INTRODUCTION
Andrographis paniculata (Burm. f) Nees (Acanthaceae) is traditionally used for the treatment of skin diseases. This medicinal plant contains major bioactive components such as diterpenoids, flavonoids, and polyphenols (Xu et al., 2010). Andrographolide is the major diterpenoid in A. paniculata, making up about 0.5%–6% of the dried leaf extract (Chao et al., 2021; Loureiro Damasceno et al., 2021. Deoxyandrographolide, neoandrographolide, isoandrographolide, and 14-deoxy-11,12-didehydroandrographolide are the other main diterpenoids in this plant (Liu et al., 2020). Studies discovered that diterpene andrographolides induced significant stimulation of antibody production and delayed the hypersensitivity response toward sheep red blood cells immunized mice (Singh, 2016). The study suggested that other compounds besides andrographolides existing in the crude extracts might as well contribute to immunostimulation. The extract also shows potent cell differentiation activity in mouse myeloid leukemia cells (Bello et al., 2018; Pfisterer et al., 2010). Andrographolide, the main compound in the A. paniculata leaf extract, is reported to inhibit the inflammatory mediator, thus providing anti-inflammatory activity through its ability to suppress vasodilation (Rahmi et al., 2022). Andrographolide is helpful in skin problems, such as dermatitis, skin irritation, skin redness, dry skin, and rashes (Hossain et al., 2021). The antimicrobial property of andrographolide is beneficial for oily skin treatment. Other than inhibiting the production of nitric oxide, andrographolide is able to reduce endothelin levels (Lin et al., 2017; Shu et al., 2020). Endothelin is involved in a number of processes, including the production of melanin. These findings suggested that andrographolide from A. paniculata might have the ability to modulate the melanogenesis mechanism by elevating or preventing the tyrosinase (TYR) oxidation process. In addition, no study regarding the A. paniculata leaf extract particularly focuses on melanogenesis activity. Therefore, this study believed that the A. paniculata leaf extract might have the potential to be used as an ingredient in cosmeceutical products to treat skin problems. The A. paniculata leaf extract has been used in various cosmetic products as an ingredient. Nevertheless, there are no scientific reports on the whitening or lightening effect of the A. paniculata leaf extract. Studies reported melanin production can be activated by reactive oxygen species (ROS) (Denat et al., 2014; Kami?ski et al., 2022). Based on this finding, this study was used to evaluate the potential of the antimelanogenic property of the A. paniculata leaf extract. The function of epidermal melanocytes of the skin is melanin synthesis. The main key to melanin production is protecting the skin from UVA and UVB radiation. However, exposure to continuous UV radiation results in constant melanin production. This accumulation of melanin will lead to hyperpigmentation skin problems (Lee, 2021).
α-Melanocyte-stimulating hormone (α-MSH) is a well-known melanogenesis inducer (Singh and Mukhopadhyay, 2014). The expression of microphthalmia-associated transcription factor (MITF) will be enhanced and induced by α-MSH binding to melanocortin 1 receptor (Oh et al., 2022). Consequently, cyclic AMP will act and elevate the expression of TRY melanogenesis-related enzymes such as tyrosinase-related protein-1 (TRP-1) and tyrosinase-related protein-2 (TRP-2). Hyperpigmentation problems relate to nonesthetically pleasing issues, such as freckles, melasma, and age spots (dos santos Videira et al., 2013). The essential enzymes in melanin regulation are TYR and TRP-1 and TRP-2 (Xue et al., 2018). The main role of TYR in melanin synthesis is being a copper-containing glycoprotein (Pillaiyar et al., 2017).
Jeon et al. (2018) reported that TYR incorporates in the catalysis of four different reactions. Firstly, tyrosine is hydroxylated to 3,4-dihydroxyphenylalanine (DOPA). Secondly, DOPA is oxidized into dopaquinone. Thirdly, dopaquinone oxidizes into dopachrome. Finally, dopachrome develops as either indole 5,6-quinone-2-carboxylic acid dihydroindolizine carboxylic acid (DHICA) or dihydroindolizine. Meanwhile, TRP-1 and TRP-2 play important roles in melanogenesis synthesis.
The catalysis of DHICA oxidation is done by TRP-1 and simultaneously TRP-2 responsible on catalyzation of dopachrome converted to DHICA (Sato et al., 2016). A specific transcription factor, MITF, is responsible for the regulation of melanogenesis enzymes (Kawakami and Fisher, 2017). MITF is important in the activation of diverse signaling pathways and is controlled by mitogen-activated protein kinases (MAPKs) (Cargnello and Roux, 2011; Munshi and Ramesh, 2013). MAPKs are constituted of protein serine/threonine kinases. They mostly participate in the signal transduction pathway, promoting cellular growth activities, such as differentiation and proliferation (Hu Frisk et al., 2018; Ngeow et al., 2018). The main three known characterized subfamilies contained in the MAPK superfamily are extracellular signal-regulated protein kinases (ESRPKs), phosphorylation of p38 MAPK, and c-Jun N-terminal kinases (Olea-Flores et al., 2019). The essential role, especially in melanogenesis regulation, is played by MAPKs (Sun et al., 2020).
Furthermore, ESRPK activation is done at the 73rd serine residue by c-Kit simulation that phosphorylates MITF. MITF ubiquitination and degradation are accompanied by MITF phosphorylation at the 73rd serine (Paruchuru et al., 2022).
Moreover, the activation of p38 MAPK can escalate the synthesis of melanin (Sun et al., 2020). Melanogenesis inhibition is correlated with TYR activity downregulation by ESRPK signaling activation (Song et al., 2015). There are some well-known melanogenesis inhibition agents used, such as arbutin, linoleic acid, and kojic acid.
Arbutin is a glycosylated hydroquinone known as glycoside (Pop et al., 2009). It is commonly based on a bearberry plant extract. It has been widely used for the treatment of pigmentation problems, such as melasma and spots. Arbutin and kojic acid are greatly used as cosmetic constituents based on TYR inhibition activity (Pillaiyar et al., 2017).
