INTRODUCTION
Transient receptor potential vanilloid 1 (TRPV1) is a non-selective cation channel that is activated by noxious heat (>43°C), anandamide, protons, endogenous activators “endovanilloids,” and the highly selective agonist capsaicin (Caterina et al., 1997). This channel is a key receptor in the detection of different painful stimuli and is widely considered a promising target for pain control (Caterina et al., 1997). TRPV1 is highly expressed in dorsal root ganglia (DRGs), trigeminal ganglia, spinal cord, and other non-neuronal tissues (Caterina et al., 1997, Dinh et al., 2004). Similar to other members of TRP channels, each subunit of TRPV1 has six transmembrane domains with pore-forming hydrophobic stretch linking segment 5 (S5) and S6 (Tominaga and Tominaga, 2005). When TRPV1 is activated, sodium (Na+) and calcium (Ca+2) channels open leading to ion influx, initiation of depolarization, propagation of action potential into the spinal cord and brain then finally, different sensations such as stinging, burning, itching, or feeling of warmth (Anand and Bley, 2011). Interestingly, there is a crosstalk between TRPV1 and several receptors. A large body of evidence reported the involvement of multiple receptors [e.g., cannabinoid receptor 1 (CB1) and alpha (α)-2 adrenergic receptors] in inducing or inhibiting TRPV1 function. In this context, Wang et al. (2014) revealed the involvement of CB1 in nerve growth factor (NGF)-induced sensitization of TRPV1 in DRG neurons. Also, Alsalem et al. (2016) found that olvanil desensitizes TRPV1 in a CB1-dependent mechanism. The importance of α-2 adrenergic receptor in inhibiting TRPV1 activity was also highlighted previously (Matsushita et al., 2018). As a key receptor in pain processing, it was reported that several analgesic and anti-nociceptive compounds exerted their effects by influencing the trafficking and/or functionality of TRPV1. However, many of these drugs have undesirable effects. Nowadays, there is trend toward finding natural sources that can modulate TRPV1 without producing side effects. In this regard, Abbas (2020) reviewed 137 natural ingredients that affect TRPV1 activity in different in vivo and in vitro assays showing that natural compounds from different origins (e.g., bacterial, fungal, animal, and plant sources) could be potential sources for analgesics.
Arbutus andrachne L. (family Ericaceae) is a flowering strawberry tree growing wild in Jordan in the regions of Jarash, Ajloun, Irbid, Salt, and Amman (Oran, 2015). According to Oran (2015), A. andrachne is an edible plant that is used in traditional medicine in Jordan. Previously, we proved the anti-nociceptive effect of A. andrachne methanolic leaf extract on mechanical, thermal, and chemical models of pain in a mechanism that involves several receptors, mainly TRPV1 and CB1 receptors (Jaffal et al., 2020; 2022). We also demonstrated the anti-inflammatory and antipyretic effects of the plant extract in carrageenan-induced paw edema model and yeast-evoked pyrexia, respectively (Jaffal, 2021a). The aim of this study was to test the effect of A. andrachne methanolic leaf extract on the functionality and expression of TRPV1 using cobalt (Co+2) influx and enzyme-linked immunosorbent assay (ELISA), respectively, and to use molecular docking, a valuable tool for enhancing drug discovery, to determine the binding affinity between TRPV1 and each compound identified in the plant extract (Al-Najjar, 2018).
MATERIALS AND METHODS
Materials
Capsazepine, SR141716A, capsaicin, and yohimbine were purchased from Tocris Bioscience, Bristol, UK. Diclofenac sodium was from Novartis, Basel, Switzerland. Collagenase (from Clostridium histolyticum), poly-L-lysine, cobalt chloride (CoCl2), trypsin, Tween 20, copper sulfate, K+-Na+ tartrate, and 70 µM cell strainers were from Sigma-Aldrich, St. Louis, MO. Penicillin-streptomycin, phosphate buffered saline (PBS), Dulbecco’s modified eagle medium (DMEM), and DMEM-F12 media were provided by Euroclone, Milano, Italy. Fetal bovine serum (FBS), bovine serum albumin (BSA), and 4-2-hydroxyethyl-1-piperazineethanesulfonic acid (HEPES) buffer were bought from Biowest, Riverside, CA. Radioimmunoprecipitation (RIPA) lysis buffer (including protease inhibitors) and TRPV1 antibody were ordered from Santa-Cruz Biotechnology, Dallas, TX. 3,3′,5,5′-tetramethylbenzidine (TMB) and stop solution for TMB substrate were from RayBiotech, Norcross, GA. β-mercaptoethanol, diethyl ether, ethanol, methanol, and sodium hydroxide (NaOH) were purchased from Scharlau, Barcelona, Spain. Olive oil was bought from the Department of Agriculture, The University of Jordan, Amman, Jordan. Polystyrene uncoated 96-well plates were purchased from ExtraGene, Taichung City, Taiwan. Secondary anti-mouse conjugated to horseradish peroxidase (HRP) was from Invitrogen, Carlsbad, CA. Folin–Ciocalteu phenol reagent was from Fluka BioChemika, Buchs, Switzerland, while Na+ carbonate and Na+ bicarbonate were from Thermo Fisher Scientific, Waltham, MA. Normal saline was from McKesson, Virginia.
