INTRODUCTION
Chronic pulmonary aspergillosis (CPA) is a continuing respiratory disease that develops over many months and years. It primarily develops among individuals with previous as well as ongoing lung bruises largely in the spaces (Denning et al., 2003). CPA is distinguished by a radiological investigation of no less than one or more spaces or cavities with or without the existence of a fungal ball or nodules proceeding to pleural or parenchymal fibrosis (Denning et al., 2003; Kosmidis and Denning, 2015; Muldoon et al., 2016).
Unavoidable frequent exposure of individuals to Aspergillus fumigatus conidia leads to saprophytic colonization inside the lung cavities. It is the most persistent causative agent of CPA. They have a small diameter of 3–5 μm, which eases their penetration into the spaces of alveoli (Kwon-Chung and Sugui, 2013). This may lead to the formation of newly developed cavities with or without aspergilloma (also called a fungal ball—a composite conglomeration of tissue debris, inflammatory cells, mucus, fibrin, and fungal mycelia) or enlargement of the colonized cavity, parenchymal, and/or pleural fibrosis and local inflammation (Pasqualotto, 2010). Fungal balls develop within the pulmonary cavity that detaches from the wall of the cavity (Roberts et al., 1987). The role of immune dysregulation and genetic aberrations in the pathogenesis of CPA and its continuation remains a case for further exploration (Bongomin et al., 2017b ; Harrison et al., 2012).
Underlying structural diseases of the lung lead to CPA in an immeasurable majority of patients. According to studies, previous infection with nontuberculous mycobacteria and pulmonary tuberculosis (PTB) are the most usual major risk factors and underlying conditions which lead to the development of CPA. Other conditions such as treated lung cancer, Pneumocystis pneumonia, pneumothorax, lung abscess, asthma, lung cysts or bullae, fibrocavitary sarcoidosis, emphysema, and/or chronic obstructive pulmonary disorder (COPD), and allergic bronchopulmonary aspergillosis all pose a risk of getting CPA. All these conditions create bullae or cavities that pose a higher risk of getting CPA in such patients (Akram et al., 2021; Baku?a et al., 2019; Bongomin et al., 2017a; Denning et al., 2003, 2011; Iqbal, 2020; Jabeen et al., 2017). Unfortunately, a large number of patients are discovered with more than one of these conditions or a history of more than one of them.
CPA has come out as a fungal infectious disease of public health significance. It has been estimated in a review carried out in 2017 that over three million people worldwide have CPA. It encompassed information from 43 countries that disclosed the elevated incidence in Russia, i.e., 126.9 reports per 100,000. The Philippines and Nigeria had 78 reports per 100,000, whereas Pakistan had 70 reports per 100,000, and Vietnam had 61 reports per 100,000. The overall incidence was 22 cases per 100,000 for all countries considered in the study (Bongomin et al., 2017a). Of these cases, almost 1.2 million are considered to be due to formerly treated PTB (Denning et al., 2011), and more than 70,000 are due to sarcoidosis (Denning et al., 2013). CPA is a high-risk disease in Pakistan as it has been regarded as the most critical sequela of pulmonary TB (Denning et al., 2011). Pakistan has the highest PTB burden, which makes it fifth in the ranking among other countries (Baku?a et al., 2019). The most updated burden estimated for CPA in Pakistan was 39 reports per 100,000 individuals (Iqbal et al., 2020).
CPA is of notable apprehension for countries with an elevated burden of PTB, such as Pakistan. Diseases like CPA pose a significant financial burden and healthcare cost for both the patient and the healthcare system. The scenario becomes more intricate by the substandard infection control practices, the emergence of antifungal resistance, inadequate fungal diagnostic capabilities, and the absence of antimicrobial stewardship as well as a scarcity of essential antifungal agents (Jabeen et al., 2017). Despite the significance of CPA, there is no study available from any country or on the global level for the estimation of the cost of illness (both direct and indirect costs) associated with CPA. Therefore, in this retrospective study, we have aimed to identify the direct costing components and estimated the range of direct out-of-pocket expenditure annually incurred upon the patient diagnosed with CPA. The average salary in Pakistan is 81,800 PKR (Pakistani Rupee) per month, or around USD 498 according to the exchange rates in August 2021. Meanwhile, the Pakistan Annual Household Income per Capita reached 587.069 USD in Jun 2019, compared with the previous value of 650.644 USD in Jun 2016. In such scenario, a disease like CPA poses an extra burden on the people of low-income countries like Pakistan and has large societal impact. This study provides insight regarding the cost of illness in terms of out-of-pocket expenditure of patients suffering from pulmonary aspergillosis, which is one of the significant infectious diseases in Pakistan. Thus, our work is beneficial for the health authorities of Pakistan as well as other countries with the same problem. This study is the first of its kind to date.
