INTRODUCTION
Worldwide, the incidence rate of type 2 diabetes, a chronic metabolic disorder in which the human cells (i.e., muscle cells, adipocytes, and liver cells) do not respond functionally to insulin (insulin resistance), has been increasing significantly. The International Diabetes Federation estimates that the cost for the number of diabetes patients is expected to rise to 552 million U.S. dollars by 2030 and to 693 million U.S. dollars by 2045, with India and China being the two biggest populations (Cho et al., 2018; Whiting et al., 2011). Although various oral antidiabetic pharmaceutical agents have been employed, including metformin, α-glucosidase inhibitors (i.e., acarbose, miglitol), dipeptidyl-peptidase-4 inhibitors (i.e., saxagliptin and sitagliptin), meglitinides (i.e., nateglinide and repaglinide), SGLT-2 inhibitors (i.e., canagliflozin and dapagliflozin), sulfonylureas (i.e., chlorpropamide, tolazamide, and tolbutamide), and thiazolidinediones (i.e., rosiglitazone and pioglitazone) and their combinations, most of them cannot control the long-term glycemic issue (Satyanarayana et al., 2015; Singh et al., 2008) and also possess numerous side effects (Stumvoll et al., 2005). In fact, metformin, a biguanide first discovered in the 1940s (Garcia, 1950) and officially approved for type 2 diabetes treatment in 1995 (DeFronzo and Goodman, 1995), has remained first-line therapy for this disorder for more than 25 years (Nguyen et al., 2021a; Sanchez-Rangel and Inzucchi, 2017). Therefore, to overcome these problems, the need for finding novel medications for type 2 diabetes is crucial.
For this purpose, natural/herbal/traditional medicines prove their potential benefits. Various herbal extracts have been demonstrated to be effective in lowering the blood glucose level with insignificant side effects (Cefalu et al., 2011; Kumar et al., 2021). In Asia, these herbal medicines have long been utilized locally in ethnic groups and officially in traditional medicine hospitals in some countries such as Vietnam (Nguyen et al., 2021b; Tran et al., 2021; Venkateswaran and Pari, 2003). Among numerous herbal plants, neem (Azadirachta indica A. Juss, “sau dau” in Vietnamese), a well-known plant in South Asia and in Vietnam, has attracted increasing scientific attention due to its wide spectrum of biological activities, namely anti-inflammatory, antimicrobial, antipyretic, antioxidant, anticancer, antiulcer, spermicidal, immunomodulating, immunocontraceptive, and antidiabetic (Atawodi and Atawodi, 2009; Alzohairy, 2016; Biswas et al., 2002). These therapeutic actions come from unique phytochemical constituents of the plant, such as azadirachtins, azadirachtol, azadironolide, isoazadironolide, nimocinol, nimbin, nimolicinol, naheedin, and mahmoodin (Atawodi and Atawodi, 2009). Specifically, for type 2 diabetes treatment, neem shows potential effects in reducing the blood glucose level (El-Hawary and Kholief, 1990; Mahdi et al., 2003; Patil et al., 2022; Satyanarayana et al., 2015). For instance, in alloxan-induced diabetic rats, neem bark and leaf extracts significantly decrease rat blood glucose level and lipid peroxidation, as well as enhance the antioxidant enzyme functions like catalase, superoxide dismutase, and glutathione peroxidase in the kidney and liver tissues. Similarly, in high-fat diet-induced diabetic Charles Foster rats, the neem leaf extract significantly reduces the plasma glucose concentrations and increases the antioxidant enzyme activities in hepatic tissues for 30 days (Shrivastava et al., 2012). In streptozotocin-induced diabetic rats, chronic treatment with the neem ethanolic extract reduces the blood glucose level and ameliorates lesions of pancreatic islets (Akinola et al., 2011; Akpan et al., 2012). At the molecular level, neem extracts, at a dose of 400 mg/kg rat body weight, play an important role in normalizing the impaired levels of insulin-signaling molecules, namely insulin receptor substrate-1, insulin receptor, phospho-AktSer473, phospho-IRS-1Ser636, phospho-IRS-1Tyr632, and glucose transporter 4 (GLUT4) (Satyanarayana et al., 2015).
Although demonstrating much potential for diabetes treatment, the neem extracts have not been formulated into an actual pharmaceutical dosage form for commercial application. Thus, this work presents the development and in-vitro/in-vivo evaluation of the film-coated tablets containing the neem leaf extract, with moisture-protected ability, for the treatment of type 2 diabetes. To this end, the extracts were firstly produced and characterized, followed by extract incorporation into the moisture-resistant film-coated tablets, and were finally antidiabetic tested in in-vitro and in-vivo models, using the α-glucosidase inhibitory assay and mice glucose tolerance test, respectively.