Methods
Drugs’ preparation
The stocks of capsazepine (10 mM), capsaicin (1 mM), and SR141716A (1 mM) were initially dissolved in absolute ethanol. Yohimbine and diclofenac sodium were dissolved in normal saline. For the in vivo injection, capsaicin was dissolved in olive oil, while all other drugs and the plant extract were dissolved in normal saline. For the in vitro study, capsaicin was added to 5 mM CoCl2 solution while the drugs and the extract were solubilized in 10% DMEM.
Collection and identification of the plant
The leaves of A. andrachne were gathered from Jarash, Jordan, in March, 2019, and were authentically identified by a plant taxonomist (Prof. Sawsan Oran) in the Department of Biological Sciences, The University of Jordan, Amman, Jordan. A voucher specimen was deposited at the herbarium, Department of Biological Sciences at The University of Jordan, Amman, Jordan.
Preparing the methanolic extract from A. andrachne leaves
The leaves of A. andrachne were washed, dried, and ground with a blender and then soaked in methanol (10:1 v/w ratio) for 3 days with shaking at room temperature. The extract was filtered by Whatman filter paper and then was evaporated in a rotary evaporator at 45°C under reduced pressure. The extraction was repeated several times. Finally, the extract was preserved in the freezer in an air-tight container.
Animals
Male Wistar rats (200–250 g) were used in this study. All animals were kept in the animal house, The University of Jordan, at a temperature-controlled environment (at 22 ± 1°C) in light and dark cycles (12 hour each). Food pellets and water were provided ad libitum. The animals were allowed to adapt to the experimental conditions before starting the experiments. All experiments were approved by the Animal Ethics Committee at The University of Jordan (Ethical approval number 235/2020/19) in accordance with the “Guide for the Care and Use of Laboratory Animals,” animal research: reporting of in vivo experiments (ARRIVE) guidelines and the guidelines of the International Association for the Study of Pain for animal use in research.
Determining the effect of A. andrachne extract on TRPV1 expression
Tissue collection
In this experiment, the animals were divided into control and experimental groups. Two groups received intraplantar (ipl) injection of 30 µg capsaicin or vehicle (olive oil) into the left hind paw of animals. The dose of A. andrachne extract (100 µg/paw) was chosen in this part of the experiment as it was the effective dose in decreasing capsaicin-induced mechanical allodynia in our previous study compared to the ipl injection of 50 µg or 200 µg (Jaffal et al., 2022). A. andrachne extract (100 µg/paw) or 2.5% diclofenac sodium (positive control) in 50 µl saline were injected into the left hind paw 30 minutes prior to capsaicin injection, ipsilaterally. The injected animals were kept for 24 hours after capsaicin or vehicle injection (Chen et al., 2008a). After that, the animals were euthanized by diethyl ether to collect different tissues including the spinal cord, DRGs, brain, skin, and its underlying muscles. The tissues were preserved at −80°C for homogenization.
Protein extraction and quantification
Tissues were crushed into small pieces before homogenization. All tissues were, then, homogenized in RIPA lysis buffer supplemented with a cocktail of protease inhibitors. The samples were centrifuged at 14,000 g for 10 minutes, 4°C to collect the supernatant. Protein quantification was performed using Hartree–Lowry assay (Hartree, 1972). In brief, BSA was used as a standard to prepare a series of dilutions ranging from 0.03 to 0.15 mg/ml. Three reagents were prepared as the following: reagent A consists of 7 mM K+-Na+ tartrate, 0.81 M Na+ carbonate, and 0.5 N NaOH. Reagent B consists of 70 mM K+-Na+ tartrate and 40 mM copper sulfate, whereas reagent C is a diluent of Folin–Ciocalteau in distilled water at a ratio of 1:15. One milliliter of the standards, protein samples, or buffer was mixed with 0.90 ml of reagent A and incubated at 50°C in a water bath for 10 minutes. After cooling the samples, 0.1 ml reagent B was added to each sample, followed by incubation at room temperature for 10 minutes and then the addition of 3 ml of reagent C to the samples and incubation at 50°C for 10 minutes. The absorbance was measured at 650 nm. Protein levels were quantified according to the absorbance and standard curve.
ELISA
TRPV1 expression was tested by ELISA. Protein samples (extracted from different tissues) were diluted to 1 µg/ml final concentration in Na+ carbonate: Na+ bicarbonate buffer (pH 9.6). Polystyrene 96-well plates were coated with the antigens from different samples by adding 100 µl of each diluted sample to a well. After that, the plate was incubated with gentle shaking for 2 hours at room temperature and then was washed thrice using 400 µl PBS containing 0.1% Tween 20. During washes, the excess wash buffer was removed by tapping the plate onto paper towels. 1% BSA was used (A 100 µl per well) to block the remaining binding sites of protein. After the addition of BSA, the plate was incubated with shaking for 2 hours at room temperature. After 3 washes, 100 µl of TRPV1 primary monoclonal antibody dissolved in blocking buffer (1:100) was added to each well and the plate was kept at 4°C overnight. Then, the primary antibody was washed thrice, followed by the addition of 100 µl anti-mouse secondary antibody (conjugated to HRP) in blocking buffer (1:1,000) and incubation for 2 hours at room temperature. Antibodies from a previously utilized rat interleukin-6 ELISA kit (Source: RayBiotech) were used as a positive control to coat part of the wells and ensure the effectiveness of the coating process. The next step was the addition of 100 µl TMB solution per well and incubation of the plate for 30 minutes in the dark at room temperature. Finally, the stop solution (50 µl/well) was added, followed by measuring the absorbance at 450 nm. TRPV1 expression was expressed as a fold change by normalizing data in response to control group.
