INTRODUCTION
Vaginal inflammation, or vaginitis, is caused by a variety of infectious and noninfectious factors (Al-Marabeh et al., 2017; Vieira-Baptista et al., 2022). Bacteria, fungi, viruses, and parasites can all penetrate the vaginal area (Al-Marabeh et al., 2017; Lin et al., 2021). The three most common types of infectious vaginitis are bacterial vaginosis (BV), vulvovaginal candidiasis (VVC), and trichomonal vaginitis (Abdul-Aziz et al., 2019). Lactobacilli, which when present in sufficient numbers defend against pathogenic bacterial species, populate the healthy vaginal tracts of reproductive-aged women (Kumar et al., 2011). As a result, lactobacilli deficiency disrupts the vaginal microbiota’s homeostasis and increases the number of anaerobic organisms, which contributes to BV (Ventolini, 2016). Although the majority of cases of BV are asymptomatic, it is distinguished by the discharge of homogeneous grayish-white foul secretions, a fishy odor after intercourse or during menstruation, and an increase in vaginal pH to even more than 4.5 (Paladine and Desai, 2018). BV prevalence ranges from 8% to 51% depending on geographic region, socioeconomic class, and ethnicity (Kenyon et al., 2013). Vaginal microbiota are essential for maintaining a healthy vaginal environment, and disruption of this system has been linked to poor vaginal health and other negative consequences (Greenbaum et al., 2019; Kroon et al., 2018). Vaginal microbiota are constantly changing and are influenced by hormonal changes, sexual activity, and hygiene (Huang et al., 2014a, 2014b). Aside from that, hormone levels fluctuate at different times. As a result, the microbial population that lives in the human vaginal canal exhibits a wide range of homeostatic states (Ravel et al., 2011). Although the vaginal microbiota’s composition is constantly changing, it is heavily influenced by factors such as food, behavior, and the stages of a woman’s reproductive cycle. The vaginal microbial ecology changes significantly during the menopausal stage (Vitali et al., 2017). Several systemic and reproductive diseases have been linked to changes in female gonadal hormone levels (Zhang et al., 2018). As a result of these challenges, novel treatment approaches have emerged (Al-Ghazzewi and Tester, 2016). Probiotics, which are living microorganisms made up primarily of Lactobacillus spp., are widely used to improve digestive health. They may, however, be useful in obstetrics or treating gynecological problems to build and maintain normal vaginal microbiota to treat conditions such as BV, VVC, and PTB (Akour, 2020). The primary goal of this study was to obtain a comprehensive picture of the organization and composition of the vaginal microbial population at various stages of gynecology. Other objectives included (a) determining whether there are any links between female sex hormone levels and vaginal microbial diversity and (b) comparing the pattern of community diversity in cases of BV (an abnormal disease characterized by microbial dysbiosis or imbalance followed by vaginal discharge) to that in a healthy state.
MATERIALS AND METHODS
Patients
Between November 2020 and March 2021, researchers conducted a cross-sectional study at the General Samarra Hospital in Samarra, Salah al-Din Governorate, Iraq (Fig. 1). During normal prenatal visits to the Gynecology and Obstetrics Clinic, nonpregnant women (n = 100) between the ages of 20 and 60 years were included with the suspicion of having symptomatic and asymptomatic vaginal infections. Patients’ information, such as age, location, past abortion history, last menstrual cycle, and clinical signs and symptoms, was gathered through referral files and laboratory request forms. The Research Council Board of Samarra University’s Faculty of Medical Laboratory Science gave its approval for the project.
Sample collection and transportation
A doctor collected three high vaginal swabs from each patient and examined them for sex hormones in Amie’s transport medium. They were transported in under three hours to the Microbiology Laboratory Department of the University of Samarra’s Faculty of Medical Laboratory Sciences.
Sample processing
Following submission to the Microbiology Laboratory, the samples were promptly analyzed for potential pathogenic microbe isolation and identification using conventional laboratory procedures (Abdul-Aziz et al., 2019). One of the blood agar plates was filled with three high vaginal swabs from each patient. They were incubated aerobically for 48 hours at 37°C in a moist CO2 environment. After that, each dish was placed in a sterile container containing 10 ml of freshly cooked beef medium (Al-Kafaween et al., 2020 Al-Kafaween et al., 2020a; Khleifat et al., 2006; Khleifat et al., 2019; Qaralleh et al., 2019).
Diagnosis of BV
BV can be diagnosed clinically and/or microbiologically. The clinical diagnostic criteria published by Amsel et al. (1983)are still in use today. Three of the four criteria in Amsel’s technique, a vaginal pH of more than 4.5, the presence of clue cells in the vaginal fluid, a milky, homogeneous vaginal discharge, and the emission of a fishy odor after adding 10% potassium hydroxide to the vaginal fluid, should all be documented (Al-kafaween et al., 2021; Cheesbrough, 2000; Khleifat et al., 2021).
Identification of genital microorganisms
To identify the bacteria, scientists employed the Gram stain, colony morphology, germ tube test, motility, and biochemical tests including catalase, coagulase, DNase, indole, lactose fermentation, citrate utilization, urease, Kligler Iron, oxidase, sugar fermentation, and an automated system (Al-Kafaween et al., 2020b; Cheesbrough, 2000; Khleifat et al., 2008).
Macroscopic and colonial morphology
The Nugent scoring method was used to diagnose BV using direct Gram stain smears (Brookheart et al., 2019). The following morphotypes were found on the Gram smear: curved Gram-variable rods, small Gram-variable rods (Gardnerella vaginalis morphotypes), small Gram-negative rods (Bacteroides species morphotypes), large Gram-positive rods (Lactobacillus morphotypes), large Gram-negative rods (Bacteroides species morphotypes), and Gram-positive cocci (Mobiluncus species morphotypes). The pattern of typical vaginal flora does not include an increase in Gram-positive cocci. Normal was defined as a score of zero to three, while BV was defined as 4 to 10. Each initial positive culture yielded a single colony, which was identified using morphological characteristics (colony size, form, pigment hue, and nature). Bacterial smears stained with the Gram stain were used to analyze cellular morphological characteristics of bacterial cells, such as Gram response, form, organization, and spores. Biochemical tests were performed based on the Gram reaction (Khleifat and Abboud, 2003; Al-Kafaween et al., 2020a; Al-kafaween et al., 2021; Cappuccino and Welsh, 2018; Khleifat et al., 2021; Al-kafaween et al., 2021; Al-kafaween et al., 2020b).
 | Figure 1. An image showing the areas of sample collection in the city of Samarra, Iraq. [Click here to view] |
Biochemical tests
Indole test
This test was carried out by inoculating a peptone water medium with bacterial growth from the loop for 24–48 hours at 37oC. Drops of Kovac’s reagent were added to the indole test (p-dimethylaminobenzaldehyde in amyl alcohol). A red color ring formed at the top of the soup, indicating a good response (Brown and Smith, 2014).