MATERIALS AND METHODS
Materials
Neem leaves were collected in the Tri Ton district, An Giang province, Vietnam, in September 2016. The samples were identified by a botanical expert, and the voucher specimens were stored at the Faculty of Pharmacy, Can Tho University of Medicine and Pharmacy, Vietnam. The commercial reference drugs, namely Diamicron (gliclazide), “Diep Ha Chau,” “Pylantin,” “Dan Sam Tam That,” and “Giai doc gan Tue Linh,” were bought from Anh Ngoc drugstore, Can Tho, Vietnam. The experimental animals, Swiss albino mice, 5-week-old, weighing 18–25 g, were provided by Nha Trang Pasteur Institute, Nha Trang, Vietnam. All other chemicals, solvents, and standard substances were of pharmaceutical grade or higher.
Neem leaf extraction
The dry neem leaves (100 g) were ground to powder, moistened with ethanol (20–30% w/v) for 1 hour, and immersed in ethanol for 36 hours at room temperature. The obtained ethanolic extract was concentrated by a Rotavapor until a semisolid mass formed. Finally, the extracts were stored at 4°C for further experiments. To determine the optimal extraction conditions, the parameters of ethanol concentrations (40%, 60%, and 90%) and plant/solvent weight ratios (1/8, 1/9, and 1/10 w/w) were varied. The best extract was considered with the highest extraction efficiency [i.e., the extract mass/the initial leaves powder (~100 g)], the lowest moisture (measured by the MX-50 infrared moisture drying scale), and the strongest in-vitro α-glucosidase inhibitory activity (determined based on the procedure described in the section in-vitro α-glucosidase inhibitory assay).
Formulation of film-coated tablets containing neem leaf extract
The optimal neem leaf extract was further formulated into the film-coated tablet dosage form. To this end, the uncoated tablets were prepared by the general wet-granulation process, followed by the moisture-resistant film-coating process on the uncoated tablets (Nha et al., 2016). Briefly, the neem leaf extract (155 mg/tablet) was mixed homogeneously with the adsorbent and blended with the diluent. The choices of adsorbent [CaCO3, MgCO3, Syloid (silica), or calcium silicate] and diluent [MgCO3, microcrystalline cellulose, tricalcium phosphate, unmilled dicalcium phosphate dihydrate (Di-tab), or sodium carboxymethylcellulose (Na CMC)], as well as their ratios in the formulations, were investigated and selected based on the tablet physicochemical properties. Then, the granules were formed by grinding the mixture through a 2 mm mesh, using polyvinylpyrrolidone (PVP) in 96% ethanol as the binder. The products were dried at 60°C and passed through the 1 mm mesh. Finally, the granules were mixed with croscarmellose, aerosil, and magnesium stearate and compressed utilizing a Rimek rotary tablet press to form the uncoated tablets (round shape, 12 mm diameter, and weight 363 ± 18 mg). Each tablet contained 155 mg (42.67% w/w) of the neem leaf extract.
The uncoated tablets were then moisture-resistant film-coated with a Caleva film-coating machine, using the PVA in the solution as a moisture-resistant film polymer and a mixture of polyethylene glycol 6000, talc, titanium dioxide, and colorant as coloring agent. The PVA solution was made by dissolving the PVA powder in an 96% ethanol:water (3:1 v/v) solvent. The coloring agent was fabricated by grinding the PEG 6000 aqueous solution with talc, titanium dioxide, and colorants, followed by the addition of 5 ml distilled water to form a suspension, and the suspension was filtered through a 0.3 mm sieve to obtain the product. The coating process was conducted following the machine’s instructions, and the endpoint was set at the tablet weight gain of 3% compared to the initial uncoated tablet weight. The complete tablet formula is presented in Table 1.
Characterizations of film-coated tablets containing neem leaf extract
The film-coated tablets containing the neem leaf extract were physicochemically characterized in terms of appearance, weight uniformity, friability, disintegration time, identification, and assay (Nha et al., 2016; Nguyen et al., 2021a). Additionally, the intermediate products (i.e., granules) were tested for moisture, apparent density, and flowability to determine the optimal formulations. To this end, the friability test was conducted using a Pharma-Test tablet friability tester (60 rpm for 5 minutes); the disintegration time was tested by an ERWEKA disintegration tester (30 minutes, in water, at 37°C); and the tablet content identification was conducted by thin-layer chromatography [the mobile phase consists of MeOH-chloroform (50:50 v/v)]. For the intermediate granules, the moisture was measured by an MX-50 infrared moisture drying scale; the apparent density was determined using a Pharma-Test apparent density meter (750 taps/minutes); and the flowability was measured by the ERWEKA granulate flow tester (outlet nozzle 15 mm, nonstir).
