INTRODUCTION
Cervical cancer is an uncontrolled growth from the tissue of the cervix of the female reproductive organ, which is strongly linked to persistent infection with oncogenic types of the human papillomavirus (HPV) (Ibeanu, 2011). In 2018, it was considered the second most prevalent cancer seen in women worldwide, with about 885,193 new cases and over 311,365 deaths annually (Aoki et al., 2020). Globally, the incidence and mortality of cervical cancer are as high as 13.1 and 6.9 per 100,000, respectively (Setiawan et al., 2020). Furthermore, in recent years, the incidence of cervical cancer has been more alarming in developing countries due to a lack of effective prevention measures such as HPV vaccines and cervical screenings, which are considered expensive. On the other hand, currently, there is no effective treatment for cervical cancer, especially in the advanced stages (Hu and Ma, 2018).
The search for effective cervical cancer treatment is currently challenging owing to its complex pathogenesis (Bhat et al., 2018). The carcinogenesis of cervical cancer was driven primarily by high-risk HPV infection, which expresses oncoproteins E6 and E7 (Vandermark et al., 2012). The high-risk E6 and E7 oncoproteins can degrade p53 proteins and inhibit the retinoblastoma protein (pRb), thus inhibiting the activation of apoptotic proteins such as caspases and escaping normal cell cycle control in G1 (Jung et al., 2013). The imbalance of proapoptotic and antiapoptotic proteins leads to sustained proliferation and resistance of apoptosis, leading to malignancy (Zhou et al., 2015).
Toxic side effects are a common issue of conventional chemotherapy and radiotherapy, which could reduce patients’ quality of life (Marjanovic et al., 2019). Antineoplastic agents’ poor selectivity results in a proliferation inhibitory effect, targeting the cancer cells and rapidly growing healthy cells, such as hair cells, epithelial cells, and hematopoietic precursors (Blowman et al., 2018). The undesirable effects include loss of appetite, diarrhea, nausea and vomiting, reduced white blood cell count, and hair loss (Rodenak-Kladniew et al., 2014). Molecular biology’s current advances have introduced the development of new anticancer drugs with enhanced cancer-cell-killing function that cause less alteration to the normal cells (Sampath et al., 2018).
Medicinal properties from plant-derived natural products provide a broad range of potential sources for drug discovery and cancer chemoprevention (Bayala et al., 2014). Inexhaustible sources of natural chemical constituents, suitable chemical characteristics, and various biological and pharmacological activities have been attributed to phytochemical-based-therapeutic agents (Blowman et al., 2018; Sampath et al., 2018). Cancer therapy has widely employed phytochemicals with potential antitumor activities (Khazir et al., 2014; Murata et al., 2013). Previous research has shown essential oils (EOs) anticancer properties with various mechanisms of action. Three EOs are currently gaining research attraction due to their growing evidence of anticancer properties, namely clove oil, eucalyptus oil, and citronella oil (Blowman et al., 2018). These EOs have plants of origin that are grown abundantly in tropical regions, including Indonesia, Vietnam, Thailand, Sri Lanka, India, and Malaysia in the Asiatic regions, and also Madagascar and Tanzania in African regions (Cortés-Rojas et al., 2014; Kaur et al., 2021; My et al., 2020).
Eugenol (4-allyl-2-methoxyphenol2-methoxy-4-prop-2-enyl phenol) is an aromatic phenylpropanoid phenol which is the major bioactive constituent in clove (Syzygium aromaticum) (Bezerra et al., 2017). While well known for its culinary use for its flavor and aroma, eugenol has been documented to have medicinal properties, such as analgesic, anesthetic, antiseptic, antiviral, antimicrobial, antioxidant, anti-inflammatory, and anticancer activities (Barboza et al., 2018; Batiha et al., 2020). A recent study reveals eugenol cytotoxicity against cervical carcinoma (HeLa cell lines) that can be explored in advance as an antineoplastic agent (Das et al., 2018; Permatasari et al., 2021).
1,8-Cineole (1,3,3-trimethyl-2-oxabicyclo[2.2.2]octane), which is also known as eucalyptol, is a cyclic ether monoterpene as a major constituent of eucalyptus EO. Traditional usages of eucalyptol are food flavoring agents, airway diseases’ symptom relievers, and aromatherapy (Rodenak-Kladniew et al., 2014). Eucalyptol is known to have diverse biological and pharmacological applications, such as antibacterial, antifungal, anti-inflammatory, antioxidant, and hypolipidemic (Rodenak-Kladniew et al., 2020). Recently, 1,8-cineole has been shown to possess anticancer properties (Sampath et al., 2018).
Another EO with potential as an anticancer agent is citronella oil. Cymbopogon nardus or citronella oil’s main components are citronellal (3,7-dimethyl-6-octenal) and geraniol ((E)-3,7-dimethyl-2,6-octadien-1-ol), monoterpene aldehydes, and monoterpene alcohols (Bayala et al., 2020). Previously, citronella oil was particularly known for its antioxidant, anti-inflammatory, and antimicrobial properties (De Toledo et al., 2016; Ka?ániová et al., 2017; Pontes et al., 2019). Recent studies have shown that citronella oil exhibits antiproliferative properties against cancer cells (HeLa, LNCaP, and MDA-MB-231 cell lines) in vitro, which are thought to be derived from the activity of citronellal and geraniol (Bayala et al., 2020; Ho et al., 2020; Kim et al., 2012; Zhang et al., 2018).
Resisting apoptosis, sustaining the proliferation cycle, and evading cellular arrest are some of the cancer hallmarks (Hanahan and Weinberg, 2011). Therefore, the search for anticancer therapeutics is focused on promoting cancer cells’ apoptosis and induced cellular arrest. This research aimed to evaluate antitumor activities of three EOs, i.e., S. aromaticum, Melaleuca cajuputi, and C. nardus, toward cervical carcinoma (HeLa cell line) viability.
MATERIALS AND METHODS
HeLa cell culture preparation
HeLa (Henrietta Lacks) human cervical carcinoma cell lines were obtained from the American Type Culture Collection (ATCC) and stored in the Biomedical Laboratory, Faculty of Medicine, Universitas Brawijaya (Malang, East Java, Indonesia). Cells were then incubated at 5% CO2 at 37°C, pH 7.2–7.4. The culture was made in Dulbecco’s modified Eagle’s medium (Invitrogen, MA, USA) with 10% (v/v) fetal bovine serum and antibiotics (100 IU/ml penicillin, 100 μl/ml streptomycin) (Das et al., 2018). HeLa cells were grown and routinely harvested with a trypsin-ethylenediaminetetraacetic acid (EDTA) [CH2N(CH2CO2H)2]2 solution (Permatasari et al., 2021).
