INTRODUCTION
Over the last few years, phytonutrients derived from plants have emerged as one of the leading dietary supplements to prevent or even cure chronic diseases. Curcumin (CU) is an active pharmaceutical and nutraceutical compound, which is extracted from dried turmeric rhizomes and is widely used in the cosmetics and the nutraceutical industries dietary supplements. CU exhibits various bioactive properties; e.g., it exhibits potent antioxidant, anti-inflammatory, antitumor, and anticardiogenic properties, which make it relevant in clinical utilization (Hewlings and Kalman, 2017; Rathore et al., 2020). Its therapeutic and nutraceutical value has caused CU to be introduced in functional foods and phytomedicine. However, free CU has limitations in terms of its usage or practical applications in both food processing and medical remedies owing to its insolubility in aqueous solutions, which results in poor absorption, rapid metabolism, rapid systemic elimination, and susceptibility to heat, light, and extreme acidity (Kharat et al., 2017; Lopresti, 2018).
Several methods have been researched to increase the solubility, stability, and bioavailability of CU, to control the release of bioactive compounds. Microencapsulation is a widely introduced technology among those attempts (Chen et al., 2020; Liu et al., 2020; Mehryar et al., 2021; Meng et al., 2021; Singh et al., 2021). Several reports showed that the encapsulation with various materials enhanced the solubility of CU and resulted in a homogeneous CU suspension (Ang et al., 2019; Singh et al., 2021). It was found that CU was released from a nanoemulsion in a phosphate buffer saline (PBS) solution much faster than that from a free CU suspension (Hasan et al., 2019). Furthermore, the release profile in a simulated gastric digestive environment showed that more than 90% of CU was released from the developed micro- to nanocapsules system (Debnath et al., 2018; Prasad et al., 2014; Rezaei and Nasirpour, 2019).
Natural and biodegradable polymers have received great attention to replace synthetic polymers, as they are safer for human health (Lopresti, 2018). Chitin, a natural copolymer of β-(1-4)-linked D-glucosamine and N-acetyl-D-glucosamine, is the second most abundant polysaccharide in nature, has excellent biocompatibility, high membrane permeability, biodegradability, and antimicrobial activity, and is nontoxic (Singh et al., 2021). Chitosan (CS) has low solubility and significant swelling in an aqueous environment that causes rapid drug release. For instance, studies have shown that CS cannot pass the colonic epithelial cells owing to its high solubility in gastric fluid, which results in some capsules bursting and releasing the enclosed drug in the stomach before it reaches the target sites (El-banna et al., 2019). CS-coated nanoparticles loaded with CU were shown to make the CU more bioavailable in Swiss mice (Marin et al., 2017). CS derivatives exhibit hydrophilic properties; CS, with a pKa = 6.4, rapidly precipitates in media at biological pH (pH = 7.4) owing to strong inter- and intramolecular hydrogen bonding (Pasanphan et al., 2014). Therefore, the idea of modifying the hydrophilic and hydrophobic segments of CS has focused on developing amphiphilic core-shell nanoparticles, with hydrophilic shells and hydrophobic cores that can trap drugs. Several studies focused on forming the nanoparticles by self-assembly in an aqueous solution to efficiently deliver hydrophobic drugs and to control the rate of release of the drugs (Liu et al., 2020; Motiei and Kashanian, 2017; Stanic, 2018). CS has no amphiphilic properties and cannot form micelles in water, though it has reactive amine and hydroxyl groups, which can be modified. Polymer coating is a promising method to modify the surface characteristics of the micelles to improve their widespread application (Li et al., 2017). Recent studies have used interpolymer complexes to produce micro- or nanocapsules for chemical crosslinking (Sanidad et al., 2019; Stanic, 2018). CS micro- and nanogels have been produced with polymer pairs, selected to form interpolymer complexes (Motiei et al., 2018, 2020; Rattanawongwiboon et al., 2018). CU-loaded nanoparticles prepared from dextran sulfate-CS were shown to have slow release of CU and were better able to cause apoptosis in cancer cells in a cell culture model (Anitha et al., 2011).
