INTRODUCTION
Colorectal cancer (CRC) is one of the most frequent malignancies in humans, which is related to cancer mortality numbers in the world (Huanga et al., 2019). In 2012, CRC was diagnosed worldwide in 614,000 women and 746,000 men (Kuipers et al., 2015). CRC is spreading rapidly because of unhealthy diets, aging, and sedentary lifestyles. Surgery and chemotherapy are considered the conventional treatments for CRC. Chemotherapeutics can cause DNA deterioration or activate numerous signaling pathways, such as stopping cell cycle, global translation inhibition, and DNA recombination, which finally lead to cancer cell death (Woods and Turchi, 2013). However, treatment with chemotherapeutic drugs in CRC patients has different outcomes depending on the subtype of cancer, as revealed in many studies, including Malignant pleural effusion (MPE) studies. So far, there have been many problems associated with chemotherapy, mainly drug resistance, the effects of cytotoxicity, and adverse reactions. Accordingly, natural products including plants, animals, marine organisms, and microorganisms still considered to be well tolerated and have less toxicity are investigated for the identification of potential chemotherapeutic agents (Mao et al., 2017; Singh et al., 2008). Previous reports revealed that drugs extracted from natural products have broad application prospects in the treatment of CRC (Aires et al., 2013; Johnson and Mukhtar, 2007).
Historically, the major contribution of natural metabolites and their derivatives has been achieved in pharmacotherapy, particularly in cancer and infectious diseases (Atanasov et al., 2021). A large number of plants, animals, marine organisms, and microorganisms are wealthy in amazing chemical diversity, which makes nature an exceptional resource of new biologically active compounds. From January 2008 to December 2013, a total of 25 natural products and natural product-derived drugs were approved in pharmaceutical markets (Butler et al., 2014).
Different natural metabolites, such as polyphenols, alkaloids, polysaccharides, diterpenoids, and unsaturated fatty acids, are involved directly or indirectly in about half of all currently used anticancer medications (Newman and Cragg, 2016). The ongoing development of such products has opened up a new avenue for cancer prevention and treatment. Natural products are now regarded as a prolific exporter of novel anticancer medications.
Euphorbia covers a great diversity of spurge-like plants (family Euphorbiaceae), with over 2,000 species (Ernst et al., 2015). Great phytochemical varieties including terpenoids (Cao et al., 1992; Liu et al., 2002), phenolics (Duarte et al., 2008; Mueller and Pohl, 1970), and tannins (Giordani et al., 2001; Yashida et al., 1994) were discovered in the Euphorbiaceae family. So far, the same family encloses a broad spectrum of therapeutic activities like antimalarial (Zhou et al., 2016) and anticancer (Guo et al., 2018; Fu et al., 2016) activities against human gastric cancer cell lines of both multidrug-resistant and nonresistant. On the other hand, Euphorbia aegyptiaca showed anti-inflammatory activity related to its saponins, coumarins, flavonoids, tannins, sterols, and triterpenes content (Abo-dola and Lotfi, 2016). So far, flavonoids isolated from Euphorbia tirucalli possess anti-inflammatory and analgesic properties (Prabha et al., 2008).
Euphorbia pseudocactus Berger has a multibranched stem (candelabra spurge) that looks like a candelabrum, which is a dwarf, and the plant is distributed along South Africa’s subtropical coast. It thrives in thorny bushlands and savannah, where it frequently forms colonies. It was reported that new abietane terpenoids which possess great antimicrobial activity have been identified in the E. pseudocactus hexane fraction (Abdel?Monem and Abdelrahman, 2016).
The limited review on E. pseudocactus drives us to explore new pharmacological activities and investigate the chemistry of this species. In this study, the ethyl acetate (EtOAc) fraction of E. pseudocactus was investigated for isolation and identification of pure compounds, and the new biological activity of the isolated compounds was investigated using in vitro colorectal cytotoxic activity.