Nonetheless, an adverse reaction is the main concern in using these agents. Those with overly sensitive skin may suffer from redness and irritation. Kojic acid was reported to be able to cause irritation to the skin and cause serious skin problems, such as dermatitis and skin cancer (Chang et al., 2010). Exogenous ochronosis and perdurable depigmentation were reported after long-term use of arbutin (Bhattar et al., 2015; Sunkara et al., 2020).
Consequently, for the best interest of the public use of these agents, a new future ingredient should be found and extracted from natural-based ingredients or plants, which importantly will cause no harm or side effects on the skin.
The current study focused on the investigation of finding a potential melanogenesis inhibitor compound from this medicinal plant. In our study, we discovered the antimelanogenesis effect of the A. paniculata leaf extract and andrographolide on α-MSH-induced melanogenesis in B16F1 mouse melanoma cells. Molecular mechanisms involved in this process were also discovered in this study. Many previous studies on the A. paniculata leaf extract have mainly determined its antimicrobial, anti-inflammatory, and antioxidant activities.
Nevertheless, in this current research, we conducted a study on melanogenesis using the A. paniculata leaf extract. The results determined from this study will be applied as primary research evidence and can be used in the development of cosmetic ingredients.
MATERIALS AND METHODS
Materials and reagents
The rabbit polyclonal MITF antibody, rabbit polyclonal TYR antibody, and rabbit polyclonal TRP-2 antibody were purchased from Cusabio (Houston, TX, USA). The Folin–Ciocalteu reagent, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma,88,415), 100% TritonTM X-100 (Sigma, T8,787), 3,4-dihydroxy-L-phenylalanine (L-DOPA) (Sigma, D9,628), kojic acid, α-MSH, and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (USA). Dulbecco’s modified eagle medium (DMEM), penicillin–streptomycin, and fetal bovine serum (FBS) were supplied by Invitrogen (Thermo Fisher Scientific, Waltham, MA). Sodium carbonate was obtained from Fisher Scientific (Pittsburgh, PA). High Performance Liquid Chromatography -grade methanol (MeOH) and 95% ethanol were purchased from QRëC Reagent Chemical. Purified water was prepared using a Barnstead E-Pure apparatus. The single-use syringe filter (0.45and 0.2 μm) was purchased from Sartorius, Malaysia. Andrographis paniculata dried and ground was purchased from Herba Bagus Sdn. Bhd., Johor, Malaysia. The dried leaves were cooled in cold storage to prevent microbial growth.
Andrographis paniculata leaf extraction
The dried leaves (200 g) of A. paniculata were deposited at Forest Research Institute Malaysia, Kuala Lumpur, Malaysia (SBID 002/12). The A. paniculata plant was cultivated in a climate-controlled area and air-dried at room temperature. The dried leaves were ground into powder form and passed through a 30-mesh sieve. Extraction was conducted using DionexTM ASETM 100 (Thermo Fisher, Waltham, MA). Briefly, 3 g of the sample was mixed with diatomite to remove the remaining moisture before extraction. The obtained sample was then packed into a 10 ml stainless steel vessel extracted with ethanol (99%) at 1,500 psi (10 MPa). The extraction was performed under extraction conditions at a temperature of 60°C under a cycle number of 3 for 5 minutes extraction time. Upon completion, the excess solvent was evaporated using a rotary evaporator (Heidolph MX07R−20, PolyScience, USA), and the extraction liquid sample was oven-dried to a constant weight. The extracts were dissolved in DMSO for further experiments.
Cell culture
The B16F1 melanoma cell line was preserved in DMEM supplemented with 10% FBS and 1% penicillin–streptomycin. The cells were incubated at 37°C in a 5% CO2 incubator.
α-MSH and A. paniculata leaf extract treatment
The B16F1 melanoma cells were seeded in six-well plates at a density of 1.5 × 106 cells per well. The wells were supplemented with FBS (10%) and penicillin–streptomycin (1%) in DMEM. The medium was replaced with a fresh medium after 24 hours incubation. This medium was boosted with α-MSH of a 5 nM concentration, and the A. paniculata leaf extract at different concentrations (100, 50, 25, 12.5, 6.25, and 3.13 μg/ml) was prepared using this medium. The treatment was done under incubation for about 48 hours. The DMEM solution only was used as the negative control in this treatment. The positive controls, α-MSH (5 nM), arbutin (2 mM), and kojic acid (800 μM), were used comparably with the treatment of the A. paniculata leaf extract.
Cell viability assay
Cell viability using the MTT colorimeter assay as described by Hamid et al. (2012) was followed with minor modifications to determine the viability of the B16F1 melanoma cells using the A. paniculata leaf extract. After incubation for 48 hours, the medium was replaced by the MTT solution and incubated for 90 minutes at 37°C. Then, the solution was substituted with an isopropyl alcohol hydrochloride solution and incubated for 30 minutes at room temperature. The solution was collected and centrifuged for 5 minutes at 13,000 rpm. The supernatant solution was harvested, and the absorbance was determined using an ELISA plate reader at 570 nm. The values were analyzed and compared with the control cells.
Mushroom TYR inhibition assay
An in vitro mushroom TYR inhibition assay was carried out as previously described by Qiao et al. (2012) and Han et al. (2015), with moderate modifications. About 140 μl of the A. paniculata leaf extract at a proper concentration was made. A sodium phosphate buffer (pH 6.8) at 10 mM was used in sample preparation and was transferred to a 96-well plate. Approximately 40 μl was poured into each well of 10 μg/ml mushroom TYR in the phosphate buffer at a concentration of 10 mM. The 96 wells were then incubated for about 10 minutes at room temperature. The sample was then added with approximately 20 μl of 10 mM L-DOPA in the phosphate buffer at a concentration of 10 mM. The sample in the 96 wells was incubated for 30 minutes. Absorbance reading was determined using a microplate reader (PerkinElmer, Waltham, MA) at 405 nm. The activity of TYR (%) was determined using
where A is the absorbance reading of the sodium phosphate buffer for the untreated mushroom TYR with each sample, B is the absorbance reading of the sodium phosphate buffer for the untreated mushroom TYR with no sample, and C is the absorbance reading of the sodium phosphate buffer for the treated mushroom TYR with each sample.