Polyethylene glycol (PEG) is hydrophilic and water-soluble and is often used as a biomedical material. Its good biocompatibility makes it widely applied in the biomedical and pharmacology fields (Cho et al., 2011). Using a Schiff base reaction, several routes have been used for conjugating PEG onto CS beads (Prasad et al., 2017). Chemical crosslinks can be used to connect the polymers; the resulting micro-/nanocapsules can be made more stable. CU in poly-lactide-co-glycolide nanoparticles coated with CS and PEG were more effective in cell culture models to treat metastatic pancreatic cancer cells (Arya et al., 2018).
One method of crosslinking water-soluble polymers into capsules is to use radiation-induced crosslinking. The preparation of micro- to nanocapsules by radiation can be conducted at room temperature without initiators or catalysts. Therefore, it is a green and convenient method for making biomaterials on the micro- or nanoscale, including for drug delivery capsules. Although chemical modifications of CS have been widely reported, not many current reports have looked at radiation-induced graft copolymerization. To the best of the authors’ knowledge, a limited number of previous studies have focused on the preparation of amphiphilic polymer-based nanoparticles by grafting PEG moieties onto CS and some application of radiation-induced crosslinking, i.e., by Desai and Park (2006), Pasanphan et al. (2014), Rattanawongwiboon et al. (2018), and Rattanawongwiboon et al. (2020). However, their objectives were only to encapsulate essential oils. There is no report on microencapsulation of CU by radiation-induced crosslinking of those materials. We hypothesized that the shell of a CU-loaded microcapsule produced via gamma radiation-induced crosslinking between CS and PEG diacrylate (PEGDA) exhibits high durability to a variety of pH conditions and maintains the constant release of CU while it passes through the gastrointestinal tract.
MATERIAL AND METHODS
Chemicals
CS flakes from a shrimp shell (molecular weight of 400–500 kDa, degree of deacetylation more than 95% analyzed via nuclear magnetic resonance spectroscopy, and viscosity 260 cPs) were purchased from Bio 21 Co., Ltd. (Thailand). PEGDA (Mn = 575, Lot no. HNUGLJN) was purchased from Tokyo Chemical Industry, Co., Ltd. CJTaban, Japan. CU (Lot no. FKANH-QR) was purchased from Tokyo Chemical Industry Co., Ltd., CJTaban (Japan). NaOH was supplied by Merck (Germany). Ethanol (AR grade) was purchased from Sigma-Aldrich, Co., Ltd. (USA). Acetic acid was purchased from QReC, New Zealand. Phosphate buffer tablets were purchased from Biotech (USA). All chemicals were used as received without being further purified.
Instruments
Gamma irradiation was conducted at the Thailand Institute of Nuclear Technology (TINT, Public Organization) using the 60Cobalt gamma irradiator (gamma chamber 5,000, BRIT, India) with a dose rate of 2.59 kGy/hour. Fourier transform infrared spectroscopy (FTIR, ThermoScientificTM, Model NicoletTM iS5) spectra were recorded to prove the successful crosslink forming. The size variation and morphology of the CS and CS–PEGDA beads were analyzed by scanning electron microscopy (SEM) (FEI, Quanta 250) using an acceleration voltage of 10 kV. For cross-sectional analysis, the samples were subjected to freeze-drying (Christ, Alpha2-4 LSC basic) and cut with a stainless-steel razor blade. All samples were sputter-coated with gold in a vacuum before being scanned. A UV-vis spectrophotometer (Biometrics, UV800) was used to measure the amount of CU.
Preparation of CS beads
CS flakes (2 g) were dissolved in aqueous acetic acid (1% v/v, 100 ml) with continuous magnetic stirring to form the CS solution (2% w/v). The CS solution was gently dropped into an NaOH solution (2 M) using a syringe and then left overnight. The beads were then washed several times with distilled water to obtain the final CS beads.