Methyl red test
Select bacterial colonies were injected into tubes of an MR-VP broth, which were then incubated at 37°C for 24–48 hours. After that, it was given five drops of methyl red reagent. Good resultant full glucose hydrolysis is indicated by the presence and observation of a red color (Brown and Smith, 2014).
Voges–Proskauer test
Selected bacterial colonies were injected into the MR-VP broth tubes, which were then incubated at 37°C for 24–48 hours. After that, 0.6 ml alpha naphthol (reagent A) and 0.2 ml 40% KOH solution were added to the result (reagent B). The presence of acetoin or acetyl-methyl-carb after 15 minutes indicates a good result owing to partial hydrolysis of glucose, which produces acetoin or acetyl-methyl-carbinol (Brown and Smith, 2014).
Citrate utilization test
The bacterial colonies were inoculated and cultured for 24–48 hours at 37°C after the Simmons citrate slants were autoclave-sterilized. The organisms were able to use citrate as their only carbon source, as evidenced by the shift in medium color from green to blue (Abboud et al., 2010; Cappuccino and Welsh, 2018; Tarawneh et al., 2009).
Urease test
By inoculating the urea medium with bacterial growth, this test was done. At 37°C, the tubes were cultured for 24–48 hours. A good outcome was signified by the medium becoming pink (Al-Asoufi et al., 2017; Cappuccino and Welsh, 2018; Khleifat et al., 2021; Al-kafaween et al., 2020a).
Motility test
This test was carried out by stabbing tested bacteria into a tube containing a semisolid medium and incubating it at 37°C for 24–48 hours. The spread of development beyond the stab line was a sign of a successful outcome (Al-Kafaween et al., 2020b; Tille, 2015).
Catalase test
Catalase is an enzyme that catalyzes the oxidation of hydrogen peroxide to produce oxygen. The chosen bacterial colonies were streaked on a nutritional agar medium and cultured at 37°C for 24 hours, after which the growth was transferred to a clean slide with a drop of 3% H2O2. A good outcome is indicated by the production of gas bubbles (Cappuccino and Welsh, 2018).
Oxidase test
This test requires the presence of a bacterial oxidase enzyme that catalyzes the transfer of electrons between electron donors in the bacteria and a redox dye (tetramethyl-p-phenylenediamine dihydrochloride), which was reduced to a deep purple color. A strip of filter paper was soaked in a small amount of freshly prepared reagent, and with a sterile wooden stick, the colony to be tested was picked up and spread on the filter paper. A bright deep purple color developed within 5–10 seconds, indicating a good outcome (Cappuccino and Welsh, 2018).
VITEK 2 compact system
This device was used to confirm isolates after diagnosing them in the usual ways. It is used to diagnose most types of bacteria through a diagnostic kit for the device. The kit contains 47 pits inoculated with a bacterial suspension of 24 hours and incubates for 24 hours, and the device records the color changes that occur as a result of the growth of bacteria.
Antibiotic susceptibility testing
All bacteria isolates were tested for susceptibility using the Clinical and Laboratory Standards Institute (CLSI) 2020 modified disc diffusion technique. Antibiotic resistance was evaluated using the CLSI 2020 standards and the six antibiotic discs AMC, AZM, DA, CN, LEV, and MET. The inoculums for this test were made by diluting the suspension with 0.5 McFarland standard tubes after adding the isolated colonies formed on a blood agar plate to 5 ml of a nutrient broth and incubating at 35°C for 18 hours (Tarawneh et al., 2021; Tarawneh et al., 2022). A swab of the growth was taken from a diluted solution and streaked across the surface of the medium multiple times on Muller-Hinton agar plates. Using a pair of sterile forceps, antibiotic discs were placed on each plate. The discs were positioned 15 mm from the plate’s edge on a 15 cm plate. To ensure even contact with the medium, each disk was gently pushed into place. For 18–24 hours, the plates were kept at 37°C in an incubator. A ruler was used to measure the diameter of each zone (including the disc’s diameter), and the results were recorded in mm. The results were then interpreted according to CLSI (Al-Essa et al., 2021; Al-kafaween et al., 2021; Al-Kafaween et al., 2020a, 2020b; Clinical and Laboratory Standards Institute, 2017; Khleifat and Abboud, 2003; Mahmoud et al., 2020; Tarawneh et al., 2021, 2022).
Sex hormones assay
Estradiol and progesterone were measured using the Estradiol Test System and the Progesterone Test System (Monobind Inc., USA). These were performed in microplate wells, and the absorbance at 450 nm was recorded after 30 minutes of adding the stop solution according to the manufacturer’s instructions.
Statistical analysis
The results were statistically analyzed using the SPSS statistical program by applying the analysis of variances test and chi χ2 test, and the significant differences were compared using the Ducum test with multihull rouge probability levels of 0.05 and 0.01.
RESULTS AND DISCUSSION
Isolation and identification
In Samarra, Salah al-Din Governorate, Iraq, 100 high vaginal swabs were isolated from infected and healthy women and compared based on bacterial type (Fig. 1). The results showed that only 95 of the samples were positive cultures (95%), where 70 (70%) were infected and 25 (25%) were healthy, while 5 (5%) samples were negative cultures where 1 (1%) was infected and 4 (4%) were healthy. As shown in Figure 1, the statistical analysis revealed no significant differences in infection between infected and healthy people. There is only one reason for do not grow in negative samples because the patient may have taken antibiotics before they were given the sample, despite the fact that the verification of antibiotic doesn’t quite absorbed before the sample is taken or may return the cause of another type of influential factors of infection, which is required to do some other techniques to identify such as virus, mycoplasma, chlamydia, and others. Our findings are consistent with previous studies in Iraq and Afghanistan (Razzak et al., 2011; Wójkowska-Mach et al., 2021), which revealed that 95% and 91.32%, respectively, of infected-healthy women had vaginitis and the results were bacterial culture positives and 5 samples gave culture negatives.