The calibration curve’s linearity range of kojic acid was prepared between 0.00 and 90.00 μg/ml (Figure S1). All absorbance values were measured at 405 nm. Estimating the IC50 values for mushroom TYR inhibition (%) of the A. paniculata leaf extract used the nonlinear regression plot derived from the plotted data using GraphPad Prism version 9.0.0 for Windows, GraphPad software.
Secreted melanin assay
The secreted melanin assay was carried out as previously described by Kim et al. (2016), with slight modifications, to determine the melanin secretion for the A. paniculata leaf extract on the B16F1 melanoma cells. The medium of the culture solution was collected and centrifuged for 10 minutes at 10,000 rpm. The absorbance was determined using a microplate reader (PerkinElmer) at 405 nm.
The activity of the secreted melanin (%) was determined using
where A is the absorbance reading prior to incubation for the medium supplemented with each sample, B is the absorbance reading after 48 hours of incubation for the untreated medium with each sample, and C is the absorbance reading after 48 hours of incubation for the medium supplemented with each sample.
Intracellular melanin assay
The intracellular melanin assay was carried out as previously reported by Oh et al. (2011), with slight modifications. After 48 hours of cell culturing, the cells were harvested and washed twice with potassium-buffered saline (PBS) and lysed with 200 μL of trypsin. Cell pellets were obtained from centrifugation at 1,000 rpm for 10 minutes and lysed with 1 ml of a water: 1N NaOH: DMSO = 7:2:1 solution for 24 hours at 37°C to solubilize the melanin, and 200 μl portions of cell fractions were transferred to 96-well plates. The absorbance of the supernatant was determined by using an ELISA plate reader at 405 nm.
DOPA staining (TYR zymography)
The L-DOPA staining, TYR zymography, assay was performed after 48 hours of cell incubation as previously reported by Lin et al. (2011) and Di Petrillo et al. (2016), with slight modifications. PBS was used to wash the cultured cells. The radioimmunoprecipitation assay (RIPA) lysis buffer was supplemented with an inhibitor of protease and used to harvest the cultured cells. Similar to the intracellular TYR assay, the amount of protein was also equilibrated in this analysis. Every sample was homogenized using a zymogram sample buffer. Each sample was filled into an 8% gel of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Next, the clear gel was then immersed two times for about 30 minutes in a sodium phosphate buffer at a concentration of 0.1 M. Then, the gel stained in the buffer was added with 0.1 M L-DOPA at 37°C for 1 hour. The dark melanin-containing band was visualized as the TYR activity of the sample.
Western blotting
Western blotting analysis was performed as previously described by Sato et al. (2011), with moderate modifications. The B16F1 melanoma cells were seeded in six-well plates at a density of 1.5 × 106 cells per well. The wells were supplemented with FBS (10%) and penicillin–streptomycin (1%) in DMEM. The medium was replaced with a fresh medium after 24 hours incubation. The new medium was added with α-MSH (5 nM), and the A. paniculata leaf extract at different concentrations (100, 50, 25, 12.5, 6.25, and 3.13 μg/ml) was prepared using this medium. The treatment was done under 48 hours incubation. The DMEM solution was used as the negative control. The positive controls, α-MSH (5 nM), arbutin (2 mM), and kojic acid (800 μM), were used comparably with the treatment of the A. paniculata leaf extract. PBS was used to wash the cultured cells. The RIPA lysis buffer supplemented with an inhibitor of protease was used to harvest the cultured cells.
Next, the cells were incubated at 4°C for about 20 minutes. Then, the cell lysates were harvested and centrifuged at 12,000 rpm for about 10 minutes. The supernatant solution was harvested, and the protein concentrations were quantified using the Bradford assay. About 20 μg of protein in each cell lysate sample was boiled for 5 minutes at 95°C in a Laemmli loading buffer (4% SDS, 10% 2-mercaptoethanol, 0.125 M Tris-HCl, 0.2% bromophenol blue, and 0.125 M Tris-HCl). All the ratios for the samples and loading buffer were 1:1. Then, gel electrophoresis of these samples was carried out. The gel was transferred to a polyvinylidene difluoride membrane. A Tris-buffered saline-Tween 20 solution (TBST) containing 5% nonfat dry milk was used to block the membranes. The expression bands of primary antibodies, MITF (60 kDa), TYR (75 kDa), and TRP-2 (55 kDa), were detected in this membrane, respectively, with the rabbit polyclonal MITF antibody (dilution 1:1,000), rabbit polyclonal TYR antibody (dilution 1:1,000), and rabbit polyclonal TRP-2 antibody (dilution 1:1,000). The membrane was incubated with primary antibodies for 24 hours at 4°C. Next, the membrane was washed with TBST and incubated with secondary antibodies, horseradish peroxidase-conjugated anti-rabbit IgG (dilution 1:1,000), for 1 hour at room temperature. The immunoblot results were visualized using an enhanced chemiluminescence solution system (PerkinElmer). A loading control, β-actin (43 kDa), was assessed in this assay.
Statistical analysis
Student’s t-test was used for statistical significance. The results were interpreted as the mean ± SD of all the data from the replicated experiments.
RESULTS AND DISCUSSION
Cytotoxicity on B16F1 melanoma cells by A. paniculata leaf extract
The cytotoxicity assay by the A. paniculata leaf extract was investigated on the B16F1 melanoma cells by treating with different concentrations of the A. paniculata leaf extract in the presence of α-MSH. The MTT assay was used to determine the cytotoxicity effect of the A. paniculata leaf extract on the B16F1 cells. The MTT assay results revealed that the A. paniculata leaf extract at a concentration below 50 μg/ml in the presence of α-MSH had no significant effect on cell viability (Fig. 1). Consequently, the A. paniculata leaf extract at concentrations of 25, 12.5, 6.25, and 3.13 μg/ml was used for future experiments.
