INTRODUCTION
Cancer is one of the health problems that impose huge economic and health burdens on the world (Ma and Yu, 2006). Cancer is considered the second cause of death in the world (Ma and Yu, 2006). There are several types of cancer. Melanoma is a malignant tumor that occurs in melanocytes, the cells that make the melanin pigment. Melanoma is considered a problem that affects the homeostatic balance of skin cells. In the skin, there are components that play key roles in tumor development, such as dermal fibroblasts, epidermal keratinocytes, inflammatory and endothelial cells, thus contributing to the control of melanocyte proliferation in normal skin cells (Serrone and Hersey, 1999).
Colorectal adenocarcinoma is another type of cancer that generates from epithelial cells (Le Marchand et al., 1997). It was noted that the invasion of carcinoma occurs through muscular mucosa into the submucosa and is characterized by the presence of desmoplasia represented by fibrous proliferation around tumor cells and necrotic debris in the lumina (Le Marchand et al., 1997). The causes of colorectal cancer pathogenesis are diverse and can include lifestyle, diet, genetic factors, and several diseases such as inflammatory bowel diseases (Niederreiter et al., 2018). Notably, genetic alterations are the direct leader of colorectal cancer, causing the neoplastic formation of the epithelium and stimulating the malignant stages (Niederreiter et al., 2018). Unfortunately, cancer treatment is associated with several side effects urging the need to find natural sources for treating cancer or alleviating its consequences. In this regard, plants have been considered a powerful source of therapeutics since ancient times. Plants produce a large number of chemical compounds that can have different medicinal values (Gurib-Fakim, 2006). Jordan has significant numbers of medicinal plants; many of these plants are used in the pharmaceutical industry and folk medicine (Oran and Al-Eisawi, 1998; Oran, 2014). According to Oran (2014), there are 363 medicinal plants in Jordan. Several researches were conducted to screen the medicinal herbs that have potential against cancer in Jordan. For instance, cooked lentils demonstrated enhanced chemoprevention against colorectal carcinogenesis (Afifi-Yazar et al., 2011), while Ononis hirta caused apoptosis in different cancerous cell lines (Afifi-Yazar et al., 2011). Furthermore, different Salvia species exerted antiproliferation against cancerous cell lines (Afifi-Yazar et al., 2011). These medicinal plants that have antineoplastic agents are examples of some precious plants from the Jordanian flora. More studies are needed to assess the effect of medicinal plants that were not previously explored to discover and benefit from their therapeutic values. Ajuga chia, Micromeria myrtifolia, Micromeria nervosa, Origanum dayi, Salvia palaestina, Varthemia iphionoides, and Bongardia chrysogonum are some of the plants that grow in Jordan (Oran and Al-Eisawi, 1998). These plants contain valuable therapeutic constituents and are used in Jordanian traditional medicine (Oran and Al-Eisawi, 1998). Several studies were conducted on these plants and proved multiple biological activities in different cell lines (Abdelwahab et al., 2015; Abdollahi-Ghehi et al., 2019; Abuhamdah et al., 2017; Formisano et al., 2014; Yacob et al., 2016; Yarmolinsky et al., 2015; Yousef et al., 2018). However, none of the previous studies, to the best of our knowledge, determined the effect of the extracts from these plants on colorectal cancer cell lines. Thus, the aim of this study was to prepare the ethanolic extract from these plants and assess their effect on Caco2, WM136-1A, and control cell lines.
MATERIALS AND METHODS
Collection of plants
The aerial parts of V. iphionoides, M. myrtifolia, M. nervosa, O. dayi, A. chia, and S. palaestina in addition to the corms of B. chrysogonum were collected (2019–2020) and were completely dried. The plant species were taxonomically identified by Prof. Sawsan Oran, Department of Biological Sciences, University of Jordan, Amman, Jordan. The voucher specimens were deposited at the Jordan University Herbarium, Amman, Jordan, and were labeled as follows: voucher specimens for V. iphionoides (JU-31), M. myrtifolia (JU-23), M. nervosa (JU-27), O. dayi (JU-29), A. chia (JU-11), S. palaestina (JU-30), and B. chrysogonum (JU-37).