In-vitro α-glucosidase inhibitory assay
The neem leaf extract (30 mg/ml), the film-coated tablets containing the neem leaf extract (at a dose equivalent to 30 mg/ml of the extract), and the reference product/positive control Glucobay (acarbose) (5 and 10 mg/ml) were evaluated in-vitro for their antidiabetic properties using the standard α-glucosidase inhibitory assay at 37°C, pH 7.0, with slight modifications (Kazeem et al., 2013). For this, the test samples (0.15 ml) were mixed with 2.4 ml 0.1 M phosphate buffer pH 7.0 and 0.15 ml of the α-glucosidase (0.5 U/ml, one unit is the required amount of α-glucosidase to catalyze the substrate 4-nitrophenyl β-D-glucopyranoside (pNPG) to form 1 μmol of p-nitrophenol (product) per minute) for 30 minutes at 37°C. Then, 0.3 ml of pNPG, at a concentration of 2 mM, was added to the mixture to start the reaction, followed by another incubation of 30 minutes. Finally, the reaction was halted with the addition of 3.0 ml Na2CO3 (0.2 M), and the solution was UV-Vis spectroscopically (Jasco V-730, Japan) measured at 405 nm using a multiwell plate reader. The blank was processed with the same protocol, with the sample being ethanol, the solvent for extracting the neem leaf. The percentages of α-glucosidase inhibition of the samples were calculated according to
where ABlank and ASample are the absorbance values of the blank and the test samples, respectively.
In-vivo mice glucose tolerance test
The neem leaf extract, the film-coated tablets containing the neem leaf extract, and the reference product/positive control Diamicron (gliclazide) were evaluated in-vivo for their antidiabetic properties on Swiss albino mice using the standard glucose tolerance test with some adjustments (Pedro et al., 2020). Prior to the experiments, mice were housed at room temperature (25 ± 1°C) with controlled humidity and a 12 hours light and dark cycle and fed a standard diet and water ad libitum. All in-vivo experiments were ethically approved by the Ethics Committee of Can Tho University of Medicine and Pharmacy, Code CTUMP-421.
The glucose tolerance tests were conducted orally on overnight (16 hours) fasted mice. The mice were divided into four groups, each with six mice including three males and three females. Group I was the negative control group in which mice were fed water only. Group II was the reference group, and the mice received Diamicron (20 mg/kg body weight) orally. Groups III and IV were the tested groups in which the mice were orally treated with the neem leaf extract (200 mg/kg body weight) and the film-coated tablets containing the neem leaf extract (at a dose equivalent to 200 mg/kg of the extract), respectively. Glucose (2 g/kg body weight) was administered orally after 1 hour of the test sample intake. The mice blood samples were collected from the tail vein at 0, 30, 60, and 120 minutes after the glucose load. The blood glucose levels (mg/dl) were analyzed using the EasyGluco Infopia blood glucose meter. The doses of neem leaf extract, Diamicron, and intake glucose were determined based on our preliminary study and the literature (Lali?-Popovi? et al., 2013; Pedro et al., 2020).
Statistical analysis
All quantitative experiments were carried out in triplicate. The data were presented in terms of mean ± standard deviation (SD). Significant differences between data were analyzed using Student’s t-test and one-way analysis of variance, with a p-value less than 0.05 for meaningful comparisons.
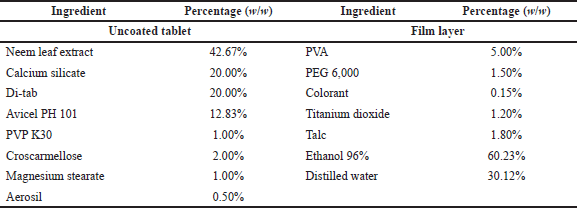 | Table 1. Formulation of film-coated tablets containing neem leaf extract. [Click here to view] |
RESULTS AND DISCUSSION
Neem leaf extraction
According to the previous study, the immersion method utilizing ethanol as the extracting solvent is the optimal condition for neem leaf extraction (Hashim et al., 2021). Thus, in our work, the neem leaves were extracted using the simple immersion method with varied experimental conditions. Table 2 shows nine extracts with different ethanol concentrations (40%, 60%, and 90% v/v) and plant/solvent weight ratios (1:8, 1:9, and 1:10 w/w). To this end, the extraction conditions significantly affected the extract moisture, extraction efficiency, and extract in-vitro α-glucosidase inhibitory activity. An increase in the ethanol (the extraction solvent) concentration from 40% to 90% decreases the extract moisture from approximately 14% to less than 10% and enhances the extraction efficiency from about 9.5% to 11.5%. On the other hand, an increase in the plant/solvent weight ratio from 1:8 to 1:10 increases both the extract moisture and the extraction efficiency. Moreover, the extract α-glucosidase inhibitory action was proportionally correlated with its extraction efficiency, as a higher efficiency resulted in a higher α-glucosidase inhibitory percentage.