Other investigators, however, reported a lower percentage of infections from Erbil and Basrah (Iraq) reporting rates of 68.3% and 67.6%, respectively (Khalid and Yassin, 2017; Lhwak and Abbas, 2018). The high rate of positive BV cultures in our study is most likely due to the fact that we chose only women with complaints of abnormal vaginal discharge and vaginitis, and the differences between our results and others could be due to sample size, and our target populations were selected by physicians of only women patients with symptoms of vaginitis such as abnormal vaginal discharge, itching, burning, and lower abdominal pain.
The chi square (χ2) test was used to find out statistical differences. p values less than 0.010 were considered statistically significant (*), whereas those more than > 0.010 were not. Statistical analysis showed that in Table 1 the incidence of microorganisms isolated showed culture positive 70 (70%), 12 (12.63%) were Gram-positive bacteria, while Gram-negative bacteria were 58 (61.05%) in infected women significantly higher compared to that from healthy women 15 (15.78%) were Gram-positive bacteria, while Gram-negative bacteria were 10 (10.52%) (p < 0.051). Our findings contradicted the findings of Essa and Hussein (2018) in Mosul (Iraq) and Erbil (Iraq), who found that the majority of vaginitis infections had Gram-positive bacterial infection.
 | Table 1. The incidence of Gram-positive and Gram-negative bacteria in vaginal infection in relation to infected and healthy women. [Click here to view] |
 | Figure 2. Distribution of women patients with vaginitis in relation to infected and healthy women. Pearson’s chi square = 6.649, p value = 0.010. [Click here to view] |
The number and percentage of microorganisms isolated from 95 patients with vaginitis and the distribution of isolated microorganisms among infected and healthy women with vaginitis are shown in Table 2 and Figure 2. The most common organisms isolated belonged to Gram-negative bacteria and were Escherichia coli at 16 (16.84%): in infected women 9 (9.47%) and healthy women 7 (7.36%). Other results from Pakistan (13.7%) that were consistent with ours showed the same type of bacteria and the same percentage (Mumtaz et al., 2008). The fact that this bacterium is part of the normal fecal flora and that different virulence factors contribute to their pathogenicity may explain the high prevalence of these bacteria in bacterial vaginitis, and the difference in results may be explained by the number of samples taken and the period (year) of the study. The second most common isolated microorganisms from women with vaginitis were Proteus vulgaris at 8 (8.42%), Acinetobacter at 7 (7.36%), Proteus mirabilis at 7 (7.36%), Lactobacillus at 6 (6.31%), and Citrobacter at 6 (6.31%). The highest percentages of the isolates belonged to Gram-positive bacteria and were Staphylococcus saprophyticus at 5 (5.26%) and Staphylococcus aureus at 4 (4.21%) followed by Enterococcus faecalis at 4 (4.21%), Bacillus stearothermophilus at 3 (3.15%), Bacillus megaterium at 3 (3.15%), Enterococcus faecium at 1 (1.05%), and Leuconostoc pseudomesenteroides at 1 (1.05%). A study in Al-Diwaniya revealed high prevalence of isolated S. aureus (5.6%) and S. saprophyticus (4.8%) (Ranjit et al., 2018). These findings contradicted a previous study conducted in Mosul, Iraq, which found that the prevalence of S. saprophyticus infection was 1.9 % (Khudhur Mohammed and Jawad, 2019). Most female vaginal infections are caused by bacteria that are endogenous to the female genital tract, as evidenced by the results presented above (Arianpour et al., 2009).
The composition of the vaginal ecosystem is not static but changes over time and in response to endogenous and exogenous influences (Eschenbach et al., 2001). The vaginal canal of women in labor has a vital role in the health of their newborns, including establishing their early gut flora (Gabriel et al., 2018). Pathogens found in the blood of newborns with early-onset sepsis were found to be significantly linked to maternal vaginal infections with E. coli or S. aureus in a recent study (Kim et al., 2017). The relation between the age of the patient and the highest percentage of infection was detected in the age group <30 years at 36 (37.89%) while the least infected age group was 60 and above at 2.1%. In Figure 3, statistical analysis showed that in a positive culture the incidence of infection in the age group < 30 years was significantly higher compared to other age groups at p < 0.008. Similar results (Dhabhai et al., 2022) from India showed the highest prevalence of infection in ages > 30 years. The findings of this study agreed with those of Lamichhane et al. (2014) from Nepal, who found the highest prevalence of infection in the age group 20–29 years. Vaginal infections were found to be most common in young, sexually active females. The different patterns of infection observed in this study could be explained by current conditions in each country, such as health education, cleanliness, and medical coverage.
