INTRODUCTION
Sleep, as well as food and shelter, is a significant necessity for all humans, and in a demanding environment, psychological stresses often contribute to bad sleep habits that abrupt their biological clock. Nearly 25% of the adults are affected by insomnia, although 10% are chronic (Levenson et al., 2015). Sleeplessness is categorized into four major types: irregular waking, early morning awakening, low sleep quality, and sleep difficulties (Krystal et al., 2019). Recently, the buccal supply of medicines has become an efficient and secure substitute to other traditional medication paths. Administration into the buccal cavity easily releases the loaded drug used either for local or systemic effects. This route of administration is especially beneficial for pediatric and geriatric patients, as some individuals have difficulty swallowing conventional solid oral doses. Additionally, the buccal route is advantageous for those drugs that become unstable in the gastrointestinal tract and induce nausea and vomiting (Krystal et al., 2019). Several types of oral dosage, including tablets and mucoadhesive films, oral disintegrating tablets, hydrogels, and rapidly dissolving films, are now available commercially.
Zaleplon (ZLP) has a high affinity for the gamma-aminobutyric acidA (GABAA) receptor (subunit α1) in the brain, stimulating GABA activity more selectively than benzodiazepines, which is a short-acting hypnotic used for sleep induction (Richter et al., 2020). ZLP significantly reduces the time required to fall asleep by improving sleep latency and may therefore facilitate sleep induction rather than sleep maintenance. ZLP is affected primarily by extensive hepatic first-pass metabolism, which results in its low bioavailability (30%) and a short elimination half-life (1 hour) (Dudhipala and Janga, 2017). This issue can be avoided by incorporating ZLP into a mucoadhesive disk for buccal delivery. Therefore, buccal delivery has always been an attractive option because it bypasses the devastating metabolism associated with other conventional oral routes, resulting in increased bioavailability (Singh et al., 2017). Extending the half-life of drugs with a short half-life reduces frequent dosing and, in this study, prevents early morning awakening without middle-of-the-night dosing. An increase in the dissolution rate of these drugs can also improve the oral bioavailability of these drugs, which improves both therapeutic effectiveness and patient compliance (Pagel et al., 2018).
The interest in developing novel buccal drug delivery systems has risen significantly in recent years. Numerous studies demonstrate that novel buccal formulations containing lidocaine have gained popularity, including mucoadhesive films (Eleftheriadis et al., 2020; Padula et al., 2013), bilayered tablets (Kottke et al., 2020), and several disks and patches (Abu-Huwaij et al., 2007; Pather et al., 2008; Yildir et al., 2018).
Mucoadhesive polymers that adhere to the gastric mucin or buccal mucosa are advantageous for drug administration because they increase the contact time of the drug with the absorbing membrane. Due to the questionable biodegradability of synthetic polymers, natural gums have been extensively explored as pharmaceutical excipients mainly for achieving mucoadhesion properties. Moringa gum is a natural gum derived from the Moringa oleifera plant, belonging to the Moringaceae family. Moringa oleifera gum contains L-arabinose, D-galactose, D-glucuronic acid, L-rhamnose, D-mannose, and D-xylose in the mole ratio of 14.5 : 11.3 : 3 : 2 : 1 : 1, respectively. The generalized primary structure of M. oleifera gum is shown in Figure 1, in line with several studies (Badwaik et al., 2020; Grewal et al., 2019).
The gum has been studied for its potential use in pharmaceuticals as a binder, disintegrant, and release retardant (Panda, 2014). Moringa gum’s side chains contain hydroxyl groups that interact with mucus, enhancing its mucoadhesive properties. Moringa gum has already been grafted with N-vinyl-2-pyrrolidone to improve its bioadhesion (Rimpy et al., 2017). The current study is the first to report Moringa gum grafting to improve its mucoadhesive properties for buccal administration. The mucoadhesive property of the modified Moringa gum was compared to that of the unmodified Moringa gum using buccal disks containing ZLP as the test drug.
This study aims to formulate a ZLP mucoadhesive buccal disk that would allow for pulsatile release via the buccal route, while avoiding extensive hepatic metabolism, increasing bioavailability, and avoiding early morning awakening. A review of the literature reveals that no previous experiments involving the development of a ZLP mucoadhesive disk have been carried out (Di Benedetto et al., 2020).
MATERIALS AND METHODS
Materials
ZLP was an ex gratis gift sample from Unichem Laboratories Ltd., Goa, India. Moringa gum was purchased from Yarrow Chem Products, Mumbai, India. Acrylamide and ceric ammonium nitrate (CAN) were purchased from VS Corporation, Chandigarh, India. Other ingredients, such as ethanol, di-calcium phosphate, PEG-4000, lactose, hydroxy propyl methyl cellulose (HPMC) K15, microcrystalline cellulose, magnesium stearate, acetone, as well as methanol, were purchased from Loba Chemie Pvt. Ltd., Mumbai, India.
Methodology
Characterization of ZLP
Drug identification by Fourier transformed infrared (FTIR)
The comparison between the obtained drug sample’s FTIR spectrum with the standard FTIR spectrum of the pure drug and excipients used was performed. The ingredients were analyzed for the interactions and compared with the reported literature (Ahuja et al., 2010).
Differential scanning calorimeter (DSC) study of the drug
The drug’s thermograms were determined by using a DSC (Perkin Elmer). In a standard aluminum pan, a few mg of the finely powdered sample was sealed and heated at 10°C/minute between 40°C and 350°C. The experiment was carried out in a nitrogen environment, with a flow rate of 50 ml/minute (Ahuja et al., 2010).
X-ray diffractometer (XRD) of the drug
Powder XRD studies were used to characterize the drug’s solid state. The diffraction pattern was traced using an XRD (Xpert PRO) at room temperature from 0° to 80° (2?) diffraction angle with a scanning speed of 0.05 minutes−1 (Ahuja et al., 2010).