Since the neem leaf extract is intended to be adsorbed into the adsorbent for tablet compression, its moisture is important, and the lower the moisture, the higher the adsorbed capacity (Rongsriyam et al., 2006). The 90% ethanol, with less water content in the solvent, yields an extract with lower moisture than the less concentrated ethanol. Additionally, due to the fact that higher extraction efficiency results in more chemical contents in the extract, it is obvious that the extract α-glucosidase inhibitory action was proportionally correlated with its extraction efficiency. Therefore, the condition that gives high extraction efficiency is beneficial. For this, the 90% ethanol and the plant/solvent weight ratio of 1:10 constituted the optimal condition in our study, which yielded moisture content of 9.57% ± 0.19%, efficiency of 12.43% ± 0.32%, and a corresponding α-glucosidase inhibitory action of 40.43% ± 3.89%. Compared to other studies, this extraction efficiency was somehow moderate, as Malaysian neem leaf ethanolic extraction possesses efficiency of 22% (Hashim et al., 2021), and the Nepali neem leaf only gives 5.75% of the mass when being extracted with methanol (Lee et al., 2017). These differences reconfirm the impact of geographic variations on the physicochemical properties of neem (Jessinta et al., 2014).
Formulation and characterizations of film-coated tablets containing neem leaf extract
The optimal neem leaf extract was further incorporated into the film-coated tablets for the in-vitro/in-vivo antidiabetic evaluations. To this end, we first determined the best adsorbent for adsorbing the extract. Among the four common adsorbents, the required extract/adsorbent weight ratios to completely adsorb the extract of CaCO3, MgCO3, Syloid (silica), and calcium silicate were 1:2.5, 1:1, 1:0.6, and 1:0.5, respectively. Thus, calcium silicate was considered the optimal adsorbent, in our case, due to its affordable price and high efficacy in adsorbing the extract. Another study also suggests that calcium silicate is a potential adsorbent for plant extraction due to its high porosity and large specific surface area (Wang et al., 2020).
We next selected the optimal diluent (Table 3). From the five investigated diluents (MgCO3, microcrystalline cellulose, tricalcium phosphate, Di-tab, and Na CMC), MgCO3 yielded unflowable granules and high-friability tablets (36.69% ± 0.32%), which were not suitable for the film-coated tablets; and Na CMC resulted in incomplete tablet disintegration. Interestingly, although Di-tab (dicalcium phosphate dihydrate) is a water-insoluble excipient that has been popularly utilized in controlled-release formulations (i.e., the matrix-type tablets) (Zhang and Schwartz, 2000), in our case, Di-tab yielded tablets with the shortest disintegration time (2.65 ± 0.04 minutes) with appropriate friability (0.05% ± 0.005%). This could be due to the high amount of the extract in the tablet (>40%) since an incorporated drug quantity of more than 5% w/w resulted in rapid tablet disintegration and burst release (Mulye and Turco, 2008). Thus, due to its inexpensiveness, ease of manufacturing, and ability to disintegrate the tablet fast, Di-tab has been selected as an optimal diluent for the next investigations.
After selecting the optimal diluent, we varied the ratios of the diluent (Di-tab: 20% and 29.5% w/w), binder (PVP K30: 1% and 2% w/w), and disintegrant (croscarmellose: 1%, 2%, and 3% w/w) to obtain the best tablets with suitable properties, in terms of friability and disintegration time (Table 4). Since the uncoated tablets need to possess low friability of <0.5% to be appropriate for film-coating and a short disintegration time of <5 minutes to be completely broken down in the digestive system, the amount of binder and disintegrant should be carefully considered. To this end, all formulas possess suitable friability of <0.5% and disintegration time of <5 minutes (Table 4). Notably, the tablet friability was mainly correlated to the binder (PVP K30) amount (i.e., the more the PVP K30 amount, the less the tablet friability). On the other hand, the tablet disintegration time was affected by both the binder and disintegrant (croscarmellose) amounts, as an increase in the PVP K30 amount resulted in longer disintegration times, whereas the croscarmellose amount reduced this time significantly. Since PVP K30 enhances tablet hardness, it takes more time for the solvent to diffuse into the tablet cores and disintegrate them (Paramita et al., 2020). Croscarmellose, a superdisintegrant, aids tablet disintegration by rapid swelling and “wicking” (i.e., the material–air/material–material interface is spontaneously replaced by the material–water interface, consequently maintaining the capillary flow) when interacting with water (Desai et al., 2014). Conclusively, formula 2 (20% Di-tab, 1% PVP K30, and 2% croscarmellose) was selected for further investigation.