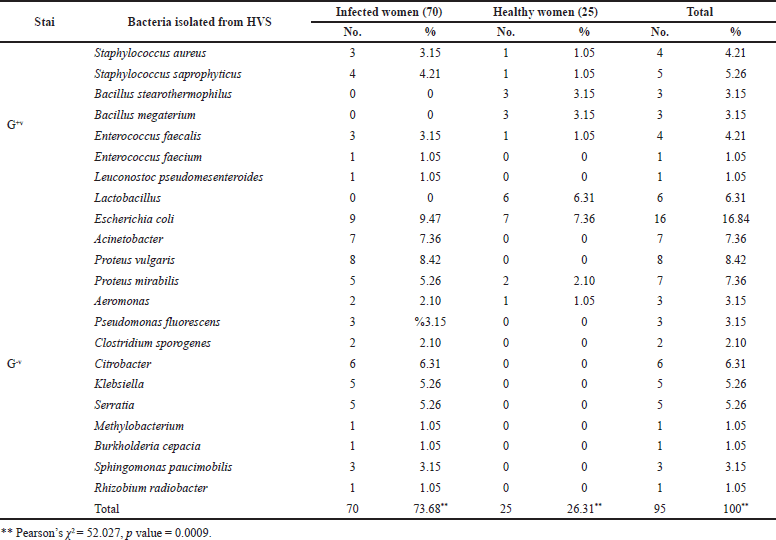 | Table 2. Frequency of microorganisms from vaginal samples in infected and healthy women. [Click here to view] |
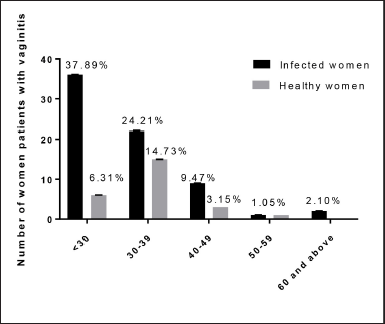 | Figure 3. Relation between age groups and positive culture of vaginal swabs in infected and healthy women. **Pearson’s χ2 = 8.299, p value = 0.008. [Click here to view] |
Antibiotic sensitivity
Nineteen –five isolated thereafter were subjected to susceptibility testing according to the CLSI 2020 guidelines using different antibiotics, namely, levofloxacin, azithromycin, amoxicillin, clavulanic acid, clindamycin, metronidazole, and gentamicin, as shown in Table 3. Gram-positive bacteria like S. aureus, S. saprophyticus, and B. stearothermophilus were highly sensitive to levofloxacin, azithromycin, and gentamicin but highly resistant to amoxicillin-clavulanic acid, clindamycin, and metronidazole. The bacterium E. faecalis was found to be sensitive to levofloxacin, clindamycin, and gentamicin but not to amoxicillin-clavulanic acid, metronidazole, or azithromycin. Enterococcus faecium was found to be sensitive to amoxicillin-clavulanic acid, metronidazole, clindamycin, and gentamicin but sensitive to levofloxacin and azithromycin.
Gram-positive bacteria S. aureus, S. saprophyticus, and B. stearothermophilus showed high sensitivity to levofloxacin, azithromycin, and gentamicin and high resistance to amoxicillin-clavulanic acid, clindamycin, and metronidazole. Enterococcus faecalis showed high sensitivity to levofloxacin, clindamycin, and gentamicin and high resistance to amoxicillin-clavulanic acid, metronidazole, and azithromycin. Enterococcus faecium showed high sensitivity to levofloxacin and azithromycin and high resistance to amoxicillin-clavulanic acid, metronidazole, clindamycin, and gentamicin. Gram-negative bacterial isolates: E. coli was intermediately resistant to levofloxacin and highly resistant to amoxicillin-clavulanic acid, metronidazole, azithromycin, clindamycin, and gentamicin. Acinetobacter, P. fluorescens, Methylobacterium, P. vulgaris, and Sphingomonas paucimobilis were found to be highly sensitive to levofloxacin and highly resistant to amoxicillin-clavulanic acid, metronidazole, azithromycin, clindamycin, and gentamicin. Proteus mirabilis was sensitive to levofloxacin and gentamicin but resistant to amoxicillin-clavulanic acid, metronidazole, clindamycin, and azithromycin.
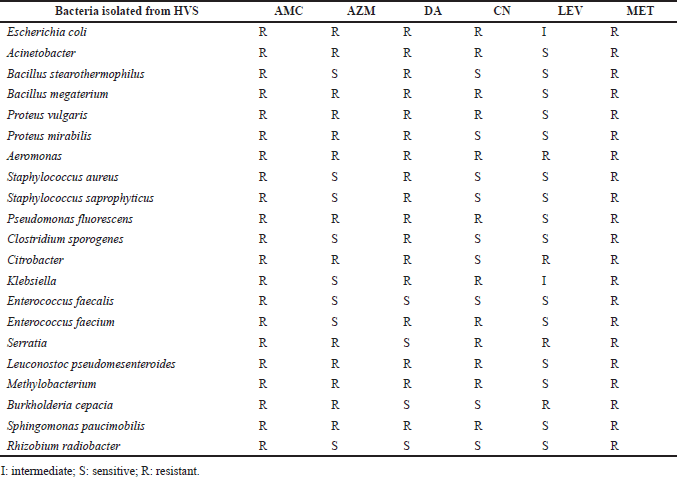 | Table 3. Antibiotic susceptibility patterns of the bacteria isolated from vaginitis cases. [Click here to view] |
Aeromonas showed high resistance to amoxicillin-clavulanic acid, metronidazole, levofloxacin, gentamicin, clindamycin, and azithromycin. Clostridium sporogenes showed high sensitivity to levofloxacin, gentamicin, and azithromycin and high resistance to amoxicillin-clavulanic acid, metronidazole, and clindamycin. Citrobacter was sensitive to gentamicin but resistant to amoxicillin-clavulanic acid, metronidazole, levofloxacin, clindamycin, and azithromycin. Klebsiella was highly sensitive to azithromycin, intermediate to levofloxacin, and extremely resistant to amoxicillin-clavulanic acid, metronidazole, gentamicin, and clindamycin. Serratia was sensitive to clindamycin but resistant to amoxicillin-clavulanic acid, levofloxacin, metronidazole, gentamicin, and azithromycin. Burkholderia cepacia was sensitive to clindamycin and gentamicin but resistant to amoxicillin-clavulanic acid, levofloxacin, metronidazole, and azithromycin. Rhizobium radiobacter was extremely sensitive to clindamycin, levofloxacin, gentamicin, and azithromycin but extremely resistant to amoxicillin-clavulanic acid and metronidazole. All of the isolated bacteria were sensitive to levofloxacin but resistant to metronidazole and amoxicillin-clavulanic acid.
We believe that the varied susceptibility of bacteria to the investigated antibacterials is due to the random and widespread administration of these antibacterials, which leads to the development of bacterial resistance and virulence factors with a high ability to avoid antibacterial effects (Brocklehurst et al., 2013; McDonald et al., 2003). These findings suggest an increase in resistance to antibacterial drugs commonly used to treat vaginitis, as well as a public health concern about the rate at which bacteria develop resistance to antibacterial treatments. As a result, bacteriological culture and sensitivity testing must be used on a regular basis in the treatment of bacterial vaginitis. Furthermore, appropriate healthcare measures that keep women’s vaginas healthy and human public health are required.