Scanning electron microscopy (SEM) of the drug
SEM (ZEISS, EVO 18, China) was used to examine the drug’s surface morphology. The powdered sample, under a high vacuum, was mounted on double-sided adhesive carbon tape and sputter-coated with a thin film of gold. At various magnifications, the photomicrographs were taken with a 20 kV accelerating voltage (Ahuja et al., 2010).
Modification of Moringa gum by the grafting technique
Microwave-assisted synthesis of polyacrylamide grafted Moringa gum using CAN as a free radical initiator
1 g of Moringa gum was dissolved in 40 ml distilled water. In 10 ml distilled water, an appropriate amount (5 ml) of acrylamide was dissolved and added to the above solution. They were thoroughly mixed before being transferred to the reaction vessel (250 ml beaker), where a catalytic amount of CAN (0.1 g w/w) was added. The reaction vessel was placed on the turntable of a domestic microwave oven (Samsung, MW71E, India), and microwave irradiation was performed at 800 W power. The microwave irradiation was stopped at one set of boiling (~65°C), and cooled by immersing the reaction vessel in cold water on a regular basis (Salaheldeen et al., 2014).
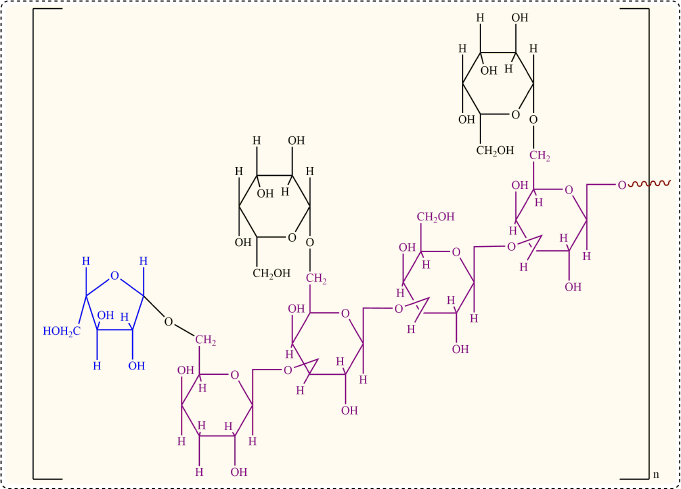 | Figure 1. Chemical structure of M. oleifera gum. [Click here to view] |
The microwave irradiation cooling cycle was repeated until a gel-like mass was formed, or until a total of 3 minutes of irradiation time was used (in the absence of gelling). The reaction vessel and its contents were then cooled and left alone to allow the grafting reaction to complete. Later, an excess of acetone (30% v/v) was poured into the gel-like mass in the reaction vessel (Duan et al., 2019). The graft copolymer precipitate was collected, dried at 40°C, pulverized, and sieved with a #100 sieve (Kokilambigai et al., 2017; Shi et al., 2019; Singh et al., 2017). This was followed by purification (Panda, 2014).
The grafting efficiency (percentage GE) of this microwave-assisted synthesized grafted Moringa gum was determined as follows:
Characterization of pure Moringa gum and modified Moringa gum
The swelling index (Grewal et al., 2019), total ash, angle of repose (AR) (Elkhodairy et al., 2014), bulk and tap densities, Hausner’s ratio (HR) (Elkhodairy et al., 2014), Carr’s compressibility index (Kumar and Rai, 2012), infrared spectral analysis (Singh and Kumar, 2018), DSC analysis (Rimpy et al., 2017), XRD (Rimpy et al., 2017), and SEM (Singh and Kumar, 2018) parameters of pure and modified Moringa gum were evaluated.
Swelling index
1 g of gum was blended with 96% ethanol (sufficient to damp the gum) and then 25 ml of purified water was added into a graduated cylindrical cylinder. The solution was shaken 1 hour per hand per 10 minutes and allowed to stand for 5 hours. The volume of the enlarged gum was then evaluated (Grewal et al., 2019). The swelling index was represented as 1 g of the prescription after swelling, as volume in ml:
where V1 = the initial volume of material before hydration and V2 = the final volume of hydrated material.
Total ash
The ash concentration was obtained after combustion in a furnace at 450°C to measure the remaining residue. The ash was boiled for 5 minutes using 25 ml 2M hydrochloric acid solution, and the insoluble material was filtered, cleaned, and ignited by hot water and the resulting weight was established. We then measured the proportion of insoluble acid ash (Elkhodairy et al., 2014).
Angle of repose
The static rest angle “REAT” was calculated by a set funnel and standing cone system. A funnel was tightened on a flat plane with its tip 2 cm over a graphical paper. The powders were slowly pumped into the funnel before the cone apex reached the edge of the funnel (134) (Elkhodairy et al., 2014). The median diameters of the base of the powder cones were determined and the tangent of the rest angle using the equation was measured as follows:
θ = tan–1 h/r
where θ = AR, h = height of granules, and r = radius of granules.
Bulk and tap densities
2 g was put in a 10 ml measuring cylinder for each of the powder samples and the volume, Vb, was observed without taping each of the samples. The filled volume, Vt, was read after 100 taps on the table. The bulk and tap densities were determined by the following equations according to the ratio of weight and volume (Vb and Vt, respectively) (Elkhodairy et al., 2014):
Bulk density (BD) = M/Vb
Tapped density (TD) = M/Vt
where M = weight of powder, Vb = volume occupied by powder without tapping, and Vt = volume occupied by powder after tapping.
Hausner’s ratio
HR is an indirect powder flow facility table. The ratio of density to mass density was determined according to the following equation of the specimens (134–135) (Elkhodairy et al., 2014):
Hausner’s ratio = TD/ BD
Carr’s compressibility index
Carr’s compressibility index specified the compression index of the powder blend. It is the straightforward measure for the measurement of the powder BD and TD and the rate it is packed (Kumar and Rai, 2012). The equation was determined as follows:
Carr’s index = TD – BD/ TD ×100
Infrared spectral analysis of pure Moringa gum and modified Moringa gum
The Moringa gum and gum powder samples were triturated using potassium bromide agate and morter with an IR (IR Hydraulic Press CAP-15T, PCI Analytics, Mumbai, India) pressing at an output of 75 kg/cm2 per 30 seconds. At 75 kg/cm2, the mixture was compressed into dishes. In FTIR (IR affinity I, Shimadzu) in the range of 4,000–400 cm−1 was registered using the FTIR disk spectrum (Singh and Kumar, 2018).