Synthesis of CS–PEGDA beads by radiation-induced crosslinking
CS–PEGDA beads were prepared via radiation-induced crosslinking as previously described by Rattanawongwiboon et al. (2020). The brief process is demonstrated in Figure 1. CS beads were left overnight in a PEGDA solution (with a concentration of 2% (w/v), 20 ml) at room temperature. Samples were then gamma-irradiated at 25 kGy. After irradiation, the samples were washed with distilled water to repeatedly remove excess PEGDA and leave only CS–PEGDA beads. The CS–PEGDA beads were stored in PBS at pH 7.4. To confirm the successful crosslink formation, FTIR spectra were acquired as described by Rajendran and Basu (2009). To prepare the individual samples, the beads were crushed in a mortar with a pestle. The crushed beads were mixed with potassium bromide (Merck, IR spectroscopy grade) in the proportion 1:100 and dried at 40°C. The crushed beads were compressed to a 12 mm semitransparent disk at a pressure of 10 tons for 2 minutes. The FTIR spectra were collected in the range of 650–4,000 cm1 in an attenuated total reflection mode with 16 scans at a resolution of 2 cm−1. The yield (%) of the CS–PEGDA beads was calculated as the ratio between the final weight of the dried CS–PEGDA beads and the initial weight of starting materials, as shown in
where W(bead) is the final weight of the dried CS–PEGDA beads and W(CS–PEGDA) is the initial weight of the CS and PEGDA materials.
Porosity of CS–PEGDA beads
The porosity (?) of the CS–PEGDA beads was calculated via the CS density and the weight of the samples before and after drying at room temperature for 48 hours, using
where Ww is the weight of CS–PEGDA beads before drying, Wd is the weight ρw of dried CS–PEGDA weight, the density of water (1.0 g/cm3), and ρCS is the density of CS (0.87 g/cm3).
Degree of swelling of CS–PEGDA beads
The dried sample CS–PEGDA beads were weighed and then soaked in water (Tai et al., 2010). After 24 hours, the beads were taken out, dried using filter paper, and then weighed. The degree of swelling in the beads was determined with
where Wd and Ws are the weights of the dried and swollen beads, respectively.
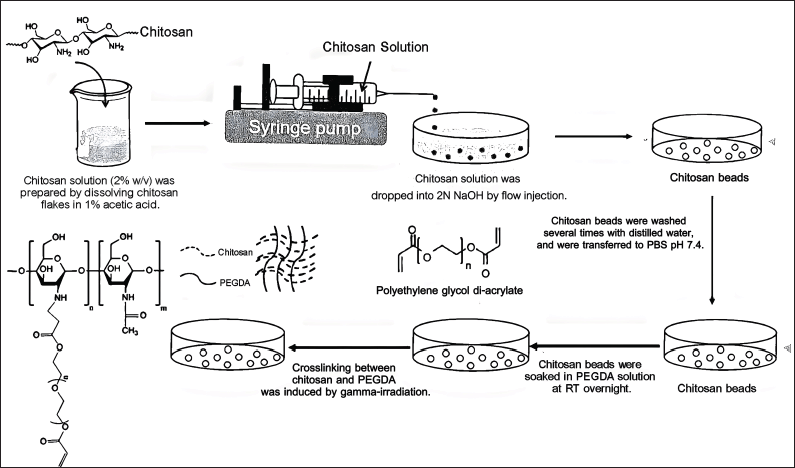 | Figure 1. The process of preparation of CS–PEGDA radiation-induced crosslinking beads for CU microencapsulation. [Click here to view] |
In vitro assessment of CS–PEGDA beads dissociation and morphology in simulated gastrointestinal tract
A model of a gastrointestinal tract was tested to reveal the effects of varying pH on the dissociation and morphology of the CU-loaded CS–PEGDA beads. This reveals the retention and the release of CU from the beads in different parts of the gastrointestinal tract. Herein, we focused only on the different pH values, although, in vivo, other effects involve different enzymes while they pass through the gastrointestinal tract. This experiment included the mouth phase (simulated with pH = 6.8), the stomach phase (simulated with pH=1.5), and the small intestine phase (simulated with pH=7.0) in accordance with a previous study(Hasan et al, 2019; Tai et al., 2019). The simulated digestion process was conducted in a shaking water bath at 37°C. 1 mg of fresh CS–PEGDA beads was mixed with 1 ml of PBS at different pH values in a test tube, i.e., pH 6.8, 1.5, and 7.0, representing the mouth, stomach, and small intestine conditions, respectively. At time intervals of 30 minutes, lasting for 4 hours, observations of the bead dissociation. In addition, some beads were collected to observe the morphology.