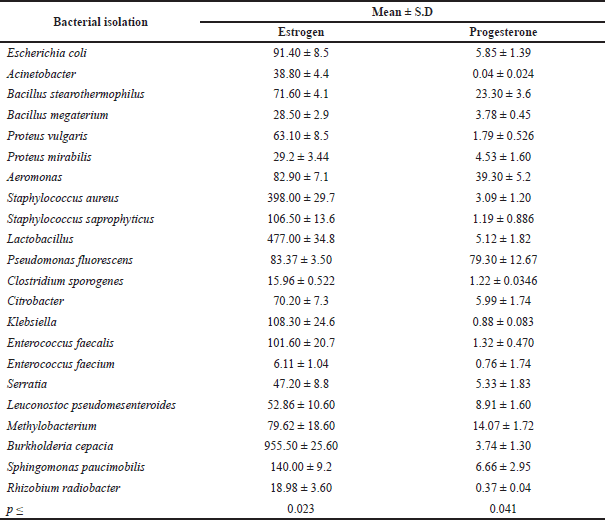 | Table 4. The relation between the isolated bacteria and sex hormone levels (estrogen and progesterone). [Click here to view] |
The relation between age of patient and sex hormones levels (estrogen and progesterone)
The statistical analysis showed significant differences between the age groups of women and sex hormone levels (estrogen and progesterone). The high rate of estrogen was in the age group of 30–39 years, while the high rate of progesterone was in the age of 40–49 years; the estradiol level was negatively correlated with age, as shown in Figure 4. This result is in agreement with another result (Lei et al., 2021), where it was found that the estradiol level was negatively correlated with age. It is important to mention that sex hormone levels are affected by many factors, including age and health status, among which age is considered the main factor (Del Río et al., 2018). According to our research’s epidemiological data, approximately 20% of girls would therefore experience severe depression at some point in their lives. Premenstrual, perinatal, and premenopausal phases, for example, can all lead to or worsen depression in some women (Bromberger and Epperson, 2018). According to new research, sex hormones play a role in the physiological and pathological development of depression in women (Lei et al., 2021; Slavich and Sacher, 2019). Nonetheless, more research into the relationship between depression and female sex hormones is required.
The relation between isolated bacteria and sex hormone levels (estrogen and progesterone)
As shown in Table 4, statistical analysis revealed significant differences in the type of bacteria isolated and sex hormone levels (estrogen and progesterone), indicating that sex hormone levels vary depending on the type of bacteria. Burkholderia cepacia and Lactobacillus had the highest estrogen rates, while P. fluorescens and Aeromonas had the highest progesterone rates. This study’s findings are similar to previous studies in that they tend to establish a link between hormone levels and vaginal bacteria diversity (Kaur et al., 2020). Hormonal contraception has been linked to a lower risk of presenting BV in the same age group of women because it increases estrogenic stability (Romero et al., 2014). In postmenopausal women, a decrease in estrogen causes a rise in pH, allowing gut bacteria to proliferate (Neggers et al., 2007). Although most studies on the link between contraception and BV have been cross-sectional, a recent longitudinal study of 3,077 reproductive-age women examined whether hormonal contraception use was associated with BV diagnosis over the course of a year (Riggs et al., 2007). Injectable hormonal contraceptives (Depo-Provera) may aid in preventing pH imbalances (Brabin et al., 2005). Vaginal pH is known to have an impact on vaginal flora. Estradiol and progesterone also regulate the innate and adaptive immunity of the female reproductive tract (Beagley and Gockel, 2003). Researchers examined estrogen and progesterone levels in women at various stages of their reproductive and menopausal lives to see if there was a relationship between circulating gonadal hormones and vaginal microbial diversity. The main objective of this data collection was to generate a preliminary framework and hypothesis to explain observed differences in microbial diversity across a variety of reproductive and menopausal (sub)phases. This study’s findings appear to suggest a link between hormone levels and microbial diversity in the vaginal canal. For example, gonadal maturation causes a rise in gonadal hormone levels during the start of puberty (Tanner stage II) (Auriemma et al., 2021). In this period, vaginal bacterial diversity is also increased, as previously mentioned. Although gonadal hormone levels grow during puberty (Tanner stages II–V), bacterial diversity decreases. The surge in hormone levels that occurs at the start of puberty is likely to result in increased glycogen accumulation in the vaginal walls. As a result, vaginal bacteria will have an ample supply of resources to grow and multiply. As gonadal maturation continues, hormone-stimulated glycogen synthesizes acids in the development of the Lactobacillus community in the vaginal canal. Lactic acid lowers the pH of the vaginal environment, preventing the growth of other anaerobic bacteria. As a result, despite increased progesterone/estrogen levels, the latter stages of puberty are characterized by limited bacterial diversity.
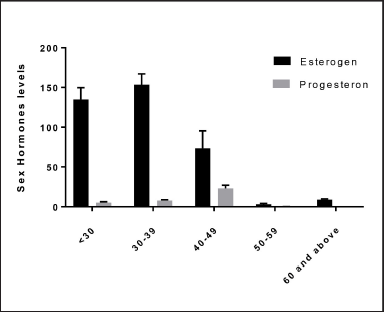 | Figure 4. The relation between the age of women and sex hormone levels (estrogen and progesterone). p ≤ 0.050 estrogen, p ≤ 0.037. [Click here to view] |
This hormone surge causes an overabundance of glycogen to be deposited in the vaginal epithelial walls, resulting in a rise in microbial diversity (Auriemma et al., 2021; Mirmonsef et al., 2014). The hostile reaction triggered by Lactobacillus growth is likely to contribute to a vaginal environment that is less varied and stable. Interestingly, prior research has found that the variety of vaginal bacteria is reduced during the first trimester. Trimester of preterm deliveries, despite the increased bacterial diversity in terms of results (Haque et al., 2017). Given the link between hormone levels and vaginal microbial variety, the absence of vaginal microbial diversity in preterm pregnancies is most likely due to a severe hormonal imbalance and/or an impaired mother immunological response. Furthermore, the vaginal microbiome of pre- and perimenopausal women is less varied, with Lactobacillus dominating. However, the postmenopausal group has a higher level of variety. Menopause is marked by a drop in the levels of circulating gonadal hormones (Yun et al., 2022). As a result, reduced hormone levels in the postmenopausal stage are likely to lead to a drop in Lactobacillus colonization and, as a result, an increase in anaerobic microbial communities in the vaginal canal. Surprisingly, hormone surge periods are linked with greater vaginal microbial diversity. During these times, the vaginal microbiota’s composition shifts from anaerobic bacteria to a Lactobacillus-dominated community (puberty, pregnancy, and menopause) according to previous experimental findings. Estrogen hormone replacement treatment has been shown to enhance Lactobacillus colonization in the vaginal environment in postmenopausal women (Vitali et al., 2017).
