INTRODUCTION
In perfumery industries, aldehydes have been used for different fragrance notes. They are mainly derived from natural and anthropogenic sources. A number of aldehydes can also be synthesized chemically (O’Brien et al., 2005). In these, aldehydes C8–C13 are important bioactive fragrant compounds used in various perfumery products (Surburg and Panten, 2005). Aldehydes are important ingredients in the legendary perfume Chanel No. 5 and are mostly perceived as pleasant smells by humans. Among them, C9 aldehyde (nonanal) is widely used in perfumery products for its rose-like fragrance. In trace amounts, it adds floral notes to a broad range of fragrance types. Cinnamon, lemongrass, milkweed, citrus, and rose essential oils contain C9 (Omonov et al., 2014; Otienoburu et al., 2012; Park et al., 2020). Nonanal is an important signal volatile of tobacco plants which attracts oviposition in female moths, Helicoverpa assulta (Wang et al., 2020b). Moreover, nonanal is an important skin odorant of various animals such as birds and mammals in addition to humans (Haze et al., 2001). Recently, Kim et al. (2019a) found that aldehydes, nonanal (C9) and decanal (C10), exhibited markedly different electroencephalographic (EEG) activity according to nostril difference.
In aromatherapy, pleasant odors have been used to improve the psychophysiological functions of humans. Olfaction and emotion are highly interconnected (Hou et al., 2020). Odors are abundant in indoor air due to the release from room fresheners, cleaning solutions, insecticides, cosmetics, and scented candles (Bartsch et al., 2016; Wolkoff and Nielsen, 2017). In general, it is not easy to generalize that all pleasant odors produce positive psychophysiological effects on humans via inhalation. Functional changes in the brain induced by odor inhalation are highly linked to the alteration of electrical activity in neuronal cells through the olfactory system. Therefore, it is necessary to figure out the influence of fragrance inhalation on brain functions (Kutlu et al., 2008).
Brain waves, including delta, theta, alpha, beta, and gamma, are considered to have relationships with specific functions of the brain. Brain wave activities are measured using various electrophysiological techniques. Among them, EEG is a widely used technique to monitor changes in brain wave activity induced by odor inhalation (Iijima et al., 2009; Kim et al., 2018; Sowndhararajan and Kim, 2016). Previous studies have shown that the EEG activity of humans is greatly altered during exposure to odor molecules (Koomhin et al., 2020; MacDonald, 2015). The EEG technique is a noninvasive approach, and it does not require the active participation of subjects (Skoric et al., 2015).
In our previous study, aldehyde C9 odor exposure exhibited a significant change only for absolute mid-beta wave activity at the left parietal region (P3) via binasal inhalation when compared with no odor exposure (Kim et al., 2019a). However, previous studies have found that the EEG recording time for determining the influence of exposure to different odors can be varied at every second. In addition, EEG readings have been exhibited to reveal nonstationary behavior in various contexts. Hence, it is important to develop a method to analyze nonstationary data (Krystal et al., 1999; Sowndhararajan and Kim, 2016). Recently, Kim et al. (2019b) reported that aldehyde C10 (decanal) odor showed considerable variations in EEG power spectra values at a specific time interval and found that absolute waves significantly decreased during the first 13 seconds period. Time series analysis is an appropriate method to determine the nonstationary behavior of EEG data using evenly spaced time intervals to identify an exact pattern in EEG data (Gao et al., 2020; Wang et al., 2020). Therefore, this study aimed to evaluate the olfactory stimulation influence of aldehyde C9 fragrance on the EEG activity of humans with respect to analysis time.
MATERIALS AND METHODS
Materials
Nonanal [CH3(CH2)7CHO] (CAS No. 124-19-6) was procured from Sigma (St. Louis, MO).
Subjects
This study was performed according to the Declaration of Helsinki on Biomedical Research Involving Human Subjects. The Ethical Committee from Kangwon National University, Chuncheon, Republic of Korea (IRB No. KWNUIRB-2017-05-001-003), approved the EEG study protocol. A total of 20 healthy volunteers (10 men and 10 women) aged 20–30 years participated in this study. The participants were selected based on the protocol described by Kim et al. (2019b). The selection criteria for participants were being nonsmokers and right-handed. In this study, participants with olfactory diseases or abused drugs were not included. Furthermore, participants who were unable to distinguish the well-known aroma types were excluded from this study. The participants were not allowed to consume alcohol or any medications from 2 days prior to the experiment. Informed consent was received from all the participants before they participated in the EEG study.
