INTRODUCTION
A cluster of cases with pneumonia caused by an unspecified etiology was detected in Wuhan, Hubei Province, China, in December 2019 (Fiani et al., 2020; Guan et al., 2020). The pathogen was then identified as a novel enveloped virus, the positive-stranded beta coronavirus that was first named the 2019-novel coronavirus as per the World Health Organization (WHO). Later, the Coronavirus Study Group at its International Committee came forward to name it severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). Finally, the WHO officially called the disease coronavirus disease 2019 (COVID-19) (Guo et al., 2020). In March 2020, after its global spread, the WHO declared COVID-19 a pandemic because of its rapid transmition rate, increasing mortality rate, and limited treatment options (Fiani et al., 2020; Halpin et al., 2020). As of October 23, 2020, there have been 41,712,300 laboratory-confirmed cases detected worldwide, with at least 1,137,400 deaths reported. The number of COVID-19 cases is exponentially increasing with a low to moderate mortality rate internationally (Fiani et al., 2020). Now, seven human coronaviruses have been identified, among which are the two most recognized coronaviruses, SARS-CoV and the Middle East respiratory syndrome-CoV (MERS-CoV), both of which had outbreaks in 2003 and 2012, respectively (Chang et al., 2020).
SARS-CoV-2 and SARS have similar signs and symptoms and structural homology leading many scientists to assume that a person infected with SARS-CoV-2 can have abnormal symptoms like SARS (Vitti-Ruela et al., 2020). Several studies highlighted that long-term sequelae were observed in those who survived SARS and other viral infections such as infectious mononucleosis and measles (Yelin et al., 2020). Therefore, concerns on whether or not COVID-19 can cause long-term impact are rising since any aspect of the disease is still under investigation. Several studies that have been done found some possible sequelae for COVID-19: pulmonary sequelae, musculoskeletal sequelae, neurological sequelae, cardiac sequelae, and psychological sequelae (Biehl and Sese, 2020; Disser et al., 2020; Fiani et al., 2020; Frija-Masson et al., 2020; Mitrani et al., 2020). It is from the WHO that a person with COVID-19 usually takes from 2 to 6 weeks to recover, but for some people, symptoms may reoccur in weeks or months, although they are not infectious to others during this period. Those symptoms include cough, congestion in-breath, loss of taste and smell, abdominal pain, headache, and diarrhea (Tenforde et al., 2020). Some of the risk factors attributed to the long-term symptoms are older age, high blood pressure, obesity, and mental health conditions (Sudre et al., 2020).
Although there are many studies to further understand the nature of this disease, there is still so much with very limited information, especially when it comes to sequelae of post-COVID-19 infection. Hence, with all the evidence provided through researches, we would like to compile all the information on post-COVID-19 infection sequelae with the following questions for this systemic review.
- Do all COVID-19 cases cause post-COVID-19 sequelae?
- What are the sequelae of post-COVID-19?
- What are the systems involved in post-COVID-19 sequelae?
- What are the comorbidities that post-COVID-19 patients have?
METHODS
Search strategy
We searched the literature in October 2020 to find the published articles that analyzed the post-COVID-19 sequelae. A systematic literature search using Cochrane Library, PubMed, ProQuest, and Springer on published articles reported the post-COVID-19 sequelae. The search was under the preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2009 criteria checklist. The search terms used were “COVID-19” or “SARS-CoV-2 infection” or “post-COVID-19 consequences” or “sequelae” or “survivor.” The search terms used in this review were broad to enclose all possibilities for various studies applied. The combination of keywords from the four databases mentioned identified 414 references, 226 references, 423 references, and 219 references, respectively. Microsoft Excel was used to create a library of references collected from the databases. A total of 1,282 references were downloaded and exported to Microsoft Excel. 31 duplicates were detected and removed. Some searches were found through cross-referencing the published papers and taken outside the mentioned databases. We followed nil restrictions with the date of publication.
Inclusion and exclusion criteria
Inclusion criteria:
- Full-text articles.