Assessing the effect of A. andrachne extract on the functionality of TRPV1 in DRG neurons
Isolation and culture of DRG neurons
The isolation and culture of DRG neurons was performed as reported by Alsalem et al. (2019). All DRG neurons (cervical, thoracic, lumbar, and sacral) were extracted after decapitating the rat. After collection, the DRG neurons were placed at 37°C in DMEM-F12 supplemented with 0.25% collagenase for half an hour with shaking. After that, the neurons were incubated for 7 minutes in DMEM-F12 media containing penicillin/streptomycin (1:200), 1% HEPES buffer, and 0.25% trypsin. The neurons were triturated (50 times) using a Pasteur pipette and then were triturated with thin-flame polished pipette for another 50 times. The neurons were centrifuged (at 400 g for 20 minutes) to collect the pellet. DMEM supplemented with 10% FBS was used to resuspend the pellet, followed by 50 times trituration using thin-flame polished pipette. Finally, the solution that contains DRG neurons was filtered using 70 µM cell strainer and was distributed in the 96-well plate, previously coated with poly-L-lysine. The neurons were cultured at 37°C in a humidified atmosphere containing 5% CO2 for 24 hours.
Co+2 influx assay
Colorimetric Co+2 influx assay was used to test the activity of TRPV1 in different groups (Alsalem et al., 2019; Sántha et al., 2010). The principle of this assay is that TRPV1 is a non-selective cation channel that allows influx for cations (including Co+2) when the channel is activated (Wood et al., 1988). 10 µM capsaicin was added to DRG neurons to induce Co+2 influx. Different concentrations of the A. andrachne extract (0.5, 1, and 2 mg/ml) were added to DRG neurons for various periods of time (1, 2, 3, and 4 hour incubation) to determine the effective concentration and incubation period in alleviating capsaicin-induced Co+2 influx. Based on the results, the conditions that were chosen for elucidating the mechanism of action of A. andrachne were 1 mg/ml A. andrachne extract with 1 hour incubation. All antagonists were added 30 minutes prior to 1 mg/ml A. andrachne extract which was added 1 hour before capsaicin. The antagonists included 10 µM capsazepine (a selective TRPV1 antagonist), 10 µM SR141716A or rimonabant (a selective CB1 antagonist), and 20 µM yohimbine (a selective α-2 adrenoceptor antagonist). A positive control group was used for this part of the experiment (10 µM capsazepine + 10 µM capsaicin). In more detail, the protocol was conducted as per the following: capsaicin was dissolved in 5 mM CoCl2 solution and then added to DRG neurons for 2 minutes, followed by the lysis of neurons with a lysis buffer prepared without chelating agents, the addition of 2-mercaptoethanol (5%) to each well and incubation for 5 minutes. The color complex was detected by spectrophotometer at 475 nm wavelength.
Molecular docking
The X-ray crystallographic structure of TRPV1 (PDB ID: 5IS0, resolution: 3.43 Å) was selected from Protein Data Bank (Gao et al., 2016). The crystal structure was co-crystallized with capsazepine (Walpole et al., 1994). The molecular docking tool AutoDock 4.2 (The Scripps Research Institute, San Diego, CA) was used to study the intermolecular interactions and binding energies of the proposed compounds found in A. andrachne leaf extract (Morris et al., 2009).
Ligand and protein preparation
TRPV1 X-ray crystallographic structure was used in this part of the study. The two-dimensional (2D) chemical structures of A. andrachne constituents were generated by available chemical directories (ACD)/ChemSketch software (ACD, 2018). ChemSketch software was used to save all compounds (as .mol files) to be converted into .pdb files that can be uploaded in AutoDockTools (Morris et al., 2009). After the addition of Gasteiger charges, the files were converted into AutoDock.pdbq format to indicate all possible rotatable bonds (Morris et al., 2009). According to the co-crystallized ligand (capsazepine), the intermolecular interactions involved chains B and C but not D and E. Kollman’s charges and polar hydrogens were added to the amino acids and the charged protein was solvated using addsol utility in AutoDockTools (Morris et al., 2009).
Docking simulation
In Jaffal et al.’s (2020) study, the compounds in A. andrachne methanolic leaf extract were identified using liquid chromatography–mass spectrometry (LC–MS) analysis. These compounds were considered for molecular docking studies (Jaffal et al., 2021b). AutoDockTools and AutoGrid 4 were used to limit the search area and to create a set of grid maps, respectively (The Scripps Research Institute, San Diego, CA) (Morris et al., 2009). A grid box (having 22.5, 22.5, and 22.5 Å as x, y, and z dimensions, respectively) was then utilized to select the area to be mapped in the protein structure. Docking simulations were carried out using the Lamarckian genetic algorithm parameters (100 runs per simulation). The output information of the free energy of binding and docked coordinates was obtained from AutoDock in the docking log file (.dlg) format (Morris et al., 2009).