Encapsulation study of CU in CS–PEGDA beads
The method has been adapted according to a previous study conducted by Rattanawongwiboon et al. (2020). CS–PEGDA (2 mg) was irradiated with a dose of 25 kGy and was placed in an Eppendorf tube. The ethanolic CU solution (2 mg/ml) at a volume of 1 ml was added to the sample tube. To induce encapsulation, the mixer was agitated at 60 rpm for 24 hours. CU-encapsulated CS–PEGDA beads were isolated by centrifugation at 1,000 rpm for 30 minutes. The supernatant was collected, and a quantitative analysis of CU was conducted using a UV-vis spectrophotometer at the wavelength of 425 nm. The efficiency of encapsulation (%EE) of CU in CS–PEGDA beads was calculated using
where A0 and A are the absorbance of the initial amount of CU and the amount of CU in the supernatant at 425 nm, respectively.
Release of CU from CS–PEGDA beads
The release profiles of CU from CS–PEGDA beads were obtained in the three media at pH values 6.8, 1.5, and 7.0, simulating the conditions in the mouth, stomach, and small intestine, respectively. A known amount of fresh CU-encapsulated CS–PEGDA beads was suspended in 10 ml of PBS (pH 7.4) and incubated in a thermal controlled (37 ± 0.5°C) agitated water bath at 80 rpm. A constant volume (1 ml) of PBS was withdrawn at different time intervals (30 minutes), which was later preplaced with 1 ml of new PBS buffer, to maintain the total volume. The observation was performed over 6 hours, and the cumulative amount of CU released from the CS–PEGDA beads into the PBS solution was measured using a UV-vis spectrophotometer at 425 nm. The CU cumulative release experiments were performed in triplicate. The percentage of CU released was calculated by
where A represents the initial absorption (at 425 nm), which represents the CU in the beads, and B is the absorption at different times, which represents the amount of CU in the solution that has been released.
Statistical analysis
The data, which were collected in triplicate, were summarized as mean ± SD and analyzed using one-way analysis of variance; statistical significance was defined as p < 0.05. The mean and standard deviation (SD) were calculated using SPSS version 28 (SPSS Inc., USA) statistical analyses, and Microsoft Excel 2019 was used.
RESULTS AND DISCUSSION
Morphological characterization, size of beads, porosity, and yield of CS–PEGDA beads
The scanning electron micrographs of the CS beads, CS–PEGDA beads, and CU loaded CS–PEGDA beads are shown in Figures 2 and 3. The beads had spherical shapes and showed surface indentations, probably caused by partial collapse of the polymer structure during drying, but no cracking was observed. The mean particle size of the CS bead ranged from 100 to 180 μm (mean ± SD = 120 ± 26.50 μm), whereas the mean particle size of the radiation-induced crosslinking CS–PEGDA ranged from 60 to 150 μm (mean ± SD = 90 ± 18.50 μm). Under the preparation conditions of CS–PEGDA as previously described, the yield of CS–PEGDA ranged between 34.11% and 59.64% (mean ± SD = 43.94 ± 0.10%). By calculating the percentage of porosity of the CS–PEGDA, the porosity of the bead is 25.70± 0.06% (as shown in Fig. 3B). Our result reveals that the yield is a little bit higher than those reported in previous studies, which obtained a maximum yield in the range of 30%–40%. The explanation is that higher concentrations of PEGDA result in its undergoing homopolymerization and or self-crosslinking higher concentration during irradiation (Li et al., 2017; Pasanphan et al., 2014). Some beads transformed into a gel-like structure, resulting in a low percentage of CS–PEGDA.