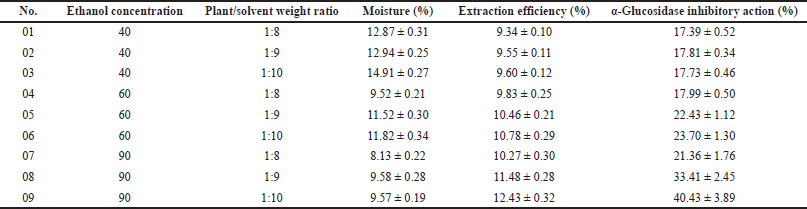 | Table 2. Effects of ethanol concentration (40%, 60%, and 90% v/v) and plant/solvent weight ratio (1:8, 1:9, and 1:10 w/w) on neem leaf extract moisture (%), extraction efficiency (%), and α-glucosidase inhibitory action (%). The results are expressed in mean ± SD (n = 3). [Click here to view] |
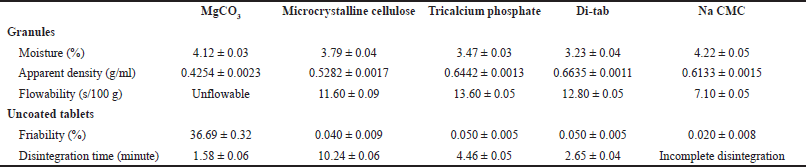 | Table 3. Effects of different diluent types on the properties of the uncoated tablets/granules containing neem leaf extract. Data are expressed in terms of mean ± SD (n = 3). Na CMC: sodium carboxymethylcellulose. [Click here to view] |
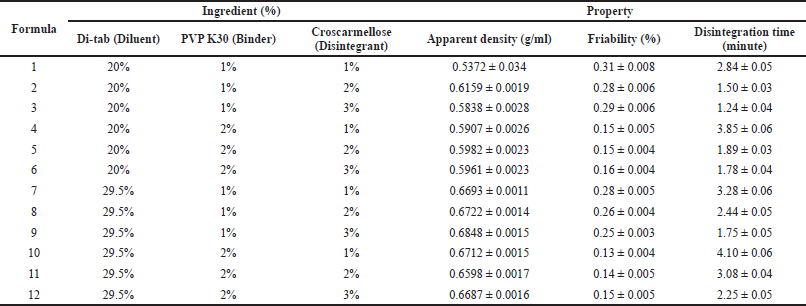 | Table 4. Effects of diluent (Di-tab: 20% and 29.5%), binder (PVP K30: 1% and 2%), and disintegrant (croscarmellose: 1%, 2%, and 3%) on the properties of the uncoated tablets/granules containing neem leaf extract. Data are expressed in terms of mean ± SD (n = 3). [Click here to view] |
Finally, the optimal uncoated tablets were film-coated to yield the complete products. Due to the fact that the tablets contain a large amount of neem leaf extract, it should be moisture-protected. For this, we first investigated the optimal moisture-resistant film-forming agent. Among various film-coating excipients of PVA, Hydroxypropyl methylcellulose (HPMC), and Eudragit E100, PVA was the optimal one since HPMC and Eudragit E100 produced wet and sticking tablets (data not shown). Thus, PVA was selected due to its low cost and moisture-lowering properties. Additionally, a 5% PVA ethanolic solution was the suitable agent, as higher percentages of PVA yielded an uncoatable solution. Moreover, the optimal spraying pressure and speed were 5 psi and 5%, respectively, and higher values produced wet and sticking tablets. Summing up, the best coating condition was using a 5% PVA ethanolic solution, with a spraying pressure of 5 psi and a spraying speed of 5%.
The complete film-coated tablets containing the neem leaf extract were then evaluated for their moisture protection ability in comparison with the commercial products. Expectedly, compared to the other film-coated tablets containing herbal extracts, like “Diep Ha Chau” (Phyllanthus urinaria extract), “Pylantin” (P. urinaria extract), “Dan Sam Tam That” (Panax notoginseng and Salviae miltiorrhizae extracts), and “Giai doc gan Tue Linh” (Eurycoma longifolia and Solanum procumbens extracts), our products possessed similar moisture of <10% in the ambient temperature of 25°C and 75% humidity for >6 months. This indicates that the PVA film-forming layer is effective in moisture-protecting the tablets.
In-vitro/in-vivo antidiabetic evaluation of film-coated tablets containing neem leaf extract
Neem leaf extracts have been proved to possess strong antidiabetic activities in both in-vitro and in-vivo studies (Akinola et al., 2011; Akpan et al., 2012; Hashim et al., 2021; Kazeem et al., 2013; Lee et al., 2017; Shrivastava et al., 2012). The main component contributing to neem antidiabetic actions is the 3-deacetyl-3-cinnamoyl-azadirachtin (Jalil et al., 2013). Nevertheless, to the best of our knowledge, no research has been reported on the formulations and in-vitro/in-vivo antidiabetic evaluations of the film-coated tablets containing the neem leaf extract. Thus, we further tested the tablets’ antidiabetic properties in the in-vitro α-glucosidase inhibitory assay and in-vivo mice glucose tolerance test.