Preparation of plant extracts
Plants were allowed to dry for 5 weeks at room temperature (24°C–26°C) and then were powdered. 100 g of each powdered plant was dissolved in 1,000 ml of 100% ethanol separately. The mixture was placed in a shaker for 72 hours at 40°C. After that, the extracts were filtered using Whatman filter papers. The solutions were concentrated using a rotary evaporator at 50°C. Then, the extracts were stored at –20°C.
Cell culture
Fibroblast, colorectal cancer (Caco-2), and human melanoma (WM136-1A) cell lines from The National Cancer Institute (NCI) of United States were used in this study. The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM), supplemented with 10% heat-inactivated fetal bovine serum, 50 IU/ml penicillin, 50 μg/ml streptomycin, and 20 μM L-glutamine. The cells were incubated at 37°C under conditions of 5% carbon dioxide (CO2) and 95% humidity conditions. 1 × 104 cells/ml were seeded in 24-well plates. Each of the seven plants’ extracts was dissolved in a growth medium to treat the cells with 200, 100, 50, 25, 12.5, and 6.25 μg/ml concentrations. After treatment, the cells were incubated for 72 hours at 37°C, 5% CO2, and 95% humidity conditions. Each experiment was repeated at least thrice.
Cell viability assay
The 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma-Aldrich, St. Louis) was used to assess the viability of the cells. Control cells were treated with dimethylsulfoxide (DMSO) or media (Lau et al., 2004). 40 μl of MTT was added to every well and was incubated for 2 hours at 37°C. Then, the MTT solution was removed and DMSO was added. A plate reader was used to measure the absorbance at 570 and 630 nm. Data were collected from three independent experiments. The percentage of surviving cells was detected by using the following formula:
Survival % = 100 – [(AC – AT) / AC] × 100%
where AC represents the absorbance of the control group and AT represents the absorbance of the treated group. Doxorubicin was used as a positive control. Then, IC50 values were calculated for all groups.
Statistical analysis
Data are shown as mean ± standard deviation (SD). The one-way analysis of variance was used for the statistical analysis using Dunnett’s test as a post-hoc test. p < 0.05 was considered significant. GraphPad Prism, version 5.02, was used to perform the statistical analysis and draw the figures.
RESULTS AND DISCUSSION
Drugs derived from plants can be good candidates for the treatment of cancer because they are natural, available, and nontoxic (Greenwell and Rahman, 2015). In the present study, the cytotoxicity of the crude ethanolic extracts of selected medicinal plants from Jordan was evaluated. The present study was conducted to evaluate the antiproliferative effect of A. chia, B. chrysogonum, M. myrtifolia, M. nervosa, O. dayi, S. palaestina, and V. iphionoides medicinal plants that grow wild in Jordan. The cytotoxicity of the selected plant extracts on the fibroblast, human melanoma, and colon cancer cell lines was determined by the cell viability assay using the MTT assay. Varthemia iphionoides is a plant in the family Asteraceae. In Jordanian folk medicine, the aqueous extract of V. iphionoides is used to treat gastrointestinal disorders and diabetes mellitus (Oran and Al-Eisawi, 1998). Our findings showed that the ethanolic extract of V. iphionoides did not exhibit antiproliferative activity against Caco-2 and WM1361-A cell lines (data not shown). The results are in agreement with an earlier report that revealed the weak antiproliferative effect of V. iphionoides on the adenocarcinomic human alveolar basal epithelial cells (A549) and melanoma cells (BG) cancer cells (Yarmolinsky et al., 2015). In fact, it is well known that the cytotoxic effect of an extract can differ according to the type of cell line and solvent that was used in extraction. For instance, a previous study documented slight cytotoxicity for V. iphionoides extract (ethyl acetate extract) compared to the high cytotoxic effect of the hexane extract of V. iphionoides against the HL-60 human leukemia cell line (Al-Dabbas et al., 2006). Additionally, V. iphionoides from the Judea region showed a moderate antiproliferative effect against the SKOV3 cell line (ovarian carcinoma cells). Notably, the most active components of the ethanolic extract of V. iphionoides against leukemia were flavonoids and polyphenols (Yarmolinsky et al., 2015).