Experimental design
A single-group pretest and posttest experimental design was used in the study (20 participants). The participants were told that the purpose of the study was to evaluate the effect of inhalation of an odor molecule on EEG activity. During EEG recordings, the participants were asked to sit quietly, keep their eyes closed, and breathe normally under awakening conditions.
EEG recordings and fragrance administration
A QEEG-8 system was used to record the EEG readings (LXE3208, LAXTHA Inc., Daejeon, Republic of Korea). The EEG data were recorded from eight-channel electrodes according to the International 10–20 System. The EEG recording conditions and administration of odor were followed according to the method described previously by Kim et al. (2019b).
Data analysis
The mean power spectra values were calculated for 25 EEG indicators, and the values were expressed as microvolts squared (μV2) (Seo et al., 2016). We analyzed the EEG data for every second by splitting the total 30 seconds EEG data (from 5 to 35 seconds). SPSS 23 (SPSS, Inc., Chicago, IL) was used for statistical analysis (Kim et al., 2019b).
RESULTS
In this study, we investigated the olfactory stimulation effect of exposure to undiluted aldehyde C9 on the EEG activity of humans. The EEG power spectra changes between no odor exposure and C9 odor exposure were examined from eight electrode sites in the prefrontal, frontal, temporal, and parietal regions. Primarily, EEG power spectrum changes were analyzed between no odor exposure and C9 odor exposure for a total of 30 seconds, and then we split the EEG data into 30 separate seconds and analyzed changes during no odor and C9 odor for every second. In the results, we presented significant changes (p < 0.05) in absolute brain wave activity in relation to time series analysis. In a total of 30 seconds of EEG analysis time, a significant decrease in absolute mid beta (AMB) wave activity at the P3 site was observed due to the exposure to C9 odor (8.8988–7.7534 μV2). The significant change in AMB wave activity due to the exposure to C9 odor is presented in Table 1. Figure 1 shows the topographical mapping of AMB power spectrum change during the exposure to C9 odor when compared to that of no odor exposure.
Table 2 shows the significant changes (p < 0.05) of absolute power spectra values between no odor exposure and C9 odor exposure based on different analysis times. In time series analysis, C9 odor exposure exhibited significant changes in all absolute waves with the exception of absolute gamma at a specific time interval when compared to that of no odor exposure. However, no significant change in absolute wave activity was observed during 6~8, 11~13, 15~16, 18~23, 28~30, and 33~35 seconds during the exposure to C9 odor. The absolute power spectra such as alpha, beta, slow alpha, low beta, and high beta activities markedly decreased in the first 6-second period. Significant differences in absolute alpha (AA), absolute slow alpha (ASA), and AMB wave activities were observed during different analysis times (Figs. 2 and 3).
 | Table 1. Significant changes of EEG power spectrum values during no odor and C9 odor exposures in total 30 seconds. [Click here to view] |
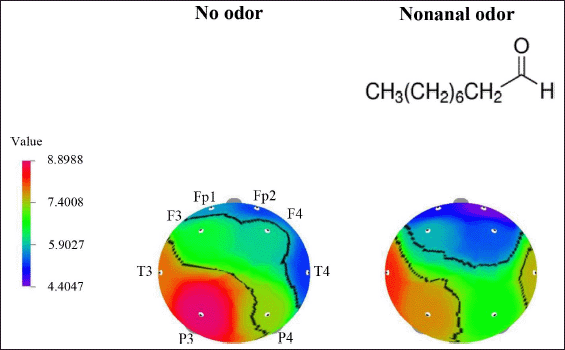 | Figure 1. The topographical mapping of AMB activity due to no odor and C9 odor exposure in total 30 seconds recording time. [Click here to view] |
The EEG data analysis revealed that absolute theta (AT) wave activity significantly increased during 8~9 seconds (16.5963–26.6732 μV2 at T3 and 16.0215–27.5788 μV2 at Fp2) and 25~26 seconds (19.7777–31.4728 μV2 at F3; 17.3160–28.1636 μV2 at F4; and 9.3947–14.3709 μV2 at T4) and decreased during 26~27 seconds (26.0982–16.7358 μV2 at T3) and 32~33 seconds (17.3059–14.1615 μV2 at T4) during the exposure to C9 odor (Fig. 4). Additionally, significant decreases in absolute beta (AB), low beta, mid beta, and high beta were observed at certain times due to the exposure of C9. AB wave activity significantly decreased during 5~6 seconds (P4), 16~17 seconds (Fp1), and 27~28 seconds (Fp1, Fp2, F3, P3, and P4), and absolute low beta (ALB) wave significantly decreased during 5~6, 14~15, 30~31, and 31~32 seconds (Figs. 5 and 6). Absolute high beta (AHB) also significantly decreased during 27~88 seconds during C9 odor inhalation when compared to that of no odor inhalation. Furthermore, AMB wave activity significantly decreased during 5~6, 9~10, 16~17, 27~28, and 30~31 seconds.
