herb extract was 5,000 mg/kg BW, which suggested that the extract was relatively safe or exhibited low toxicity (Abere et al., 2012; Arrigoni-Blank et al., 2004). Similarly, acute toxicity tests performed by Waty et al. (2017) found that the LD50 of the methanol extract of the plant was more significant than 4,000 mg/kg BW in Deutsch Denken Yoken mice and there was no sign of toxicity on the skin and hair, respiration system, defecation, feed intake, and behavior. In addition, the highest tested dose (4,000 mg/kg BW) did not cause mortality. The safety of the P. pellucida herbs is also supported by the findings of a histopathological study on white rats, which statistically declared that the tested extract was nontoxic in the biological system tested (Beltran-Be et al., 2013). Cytotoxicity effects on human embryonic kidney 293 cells (HEK 293) have also been observed by Pappachen and Chacko (2013). The results showed that there was no sign of toxicity at the lowest (6.25 μg/ml) to the highest (100 μg/ml) tested dose. Further research was done by Hsuuw and Chan (2015) to distinguish the apoptotic effects of dillapiole isolated from P. pellucida on rats’ oocyte maturation, in vitro fertilization (IVF), and development before and after implantation. Dillapiole significantly impaired rat oocyte maturation, lowered the IVF rate, and inhibited the development of embryos in tested animals. The findings implied that P. pellucida-based products are not suggested for pregnant women and need further research. In vitro toxicity tests have been carried out on monkey kidney cells with an lethal concentration 50% of 70 μg/ml (Mengome et al., 2010).
Extraction technique development
Designing environmentally friendly and sustainable natural product extraction processes is a research topic currently attracting attention in the interdisciplinary fields of applied pharmaceutical sciences, chemistry, biology, and technology (Ahmad et al., 2017a; Mediani et al., 2022; Mun’im et al., 2017). Three main approaches have been identified to design and demonstrate green extraction on a laboratory and industrial scale to reach an optimal consumption of raw materials, solvents, and energy: (1) improving and optimizing existing processes; (2) using nondedicated equipment; and (3) innovation in processes and procedures as well as the discovery of alternative solvents (Chemat et al., 2012).
The application of “green extraction” on the P. pellucida herb has been reported in a few studies. Gomes et al. (2022) extracted this herb with 99.8% ethanol using indirect ultrasounds assisted technique for 40 minutes to obtain crude extract. The extraction time was relatively low compared to the conventional methods that usually need longer time (Ahmad et al., 2017b).
Moreover, ultrasound-assisted extraction does not involve high temperatures that can cause degradation of desired bioactive compounds in the extracts, decreasing the bioactivity (Kumar et al., 2021). Therefore, using ultrasounds to extract thermolabile compounds in P. pellucida could be an alternative method for the chemical, cosmetics, and pharmaceutical industries to obtain the compounds of interest.
Another technique studied for extracting the P. pellucida herbs was MAE. Extraction of phenolic constituents from this plant using MAE with solvent 95% ethanol was carried out to determine the impact of extraction duration (5–25 minutes) and temperature (65°C–145°C) on the extraction yield and the total phenolic content (TPC). The optimum condition reached 15 minutes at 145°C (Hashim et al., 2020). A similar study was conducted by Mun’im et al. (2017). They investigated the efficiency of some parameters, including sample ratio, extraction time, ethanol concentration, and microwave power for extracting phenolic and flavonoid compounds. The ideal MAE parameters for TPC [49.78 mg gallic acid equivalent (GAE)/g extract] were 80% ethanol as solvent, 1:12 solid-to-solvent ratio, extraction time of 2 minutes, and microwave power of 30% watts, while for total flavonoid content these were 80% ethanol as solvent, 1:12 solid-to-solvent ratio, time of 2 minutes, and microwave power of 70% watts.