METHODOLOGY
Study design and settings
A retrospective study design was carried out on our previously published study (Akram et al., 2021) to identify direct costing components and patients’ out-of-pocket expenditures for the management of CPA. This study was carried out at Gulab Devi Chest Hospital, a tertiary care hospital situated in Lahore, Pakistan. Patients who got enrolled for their treatment at the hospital from the period of January 1, 2017, to December 30, 2019, were incorporated in this study: (1) patients at age 18 years or above; (2) those with a confirmed diagnosis of CPA incorporating all types, i.e., chronic fibrosing pulmonary aspergillosis, subacute invasive pulmonary aspergillosis (SAIA), and chronic cavitary pulmonary aspergillosis (CCPA), as well as simple aspergilloma; (3) patients who had a chest X-ray or/and CT scan indicative of aspergillosis; (4) patients with positive histopathology of lungs proposing the existence of Aspergillus spp. and/or positive sputum cultures and/or bronchoalveolar lavage (BAL) for Aspergillus spp.; and (5) patients who did not have complete records and those with additional types of lung aspergillosis different than the chronic one were not included in the study. Individuals who represent cultures with positive results but had colonization only were also not included in the study as the colonization of microbes does not result in the disease (Dani, 2014).
Sample size
A total of 528 cases were assessed who had confirmation of the diagnosis of aspergillosis. From isolated aspergillosis records, 218 cases met our inclusion criteria.
Data collection
The hospital’s computerized record system and patients’ files were reviewed for information on their demographic characteristics, including age, gender, smoking status, symptoms, associated conditions, other respiratory conditions, and types of CPA encountered resource utilization and clinical and treatment-related characteristics.
Cost calculation
According to the European Respiratory Society (ERS) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guideline for the management of CPA (Denning et al., 2016), the direct costing components for the patients identified were (1) length of hospital stay cost, (2) initial laboratory and diagnostic testing cost, (3) follow-up laboratory and diagnostic costs, and (4) medication cost. To calculate the cost of each component, the number of patients and their frequency of utilizing the resources were added with the unit cost of the resource in each component.
As the hospital belongs to the government sector, the cost associated with each component was endured by the government, and the resources of each component were offered free of cost to patients except for the cost of stay at the hospital. Hence, to illustrate the out-of-pocket expenditures endured by the patient diagnosed with CPA, the range of unit cost of each resource was acquired from different private pharmacies, laboratories, and diagnostic centers. The minimal and the maximal out-of-pocket expenditures for each component were calculated accordingly, depending on the range of unit cost of each resource utilized. The unit cost range for each resource is shown in Table 1.
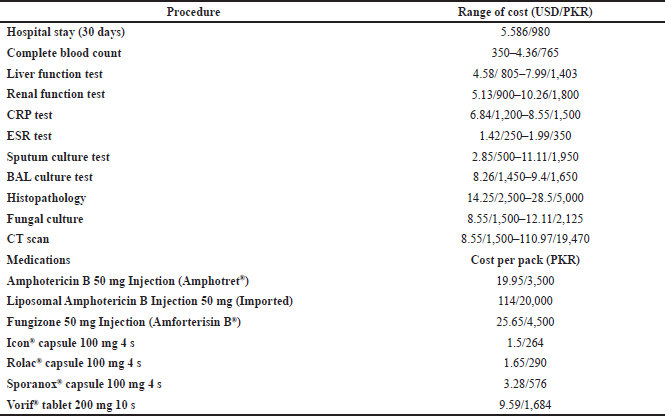 | Table 1. Unit costs of procedures and most commonly used drugs for CPA. [Click here to view] |
Length of stay cost
Length of stay was categorized into five categories, i.e., <10, 10–19, 20–29, 30–59, and >60 days. The cumulative length of stay (LOS) cost was calculated by multiplying the cost of a 30 days’ stay at the hospital by the number of patients and the frequency of resource utilization. The cost of 30 days was multiplied once in categories less than 30 days because even if the patient stayed for less than 30 days, he had to pay the cost for the entire month. The cost of 30 days was multiplied twice for the category 30–59 and thrice for category >60 days. The mean LOS cost was calculated by dividing the cumulative LOS cost by the number of patients.
Initial laboratory and diagnostic cost
The resources utilized for laboratory and diagnostic testing were the C-reactive protein (CRP) test, erythrocyte sedimentation rate (ESR) test, sputum culture test, BAL culture test, histopathology, fungal culture test, and CT scan. Regular tests, such as blood, liver, and renal tests, were also included in the estimation of this component. The minimum initial laboratory and the diagnostic costs were calculated by adding the lower limit of the range of unit cost of each resource, whereas the maximum initial laboratory and the diagnostic cost were calculated by adding the upper limit of the range of unit cost of each resource. The cumulative minimum was calculated by multiplying the minimum initial laboratory and diagnostic cost with the number of patients and their frequency of utilization. The cumulative maximum was calculated by multiplying the maximum initial laboratory and diagnostic cost with the number of patients and their frequency of utilization.