EO preparation
Syzygium aromaticum, M. cajuputi, and C. nardus plant samples were obtained from PT, Jeeva Aroma Nusantara Institute (Surabaya, East Java, Indonesia), in July 2021. The aerial parts of the samples were grounded and subjected to Soxhlet extraction. EOs were extracted in a Soxhlet by diluting 10 g of each grounded plant with a solvent of 150 ml n-hexane at 150°C (Butt et al., 2019). The extraction process was continued until the mixture returned to its original solvent color. EOs were collected, and the solvent was removed by a Soxhlet extractor at 150°C.
Cell viability assay
HeLa cells were seeded in 24-well plates and treated with different concentrations of each EO from three plants (S. aromaticum, M. cajuputi, and C. nardus) at doses of 0, 30, 60, and 120 µg/ml. The 0 µg/ml dose served as a negative control for each group. Cells were incubated at 37°C for 24 hours treatment in RPMI-1640. The concentration determination was based on the previous research, with some modifications. The current research used different delivery methods, of which the EOs were encapsulated (Permatasari et al., 2021).
Trypan blue exclusion assay
Cytotoxic activity of the EOs was examined by the trypan blue exclusion test (Sinha et al., 2011). The trypan blue assay aimed to estimate the number of viable and dead cells within a population. After 24 hours of treatment, the cell culture medium was discarded from each plate. Trypsinization was performed by administering 250 μl of trypsin-EDTA 0.25% for 10 minutes. Cells were then suspended, 20 µl of which was taken. Subsequently, cells were stained with 250 μl 0.1% trypan blue dye. Next, 10 µl of cell suspension was subjected to a hemocytometer for cell quantification and observed under a microscope to calculate its viability. Viable cells did not get stained nor emit fluorescence under a light microscope. Conversely, dead cells were stained dark blue. Cell calculations were carried out at four repetitions of each sample to obtain the average value. The half-maximal inhibitory concentration (IC50) was derived from the cell calculation (Gupta et al., 2019; Muthukrishnan et al., 2018).
Cell apoptosis assay
The number of cells that undergo apoptosis induced by S. aromaticum, M. cajuputi, and C. nardus EOs was measured by the flow cytometry method using Annexin V (AV)–fluorescein isothiocyanate (FITC)–propidium iodide (PI) staining (Sigma). First, the cells were washed twice with a cold cell staining buffer and then resuspended in an AV binding buffer at a concentration of 0.25–1.0 × 107 cells/ml. After that, 100 μl of cell suspension was transferred to a 5 ml test tube, and a mixture with a concentration of 20 μg/ml was made by adding 5 μl of FITC AV and 10 μl of PI solution. The cells were then vortexed slowly and incubated for 15 minutes at room temperature (25°C) in the dark. Finally, 400 μl of AV binding buffer was added to each tube and then analyzed with a flow cytometer according to the standard machine settings. The data were analyzed using the Cell Pro-Quest software on a computer connected to a flow cytometer to measure the percentage of apoptosis in the HeLa cells (Crowley et al., 2016; Rieger et al., 2011).
Statistical analysis
One-way analysis of variance (ANOVA) test, followed by post-hoc Tukey’s multiple comparison test, was adopted for the statistical evaluation of the result’s data. Cytotoxic activity was presented as a percentage and calculated using the following formula of Sithole and Mukanganyama (2017):
To evaluate the correlation of EO doses and HeLa cells’ viability, Pearson’s correlation test was also performed. Significant differences were established at p ≤ 0.05. Statistical analysis and graphical representation were accomplished using GraphPad Prism 9 (GraphPad Software, San Diego, USA).
RESULTS
Cytotoxic activity in a dose-dependent pattern
To investigate the cytotoxic ability of three EOs from S. aromaticum, M. cajuputi, and C. nardus against human cervical cancer cells, HeLa cells received treatment of three different EOs for 24 hours at doses of 30, 60, and 120 μg/ml, with the negative control of that of 0 μg/ml. Following 24 hours of treatment, a trypan blue exclusion test was performed to determine the viability of the HeLa cell line. Figure 1 shows the microscopic view of the aftertreatment HeLa cell line. The viability of the HeLa cell lines decreased after treatment with the three EOs following a dose-dependent pattern, shown by the increasing clear zone. Three EOs showed potent antiproliferative activity, as depicted by a significant decline in HeLa cells’ viability at doses of 30, 60, and 120 μg/ml, at 24 and 48 hours after treatment compared to the control (p-value < 0.0001). At the maximum dose of 120 μg/ml, the administration of all three EOs was able not only to inhibit proliferation but also to reduce the HeLa cells number into a minimum amount, of which almost no cancer cells were viable at the end of 48 hours. In brief, all three EOs caused a significant reduction in the number of viable HeLa cell lines, indicating their potent cytotoxic effects. Figure 2 shows the cytotoxic activity of the three plant EOs.
Comparison of all three EOs’ efficacy to reduce HeLa cells’ viability
The efficacies of the three EOs were compared at doses of 30, 60, and 120 μg/ml at 24 and 48 hours after treatment. Figure 3 shows the comparison of treatment using three EOs toward viable cells number after 24 hours at a similar dose. At the lowest administration dose of 30 μg/ml, the three EOs had an insignificant difference in viable cell number (p = 0.4468). Meanwhile, when the cells were treated with a dose of 60 μg/ml, S. aromaticum showed the highest efficacy, while M. cajuputi had the lowest. At the maximum dose of 120 μg/ml, the EOs from S. aromaticum also displayed the highest cytotoxic activity, as demonstrated by the least viable cell contrast to that of the other plants.
At 48 hours after treatment, S. aromaticum at a dose of 30 μg/ml also showed the highest efficacy, while the efficacy of M. cajuputi was similar to that of C. nardus (Fig. 4). At doses of 60 and 120 μg/ml, S. aromaticum was still the highest and comparable to M. cajuputi, while C. nardus still had the lowest efficacy. We can see that the S. aromaticum EO demonstrated the most potent efficacy at higher doses of 60 and 120 μg/ml when compared to the other two EO sources.
IC50 determination
The half-maximal IC50 was extracted to measure the efficacy of the plants’ EOs as an anticancer agent to induce 50% population death of HeLa cells. Table 1 summarizes the IC50 values of the three Eos’ extract (S. aromaticum, M. cajuputi, and C. nardus) on the HeLa cervical cancer cell line. All three EOs showed potent activity in inhibiting the proliferation of cancer cells. The S. aromaticum extract yielded lower IC50 values after 48 hours incubation, which represented a more potent inhibitory effect after longer treatment. In addition, S. aromaticum EO was most potent in inducing cell death, as it exhibited the lowest 48 hours IC50 among the others. On the other hand, treatment with M. cajuputi and C. nardus suppressed the HeLa cell line with lower IC50 values at 24 hours in comparison to after 48 hours of incubation. The IC50 values of all three EOs were below the lowest dose used in the current research of 30 μg/ml. Therefore, future research should be performed at lower than 30 μg/ml to examine the optimized dose of each EO.