- English language articles.
- Articles from the mentioned databases.
- Article on post-COVID-19 sequelae in adults.
Exclusion criteria:
- Articles on post-COVID-19 sequelae in children.
- Articles on post-COVID-19 sequelae in pregnant mothers.
- Animal studies.
Primary identification, eligibility criteria, and study selection
Articles with titles and abstracts which did not fit our main scope were eliminated. Any articles that were not full-text were excluded. After all full-text articles were gathered, the eligibility of each article was further assessed according to the criteria stated earlier. Such process of study selection was done separately with the workload divided equally between four researchers. The title with abstract followed by the full-text review was carried out through four independent researchers, and the end number of articles collected by the researchers was added up during the final process of data selection. Any differences between the researchers were discussed thoroughly to reach a mutual agreement and were reviewed by a fifth reviewer. The final analysis of the total articles combined had to be relevant and answer the research questions as stated before. After excluding the full-texted article irrelevant to the topic of studies, 36 full-text English articles were selected for the qualitative analysis. In this systematic review, other than clinical trials, we also included clinical and narrative reviews together with case reports and letters to the editor. Data from relevant studies were extracted, organized, and summarized according to the research questions.
RESULTS
Study characteristics
The flowchart for the article searches and the screening process of this study is shown in Figure 1. Of 1,328 studies retrieved from the databases, 1,251 duplicates were removed, and 130 unique articles were selected. After further curation and application of the exclusion criteria, 94 articles were excluded. Finally, 36 relevant studies were selected.
Presence of post-COVID-19 infection sequelae
Prevalence of patients with post-COVID-19 sequelae was found in 11 articles out of 36 involved in the study. In total, 1,520 survivors were included in this study, from whom 877 (57.7%) survivors were seen having post-COVID-19 sequelae. Most studies reported more than 50% in the prevalence of post-COVID-19 sequelae in survivors, as shown in Table 1. Only one study did not report any comorbidities in their patients (Chang and Park, 2020), whereas the rest of the studies reported comorbidities in patients with heart disease, hypertension, lung disease, and diabetes mellitus being recorded in almost all articles. Only two studies did not report any lung disease among their patients (Huang et al., 2020a, 2020b; Zhao et al., 2020).
Systems involved and consequences in COVID-19 survivors
From 36 articles included in the study, 11 articles reported post-COVID-19 sequelae among the survivors. Four articles recorded sequelae involving the respiratory system: with an impaired capacity of diffusion and ventilator defects (Mo et al., 2020), with pulmonary fibrosis (Zhao et al., 2020), with vocal fold paresis and paralysis (Helding et al., 2020), and with pulmonary fibrosis (Kamal et al., 2020). Nervous system involvement was found in studies (Chang and Park, 2020; Chaumont et al., 2020; Lahiri and Ardila, 2020), all of which reported obsessive-compulsive disorder and stroke, cognitive impairment and premature onset of dementia, posttraumatic stress disorder, memory deficit, and frontal syndrome, respectively, as shown in Table 2.