The?co-crystallized?ligand?(capsazepine) was successfully re-docked against 5IS0 crystal structure with a root-mean-square deviation (RMSD) of of 1.55 Å and free energy of binding of -8.5 Kcal/mol. Molecular docking simulations that showed RMSD values of less than 2.0Å are believed to have performed effectively (*). Similar parameters were used to dock plant constituents within the active site (Hevener et al., 2009).
Statistical analysis
The normality test was performed using the Shapiro–Wilk test. The statistical significance of difference between groups was examined by one-way analysis of variance (ANOVA), followed by the suitable post-hoc test (Tukey’s or Dunnett’s test) using GraphPad Prism version 7. p < 0.05 was considered significant. Data were presented as mean ± standard error of mean (SEM). The data of using different concentrations and incubation times of A. andrachne extract in capsaicin-induced Co+2 uptake experiment were analyzed using two-way ANOVA considering concentration and time as main factors. Sidak test was used as a post-hoc test for the two-way ANOVA test
RESULTS AND DISCUSSION
The identification of drug targets is fundamental for the development of novel pharmaceutical compounds. The docking models provide information about the binding energy, binding affinity, orientation of ligand–receptor interactions, and function of compounds. Thus, the aim of this work was to examine the in vitro effect of A. andrachne extract on the inhibition of the activity and/or expression of the pain receptor (TRPV1) and to use molecular docking tools to identify the active constituents in this action. The results proved the expected effect and mechanism of action.
Figure 1 shows a fold change in TRPV1 protein expression in different tissues collected from animals that received various treatments. The ipl injection of 30 µg capsaicin markedly elevated TRPV1 protein expression in the skin of inflamed paw, DRGs, spinal cord, and brain of animals dissected 24 hour post-capsaicin injection. In this context, Jeffry et al. (2009) found that the intrathecal (i.t) injection of the TRPV1 agonist (resiniferatoxin, RTX) for 20 days caused ablation of TRPV1-expressing central nerve terminals in the spinal cord but not DRGs or peripheral terminals in paw skin. Additionally, the ipl injection of 10 μg capsaicin caused a reversible reduction in TRPV1-expressing terminals, measured after 3 days of injection (Wang et al., 2020). The ipl injection of A. andrachne leaf extract prior to capsaicin did not change the effect of capsaicin on TRPV1 expression in any of the tissues. The effect of the plant extract was similar to the results found in the positive control group that received diclofenac sodium. However, this finding is not necessarily a contradiction to the anti-nociceptive and anti-allodynic effects that were shown by the extract (Jaffal et al., 2020; 2022). The finding that diclofenac sodium exhibited prominent anti-nociceptive effects in vivo with no change in TRPV1 expression compared to capsaicin-treated group in the current study is a supportive evidence for this point. Furthermore, accumulating evidences revealed that the chronic treatment with morphine increased TRPV1 expression in DRGs, sciatic nerve, and the spinal cord (Chen et al., 2008a; Vardanyan et al., 2009).
On the other hand, capsaicin-induced Co+2 uptake assay was used to determine the effect of the plant extract on TRPV1 functionality. Figures 2 and 3 show the fold change in Co+2 influx in cultured DRG neurons treated with different treatments prior to capsaicin. Treating DRG neurons with 10 µM capsaicin for 2 minutes produced a 2.7-fold increase in Co+2 influx compared to control group. This effect decreased in the group incubated with 10 µM capsazepine prior to capsaicin (data not shown).
As illustrated in figure 2, the effect of different concentrations of A. andrachne leaf extract (0.5, 1 and 2 mg/ml) on capsaicin-evoked Co+2 influx was determined after 1, 2, 3, and 4 hour of adding the extract to DRG neurons (Fig. 2). All concentrations of A. andrachne extract produced a significant reduction in capsaicin-induced Co+2 influx at all time points. Pre-incubating DRG neurons with 1 mg/ml A. andrachne extract for 1 hour exhibited maximum reduction in capsaicin-induced Co+2 influx. Additionally, the role of TRPV1, CB1, and α-2 adrenergic receptors in mediating the inhibitory effect of A. andrachne extract on capsaicin-induced Co+2 influx was investigated by applying different antagonists 30 minutes prior to 1 mg/ml A. andrachne extract. The findings of this study showed that the antagonists of CB1 and TRPV1 receptors, but not α-2 adrenergic receptor, inhibited the effect of the plant extract on capsaicin-induced Co+2 influx in DRG neurons (Fig. 3). With respect to TRPV1 functionality, the results of the current study showed that A. andrachne methanolic extract decreased capsaicin-induced Co+2 influx. The effect of the extract on the activity of TRPV1 can be attributed to the effectiveness of the methanolic solution in extracting several plant constituents that play crucial roles in pain responses, particularly Ca+2-mediated responses (Panda et al., 2009). Many of these compounds are unlikely to be extracted by other solvents like petroleum ether (Panda et al., 2009). Importantly, there are strong lines of evidence that show the involvement of CB1 receptor in the functionality of TRPV1 in different models. Using Ca+2 imaging and whole-cell patch clamp techniques, Fenwick et al. (2017) found that the endocannabinoid arachidonoylethanolamine displayed excitatory activity on TRPV1 receptor in vagal afferent neurons and that palmitoylethanolamide-evoked Ca+2 responses were inhibited by TRPV1 antagonist (Fenwick et al., 2017). Also, Patwardhan et al. (2006) found that WIN 55,212 had anti-hyperalgesic effects by inhibiting TRPV1 activity in a Ca+2-calcineurin-dependent mechanism. Additionally, Millns et al. (2001) reported that the cannabinoids have an inhibitory effect on capsaicin-induced Ca+2 responses in DRG neurons, while Alsalem et al. (2016) found that olvanil desensitizes TRPV1 in a CB1-dependent mechanism.