A significant decrease in AA and ASA activity was observed at similar analysis times during 5~6, 9~10, and 10~11 seconds and increased during 13~14 seconds. However, AA significantly increased during 17~18 seconds, while ASA significantly decreased at the same period during C9 odor inhalation. On the other hand, absolute fast alpha (AFA) significantly increased during 23~24 seconds and decreased during 24~26 seconds. In particular, AA wave significantly decreased at the Fp1, Fp2, F3, F4, and T4 regions during the period of 5~6 seconds. However, no significant change in AA and ASA activity was observed during the last 17 seconds of time series analysis (Table 2). Furthermore, C9 odor had no effect on absolute gamma wave activity. In the EEG time series analysis, significant changes were observed in more EEG indicators such as AA, AB, ASA, ALB, and AMB during the first second of analysis time (5~6 seconds) than in other periods. Furthermore, the F4 region was highly sensitive to C9 odor, followed by the Fp1, F3, T4, and P3 regions. According to time series analysis, exposure to C9 odor exhibited nonstationary EEG power spectra activity.
DISCUSSION
It is well known that odor molecules affect brain wave activities, thereby producing changes in the psychophysiological functions of humans. Brain wave modifications can be effectively measured by EEG (Angelucci et al., 2014; Ko et al., 2021). In the present study, only AMB wave activity significantly changed in the left parietal region during the exposure to C9 odor in a total of 30 seconds analysis time. On the other hand, all absolute wave activities significantly changed in different regions at different time points due to the exposure to C9 odor. However, no significant change in AG wave activity was observed during the exposure to C9 odor (Table 2).
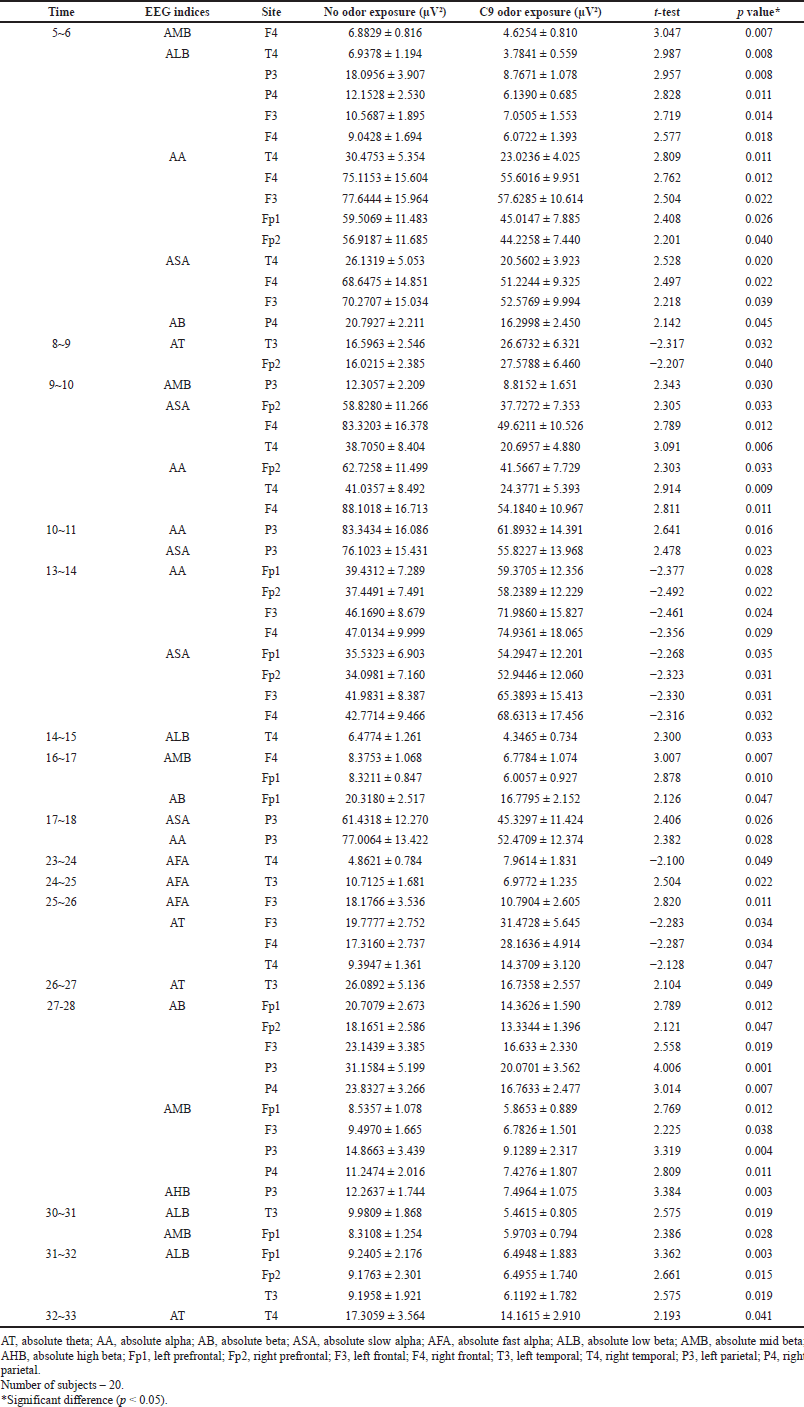 | Table 2. Significant changes of EEG power spectrum values at different time series during no odor and C9 odor exposures. [Click here to view] |
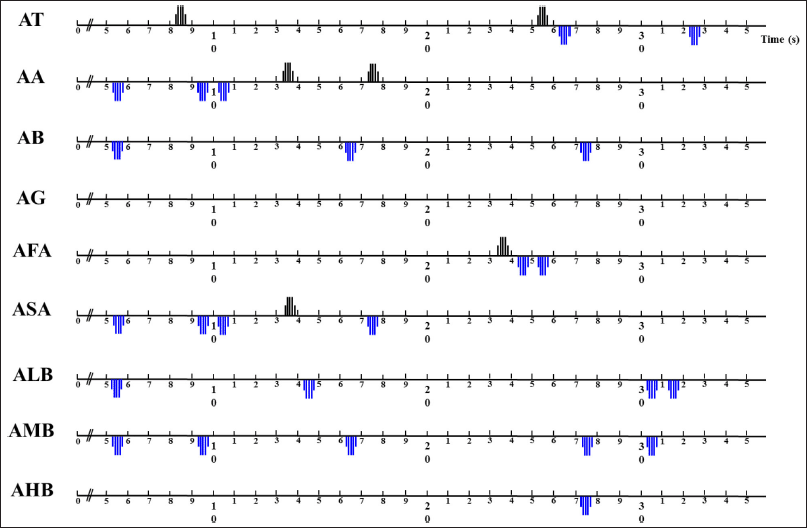 | Figure 2. Significant increase and decrease in absolute brain waves due to the inhalation of C9 odor in relation to analysis time. [Click here to view] |
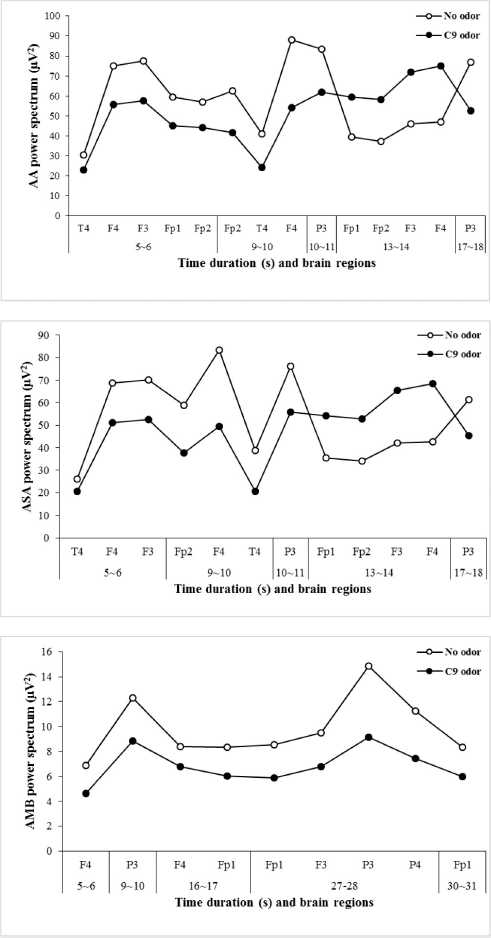 | Figure 3. Significant changes in AA, AFA, and AMB waves due to the inhalation of C9 odor in relation to analysis time. [Click here to view] |