In the in-vitro study, the neem leaf extract, at a concentration of 30 mg/ml, demonstrated acceptable α-glucosidase inhibitory activity (43.43%) in comparison with the commercial drug Glucobay (acarbose) (Fig. 1). The neem leaf extract action in our result was lower than that in the previous study in Nigeria, which showed that the extract’s IC50 value, on a similar assay, was 8.7 mg/ml (Kazeem et al., 2013). This, again, suggests that the cultivation geography significantly affects the biological properties of neem. Surprisingly, although the extraction efficiency of the neem leaf ethanolic extract was only 2.25% in the Nigerian study (Kazeem et al., 2013), lower than that in our work (12.43%), the Nigerian neem showed better α-glucosidase inhibitory activity than our neem extract. Thus, relationships between the plant’s extract mass and its biological activities need to be critically investigated, as they might not be proportionally correlated. In terms of the tablet efficacy, the film-coated tablets containing equivalent amounts of the neem leaf extract demonstrated a similar α-glucosidase inhibitory percentage (43.33%) compared to the respective extract (43.43%). This indicates that the formulating processes and the tablet excipients did not significantly affect the extract activity.
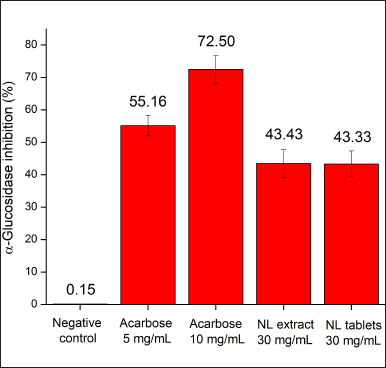 | Figure 1. In-vitro α-glucosidase inhibitory percentages of the neem leaf extract (NL extract, 30 mg/ml) and the film-coated tablets containing neem leaf extract (NL tablets, 30 mg/ml equivalent to the extract) in comparison with the negative control and the reference drug Glucobay (acarbose 5, 10 mg/ml) (n = 3). [Click here to view] |
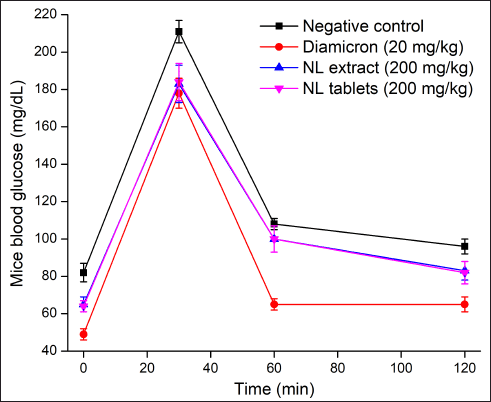 | Figure 2. In-vivo mice glucose tolerance tests on the neem leaf extract (NL extract, group III) and the film-coated tablets containing neem leaf extract (NL tablets, group IV) in comparison with the negative control (water, group I) and the reference drug Diamicron (gliclazide 20 mg/kg, group II) (n = 3). [Click here to view] |
In the in-vivo study (Fig. 2), compared to the negative control group (i.e., mice fed water), mice fed the commercial drug Diamicron (20 mg/kg), the neem leaf extract (200 mg/kg), and the film-coated tablets containing the neem leaf extract (200 mg/kg, equivalent to the extract concentration) had significantly lower blood glucose levels. These data reconfirmed the antidiabetic effects of the neem leaf extract. Additionally, the film-coated tablets could preserve these effects without any interference from the formulation processes and the excipients. Regarding the differences in the blood glucose patterns between our tablets and the reference, Diamicron, it is possible that the neem leaf extract and Diamicron possessed different mechanisms of action. The neem leaf extract acts as an α-glucosidase inhibitor, whereas Diamicron belongs to the sulfonylurea group. Thus, the extract was effective in managing the post-diet fast sugar (i.e., 30 minutes), but not the long-term sugar (i.e., 60 and 120 minutes), leading to a higher long-term sugar compared to the Diamicron effects.
CONCLUSION
For the first time, moisture-resistant film-coated tablets containing the neem (A. indica A. Juss) leaf extract were developed and evaluated in-vitro/in-vivo for diabetes treatment. The optimal formula consists of 20% calcium silicate as adsorbent, 20% Di-tab as diluent, 1% PVP K30 as binder, 2% croscarmellose as disintegrant, and 5% PVA as film-coating agent, which possesses a suitable disintegration time of <5 minutes and a moisture-resistant property comparable to the commercial products. The tablets could well preserve the neem leaf extract’s antidiabetic activities in both the in vitro α-glucosidase inhibitory assay and in-vivo mice glucose tolerance test. Conclusively, the moisture-resistant film-coated tablets containing the neem leaf extract could be further investigated, especially in subclinical and clinical trials, to become a potential antidiabetic pharmaceutical product.
AUTHORS’ CONTRIBUTIONS
NNTN and DTP contributed to the concept and design; NNTN, XCD, KNN, and TNVN contributed to data acquisition; NNTN, TTDN, TTYL, TCTL, TTTN, and DTP contributed to data analysis/interpretation; NNTN, KNN, and DTP drafted the manuscript; NNTN and DTP critically revised the manuscript; NNTN and KNN conducted the statistical analysis; DTP acquired the funding; DTP supervised the study; and DTP gave final approval of the study.