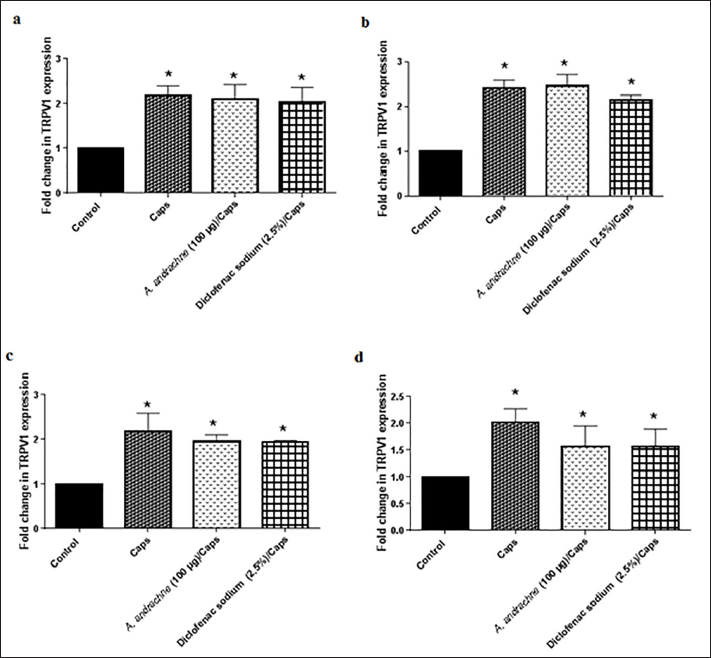 | Figure 1. Effect of A. andrachne leaf extract on capsaicin (Caps)-induced TRPV1 expression in the skin (a), DRGs (b), spinal cord (c), and brain (d). Data presented as mean ± SEM, n = 6. * Significant when compared to the control group. One-way ANOVA, followed by Tukey’s post-hoc test. [Click here to view] |
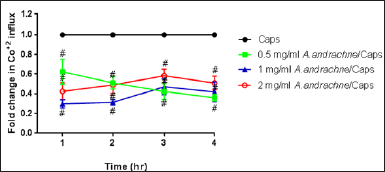 | Figure 2. Effect of A. andrachne extract on capsaicin (Caps)-induced Co+2 influx in cultured DRG neurons. Data are presented as mean ± SEM, n = 8. # Significant when compared to Caps-treated group, p < 0.05. Two-way ANOVA, followed by Sidak test. [Click here to view] |
In other part of the experiments, the 17 compounds that were identified in A. andrachne leaf extract by using LC–MS were docked against TRPV1. The list of top compounds with the lowest binding energies is presented in Table 1 and Figure 4. The results showed that the top compounds with the lowest estimated binding energies were α-tocopherol (a type of vitamin E), ursolic acid (a pentacyclic triterpenoid and a hydroxy-monocarboxylic acid) and β-sitosterol (a phytosterol) having −10.53, −10.49, and −9.76 Kcal/mol binding energies, respectively. A conserved intermolecular interaction was found between these compounds and capsazepine. Importantly, the docked compounds in addition to capsazepine fit in the same pocket of TRPV1 receptor. Figure 5 shows that the docked α-tocopherol performed hydrogen bond interactions with Ser512 and Leu553, whereas ursolic acid exhibited interactions with Glu570, Ala665 amino acids. On the other hand, β-sitosterol had one hydrogen bond interaction with Phe543. Molecular docking tools used in the current study showed that α-tocopherol, ursolic acid and β-sitosterol fit in the same pocket of TRPV1 receptor similar to capsazepine, indicating that these compounds are the active ingredients responsible for the effect of the extract in decreasing capsaicin-induced Co+2 influx. Similarly, Shukla et al. (2014) reported the interaction between capsazepine or derivatives of capsazepine (CPZ-30, CPZ-33, and CPZ-34) and tumor necrosis factor-alpha (TNF-α). The authors proved the existence of hydrogen bonds between these compounds and TNF-α, indicating the high structural stability and the possibility of an inhibitory interaction. Likewise, the interaction between capsazepine and carbonic anhydrase isoenzymes was identified through molecular docking (Xuan-yi et al., 2015).