DSC analysis of pure Moringa gum and modified Moringa gum
Moringa gum and grafted Moringa gum thermograms were obtained with a differential calorimeter scanning (Q10, TA systems, USA). A small amount (mg) of fine powder of the sample was sifted into a regular aluminum pan and heated at a rate of 40°C–350°C at 10°C/minute. The inert nitrogen atmosphere (50 ml/minute) analysis was carried out.
XRD of pure Moringa gum and modified Moringa gum
For the solid characterization of Moringa gum and grafted Moringa gum, powder XRD experiments were carried out. The diffractometer configuration was traced from 0°C to 80°C (2 pounds) with a 0.05 minutes to 1.1 scanning speed at room temperature by using XRDs (Miniflex II, Rigaku, Japan) (Rimpy et al., 2017).
SEM of pure Moringa gum and modified Moringa gum
The Moringa gum and grafted Moringa gum surface morphologies were analyzed by SEM (ZEISS, EVO 18, China). The powdered sample was fixed on a double-sided adhesive carbon tape set on a holder and covered in a thin, high-vacuum gold film. At a various magnifications, the photomicrographs had a 20 kV acceleration voltage (Singh and Kumar, 2018).
Formulation of ZLP loaded-buccal disks of Moringa gum and grafted Moringa gum
Buccal disks were made in six batches, each with a different amount of gum or gum copolymer and lactose. To summarize, Moringa gum or grafted Moringa gum (60 mg w/w), ZLP (10 mg w/w), PEG-4000 (25 mg w/w), lactose (45 mg w/w), dicalcium phosphate (45 mg w/w), HPMC K15M (15 mg w/w), microcrystalline cellulose (MCC) (45 mg w/w), talc (1.5 mg w/w), and magnesium stearate (3.5 mg w/w) were homogeneously mixed with the aid of a mortar and pestle, followed by compression in an IR hydraulic press (Hydraulic Press, CAP-15T) under a pressure of 50 kg/cm2 for a dwelling time of 30 seconds (Table 1).
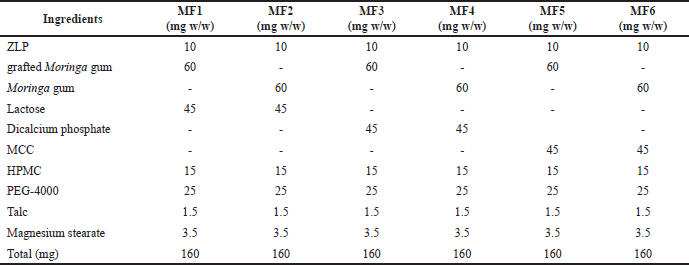 | Table 1. Composition of different batches of ZLP-loaded buccal disks. [Click here to view] |
Evaluation of ZLP loaded-buccal disks
For evaluating ZLP loaded-buccal disks, several physical parameters include weight variation test for uniformity (Jangra et al., 2013; Li and Castillo, 2020; Rani et al., 2012), hardness test for crushing strength, friability test for durability, thickness test, and a percentage drug content for the tablet was performed.
Weight variation test
Ten disks were selected and weighed individually. Then the average weight and standard deviation (SD) was calculated. The test passes when not more than two disks deviate from average weight (Jangra et al., 2013; Li and Castillo, 2020; Rani et al., 2012).
Hardness
Hardness or crushing strength is the force required to break a disk in a diametric compression which was measured using Monsanto disks hardness tester. It is expressed in kg/cm2 (Koirala et al., 2021).
Disk friability
A preweighed sample (W1) of 10 buccal disks was placed in the drum of a disk friability test apparatus (Remi Equipment’s, Mumbai, India). The drum was rotated at 25 rpm for 4 minutes and the buccal disks were reweighed (W2) (Koirala et al., 2021). The (%) friability was calculated using the following equation:
where
W1 = Preweighed sample of buccal disks; and
W2= Reweighed sample of buccal disks.
Thickness
Ten randomly selected buccal disks were subjected to thickness testing by using the digital Vernier Caliper (YAMAYO digital caliper). Values are reported as mean ± SD (Koirala et al., 2021).
Percentage drug content
Drug content was determined by dissolving 1 disk (equivalent to 10 mg of drug) in methanol and kept in an ultrasonicator for 20 minutes. The volume was adjusted to 100 ml with distilled water. The solution was filtered through Whatman filter paper no. 41, suitably diluted, and absorbance was measured at 254 nm using a UV spectrophotometer (Dhiman et al., 2022).
Several additional parameters are discussed below
Mucoadhesive strength
The mucoadhesive strength of the formulated disks was determined with a texture analyzer by recording the force needed to separate the disks from freshly excised goat’s gastric mucosal tissue (Rathee et al., 2011). In a nutshell, gastric mucosal tissues from a goat were obtained from a local slaughterhouse and used right away. A cyanoacrylate adhesive tape fixed the test disks to the cylindrical probe (diameter 10 mm). The cylindrical probe (with attached tablet) was lowered down (speed maintained 0.5 mm/second) for making the contact of test disks and mucosal tissue. A force (1 N) was applied to the probe for a specified time (60 seconds). The probe was then moved upward (15 mm distance) with 0.5 mm/seccond speed. The mucoadhesive strength was obtained, i.e., the maximum force was required to separate the disks from the mucosal surface (Shaikh et al., 2011).