In terms of solvent optimization, ionic liquid-based microwave-assisted methods (IL-MAE) were studied to extract the P. pellucida herbs. The use of 1-ethyl-3-methylimidazolium bromide (EMIMBr) as a solvent for attracting optimum polyphenolic compounds has been demonstrated by Ahmad et al. (2017c). The study showed that using EMIMBr MAE on the following parameters, a microwave power of 30% watts, 10 minutes of extraction time, 14:1 (ml/g) liquid-to-solid ratio, and EMIMBr concentration of 0.7 mol/l yielded the highest TPC (13.750 μg GAE/g) compared to conventional organic solvent n-hexane and ethyl acetate (3.408 and 7.823 μg GAE/g, respectively). The application of ionic liquids, including 1-butyl-3-methyl imidazolium bromide ([BMIM]Br) and 1-butyl-3-methyl imidazolium chloride ([BMIM]Cl), on polyphenolic compounds extraction from the P. pellucida herbs was also demonstrated. The yield of TPC obtained using [BMIM]Cl as solvent was 18.287 μg GAE/g with parameter concentration of solvent 0.7 mol/l, 14 ml/l liquid–solid ratio, and 270-watt microwave power for 10 minutes. For the [BMIM]Br solvent, the highest TPC measurement was 15.734 μg GAE/g obtained from parameter concentration of solvent 0.7 mol/l, 14 ml/l liquid–solid ratio, and 270-watt microwave power for 15 minutes (Ahmad et al., 2017c).
Metabolite profiling analysis of the P. pellucida herbs was conducted by comparing the metabolite profile of extracts obtained from IL-MAE and maceration with 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM]BF4) and ethyl acetate as a solvent, respectively. The total ion chromatogram from ultra high-performance liquid chromatography with quadrupole time-of-flight mass spectrometry/mass spectrometry suggested that there were differences between organic and ionic liquid solvents [BMIM]BF4 in their metabolite profiles (Ahmad et al., 2018). This may imply that using ionic liquids such as [BMIM]BF4 can be opted to attract a class of compounds like polyphenols or to get some kinds of enriched P. pellucida extract.
Modern extraction techniques like MAE can efficiently extract valuable phytochemicals. MAE is thought to be more environmentally friendly and consumes less time and solvent since there is minimal to no carbon dioxide (CO2) emission. This method has successfully demonstrated the extraction of flavonoid and phenolic compounds from the P. pellucida herbs and other plants (Ahmad et al., 2018; Chemat et al., 2019; Zhang et al., 2018). Dipole rotation (reversal of dipoles) and ionic conduction (movement of charged ions present in the solute and solvent) are the two methods by which MAE transfers energy (Kubrakova and Toropchenova, 2008). In terms of microwave extraction, polar solvents are thought to be more efficient in absorbing electrical energy. The effectiveness of a solvent in a microwave environment, however, depends on the dielectric constant and the dissipation factor, both of which are expected to be high for solvents used to effectively influence microwaves. The efficiency of the process is further increased by the careful selection of an appropriate solvent to interact with the metabolite constituents to be extracted. Microwave energy absorbed by biological materials causes the build-up of pressure within the cellular material, eventually leading to the splitting of the cellular structure with the release of its chemical constituents (Kratchanova et al., 2004; Routray and Orsat, 2012).
Prospect and economic promising
Peperomia pellucida-based medicines have been used empirically by the communities in Indonesia, Bolivia, Brazil, Guyana, the Amazon, the Philippines, Bangladesh, and South America to treat many kinds of diseases (de Albuquerque et al., 2007; Heyne, 1988; Majumder, 2011; Nwokocha et al., 2012). Even though it has great economic potential, there is very limited data available about this plant’s commercial products, at least until now. The previous section of this review has described the current development of extraction methods to increase the yield of polyphenolic content and flavonoid content of this herb using IL-MAE. Such a technique has a high possibility of being transferred to an industrial scale. It is because the time, energy use, high extraction yield, small amount of solvent, low economic costs, and eco-friendliness meet the criteria of the green extraction concept.
From the pharmaceutical industry perspective, P. pellucida has a high potential to be developed into herbal medicines with indications related to its pharmacological activities, as discussed in the previous section of this review. The most likely product to be produced with the currently available data (effective extraction method, pharmacological activity, and toxicity data) is health supplements. The health supplements could be used as a complementary therapy for hypertension, diabetes, or inflammation patients. Health supplements are regulated as food by the United States Food and Drug Administration, making it more feasible to be manufactured since the regulation for food is not as strict as the regulation for drugs (Commissioner, 2022). However, future development of P. pellucida as a herbal medicine should probe deeply into the formulation and clinical data trial so that the herbal medicine could be used in primary healthcare.