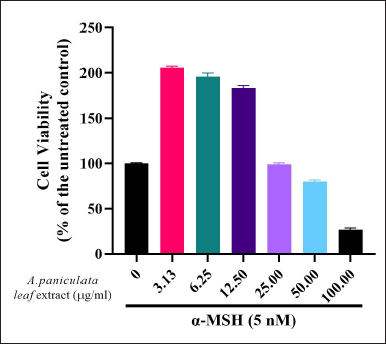 | Figure 1. Cell viability effect of A. paniculata leaf extract on B16F1 melanoma cells was analyzed by MTT assay. Cells were treated with A. paniculata leaf extract and α-MSH (5 nM) simultaneously. [Click here to view] |
Mushroom TYR activity inhibition by A. paniculata leaf extract
The A. paniculata leaf extract was applied in the mushroom TYR inhibition assay to establish the TYR activity inhibitory effect. The ideal standard used in this assay was kojic acid, known as a recognized inhibitor (Fig. 2). The A. paniculata leaf extract showed inhibition in the TYR enzyme at 50 μg/ml of about 74.361% ± 0.026% (Table 1). This result is moderately relative to the standard at 50 μg/ml of kojic acid of about 76.193% ± 0.005% TYR inhibition. This finding indicated that the A. paniculata leaf extract has the ability to inhibit TYR activity and melanin synthesis. The main structures of the inhibitor similar to DOPA or tyrosine are from a phenol or catechol derivative (Panzella and Napolitano, 2019). The A. paniculata leaf extract has the potential to mimic the amino acid tyrosine extract with the catalytic site binding of the TYR enzyme that blocks pigment formation in skin cells as this study showed inhibition in TYR activity. Therefore, the A. paniculata leaf extract can reduce melanogenesis activity.
Andrographis paniculata leaf extract inhibition effect in B16F1 melanoma cells on melanin synthesis
The essential factor in increasing the production of melanin and regulating melanogenesis is α-MSH (Buscà and Ballotti, 2000). Hence, this study evaluated the A. paniculata leaf extract on melanin inhibition in the α-MSH-induced B16F1 melanoma cells. The secreted melanin content elevated twofold once with α-MSH treatment, as in Figure 3. Simultaneously, the A. paniculata leaf extract had a morphological outcome on the B16F1 melanoma cells, as shown in Figure 4. Melanin secretion reduction comparable with the untreated control was demonstrated by arbutin, indicating its impressive antimelanogenic activity. In the meantime, remarkable reduction of melanin secretion was also shown by kojic acid. The spectacular inhibitory effect of the A. paniculata leaf extract on melanin secretion at 25 μg/ml was more powerful than that of kojic acid and arbutin in this condition of the experiment. The A. paniculata leaf extract was shown to dose-dependently reduce melanin content secretion. The powerful inhibitory effect on melanin secretion at 25 μg/ml indicated potency reduction was more effective than that of arbutin (2 and 4 mM) and kojic acid (400 and 800 μM).
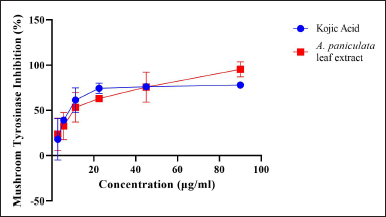 | Figure 2. Mushroom tyrosinase activity by A. paniculata leaf extract. [Click here to view] |
Intracellular TYR activity reduced by A. paniculata leaf extract
TYR direct inhibition or also the expression of gene suppression encouraged the reduction of the protein level in skin cells. This can be established by TYR activity reduction in melanocytes. In the prior experiment, the A. paniculata leaf extract had an outstanding TYR inhibitory activity. Next, the experiment to determine the effect on the intracellular TYR activity of the A. paniculata leaf extract was examined. Cell lysates were obtained from the B16F1 melanoma cells treated with different concentrations of the A. paniculata leaf extract. This was used as the source of TYR. The key enzyme that manages melanin pigment formation in humans and animals is TYR (Han et al., 2015). Then, the B16F1 melanoma cells were treated in the presence of α-MSH with different concentrations of the A. paniculata leaf extract, arbutin (2 and 4 mM), and kojic acid (400 and 800 μM). Afterward, TYR activity was measured. From the results, the A. paniculata leaf extract was inhibited and showed a concentration-dependent behavior as in the α-MSH-induced melanocytes by the activity of the TYR enzyme. This experiment exhibited that TYR enzyme activity inhibition by the A. paniculata leaf extract was more significant than that by arbutin (2 and 4 mM) and kojic acid (400 and 800 μM), as shown in Figure 5. T TYR activity was suppressed by 45.4% at a concentration of 25 μg/ml of the A. paniculata leaf extract.
 | Table 1. Tyrosinase inhibition of A. paniculata leaf extract in mushroom TYR activity. Kojic acid was used as a positive control. [Click here to view] |
TYR zymography of A. paniculata leaf extract showed positive suppresive effect on the intracellular TYR activity
TYR activity assessment was done after SDS-PAGE protein separation. Molecular weight was used as the basis for TYR separation on a gel. The gel could demonstrate its enzymatic process in the order that it could oxidize the L-DOPA solution when exerted on the gel. Therefore, the dark color of DOPA quinone will be formed (Sato et al., 2008). TYR zymography was carried out with the purpose of identifying TYR activity. The A. paniculata leaf extract showed its role in TYR degradation of the α-MSH-activated B16F1 melanoma cells. TYR activity was suppressed initially at the concentration of 6.25 μg/ml (Fig. 6). Nearly none of the activated TYR remained at the concentration of 25 μg/ml. The results showed highly demonstrated intracellular TYR comparable with TYR zymography. As shown, the result suggested that at the concentration of 25 μg/ml the A. paniculata leaf extract is equivalent to arbutin (2 and 4 mM), which is a known great TYR inhibitor.