Follow-up cost
Patients underwent a CRP test, ESR test, and CT scan for the follow-up of the progress of their disease. Follow-up duration was categorized into seven categories, i.e., 0–3 months, 0–6 months, 0–9 months, 0–12 months, 0–15 months, 0–18 months, and >0–18 months. The minimum and maximum follow-up costs for each duration were calculated by adding the lower and upper limits of the unit cost of these three resources and multiplying with the frequency of resource utilization. The cumulative minimum and maximum follow-up costs were obtained by multiplying the range of unit costs of these three resources with the number of patients utilizing them and their frequency of utilization.
Medication cost
Patients were prescribed antifungals which included itraconazole, amphotericin B, and voriconazole. cost estimated for the top brands of antifungals prescribed for treating fungal infections. The duration of therapy was categorized into three categories, i.e., <6 months, 6 months, and >6 months. The medication cost was estimated by multiplying the frequency of utilization of therapy with the unit cost of the therapy and the duration in which the therapy was continued. The cumulative cost was calculated by multiplying the medication cost by the number of patients utilizing the medication in the specific duration.
Statistical analysis
An Excel sheet was utilized to tabulate all data, and each variable was assigned a specific code. Descriptive statistical analyses were performed to determine the means, percentages, and frequencies by using Statistical Package for the Social Sciences (version 15). The variables which were recorded on admission were put to univariate analysis: total days of hospital stay, laboratory and diagnostic data, and demographic characteristics.
RESULTS
Demographic characteristics
The total number of cases which were reviewed was 521. They had a confirmed diagnosis of aspergillosis. Only 218, i.e., 41.84%, of cases met our inclusion criteria. The participants had a mean age of 45.75 ± 6.26 years, and the number of males was 160, i.e., 73.4% (Table 2). Out of the total 218 cases of CPA, 122, i.e., 56%, had simple aspergilloma, whereas 68 cases, i.e., 31.2%, had CCPA, and 28, i.e., 12.8%, were diagnosed with SAIA. Many nonspecific manifestations which were clinically important were seen in the present study, one of which was cough seen in 207 patients, i.e., 95%, and fatigue seen in 202 patients, i.e., 92.7% (Table 2).
Associated lung diseases
The most common lung disease which was associated with PTB was present in 137 patients (62.8%). Of these 137 patients, those with previous tuberculosis were 96 patients (70%) and those with active tuberculosis were 41 patients (29.9%) (Table 2). Those with simple aspergilloma were 122 patients, and out of those, 20 patients (16.4%) showed active tuberculosis. Out of these, two patients (10%) were suffering from underlying COPD. Among 68 patients with CCPA and 28 patients with SAIA, active TB was seen in 14 patients (20.6%) and 7 patients (25%). Among these, 1 patient out of 14 patients (7.1%) and 1 patient out of 7 patients (14.3%) had COPD, whereas pulmonary sarcoidosis was observed in 46 patients (21.1%).
Patient’s out-of-pocket expenditures
Length of stay cost
The mean LOS ± SD in the hospital was 18.5±10.9 d for patients hospitalized due to CPA. The numbers of patients in each category, i.e., <10, 10–19, 20–29, 30–59, and >60 days, were 16 (7.33%), 124 (56.88%), 20 (9.17%), 57 (26.14%), and 1 (0.45%), respectively. The frequency of resource utilization was 1, 1, 1, 2, and 3, respectively, whereas the cumulative LOS cost was USD 89.65, USD 694.79, USD 112.06, USD 638.76, and USD 16.81, and the mean LOS cost was USD 5.60, USD 5.60, USD 5.60, USD 11.21, and USD 16.81. The total cumulative LOS cost was USD 1473.63, whereas the total mean LOS cost was USD 6.76, respectively (Table 3).
Initial and follow-up laboratory and diagnostic cost
The range of initial laboratory and diagnostic cost was from USD 62.44 to USD 203.27, whereas the range of cumulative cost was from USD 13,611.92 to USD 44,314.84 (Table 4).
Follow-up cost
The numbers of patients in the follow-up durations of 0–3 months, 0–6 months, 0–9 months, 0–12 months, 0–15 months, 0–18 months, and >0–18 months were 13 (5.96%), 51 (23.39%), 118 (54.12%), 15 (6.88%), 10 (4.58%), 9 (4.13%), and 2 (0.92%) with the frequencies of resource utilization being 1, 2, 3, 4, 5, 6, and 7, respectively. The cumulative minimum follow-up laboratory and diagnostic costs for each duration were USD 278.73, USD 2186.95, USD 7590.01, USD 1286.44, USD 1072.03, USD 1157.80, and USD 300.17, whereas the cumulative maximum follow-up laboratory and diagnostic costs for each duration were USD 1887.55, USD 14810.03, USD 51399.51, USD 8711.78, USD 7259.82, USD 7840.60, and USD 2032.75, respectively (Table 5).