EOs’ proapoptotic activity
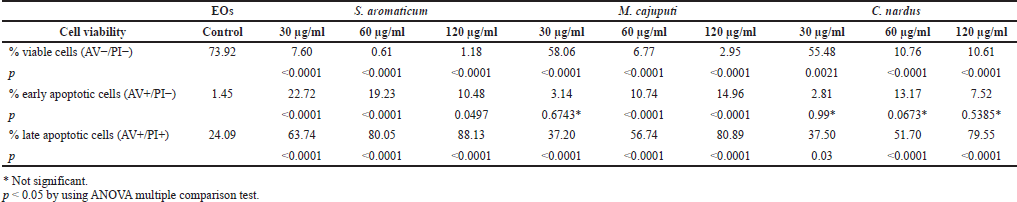
The proapoptotic activity measurement of the three EOs was examined using flow cytometry with AV and PI following the administration of four doses EOs (30, 60, and 120 μg/ml and 0 μg/ml as the control). Cells that failed to express both AV and PI (AV−/PI−) were regarded as healthy cells. Cells only expressing AV but not PI (AV+/PI−) were measured as cells that underwent early apoptosis. On the contrast, cells expressing both AV and PI (AV+/PI+) were measured as cells that underwent late apoptosis or necrosis due to the three EOs. Table 2 presents the AV/PI analysis as a percentage of viable, early, and late apoptotic cells.
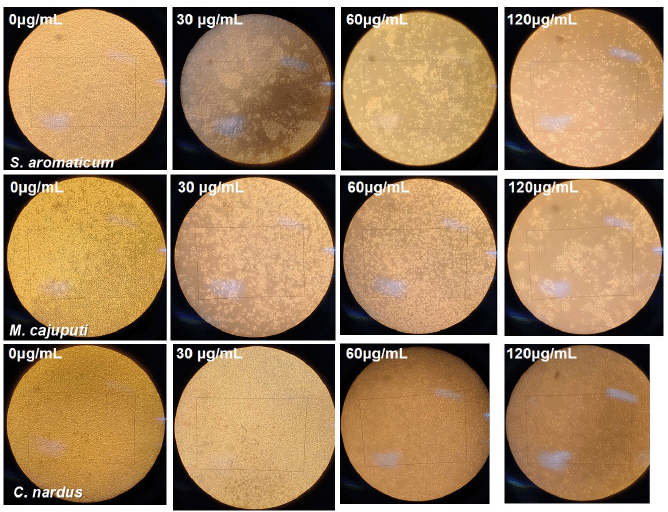 | Figure 1. Microscopic feature of cytotoxic activity assessment from the treatment at 0, 30, 60, and 120 μg/ml of S. aromaticum, M. cajuputi, and C. nardus under a light microscope. Escalating concentration was associated with a reduced number of viable cervical cancer cells. [Click here to view] |
The AV/PI assay scatter plots are shown in Figure 5. The negative control displayed negative results for both Annexin and PI (AV−, PI−), which indicated that the cancer cells were still viable as they did not receive any treatments. Meanwhile, at the lowest dose of 30 ug/ml, the populations treated with EOs from M. cajuputi and C. nardus still dominated with viable cells: negative results for both probes (AV−/PI−) as of the negative control. Meanwhile, increasing dosage shifted the expression into positive results of both AV and PI. Cells treated with S. aromaticum EOs consistently demonstrated AV+/PI+. Here, we can see that EOs’ treatment induced the necrotic cell stage of the HeLa cancer cells.
Syzygium aromaticum EO treatment significantly decreased viable cell population (AV−/PI− HeLa cells) (p < 0.0001), with a noticeable increment in both early apoptotic (AV+/PI−) and late apoptotic (AV+/PI+) HeLa cells (p < 0.0001; two ANOVA tests) at a dose of 30 ug/ml compared to control. Afterward, the percentage of AV−/PI− HeLa cells had a slight decrease trend although not significant at the 60 ug/ml dose, followed by a slight increase at the 120 ug/ml dose. Likewise, there was a decline in a dose-dependent relationship of the percentage of AV+/PI− HeLa (p < 0.01; 30 ug/ml compared to 120 ug/ml). On the other hand, there was a rise in the late apoptotic cell percentage (AV+/PI+) reciprocal to the dose increment (p < 0.001), with the exception of a slight increase between the doses of 60 ug/ml and 120 ug/ml (Fig. 6A).
A distinct trend can be inferred from the administration of M. cajuputi EO. The percentage of AV−/PI− HeLa cells was diminished significantly in respect to the dose (p < 0.0001), with a special case of an insignificant decrease between the doses of 60 and 120 ug/ml. On the other hand, there was a positive trend in the percentage of AV+/PI− and AV+/PI+ correspondingly to the dose (Fig. 6B).
Comparatively, C. nardus EO significantly decreased the number of viable HeLa cells (AV−/PI−) following dose escalations (p < 0.05, two-way ANOVA test). Dose increments of C. nardus EO increased the percentage of AV+/PI+ significantly (p < 0.05, two-way ANOVA test), while there was insignificance in the percentage of AV+/PI− HeLa cells (Fig. 6C).
DISCUSSION
Cervical cancer progression was driven primarily by high-risk HPV infection, which expresses oncoproteins E6 and E7 (Ibeanu, 2011). The E6 and E7 oncoproteins have the ability to degenerate p53 proteins and hinder the expression of pRb, thus suppressing the activation of apoptotic proteins such as caspases and escaping normal cell cycle control in G1 (Wang et al., 2004).
Cancer therapy conventional modalities are surgery, radiotherapy, chemotherapy, and combinative treatment (Shahneh et al., 2013). Apoptosis, a programmed cell death mechanism, plays a role as the protective means against cancer development (Gong et al., 2019). Nonetheless, cancer hallmarks include evasion and resistance to apoptosis (Fouad and Aanei, 2017). The imbalance of proapoptotic and antiapoptotic proteins leads to sustained proliferation and resistance of apoptosis, leading to malignancy (Zhou et al., 2015). Apoptosis disruption due to oncogenic mutations leads to initiation and progression of tumors (tumorigenesis) and also drug resistance, which results in chemotherapy failure (Gong et al., 2019; Shahneh et al., 2013). Therefore, a promising approach in the development of cancer therapies is to induce apoptosis and interfere with the cell cycle (Sampath et al., 2018).