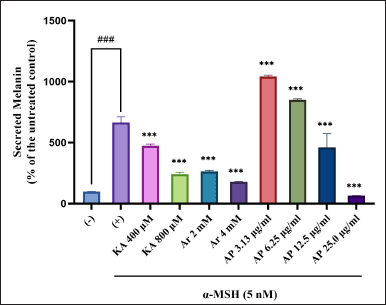 | Figure 3. Melanin production inhibition effect in melanocytes of A. paniculata leaf extract. The results represent the mean ± SD of triplicate samples. ###Statistically significant (p < 0.001) compared to control group. ***Statistically significant (p < 0.001) compared to α-MSH group. [Click here to view] |
Immunoblot showed that A. paniculata leaf extract downregulated TYR, TRP-2, and MITF expressions
To evaluate the A. paniculata leaf extract in regulating the protein expression of melanogenic enzymes like TYR, immunoblotting or Western blot analysis was carried out using different concentrations of the A. paniculata leaf extract. The loading control used in this study was β-actin. Intracellular TYR activity intensification for similar conditions corresponded to this, as the result showed when treated with α-MSH at the 75 kDa TYR enhancement marker. Treatment induced by α-MSH showed that the level of TYR protein was elevated and the induction was inhibited significantly by the A. paniculata leaf extract, as shown in Figure 7. The inhibition relatively approached at 6.25 μg/ml, 12.5 μg/ml and 25 μg/ml The regulation possibly exerted at TYR gene expression level and A. paniculata leaf extract was demonstrated depleted the intracellular TYR activity. The result showed that TYR protein decreased similarly to kojic acid (800 μM) starting at 6.25 until 25 μg/ml treatment of the A. paniculata leaf extract. TYR was presented as a crucial enzyme in ideal melanin production (Zhu et al., 2015).
The effects of the A. paniculata leaf extract on the protein expression level of TRP-2 were evaluated by western blot analysis. The result found that the A. paniculata leaf extract suppressed the expression level of TRP-2 slightly approached at 3.13 μg/ml and 6.25 μg/ml simultaneously in the B16F1 melanoma cells stimulated by α-MSH (Fig. 8). In the meantime, the A. paniculata leaf extract significantly suppressed TRP-2 at 25 μg/ml.
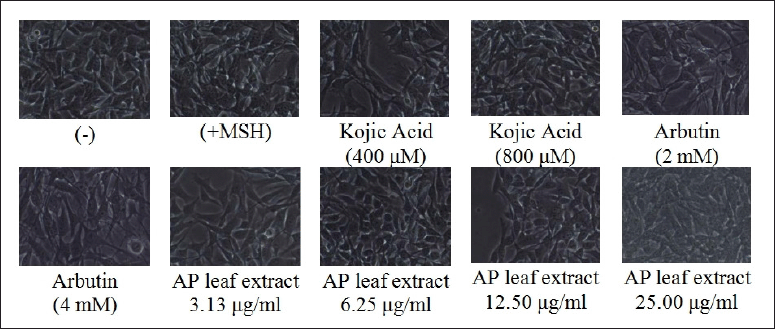 | Figure 4. Representative morphology of B16F1 melanoma cells from Figure 3 by an inverted phase-contrast microscope. [Click here to view] |
MITF is the main component in the regulation of tyrosinase-related proteins which incorporate TRP-1 and TRP-2. The regulation happens throughout the melanogenesis process in mammalian cells. As MITF is the main transcription factor in modulating the expression of the major melanogenic genes, the effectiveness of the A. paniculata leaf extract was evaluated on MITF expression. The result, as shown in Figure 9, demonstrated that the A. paniculata leaf extract significantly reduced the MITF expression level, which indicated that the A. paniculata leaf extract had significant inhibition activity towards MITF protein expression. In western blot analysis, in Figure 9, MITF was downregulated in a dose-dependent manner and significantly reduced at 25 μg/ml.
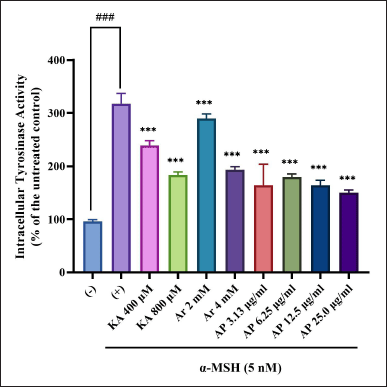 | Figure 5. Intracellular tyrosinase activity of B16F1 melanoma was inactivated by A. paniculata leaf extract. Intracellular tyrosinase activity reduction was discovered. The results represent the mean ± SD of triplicate samples. ### Statistically significant (p < 0.001) compared to control group. ***Statistically significant (p < 0.001) compared to α-MSH group. [Click here to view] |
 | Figure 6. Decreased tyrosinase activity by A. paniculata leaf extract confirmed through the result of tyrosinase zymography. [Click here to view] |
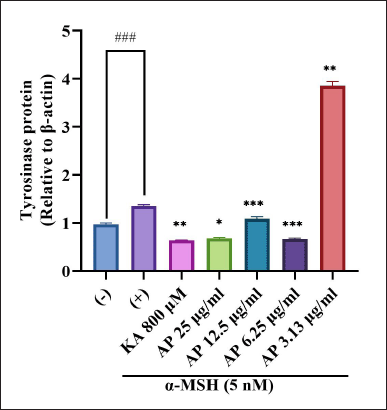 | Figure 7. Effects of A. paniculata leaf extract in α-MSH-induced B16F1 melanoma cells on expression levels of tyrosinase were analyzed by different concentrations of Andrographis paniculata leaf extract (AP; 3.13, 6.25, 12.50, and 25.00 μg/ml) or kojic acid (KA; 800 μM) for about 48 hours in α-MSH presence. The results represent the mean ± SD of triplicate samples. ### Statistically significant (p < 0.001) compared to control group. Statistically significant at ***p < 0.001, **p < 0.01, and *p < 0.05 compared to α-MSH group. [Click here to view] |
As prior studies expressed, TYR and TRP-2 are incorporated into the pathway of melanogenesis for moderate essential responses to melanogenesis (Jin et al., 2014; Xue et al., 2018). The hypopigmentation effect of the A. paniculata leaf extract as possibly the outcome of MITF gene expression was downregulated and suppressed the gene and protein expression of TYR, TRP-1, and TRP-2.
A copper-containing enzyme, TYR, is the major component in the control of melanin production (Lajis et al., 2012; Zolghadri et al., 2019). Therefore, hyperpigmentation can be treated by a compound that can control TYR and activity (Briganti et al., 2003; Lim et al., 2019). Moreover, previous studies reported melanin production and hyperpigmentation are elevated as the generation of ROS increases (Kami?ski et al., 2022; Qiao et al., 2012). Hence, this study was conducted using the A. paniculata leaf extract known for its potency in antioxidant activity. The A. paniculata leaf extract exhibited an antimelanogenic effect in a concentration-dependent mode based on the result of this study. This antimelanogenic possession was applied without particularly affecting the cytotoxicity of the B16F1 melanoma cells.