CONCLUSION
The study showed that microbiome diversity in the vagina VMB depends on the level of hormones in women in the Iraq region. The high rate of estrogen was in the age group 30–39 years, while the high rate of progesterone was in the age group 40–49 years; the estradiol level was negatively correlated with age. The statistical analysis showed significant differences between the age groups of women and sex hormone levels (estrogen and progesterone). As it is known, the formation of vaginal microbiota is completely continuous, but it may be affected by several factors such as diet, behavior, psychological state, and other requirements of life.
ACKNOWLEDGMENTS
The authors thank the staff members of the Faculty of Health Sciences, Department of Sciences, and Faculty of Pharmacy at UniSZA, Malaysia, Mutah University, Jordan, and Al-Zaytoonah University, Jordan, respectively.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
FUNDING
This work was supported by Universiti Sultan Zainal Abidin (UniSZA) (Grant No. UniSZA/2017/DPU/41) and the Deanship of Scientific Research at Mutah University, Jordan (Grant No. 316/2020 and 388/2021).
AUTHORS’ CONTRIBUTION
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVAL
The ethical statement for this study was approved by the Ethical Committee of Samarra Hospital [Reference No. 2020/20] in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All experiments were performed in accordance with the approved guidelines.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abboud MM, Aljundi IH, Khleifat KM, Dmour S. Biodegradation kinetics and modeling of whey lactose by bacterial hemoglobin VHb-expressing Escherichia coli strain. Biochem Eng J, 2010; 48(2):166–72. CrossRef
Abdul-Aziz M, Mahdy MAK, Abdul-Ghani R, Alhilali NA, Al-Mujahed LKA, Alabsi SA, Al-Shawish FAM, Alsarari NJM, Bamashmos W, Abdulwali SJH, Al Karawani M, Almikhlafy AA. Bacterial vaginosis, vulvovaginal candidiasis and trichomonal vaginitis among reproductive-aged women seeking primary healthcare in Sana’a city, Yemen. BMC Infect Dis, 2019; 19(1):1–10. CrossRef
Akour A. Probiotics and COVID-19: is there any link? Lett Appl Microbiol, 2020; 71(3):229–34. CrossRef
Al-Asoufi A, Khlaifat A, Tarawneh AA, Alsharafa K, Al-Limoun M, Khleifat K. Bacterial quality of urinary tract infections in diabetic and non diabetics of the population of Ma’an province, Jordan. Pak J Biol Sci, 2017; 20(4):179–88. CrossRef
Al-Essa L, Abunaja MH, Hamadneh L, Abu-Sini M. Mapping the intensive care unit environment and health care workers for methicillin-resistant Staphylococcus aureus with mecA gene confirmation and antibacterial resistance pattern identification in a district hospital in Amman. Kuwait Med J, 2021; 53(3):265–70.
Al-Ghazzewi F, Tester R. Biotherapeutic agents and vaginal health. J Appl Microbiol, 2016; 121(1):18–27. CrossRef
Al-Kafaween M, Mohd Hilmi AB, A Nagi Al-Jamal H, A Elsahoryi N, Jaffar N, Khairi Zahri M. Pseudomonas aeruginosa and Streptococcus pyogenes exposed to Malaysian trigona honey in vitro demonstrated downregulation of virulence factor. Iran J Biotechnol, 2020a; 18(4):e2542.
Al-kafaween MA, Mohd Hilmi AB, Nagi Al-Jamal HA, Jaffar N, Al-Sayyed H, Zahri MK. Effects of selected malaysian kelulut honey on biofilm formation and the gene expression profile of Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli. Jordan J Pharma Sci, 2021; 14(1).
Al-kafaween MA, Hilmi AB, Jaffar N, Al-Jamal HA, Zahri MK, Jibril FI. Antibacterial and antibiofilm activities of Malaysian Trigona honey against Pseudomonas aeruginosa ATCC 10145 and Streptococcus pyogenes ATCC 19615. Jordan J Biol Sci, 2020b; 13(1):69–76.
Al-Marabeh S, Khalil E, Khanfar M, Al-Bakri AG, Alzweiri M. A prodrug approach to enhance azelaic acid percutaneous availability. Pharm Dev Technol, 2017; 22(4), 578-586. CrossRef
Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med, 1983; 74(1):14–22. CrossRef
Arianpour N, Safari A, Hatami F. Bacteria isolated from post-partum infections. Journal of Family and Reproductive Health, 2009; 63–6.
Auriemma RS, Scairati R, Del Vecchio G, Liccardi A, Verde N, Pirchio R, Pivonello R, Ercolini D, Colao A. The vaginal microbiome: a long urogenital colonization throughout woman life. Front Cell Infect Microbiol, 2021; 11:613. CrossRef
Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol, 2003; 38(1):13–22. CrossRef
Brabin L, Roberts SA, Fairbrother E, Mandal D, Higgins SP, Chandiok S, Wood P, Barnard G, Kitchener HC. Factors affecting vaginal pH levels among female adolescents attending genitourinary medicine clinics. Sex Transm Infect, 2005; 81(6):483–7. CrossRef
Brocklehurst P, Gordon A, Heatley E, Milan SJ. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev , 2013; (1):CD000262. CrossRef
Bromberger JT, Epperson CN. Depression during and after the perimenopause: Impact of hormones, genetics, and environmental determinants of disease. Obstet Gynecol Clin, 2018; 45(4):663–78. CrossRef
Brookheart RT, Lewis WG, Peipert JF, Lewis AL, Allsworth JE. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol, 2019; 220(5):476.e1–11. CrossRef
Brown A, Smith H. Benson’s microbiological applications, laboratory manual in general microbiology, short version, McGraw-Hill Education, New York, NY, 2014.