Mucoadhesion time
The adhesion of the formulated disks was carried out by determining the mucoadhesion time to the goat’s gastric mucosa (Reddy et al., 2011). The gastric mucosal tissue was first pasted using cyanoacrylate tape onto a slide (glass). On the surface of the test disks, a drop (pH 6.8) was poured, making the disks wet so that they could adhere to the mucosal tissue. A beaker buffer medium of pH 6.8 (200 ml) was filled, and the temperature was maintained to 37°C ± 0.5°C. The slide with the pasted disks on mucosal tissue was kept inside the beaker. The stirrer was allowed to rotate at 50 rpm and the time required for the disks to detach from the goat’s gastric mucosal tissue was recorded as mucoadhesion time (Shaikh et al., 2011).
In-vitro swelling percentage (SP) of tablet formulation in (pH 6.8)
In a petri dish, individual disks were soaked in buffer solutions (pH 6.8) after weighing. After the specified time (2, 4, 6, 8, 12, and 24 hours), the disks (swollen) were removed and dried superficially using blotting paper. The weight of the swollen tablet was then noted, and the SP calculation was done using the following formula:
where wf is the final weight of the sample after swelling and wi is the initial weight of the sample.
Also, the physical observation of the disk’s swelling was recorded by measuring the diameter of the disks using graph paper stuck to the base of a petri dish (Grewal et al., 2019).
In-vitro drug dissolution studies
Dissolution studies of various mixtures and marketed formulations (control) were carried out in 900 ml of phosphate buffer (pH 6.8) at 37°C ± 5°C and a stirrer rotation speed of 50 rpm using a United States Pharmacopoeia dissolution apparatus equipped with a paddle stirrer (Type II). At 1, 2, 4, 6, 8, 10, and 12 hours, a 5ml aliquot of dissolution medium was removed and replaced with the same amount of buffer using a pipette. The samples were appropriately prepared and analyzed spectrophotometrically at a wavelength of 254 nm (Jangra et al., 2013; Rani et al., 2012).
Mathematical models for drug release
The cumulative amount of ZLP released from formulated disks at various time intervals was fitted to zero-order kinetics, first-order kinetics, the Higuchi model, the Hixon–Crowel model, and the Korsmeyer–Peppas model to characterize the mechanism of drug release.
Data analysis
The results of the one-way analysis of variance (ANOVA) (Sigma Stat 3.5 software) were analyzed using the student’s t-test. The difference was considered statistically significant if it was less than the probability level of 0.05.
Stability studies for the optimized batch
For the determination of the effect of temperature, humidity, moisture, and light on the drug’s quality, stability studies were carried out. These findings are used to determine the storage conditions, the test period, and shelf life (Li and Castillo, 2020). The optimized formulation (F3) was stored in a stability chamber at 40°C ±2°C and 75% ± 5% relative humidity for a period of 1 month. The samples were analyzed for various parameters after 1 month by spectrophotometer at 254 nm.
RESULTS AND DISCUSSION
Characterization of the drug
Drug identification by FTIR
The IR spectrum of the drug clearly shows the peaks of the various functional groups present (Ahuja et al., 2010). The IR spectrum exhibits characteristic bands of the stretching vibration of C-H stretch vibrations at 3,087.7 cm−1 and 2,933.7 cm−1. It also exhibits characteristic bands of stretch vibrations of –C=N (alcohol) at 1,614.4 cm−1 and 1,223.5 cm−1 for C-N. It shows stretch bands of –C=C- (aromatic) at 1,576.3 cm−1. ZLP exhibits strong absorption peaks at 2,232.7 cm−1 and 1,651 cm−1 suggesting the existence of cyanide and amide carbonyl groups, respectively. The IR spectrum of a pure drug (ZLP) obtained is shown in Figure 2 (Singh and Kumar, 2018).
Differential scanning calorimeter
In the case of pure drug, a sharp endotherm was observed at 184.94°C (Fig. 3) (Rimpy et al., 2017). The observed peak was compared to the drug’s standard peak, which indicates the purity of the drug.
SEM studies of the pure drug (ZLP)
The drug particles show a spherical shape, smooth surface, and crystalline geometry as observed both at 2,000 and 8,000× (Fig. 4) (Rimpy et al., 2017).
XRD of the drug
From the diffractogram of the drug, sharp peaks can be observed at 2θ = 10, 14, 16, 18, 21, 22, 25, 26, and 29, which show its crystalline nature in the pure form shown in (Fig. 5) (Ahuja et al., 2010). This shows that the drug, which was in crystalline form, has reduced to an amorphous form.
Modification of Moringa gum by grafting technique: synthesis of grafted Moringa gum
The properties of Moringa gum were modified via microwave-assisted graft copolymerization of acrylamide onto Moringa gum (Elkhodairy et al., 2014). Moringa gum is composed of galactose (25.9%), rhamnose (5.6%), and traces of uronic acid with pendent hydroxyl groups. When polysaccharides are microwave irradiated, the pendant hydroxyl groups exhibit localized rotation, resulting in dielectric heating and increasing the reaction rates at these groups (Elkhodairy et al., 2014). Additionally, microwave irradiation decreases the activation Gibbs energy of the reactions, resulting in faster reaction rates. Additionally, CAN, which was used as a free radical initiator in the reaction, generates free radicals that abstract hydrogen atoms from polysaccharides molecules and generate macro radicals (Elkhodairy et al., 2014). These free radicals then reacted with the acrylamide monomer to form acrylamide free radicals, initiating a chain reaction that results in the formation of Moringa gum-g-poly (acrylamide) and poly(acrylamide) homopolymers.
Graft copolymer synthesis is a combination of microwave-assisted and conventional methods, in that it is based on a free radical mechanism that uses microwave radiation in conjunction with a chemical-free radical initiator (CAN) to create extra radical sites on the Moringa gum backbone. By varying the concentrations of CAN and acrylamide (monomer), different grades of the graft copolymer were formed. In each case, the reaction mixture was microwave irradiated until it solidified into a viscous gel-like mass. The optimized grade was determined by its higher grafting percentage. When the microwave power is kept at 800 W, the grafting is optimized with a 5 g acrylamide and a 0.1 g CAN in the reaction mixture (~50 ml). The optimal batch with a concentration of CAN is an electron-deficient molecule. As a result, it borrows electrons from Moringa gum’s alcoholic oxygen to form a new bond. The optimal calculated parameters were 1.0 g Moringa gum, 0.1 g CAN, and 5.0 g acrylamide, which resulted in a graft copolymer with a GE of 65%. This bond is more polar (than the OH bond), and it breaks easily in the presence of microwave irradiation, forming a free radical site on the backbone of Moringa gum.