M. myrtifolia plant belongs to the family Lamiaceae. Earlier reports proved that the essential oils of M. myrtifolia have demonstrated antifungal, antioxidant, and antiviral activity (Formisano et al., 2014). The current research showed that the ethanolic extract of M. myrtifolia did not have any toxicity on the proliferative rate of different cancer cells (data not shown). To the best of our knowledge, there are no publications regarding the effect of M. myrtifolia extract on the proliferation of cancer cell lines.
M. nervosa plant belongs to the family Lamiaceae. Our results show that the ethanolic extract of M. nervosa exhibited good antiproliferative activity against Caco-2 with IC50 = 98 ± 4.7 (Fig. 1) but not the melanoma or fibroblast cell lines. According to Abdelwahab et al. (2015), the acetone extract of M. nervosa showed cytotoxicity against the human liver hepatocellular carcinoma (SNU-398, HepG2), ovary adenocarcinoma (OVCAR-3, SK-OV-3), human colon cancer (COLO 205, HCT-116), and stomach gastric carcinoma (MKN-28, NCI-N87) cell lines. It is suggested that the cytotoxicity of M. nervosa can be attributed to diterpenes and flavonoids (Abdelwahab et al., 2015).
Origanum dayi is a plant that belongs to the Lamiaceae family (Dudai et al., 2003). This plant is rich in 50 volatile components such as α-terpineol, 1,8-cineole, and terpinen-4-ol and has a wide margin of biological effects (Dudai et al., 2003). The data of the present study demonstrated that the ethanolic extract of O. dayi had an antiproliferative effect against the against Caco2 cell line after 72 hours cell line after 72 hours of treatment compared to the nontreated cells (control group) with IC50 = 73.6 ± 3.4 μg/ml and WM1361A with IC50 = 245 ± 3.6 μg/ml (Figs. 2 and 3). Previously, we found that treating the MCF7 and T47D cell lines with O. dayi ethanolic extract had a promising anticancerous effect on these cell lines (Yousef et al., 2018). Additionally, the use of O. dayi extract had antitumor activity against HepG2 human hepatocellular carcinoma cells, as reported in earlier studies (Thoppil et al., 2013).
Ajuga chia belongs to the Lamiaceae family and includes many species. In this study, the ethanolic extract of A. chia showed an antiproliferative effect against the WM1361A cell line with IC50 = 400 ± 5.2 μg/ml (Fig. 4). This result is in agreement with the work of Sadati et al. (2012) who documented the cytotoxic effect of Ajuga chamaecistus spp. tomentella on the T47D, Caco-2, and HT-29 cancer cell lines. It is worth mentioning that many compounds were isolated from Ajuga species, such as neo-clerodane-diterpenes, phytoecdysteroids, diterpenoids, triterpenes, specific sterols, flavonoids, and essential oils (Yacob et al., 2016, Jaffal et al., 2019). Phytoecdysteroids that are found in Ajuga species have demonstrated various pharmacological and biological activities (Yacob et al., 2016, Jaffal et al., 2019) (Yacob et al., 2016).