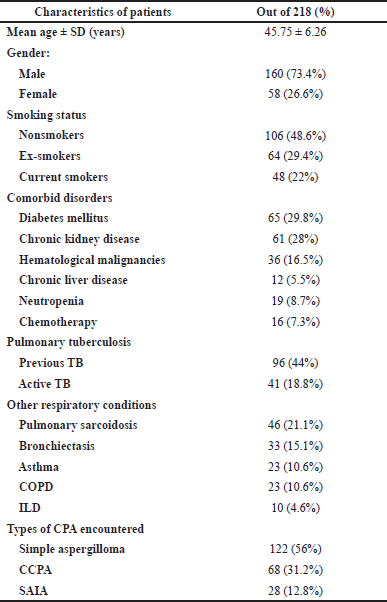 | Table 2. Demographics and disease features of the study group. [Click here to view] |
Medication cost
Itraconazole was prescribed to 152 (69.7%), amphotericin B was prescribed to 51 (23.4%), and voriconazole was prescribed to 15 (6.9%) patients.
The three most commonly prescribed brands selected for itraconazole were Icon® Cap 100 mg 4’s, Rolac® Cap 100 mg 4’s, and Sporanox® Cap 100 mg 4’s. The frequency of utilization was 200 mg twice daily. The cumulative cost of each brand selected is given in Table 6. The brands selected for amphotericin B were Amphotericin B 50 mg injection (Amphotret®), Liposomal Amphotericin B Injection 50 mg imported, and Fungizone 50 mg injection (Amforterisin B®). The frequency of utilization was 0.7–1.0 mg/kg/day. The cumulative cost of each brand selected is given in Table 6. The brand available for voriconazole in Pakistan is Vorif® Tab 200 mg 10’s. The frequency of utilization was 200 mg twice daily. The cumulative cost of the selected brand is given in Table 6.
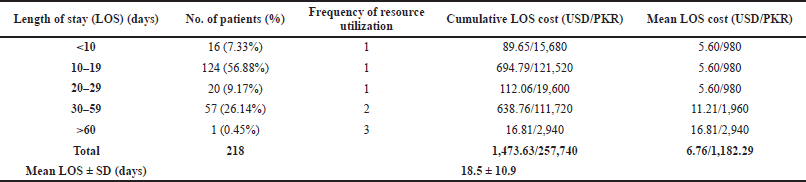 | Table 3. Length of hospital stay cost. [Click here to view] |
 | Table 4. Initial laboratory and diagnostic cost. [Click here to view] |
Univariate analysis, sensitivity analysis, and correlation
According to our study, the major component of patients’ direct out-of-pocket expenditures came from medication cost, the range of percentage of which was 53.75%–98.97%, followed by the initial laboratory and diagnostic cost, i.e., 0.17%–34.75%, for the management of CPA (Table 7). The range of length of hospital stay cost and follow-up cost were the minor components of direct out-of-pocket expenditure of the patient, i.e., 0.014%–3.32% and 0.86%–12.72% (Table 7). Direct out-of-pocket expenditure has exhibited a moderately positive correlation with length of hospital stay cost and follow-up cost, whereas a very strong positive correlation was found with medication cost and initial laboratory and diagnostic cost. One-variable sensitivity analysis has also been performed to analyze the effect of cost components on the total out-of-pocket expenditure of CPA patients. The medication cost has a large effect on the total out-of-pocket expenditures of patients, according to our sensitivity analysis (Supplementary Data).
DISCUSSION
In this study, we estimated the minimum and maximum direct out-of-pocket expenditure of CPA per patient annually as USD 83.53 and USD 58,550 (PKR is equal to USD 0.0057, as of 05 Feb 2022) when managed according to the ERS and ESCMID guidelines at a tertiary care hospital in Pakistan. The mean length of stay cost varies between USD 5.56 and USD 16.67. The mean initial laboratory and diagnostic cost stretched from USD 62.44 to USD 203.27. The mean follow-up cost extended from USD 21.26 to USD 1007.74. The mean medication cost ranged between USD 89.80 and USD 115,896.47 (Table 7). The medication cost showed significant variation as local medicines have lower prices than imported medicines. Both local and international brands are utilized for the treatment of CPA, which depends upon the patient’s range of income and facilities.