Data from the SEM micrographs showed that the synthesis of CS–PEGDA via radiation weakly affected the diameter of the beads. This seems to correlate with the previous experiment. Rattanawongwiboon et al. (2020) have previously attributed this phenomenon to the CS beads being formed when CS drops into an NaOH solution, as shown in Figure 1. The CS beads were then soaked in a PEGDA solution, where PEGDA diffused into the CS beads. The crosslinking reactions, from the gamma irradiation, occurred inside the CS beads, resulting in the formation of crosslinked CS–PEGDA beads, but this did not substantially change the CS bead size. Another report by Pasanphan et al. (2014) describes that the CS+ terminal is located on the surface of colloidal CS particles while the grafting material, PEGDA, is coated on their CS+ surfaces and forms the shell during graft copolymerization. Our electron micrograph data showed that the crosslinked CS–PEGDA complex induced by irradiation enabled the surface of the beads to be packed tighter and be smoother than the surface of the CS beads (Fig. 2C). As a result, the size of the CS–PEGDA bead is more compact than that of the CS beads.
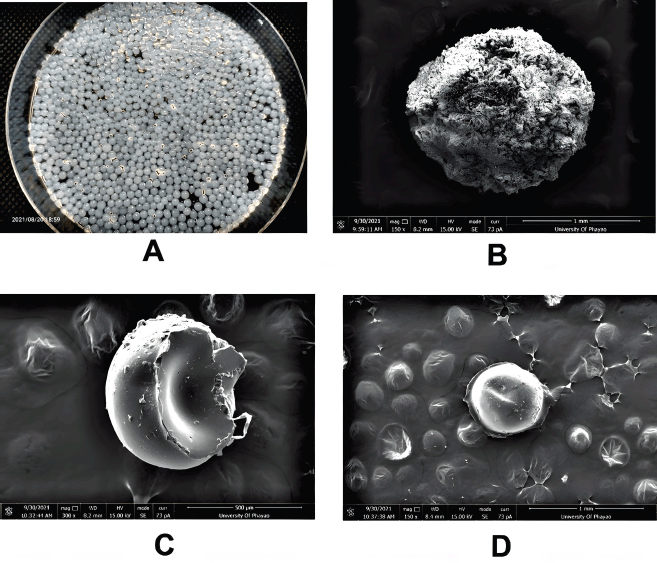 | Figure 2. (A) Fresh CS beads prepared by dropping chitosan solution (2% w/v in 1% acetic acid) into 2-M NaOH. (B, C, and D) Scanning electron micrograph of the CS bead, radiation-induced CS–PEGDA crosslinked bead, and CU loaded CS–PEGDA crosslinked beads after being air-dried, respectively. The surface of the PEGDA-coated CS bead is smoother than the CS bead, and the size of the bead is more compact. Indentation of the bead caused by drying may be observed, but there is no cracking on the surface of the bead. [Click here to view] |
Success of CS–PEGDA beads by radiation-induced synthesis
The FTIR spectra, shown in Figure 4, confirmed that CS beads conjugated with PEGDA were successfully produced.