MATERIALS AND METHODS
General
The NMR spectra were recorded on a Bruker Nuclear Magnetic Resonance (NMR) spectrometer operating at 400 MHz for 1H and 100 MHz for 13C. The NMR spectra chemical shift values were recorded in δ (ppm). Solvents used include Dimethyl sulfoxide (DMSO), CDCl3, and Methanol (MeOD). Silica gel 60 (Sigma-Aldrich Chemicals, Darmstadt, Germany) and, for column chromatography (CC), Sephadex LH-20 (E-Merck) were utilized. Thin-layer chromatography (TLC) plates (Fluka precoated silica gel, F254) were used for monitoring the fractions from CC. The cell lines used in the cytotoxicity assay (LS-174T and LS-513) were acquired from Nawah Scientific Inc. (Cairo, Egypt).
Cell culture
CRC cell lines LS-174T and LS-513 (cecum) were kept in Roswell Park Memorial Institute (RPMI) media complemented by 100 mg/ml of streptomycin (100 units/ml).
Cytotoxic assay
The cell viability was determined using the sulforhodamine B (SRB) assay. In 96-well plates, aliquots of 100 μl of cell suspension (5 × 103 cells) were incubated in full medium for 24 hours. Other aliquots of 100 μl media containing drugs and doxorubicin (positive control) at different concentrations (0.01, 0.1, 1, 10, and 100 μg/ml) were added to the cells. After 72 hours of drug treatment, the cells were fixed by replacing the medium with 150 μl of 10% Trichloroacetic acid (TCA) and incubating for 1 hour at 4°C. The TCA solution was taken away, and the cells were washed with distilled water five times, and then after adding aliquots of 70 μl SRB solution (0.4 % w/v), plates were incubated in a dark area for 10 minutes at room temperature. The plates were washed with 1% acetic acid three times and air-dried overnight. After that, a protein-bound SRB stain was dissolved in 150 μl of TRIS (10 mM). The absorbance at 450 nm was measured using a BMG LABTECH®-FLUOstar Omega microplate reader (Ortenberg, Germany) (Allam et al., 2018; Skehan et al., 1990).
Plant material
Euphorbia pseudocactus aerial parts were gathered in October 2014 from Cactus Farm in Tahanop, Shebin El-Qanater, Qalubiya, Egypt. The authentication of the collected plant was confirmed by Dr. Abdelhalim Mohamed (Phytotaxonomy Department, Agricultural Research Institute, Cairo, Egypt). The Pharmacognosy Department Herbarium, Faculty of Pharmacy, Beni-Suef University, received a voucher specimen (BUPD-62).
Extraction and isolation
Euphorbia pseudocactus aerial parts (7 kg) were extracted with methanol, filtered, and evaporated under reduced pressure to get a crude extract (80 g) .The dried extract was suspended in 500 ml of distilled water and partitioned into n-hexane (500 ml × 3), dichloromethane (500 ml × 3), EtOAc (500 ml × 3), and n-butanol (500 ml × 3). Evaporation of the collected extractives under reduced pressure using a rotary evaporator yielded (9 g) n-hexane, (2.1 g) dichloromethane, (2.3 g) EtOAc, and (6 g) n-butanol (Altemimi et al., 2017).
The EtOAc fraction (2.3 g) was chromatographed on a silica gel column ((70 g, 130 × 2.5 cm), using solvent system CH2Cl2 and MeOH of 5% increasing polarity, six main subfractions were collected and evaporated under reduced pressure. Fraction A (0.2 g) was chromatographed over a silica gel column (10 g, 20 × 1.5 cm) and eluted with 90% CH2Cl2/MeOH and then purified over Sephadex LH-20 using 100% MeOH to get compound 1 (50 mg). Fraction B (0.2 g) was chromatographed over a silica gel column (10 g, 20 × 1.5 cm), using 85% CH2Cl2/MeOH, followed by purification over ‘Sephadex LH-20 eluted with 100% MeOH to get compound 2 (30 mg). Fraction C (0.8 g) was chromatographed on a silica gel column (40 g, 75 × 2.5 cm), eluted with 80% CH2Cl2/MeOH, followed by Sephadex LH-20 using 100% MeOH for purification of compound 3 (100 mg). Fraction D (0.3 g) was chromatographed over a silica gel column (15 g, 30 × 1.5 cm), eluted with 80% CH2Cl2/MeOH, with further purification using Sephadex LH-20, and eluted with 100% MeOH to get compound 4 (50 mg) and compound 5 (70 mg). Fraction E (0.2 g) was chromatographed using a silica gel column (10 g, 20 × 1.5 cm), eluted with 70% CH2Cl2/MeOH and then Sephadex LH-20 using 100% MeOH yielding compound 6 (50 mg) and compound 7 (30 mg). Finally, fraction F (0.2 g) was chromatographed over a silica gel column (10 g, 20 × 1.5 cm), eluted with 65% CH2Cl2/MeOH, followed by Sephadex LH-20 using 100% MeOH to get compound 8 (20 mg).