A review carried out in 2017 revealed a high incidence of CPA in Pakistan (70 cases per 100,000) (Bongomin et al., 2017a). CPA is a high-risk disease in Pakistan as it has been regarded as the most critical sequela of pulmonary TB (Denning et al., 2011). Pakistan has been ranked fifth among countries that have elevated pulmonary TB burdens (Baku?a et al., 2019). The most up-to-date estimate of the burden of CPA is 39 cases per 100,000 individuals (Iqbal et al., 2020). This information renders CPA an ailment of vital concern for the elevated burden of pulmonary tuberculosis in countries such as Pakistan. This data makes CPA a disease of significant concern for the high pulmonary TB burden in countries like Pakistan. There has been a paucity of studies on the cost of CPA both nationally and internationally, so we compared the outcomes of our study with the cost incurred on the chronic respiratory diseases, i.e., TB and asthma, in Pakistan to illuminate the direct out-of-pocket expenditure of CPA, a significant respiratory disease in Pakistan. This study is unique and the first of its kind in the sense that it has undertaken the cost estimation of CPA.
According to an analysis study on pharmacoeconomic care carrier out on control of tuberculosis in Pakistan, the annual mean cost for tuberculosis patients was about USD 176.26 incurred directly upon the patients (Iqbal et al., 2014), which is far less than the mean direct cost of our study. In another study carried out in the Quetta city of Pakistan, the out-of-pocket expenditures given by the patient per month were calculated to be USD 67.01 (Haq et al., 2015), which is also lower than our study. This difference can be attributed to the lengthy duration of follow-up and the high cost of medication required in the management of CPA as compared to TB. According to another recent study carried out to recognize important factors in Pakistan which are giving rise to the catastrophic cost of tuberculosis care, the range of the total TB-related costs was USD 181.69–USD 552.72, out of which the range of out-of-pocket treatment costs incurred by TB was USD 1.70–USD 34.01 (Ikram et al., 2020). This range of medical costs is also quite below the range of costs incurred in the current study for CPA. The reason may be the extensive initial laboratory and diagnostic assessment required for the diagnosis of CPA and its type, the follow-up cost, and the medication cost of CPA in comparison to TB.
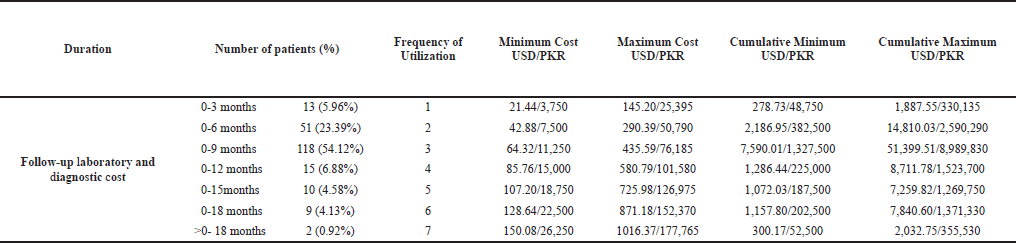 | Table 5. Follow-up laboratory and diagnostic cost. [Click here to view] |
In the present study, the mean initial laboratory and diagnostic cost per patient ranged from USD 51.04 to USD 181.75, and the mean medication cost varied between USD 89.80 and USD 115,896.47. Compared to a study (Razzaq et al., 2018) carried out to estimate the household expenditure for TB in Karachi, Pakistan, the median direct costs are borne by patients during the prediagnostic and diagnostic phases which were estimated to be USD 70.6 and USD 55, respectively. The median direct cost during the treatment phase was USD 12. The prediagnostic and diagnostic cost estimated in this study is comparable to the initial laboratory and diagnostic cost in our study, but the treatment cost largely varies for CPA and TB. This is due to the elevated unit cost of medications and the lengthy duration of treatment required in CPA. The median cost of hospitalization was estimated at USD 109.5, and the mean length of stay was calculated to be 6.5 days, whereas the mean length of stay cost in our study ranged between USD 5.56 and USD 16.67, and the mean LOS was calculated to be 18.5 days.
The prevalence of asthma, another chronic respiratory disease in Pakistan, is expanding annually by 5%; hence, the cost is also on the rise. Almost 20 million persons have asthma, of which 12% are the adult population (Song et al., 2014). It is also shown that about 5%–7% of the total population is presently suffering, where almost greater than 5% are children (Ashraf et al., 2008). An analysis carried out in Karachi presented asthma prevalence of about 18% in different age groups (Shahzad et al., 2006). The Global Initiative for Asthma statistics presented a prevalence of about 4%–5% of the patients suffering from asthma in Pakistan (Masoli et al., 2004). According to a cross-sectional study (Batool et al., 2017) carried out in elderly asthmatic patients, the mean out-of-pocket expenditure calculated for inpatients turned out to be PKR 1,128, whereas in outpatients it turned out to be PKR 854. Medication was the vital source of out-of-pocket expenditure, i.e., 43%, followed by lab investigations at 24%, which is similar to our findings while estimating the mean direct cost for CPA where medication cost is the major component of the mean direct cost, followed by laboratory and diagnostic testing cost. A recent study also found similar results, which were used to find out the direct cost related to acute exacerbation of asthma (AEXA) in asthmatic individuals. The majority of the cost was based on the medication cost, i.e., 52.38%, whereas the cost of lab investigations was followed by medication cost. However, the median cost related to AEXA that came under the government and patients was USD 105.00 and USD 22.50 per episode (Iqbal, 2020).