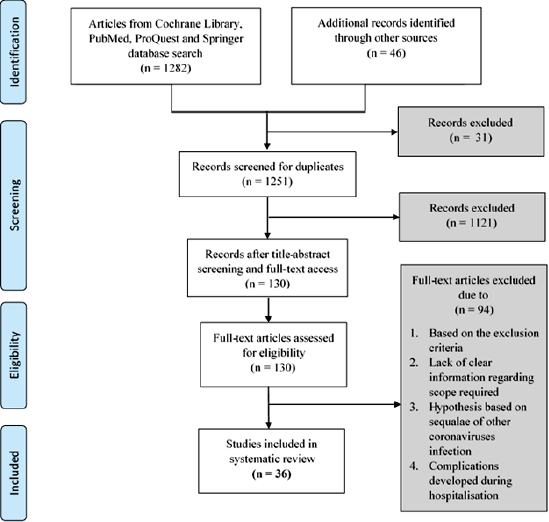 | Figure 1. The PRISMA flow diagram. [Click here to view] |
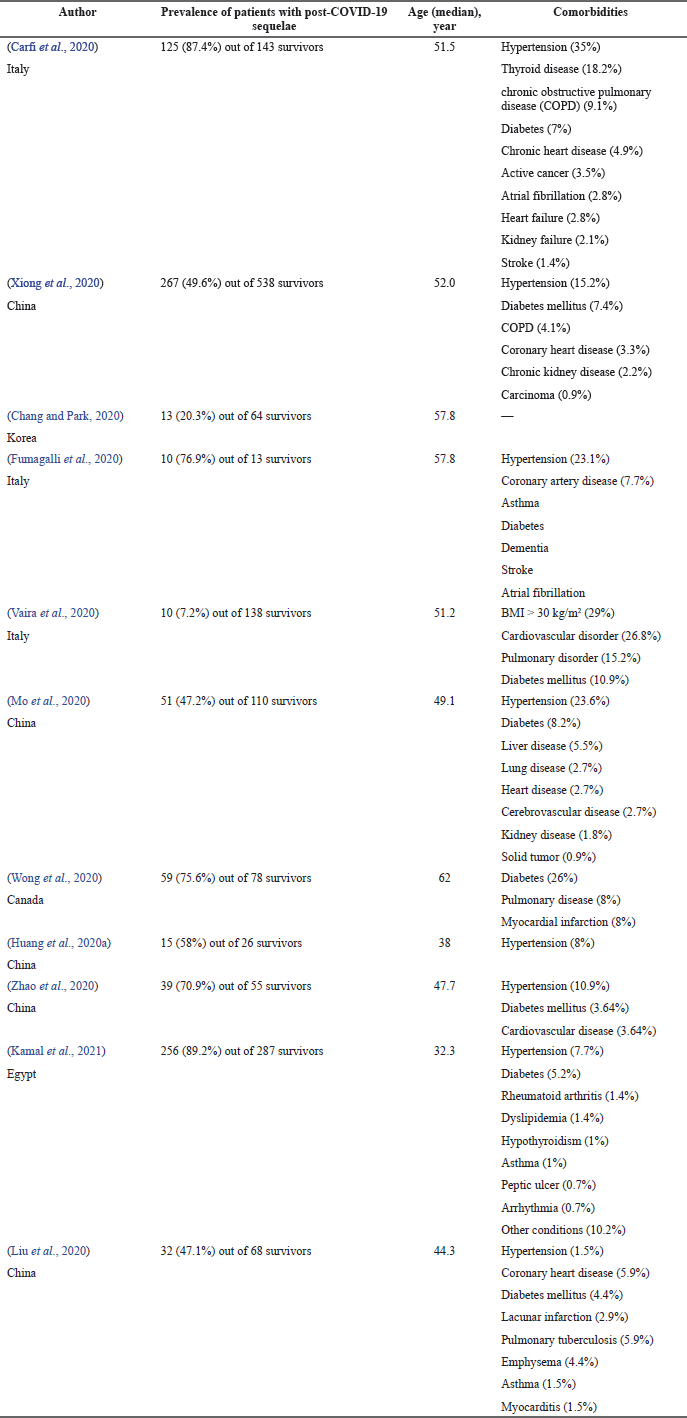 | Table 1. The prevalence of patients with post-COVID-19 sequelae. [Click here to view] |
Symptoms in COVID-19 survivors
Respiratory symptoms
Table 3 showed that seven out of eight articles recorded more than three respiratory symptoms experienced by COVID-19 survivors, except for Wong et al. (2020) that showed only two respiratory symptoms, which were cough (23%) and dyspnea (50%). Goërtz et al. (2020) and Carfi et al. (2020) recorded that all five respiratory symptoms were present in survivors. Among the five symptoms, the prevalence of dyspnea in an article by Goërtz et al. (2020) was the highest (71.0%).