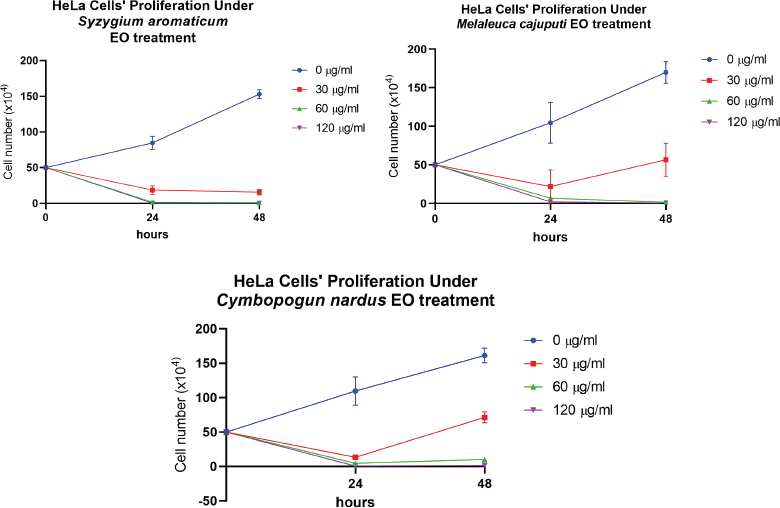 | Figure 2. Comparison of the antiproliferative effects of A) M. cajuputi, B) S. aromaticum, and C) C. nardus EOs in the HeLa cell line at 0, 30, 60, and 120 μg/ml. Values are presented as the mean ± SD from eight replications. [Click here to view] |
EOs refer to secondary metabolites that plants generate to protect themselves against pests and predators and increase appeal to pollinators or promote seed dissemination. These oils can be obtained from numerous parts of plants, for instance, roots and aerial parts: stems, leaves, flowers, fruits, and seeds, according to their species (Mugao et al., 2020). EOs have been known for their anticancer properties with a wide range of mechanisms, such as their role in cancer chemoprevention and the ability to interfere with established tumor cells and interact with the tumor microenvironment (Bayala et al., 2014; Blowman et al., 2018). Several plant-derived antineoplastic agents have been documented to cause cell cycle arrest and induce both intrinsic and extrinsic apoptosis pathways. Moreover, cancer cell target specificity and less toxicity toward healthy cells have been observed in EOs application as a single agent (Blowman et al., 2018).
This study established the potential anticancer activity of EOs from three plants of S. aromaticum, M. cajuputi, and C. nardus for human cervical cancer. The HeLa cervical cancer cell line was treated with one plant EO for 24 and 48 hours before being assessed for death and viable cells calculation. The cytotoxic activity, represented by the IC50 value, was examined at two time points, at 24 and 48 hours, since the EOs may exert different half-lives. In addition, the IC50 was further evaluated at 48 hours owing to the average doubling time of HeLa cells which is approximately in the range of 33–35 hours (Sato et al., 2016). All EOs explored in this research manifested cytotoxic activity to suppress the proliferation and viability of cervical cancer cells, as depicted by the significant reduction of HeLa cell viability correspondingly to the EO dose augmentation. Syzygium aromaticum EO demonstrated a more potent inhibitory effect after a longer incubation period, and it showed the lowest IC50 in 48 hours treatment. The two other EOs displayed lower IC50 in 24 hours compared to 48 hours treatment. The EO from C. nardus exhibited the lowest IC50 after 24 hours incubation, showing the most potent activity compared to the other two.
The cell death phase, subsequent to the exposure to anticancer activity from the EOs, is portrayed by flow cytometry analysis of AV/PI staining. Cells in the viable-, early-, and late-stage apoptotic states were identified using AV/PI. Membrane permeability affects PI’s ability to enter a cell. Cells in the viable or in the early apoptotic state with the plasma membrane in an intact state prevent the PI staining penetration inside. On the contrary, a decrease in plasma and nuclear membrane integrity enables PI penetration into the nucleus and DNA staining of late apoptotic and necrotic cells (Chothiphirat et al., 2019; Rieger et al., 2011). During the late phase of apoptosis, such as secondary necrotic cells, there is an increase in cell permeabilization (Poon et al., 2009).
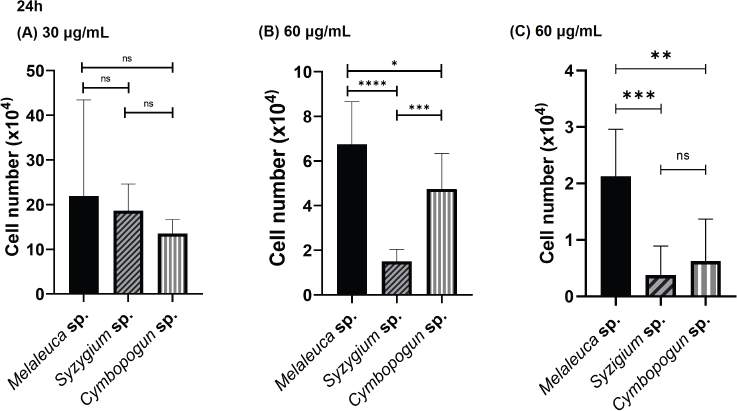 | Figure 3. Comparison of cytotoxic effects of S. aromaticum, M. cajuputi, and C. nardus EOs at 24 hours after treatment against the HeLa cervical cancer cell line investigated by incubating HeLa cells with each extract in 12-well plates for 24 hours. (A) 30 μg/ml, (B) 60 μg/ml, and (C) 120 μg/ml. Values are presented as the mean ± SD from eight replications. Statistical one-way ANOVA and Tukey’s multiple comparison test were carried out. ns: not significant, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. [Click here to view] |
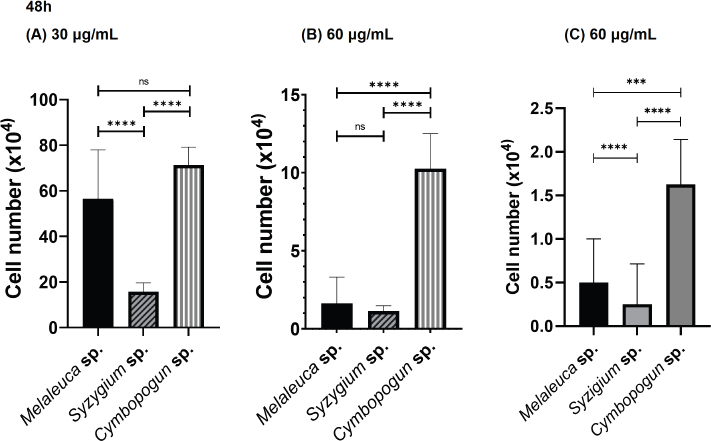 | Figure 4. Comparison of cytotoxic effects of S. aromaticum, M. cajuputi, and C. nardus EOs at 48 hours after treatment against the HeLa cervical cancer cell line investigated by incubating HeLa cells with each extract in a 12-well plate for 48 hours. (A) 30 μg/ml, (B) 60 μg/ml, and (C) 120 μg/ml. Values are presented as the mean ± SD from eight replications. Statistical one-way ANOVA and Tukey’s multiple comparison test were performed. ns: not significant, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. [Click here to view] |
 | Table 1. The EOs dose to cause half-maximal inhibitory effect (IC50) on HeLa cervical cancer cell line in 24 and 48 hours. [Click here to view] |
There is an identical outcome of the administration of three EOs to the cervical cancer cells: exposure to EO treatment decreased the population of viable cervical cancer cells (AV−/PI−). In comparison to the control group, all HeLa cervical cancer cell cultures incubated with EOs underwent early- and late-stage apoptosis, as displayed by the increment of cells exhibiting AV+/PI− and AV+/PI+. Late apoptotic, necrotic cells, with double-positive AV/PI (AV+/PI+), dominate the population of all three EOs, and fewer cells appear in early apoptotic cells. The number of late-stage apoptotic cells increased following the increase in concentration exposure of S. aromaticum, M. cajuputi, and C. nardus. Therefore, the EOs demonstrated a potent activity in inducing late-stage apoptosis in a dose-dependent relationship of the cervical cancer cell. This study discovered the potent anticancer activity of the three EOs.