The results revealed that the changes mainly occurred in AA, ASA, and AMB wave activities when compared with other brain waves (Fig. 3). In general, the neuronal electrical activity of the brain produces time-dependent potential alterations on the scalp. Masago et al. (2000) found that lavender, eugenol, and chamomile odors produced a significant decrease in alpha 1 wave activity during the first 10-second period of odor inhalation. The authors found that the significant decrease in alpha 1 activity was observed during the exposure to lavender, eugenol, and chamomile aromas in which subjects felt comfortable. The significant decrease was stable only due to the inhalation of lavender odor, but the decrease was unstable during the inhalation of other kinds of odors. Previously, some researchers used the time-frequency analysis approach to evaluate EEG signals in regard to the time as well as frequency domains simultaneously. Huart et al. (2013) used time-frequency analysis of EEG signals to evaluate olfactory functions in patients. Time-frequency analysis was also used to study EEG power spectra change induced by olfactory stimuli, phenyl ethyl alcohol, and eucalyptol, and this approach reliably discriminates patients with olfactory impairments from healthy subjects (Schriever et al., 2017). A recent study showed that absolute waves markedly decreased in the first 13-second period due to the exposure to C10 odor. Later, AA, ASA, and AFA wave activities significantly increased. In addition, the odor inhalation of C10 mainly showed changes especially in the left frontal site (F3) when compared to that of other sites (Kim et al., 2019b). A functional magnetic resonance image study demonstrated that the time course to activate the human primary olfactory cortex induced by odor molecules is 30~40 seconds (Sobel et al., 2000). In addition, Boesveldt et al. (2009) used a time series approach for magnetoencephalography data to detect olfactory dysfunction in patients with Parkinson’s disease and healthy individuals.
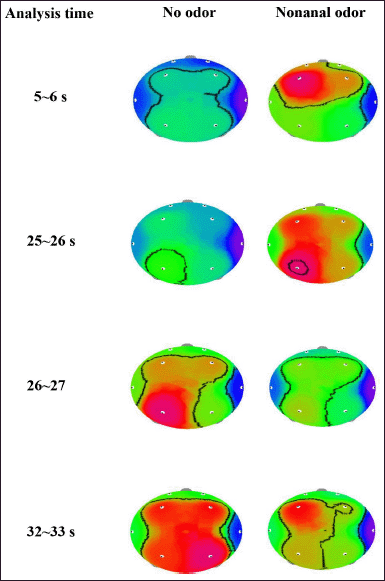 | Figure 4. The topographical mapping of AT activity due to the inhalation of C9 odor in relation to analysis time. [Click here to view] |
Among different brain waves, alpha and beta waves play a vital role in cognitive performance in humans. According to previous studies, a significant decrease in the alpha wave was observed during emotional tension and stress states and a significant increase was noticed during relaxation as well as calmness states of the brain (Iijima et al., 2009; Lorig and Schwartz, 1988; Sayorwan et al., 2012). The data of the present study indicated that AA waves (AA and ASA) decreased due to the exposure to C9 odor. However, the AA wave markedly increased during 13~14 and 17~18 