Cardiovascular-related symptoms
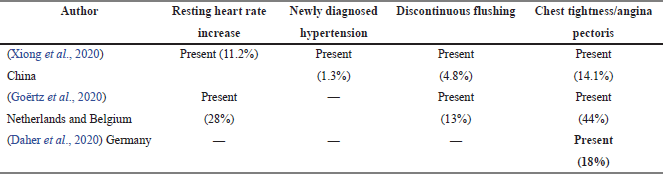
Based on Table 4, Xiong et al. (2020) recorded that all four symptoms were present in COVID-19 survivors. Among the four symptoms, chest tightness was the highest.
Psychological symptoms
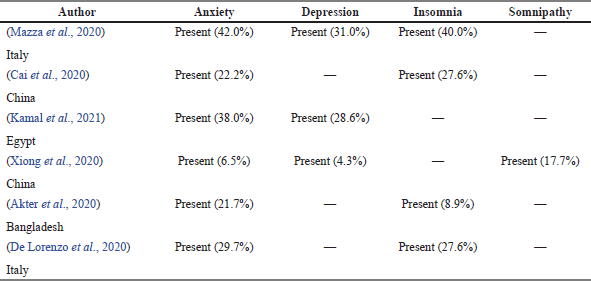
As shown in Table 5, 6 out of 36 articles in the study mentioned survivors of COVID-19 were having psychological symptoms during some period after becoming free from the virus. Two articles stated that more than three psychological symptoms were experienced by the survivors. Meanwhile, only articles by Xiong et al. (2020) stated that the survivors experienced somnipathy which is 17.7% of the population study. Among the six articles, anxiety was the highest symptoms percentage reported compared to the other symptoms.
Neurological symptoms
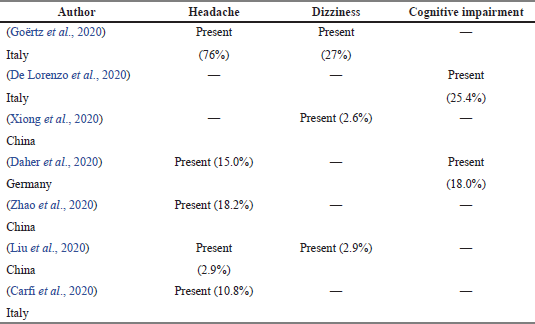
Of the 36 articles, 7 articles mentioned the prevalence of neurological symptoms in COVID-19 survivors occurring around 3 countries. Headache was the highest neurological symptom reported, as shown in Table 6.
Musculoskeletal symptoms
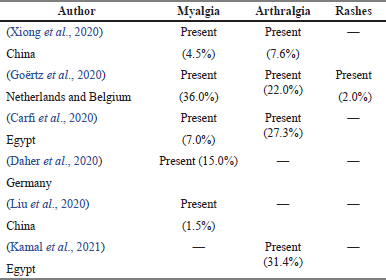
As shown in Table 7, 6 articles from a total of 36 showed the prevalence of musculoskeletal symptoms in survivors of COVID-19. The most important symptom reported in all the articles was myalgia. Meanwhile, Goërtz et al. (2020) reported having three musculoskeletal symptoms, which are myalgia, arthralgia, and also skin rashes, with a percentage of 2% among the population study.
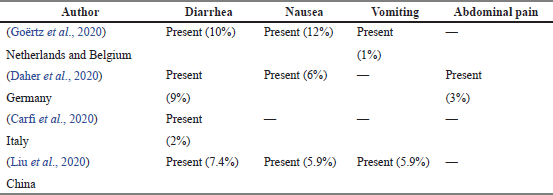
Gastrointestinal symptoms
Table 8 shows a total of four articles indicating the prevalence of gastrointestinal symptoms experienced by COVID-19 survivors. Survivors experienced three symptoms such as diarrhea, nausea, and vomiting. All the articles revealed survivors experienced diarrhea post-COVID-19. On the contrary, only one article found that survivors experienced abdominal pain with a prevalence of 3% (Daher et al., 2020).