The exploration of anticancer treatment is currently concentrated on agents that induce cell apoptosis. Nevertheless, as tumor cells develop various strategies to evade apoptosis during tumorigenesis, chemotherapeutic development which enhances necrotic cell death has been appreciated (Yu et al., 2020). Necroptosis is considered to be a novel necrotic cell death programmed form, which resembles the apoptosis mechanism and necrosis morphology. Necroptosis came up as a novel phase to be targeted in the search for anticancer therapy (Gong et al., 2019). These research findings suggested the potential antineoplastic agent of EOs with late apoptotic/necrotic activity for cervical cancer.
Previous research revealed apoptosis-inducing activity against human cervical cancer of eugenol from S. aromaticum EO with the proposed mechanism from the expression upregulation of proteins related to cell cycle arrest and apoptosis such as caspase-3 and p53 (Permatasari et al., 2019). Eugenol has demonstrated potent activity in altering the epithelial-mesenchymal transition pathway in metastasis development by repressing transcription factors and mesenchymal cell markers. At the same time, it also enhances the expression of epithelial cell markers of HeLa cervical cancer, which indicates a less invasive property (Permatasari et al., 2021). In breast cancer, eugenol-treated cancer cells displayed a greater expression of proteins involved in cell death apoptosis: caspase-3, caspase-7, and caspase-9. Therefore, eugenol can serve as a potent cytotoxic compound with the ability to trigger caspase-mediated cell death in breast cancer cells (Abdullah et al., 2018). Moreover, eugenol, incorporated into the other active agents, displays a synergistic antitumor activity. Amarogentin and eugenol combined impeded cells from proliferating and forming colonies, while also inducing apoptosis. The antiproliferative mechanism was proposed through the means of downregulating the expression of cyclinD1 and upregulating cell cycle inhibitors (Pal et al., 2018).
 | Table 2. AV/PI staining flow cytometry results to evaluate the HeLa cells after treatment with EOs. [Click here to view] |
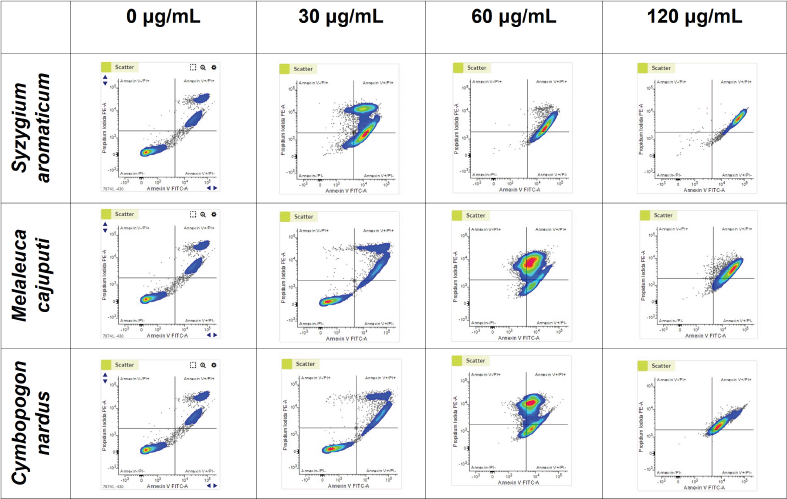 | Figure 5. Flow cytometry scatter plots of AV/PI assays. HeLa cells were incubated separately with the EOs of S. aromaticum, M. cajuputi, and C. nardus at concentrations of 0, 30, 60, and 120 μg/ml. [Click here to view] |
Anticancer activity of eucalyptol, the main component of M. cajuputi, has been observed in other types of malignancies. Eucalyptol induced apoptosis in human skin carcinoma (A431) cell lines by mitochondrial apoptotic proteins activation. Cells receiving eucalyptol treatment demonstrated a shift of expression of the mitochondrial pro- and antiapoptotic proteins in proportion to the dose, such as Bcl-2 and mitochondrial Cyt-C downregulation with upregulation of proapoptotic factors such as Bax, p53, caspase (Sampath et al., 2018). Furthermore, eucalyptol also displayed a potent ability to suppress human colorectal cancer proliferation by means of inducing apoptosis. Administration of eucalyptol was linked with silencing survivin and Akt. It promotes the expression of p38, which subsequently leads to cleaved caspase-3 and ultimately causes apoptosis (Murata et al., 2013). Eucalyptol also showed antineoplastic potential effects when combined with other drugs. In combination with antisenescence drugs to treat hepatocellular cancer, eucalyptol induces G0/G1 arrest and apoptosis in HepG2 cells by oxidative stress and the other pathways. Eucalyptol also enhances the sensitivity of cancer cells toward antisenescence drugs (Rodenak-Kladniew et al., 2020). Together with simvastatin, eucalyptol increased G0/G1 cell cycle arrest in human small cell lung cancer (Rodenak-Kladniew et al., 2020).
Citronellal is the major constituent of C. nardus EOs. Citronellal and geraniol, monoterpene alcohol, and monoterpene aldehyde, respectively, were recognized for their antiproliferative activities (Bayala et al., 2020). Citronellal demonstrates a potent ability to promote transient calcium signaling and activate human olfactory receptor OR1A2, which results in phosphorylation of p38 MAPK and hinders cell proliferation (Maßberg et al., 2015). Conversely, geraniol is able to restrain AKT signaling and stimulate AMPK signaling, which consequently leads to mTOR inhibition, thus cell apoptosis and autophagy (Kim et al., 2012). It also enhances the expression of Bax and suppresses Bcl-2 expression, causing DNA damage, and cell cycle arrest at the G2/M phase in cancer cell lines (Zhang et al., 2018).