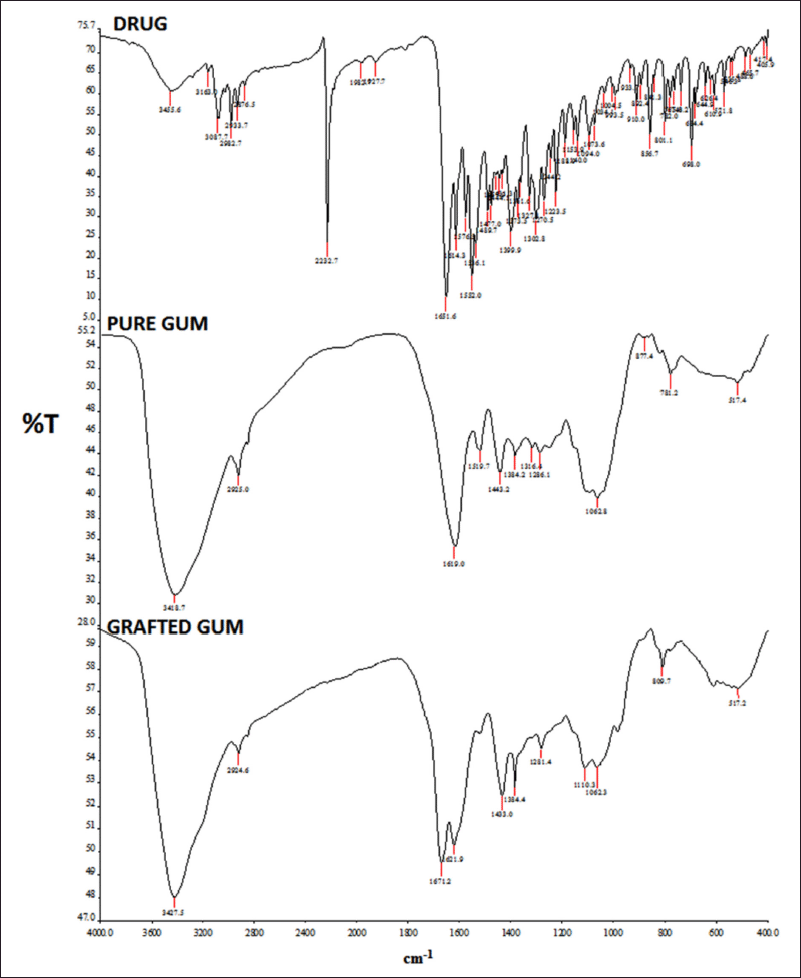 | Figure 2. FTIR spectrum of the drug (ZLP), pure gum, and grafted gum. [Click here to view] |
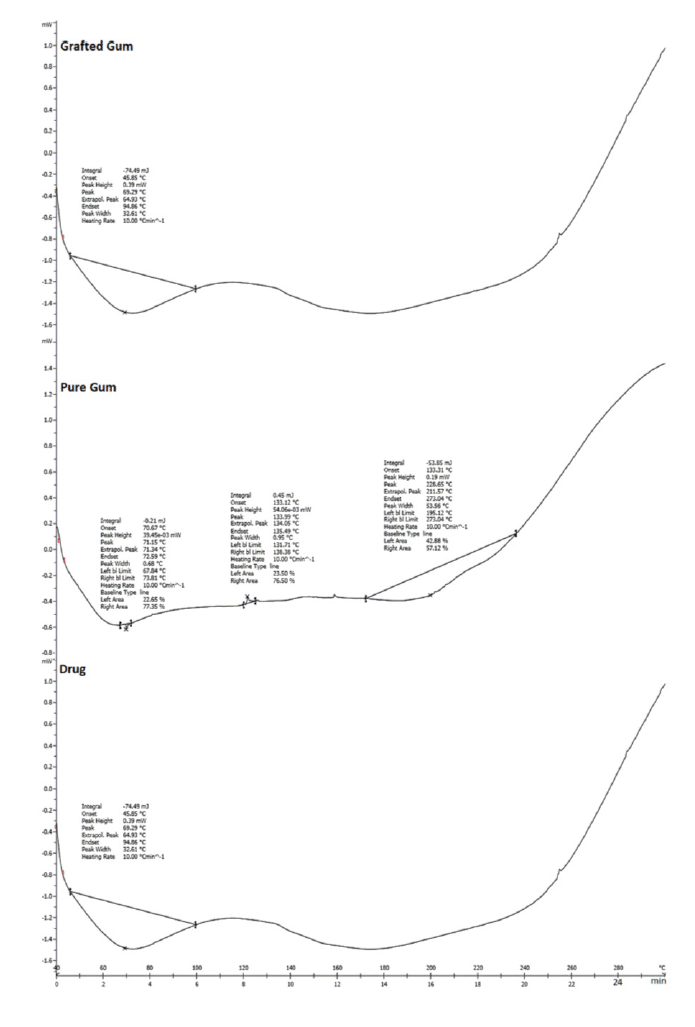 | Figure 3. DSC showing of drug (ZLP), pure gum, and grafted gum. [Click here to view] |
 | Figure 4. SEM images of pure drug showing shape and surface topology. (a) 2,000× and (b) 8,000×. [Click here to view] |
Physicochemical characterization of pure gum and modified Moringa gum
Swelling index
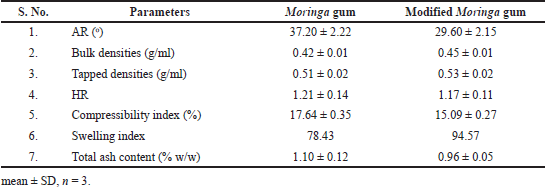
An optimized method was used to calculate the swelling index (Koirala et al., 2021). The grafted gum shows a marginally higher SP in phosphate buffer (pH 6.8) compared with pure Moringa gum (Kumar and Rai, 2012; Singh et al., 2017).