Spectral data of isolated compounds
Kaempferol (1): yellow powder, 1H NMR (400 MHz, MeOD) δ 8.10 (2H, d, J = 7.6 Hz, H-2′, 6′), 6.92 (2H, d, J = 7.6 Hz, H-3′, 5′), 6.41 (H, s, H-8), 6.20 (H, s, H-6).13C NMR (100 MHz, MeOD) δ 146.65 (C-2), 135.74 (C-3), 175.97 (C-4), 159.14 (C-5), 97.86 (C-6), 164.17 (C-7), 93.06 (C-8), 161.10 (C-9), 103.14 (C-10), 122.32 (C-1′), 129.28 (C-2′, 6′), 114.90 (C-3′, 5′), 156.85 (C-4′).
Ethyl gallate (2): off-white powder, 1H NMR (400 MHz, MeOD) δ 7.08 (2H, d, J = 2.5 Hz, H-2, 6), 4.26 (2H, q, J = 7.1 Hz, H-8), 1.32 (3H, t, J = 7.1 Hz, H-9).
Gallic acid (3): yellowish white needles, 1H NMR (400 MHz, MeOD) δ 7.08 (1H, s, OH-4), 6.64 (2H, s, H-2, 6).13C NMR (100 MHz, MeOD) δ 170.49 (C-7), 146.24 (C-3, 5), 139.18 (C-4), 121.64 (C-1), 110.33 (C-2, 6).
Kaempferol-3-O-β-D-glucoside “astragalin” (4): yellow powder, 1H NMR (400 MHz, MeOD) δ 8.07 (2H, d, J = 8 Hz, H-2′, 6′), 6.91 (2H, d, J = 6.8 Hz, H-3′, 5′), 6.42 (1H, s, H-8), 6.22 (1H, s, H-6), 5.27 (1H, d, J = 6 Hz, H-1?), 3.23–3.73 (m, 6H of glucose). 13C NMR (100 MHz, MeOD) δ 157.67 (C-2), 134.05 (C-3), 178.10 (C-4), 161.67 (C-5), 98.53 (C-6), 164.69 (C-7), 93.37 (C-8), 157.10 (C-9), 104.31 (C-10), 121.38 (C-1′), 130.88 (C-2′, 6′), 114.67 (C-3′, 5′), 160.17 (C-4′), 102.70 (C-1″), 74.33 (C-2″), 77.01 (C-3″), 69.94 (C-4″), 76.63 (C-5″), 61.21 (C-6″).
Astragalin-6″-gallate (5): yellow powder, 1H NMR (400 MHz, DMSO) δ 7.94 (2H, d, J = 8.8 Hz, H-2′, 6′), 6.77 (2H, d, J = 8.8 Hz, H-3′, 5′), 6.40 (1H, s, J = 2 Hz, H-8), 6.20 (1H, s, J = 2 Hz, H-6), 5.46 (1H, d, J = 7.2 Hz, H-1″), 3.17–3.43 (m, 4H of H-2″, H-3″, H-4″ & H-5″), 4.26 (2H, d, H-6″), 6.92 (2H, s, H-2?, 6?) 13C NMR (100 MHz, DMSO) δ 156.87 (C-2), 133.60 (C-3), 177.76 (C-4), 161.61 (C-5), 99.24 (C-6), 164.73 (C-7), 94.21 (C-8), 157.23 (C-9), 104.38 (C-10), 121.09 (C-1′), 131.23 (C-2′, 6′), 115.54 (C-3′, 5′), 160.39 (C-4′), 101.89 (C-1″), 74.54 (C-2″), 74.54 (C-3″), 69.75 (C-4″), 76.60 (C-5″), 63.21 (C-6″), 119.76 (C-1?), 109.00 (C-2?, 6?), 145.93 (C-3?, 5?), 138.87 (C-4?), 166.11 (C-7?).