Regarding raw material supply, this plant is abundant in nature and is even considered a weed by local farmers. Utilizing this plant as a basic material in herbal medicine has several advantages. Firstly, concerning its nature that it can grow easily, it will not be challenging to cultivate so the reproducibility of the products can be maintained. Secondly, it can be cultivated with several modification methods, from hydroponic techniques, which can overcome the limited land issue, to biotechnology techniques to increase the yield of the compounds of interest. For instance, the polyphenolic content and the volatile oils are the compounds responsible for biological activities in the extract that can be improved by inoculating P. pellucida with Enterobacter asburiae and Klebsiella variicola (Alves et al., 2022). Nevertheless, postharvest management for this plant would be challenging since the herb contains a lot of water, so it is easy to rot if not handled properly.
Interestingly, the Philippine Patent has been granted to the National Integrated Research Program on Medicinal Plants, Institute of Herbal Medicine, the University of the Philippines Manila, for ulasimang bato, the local name for P. pellucida, as an antihyperuricemic agent. The use of this plant as an antihyperuricemic agent has successfully passed clinical trials phases 1–3, and it is now available for commercial production and ready for public consumption as a medicine (Sanchez, 2020). This initial information rationalizes that the use of P. pellucida as a therapeutic agent in the treatment of hyperuricemia and the treatment of other various diseases is very possible.
CONCLUSION
The data presented in the review indicate that the herbs of P. pellucida are highly likely to be developed into herbal medicines. The research progress on the developments of extraction techniques for the plant in terms of yield improvement with green extraction approaches raises its chance of being produced on large scale. Combining the right extraction technique with available data on the phytochemical, pharmacology, and toxicity properties of P. pellucida could increase the economic value of this plant to become a commercial herbal medicinal product. In addition, utilizing this weed plant as a herbal medicine, apart from increasing self-reliance in terms of drug supply, can also improve the economy of the farmers in the future as well as enhance the value of the plant.
LIST OF ABBREVIATIONS
ACE: Angiotensin-converting enzyme; [BMIM]BF4: 1-Butyl-3-metylimidazolium tetrafluoroborate; [BMIM]Br: 1-Butyl-3-metylimidazolium bromide; [BMIM]Cl: 1-Butyl-3-methylimidazolium chloride; BW: Body weight; EMIMBr: 1-Ethyl-3-methylimidazolium bromide; GAE: Gallic acid equivalent; IL-MAE: Ionic liquid-based microwave-assisted extraction; IVF: In vitro fertilization; LD50: Lethal dose fifty percent; MAE: Microwave-assisted extraction; MIC: Minimal inhibitory concentration; P. pellucida: Peperomia pellucida L. Kunth; TPC: Total polyphenolic content; UV: Ultraviolet.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abere TA, Agoreyo FO, Eze GI. Phytochemical, antimicrobial and toxicological evaluation of the leaves of Peperomia pellucida (L.) HBK (Piperaceae). J Pharm Allied Sci, 2012; 9:1637–52.