Powder flow properties
The BD, TD, AR, HR, and Carr’s index of pure gum as well as modified Moringa gum are shown in Table 2. Dried gum powder shows excellent flow properties and compressibility, making them suitable for direct compression tableting (Singh et al., 2017).
Ash content
The total ash content of pure Moringa gum and modified Moringa gum is presented in tabular form (Table 2).
PRECOMPRESSION PARAMETERS
HR was <1.13, indicating that all powder blends, i.e., MF1–MF6 have good flow properties. The compressibility index of powder blend MF1–MF6 was between 6% and 12%, indicating good flow properties. The various formulations showed an AR between 25°C and 30°C, which indicates that all formulation blends have good flow properties (Fig. 6).
Postcompression parameters
The weight variation, tablet thickness, hardness, friability, and drug content have all been evaluated and illustrated as postcompression parameters (Fig. 7). The mucoadhesion time and mucoadhesion strength are shown in Figure 8.
In line with a previous study (Grewal et al., 2019), the introduction of a new functional group by grafting resulted in enhanced charge density (anionic charge) on modified Moringa gum, making it susceptible to bind to the opposite charge present on the mucin layer, thus increasing the mucoadhesion strength and mucoaadhesive time. Compared to the formulation (MF2, MF4, and MF6) (6 hours), the formulations containing modified Moringa gum (MF1, MF3, and MF5) required a longer time (12 hours) for complete detachment. The possible reason might be that the modified Moringa gum has uronic acid molecules containing –COO− groups, while the mucosal mucin protein surface has –NH3+ groups, both carrying opposite charges.
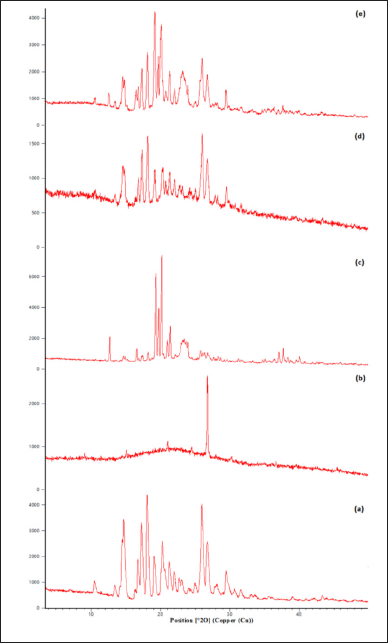 | Figure 5. XRD diffractogram showing (a) drug, (b) pure gum, (c) modified gum, (d) drug and pure gum physical mixture, and (e) drug and modified gum. [Click here to view] |
 | Table 2. Physicochemical characterization of pure gum and modified Moringa gum. [Click here to view] |
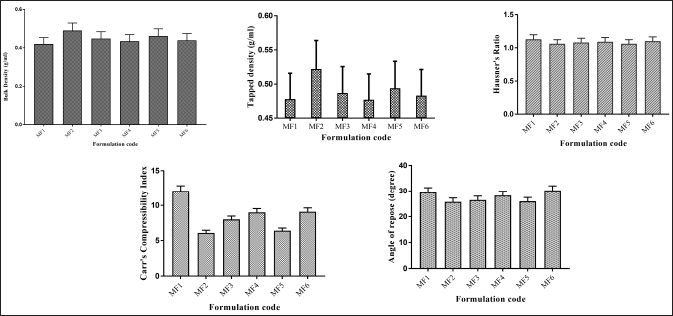 | Figure 6. Precompression parameters of ZLP buccal disks using Moringa gum and grafted Moringa gum. *(mean ± SD; n = 3). [Click here to view] |
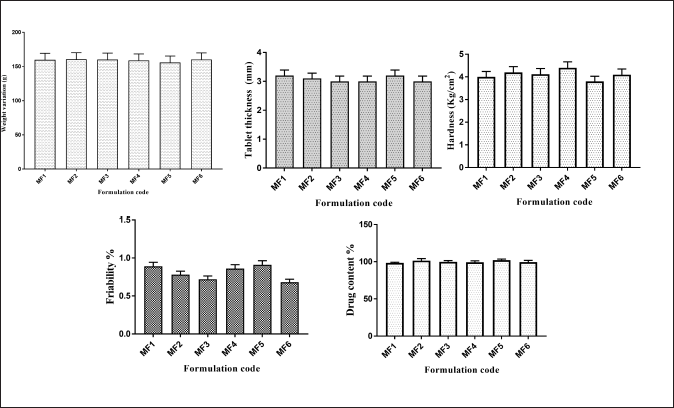 | Figure 7. Postcompression parameters of ZLP buccal disks using grafted Moringa gum. (mean ± SD; n = 3). [Click here to view] |
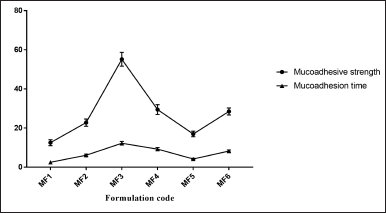 | Figure 8. Mucoadhesive strength and mucoadhesive time of ZLP buccal disks using grafted Moringa gum. a(mean ± SD; n = 3). [Click here to view] |
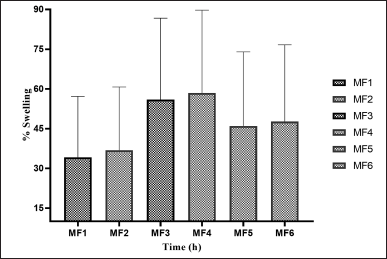 | Figure 9. SPs of ZLP buccal disk in pH 6.8 a(mean ± SD; n = 3). [Click here to view] |
In-vitro SP of buccal disks in (pH 6.8)
The weight gain and swelling were proportional. The SPs of ZLP buccal disks (pH 6.8) are shown in Figure 9.