ACKNOWLEDGMENTS
The authors thank Can Tho University of Medicine and Pharmacy, and the Department of Science and Technology in An Giang province for supporting this research.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
FUNDING
There is no funding to report.
ETHICAL APPROVAL
The research ethics were approved by Can Tho University of Medicine and Pharmacy, Vietnam, Code CTUMP-421.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Akinola OB, Omotoso OG, Dosumu OO, Akinola OS, Olotufore F. Diabetes-induced prefrontal nissl substance deficit and the effects of neem-bitter leaf extract treatment. Int J Morphol, 2011; 29:850–6. CrossRef
Akpan HD, Ekaidem IS, Usoh IF, Ebong PE, Isong NB. Effect of aqueous extract of Azadirachta indica (Neem) leaves on some indices of pancreatic function in alloxan-induced diabetic wistar rats. Pharmacologia, 2012; 3:420–5; doi:10.5567/PHARMACOLOGIA.2012.420.425 CrossRef
Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evid Based Complement Alternat Med, 2016; doi:10.1155/2016/7382506 CrossRef
Atawodi SE, Atawodi JC. Azadirachta indica (neem): a plant of multiple biological and pharmacological activities. Phytochem Rev, 2009; 83(8):601–20; doi:10.1007/S11101-009-9144-6
Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological activities and medicinal properties of neem (Azadirachta indica). Curr Sci, 2002; 82:1336–45. CrossRef
Cefalu WT, Stephens JM, Ribnicky DM. Diabetes and herbal (Botanical) medicine. Herb Med Biomol Clin Asp Second Ed, 2011; 405–18; doi:10.1201/b10787-20 CrossRef
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract, 2018; 138:271–81; doi:10.1016/J.DIABRES.2018.02.023 CrossRef
DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The multicenter metformin study group. N Engl J Med, 1995; 333:541–9; doi:10.1056/NEJM199508313330902 CrossRef
Desai PM, Er PXH, Liew CV, Heng PWS. Functionality of disintegrants and their mixtures in enabling fast disintegration of tablets by a quality by design approach. AAPS PharmSciTech, 2014; 15:1093; doi:10.1208/S12249-014-0137-4 CrossRef
El-Hawary ZM, Kholief TS. Biochemical studies on hypoglycemic agents (I) effect of Azadirachta indica leaf extract. Arch Pharmacal Res, 1990; 131(13):108–12; doi:10.1007/BF02857845
Garcia EY. Flumamine, a new synthetic analgesic and anti-flu drug. J Philipp Med Assoc, 1950; 26:287–93. CrossRef
Hashim N, Abdullah S, Hassan LS, Ghazali SR, Jalil R. A study of neem leaves: identification of method and solvent in extraction. Mater Today Proc, 2021; 42:217–21; doi:10.1016/J.MATPR.2020.11.726 CrossRef
Jalil A, Ashfaq UA, Shahzadi S, Javed MR, Rasul, I, Rehman S, Shah M, Masoud MS. Screening and design of anti-diabetic compounds sourced from the leaves of neem (Azadirachta indica). Bioinformation, 2013; 9:1031; doi:10.6026/97320630091031
Jessinta S, Azhari HN, Saiful NT, Abdurahman HN. Impact of geographic variation on physicochemical properties of neem (Azadirachta indica) Seed Oil. Int J Pharm Sci Res, 2014; 5:4406–13. CrossRef
Kazeem MI, Dansu TV, Adeola SA. Inhibitory effect of Azadirachta Indica A. juss leaf extract on the activities of α-amylase and α-glucosidase. Pakistan J Biol Sci, 2013; 16:doi:10.3923/pjbs.2013.1358.1362 CrossRef
Kumar S, Mittal A, Babu D, Mittal A. Herbal medicines for diabetes management and its secondary complications. Curr Diabetes Rev, 2021; 17:437–56. doi:10.2174/1573399816666201103143225 CrossRef
Lali?-Popovi? M, Vasovi? V, Milijaševi? B, Golo?orbin-Kon S, Al-Salami H, Mikov M. Deoxycholic acid as a modifier of the permeation of gliclazide through the blood brain barrier of a rat. J Diabetes Res, 2013; doi:10.1155/2013/598603 CrossRef
Lee JW, Ryu HW, Park SY, Park HA, Kwon OK, Yuk HJ, Shrestha KK, Park M, Kim JH, Lee S, Oh SR, Ahn KS. Protective effects of neem (Azadirachta indica A. Juss.) leaf extract against cigarette smoke- and lipopolysaccharide-induced pulmonary inflammation. Int J Mol Med, 2017; 40:1932–40. doi:10.3892/IJMM.2017.3178/HTML CrossRef