Abere TA, Okpalaonyagu SO. Pharmacognostic evaluation and antisickling activity of the leaves of Peperomia pellucida (L.) HBK (Piperaceae). Afr J Pharm Pharmacol, 2015; 9:561–6. CrossRef
Adhitia AM, Octaviani AN, Rissyelly, Basah K, Mun’im A. Effect of gamma irradiation on angiotensin converting enzyme inhibition, antioxidant activity, total phenolic compound and total flavonoid of Peperomia pellucida herbs extract. Pharmacog J, 2017; 9(2):244–8. CrossRef
Ahmad I. Penentuan nilai persentase eritema dan pigmentasi ekstrak herba Suruhan (Peperomia pellucida L.) secara in vitro. J Sains Kes, 2015; 1:90–5. CrossRef
Ahmad I, Ambarwati N, Elya B, Omar H, Mulia K, Yanuar A, Negishi O, Mun A. A new angiotensin-converting enzyme inhibitor from Peperomia pellucida (L.) Kunth. Asian Pac J Trop Biomed, 2019; 9:257–62. CrossRef
Ahmad I, Mulia K, Yanuar A, Mun’im A. Metabolite profiling analysis of conventional and non-conventional extraction methods on secondary metabolite from Peperomia pellucida (L.) Kunth using UPLC-QToF-MS/MS system. J Young Pharm, 2018; 10:S40–4. CrossRef
Ahmad I, Rissyelly R, Kurniawan A, Munim A. Screening of extraction method for alkaloid enrichment of Peperomia pellucida (L.) Kunth. Asian J Pharm Clin Res, 2017a; 10:214. CrossRef
Ahmad I, Yanuar A, Mulia K, Mun’im A. Application of ionic liquid as a green solvent for polyphenolics content extraction of Peperomia pellucida (L) Kunth herb. J Young Pharm, 2017b; 9:486–90. CrossRef
Ahmad I, Yanuar A, Mulia K, Mun’im A. Extraction of polyphenolic content from Peperomia pellucida (L) Kunth herb with 1-ethyl-3-methylimidazolium bromide. Indian J Pharm Sci, 2017c; 79(6):1013–7. CrossRef
de Albuquerque UP, Monteiro JM, Ramos MA, de Amorim ELC. Medicinal and magic plants from a public market in northeastern Brazil. J Ethnopharmacol, 2007; 110:76–91. CrossRef
Alves NSF, Kaory Inoue SG, Carneiro AR, Albino UB, Setzer WN, Maia JG, Andrade EH, da Silva JK. Variation in Peperomia pellucida growth and secondary metabolism after rhizobacteria inoculation. PLoS ONE, 2022; 17:e0262794. CrossRef
Anonim. USDA plants database, 2015. Available via https://plants.usda.gov/home/classification/72211 (Accessed 11 August 2022).
Arrigoni-Blank M de F, Dmitrieva EG, Franzotti EM, Antoniolli AR, Andrade MR, Marchioro M. Anti-inflammatory and analgesic activity of Peperomia pellucida (L.) HBK (Piperaceae). J Ethnopharmacol, 2004; 91:215–8. CrossRef
Awe FA, Giwa-Ajeniya AO, Akinyemi AA, Ezeri GNO. Phytochemical analysis of Acalypha wilkesiana, Leucaena leucocephala, Pepperomia pellucida and Sena alata leaves. Inter J Eng Sci, 2013; 2:41–4.
Aziba PI, Adedeji A, Ekor M, Adeyemi O. Analgesic activity of Peperomia pellucida aerial parts in mice. Fitoterapia, 2001; 72:57–8. CrossRef
Bayma JD, Arruda MS, Müller AH, Arruda AC, Canto WC. A dimeric ArC 2 compound from Peperomia pellucida. Phytochemistry, 2000; 55:779–82. CrossRef
Beltran-Be KS, Co EL, Gaspi SAD, Matibag JLR, Su GLS. Enzyme activity and histopathology of rat liver treated with crude methanolic extract of Peperomia pellucida (L.) HBK. J Bio Sci, 2013; 13:183–95. CrossRef
Bialangi N, Mustapa MA, Salimi YK, Widiantoro A, Situmeang B. Antimalarial activity and phytochemical analysis from Suruhan (Peperomia pellucida) extract. J Pendidik Kim, 2016; 8:183–7.
Bojo AC, Garcia EA, Pocsidio GN. Antibacterial activity of Peperomia pellucida (L.) HBK (Piperaceae). Asia Life Sci, 1995; 3(1):35–44.