In-vitro dissolution study
The in-vitro dissolution study of ZLP mucoadhesive buccal disk (MF1–MF6) with control (marketed formulation) is shown in Figure 10. The optimized batch (MF3) showed a drug release of 95.22% in 12 hours.
Drug release kinetics and mechanism
Data from ZLP buccal disks drug release kinetics fitted to zero order, first order, Korsmeyer–Peppas, Higuchi, and Hixon–Crowel release models are shown (Fig. 11). The “n” values obtained ranged from 0.54 to 0.80. The best-fitted model, as depicted in, was found to be the Korsmeyers–Peppas model. The mechanism involved in the Korsmeyers–Peppas model was found to be a combination of diffusion and erosion-based drug release.
ANOVA- analysis of variance
The analysis of variance indicated that swelling behavior, mucoadhesion strength, mucoadhesion time, and %CDR (12 hours) of ZLP-loaded buccal disks were found to be significant (p < 0.05) and adequate, without a significant lack of fit.
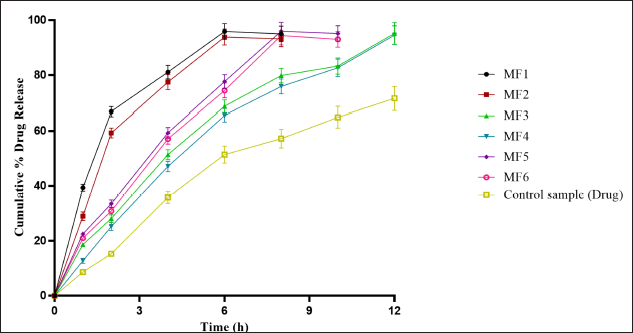 | Figure 10. In-vitro drug release data of drug-loaded formulation (MF1–MF6) a(mean ± SD; n = 3). [Click here to view] |
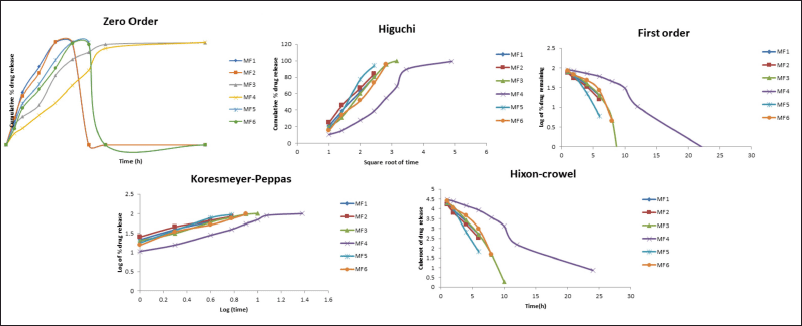 | Figure 11. Drug release model of ZLP buccal disks. [Click here to view] |
 | Table 3. Stability data of the optimized batch of ZLP buccal disks. [Click here to view] |
Stability study for optimized batch (MF3)
The accelerated stability studies’ results showed no appreciable modification observed in hardness, drug content, and cumulative percentage drug release in the stored tablets under specified conditions (Table 3) (Li and Castillo, 2020).
CONCLUSION
This study aimed to develop and evaluate ZLP buccal disks made of grafted Moringa gum polymer. Additionally, a modified polymer was used to enable controlled release of ZLP via the buccal route, avoiding extensive hepatic metabolism, increasing bioavailability, and avoiding early morning awakening without the use of a middle-of-the-night dose. Moringa gum was modified using graft copolymer synthesis. This method combines microwave and traditional methods of synthesis. It is based on the free radical mechanism. It utilizes microwave radiation with a radical initiator (CAN) to make free radical sites on the backbone of Moringa gum. ZLP buccal disks were developed to investigate the grafted Moringa gum further. The disks containing grafted Moringa gum demonstrated a longer mucoadhesion time when compared to unmodified Moringa gum. Based on the findings of this study, modified Moringa gum (optimum batch MF3) proved to have excellent potential as a mucoadhesive polymer that can be effectively used to formulate modified ZLP buccal drug delivery systems able to extend the release of the drug, thereby avoiding early hours awakening, improving sleep quality, and enhancing its bioavailability.