Mahdi AA, Chandra A, Singh RK, Shukla S, Mishra LC, Ahmad S. Effect of herbal hypoglycemic agents on oxidative stress and antioxidant status in diabetic rats. Indian J Clin Biochem, 2003; 18:8–15. doi:10.1007/BF02867361 CrossRef
Mulye NV, Turco SJ. Use of dicalcium phosphate dihydrate for sustained release of highly water soluble drugs. 2008; 20:2621–632. doi:10.3109/03639049409042666 CrossRef
Nguyen NNT, Pham DT, Nguyen DT, Trinh TTL. Bilayer tablets with sustained-release metformin and immediate-release sitagliptin: preparation and in vitro/in vivo evaluation. J Pharm Investig, 2021a; 515(51):579–86. doi:10.1007/S40005-021-00533-Z CrossRef
Nguyen PH, De Tran V, Pham DT, Phong Dao TNP, Deway RS. Use of and attitudes towards herbal medicine during the COVID-19 pandemic: a cross-sectional study in Vietnam. Eur J Integr Med, 2021b; 101328. doi:10.1016/j.eujim.2021.101328
Nha TNN, Duy TP, Ngoc MT. Formulation and evaluation of low floating lag time metformin hydrochloride 500 mg sustained release floating tablet. Asian J Pharm, 2016; 10:S497–S503. CrossRef
Paramita DP, Soeratri W, Widjaja B, Nuruddin NM, Setyawan D. Optimization of Povidone K-30 and sodium starch glycolate on levofloxacin tablet by factorial design. J ILMU DASAR, 2020; 21:35–42. doi:10.19184/jid.v21i1.10220 CrossRef
Patil SM, Shirahatti PS, Ramu R. Azadirachta indica A. Juss (neem) against diabetes mellitus: a critical review on its phytochemistry, pharmacology, and toxicology. J Pharm Pharmacol, 2022; 74:681–710. doi:10.1093/JPP/RGAB098 CrossRef
Pedro PF, Tsakmaki A, Bewick GA. The glucose tolerance test in mice. Methods Mol Biol, 2020; 2128:207–16. doi:10.1007/978-1-0716-0385-7_14
Rongsriyam Y, Trongtokit Y, Komalamisra N, Sinchaipanich N, Apiwathnasorn C, Mitrejet A. Formulation of tablets from the crude extract of Rhinacanthus nasutus (Thai local plant) against Aedes aegypti and Culex quinquefasciatus larvae: a preliminary study. Southeast Asian J Trop Med Public Health, 2006; 37:265–71. CrossRef
Sanchez-Rangel E, Inzucchi SE. Metformin: clinical use in type 2 diabetes. Diabetologia, 2017; 60:1586–93. doi:10.1007/S00125-017-4336-X CrossRef
Satyanarayana K, Sravanthi K, Shaker IA, Ponnulakshmi R. Molecular approach to identify antidiabetic potential of Azadirachta indica. J Ayurveda Integr Med, 2015; 6:165. doi:10.4103/0975-9476.157950 CrossRef
Shrivastava A, Chaturvedi U, Sonkar R, Khanna AK, Saxena JK, Bhatia G. Antioxidant effect of Azadirachta indica on high fat diet induced diabetic Charles foster rats. Appl Biochem Biotechnol, 2012; 167:229–36. doi:10.1007/S12010-012-9681-0 CrossRef
Singh SK, Rai PK, Jaiswal D, Watal G. Evidence-based critical evaluation of glycemic potential of Cynodon dactylon. Evid Based Complement Alternat Med, 2008; 5:415–20. doi:10.1093/ECAM/NEM044 CrossRef
Stumvoll M, Goldstein BJ, Van Haeften TW. In: Lancet. Type 2 diabetes: principles of pathogenesis and therapy. London, UK, 365:pp 1333–46, 2005. doi:10.1016/S0140-6736(05)61032-X
Tran VD, Pham DT, Cao TTN, Bahlol M, Dewey,RS, Le Mh, Nguyen VA. Perspectives on COVID-19 prevention and treatment using herbal medicine in Vietnam: a cross-sectional study. Ann Ig, 2021; 34(5):515–31. doi:10.7416/AI.2021.2484 CrossRef
Venkateswaran S, Pari L. Effect of Coccinia indica leaves on antioxidant status in streptozotocin-induced diabetic rats. J Ethnopharmacol, 2003; 84:163–8. doi:10.1016/S0378-8741(02)00294-5 CrossRef
Wang F, Zhang Y, Mao Z. High adsorption activated calcium silicate enabling high-capacity adsorption for sulfur dioxide. New J Chem, 2020; 44:11879–86. doi:10.1039/D0NJ01874K CrossRef
Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract, 2011; 94:311–21. doi:10.1016/J.DIABRES.2011.10.029
Zhang YE, Schwartz JB. Effect of diluents on tablet integrity and controlled drug release. 2000; 26:761–5. doi:101081/DDC-100101295