Characteristic peaks for CS of O–H and C–H stretching vibrations and N–H bending appear at around 3,300–3,500, 3,000, and 1,600 cm−1, respectively. The peaks of N–H bending were transformed into amide I and amide II and match the peaks of a previous study conducted by Ji et al. (2020). For PEGDA, peaks around 3,000 and 2,800 cm−1 are characteristic of the alkyl C–H stretching and O–C=O acrylate group stretching. The results match those derived from the previous experiments (Rattanawongwiboon et al., 2018; Shameli et al., 2012; Wang et al., 2016). The FTIR spectra of CS–PEGDA and CS are similar, though an additional peak at 1,730 cm-1 is seen for PEGDA for the O–C=O bond stretching, Rattanawongwiboon et al. (2020). The presence of characteristic peaks of CS and PEGDA implies that the CS crosslinked with the PEGDA bifunctional vinyl groups.
Swelling degree of the CS–PEGDA beads
CS swells to a high degree owing to its rapid water adsorption that greatly limits its ability to control drug release (Motiei and Kashanian, 2017). The results of swelling studies are shown in Figure 5, where it can be seen that the pH caused varying degrees of swelling. The ratio of swelling was 85.23 ± 12.96%, 73.24 ± 17.66%, and 110.48 ± 15.68% in media with pH values 6.8, 1.5, and 7.0, respectively. No signs of deterioration of the beads were seen in the simulated gastrointestinal tract conditions over a period of 6 hours. The swelling at pH 7.0 was considerably larger than those at pH 1.5 and pH 6.8. This result seems to match those obtained from the SEM micrographs (Fig. 3, porosity and durability of the bead) and those obtained from the assessment of in vitro dissociation of the beads in the simulated gastrointestinal tract (Fig. 6). This implies that the synthesized CS–PEGDA beads are strong and durable enough to remain in the gastrointestinal tract and could keep sustained release of CU, as shown in the releasing profile below. In addition, CS–as shown in the result above, the high microporosity of the CS–PEGDA beads affects their swelling degree, which reflects their releasing profile.
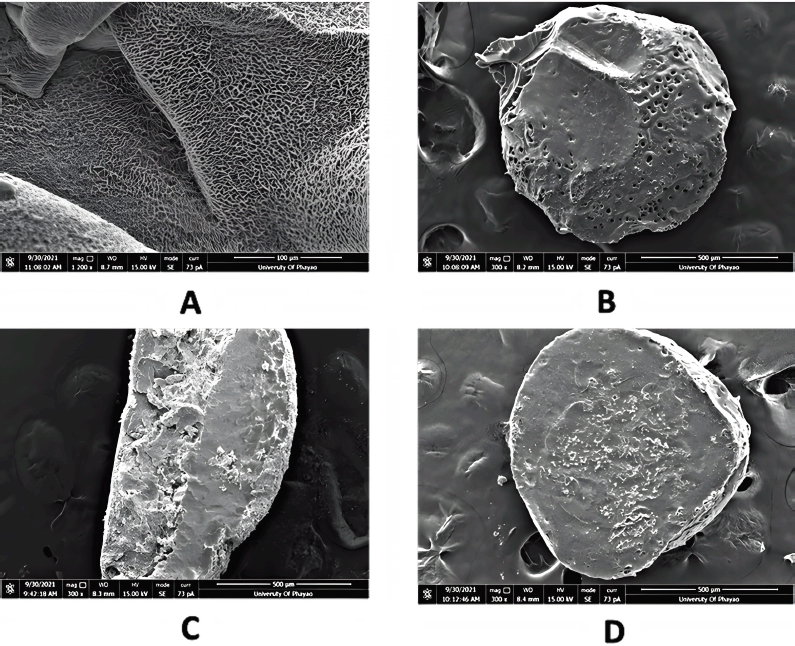 | Figure 3. Electron micrographs of (A) crosslinking fabrication of the CS and PEGDA on the surface of the bead, (B) porosity forming on the CS–PEGDA bead, (C) cross-section of the CS–beads, and (D) cross-section of the crosslinked CS–PEGDA beads. Note that the crosslinked CS–PEGDA bead is more durable to the razor cutting force. [Click here to view] |