Kaempferol-3-O-rutinoside “nicotiflorin” (6): yellow powder, 1H NMR (400 MHz, MeOD) δ 8.07 (2H, d, J = 8.4 Hz, H-2′, 6′), 6.90 (2H, d, J = 8.4 Hz, H-3′, 5′), 6.39 (1H, s, H-8), 6.21 (1H, s, H-6), 5.12 (1H, d, J = 6.4 Hz, H-1″), 4.54 (1H, s, H-1?), 3.27–3.82 (m, 6H of glucose and 4H of rhamnose), 1.14 (3H, d, J = 6 Hz, H-6?). 13C NMR (100 MHz, MeOD) δ 158.0 (C-2), 134.11 (C-3), 177.95 (C-4), 161.56 (C-5), 98.71 (C-6), 164.96 (C-7), 93.62 (C-8), 157.15 (C-9), 104.17 (C-10), 121.35 (C-1′), 130.98 (C-2′, 6′), 114.75 (C-3′, 5′), 160.09 (C-4′), 103.25 (C-1″), 75.78 (C-2″), 74.35 (C-3″), 70.04 (C-4″), 76.73 (C-5″), 67.18 (C-6″), 101.02 (C-1?), 70.67 (C-2?), 70.89 (C-3?), 72.49 (C-4?), 68.33 (C-5?), 16.52 (C-6?).
Ellagic acid (7): yellow powder, 1H NMR (400 MHz, DMSO) δ 7.46 (2H, s, H-5, 5′).13C NMR (100 MHz, DMSO) δ 159.63 (C-7), 148.61 (C-4), 140.23 (C-3), 136.84 (C-2), 112.81 (C-1), 110.64 (C-5), 107.96 (C-6).
1,2,3,4,6-pentagalloylglucose (8): yellow powder, 1H NMR (400 MHz, MeOD) δ 4.29–5.45 (6H, glc. H-2,3,4,5,6), 6.39 (1H, d, glc. H-1), 6.68, 6.71, 7.03, 7.07, 7.12 (each 2H, s, galloyl H). 13C NMR (MeOD, 100 MHz): δ 63.63 (glc. C-6), 70.23 (glc. C-4), 72.68 (glc. C-2), 73.94 (glc. C-3), 74.79 (glc. C-5), 94.0 (glc. C-1), 108.81, 108.92, 109.01, 109.05, 109.57, 110.31 (galloyl C-2, C-6), 119.20, 119.64, 119.78, 119.90 (galloyl C-1), 138.50, 138.53, 138.62, 138.69, 138.73 (galloyl C-4), 145.03, 145.05, 145.06, 145.11, 145.14 (galloyl C-3, C-5), 166.09, 166.63, 166.85, 167.16, 168.76 (–COO–).
RESULTS AND DISCUSSION
Identification of isolated compounds
Eight phenolic compounds were isolated and identified for the first time from the EtOAc fraction of the E. pseudocactus aerial parts. Structural elucidation of the isolated compounds was carried out by comparing NMR spectral data with previous reports. Consequently, the isolated and identified compounds (Fig. 1) were kaempferol (1) (Wu et al., 2012), ethyl gallate (2) (Ooshiro et al., 2009), gallic acid (3) (López-Martínez et al., 2015), kaempferol-3-O-β-D-glucoside “astragalin” (4) (Abdelkader et al., 2021; Ghareeb et al., 2018), astragalin-6″-gallate (5) (Nugroho et al., 2014), kaempferol-3-O-rutinoside “nicotiflorin” (6) (Orhan et al., 2007), ellagic acid (7) (Ghareeb et al., 2018), and 1,2,3,4,6-pentagalloylglucose (8) (Deiab et al., 2015). The isolated compounds’ NMR spectra are provided in the supplementary file section.