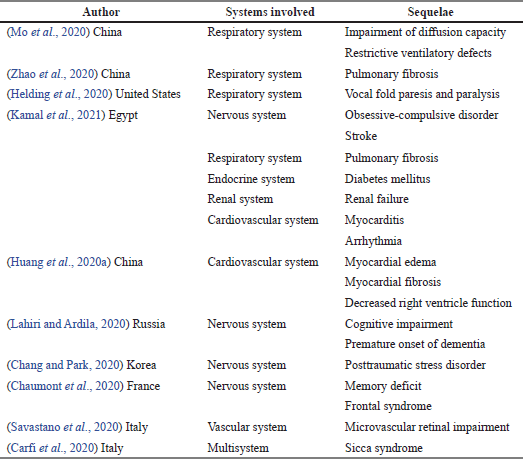 | Table 2. Systems involved and sequelae in COVID-19 survivors. [Click here to view] |
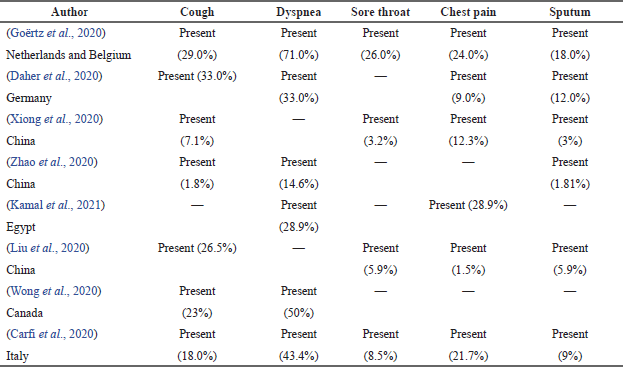 | Table 3. Prevalence of respiratory symptoms in COVID-19 survivors. [Click here to view] |
 | Table 4. Prevalence of cardiovascular-related symptoms in COVID-19 survivors. [Click here to view] |
 | Table 5. Prevalence of psychological symptoms in COVID-19 survivors. [Click here to view] |
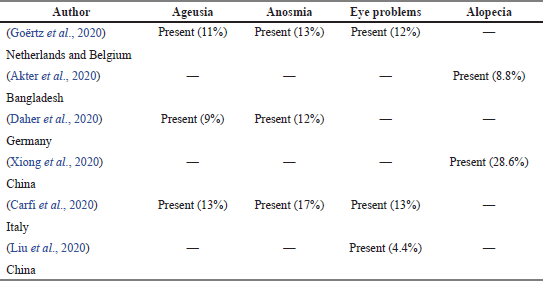
Other symptoms
Table 9 shows a total of six articles that reported other symptoms, which are loss of smell (anosmia) and taste (ageusia), loss of hair (alopecia), and problems related to the eyes in post-COVID-19 survivors. Carfi et al. (2020) and Goërtz et al. (2020) reported the survivors had experience with ageusia, anosmia, and eye problems, while Akter et al. (2020) and Xiong et al. (2020) reported the survivors experienced only alopecia.