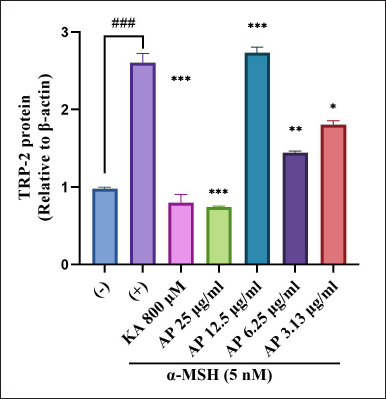 | Figure 8. Effects of A. paniculata leaf extract in α-MSH-induced B16F1 melanoma cells on expression levels of TRP-2 were analyzed by different concentrations of A. paniculata leaf extract (3.13, 6.25, 12.50, and 25.00 μg/ml) or kojic acid at 800 μM and arbutin at 2 mM for about 48 hours in α-MSH presence. The results represent the mean ± SD of triplicate samples. ### Statistically significant (p < 0.001) compared to control group. Statistically significant at ***p < 0.001, **p < 0.01, and *p < 0.05 compared to α-MSH group. [Click here to view] |
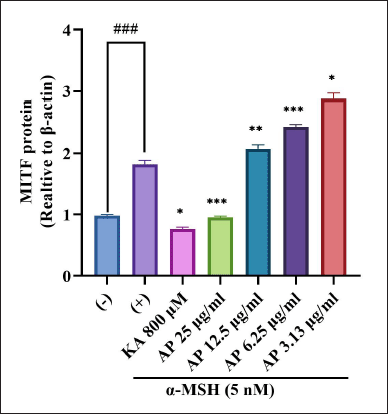 | Figure 9. Effects of A. paniculata leaf extract in α-MSH-induced B16F1 melanoma cells on expression levels of MITF were analyzed by different concentrations of A. paniculata leaf extract (3.13, 6.25, 12.50, and 25.00 μg/ml) or kojic acid at 800 μM and arbutin at 2 mM for about 48 hours in α-MSH presence. The results represent the mean ± SD of triplicate samples. ### Statistically significant (p < 0.001) compared to control group. Statistically significant at ***p < 0.001, **p < 0.01, and *p < 0.05 compared to α-MSH group. [Click here to view] |
Furthermore, there was a study that found andrographolide in A. paniculata-induced apoptosis in HT-29 cells, which seemed to be linked with the augmented intracellular ROS level (Khan et al., 2018). MITF engagement in regulating genes such as TYR, TRP1 and TRP 2 expression as MITF main transcription factor in melanogenic activity (Kim et al., 2013; Villareal et al., 2017). In this state, it plays crucial roles in melanin derivation from tyrosine.
CONCLUSION
The A. paniculata leaf extract suppressed the enzyme expression of TYR and MITF in a concentration-dependent mode. The results from our study indicated the significantly reduced protein expression of TRP-2 in certain concentration conditions. It can suppress melanin production. Thus, the A. paniculata leaf extract at 25 μg/ml is highly possibly applicable being a powerful natural and harmless ingredient as a skin lightening agent as the extract possesses a melanin production inhibition property. The A. paniculata leaf extract could represent novelty for antimelanogenesis potential through TYR and MITF and its related protein inhibitions. The A. paniculata leaf extract is safe and reliable for application as an antimelanogenic agent for hyperpigmentation control.
FUNDING
This work was supported by the NKEA Research Grant Scheme from the Ministry of Agriculture and Agro-Based Industry, Malaysia (MOA), and also the Ministry of Higher Education (MOHE), Malaysia (R.J130000.7909.4H020).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Bello OA, Ayanda OI, Aworunse OS, Olukanmi BI. Exploring the mechanism of cytotoxic and anti-inflammatory property of andrographolide and its derivatives. Pharmacogn Rev, 2018; 1:56–65. CrossRef
Bhattar PA, Zawar VP, Godse KV, Patil SP, Nadkarni NJ, Gautam MM. Exogenous ochronosis. Indian J Dermatol, 2015; 60(6):537–43. CrossRef
Briganti S, Camera E, Picardo M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res, 2003; 16:101–10. CrossRef
Buscà R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res, 2000; 13(2):60–9. CrossRef
Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev, 2011; 75(1):50–83. CrossRef
Chang MS, Choi MJ, Park SY, Park SK. Inhibitory effects of hoelen extract on melanogenesis in B16F1 melanoma cells. Phytother Res, 2010; 24:1359–64. CrossRef
Chao WW, Kuo YH, Lin BF. Isolation and identification of Andrographis paniculata (Chuanxinlian) and its biologically active constituents inhibited enterovirus 71-induced cell apoptosis. Front Pharmacol, 2021; 12:1–11. CrossRef
Denat L, Kadekaro AL, Marrot L, Leachman SA, Abdel-Malek ZA. Melanocytes as instigators and victims of oxidative stress. J Invest Dermatol, 2014; 134(6):1512–8. CrossRef
Di Petrillo A, González-Paramás AM, Era B, Medda R, Pintus F, Santos-Buelga C, Fais A. Tyrosinase inhibition and antioxidant properties of Asphodelus microcarpus extracts. BMC Complement Altern Med, 2016; 16:1–9. CrossRef
dos santos Videira IF, Moura DFL, Magina S. Mechanisms regulating melanogenesis. An Bras Dermatol, 2013; 88:76–83. CrossRef
Hamid MA, Sarmidi MR, Park CS. Mangosteen leaf extract increases melanogenesis in B16F1 melanoma cells by stimulating tyrosinase activity in vitro and by up-regulating tyrosinase gene expression. Int J Mol Med, 2012; 29:209–17.