Cytotoxic activity of the isolated compounds
Colorectal cytotoxic efficacy of the isolated phenolic compounds was tested against two human carcinoma cell lines, LS-174T and LS-513 (cecum), using the SRB assay and doxorubicin as the positive control with IC50 values of 4.50 and 5.23 μg/ml against the LS-174T and LS-513 cell lines, respectively. Results revealed that gallic acid possessed colorectal cytotoxic activity with IC50 values of 18.27 and 50.11 μg/ml against the LS-174T and LS-513 cell lines, respectively; also, ethyl gallate showed a moderate cytotoxic activity with IC50 value of 25.42 μg/ml against the LS-174T cell line, while the other phenolic compounds showed nonsignificant results. IC50 values and percent of carcinoma cells viability in relation to varying concentrations of gallic acid and ethyl gallate are summarized and shown in Figure 2.
CRC is third among the most frequent cancers and is the fourth leading cause of cancer-related deaths. The majority of CRC cases are found in Western countries, and its prevalence is increasing year by year (Mármol et al., 2017).
The great importance of the genus Euphorbia is because of its diverse phytochemicals, which include phenolics (Duarte et al., 2008) such as tannins (Giordani et al., 2001) and terpenoids (Liu et al., 2002). Previous reports highlight the chemical profile and biological importance of the hexane and dichloromethane fractions of E. pseudocactus; however, nothing could be traced concerning the chemistry and biology of the EtOAc fraction of the same species, which is considered our main issue in this study.
Phenolic compounds are one of the secondary metabolites which are not essential for plant growth, development, or reproduction, but they are efficient antioxidants and play a role in plant–environment interactions. Due to their effect as antioxidants (Martin and Appel, 2009) and anticancer activities (Harris et al., 2007), phenolics are also important components of the human healthy diet. Many classes of phenolics can be distinguished depending on the number of phenol rings and the structural features that join these rings (Stalikas, 2007).