 | Table 6. Prevalence of neurological symptoms in COVID-19 survivors. [Click here to view] |
 | Table 7. Prevalence of MSSK symptoms in COVID-19 survivors. [Click here to view] |
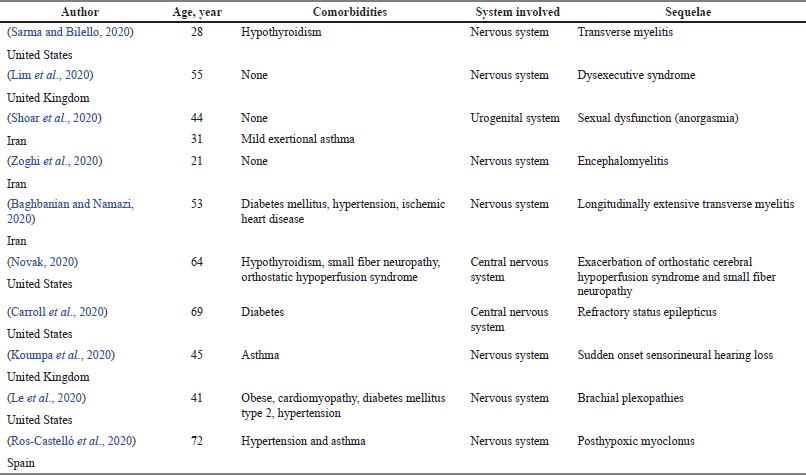
Table 10 shows case reports reported on post-COVID-19 sequelae along with their age and comorbidities. Nine out of 10 articles (81.8%) revealed the nervous system was involved as a sequela of COVID-19, followed by the urogenital and respiratory systems with 1 article each (9.1%). Out of all the articles, only four studies reported patients with no comorbidities, while the remaining articles reported various comorbidities. The most prevalent comorbidities reported were diabetes mellitus, hypertension, and asthma (25%), followed by hypothyroidism (16.7%), and obesity, ischemic heart disease, cardiomyopathy, small fiber neuropathy, and orthostatic hypoperfusion syndrome (8.3%). The youngest patient reported to have a sequela was 21 years old, and the oldest was 76 years old. Both of them involved the nervous system with encephalomyelitis and posthypoxic myoclonus, respectively.
DISCUSSION
Though there was limited information on COVID-19 disease and its sequelae, reports on post-COVID-19 sequelae have emerged. This study identified 36 relevant articles, although we cannot rule out that there were other reports that our research may have missed, from which we can conclude that there was indeed the presence of post-COVID-19 sequelae where the systems involved differ between each patient although not all patients who survived COVID-19 experience persistent symptoms nor develop sequelae.
From our study, we found that 57.7% of survivors had developed post-COVID-19 sequelae. Kamal et al. (2021) reported the highest prevalence of post-COVID-19 sequelae, 89.2%, while Vaira et al. (2020) had the lowest, 7.2%. 11 case reports showed 12 patients who had developed post-COVID-19 sequelae, while the rest of the studies reported on different symptoms developing post-COVID-19. Researchers suggested the range of symptoms may develop from acute viral infection/after some time after infection. During other coronaviruses infection outbreaks, such as the SARS-CoV epidemic in 2003 and MERS-CoV in 2012, sequelae were reported through several studies (Chan et al., 2003; Park et al., 2018; Troyer et al., 2020). A few studies also found that the most common symptoms patients may have post-COVID-19 include fatigue, dyspnea, cough, arthralgia, and chest pain (Banda et al., 2020; Carfi et al., 2020; Keefe and Cellai, 2020) with more serious complications such as myocardial inflammation, pulmonary function abnormalities, acute kidney injury, memory impairment, depression, and anxiety (Banda et al., 2020; Huang et al., 2020b; Peleg et al., 2020; Puntmann et al., 2020).
There was a limited amount of research that emphasized the sequelae of COVID-19 to our knowledge (Mo et al., 2020). The previous study stated that about 90% of COVID-19 survivors along with post-COVID-19 sequelae also have other symptoms such as pulmonary fibrosis, stroke, and renal failure, which may vary from a mild to severe scale (Kamal et al., 2021). From this research, we found that many sequelae arise post-COVID-19 in different systems. Most of our findings showed that COVID-19 survivors had lung-related sequelae. Previous studies showed pneumonia in survivors with coronavirus resulted in damaged lungs. The common sequelae were impaired lung function, which may last for months or years (Mo et al., 2020). We also discovered that survivors experienced vocal fold paresis and paralysis, which was caused by a short period of intubation and injury to the vagus nerve (Helding et al., 2020). Other than that, cardiovascular sequelae such as myocarditis, arrhythmia, and myocardial edema were recorded in two articles. In a recent study, myocardial edema was the major image manifestation noted. Two mechanisms are probably involved in post-COVID-19 myocardial sequelae. First, myocardial inflammation can be caused directly through the angiotensin-converting enzyme 2 (ACE2) receptor binding domain of spike protein-coding S, which is similar to SARS-CoV-2 and SARS-CoV. Second, cytokine storm by the immune response may cause indirect injury (Kamal et al., 2021).