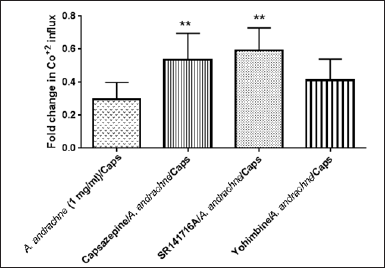 | Figure 3. Effect of different antagonists on the decrease of capsaicin (Caps)-induced Co+2 influx mediated by 1 mg A. andrachne extract. Data are presented as mean ± SEM, n = 8. ** Significant when compared to 1 mg/ml A. andrachne-treated group, p < 0.05. One-way ANOVA, followed by Dunnett’s post-hoc test. [Click here to view] |
Interestingly, previous studies have examined the activity of some compounds against TRPV1 receptor (Crouzin et al., 2007, Zhang et al., 2011). In more detail, it was reported that ursolic acid (100 μM) showed inhibition of capsaicin-induced Ca+2-flux in TRPV1 in mammalian cells stably expressing human TRPV1 receptor (Zhang et al., 2011). Furthermore, Di et al. (2020) demonstrated that ursolic acid inhibited TRPV1-evoked Ca+2 responses as a defensive mechanism against ototoxicity induced by the anti-tumor agent cisplatin. In addition, the anti-nociceptive effects of ursolic acid from Agastache mexicana aerial parts involved antagonizing TRPV1 receptor (Verano et al., 2013).
Crouzin et al. (2007) demonstrated that α-tocopherol protected against Fe+2-induced oxidative stress in hippocampal neurons by decreasing Ca+2 entry through TRP channels. Further studies are needed to examine the effect of β-sitosterol on capsaicin-induced Ca+2 entry.
Importantly, the results of this study highlight the mechanism of action of ursolic acid, α-tocopherol and β-sitosterol (compounds on A. andrachne) in exerting their analgesic effects as reported in previous studies. In brief, it was documented that the amelioration of mechanical alldoynia in different animal models was also mediated by α-tocopherol (Tiwari et al., 2009) and ursolic acid (Bhat et al., 2016), Also, both compounds reduced the levels of prostaglandin E2 (PGE2) (Chen et al., 2008b; Sakamoto et al., 1991). Moreover, α-tocopherol showed analgesic effects in neuropathic pain, formalin-induced paw licking, and writhing tests (Chen et al., 1998; Kim et al., 2006; Juaira et al., 2018). The analgesic effect of β-sitosterol in tail flick and hot plate was also documented (Sakul and Okur, 2021).
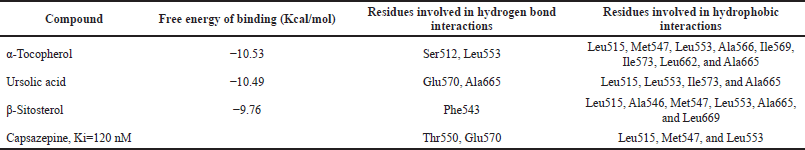 | Table 1. Top compounds in A. andrachne leaf extract having the lowest free energy of binding with the co-crystallized ligand (capsazepine). [Click here to view] |
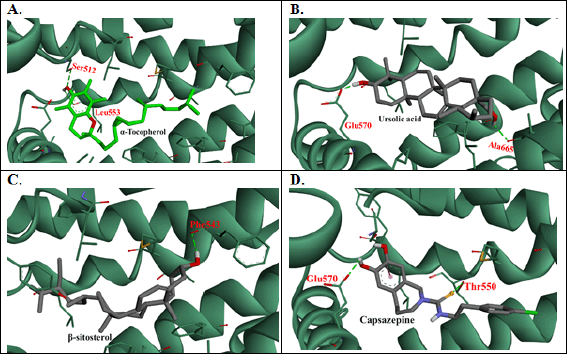 | Figure 4. 3D intermolecular interactions’ representations between TRPV1 (PDB ID: 5IS0) and (A) α-tocopherol, (B) ursolic acid, (C) β-sitosterol, and (D) Capsazepine. Prepared by Biovia DiscoveryStudio visualizer. [Click here to view] |
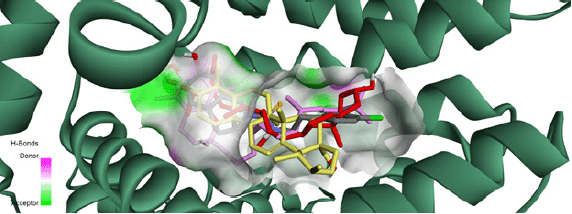 | Figure 5. Solid ribbon representation of TRPV1 receptor showing the co-crystallized capsazepine (gray), α-tocopherol (pink), ursolic acid (yellow), and β-sitosterol (red) binding in the same pocket. [Click here to view] |
CONCLUSION
Taken together, the data of the current research proved that A. andrachne methanolic leaf extract decreased capsaicin-induced Co+2 influx. Also, molecular docking tools demonstrated that α-tocopherol, ursolic acid, and β-sitosterol (similar to capsazepine) fit in the same pocket of TRPV1 receptor indicating that these compounds are the active ingredients responsible for the effect of the extract in decreasing capsaicin-induced Co+2 influx. These findings are promising and can open a gate toward developing new analgesics as A. andrachne can be a good candidate.