Chemat F, Abert-Vian M, Fabiano-Tixier AS, Strube J, Uhlenbrock L, Gunjevic V, Gunjevic V, Cravotto G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends in Analyt Chem, 2019; 118:248–63. CrossRef
Chemat F, Vian MA, Cravotto G. Green extraction of natural products: Concept and principles. Inter J Mol Sci, 2012; 13:8615–27. CrossRef
Commissioner Office of the Commissioner. Dietary supplements. FDA, 2022. Available via https://www.fda.gov/consumers/consumer-updates/dietary-supplements (Accessed 1 August 2022),
Fakayode AE, Imaghodor FI, Fajobi AO, Emma-Okon BO, Oyedapo OO. Phytonutrients, antioxidants and anti-inflammatory analysis of Peperomia pellucida. J Med Pharm Allied Sci, 2021; 10:3517–23. CrossRef
Gini TG, Jothi GJ. Preliminary phytochemical screening of whole plant extracts of Peperomia pellucida (Linn.) HBK (Piperaceae) and Marsilea quadrifolia Linn. (Marsileaceae). Inter J Pharmacog Phytochem Res, 2013; 5:200–14.
Gomes PWP, Barretto H, Reis JDE, Muribeca A, Veloso A, Albuquerque C, Teixeira A, Braamcamp W, Pamplona S, Silva C, Silva M. Chemical composition of leaves, stem, and roots of Peperomia pellucida (L.) Kunth. Molecules, 2022; 27:1847. CrossRef
Hamzah RU, Odetola AA, Erukainure OL, Oyagbemi AA. Peperomia pellucida in diets modulates hyperglyceamia, oxidative stress and dyslipidemia in diabetic rats. J Acute Disease, 2012; 1:135–40. CrossRef
Hashim MR, Mohamad Z, Ngadi N, Jusoh YMM, Zakaria ZY, Jusoh M. Microwave-assisted extraction and phytochemical analysis of Peperomia Pellucida for treatment of dengue. Chem Eng Transactions, 2020; 78:409–14.
Heinrich M, Koehler I, Rimpler H, Bauer R. Bioactive compounds from the Mixe Indian Medicinal Plant Peperomia pellucida. Rev Soc Quim Mexico, 1998; 42:245–8.
Heyne K. Tumbuhan berguna Indonesia. Yayasan Sarana Wana Jaya, Jakarta, Indonesia,? 1988.
Ho KL, Yong PH, Wang CW, Kuppusamy UR, Ngo CT, Massawe F, Ng ZX. Peperomia pellucida (L.) Kunth and eye diseases: A review on phytochemistry, pharmacology and toxicology. J Integr Med, 2022; 20:292–304. CrossRef
Hsuuw YD, Chan WH. Apoptotic effects of dillapiole on maturation of mouse oocytes, fertilization and fetal development. Drug Chem Toxicol, 2015; 38:469–76. CrossRef
Idris OO, Olatunji BP, Madufor P. In vitro antibacterial activity of the extracts of Peperomia pellucida (L). British Microbiol Res J, 2016; 11(4):1–7. CrossRef
Kartika IGAA, Insanu M, Safitri D, Putri CA, Adnyana IK. New update: Traditional uses, phytochemical, pharmacological, and toxicity review of Peperomia Pellucida (L.) Kunth. PharmacologyOnline, 2016; 2:30–43.
Khan A, Rahman M, Islam S. Isolation and bioactivity of a xanthone glycoside from Peperomia pellucida. Life Sci Med Res, 2010; 2010:1–6.
Khan MR, Omoloso AD. Antibacterial activity of Hygrophila stricta and Peperomia pellucida. Fitoterapia, 2002; 73:251–4. CrossRef
Kratchanova M, Pavlova E, Panchev I. The effect of microwave heating of fresh orange peels on the fruit tissue and quality of extracted pectin. Carbohydr Polym, 2004; 56:181–5. CrossRef
Kubrakova IV, Toropchenova ES. Microwave heating for enhancing efficiency of analytical operations (Review). Inorg Mater, 2008; 44:1509–19. CrossRef
Kumar K, Srivastav S, Sharanagat VS. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason Sonochem, 2021; 70:105325. CrossRef
Kurniawan A, Saputri FC, Rissyelly, Ahmad I, Mun’im A. Isolation of angiotensin converting enzyme (ACE) inhibitory activity quercetin from Peperomia pellucida. Inter J PharmTech Res, 2016; 9:115–21.