 | Table 8. Prevalence of gastrointestinal symptoms in COVID-19 survivors. [Click here to view] |
 | Table 9. Prevalence of other symptoms in COVID-19 survivors. [Click here to view] |
COVID-19 survivors could develop neurological sequelae, and acute respiratory distress syndrome (ARDS) was the main pulmonary manifestation. It was found that there was sufficient evidence where a significant percentage of ARDS survivors also have cognitive impairment in the long term. There was a decline in higher brain functions following ARDS due to several factors. One of the notable factors was the blood–brain barrier acute injury responsible for cognitive impairment after recovering ARDS in COVID-19 survivors (Lahiri and Ardila, 2020).
From 36 articles included in the study, a total of 22 articles reported the system involved in post-COVID-19 sequelae among the survivors. The most affected system reported in 13 articles on post-COVID-19 sequalae was the nervous system. Nervous system involvement was found in Chang and Park (2020); Chaumont et al. (2020); Kamal et al. (2021); and Lahiri and Ardila (2020), all of which reported obsessive-compulsive disorder and stroke, cognitive impairment and premature onset of dementia, posttraumatic stress disorder, memory deficit and frontal syndrome, respectively. The uncertainties of the etiology of these neurological symptoms should be further assessed. Some of the symptoms recorded are unclear, especially lack of consciousness, headache, and dizziness. These symptoms may be developed because of respiratory failure with extreme hypoxia. The day immune condition triggered by the infection, especially the abnormally high inflammation known as the cytokine storm, could result in other symptoms. Cytokine storms with acute neurovascular pathologies were seen in COVID-19 patients without vascular risk factors. Meanwhile, four articles recorded sequelae involving the respiratory system (Mo et al., 2020), pulmonary fibrosis (Zhao et al., 2020), vocal fold paresis and paralysis (Helding et al., 2020), and pulmonary fibrosis (Kamal et al., 2021). Infection with SARS-CoV-2 causes fibroproliferation that brings massive damage to the alveolar epithelial and endothelial cells. This may impose chronic alveolar remodeling and result in lung fibrosis or pulmonary hypertension (Frija-Masson et al., 2020). Infection with SARS-CoV-2 causes a strong and seemingly uncontrolled inflammatory reaction that most likely contributes to the underlying pathology of the COVID-19 tissue damage already caused by the viral infection. The high concentration of proinflammatory mediators dubbed “the cytokine storm” damages the respiratory, hepatic, and renal processes resulting in multiorgan system dysfunction and/or death mediated by tumor necrosis factor.
 | Table 10. Case report on post-COVID-19 sequelae. [Click here to view] |
Out of 36 articles, we gathered a total of 17 articles (47.2%) that reported survivors with various comorbidities. Diabetes mellitus was present in 12 articles (70.6%) followed by hypertension and those with respiratory diseases (11 articles, 64.7%) with survivors having heart diseases following closely behind (10 articles, 58.8%). Less common ones include obesity, renal diseases, thyroid diseases, neurological diseases, and malignancy. In comparison to our results, Yu et al. (2020) also found that hypertension, diabetes, and heart diseases were the most common preexisting illnesses in COVID-19 patients. Researchers suggest individuals with comorbidities who are infected with COVID-19 are at a higher risk of complications and poor outcomes (Albitar et al., 2020; Muniyappa and Gubbi, 2020). This is parallel to a study that found comorbidities such as hypertension and obesity are some of the risk factors attributed to the persistence of symptoms after SARS-CoV-2 infection (Sudre et al., 2020). In addition, it has been shown that treatment of diabetes mellitus and hypertension using ACE inhibitors and angiotensin II type I receptor blockers causes an augment of ACE2 expression, which subsequently raises the risk of poor prognosis (Fang et al., 2020).