ACKNOWLEDGMENT
The authors acknowledge the Deanship of Scientific Research, The University of Jordan, for the financial support (235/2020/19).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The Animal Ethics Committee at The University of
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abbas, M. Modulation of TRPV1 channel function by natural products in the treatment of pain. Chemico-Biol Interact, 2020; 330:109178. CrossRef
ACD. Chemistry software for analytical and chemical knowledge management, 2018. Avaiable via https://www.acdlabs.com
Al-Najjar, BO. Synthesis, molecular docking and antioxidant evaluation of benzylidene ketone derivatives. Jordan J Biol Sci, 2018a; 11(3):307–13.
Alsalem M, Haddad M, Aldossary SA, Kalbouneh H, Azab B, Dweik A, Imraish A, El-Salem K. Effects of dual peroxisome proliferator-activated receptors α and γ activation in two rat models of neuropathic pain. PPAR Res, 2019; 2019:1–9. CrossRef
Alsalem M, Millns P, Altarifi A, El-Salem K, Chapman V, Kendall DA. Anti-nociceptive and desensitizing effects of olvanil on capsaicin-induced thermal hyperalgesia in the rat. BMC Pharmacol Toxicol, 2016; 17(1):31–42. CrossRef
Anand P, Bley K. Topical capsaicin for pain management, therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8 patch. Br J Anaesth, 2011; 107:490–502. CrossRef
Bhat RA, Lingaraju MC, Pathak NN, Kalra J, Kumar D, Tandan SK. Effect of ursolic acid in attenuating chronic constriction injury-induced neuropathic pain in rats. Fundam Clin Pharmacol, 2016; 30(6):517–28. CrossRef
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor, a heat-activated ion channel in the pain pathway. Nature, 1997; 389:816–24. CrossRef
Chen Y, Geis C, Sommer C. Activation of TRPV1 contributes to morphine tolerance, involvement of the mitogen-activated protein kinase signaling pathway. J Neurosci, 2008a; 28(22):5836–45. CrossRef
Chen Z, Ma C, Song B. The analgesic effect of vitamine E and its mechanism. Chinese Pharm J, 1998; 33:285–8.
Chen G, Shen Y, Duan H. Anti-tumor effect and its mechanisms of ursolic acid on human esophageal carcinoma cell Eca-109 in vivo. Chinese J Cancer Res, 2008b; 20(3):205–10. CrossRef
Crouzin N, Dejesusferreira M, Cohensolal C, Aimar R, Vignes M, Guiramand J. α-tocopherol-mediated long-lasting protection against oxidative damage involves an attenuation of calcium entry through TRP-like channels in cultured hippocampal neurons. Free Radic Biol Med, 2007; 42(9):1326–37. CrossRef
Di Y, Xu T, Tian Y, Ma T, Qu D, Wang Y, Lin Y, Bao D, Yu L, Liu S, Wang A. Ursolic acid protects against cisplatin?induced ototoxicity by inhibiting oxidative stress and TRPV1?mediated Ca2+?signaling. Int J Mol Med, 2020; 46(2):806–16. CrossRef
Dinh QT, Groneberg DA, Peiser C, Mingomataj E, Joachim RA, Witt C, Arck PC, Klapp BF, Fischer A. Substance P expression in TRPV1 and trkA-positive dorsal root ganglion neurons innervating the mouse lung. Respir Physiol Neurobiol, 2004; 144:15–24. CrossRef
Fenwick AJ, Fowler DK, Wu S, Shaffer FJ, Lindberg JE, Kinch DC, Peters JH. Direct anandamide activation of TRPV1 produces divergent calcium and current responses. Front Mol Neurosci, 2017; 10:200. CrossRef
Gao Y, Cao E, Julius D, Cheng Y. TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature, 2016; 534(7607):347. CrossRef
Hartree EF. Determination of protein, a modification of the lowry method that gives a linear photometric response. Anal Biochem, 1972; 48(2):422–7. CrossRef
Hevener KE, Zhao W, Ball DM, Babaoglu K, Qi J, White SW, Lee RE. Validation of molecular docking programs for virtual screening against dihydropteroate synthase. J Chem Inf Model, 2009; 49(2):444–60. CrossRef
Jaffal SM, Al-Najjar BO, Abbas MA. Ononis Spinosa alleviated capsaicin-induced mechanical allodynia in a rat model through transient receptor potential vanilloid 1 modulation. Korean J Pain, 2021b; 34(3):262–70. CrossRef
Jaffal SM, Oran SA, Alsalem M. Anti-nociceptive effect of Arbutus andrachne methanolic leaf extract mediated by CB1, TRPV1 and PPARs in mouse pain models. Inflammopharmacology, 2020; 37:189–95. CrossRef
Jaffal SM, Oran SA, Alsalem M. Alleviation of capsaicin-induced mechanical allodynia by Arbutus andrachne L. methanolic leaf extract in male rats. Mutah Lil Buhuth wad-Dirasat. Nat Appl Sci Ser, 2022; 37(2):31–49.