Cappuccino J, Welsh C. Microbiology a laboratory manual. Pearson Education Limited, England, UK, 2018.
Cheesbrough M. Examination of urogenital specimens. In: District Laboratory Practice in Tropical Countries, Cambridge University Press, Cambridge, UK, pp 93–4, 2000.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, Clinical and Laboratory Standards Institute, Wayne, PA, 2017.
Dhabhai N, Chaudhary R, Wi T, Mburu G, Chowdhury R, More D, Chatterjee L, De D, Kabra R, Kiarie J, Habib N, Dang A, Dang M, Mazumder S. Prevalence of reproductive tract infections including sexually transmitted infections among married women in urban and peri-urban mid to low socioeconomic neighbourhoods of Delhi, North India: an observational study protocol. BMJ Open, 2022; 12(3):e059583. CrossRef
Del Río JP, Alliende MI, Molina N, Serrano FG, Molina S, Vigil P. Steroid hormones and their action in women’s brains: the importance of hormonal balance. Front Public Health, 2018; 6:141. CrossRef
Eschenbach DA, Patton DL, Hooton TM, Meier AS, Stapleton A, Aura J, Agnew K. Effects of vaginal intercourse with and without a condom on vaginal flora and vaginal epithelium. J Infect Dis, 2001; 183(6):913–8. CrossRef
Essa MA, Hussein FH. Isolation and identification of some microorganisms causing vaginitis and cervicitis and relationship of risk factors with these infections. Rafid J Sci, 2018; 27(3), 77-94. CrossRef
Gabriel I, Olejek A, Stencel-Gabriel K, Wielgo? M. The influence of maternal vaginal flora on the intestinal colonization in newborns and 3-month-old infants. J Matern Fetal Neonatal Med, 2018; 31(11):1448–53. CrossRef
Greenbaum S, Greenbaum G, Moran-Gilad J, Weintraub AY. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am J Obstet Gynecol, 2019; 220(4):324–35. CrossRef
Haque MM, Merchant M, Kumar PN, Dutta A, Mande SS. First-trimester vaginal microbiome diversity: a potential indicator of preterm delivery risk. Sci Rep, 2017; 7(1):1–10. CrossRef
Huang B, Fettweis JM, Brooks JP, Jefferson KK, Buck GA. The changing landscape of the vaginal microbiome. Clin Lab Med, 2014a; 34(4):747–61. CrossRef
Huang H, Song L, Zhao W. Effects of probiotics for the treatment of bacterial vaginosis in adult women: a meta-analysis of randomized clinical trials. Arch Gynecol Obstet, 2014b; 289(6):1225–34. CrossRef
Khalid HM, Yassin NA. Distribution of extended spectrum β-lactamase genes among Proteus mirabilis isolated from clinical specimens in Duhok city, Kurdistan region, Iraq. Sci J Univ Zakho, 2017; 5(1):1–6. CrossRef
Kaur H, Merchant M, Haque MM, Mande SS. Crosstalk between female gonadal hormones and vaginal microbiota across various phases of women’s gynecological lifecycle. Front Microbiol, 2020; 11:551. CrossRef
Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol, 2013; 209(6):505–23. CrossRef
Khleifat KM, Qaralleh H, Al-limoun MO, Al-khlifeh EM, Aladaileh SA, Tawarah N, Almajali IS. Antibacterial and antioxidant activities of local honey from Jordan. Trop J Nat Prod Res, 2021; 5(3):470–47. CrossRef
Khleifat KM, Matar SA, Jaafreh M, Qaralleh H, Al-limoun MO, Alsharafa KY. Essential oil of Centaurea damascena aerial parts, antibacterial and synergistic effect. J Essent Oil Bear Plants, 2019; 22(2):356–67. CrossRef
Khleifat K, Abboud M, Al-Shamayleh W, Jiries A, Tarawneh K. Effect of chlorination treatment on gram negative bacterial composition of recycled wastewater. Pak J Biol Sci, 2006; 9:1660–8. CrossRef
Khleifat K, Abboud MM. Correlation between bacterial haemoglobin gene (vgb) and aeration: their effect on the growth and α-amylase activity in transformed Enterobacter aerogenes. J Appl Microbiol, 2003; 94(6):1052–8. CrossRef
Khleifat KM, Tarawneh KA, Ali Wedyan M, Al-Tarawneh AA, Al Sharafa K. Growth kinetics and toxicity of Enterobacter cloacae grown on linear alkylbenzene sulfonate as sole carbon source. Curr Microbiol, 2008; 57(4):364–70. CrossRef
Kim JY, Sung JH, Chang KH, Choi SJ, Oh SY, Roh CR, Kim JH. Abnormal vaginal colonization by gram-negative bacteria is significantly higher in pregnancy conceived through infertility treatment compared to natural pregnancy. J Matern Fetal Neonatal Med, 2017; 30(5):556–61. CrossRef
Khudhur Mohammed T, Jawad KMWMA. Isolation and Identification of Candida albicans in different clinical samples. Al-Nisour J Med Sci, 2019;1(1).
Kroon SJ, Ravel J, Huston WM. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil Steril, 2018; 110(3):327–36. CrossRef
Kumar N, Behera B, Sagiri SS, Pal K, Ray SS, Roy S. Bacterial vaginosis: etiology and modalities of treatment—a brief note. J Pharm Bioallied Sci, 2011; 3(4):496. CrossRef
Lamichhane P, Joshi DR, Subedi YP, Thapa R, Acharya GP, Lamsal A, Upadhaya S. Study on types of vaginitis and association between bacterial vaginosis and urinary tract infection in pregnant women. IJBAR, 2014; 5(06):305–7.