Study limitation
There are some aspects that need further attention in the application of EOs. Being highly volatile, EOs’ application differs from a common plant crude extract. As a result, EOs need some modification regarding their drug delivery, such as developing an encapsulation method, to optimize their utilization in the targeted cells. This study made use of whole EOs rather than isolation of specific bioactive compounds from each EO. Exploration of the constituents of the volatile compounds from the EOs of each plant still has not been conducted. Isolating individual compounds is considered to be essential as they may possess distinct uses and medicinal properties. Therefore, the authors recommend the analysis of chemical composition and isolating specific compounds.
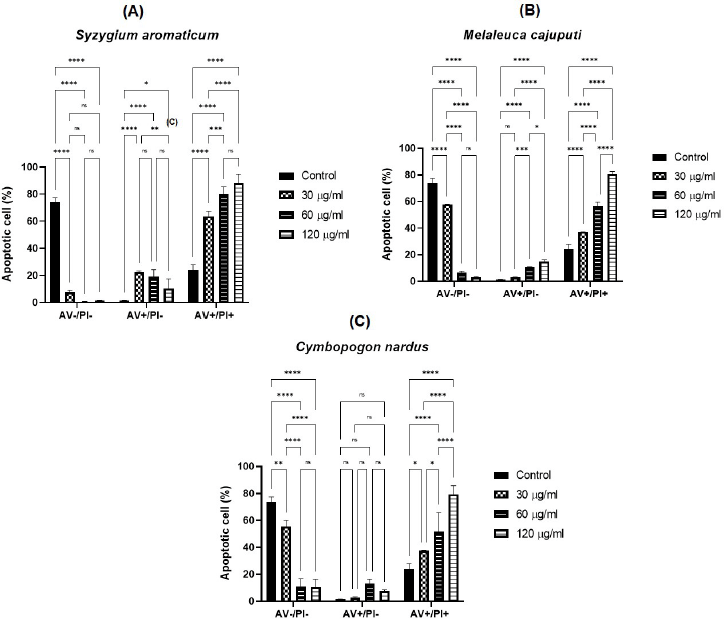 | Figure 6. Flow cytometric analysis of AV/PI. EOs of (6A) S. aromaticum, (6B) M. cajuputi, and (6C) C. nardus induce apoptosis in the HeLa cervical cancer cell line. AV(−) PI(−), AV(+) PI(−), and AV(+) PI(+) cells served as viable cells, early apoptotic cells, and late apoptotic/necrotic cells, respectively. [Click here to view] |
This experimental study explored the cytotoxic activity of the EOs on a small population of HeLa cells. Hence, the authors recommend continuing research on these three plant EOs in a larger HeLa cell population. It was also necessary to study the efficacy of the EOs in animal model studies to explore the pharmacology of their bioactive compounds.
In the instance of study significance, this study revealed the potential of the three aforementioned EOs to halt the proliferation and induce apoptosis of HeLa cell cultures. While prior research on the other types of cancer has revealed some possible mechanisms for the cytotoxic activity of each EO, this study has not yet explored possible pathways and mechanisms in HeLa cells due to the absence of mechanism investigation and an in vitro adverse drug reaction diagnostic test. Further study is required to fill the gap. There is an inadequate exploration of factors contributing to the reduced viability of HeLa cells. For that reason, the toxic side-effect possibilities could not be assessed extensively. Upcoming research may focus on the evaluation of the expression of proteins linked with apoptosis and cell cycle arrest in the cervical cancer cell lines and exploration of any possible toxic side effects from treatment with EOs.
CONCLUSION
This study reveals the cytotoxic activity of EOs from three plants, S. aromaticum, M. cajuputi, and C. nardus, to induce death in the HeLa human cervical cancer cell line. EOs’ treatment significantly decreased the number of viable HeLa cells, with a rise in cytotoxic activity respective to the dose increment. At 24 hours of treatment, C. nardus EOs stand as the most potent EO, while S. aromaticum EOs are the most potent at 48 hours. Flow cytometry analysis indicated EOs treatment’s positive effect in triggering late-stage apoptosis in cervical cancer cells proportionately with the concentration. These findings suggest these EOs have potential anticancer properties that could be further explored as a combination with antineoplastic drugs for cervical cancer therapy.
ACKNOWLEDGMENTS
The authors express gratitude to the Biochemistry Department, Faculty of Medicine, Brawijaya University, East Java, Indonesia. They would also like to thank PT Jeeva Aroma Nusantara for EOs’ provision.
CONFLICTS OF INTEREST
The authors disclosed no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
FUNDING
This research was financially supported by “Hibah Lembaga Penelitian dan Pengabdian Masyarakat (LPPM),” Brawijaya University, 2021.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
PUBLISHER’S NOTE
This journal stays in a neutral position in regard to jurisdictional claims within the published institutional affiliation.
REFERENCES
Abdullah ML, Hafez MM, Al-Hoshani A, Al-Shabanah O. Anti-metastatic and anti-proliferative activity of eugenol against triple negative and HER2 positive breast cancer cells. BMC Complement Altern Med, 2018; 18:1–11; doi:10.1186/S12906-018-2392-5. CrossRef
Aoki ES, Yin R, Li K, Bhatla N, Singhal S, Ocviyanti D, Saika K, Suh M, Kim M, Termrungruanglert W. National screening programs for cervical cancer in Asian countries. J Gynecol Oncol, 2020; 31:e55; doi:10.3802/JGO.2020.31.E55. CrossRef
Barboza JN, da Silva Maia Bezerra Filho C, Silva RO, Medeiros JVR, de Sousa DP. An overview on the anti-inflammatory potential and antioxidant profile of eugenol. Oxid Med Cell Longev, 2018; 2018:3957262; doi:10.1155/2018/3957262. CrossRef
Batiha GE, Alkazmi LM, Wasef LG, Beshbishy AM, Nadwa EH, Rashwan EK. Syzygium aromaticum L. (Myrtaceae): traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules, 2020; 10:202; doi:10.3390/biom10020202. CrossRef
Bayala B, Bassole IHN, Gnoula C, Nebie R, Yonli A, Morel L, Figueredo G, Nikiema JB, Lobaccaro JM, Simpore J. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS One, 2014; 9:1–11; doi:10.1371/journal.pone.0092122. CrossRef
Bayala B, Coulibaly AY, Djigma FW, Nagalo BM, Baron S, Figueredo G, Lobaccaro JA, Simpore J. Chemical composition, antioxidant, anti-inflammatory and antiproliferative activities of the essential oil of Cymbopogon nardus, a plant used in traditional medicine. Biomol Concepts, 2020; 11:86–96; doi:10.1515/bmc-2020-0007. CrossRef
Bezerra PD, Militão GCG, de Morais MC, de Sousa DP. The dual antioxidant/prooxidant effect of eugenol and its action in cancer development and treatment. Nutrients, 2017; 9(12):1367; doi:10.3390/nu9121367. CrossRef
Bhat MY, Gul MZ, Maurya R, Khan N, Qureshi IA, Ghazi IA. Growth inhibition and apoptosis inducing effects of Artemisia absinthium L. fractions on chronic myeloid leukemia (K562) cells. J Biol Act Prod Nat, 2018; 8:70–89; doi:10.1080/22311866.2018.1461577. CrossRef
Blowman K, Magalhães M, Lemos MFL, Cabral C, Pires IM. Anticancer properties of essential oils and other natural products. Evid Based Complement Altern Med, 2018; 2018:3149362; doi:10.1155/2018/3149362. CrossRef
Butt AS, Nisar N, Mughal TA, Ghani N, Altaf I. Anti-oxidative and anti-proliferative activities of extracted phytochemical compound thymoquinone. J Pak Med Assoc, 2019; 69:1479–85; doi:10.5455/JPMA.302643156.