Salvia species, which belong to the Lamiaceae family, are commonly used for the treatment of a large number of diseases. Salvia species are characterized by two main bioactive compounds, namely flavonoids and terpenoids (Irtegun Kandemir et al., 2022). Salvia palaestina is a common plant that is used in folk medicine by Jordanian people (Oran and Al-Eisawi, 1998). Previous studies reported many active compounds in S. palaestina such as flavonoids, ursolic acid, and modified abietane diterpenoids (Irtegun Kandemir et al., 2022). Our data demonstrated that the ethanolic extract of S. palaestina did not exhibit antiproliferative activity against Caco-2 (data not shown) but was effective against the WM1361-A cell lines (Fig. 5). In this regard, Yildirim and Kutlu (2015) revealed that S. absconditiflora had a moderate cytotoxic effect against the MCF7 and MDA-MB-231 breast cancer cell lines.
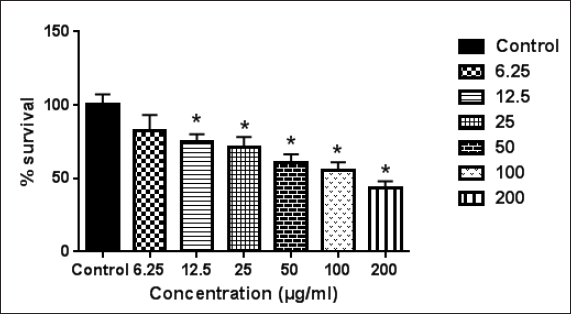 | Figure 1. Effect of M. nervosa ethanolic extract on Caco-2 cell line. Error bars represent SD. *Significant compared to the control group. [Click here to view] |
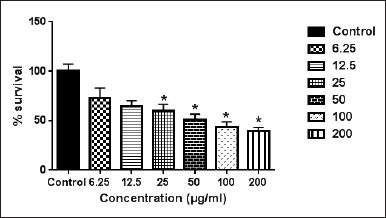 | Figure 2. Effect of O. dayi ethanolic extract on Caco-2 cell line. Error bars represent SD. *Significant compared to the control group. [Click here to view] |
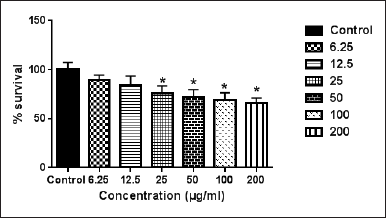 | Figure 3. The cytotoxic effect of the crude ethanolic extract of O. dayi on WM1361A cell line. Error bars represent SD. *Significant compared to the control group. [Click here to view] |
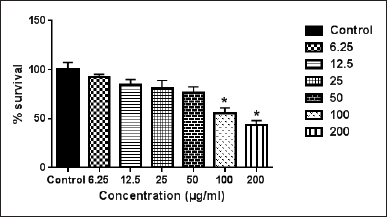 | Figure 4. The cytotoxic effect of the crude ethanolic extract of A. chia on WM1361A cell line. Error bars represent SD. *Significant compared to the control group. [Click here to view] |
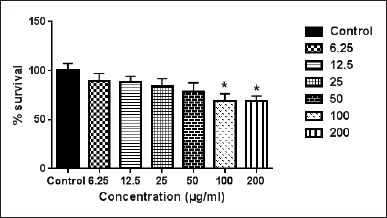 | Figure 5. The cytotoxic effect of the crude ethanolic extract of S. palaestina on WM1361A cell line. Error bars represent SD. *Significant compared to the control group. [Click here to view] |
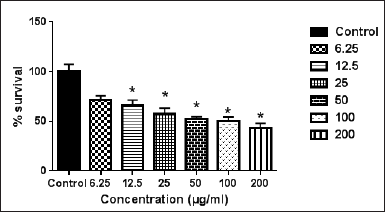 | Figure 6. The cytotoxic effect of the crude ethanolic extract of B. chrysogonum on WM1361A cell line. Error bars represent SD. *Significant compared to the control group. [Click here to view] |
B. chrysogonum plant belongs to the Berberidaceae family. B. chrysogonum is widely used in traditional medicine in Jordan as a remedy for epilepsy (Abuhamdah et al., 2017). In folk medicine in Lebanon, the corms of B. chrysogonum were used in the treatment of prostate hypertrophia (Baydoun et al., 2015). In our research, the ethanolic extract of B. chrysogonum corms demonstrated a cytotoxic effect against the WM1361A cell line with IC50 = 98.1 ± 3.2 μg/ml (Fig. 6). It was reported that the methanolic extract of B. chrysogonum exhibited a cytotoxic effect on the U266 multiple myeloma and BJAB Burkitt’s lymphoma cell lines, with IC50 = 126.3 and 114.4 μg/ml, respectively(Assaf et al., 2013). Importantly, none of the extracts had an effect on fibroblast cells at any used concentration (data not shown).