Han SM, Kim JM, Pak SC. Anti-melanogenic properties of honeybee (Apis mellifera L.) venom in α-MSH-stimulated B16F1 cells. Food Agric Immunol, 2015; 26:451–62. CrossRef
Hossain S, Urbi Z, Karuniawati H, Mohiuddin RB, Moh Qrimida A, Allzrag AMM, Ming LC, Pagano E, Capasso R. Andrographis paniculata (Burm. f.) Wall. ex Nees: an updated review of phytochemistry, antimicrobial pharmacology, and clinical safety and efficacy. Life (Basel), 2021; 11(4):348. CrossRef
Hu Frisk JMH, Kjellén L, Melo FR, Öhrvik H, Pejler G. Mitogen-activated protein kinase signaling regulates proteoglycan composition of mast cell secretory granules. Front Immunol, 2018; 9:1670. CrossRef
Jeon NJ, Kim YS, Kim EK, Dong X, Lee JW, Park JS, Shin WB, Moon SH, Jeon BT, Park PJ. Inhibitory effect of carvacrol on melanin synthesis via suppression of tyrosinase expression. J Funct Foods, 2018; 45:199–205. CrossRef
Jin KS, Oh YN, Hyun SK, Kwon HJ, Kim BW. Betulinic acid isolated from Vitis amurensis root inhibits 3-isobutyl-1-methylxanthine induced melanogenesis via the regulation of MEK/ERK and PI3K/Akt pathways in B16F10 cells. Food Chem Toxicol, 2014; 68:38–43. CrossRef
Kami?ski K, Kazimierczak U, Kolenda T. Oxidative stress in melanogenesis and melanoma development. Contemp Oncol (Pozn), 2022; 26(1):1–7. CrossRef
Kawakami A, Fisher DE. The master role of microphthalmia-associated transcription factor in melanocyte and melanoma biology. Lab Invest, 2017; 97:649–56. CrossRef
Khan I, Khan F, Farooqui A, Ansari IA. Andrographolide exhibits anticancer potential against human colon cancer cells by inducing cell cycle arrest and programmed cell death via augmentation of intracellular reactive oxygen species level. Nutr Cancer, 2018; 70(5):787–803. CrossRef
Kim MJ, Jung TK, Park H, Yoon K. Effect of hoechunyangkyeok-san extract on melanogenesis. J Cosmet Dermatol Sci Appl, 2016; 6:85–95. CrossRef
Kim SS, Kim MJ, Choi YH, Kim BK, Kim KS, Park KJ, Park SM, Lee NH, Hyun CG. Down-regulation of tyrosinase, TRP-1, TRP-2 and MITF expressions by citrus press-cakes in murine B16 F10 melanoma. Asian Pac J Trop Biomed, 2013; 3(8):617–22. CrossRef
Lajis AFB, Hamid M, Ariff AB. Depigmenting effect of kojic acid esters in hyperpigmented B16F1 melanoma cells. J Biomed Biotechnol, 2012; 2012:952452. CrossRef
Lee AY. Skin pigmentation abnormalities and their possible relationship with skin aging. Int J Mol Sci, 2021; 22(7):3727. CrossRef
Lim J, Nam S, Jeong JH, Kim MJ, Yang Y, Lee MS, Lee HG, Ryu JH, Lim JS. Kazinol U inhibits melanogenesis through the inhibition of tyrosinase-related proteins via AMP kinase activation. Br J Pharmacol, 2019; 176(5):737–50. CrossRef
Lin VC, Ding HY, Tsai PC, Wu JY, Lu YH, Chang TS. In vitro and in vivo melanogenesis inhibition by biochanin a from Trifolium pratense. Biosci Biotechnol Biochem, 2011; 75:914–8. CrossRef
Lin HC, Su SL, Lu CY, Lin AH, Lin WC, Liu CS, Yang YC, Wang HM, Lii CK, Chen HW. Andrographolide inhibits hypoxia-induced HIF-1α-driven endothelin 1 secretion by activating Nrf2/HO-1 and promoting the expression of prolyl hydroxylases 2/3 in human endothelial cells. Environ Toxicol, 2017; 32(3):918–30. CrossRef
Liu YT, Chen HW, Lii CK, Jhuang JH, Huang CS, Li ML, Yao HT. A diterpenoid, 14-deoxy-11, 12-didehydroandrographolide, in Andrographis paniculata reduces steatohepatitis and liver injury in mice fed a high-fat and high-cholesterol diet. Nutrient, 2020; 12(2):523. CrossRef
Loureiro Damasceno JPL, Silva da Rosa H, Silva de Araújo L, Furtado NAJC. Andrographis paniculata formulations: impact on diterpene lactone oral bioavailability. Eur J Drug Metab Pharmacokinet, 2022; 47(1):19–30. CrossRef
Munshi A, Ramesh R. Mitogen-activated protein kinases and their role in radiation response. Genes Cancer, 2013; 4(9–10):401–8. CrossRef
Ngeow KC, Friedrichsen HJ, Li L, Zeng Z, Andrews S, Volpon L, Brunsdon H, Berridge G, Picaud S, Fischer R, Lisle R, Knapp S, Filippakopoulos P, Knowles H, Steingrímsson E, Borden KLB, Patton EE, Goding CR. BRAF/MAPK and GSK3 signaling converges to control MITF nuclear export. In: Proceedings of the National Academy of Sciences of the United States of America, 2018; 115:E8668–77. CrossRef
Oh E, Kim HJ, Lee D, Kang JH, Kim HG, Han SH, Baek NI, Kim KT. 8-Methoxybutin inhibits α-MSH induced melanogenesis and proliferation of skin melanoma by suppression of the transactivation activity of microphthalmia-associated transcription factor. Biomed Pharmacother, 2022; 152:113272. CrossRef
Oh MJ, Hamid MA, Ngadiran S, Seo YK, Sarmidi MR, Park CS. Ficus deltoidea (Mas cotek) extract exerted anti-melanogenic activity by preventing tyrosinase activity in vitro and by suppressing tyrosinase gene expression in B16F1 melanoma cells. Arch Dermatol Res, 2011; 303:161–70. CrossRef
Olea-Flores M, Zuñiga-Eulogio MD, Mendoza-Catalán MA, Rodríguez-Ruiz HA, Castañeda-Saucedo E, Ortuño-Pineda C, Padilla-Benavides T, Navarro-Tito N. Extracellular-signal regulated kinase: a central molecule driving epithelial-mesenchymal transition in cancer. Int J Mol Sci, 2019; 20(12):2885. CrossRef
Panzella L, Napolitano A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: recent advances. Cosmetics, 2019; 6(4):57. CrossRef
Paruchuru LB, Govindaraj S, Razin E. The critical role played by mitochondrial MITF serine 73 phosphorylation in immunologically activated mast cells. Cells, 2022; 11(3):589. CrossRef
Pfisterer PH, Rollinger JM, Schyschka L, Rudy A, Vollmar AM, Stuppner H. Neoandrographolide from Andrographis paniculata as a potential natural chemosensitizer. Planta Med, 2010; 76(15):1698–700. CrossRef
Pillaiyar T, Manickam M, Namasivayam V. Skin whitening agents: medicinal chemistry perspective of tyrosinase inhibitors. J Enzyme Inhib Med Chem, 2017; 32(1):403–25. CrossRef
Pop C, Vlase L, Tamas M. Natural resources containing arbutin. determination of arbutin in the leaves of Bergenia crassifolia (L.) fritsch. acclimated in Romania. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 2009; 37:129–32.