seconds. However, the change in AA, ASA, and AFA waves was not persistent during time series analysis. Beta waves such as AB, ALB, AMB, and AHB significantly decreased at different time points during time series analysis. It is well known that beta wave activity is attenuated under the drowsiness state and increases under the awareness and concentration states of the brain (Lee et al., 2014). According to time series analysis results, reduction in beta wave activity due to C9 odor inhalation may connect with the drowsiness state of the brain.
In addition, absolute theta wave activity increased during 8~9 and 25~26 seconds and decreased during 26~27 and 32~33 seconds due to the exposure to C9 odor. Previous studies suggest that the decrease in theta wave activity is highly linked with memory formation (Greenberg et al., 2015; Razumnikova, 2007; Sowndhararajan and Kim, 2016). However, AT wave activity was not persistent during the exposure to C9 odor. The findings of this study suggest that C9 odor produced alterations in different brain waves at different brain sites based on time series analysis. From the results, we cannot predict the accurate action of C9 odor on brain function due to the unstable EEG signals during time series analysis.
Time series analysis of EEG signals also allows the measurement of durations and order of activation of each brain region (Jung et al., 2006). In this study, the results suggest that the inhalation of C9 odor highly affected the frontal and prefrontal regions when compared with the temporal and parietal regions. Particularly, C9 odor mainly affects the F4 region of the brain, followed by the F3, Fp1, T4, and P3 regions. The prefrontal and frontal sites are important functional areas in the cerebral cortex of the brain. The prefrontal and frontal regions are involved in various cognitive functions, such as decision-making skills, problem-solving, language, memory, attention, and movements (Collins and Koechlin, 2012; Sowndhararajan and Kim, 2016). Furthermore, Grabenhorst et al. (2007) stated that the medial orbitofrontal cortex region of the brain is mainly activated by odors with a pleasant smell. In olfaction, the odor molecules present in the air reach the primary olfactory cortex via olfactory receptor cells and the olfactory bulb (Simoes de Souza and Antunes, 2007). Primary olfactory regions, such as the piriform cortex, amygdala, and neighboring cortex, are mainly activated by the stimulation of odor molecules through the transmission between inhaling and odor molecules (Billot et al., 2011; Sobel et al., 2000).