Majumder P. Evaluation of taxo-chemical standardization and quality control parameters of Peperomia Pellucida (Family: Piperaceae): A multi valuable medicinal herb. J Pharm Sci Innov, 2012; 1:7–12.
Majumder P. Phytochemical, pharmacognostical and physicochemical standardization of Peperomia pellucida (L.) HBK. stem. Inter J Comprehensive Pharm, 2011; 2(8):1–4.
Majumder P, Majumder S. Preparation and characterization of some herbal ointment formulations with evaluation of antimicrobial property. Indian J Res Pharm Biotechnol, 2013; 1:385–90.
Manalo JB, Han BH, Han YH, Park MH, Anzaldo FE. Studies on ether-soluble neutral compounds of Peperomia pellucida. Arch Pharm Res, 1983; 6:133–6. CrossRef
Mediani A, Kamal N, Lee SY, Abas F, Farag MA. Green extraction methods for isolation of bioactive substances from coffee seed and spent. Sep Purif Rev, 2022; 0:1–19. CrossRef
Mengome L-E, Akue JP, Souza A, Feuya Tchoua GR, Nsi Emvo E. In vitro activities of plant extracts on human Loa loa isolates and cytotoxicity for eukaryotic cells. Parasitol Res, 2010; 107:643–50. CrossRef
Mun’im A, Nurpriantia S, Setyaningsih R, Syahdi RR. Optimization of microwave-assisted extraction of active compounds, antioxidant activity and angiotensin converting enzyme (ACE) inhibitory activity from Peperomia pellucida (L.) Kunth. J Young Pharm, 2017; 9:s73–8. CrossRef
Ng ZX, Than MJY, Yong PH. Peperomia pellucida (L.) Kunth herbal tea: Effect of fermentation and drying methods on the consumer acceptance, antioxidant and anti-inflammatory activities. Food Chem, 2021; 344:128738. CrossRef
Ngueguim FT, Khan MP, Donfack JH, Tewari D, Dimo T, Kamtchouing P, Maurya R, Chattopadhyay N. Ethanol extract of Peperomia pellucida (Piperaceae) promotes fracture healing by an anabolic effect on osteoblasts. J Ethnopharmacol, 2013; 148:62–8. CrossRef
Nwokocha CR, Owu DU, Kinlocke K, Murray J, Delgoda R, Thaxter K, McCalla G, Young L. Possible mechanism of action of the hypotensive effect of Peperomia pellucida and interactions between human cytochrome P450 enzymes. Med Aromatic Plants, 2012; 1(4):1–6. CrossRef
Oloyede GK, Onocha PA, Olaniran BB. Phytochemical, toxicity, antimicrobial and antioxidant screening of leaf extracts of Peperomia pellucida from Nigeria. Adv Environ Biol, 2011; 5:3700–9.
Ooi D, Iqbal S, Ismail M. Proximate composition, nutritional attributes and mineral composition of Peperomia pellucida L. (Ketumpangan Air) grown in Malaysia. Molecules, 2012; 17:11139–45. CrossRef
Pappachen LK, Chacko A. Preliminary phytochemical screening and in-vitro cytotoxicity activity of Peperomia pellucida Linn. Inter J Comprehensive Pharm, 2013; 4(8):1–4.
Rahman AHMM, Hossain MM, Islam AKMR. Taxonomy and medicinal uses of angiosperm weeds in the wheat field of Rajshahi, Bangladesh. Front Bio Life Sci, 2014; 2:8–11. CrossRef
Rojas-Martínez R, Arrieta J, Cruz-Antonio L, Arrieta-Baez D, Velázquez-Méndez A, Sánchez-Mendoza M. Dillapiole, isolated from Peperomia pellucida, shows gastroprotector activity against ethanol-induced gastric lesions in wistar Rats. Molecules, 2013; 18:11327–37. CrossRef
Routray W, Orsat V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess Technol, 2012; 5:409–24. CrossRef
Sanchez CM. Ulasimang bato as antihyperurecemic agent. UPM TTBDO, 2020. Available via https://ttbdo.upm.edu.ph/ulasimang-bato-as-antihyperurecemic-agent/ (Accessed 10 August 2022).