On the other hand, the ethanolic extracts of the selected medicinal plants were screened for their cytotoxicity in Caco-2, WM1361A, and fibroblast cell lines at various concentrations to detect IC50 values (μg/ml). The results of IC50 are illustrated in Table 1. The most potent extracts in the present study are the extracts that scored the lowest IC50 in the in vitro MTT assay.
In conclusion, the results of this research showed that M. nervosa, O. dayi, A. chia, and B. chrysogonum can be good candidates as anticancerous compounds. Notably, O. dayi was the most potent plant in antiproliferation effect.
 | Table 1. IC50 of different groups. [Click here to view] |
ACKNOWLEDGMENTS
The authors are thankful to the Deanship of Scientific Research at the University of Jordan for the financial support.
CONFLICT OF INTEREST:
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdelwahab MF, Hussein MH, Kadry HH. Cytotoxicity and antioxidant activity of new biologically active constituents from Micromeria nervosa grown in Egypt. Bull Fac Pharm Cairo Univ, 2015; 53(2):185–94. CrossRef
Abdollahi-Ghehi H, Sonboli A, Ebrahimi SN, Esmaeili MA, Mirjalili MH. Triterpenic acid content and cytotoxicity of some Salvia species from Iran. Nat Prod Commun, 2019; 14(5):1934578X1984272. CrossRef
Abuhamdah S, Abuirmeile AN, Thaer F, Al-Olimat S, Abdel E, Chazot PL. Anti-convulsant effects of Bongardia chrysogonum L. Tuber in the pentylenetetrazole-induced seizure model. Int J Pharmacol, 2017; 14(1):127–35. CrossRef
Afifi-Yazar FU, Kasabri V, Abu-Dahab R. Medicinal plants from Jordan in the treatment of cancer: traditional uses vs. in vitro and in vivo evaluations–Part 1. Planta Med, 2011; 77(11):1203–9. CrossRef
Al-Dabbas MM, Suganuma T, Kitahara K, Hou DX, Fujii M. Cytotoxic, antioxidant and antibacterial activities of Varthemia iphionoides Boiss. extracts. J Ethnopharmacolo, 2006; 108(2):287–93. CrossRef
Assaf AM, Haddadin RN, Aldouri NA, Alabbassi R, Mashallah S, Mohammad M, Bustanji Y. Anti-cancer, anti-inflammatory and anti-microbial activities of plant extracts used against hematological tumors in traditional medicine of Jordan. J Ethnopharmacol, 2013; 145(3):728–36. CrossRef
Baydoun S, Chalak L, Dalleh H, Arnold N. Ethnopharmacological survey of medicinal plants used in traditional medicine by the communities of Mount Hermon, Lebanon. J Ethnopharmacol, 2015; 173:139–56. CrossRef
Dudai N, Larkov O, Chaimovitsh D, Lewinsohn E, Freiman L, Ravid U. Essential oil compounds of Origanum dayi post. Flav Fragr J, 2003; 18:334–7. CrossRef
Formisano C, Oliviero F, Rigano D, Saab AM, Senatore F. Chemical composition of essential oils and in vitro antioxidant properties of extracts and essential oils of Calamintha origanifolia and Micromeria myrtifolia, two Lamiaceae from the Lebanon flora. Indus Crop Prod, 2014; 62:405–11. CrossRef
Greenwell M, Rahman PK. Medicinal plants: their use in anticancer treatment. Int J Pharm Sci Res, 2015; 6(10):4103–12.
Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med, 2006; 27(1):1–93. CrossRef
Irtegun Kandemir S, Fidan HS, Yener I, Mete N, Ertas A, Topcu G, Kolak U. Investigation of cytotoxic and apoptotic effects of 63 compounds obtained from Salvia species: promising anticancer agents. J Food Biochem, 2022; e14226. CrossRef
Jaffal S, Abbas M, Alsalem M, Al-Najjar. Evidence for the involvement of opioid receptor in Ajuga chamaepitys action in chemical and thermal models of pain in BALB/c mice. Medicinal Chemistry Research 2019, 28: 992-999. CrossRef
Lau CB, Ho CY, Kim CF, Leung KN, Fung KP, Tse TF, Chan HH, Chow MS. Cytotoxic activities of Coriolus versicolor (Yunzhi) extract on human leukemia and lymphoma cells by induction of apoptosis. Life Sci, 2004; 75(7):797–808. CrossRef
Le Marchand L, Wilkens LR, Hankin JH, Kolonel LN, Lyu LC. A case-control study of diet and colorectal cancer in a multiethnic population in Hawaii (United States): lipids and foods of animal origin. Cancer Causes and Control, 1997; 8(4):637–48.Ma X, Yu H. Global burden of cancer. Yale J Biol Med, 2006; 79(3-4):85–94. CrossRef
Niederreiter L, Adolph TE, Tilg H. Food, microbiome and colorectal cancer. Dig Liver Dis, 2018, 50(7):647–52. CrossRef
Oran S. The status of medicinal plants in Jordan. J Agri Sci Technol, 2014; 4:461–57.
Oran SA, Al-Eisawi DM. Check-list of medicinal plants in Jordan. Dirasat, 1998; 25(2):84–112.
Sadati N, Ostad SN, Karimian Z, Shams Ardekani MR, Akbarzadeh T, Hadjiakhoondi A, Khanavi M. Phytochemical study and in vitro cytotoxic effect of Ajuga chamaecistus ssp tomentella. Asian J Chem, 2012; 24:2871–4. CrossRef
Serrone L, Hersey P. The chemoresistance of human malignant melanoma: an update. Melanoma Res, 1999; 9(1):51–8. CrossRef
Thoppil RJ, Harlev E, Mandal A, Nevo E, Bishayee A. Antitumor activities of extracts from selected desert plants against HepG2 human hepatocellular carcinoma cells. Pharm Biol, 2013; 51(5):668–74. CrossRef
Yacob T, Shibeshi W, Nedi T. Antidiarrheal activity of 80% methanol extract of the aerial part of Ajuga remota Benth (Lamiaceae) in mice. BMC Compl Altern Med, 2016; 16:303.
Yarmolinsky L, Bari G, Hamias R, Maor H, Budovsky A, Wolfson M, Ben-Shabat S. Preferential anti-proliferative activity of Varthemia iphionoides (Chiliadenus iphinoides). Israel J Plant Sci, 2015; 62(4):229–33. CrossRef
Yildirim I, Kutlu T. Anticancer properties of different species of Salvia. J Biol Chem, 2015; 43(2):91–7.
Yousef I, Oran S, Bustanji Y, Al-Eisawi D, Abu-Irmaileh B. Cytotoxic effect of selected wild medicinal plant species from Jordan on two different breast cancer cell lines, MCF7 and T47D. Biol Med, 2018; 10(4):443–C. CrossRef
Yousef I, Oran S, Alqaraleh M, Bustanji Y. Evaluation of cytotoxic, antioxidant and antibacterial activities of origanum dayi, salvia palaestina and Bongardia chrysogonum plants growing wild in Jordan. Tropical Journal of Natural Product Research, 2021, 5(1), 66-70. CrossRef