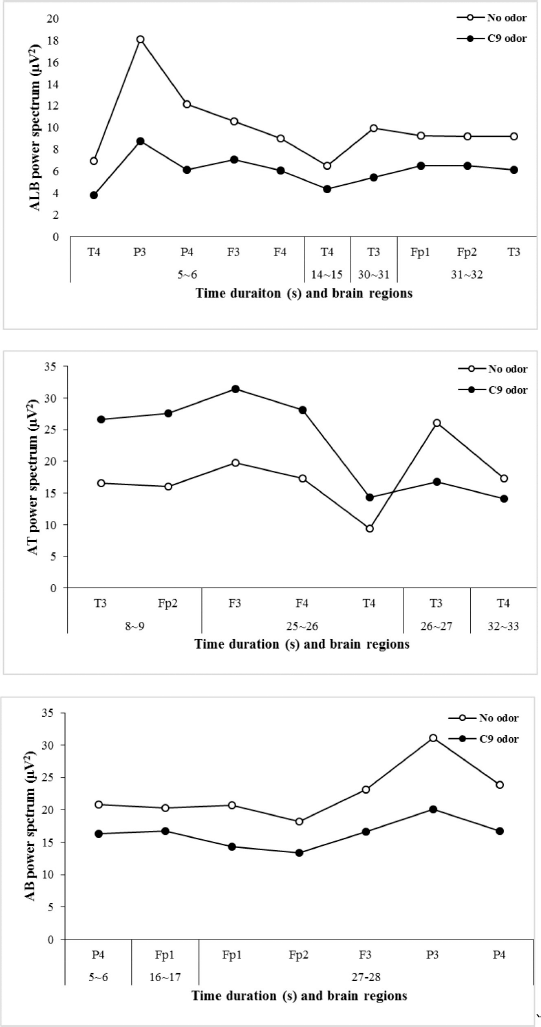 | Figure 5. Significant changes in ALB, AT, and AB waves due to the inhalation of C9 odor in relation to analysis time. [Click here to view] |
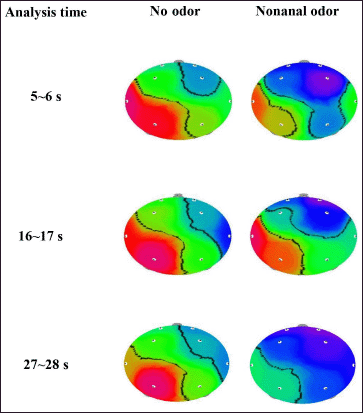 | Figure 6. The topographical mapping of AB activity due to the inhalation of C9 odor in relation to analysis time. [Click here to view] |
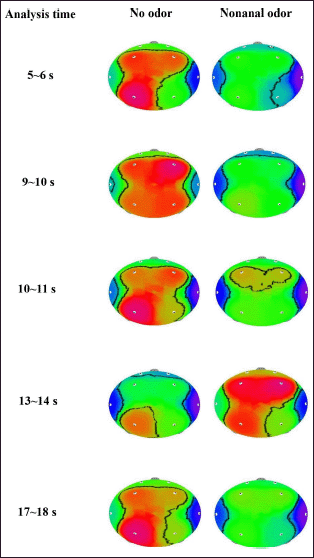 | Figure 7. The topographical mapping of AA activity due to the inhalation of C9 odor in relation to analysis time. [Click here to view] |
CONCLUSION
The results revealed that the EEG activity of C9 odor exposure in a total of 30 seconds analysis time is completely different from time series analysis. In time series analysis, AB waves markedly decreased during C9 odor exposure, and these changes may reduce the alertness state of the brain. In aromatherapy, aldehyde C9 may be used for managing cognitive activities after fixing the appropriate dosage. In addition, this study demonstrates that there was no trend in the EEG activity of C9 odor during time series analysis. Hence, further investigations are needed in association with slightly longer EEG recording time along with time series analysis.
AUTHORS’ CONTRIBUTION
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be authors as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or other conflicts of interest in this work.
FUNDING
There is no funding to report.