In vitro assessment of CS–PEGDA beads dissociation and morphology in simulated gastrointestinal tract
To determine the tolerance of the CU encapsulated into CS–PEGDA beads at different pH values, we simulated the in vitro gastrointestinal tract conditions. The results on the stability of CU encapsulated into CS–PEGDA beads confirmed its excellent tolerance to various pH values of the simulated gastrointestinal tract, i.e., in the mouth (pH 6.8), stomach (pH 1.5), and small intestine (pH 7.0), as shown in Figure 6. A previous study conducted by Worthen et al. (2019) showed that the timescales of CS dissolution highly depended on the pH. The researchers varied the pH between 2.0 and 5.0. At pH 5.0, which was the highest pH of their examination, the beads remained, and no changes were observed on the surface over the first hour. After 8 hours the beads were slightly smaller, and after 16 hours the beads became deformed; and they completely dissolved after roughly 30 hours. Based on the study of Degen and Phillips (1996), the total time of emptying the stomach from food contents is 4-5 hours and emptying 50% of chyme from the small intestine takes 2.5–3 hours. Therefore, our data show that the stability of the CU-loaded CS–PEGDA beads over 4 hours (Fig. 6B) will enable sustained release of CU along the gastrointestinal tract without bursting release before it reaches the small intestine, which is the site of the main absorption. In addition, the beads can be stabilized in the small intestine and retain constant release of CU.
The cross-section of the CS bead, as shown in the SEM micrographs (Fig. 3), revealed that the bead could not maintain its spherical shape, but the CS–PEGDA bead maintained its shape. A plausible explanation is that the CS bead was too brittle and could not stand the force of being cut by a razor blade, whereas the formation of crosslinks enabled by gamma irradiation rendered the strength to the CS–PEGDA bead to maintain its original spherical shape. A similar result has been previously reported by Rattanawongwiboon et al. (2020). As a result, the strength of the CS–PEGDA bead is reasonable according to its simulated dissociation in the solution in the gastrointestinal tract.
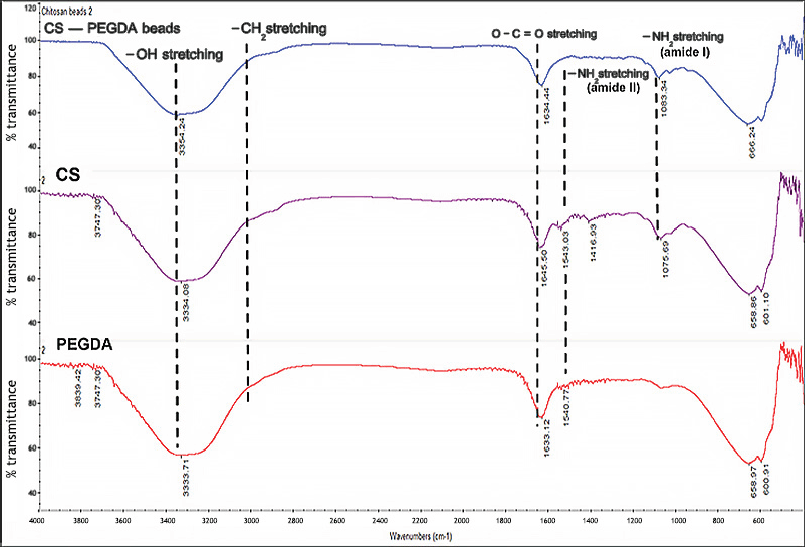 | Figure 4. FITR spectra of CS, PEGDA, and CS–PEGDA beads synthesized from 2% (w/v) of chitosan and 0.25% (w/v) of PEGDA by gamma irradiation at 25 kGy. (Note: peaks analyses were compared with calculated IR spectra of amide I and amide II studied by Ji et al., 2020.) [Click here to view] |