Jaffal SM, Oran SA, Alsalem M. Anti-inflammatory and antipyretic potential of Arbutus andrachne L. methanolic leaf extract in rats. Asian Pac J Trop Biomed, 2021a; 11(11):491–9. CrossRef
Jeffry JA, Yu SQ, Sikand P, Parihar A, Evans MS, Premkumar S. Selective targeting of TRPV1 expressing sensory nerve terminals in the spinal cord for long lasting analgesia. PLoS One, 2009; 4(9):e7021. CrossRef
Juaira T, Begum N, Yusuf MA, Patwary MA. Effect of Αlfa-tocopherol on pain and inflammation of rats. J Curr Adv Med Res, 2018; 5(1):15–8. CrossRef
Kim HK, Kim JH, Gao X, Zhou JL, Lee I, Chung K, Chung JM. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain, 2006; 122(1):53–62. CrossRef
Matsushita Y, Manabe M, Kitamura N, Shibuya I. Adrenergic receptors inhibit TRPV1 activity in the dorsal root ganglion neurons of rats. PLoS One, 2018; 13(1):e0191032. CrossRef
Millns PJ, Chapman V, Kendall DA. Cannabinoid inhibition of the capsaicin-induced calcium response in rat dorsal root ganglion neurons. Br J Pharmacol, 2001; 132(5):969–71. CrossRef
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem, 2009; 30(16):2785–91. CrossRef
Oran S. Selected wild aromatic plants in Jordan. Int J Med Plants (Photon), 2015; 108:686–99.
Panda BB, Gaur K, Kori ML, Tyagi LK, Nema RK, Sharma CS, Jain AK. Anti-Inflammatory and analgesic activity of jatropha gossypifolia in experimental animal models. Glob J Pharmacol, 2009; 3(1):1–5. Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM. The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc Natl Acad Sci, 2006; 103(30):11393–8. CrossRef
Sakamoto W, Fujie K, Nishihira J, Mino M, Morita I, Murota SI. Inhibition of PGE2 production in macrophages from vitamin E-treated rats. Prostaglandins Leukot Essent Fatty Acids, 1991; 44(2):89–92. CrossRef
?akul AA, Okur ME. Beta-sitosterol and its antinociceptive mechanism action. Ankara Universitesi Eczacilik Fakultesi Dergisi, 2021; 45(2):238–52. CrossRef
Sántha P, Oszlács O, Dux M, Dobos I, Jancsó G. Inhibition of glucosylceramide synthase reversibly decreases the capsaicin-induced activation and TRPV1 expression of cultured dorsal root ganglion neurons. Pain, 2010; 150(1):103–12. CrossRef
Shukla A, Sharma P, Prakash O, Singh M, Kalani K, Khan F, Bawankule DU, Luqman S, Srivastava SK. QSAR and docking studies on capsazepine derivatives for immunomodulatory and anti-inflammatory activity. PLoS One, 2014; 9(7):e100797. CrossRef
Tiwari V, Kuhad A, Chopra K. Tocotrienol ameliorates behavioral and biochemical alterations in the rat model of alcoholic neuropathy. Pain, 2009; 145(1):129–35. CrossRef
Tominaga M, Tominaga T. Structure and function of TRPV1. Pflügers Archiv—Eur J Physiol, 2005; 451(1):143–50. CrossRef
Verano J, González-Trujano ME, Déciga-Campos M, Ventura-Martínez R, Pellicer F. Ursolic acid from Agastache mexicana aerial parts produces antinociceptive activity involving TRPV1 receptors, cGMP and a serotonergic synergism. Pharmacol Biochem Behav, 2013; 110:255–64. CrossRef
Vardanyan, A. Wang, R. Vanderah, T. W. Ossipov, M. H. Lai, J. Porreca, F. and King, T. (2009), TRPV1 Receptor in Expression of Opioid-Induced Hyperalgesia. The Journal of Pain, 10(3), 243–252. CrossRef
Walpole CSJ, Bevan S, Bovermann G, Boelsterli JJ, Breckenridge R, Davies JW, Hughes GA, James I, Oberer L, Winter J. The discovery of capsazepine, the first competitive antagonist of the sensory neuron excitants capsaicin and resiniferatoxin. J Med Chem, 1994; 37(13):1942–54. CrossRef
Walpole CSJ, Bevan S, Bovermann G, Boelsterli JJ, Breckenridge R, Davies JW, Hughes GA, James I, Oberer L, Winter J, Wrigglesworth R. The discovery of capsazepine, the first competitive antagonist of the sensory neuron excitants capsaicin and resiniferatoxin. J Med Chem, 1994; 37(13):1942–54. CrossRef
Wang Z, McDowell T, Wang P, Alvarez R, Gomez T, Bjorling D. Activation of CB1 inhibits NGF-induced sensitization of TRPV1 in adult mouse afferent neurons. Neuroscience, 2014; 277:679–89. CrossRef
Wang S, Bian C, Yang J, Arora V, Gao Y, Wei F, Chung M-K. Ablation of TRPV1+ afferent terminals by capsaicin mediates long-lasting analgesia for trigeminal neuropathic pain. eNeuro, 2020; 7(3):1–12. CrossRef
Wood J, Winter J, James I, Rang H, Yeats J, Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J Neurosci, 1988; 8(9):3208–20. CrossRef
Xuan-yi Y, Qing-zhi L, Shao-jun C. Identification of a potential target of capsaicin by computational target fishing. Evid Based Complement Altern Med, 2015; 2015:Article ID 983951, 6. CrossRef
Zhang Y, Sreekrishna K, Lin Y, Huang L, Eickhoff D, Degenhardt C, Xu T. Modulation of transient receptor potential (TRP) channels by Chinese herbal extracts. Phytother Res, 2011; 25(11):1666–70. CrossRef