Invasive pulmonary aspergillosis, an advanced form of CPA, does not have a good prognosis. It is linked with substantial morbidity and healthcare costs as well (Koulenti et al., 2014). It has been shown that the intensive care unit LOS increases to 12 days in case of invasive aspergillosis. The period in which mechanical ventilation is given increases up to 9 days, whereas the hospital length of stay increases by 10 days in general, which ranges from 3 to 16 days which in turn depends on the underlying disease (Tong et al., 2009; Vandewoude et al., 2004). A median cost of a hospital was reported to be $52,803 by a huge retrospective cohort of US hospitals consisting of patients who were 1,603 in number and suffering from aspergillosis. Interestingly, it was found that intravenous antifungals represented only 7.2% of the hospitalization costs related to aspergillosis, whereas the initial choice of antifungal was not independently linked to crude mortality. It was also noticed that treatment with caspofungin or amphotericin B lipid complex was linked independently with a lower length of stay at the hospital (Kim et al., 2011).
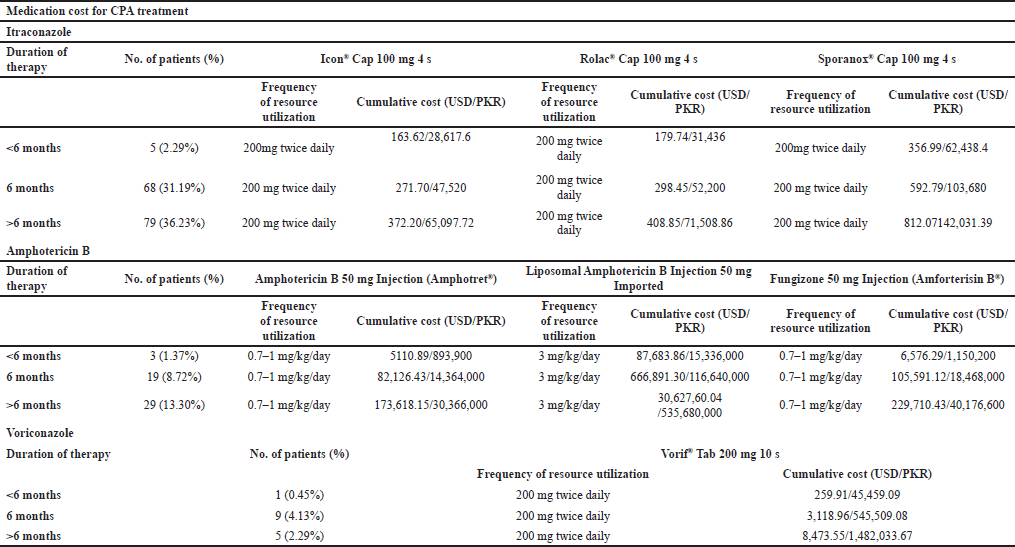 | Table 6. Medication cost for CPA treatment with various prescribed brands in Pakistan. [Click here to view] |
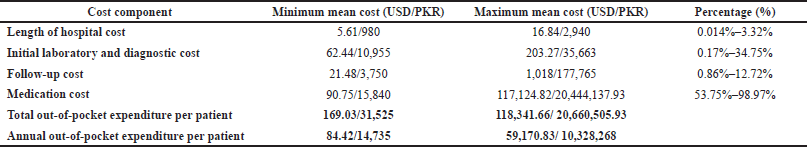 | Table 7. Cost components and total out-of-pocket expenditure. [Click here to view] |
According to another study, it was reported that patients suffering from invasive aspergillosis who did not have risk factors under the domain of “classic” represented 46% of in-hospital mortality, with mean and hospital length of stay of 15.8 and 26.9 days. The average hospital cost per patient was 76,235, with board and room accounting for the majority of the expense. It was also noticed that each one-day delay in initiating antifungal treatment was associated with the extension of hospital stay by 1.28 days and an elevation of 3.5% in the costs. Similarly, patients treated initially with fluconazole in comparison to those treated with voriconazole showed a mean elevated length of stay by 6 days and a 33% elevation in hospital costs (Baddley et al., 2013).