Lei R, Sun Y, Liao J, Yuan Y, Sun L, Liu Y, Yang X, Ma W, Yu Z. Sex hormone levels in females of different ages suffering from depression. BMC Women’s Health, 2021; 21(1):1–9. CrossRef
Lhwak NS, Abbas YA. Detection of extended spectrum β–Lactmase GeneeCTX-M-1 in Escherchia coli and Klebseilla pneumonia isolated from urinary tract infection of pregnant women in Al-Nassyriah city. Univ Thi-Qar J Sci, 2018; 2(4):92–6.
Lin YP, Chen WC, Cheng CM, Shen CJ. Vaginal pH value for clinical diagnosis and treatment of common vaginitis. Diagnostics, 2021; 11(11):1996. CrossRef
Mahmoud IS, Altaif KI, Abu Sini MK, Daoud S, Aqel NN. Determination of antimicrobial drug resistance among bacterial isolates in two hospitals of Baghdad. Jordan J Pharma Sci, 2020; 13(1).
McDonald H, Brocklehurst P, Parsons J, Vigneswaran R. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev, 2003; 1(2):CD000262. CrossRef
Mirmonsef P, Hotton AL, Gilbert D, Burgad D, Landay A, Weber KM, Cohen M, Ravel J, Spear GT. Free glycogen in vaginal fluids is associated with Lactobacillus colonization and low vaginal pH. PloS One, 2014; 9(7):e102467. CrossRef
Mumtaz S, Ahmad M, Aftab I, Akhtar N, ul Hassan M, Hamid A. Aerobic vaginal pathogens and their sensitivity pattern. J Ayub Med Coll Abbottabad, 2008; 20(1):113–7.
Neggers YH, Nansel TR, Andrews WW, Schwebke JR, Yu KF, Goldenberg RL, Klebanoff MA. Dietary intake of selected nutrients affects bacterial vaginosis in women. J Nutr, 2007; 137(9):2128–33. CrossRef
Paladine HL, Desai UA. Vaginitis: diagnosis and treatment. Am Fam Phys, 2018; 97(5):32–9.
Qaralleh H, Khleifat KM, Al-Limoun MO, Alzedaneen FY, Al-Tawarah N. Antibacterial and synergistic effect of biosynthesized silver nanoparticles using the fungi Tritirachium oryzae W5H with essential oil of Centaurea damascena to enhance conventional antibiotics activity. Adv Nat Sci, 2019; 10(2):025016. CrossRef
Ranjit E, Raghubanshi BR, Maskey S, Parajuli P. Prevalence of bacterial vaginosis and its association with risk factors among nonpregnant women: A hospital based study. Int J Microbiol, 2018; 2018:8349601. CrossRef
Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A, 2011; 108(Supplement 1):4680–7. CrossRef
Razzak MS, Al-Charrakh AH, Al-Greitty BH. Relationship between lactobacilli and opportunistic bacterial pathogens associated with vaginitis. N Am J Med Sci, 2011; 3(4):185–92. CrossRef
Riggs M, Klebanoff M, Nansel T, Zhang J, Schwebke J, Andrews W. Longitudinal association between hormonal contraceptives and bacterial vaginosis in women of reproductive age. Sex Transm Dis, 2007; 34(12):954–9. CrossRef
Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science, 2014; 345(6198):760–5. CrossRef
Slavich GM, Sacher J. Stress, sex hormones, inflammation, and major depressive disorder: extending social signal transduction theory of depression to account for sex differences in mood disorders. Psychopharmacology, 2019; 236(10):3063–79. CrossRef
Tarawneh KA, Al-Tawarah NM, Abdel-Ghani AH, Al-Majali AM, Khleifat KM. Characterization of verotoxigenic Escherichia coli (VTEC) isolates from faeces of small ruminants and environmental samples in Southern Jordan. J Basic Microbiol, 2009; 49(3):310–7. CrossRef
Tarawneh O, Alwahsh W, Abul-Futouh H, Al-Samad LA, Hamadneh L, Abu Mahfouz H, Fadhil Abed A. Determination of antimicrobial and antibiofilm activity of combined LVX and AMP impregnated in p (HEMA) hydrogel. Appl Sci, 2021; 11(18):1–11. CrossRef
Tarawneh O, Abu Mahfouz H, Hamadneh L, Deeb AA, Al-Sheikh I, Alwahsh W, Fadhil Abed A. Assessment of persistent antimicrobial and anti-biofilm activity of p-HEMA hydrogel loaded with rifampicin and cefixime. Sci Rep, 2022; 12(1):1–11. CrossRef
Tille P. Bailey & Scott’s diagnostic microbiology-E-Book. Elsevier Health Sciences, 2015.
Ventolini G. Progresses in vaginal microflora physiology and implications for bacterial vaginosis and candidiasis. Women’s Health, 2016; 12(3):283–91. CrossRef
Vieira-Baptista P, De Seta F, Verstraelen H, Ventolini G, Lonnee-Hoffmann R, Lev-Sagie A. The vaginal microbiome: V. therapeutic modalities of vaginal microbiome engineering and research challenges. J Low Genit Tract Dis, 2022; 26(1):99–104. CrossRef
Vitali D, Wessels JM, Kaushic C. Role of sex hormones and the vaginal microbiome in susceptibility and mucosal immunity to HIV-1 in the female genital tract. AIDS Res Ther, 2017; 14(1):1–5. CrossRef
Wójkowska-Mach J, Pomorska-Weso?owska M, Romanik M, Romaniszyn D. Prevalence and antimicrobial susceptibility profiles of microorganisms associated with lower reproductive tract infections in women from southern poland—Retrospective laboratory-based study. Int J Environ Res Public Health, 2021; 18(1):335 CrossRef
Yun S, Nguyen HD, Park JS, Oh C, Kim MS. The association between the metabolic syndrome and iron status in pre-and postmenopausal women: Korean National Health and Nutrition Examination Survey (KNHANES) in 2012. Br J Nutr, 2022; 127(4):630–40. CrossRef
Zhang L, Hernández VS, Swinny JD, Verma AK, Giesecke T, Emery AC, Mutig K, Garcia-Segura LM, Eiden LE. A GABAergic cell type in the lateral habenula links hypothalamic homeostatic and midbrain motivation circuits with sex steroid signaling. Transl Psychiatry, 2018; 8(1):1–14. CrossRef