Chothiphirat A, Nittayaboon K, Kanokwiroon K, Srisawat T, Navakanitworakul R. Anticancer potential of fruit extracts from vatica diospyroides Symington type SS and their effect on program cell death of cervical cancer cell lines. Sci World J, 2019; 2019:5491904; doi:10.1155/2019/5491904. CrossRef
Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pac J Trop Biomed, 2014; 4(2):90–6; doi: 10.1016/S2221-1691(14)60215-X. CrossRef
Crowley LC, Marfell BJ, Scott AP, Waterhouse NJ. Quantitation of apoptosis and necrosis by annexin V binding, propidium iodide uptake, and flow cytometry. Cold Spring Harb Protoc, 2016; 2016:953–7; doi:10.1101/pdb.prot087288. CrossRef
Das A, Harshadha K, Dhinesh Kannan SK, Raj KH, Jayaprakash B. Evaluation of therapeutic potential of eugenol-a natural derivative of Syzygium aromaticum on cervical cancer. Asian Pac J Cancer Prev, 2018; 19:1977; doi:10.22034/APJCP.2018.19.7.1977.
De Toledo LG, Ramos MA, Spósito L, Castilho EM, Pavan FR, Lopes Éde O, Zocolo GJ, Silva FA, Soares TH, Dos Santos AG, Bauab TM, De Almeida MT. Essential Oil of Cymbopogon nardus (L.) rendle: a strategy to combat fungal infections caused by Candida species. Int J Mol Sci, 2016; 17:1252; doi:10.3390/IJMS17081252. CrossRef
Fouad YA, Aanei C. Revisiting the hallmarks of cancer. Am J Cancer Res, 2017; 7:1016–36. Available via www.ajcr.us/ISSN:2156-6976/ajcr0053932
Gong Y, Fan Z, Luo G, Yang C, Huang Q, Fan K, Cheng H, Jin K, Ni Q, Yu X, Liu C. The role of necroptosis in cancer biology and therapy. Mol Cancer, 2019; 18:1–17; doi:10.1186/s12943-019-1029-8. CrossRef
Gupta R, Bhatt LK, Momin M. Potent antitumor activity of laccaic acid and phenethyl isothiocyanate combination in colorectal cancer via dual inhibition of DNA methyltransferase-1 and histone deacetylase-1. Toxicol Appl Pharmacol, 2019; 377; doi:10.1016/j.taap.2019.114631. CrossRef
Hanahan D, Weinberg RA. Leading edge review hallmarks of cancer: the next generation. Cell, 2011; 144:646–74; doi:10.1016/j.cell.2011.02.013. CrossRef
Ho Y, Suphrom N, Daowtak K, Potup P, Thongsri Y, Usuwanthim K. Anticancer effect of Citrus hystrix DC. leaf extract and its bioactive constituents citronellol and, citronellal on the triple negative breast cancer MDA-MB-231 cell line. Pharmaceuticals (Basel), 2020; 13:476; doi:10.3390/PH13120476. CrossRef
Hu Z, Ma D. The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med, 2018; 7:5217; doi:10.1002/CAM4.1501. CrossRef
Ibeanu OA. Molecular pathogenesis of cervical cancer. Cancer Biol Ther, 2011; 11:295–306; doi:10.4161/CBT.11.3.14686. CrossRef
Jung YS, Kato I, Kim HRC. A novel function of HPV16-E6/E7 in epithelial-mesenchymal transition. Biochem Biophys Res Commun, 2013; 435:339–44; doi:10.1016/j.bbrc.2013.04.060. CrossRef
Ka?ániová M, Terentjeva M, Vukovic N, Puchalski C, Roychoudhury S, Kunová S, Kl?ga A, Tokár M, Kluz M, Ivanišová E. The antioxidant and antimicrobial activity of essential oils against Pseudomonas spp. isolated from fish. Saudi Pharm J, 2017; 25:1108; doi:10.1016/J.JSPS.2017.07.005. CrossRef
Kaur H, Bhardwaj U, Kaur R. Cymbopogon nardus essential oil: a comprehensive review on its chemistry and bioactivity. J Essent Oil Res, 2021; 33:205–20; doi:10.1080/10412905.2021.1871976. CrossRef
Khazir J, Riley DL, Pilcher LA, De-Maayer P, Mir BA. Anticancer agents from diverse natural sources. Nat Prod Commun, 2014; 9:1655–69. CrossRef
Kim SH, Park EJ, Lee CR, Chun JN, Cho NH, Kim IG, Lee S, Kim TW, Park HH, So I, Jeon JH. Geraniol induces cooperative interaction of apoptosis and autophagy to elicit cell death in PC-3 prostate cancer cells. Int J Oncol, 2012; 40:1683–90; doi:10.3892/IJO.2011.1318. CrossRef
Marjanovic D, Plesinac-Karapandzic V, Rundic SS, Tomasevic A, Saric M, Miskovic I, Nidzovic B, Petrasinovic P. Acute toxicity of postoperative intensity-modulated radiotherapy and three-dimensional conformal radiotherapy for cervical cancer: The role of concomitant chemotherapy. J BUON, 2019; 24:2347–54.