The Global Action Fund for Fungal Infections added the point-of-care Aspergillus lateral-flow assay to the essential diagnostics list of the WHO, which should be capable of making the diagnosis of CPA easy, especially in settings where resources are limited (Bongomin et al., 2019). A preferred antifungal for CPA, i.e., oral/itraconazole, is obtainable in 43% of African countries, but it has a high cost (Kneale et al., 2016). The expenditures differ widely, ranging from < $1 in Uganda to $19 in Nigeria for a 400 mg per day dose. Also, the WHO has recently incorporated itraconazole into the “2017 Model List of Essential Medicines,” which are used in managing fungal infections in adults. It has been anticipated that such efforts could decrease the cost associated with diagnosis and treatment, which will encourage the treatment and screening programs in TB endemic areas complemented along with research studies.
Although very useful information regarding the direct cost components and patients’ range of direct out-of-pocket expenditure for CPA management has been drawn out from our study, it also had a few limitations, which should be taken into consideration when interpreting the results. The cost estimated in this study was direct out-of-pocket expenditures, but the indirect out-of-pocket expenditures were not estimated, which might make up half of the total economic burden of CPA, such as those for loss of productivity and informal care. Factors for this included the noncooperation of patients as well as a shortage of time. Secondly, the results generated by the study carried out in a single center cannot be generalized. In addition, standardization was not ensured as the nature of the study is retrospective. Also, the findings of this study cannot be extrapolated to the general population as it is performed in a specific site in the country. However, this kind of research is the initial cost of illness research about CPA in developing countries like Pakistan. The medication cost being identified as the most contributing cost component for CPA necessitates the development and implementation of cost-effective treatment strategies in developing countries for diseases like CPA. This study gives us a beginning thought concerning the current situation, which can pave the way for the future development of cost-effective management strategies for CPA in countries like Pakistan with a high CPA burden. Also, further multicenter studies with proper standardization should be carried out to bring under control the lack of standardization and the latest diagnostic techniques for CPA.
CONCLUSION
The most expensive cost component identified for CPA is medication cost, followed by follow-up cost. Cost-effective treatment strategies are required to optimize the cost of illness of diseases like CPA in developing countries. Direct out-of-pocket expenditure has exhibited a moderately positive correlation with length of hospital stay cost and the initial laboratory and diagnostic cost, whereas a very strong positive correlation was found with medication cost and follow-up laboratory and diagnostic cost.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
CONFLICTS OF INTEREST
The authors declare they have no potential conflicts of interest regarding the publication of the paper.
ETHICAL APPROVAL
The Institutional Review Board (IRB) of Gulab Devi Chest Hospital, Lahore, approved (No. Admin/GDEC/241/19, dated 11.07.2019) this study. Written informed consent was taken from the patients who visited the hospital for their treatment in regards to using their medical records.
REFERENCES
Akram W, Ejaz MB, Mallhi TH, Syed Sulaiman SA, Khan AH. Clinical manifestations, associated risk factors and treatment outcomes of Chronic Pulmonary Aspergillosis (CPA): experiences from a tertiary care hospital in Lahore, Pakistan. PLoS One, 2021; 16(11):e0259766. CrossRef
Ashraf M, Ullah E, Ahmed M, Zaidi AA, Zainab G, Muslim HM. Asthma care perceptions and practices among general practitioners at Bahawalpur. Pak J Chest Med, 2008; 14(3).
Baddley JW, Stephens JM, Ji X, Gao X, Schlamm HT, Tarallo M. Aspergillosis in Intensive Care Unit (ICU) patients: epidemiology and economic outcomes. BMC Infect Dis, 2013; 13(1):1–8. CrossRef
Baku?a Z, Javed H, Ple? M, Jamil N, Tahir Z, Jagielski T. Genetic diversity of multidrug-resistant Mycobacterium tuberculosis isolates in Punjab, Pakistan. Infect Genet Evol, 2019; 72:16–24. CrossRef
Batool N, Mehboob G, Bahar S. Out of pocket expenditures for treating asthma in elderly population: a case study of Pakistan institute of medical sciences government hospital, Islamabad. Pak J Public Health, 2017; 7(4):192–6. CrossRef
Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases—estimate precision. J Fungi, 2017a; 3(4):57. CrossRef
Bongomin F, Govender NP, Chakrabarti A, Robert-Gangneux F, Boulware DR, Zafar A, Oladele RO, Richardson MD, Gangneux JP, Alastruey-Izquierdo A, Bazira J. Essential in vitro diagnostics for advanced HIV and serious fungal diseases: international experts’ consensus recommendations. Eur J Clin Microbiol, 2019; 38(9):1581–4. CrossRef
Bongomin F, Harris C, Foden P, Kosmidis C, Denning DW. Innate and adaptive immune defects in chronic pulmonary aspergillosis. J Fungi, 2017b; 3(2):26. CrossRef
Dani A. Colonization and infection. Cent Eur J Urol, 2014; 67(1):86. CrossRef
Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, Ullmann AJ, Dimopoulos G, Lange C. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J, 2016; 47(1):45–68. CrossRef
Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ, 2011; 89:864–72. CrossRef
Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis complicating sarcoidosis. Eur Respir J, 2013; 41(3):621–6. CrossRef
Denning DW, Riniotis K, Dobrashian R, Sambatakou H. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis, 2003; 37(Supplement_3): S265–80. CrossRef
Harrison E, Singh A, Morris J, Smith NL, Fraczek MG, Moore CB, Denning DW. Mannose-sbinding lectin genotype and serum levels in patients with chronic and allergic pulmonary aspergillosis. Int J Immunogenet, 2012; 39(3):224–32. CrossRef
Ikram A, Ali A, Abbasi SH, Ashraf N, Wali S, Salman M, Khan MA, Syed N, Ansari JA. Is tuberculosis treatment truly free? A study to identify key factors contributing to the catastrophic cost of TB care in Pakistan. J Tuberc Res, 2020; 8(4):181–98. CrossRef
Iqbal MS, Iqbal MW, Bahari MB, Khalid SH, Iqbal MZ. a pharmacoeconomic care analysis of tuberculosis control in Pakistan. Value Health, 2014; 17(7):A594. CrossRef
Iqbal MS. The burden of illness of acute exacerbation of asthma. Asian J Pharm, 2020; 14(2).