In this study, a chemical investigation of the EtOAc fraction of the titled species was carried out, resulting in the isolation of eight phenolic and flavonoid compounds that were tested for colorectal cytotoxic activity; among the isolated phenolic constituent, gallic acid possessed colorectal cytotoxic activity. However, gallic acid (3,4,5?trihydroxybenzoic acid; GA), a natural phenolic molecule obtained from plants, has been documented to prevent the formation and progression of different cancer types (Zhang et al., 2019). This is the first report on the cytotoxic activity of gallic acid against these two cell lines.
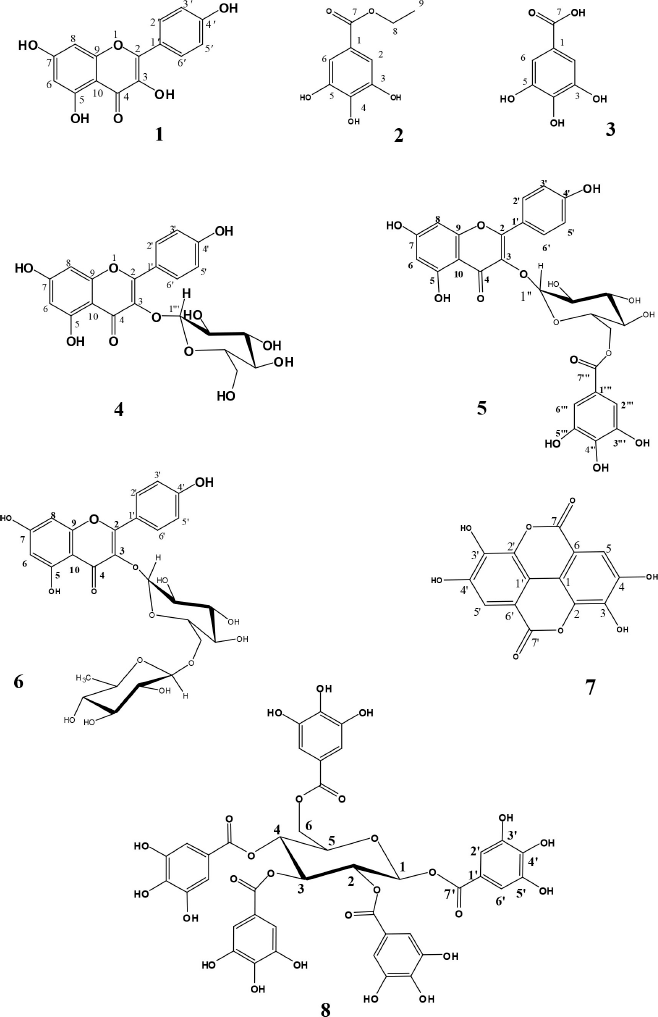 | Figure 1. Structures of phenolic compounds isolated from E. pseudocactus. [Click here to view] |
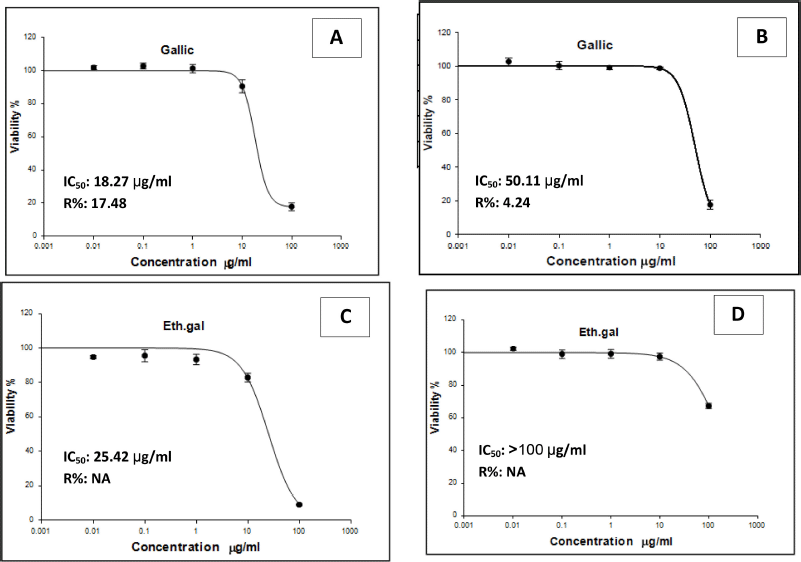 | Figure 2. IC50 values and percent of carcinoma cells viability in relation to varying concentrations of gallic acid against LS-174T (A) and LS-513 (B) and of ethyl gallate against LS-174T (C) and LS-513 (D). [Click here to view] |
CONCLUSION
In this study, the chemical investigation of the unexplored EtOAc fraction of E. pseudocactus resulted in the isolation and identification of eight phenolic compounds for the first time from E. pseudocactus. Some of the phenolic constituents showed colorectal cytotoxic activity; interestingly, gallic acid showed inhibitory activity against CRC cell lines, and we recommend submitting gallic acid for cytotoxic activity against CRC. Last but not least, E. pseudocactus could be considered a large reservoir for potential activities that could be an interesting aspect in the future.
ACKNOWLEDGMENTS
They would also like to express their gratitude to Dr. Abdel Haleem Mohammed for his assistance with plant identification and Mr. Tharout Badawy, the owner of Cactus Farm in Tahanop, Shebin El-Qanater, Qalubiya, Egypt, for supplying them with the plant.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
AUTHORS’ CONTRIBUTION
Concept and design, data acquisition, data analysis, interpretation, funding, and final approval were created by all of the authors. The original article draft and statistical analysis were cowritten by Marwa Hassan and Mona Ismail. Rabab Mohammed, Abeer Moawad, and Mohamed Zaki contributed to supervision, technical material support, and critical revision of the manuscript.