Maßberg D, Simon A, Häussinger D, Keitel V, Gisselmann G, Conrad H, Hatt H. Monoterpene (−)-citronellal affects hepatocarcinoma cell signaling via an olfactory receptor. Arch Biochem Biophys, 2015; 566:100–9; doi:10.1016/J.ABB.2014.12.004. CrossRef
Mugao LG, Gichimu BM, Muturi PW, Mukono ST. Characterization of the volatile components of essential oils of selected plants in Kenya. Biochem Res Int, 2020; 2020:8861798; doi:10.1155/2020/8861798. CrossRef
Murata S, Shiragami R, Kosugi C, Tezuka T, Yamazaki M, Hirano A, Yoshimura Y, Suzuki M, Shuto K, Ohkohchi N, Koda K. Antitumor effect of 1, 8-cineole against colon cancer. Oncol Rep, 2013; 30:2647–52; doi:10.3892/or.2013.2763. CrossRef
Muthukrishnan S, Vellingiri B, Murugesan G. Anticancer effects of silver nanoparticles encapsulated by Gloriosa superba (L.) leaf extracts in DLA tumor cells. Futur J Pharm Sci, 2018; 4:206–14; doi:10.1016/j.fjps.2018.06.001. CrossRef
My TTA, Loan HTP, Hai NTT, Hieu LT, Hoa TT, Thuy BTP, Quang DT, Triet NT, Anh TTV, Dieu NTX, Trung NT, Hue NV, Tat PV, Tung VT, Nhung NTA. Evaluation of the inhibitory activities of COVID-19 of Melaleuca cajuputi oil using docking simulation. ChemistrySelect, 2020; 5:6312–20; doi:10.1002/slct.202000822. CrossRef
Pal D, Sur S, Roy R, Mandal S, Kumar Panda C. Epigallocatechin gallate in combination with eugenol or amarogentin shows synergistic chemotherapeutic potential in cervical cancer cell line. J Cell Physiol, 2018; 234:825–36; doi:10.1002/jcp.26900. CrossRef
Permatasari HK, Effendi AB, Qhabibi FR, Fawwaz F, Subali DA. Eugenol isolated from Syzygium aromaticum inhibits HeLa cancer cell migration by altering epithelial-mesenchymal transition protein regulators. J Appl Pharm Sci, 2021; 11:49–53; doi:10.7324/JAPS.2021.110507. CrossRef
Permatasari HK, Kusuma ID, Mayangsari E. Minyak Cengkeh (Syzygium aromaticum) Menginduksi Apoptosis pada Sel Kanker Servik HeLa melalui Peningkatan Kadar Protein p53. J Kedokt Brawijaya, 2019; 30:185; doi:10.21776/ub.jkb.2019.030.03.4. CrossRef
Pontes EKU, Melo HM, Nogueira JWA, Firmino NCS, de Carvalho MG, Catunda Júnior FEA, Cavalcante TTA. Antibiofilm activity of the essential oil of citronella (Cymbopogon nardus) and its major component, geraniol, on the bacterial biofilms of Staphylococcus aureus. Food Sci Biotechnol, 2019; 28:633; doi:10.1007/S10068-018-0502-2. CrossRef
Poon IKH, Hulett MD, Parish CR. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ, 2010; 17:381–97; doi:10.1038/cdd.2009.195. CrossRef
Rieger AM, Nelson KL, Konowalchuk JD, Barreda DR. Modified Annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J Vis Exp, 2011:2597; 50; doi:10.3791/2597. CrossRef
Rodenak Kladniew B, Polo M, Montero Villegas S, Galle M, Crespo R, García De Bravo M. Synergistic antiproliferative and anticholesterogenic effects of linalool, 1,8-cineole, and simvastatin on human cell lines. Chem Biol Interact, 2014; 214:57–68; doi:10.1016/j.cbi.2014.02.013. CrossRef
Rodenak-Kladniew B, Castro MA, Crespo R, Galle M, García de Bravo M. Anti-cancer mechanisms of linalool and 1,8-cineole in non-small cell lung cancer A549 cells. Heliyon, 2020; 6:e05639; doi:10.1016/j.heliyon.2020.e05639. CrossRef
Sampath S, Subramani S, Janardhanam S, Subramani P, Yuvaraj A, Chellan R. Bioactive compound 1,8-Cineole selectively induces G2/M arrest in A431 cells through the upregulation of the p53 signaling pathway and molecular docking studies. Phytomedicine, 2018; 46:57–68; doi:10.1016/j.phymed.2018.04.007. CrossRef
Sato S, Rancourt A, Sato Y, Satoh MS. Single-cell lineage tracking analysis reveals that an established cell line comprises putative cancer stem cells and their heterogeneous progeny. Sci Rep, 2016; 6:1; doi:10.1038/SREP23328. CrossRef
Setiawan D, Andrijono, Hadinegoro SR, Meyta H, Sitohang RV, Tandy G, Perwitasari DA, Postma MJ. Cervical cancer prevention in Indonesia: an updated clinical impact, cost-effectiveness and budget impact analysis. PLoS One, 2020; 15:e0230359; doi:10.1371/JOURNAL.PONE.0230359. CrossRef
Shahneh FZ, Valiyari S, Baradaran B, Abdolalizadeh J, Bandehagh A, Azadmehr A, Hajiaghaee R. Inhibitory and cytotoxic activities of Salvia Officinalis L. extract on human lymphoma and leukemia cells by induction of apoptosis. Adv Pharm Bull, 2013; 3:51; doi:10.5681/APB.2013.009.
Sinha S, Biswas D, Mukherjee A. Antigenotoxic and antioxidant activities of palmarosa and citronella essential oils. J Ethnopharmacol, 2011; 137:1521–7; doi:10.1016/j.jep.2011.08.046. CrossRef
Sithole S, Mukanganyama S. Evaluation of the antiproliferative activity of Maerua edulis (Capparaceae)on Jurkat-T cells. J Biol Act Prod from Nat, 2017; 7:214–27; doi:10.1080/22311866.2017.1342561. CrossRef
Vandermark ER, Deluca KA, Gardner CR, Marker DF, Schreiner CN, Strickland DA, Wilton KM, Mondal S, Woodworth CD. Human papillomavirus type 16 E6 and E 7 proteins alter NF-kB in cultured cervical epithelial cells and inhibition of NF-kB promotes cell growth and immortalization. Virology, 2012; 425:53–60; doi:10.1016/j.virol.2011.12.023. CrossRef
Wang JL, Zheng BY, Li XD, Ångström T, Lindström MS, Wallin KL. Predictive significance of the alterations of p16INK4A, p14ARF, p53, and proliferating cell nuclear antigen expression in the progression of cervical cancer. Clin Cancer Res, 2004; 10:2407–14; doi:10.1158/1078-0432.CCR-03-0242. CrossRef
Yu B, Yuan B, Li JZ, Kiyomi A, Kikuchi H, Hayashi H, Hu X, Okazaki M, Sugiura M, Hirano T, Fan Y, Pei X, Takagi N. JNK and autophagy independently contributed to cytotoxicity of arsenite combined with tetrandrine via modulating cell cycle progression in human breast cancer cells. Front Pharmacol, 2020; 11:1087; doi:10.3389/FPHAR.2020.01087/FULL. CrossRef
Zhang G, Qi F, Yan Q, Zheng Z, Liu J, Chen Y. Geraniol and geranyl acetate induce potent anticancer effects in colon cancer Colo-205 cells by inducing apoptosis, DNA damage and cell cycle arrest. J BUON, 2018; 23:346–52.
Zhou R, Wei C, Liu J, Luo Y, Tang W. The prognostic value of p53 expression for patients with cervical cancer: a meta analysis. Eur J Obstet Gynecol Reprod Biol, 2015; 195:210–3; doi:10.1016/J.EJOGRB.2015.10.006. CrossRef