Iqbal N, Irfan M, Mushtaq A, Jabeen K. Underlying conditions and clinical spectrum of chronic pulmonary aspergillosis (cpa): an experience from a tertiary care hospital in Karachi, Pakistan. J Fungi, 2020; 6(2):41. CrossRef
Jabeen K, Farooqi J, Mirza S, Denning D, Zafar A. Serious fungal infections in Pakistan. Eur J Clin Microbiol, 2017; 36(6):949–56. CrossRef
Kim A, Nicolau DP, Kuti JL. Hospital costs and outcomes among intravenous antifungal therapies for patients with invasive aspergillosis in the United States. Mycoses, 2011; 54(5):e301–12. CrossRef
Kneale M, Bartholomew JS, Davies E, Denning DW. Global access to antifungal therapy and its variable cost. J Antimicrob Chemother, 2016; 71(12):3599-606. CrossRef
Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax, 2015; 70(3):270–7. CrossRef
Koulenti D, Garnacho-Montero J, Blot S. Approach to invasive pulmonary aspergillosis in critically ill patients. Curr Opin Infect Dis, 2014; 27(2):174–83. CrossRef
Kwon-Chung KJ, Sugui JA. Aspergillus fumigatus—what makes the species a ubiquitous human fungal pathogen? PLoS Pathog, 2013; 9(12):e1003743. CrossRef
Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy, 2004; 59(5):469–78. CrossRef
Muldoon EG, Sharman A, Page I, Bishop P, Denning DW. Aspergillus nodules; another presentation of chronic pulmonary aspergillosis. BMC Pulm Med, 2016; 16(1):1–9. CrossRef
Pasqualotto AC. Aspergillosis: from diagnosis to prevention. Springer, Berlin, Germany, 2010.
Razzaq S, Zahidie A, Fatmi Z. Household expenditure for tuberculosis care, its determinants and coping strategies among adults 18 years and older of Karachi, Pakistan. Eur Respir J, 2018; 52(62):3161. CrossRef
Roberts CM, Citron KM, Strickland B. Intrathoracic aspergilloma: role of CT in diagnosis and treatment. Radiology, 1987; 165(1):123–8. CrossRef
Shahzad K, Akhtar S, Mahmud S. Prevalence and determinants of asthma in adult male leather tannery workers in Karachi, Pakistan: a cross sectional study. BMC Public Health, 2006; 6(1):1–7. CrossRef
Song WJ, Kang MG, Chang YS, Cho SH. Epidemiology of adult asthma in Asia: toward a better understanding. Asia Pac Allergy, 2014; 4(2):75–85. CrossRef
Tong KB, Lau CJ, Murtagh K, Layton AJ, Seifeldin R. The economic impact of aspergillosis: analysis of hospital expenditures across patient subgroups. Int J Infect Dis, 2009; 13(1):24–36. CrossRef
ul Haq N, Sattar A, Iqbal Q, Naseem A, Bashir S, Baloch M. Cost of Illness (Out of Pocket Costs Paid by Patient) for TB in Quetta City, Pakistan. Value Health, 2015; 18(7):A839–40. CrossRef
Vandewoude KH, Blot SI, Benoit DO, Colardyn F, Vogelaers D. Invasive aspergillosis in critically ill patients: attributable mortality and excesses in length of ICU stay and ventilator dependence. J Hosp Infect, 2004; 56(4):269-76. CrossRef