ETHICAL APPROVAL
The study protocol was approved by the Ethical Committee from Kangwon National University, Chuncheon, Republic of Korea (IRB No. KWNUIRB-2017-05-001-003).
DATA AVAILABILITY
The data presented in this study are available within the article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Angelucci FL, Silva VV, Dal Pizzol C, Spir LG, Praes CE, Maibach H. Physiological effect of olfactory stimuli inhalation in humans: An overview. Int J Cosmet Sci, 2014; 36:117–23. CrossRef
Bartsch J, Uhde E, Salthammer T. Analysis of odour compounds from scented consumer products using chromatography-mass spectrometry and gas chromatography-olfactometry. Anal Chim Acta, 2016; 904:98–106. CrossRef
Billot PE, Comte A, Galliot E, Andrieu P, Bonnans V, Tatu, L, Gharbi T, Moulin T, Millot JL. Time course of odorant- and trigeminal-induced activation in the human brain: an event-related functional magnetic resonance imaging study. Neuroscience, 2011; 189:370–6. CrossRef
Boesveldt S, Stam CJ, Knol DL, Verbunt JP, Berendse HW. Advanced time-series analysis of MEG data as a method to explore olfactory function in healthy controls and Parkinson’s disease patients. Hum Brain Mapp, 2009; 30:3020–30. CrossRef
Collins A, Koechlin E. Reasoning, learning, and creativity: frontal lobe function and human decision-making. PLoS Biol, 2012; 10:e1001293. CrossRef
Gao Y, Zhao Z, Chen Y, Mahara G, Huang J, Lin Z, Zhang J. Automatic epileptic seizure classification in multichannel EEG time series with linear discriminant analysis. Technol Health Care, 2020; 28:23–33. CrossRef
Grabenhorst F, Rolls ET, Margot C, da Silva MA, Velazco MI. How pleasant and unpleasant stimuli combine in different brain regions: odor mixtures. J Neurosci, 2007; 27:13532–40. CrossRef
Greenberg JA, Burke JF, Haque R, Kahana MJ, Zaghloul KA. Decreases in theta and increases in high frequency activity underlie associative memory encoding. NeuroImage, 2015; 114:257–63. CrossRef
Haze S, Gozu Y, Nakamura S, Kohno Y, Sawano K, Ohta H, Yamazakiet K. 2-Nonenal newly found in human body vodor tends to increase with aging. J Invest Dermatol, 2001; 116:520–4. CrossRef
Hou HR, Zhang XN, Meng QH. Odor-induced emotion recognition based on average frequency band division of EEG signals. J Neurosci Methods, 2020; 334:108599. CrossRef
Huart C, Rombaux P, Hummel T, Mouraux A. Clinical usefulness and feasibility of time-frequency analysis of chemosensory event-related potentials. Rhinology, 2013; 51:210–21. CrossRef
Iijima M, Osawa M, Nishitani N, Iwata M. Effects of incense on brain function: Evaluation using electroencephalograms and event–related potentials. Neuropsychobiology, 2009; 59:80–6. CrossRef
Jung J, Hudry J, Ryvlin P, Royet JP, Bertrand O, Lachaux JP. Functional significance of olfactory-induced oscillations in the human amygdala. Cereb Cortex, 2006; 16:1–8. CrossRef
Kim M, Sowndhararajan K, Park SJ, Kim S. Effect of inhalation of isomers, (+)-α-pinene and (+)-β-pinene on human electroencephalographic activity according to gender difference. Eur J Integr Med, 2018; 17:33–9. CrossRef
Kim M, Sowndhararajan K, Choi HJ, Park SJ, Kim S. Olfactory stimulation effect of aldehydes, nonanal, and decanal on the human electroencephalographic activity, according to nostril variation. Biomedicines, 2019a; 7:57. CrossRef
Kim M, Nishi K, Sowndhararajan K, Kim S. A time series analysis to investigate the effect of inhalation of aldehyde C10 on the human EEG activity. Eur J Integr Med, 2019b; 25:20–7. CrossRef
Ko LW, Su CH, Yang MH, Liu SY, Su TP. A pilot study on essential oil aroma stimulation for enhancing slow-wave EEG in sleeping brain. Sci Rep, 2021; 11:1078. CrossRef
Koomhin P, Sattayakhom A, Chandharakool S, Sinlapasorn J, Suanjan S, Palipoch S, Na-ek P, Punsawad C, Matan N. Michelia essential oil inhalation increases fast alpha wave activity. Sci Pharm, 2020; 88:23. CrossRef