ACKNOWLEDGMENT
The authors are grateful to Chitkara University, Punjab, India, for support and institutional facilities.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
FUNDING
There is no funding to report.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abu-Huwaij R, Assaf S, Salem M, Sallam A. Potential mucoadhesive dosage form of lidocaine hydrochloride: II. In vitro and in vivo evaluation. Drug Dev Ind Pharm, 2007; 33(4):437–48. CrossRef
Ahuja M, Kumar S, Yadav M. Evaluation of mimosa seed mucilage as bucoadhesive polymer. Yakugaku Zasshi, 2010; 130(7):937–44. CrossRef
Badwaik HR, Hoque AA, Kumari L, Sakure K, Baghel M, Giri TK. Moringa gum and its modified form as a potential green polymer used in biomedical field. Carbohydr Polym, 2020; 249:116893. CrossRef
Di Benedetto M, Towt CJ, Jackson ML. A cluster analysis of sleep quality, self-care behaviors, and mental health risk in Australian university students. Behav Sleep Med, 2020; 18(3):309–20. CrossRef
Dhiman S, Babbar R, Singh TG, Anand S, Mannan A, Arora S. Development and characterization of oro-dispersible tablets of Metformin Hydrochloride using Cajanus cajan starch as a natural superdisintegrant: oro-dispersible and superdisintegrant. Int J Appl Pharm, 2022; 14(1):139–47. CrossRef
Duan M, Ma J, Fang S. Synthesis of hydrazine-grafted guar gum material for the highly effective removal of organic dyes. Carbohydr Polym, 2019; 211:308–14. CrossRef
Dudhipala N, Janga KY. Lipid nanoparticles of zaleplon for improved oral delivery by Box-Behnken design: optimization, in vitro and in vivo evaluation. Drug Dev Ind Pharm, 2017; 43(7):1205–14. CrossRef
Eleftheriadis GK, Monou PK, Bouropoulos N, Boetker J, Rantanen J, Jacobsen J, Vizirianakis IS, Fatouros DG. Fabrication of mucoadhesive buccal films for local administration of ketoprofen and lidocaine hydrochloride by combining fused deposition Modeling and inkjet printing. J Pharm Sci, 2020; 109(9):2757–66. CrossRef
Elkhodairy KA, Hassan MA, Afifi SA. Formulation and optimization of orodispersible tablets of flutamide. Saudi Pharm J, 2014; 22(1):53–61. CrossRef
Grewal P, Mundlia J, Ahuja M. Thiol modified Moringa gum–a potential bioadhesive polymer Carbohydr Polym, 2019; 209:400–8. CrossRef
Jangra S, Ahuja M, Kumar A. Evaluation of mucoadhesive property of gum ghatti. J Pharm Investig, 2013; 43(6):481–7. CrossRef
Koirala S, Nepal P, Ghimire G, Basnet R, Rawat I, Dahal A, Pandey J, Parajuli-Baral K. Formulation and evaluation of mucoadhesive buccal tablets of aceclofenac. Heliyon, 2021; 7(3):2405–8440. CrossRef
Kokilambigai KS, Seetharaman R, Kavitha J, Sowndaravel P, Lakshmi KS. Multivariate calibration technique for the spectrophotometric quantification of zaleplon in bulk drug and pharmaceutical formulations. J Pharm Sci Res, 2017; 9(6):824.
Kottke D, Majid H, Breitkreutz J, Burckhardt BB. Development and evaluation of mucoadhesive buccal dosage forms of lidocaine hydrochloride by ex-vivo permeation studies. Int J Pharm, 2020; 581:119293. CrossRef
Krystal AD, Prather AA, Ashbrook LH. The assessment and management of insomnia: an update. World Psychiatry, 2019; 18(3):337–52. CrossRef
Kumar K, Rai AK. Development and evaluation of floating microspheres of curcumin. Trop J Pharm Res, 2012; 11(5):713–9. CrossRef
Levenson JC, Kay DB, Buysse DJ. The pathophysiology of insomnia. Chest, 2015; 147(4):1179–92. CrossRef
Li LL, Castillo AL. Formulation and evaluation of a mucoadhesive buccal tablet of mefenamic acid. Braz J Pharm Sci, 2020; 56:e18575. CrossRef
Padula C, Pozzetti L, Traversone V, Nicoli S, Santi P. In vitro evaluation of mucoadhesive films for gingival administration of lidocaine. AAPS PharmSciTech, 2013; 14(4):1279–83. CrossRef
Pagel JF, Pandi-Perumal SR, Monti JM. Treating insomnia with medications. Sleep Sci Pract, 2018; 2(1):1–12. CrossRef
Panda DS. Studies on gum of Moringa oleifera for its emulsifying properties. J Pharm Bioallied Sci, 2014; 6(2):92–6. CrossRef
Pather SI, Rathbone MJ, Senel S. Current status and the future of buccal drug delivery systems. Exp Opin Drug Del, 2008; 5(5):531–42. CrossRef
Rani P, Sen G, Mishra S, Jha U. Microwave assisted synthesis of polyacrylamide grafted gum ghatti and its application as flocculant. Carbohydr Polym, 2012; 89(1):275–281. CrossRef
Rathee P, Jain M, Garg A, Nanda A, Hooda A. Gastrointestinal mucoadhesive drug. Delivery system: a review. J Pharm Res, 2011; 4(5):1488–53.
Reddy PC, Chaitanya KS, Rao YM. A review on bioadhesive buccal drug delivery systems: current status offormulation and evaluation methods. DARU J Pharm Sci, 2011; 19(6):385.
Richter G, Liao V, Ahring PK, Chebib M. The Z-drugs zolpidem, zaleplon, and eszopiclone have varying actions on human GABA A receptors containing γ1, γ2, and γ3 subunits. Front Neurosci, 2020; 14:599812. CrossRef
Rimpy, Abhishek, Ahuja M. Evaluation of carboxymethyl Moringa gum as nanometric carrier. Carbohydr Polym, 2017; 174:896–903. CrossRef
Salaheldeen M, Aroua M, Mariod A, Cheng SF, Abdelrahman MA. An evaluation of Moringa peregrina seeds as a source for bio-fuel. Ind Crop Prod, 2014; 61:49–61. CrossRef
Shaikh R, Raj Singh T, Garland M, Woolfson A, Donnelly R. Mucoadhesive drug delivery systems. J Pharm Bioallied Sci, 2011; 3(1):89–100. CrossRef
Shi Z, Jia C, Wang D, Deng J, Xu G, Wu C, Dong M, Guo Z. Synthesis and characterization of porous tree gum grafted copolymer derived from Prunus cerasifera gum polysaccharide. Int J Biol Macromol, 2019; 133:964–70. CrossRef
Singh A, Mangla B, Sethi S, Kamboj S, Sharma R, Rana V. QbD based synthesis and characterization of polyacrylamide grafted corn fibre gum. Carbohydr Polym, 2017; 156:45–55. CrossRef
Singh B, Kumar A. Radiation-induced graft copolymerization of N?vinyl imidazole onto Moringa gum polysaccharide for making hydrogels for biomedical applications. Int J Biol Macromol, 2018; 120:1369–78. CrossRef
Yildir E, Sjöholm E, Preis M, Trivedi P, Trygg J, Fardim P, Sandler N. Investigation of dissolved cellulose in development of buccal discs for oromucosal drug delivery. Pharm Develop Echnol, 2018; 23(5):520–9. CrossRef