FUNDING
The financial support from Beni-Suef University, University Performance Development Center, Support and Project Finance Office, Project ID YR4-BSU2110
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdelkader MSA, Abdelhamid RA, Abouelela ME, Rateb ME, Ahmed MH. Isolation of phenolic constituents from Rhododendron yunnanense flowers as a potent cyclooxygenase-2 and vascular endothelial growth factor receptor-2 inhibitor: phytochemical and molecular simulation studies. J Appl Pharm Sci, 2021; 11(11):87–94. CrossRef
Abdel?Monem AR, Abdelrahman EH. New abietane diterpenes from Euphorbia pseudocactus berger (euphorbiaceae) and their antimicrobial activity. Pharmacogn Mag, 2016; 12(46):346–49. CrossRef
Abo-dola MA, Lotfi MF. Anti-inflammatory activity of Euphorbia aegyptiaca extract in rats. Int J Health Sci (Qassim), 2016; 10(1):69–75. CrossRef
Aires V, Limagne E, Cotte AK, Latruffe N, Ghiringhelli F, Delmas D. Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol Nutr Food Res, 2013; 57(7):1170–81. CrossRef
Allam RM, Al-Abd AM, Khedr A, Sharaf OA. Fingolimod interrupts the cross talk between estrogen metabolism and sphingolipid metabolism within prostate cancer cells. Toxicol Lett, 2018; 291:77–85. CrossRef
Altemimi A, Lakhssassi N, Baharlouei A,Watson DG, Lightfoot DA. Phytochemicals: extraction, isolation, and identification of bioactive compounds from plant extracts. Plants, 2017; 6(4):30–42. CrossRef
Atanasov AG, Zotchev SB, Dirsch VM. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov, 2021; 20:200–16. CrossRef
Butler MS, Robertson AB, Cooper MA. Natural product and natural product derived drugs in clinical trials. Nat Prod Rep, 2014; 31(11):1612–61. CrossRef
Cao D, Su YL, Yang JS. Triterpene constituents from Euphorbia nematocypha Hand- muzz. Yao Xue Xue Bao, 1992; 27(6):445–51.
Deiab S, Mazzio E, Eyunni S, McTier O, Mateeva N, Elshami F, Soliman KF. 1,2,3,4,6-Penta-O-galloylglucose within galla Chinensis inhibits human LDH-A and attenuates cell proliferation in MDA-MB-231 breast cancer cells. Evid Based Complement Alternat Med, 2015; 2015:276946; doi:10.1155/2015/276946 CrossRef
Duarte N, Kayser O, Abreu P, Ferreira MJ. Antileishmanial activity of piceatannol isolated from euphorbia lagascae seeds. Phytother Res, 2008; 22(4):455–70. CrossRef
Ernst M, Grace OM, Saslis-Lagoudakis CH, Nilsson N, Simonsen HT, Rønsted N. Global medicinal uses of Euphorbia L. (Euphorbiaceae). J Ethnopharmacol, 2015; 176:1–91. CrossRef
Fu ZY, Han XD, Wang AH, Liu XB. Apoptosis of human gastric carcinoma cells induced by Euphorbia esula latex. World .Gastroenterol, 2016; 22(13):3564–72. CrossRef
Ghareeb TA, El-Toumy SA, Gendy H, Hagga EG. Secondary metabolites and hepatoprotective activity of Euphorbia retusa. J Archit Plan Res, 2018; 2(4), 283–91.
Giordani R, Trebaux J, Masi M, Regli P. Enhanced antifungal activity of ketoconzole by Euphorbia characias latex against candidca albicens. J Ethnopharmacol, 2001; 78(1):1–5. CrossRef
Guo X, Fu Z, Bi Y, Zheng J, Wang L, He X, Li F, Lei X, Ren Q. Chinese herbal medicine Euphorbia esula extract induces apoptosis and inhibits the proliferation, migration and invasion of multidrug resistant gastric carcinoma cells. J Biomed Eng, 2018; 35(6):244–51.