Sangsuwon C, Jirujchariyakul W, Roongruangchai K. Chemical constituents and antiamoebic of methanolic fraction from Peperomia pellucida (Linn.) Kunth. Appl Mechanics Materials, 2015; 709:417–21. CrossRef
Saputri FC, Hutahaean I, Mun’im A. Peperomia pellucida (L.) Kunth as an angiotensin-converting enzyme inhibitor in two-kidney, one-clip Goldblatt hypertensive rats. Saudi J Bio Sci, 2021; 28:6191–7. CrossRef
Saputri FC, Mun’im A, Lukmanto D, Aisyah SN, Rinandy JS. Inhibition of angiotensin converting enzyme (ACE) activity by some Indonesia edible plants. Inter J Pharm Sci Res, 2015; 6:1054–9.
Sheikh H, Sikder S, Paul SK, Hasan AMR, Rahaman M, Kundu SP. Hypoglycemic, anti-inflammatory and analgesic activity of Peperomia pellucida (L.) HBK (Piperaceae). Inter J Pharm Sci Res, 2013; 4:458–63.
Susilawati Y, Nugraha R, Krishnan J, Muhtadi A, Sutardjo S, Supratman U. A new antidiabetic compound 8,9-dimethoxy ellagic acid from Sasaladaan (Peperomia pellucida L. Kunth). Res J Pharm, Biol Chem Sci, 2017; 8:269–74.
Tarigan IM, Bahri S, Saragih A. Aktivitas antihiperurisemia ekstrak etanol herba Suruhan (Peperomia pellucida (L.) Kunth) pada mencit jantan. J Pharm Pharmacol, 2012; 1(1):37–3.
Usman LA, Ismaeel RO. Chemical composition of root essential oil of Peperomia pellucida (L.) Kunth. grown in Nigeria. J Essent Oil Bearing Plants, 2020; 23:628–32. CrossRef
Uwaya OD, Omozuwa PO, Inegbedion RE. Evaluation of in-vitro antioxidant and antidiarrheal activities of Peperomia Pellucida methanol extracts on Albino mice. J Appl Sci Environment Manag, 2021; 25:1681–8. CrossRef
Verma RS, Padalia RC, Goswami P, Chauhan A. Essential oil composition of Peperomia pellucida (L.) Kunth from India. J Essent Oil Res, 2015; 27:89–95. CrossRef
Wakhidah AZ, Novianti C, Mustaqim WA. Peperomia pellucida (L.) Kunth Piperaceae. In: Franco FM (ed.). Ethnobotany of the mountain regions of Southeast Asia, Springer International Publishing, Cham, Switzerland, pp 1–8, 2020. CrossRef
Waty DR, Saputri FC, Mun’im A. secondary metabolites screening and acute toxicity test of Peperomia pellucida (L.) Kunth methanolic extracts. Inter J PhamTech Res, 2017; 10:31–8. CrossRef
Wei LS, Wee W, Siong JYF, Syamsumir DF. Characterization of anticancer, antimicrobial, antioxidant properties and chemical compositions of Peperomia pellucida leaf extract. Acta Med Iran, 2011; 49:670–4.
Xu S, Li N, Ning MM, Zhou CH, Yang QR, Wang MW. Bioactive compounds from Peperomia pellucida. J Nat Prod, 2006; 69:247–50. CrossRef
Yunarto N, Ar Rossyid HM, Lienggonegoro LA. Effect of ethanolic leaves extract of Peperomia pellucida (L) Kunth as antimalarial and antioxidant. Media Peneliti Pengembang Kes, 2018; 28:123–30. CrossRef
Yusuf M, Wulandari I, Amelia L, Amelia L, Noviani A, Noviani A, Mun’im A. Effect of gamma irradiation on Suruhan (Peperomia pellucida (L.) Kunth) herb powder. Pharmacogn J, 2017; 9:239–43. CrossRef
Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: a comprehensive review. Chinese Med, 2018; 13:20. CrossRef