Qiao Z, Koizumi Y, Zhang M, Natsui M, Flores MJ, Gao L, Yusa K, Koyota S, Sugiyama T. Anti-melanogenesis effect of Glechoma hederacea L. extract on B16 murine melanoma cells. Biosci Biotechnol Biochem, 2012; 76(10):1877–83. CrossRef
Rahmi EP, Kumolosasi E, Jalil J, Buang F, Jamal JA. Extracts of Andrographis paniculata (Burm.f.) nees leaves exert anti-gout effects by lowering uric acid levels and reducing monosodium urate crystal-induced inflammation. Front Pharmacol, 2022; 12:787125. CrossRef
Sato K, Ando R, Kobayashi H, Nishio T. 2-Ethoxybenzamide stimulates melanin synthesis in B16F1 melanoma cells via the CREB signaling pathway. Mol Cell Biochem, 2016; 423:39–52. CrossRef
Sato K, Morita M, Ichikawa C, Takahashi H, Toriyama M. Depigmenting mechanisms of all-trans retinoic acid and retinol on B16 melanoma cells. Biosci Biotechnol Biochem, 2008; 72:2589–97. CrossRef
Sato K, Takahashi H, Toriyama M. Depigmenting mechanism of NSAIDs on B16F1 melanoma cells. Arch Dermatol Res, 2011; 303:171–80. CrossRef
Shu J, Huang R, Tian Y, Liu Y, Zhu R, Shi G. Andrographolide protects against endothelial dysfunction and inflammatory response in rats with coronary heart disease by regulating PPAR and NF-κB signaling pathways. Ann Cardiothorac Surg, 2020; 9:1965–75. CrossRef
Singh M, Mukhopadhyay K. Alpha-melanocyte stimulating hormone: an emerging anti-inflammatory antimicrobial peptide. Biomed Res Int, 2014; 2014:874610. CrossRef
Singh N. A review on herbal plants used as immunomodulators. Int J Pharm Res, 2016; 13:3602–10.
Song YS, Balcos MC, Yun HY, Baek KJ, Kwon NS, Kim MK, Kim DS. ERK activation by fucoidan leads to inhibition of melanogenesis in Mel-Ab cells. Korean J Physiol Pharmacol, 2015; 19(1):29–34. CrossRef
Sun L, Guo C, Yan L, Li H, Sun J, Huo X, Xie X, Hu J. Syntenin regulates melanogenesis via the p38 MAPK pathway. Mol Med Rep, 2020; 22(2):733–8. CrossRef
Sunkara HP, Kilaru KR, Kumar AP, Ramineni HB, Krishna PR. A case report on hydroquinone induced exogenous ochronosis. Int J Adv Med, 2020; 7:337. CrossRef
Villareal MO, Kume S, Neffati M, Isoda H. Upregulation of mitf by phenolic compounds-rich Cymbopogon schoenanthus treatment promotes melanogenesis in B16 melanoma cells and human epidermal melanocytes. Biomed Res Int, 2017; 2017:8303671. CrossRef
Xu C, Chou GX, Wang ZT. A new diterpene from the leaves of Andrographis paniculata Nees. Fitoterapia, 2010; 81(6):610–3. CrossRef
Xue L, Li Y, Zhao B, Chen T, Dong Y, Fan R, Li J, Wang H, He X. TRP?2 mediates coat color pigmentation in sheep skin. Mol Med Rep, 2018; 17(4):5869–77. CrossRef
Zhu PY, Yin WH, Wang MR, Dang YY, Ye XY. Andrographolide suppresses melanin synthesis through Akt/GSK3β/β-catenin signal pathway. J Dermatol Sci, 2015; 79(1):74–83. CrossRef
Zolghadri S, Bahrami A, Hassan Khan MT, Munoz-Munoz J, Garcia-Molina F, Garcia-Canovas F, Saboury AA. A comprehensive review on tyrosinase inhibitors. J Enzyme Inhib Med Chem, 2019; 34(1):279–309. CrossRef
SUPPLEMENTARY MATERIAL
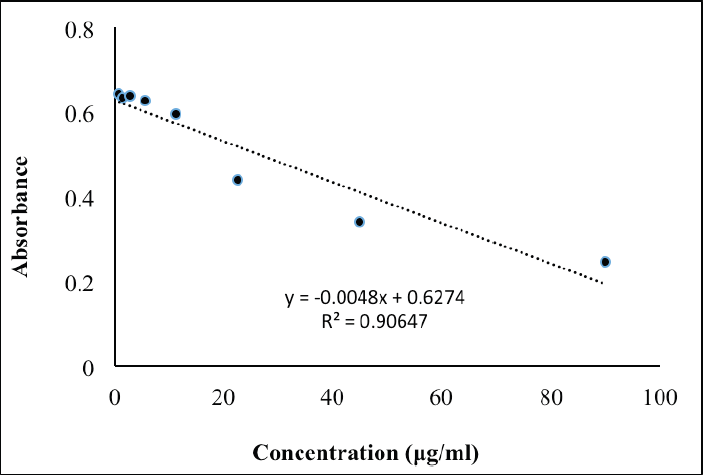 | Figure S1. Calibration curve for reference standard of kojic acid for mushroom tyrosinase assay. [Click here to view] |