Harris CS, Mo F, Migahed L, Chepelev L, Haddad PS, Wright JS, Willmore WG, Arnason JT, Bennett SA. Plant phenolics regulate neoplastic cell growth and survival: a quantitative structure-activity and biochemical analysis. Can J Physiol Pharmacol, 2007; 85(11):1124–38. CrossRef
Huanga X, Yang Z, Xie Q, Zhang Z, Zhang H, Ma J. Natural products for treating colorectal cancer: a mechanistic review. Biomed Pharmacother, 2019; 117(9):109–42 CrossRef
Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett, 2007; 255(2):170–81. CrossRef
Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T. Colorectal cancer. Nat Rev Dis Primers, 2015; 1:15065–70. CrossRef
Liu LG, Meng JC, Wu SX, Li XY, Zhoo XC, Tan RX. New macrocyclic diterpenoids from Euphorbia esula. Planta Med, 2002; 68(3):244–8. CrossRef
López-Martínez LM, Santacruz-Ortega H, Navarro R, Sotelo-Mundo R, González-Aguila G. 1H NMR investigation of the interaction between phenolic acids found in mango (Manguifera indica cv Ataulfo) and papaya (Carica papaya cv Maradol) and 1,1-diphenyl- 2-picrylhydrazyl (DPPH) free radicals. PLoS One, 2015; 10(11):e0140242. CrossRef
Mao J, Liu S, Ai M, Wang Z, Wang D, Li X, Hu K, Gao X, Yang Y. A novel melittin nano-liposome exerted excellent anti-hepatocellular carcinoma efficacy with better biological safety. J Hematol Oncol; 2017; 10(1):10–17. CrossRef
Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int J Mol Sci, 2017; 18(1):185–97. CrossRef
Martin KR, Appel CL. Polyphenols as dietary supplements: a double-edged sword. Nutr Diet Suppl, 2009; 2:1–12. CrossRef
Mueller R, Pohl R. Flavonol glycosides of Euphorbia amygdaloides and their quantitative determination at various stages of plant development. Planta Med, 1970; 18(2):114–29. CrossRef
Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod, 2016; 79(3):629–61. CrossRef
Nugroho A, Rhim TJ, Choi MY, Choi JS, Kim Y, Kim M, Park H. Simultaneous analysis and peroxynitrite-scavenging activity of galloylated flavonoid glycosides and ellagic acid in Euphorbia supine. Arch Pharm Res, 2014; 37(7):890–8. CrossRef
Ooshiro A, Hiradate S, Kawano S, Takushi T, Fujii Y, Natsume M, Abe H. Identification and activity of ethyl gallate as an antimicrobial compound produced by Geranium carolinianum. Weed Biol Manag, 2009; 9:169–72. CrossRef
Orhan DD, Ergun F, Yesilada E, Tsuchiya K, Takaishi Y, Kawazoe K. Antioxidant activity of two flavonol glycosides from Cirsium hypoleucum dc. Through bioassay- guided fractionation. Turkish J Pharm Sci, 2007; 4(1):1–14.
Prabha MN, Ramesh CK, Kuppast IJ, Mankani KL. Studies on anti-inflammatory and analgesic activities of Euphorbia tirucalli L. latex. Int J Chem Sci, 2008; 6(4):1781–87.
Singh R, Sharma M, Joshi P, Rawat DS. Clinical status of anti-cancer agents derived from marine sources. Anticancer Agents Med Chem, 2008; 8(6):603–17. CrossRef
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst, 1990; 82(13):1107–12. CrossRef
Stalikas CD. Extraction, separation, and detection methods for phenolic acids and flavonoids. J Sep Sci, 2007; 30(18):3268–95. CrossRef
Woods D, Turchi JJ. Chemotherapy induced DNA damage response: con-vergence of drugs and pathways. Cancer Biol Ther, 2013; 14(5):379–89. CrossRef
Wu Y, Qu W, Geng D, Liang J, Luo Y. Phenols and flavonoids from the aerial part of Euphorbia hirta. Chin J Nat Med, 2012; 10(1):40–2. CrossRef
Yashida T, Namba O, Kurokawa K, Amakuka Y, Liu Y, Okuda T. Tannins and related polyphenol of Euphorbiaceae plants XΙΙ, Euphorbin G and H, New dimeric hydrolysable tannin from E. chamaesyce. Chem Pharm Bull, 1994; 42(9):1803–7. CrossRef
Zhang T, Ma L, Wu P, Li W, Li T, Gu R, Dan X, Li Z, Fan X, Xiao Z. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in non?small cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol Rep, 2019; 41(3):1779–88. CrossRef
Zhou B, Wu Y, Dalal S, Cassera MB, Yue, JM. Euphorbesulins A–P, structurally diverse diterpenoids from Euphorbia esula. J Nat Prod, 2016; 79(8):1952–61. CrossRef