 | Figure 5. Swelling of CS–PEGDA beads. (A) Dried beads before soaking and (B) swollen beads after soaking in PBS buffer pH 7.4 for 24 hours. [Click here to view] |
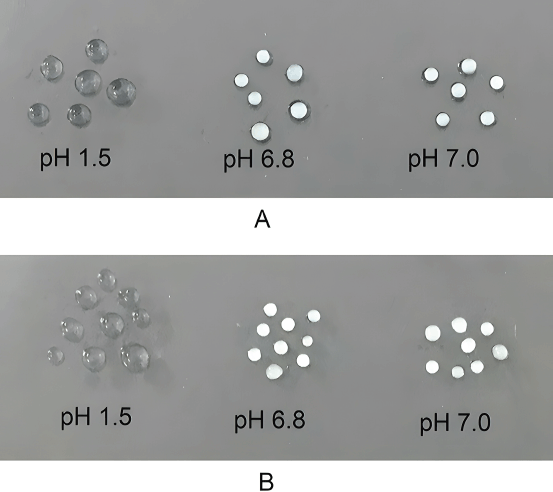 | Figure 6. Dissociation of CU loaded CS–PEGDA beads (A) at the first 30 minutes of incubation in simulated gastrointestinal fluid of pH values 1.5, 6.8, and 7.0 and (B) after incubation in simulated gastrointestinal fluid of pH values 1.5, 6.8, and 7.0 for 4 hours. The beads are durable with intact morphology with no sign of bursting. [Click here to view] |
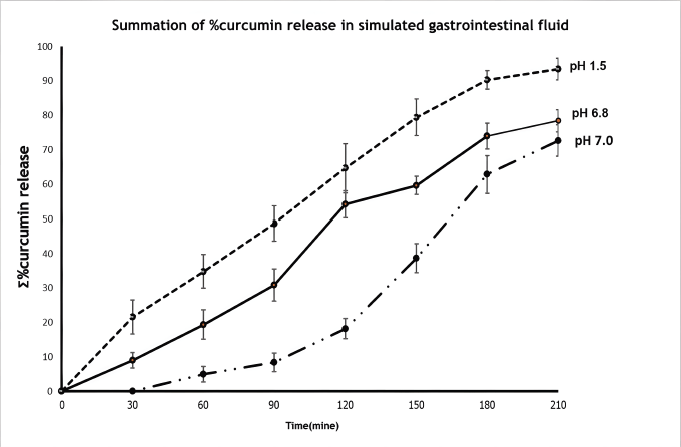 | Figure 7. CU release profile in three simulated gastrointestinal conditions. [Click here to view] |
Encapsulation Efficiency of CU in CS–PEGDA beads
As the efficiency of trapping CU into nanoparticles is important for its widespread use, the efficiency of different crosslinking/fabrication agents has been reported (Ahmad et al., 2021; Kolter et al., 2019; Mandez and Lopez, 2019; Saeed et al., 2020; Sorasitt2hiyanukarn et al., 2021; Yang et al., 2021). Our result on the encapsulation efficiency of CU in CS–PEGDA beads is 87.73 ± 1.25%, which matches the results of previous studies, which reported a range of 70%–90% depending on the conditions and the crosslinking/fabrication agents. However, our results are consistent with those reported in the studies conducted by Pasanphan et al. (2014) and Rattanawongwiboon et al. (2020), which used the same crosslinking agent, i.e., PEG, and the same method for preparing the CS–PEGDA beads. Pasanphan et al. (2014) reported that the initial concentration of the drug affected the drug encapsulation efficiency within the hybrid CS particles. We obtained the aforementioned results because we used an excessive amount of CU as the initial concentration.
Release of CU from CS–PEGDA beads
The CU-loaded CS–PEGDA beads showed CU was released over a period of 6 hours. The CU accumulation release profiles from CS–PEGDA beads are shown in Figure 7. The initial CU releases observed from all simulated conditions were in the range of 5%–20% during the first incubation. After 3–4 hours, the cumulative release rapidly increased, ranging between 20% and 90%, and complete release was noticed at the end of observation for 6 hours. The results suggest that CS–PEGDA could be suitable for the oral administration of CU.