Krystal AD, Prado R, West M. New methods of time series analysis of non-stationary EEG data: eigenstructure decompositions of time varying autoregressions. Clin Neurophysiol, 1999; 110:2197–206. CrossRef
Kutlu AK, Yilmaz E, Cecen D. Effects of aroma inhalation on examination anxiety. Teach Learn Nursing, 2008; 3:125–30. CrossRef
Lee BG, Lee BL, Chung WY. Mobile healthcare for automatic driving sleep-onset detection using wavelet-based EEG and respiration signals. Sensors, 2014; 14:17915–36. CrossRef
Lorig TS, Schwartz GE. Brain and odor: I. Alteration of human EEG by odor administration. Psychobiology, 1988; 16:281–4. CrossRef
MacDonald DB. 2015. Electroencephalography: basic principles and applications. In: Wright, JD (ed.). International encyclopedia of the social & behavioral sciences, 2nd edition, Amsterdam, Netherlands: Elsevier, pp 353–63. CrossRef
Masago R, Matsuda T, Kikuchi Y, Miyazaki Y, Iwanaga K, Harada H, Katsuura T. Effects of inhalation of essential oils on EEG activity and sensory evaluation. J Physiol Anthropol, 2000; 19:35–42. CrossRef
O’Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit Rev Toxicol, 2005; 35:609–62. CrossRef
Omonov TS, Kharraz E, Foley P, Curtis JM. The production of biobased nonanal by ozonolysis of fatty acids. RSC Adv, 2014; 4:53617. CrossRef
Otienoburu PE, Ebrahimi B, Phelan PL, Foster WA. Analysis and optimization of a synthetic milkweed floral attractant for mosquitoes. J Chem Ecol, 2012; 38:873–81. CrossRef
Park S, Kang W, Choi D, Son B, Park T. Nonanal stimulates growth factors via cyclic adenosine monophosphate (cAMP) signaling in human hair follicle dermal papilla cells. Int J Mol Sci, 2020; 2:8054. CrossRef
Razumnikova OM. Creativity related cortex activity in the remote associates task. Brain Res Bull, 2007; 73:96–102. CrossRef
Sayorwan W, Siripornpanich V, Piriyapunyaporn T, Hongratanaworakit T, Kotchabhakdi N, Ruangrungsi N. The effects of lavender oil inhalation on emotional states, autonomic nervous system, and brain electrical activity. J Med Assoc Thai, 2012; 95:598–606.
Schriever VA, Han P, Weise S, Hösel F, Pellegrino R, Hummel T. Time frequency analysis of olfactory induced EEG-power change. PLoS One, 2017; 12:e0185596. CrossRef
Seo M, Sowndhararajan K, Kim S. Influence of binasal and uninasal inhalations of essential oil of Abies koreana twigs on electroencephalographic activity of human. Behav Neurol, 2016; 2016:9250935. CrossRef
Simoes de Souza FM, Antunes G. Biophysics of olfaction. Rep Prog Phy, 2007; 70:451–91. CrossRef
Skoric MK, Ivan Adamec I, Jerbic AB, Gabelic T, Hajnšek S, Habek M. Electroencephalographic response to different odors in healthy individuals: a promising tool for objective assessment of olfactory disorders. Clin EEG Neurosci, 2015; 46:370–6. CrossRef
Sobel N, Prabhakaran V, Zhao Z, Desmond JE, Glover GH, Sullivan EV, Gabrieli JD. Time course of odorant-induced activation in the human primary olfactory cortex. J Neurophysiol, 2000; 83:537–51. CrossRef
Sowndhararajan K, Kim S. Influence of fragrances on human psychophysiological activity: with special reference to human electroencephalographic response. Sci Pharm, 2016; 84:724–51. CrossRef
Surburg H, Panten J. 2005. Common fragrance and flavor materials, preparation, properties and uses, 5th edition, Weinheim, Germany: Wiley-VCH. CrossRef
Wang C, Li G, Miao C, Zhao M, Wang B, Guo X. Nonanal modulates oviposition preference in female Helicoverpa assulta (Lepidoptera: Noctuidae) via the activation of peripheral neurons. Pest Manag Sci, 2020a; 76:3159–67. CrossRef
Wang C, Zhu M, Wang X, Wang X, Zhang H, Deng H, Cui H, Chen S, Li G. Time-frequency analysis of electroencephalogram signals in a cognitive decision-making task. Annu Int Conf IEEE Eng Med Biol Soc, 2020b; 2020:3469–72. CrossRef
Wolkoff P, Nielsen GD. Effects by inhalation of abundant fragrances in indoor air - an overview. Environ Int, 2017; 101:96–107. CrossRef