The long-term effects of COVID-19 might be due to persistent viral infection with low levels of viral shedding, delayed immune reaction, latency, or the presence of virus in reservoir organs or tissues (Jamiolkowski et al., 2020). SARS-CoV-2 also appears to be able to reinfect (To et al., 2021) and have the potential to precipitate new diseases (Kamal et al., 2021). Researches indicating the presence of sequelae even in asymptomatic cases suggest that mass screening and treatment may be needed without any biases. These recent findings urge further and in-depth research in measuring the symptom duration, fluctuation, overall functionality, and quality of life of survivors in comparison to the preinfection state (Perego et al., 2020).
CONCLUSION
Based on this systematic review, there was indeed presence of post-COVID-19 sequelae where a wide range of systems are involved, which differ between each patient. Comorbidities play a role in determining the severity of sequelae and the persistence of symptoms after SARS-CoV-2 infection. However, our review also suggests that those without any preexisting illness may also develop some degree of sequelae. COVID-19 survivors may present with different symptoms and conditions that vary from mild symptoms to severe and rare conditions. It is crucial to understand the sequelae of post-COVID-19 for prevention and help to establish pandemic control strategies and rehabilitation needs.
ACKNOWLEDGMENTS
The authors thank the Dean of the Faculty of Medicine at Universiti Kuala Lumpur Royal College of Medicine, Perak, Malaysia, for his support in doing this study as a Student Special Module (SSM) Project.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
FUNDING
There is no funding to report.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
AUTHORS’ CONTRIBUTIONS
Concept and design: Jannathul Firdous and Nang Thinn Thinn Htike; Data acquisition: Alia Afiqah Binti Zainudin; Drafting manuscript: Azizah Haziqah Binti Azizah Ariffin and Mohamad Aidel Mukhriz Bin Mohd Burhan; Critical revision of manuscript: Jannathul Firdous; Technical support: Nurin Zahirah Binti Zulhisham; Supervision: Jannathul Firdous; Final approval: Jannathul Firdous.
REFERENCES
Akter F, Mannan A, Mehedi HMH, Rob MA, Ahmed S, Salauddin A, Hossain MS, Hasan MM. Clinical characteristics and short term outcomes after recovery from COVID-19 in patients with and without diabetes in Bangladesh. Diabetes Metab Syndr, 2020; 14(6):2031–8. CrossRef
Albitar O, Ballouze R, Ooi JP, Ghadzi SM. Risk factors for mortality among COVID-19 patients. Diabetes Res Clin Pract, 2020; 166(2020):108293. CrossRef
Baghbanian SM, Namazi F. Post COVID-19 longitudinally extensive transverse myelitis (LETM)-a case report. Acta Neurol Belg, 2020; 121:1–2. CrossRef
Banda JM, Singh GV, Alser O, Prieto-Alhambra D. Long-term patient-reported symptoms of COVID-19: an analysis of social media data. medRxiv, 2020:1–4. 2020:2020.07.29.20164418. CrossRef
Biehl M, Sese D. Post-intensive care syndrome and COVID-19—implications post pandemic. Cleve Clin J Med, 2020;10:1–3. CrossRef
Cai X, Hu X, Ekumi IO, Wang J, An Y, Li Z, Yuan B. Psychological distress and its correlates among COVID-19 survivors during early convalescence across age groups. Am J Geriatr Psychiatry, 2020; 28(10):1030–9. CrossRef
Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. J Am Med Assoc, 2020; 324:603–5. CrossRef
Carroll E, Neumann H, Aguero-Rosenfeld ME, Lighter J, Czeisler BM, Melmed K, Lewis A. Post-COVID-19 inflammatory syndrome manifesting as refractory status epilepticus. Epilepsia, 2020; 61:135–9. CrossRef
Chan KS, Zheng JP, Mok YW, Li YM, Liu YN, Chu CM, Ip MS. SARS: prognosis, outcome and sequelae. Respirology, 2003; 8 Suppl(Suppl 1):S36–40. CrossRef
Chang L, Yan Y, Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus Med Rev, 2020; 34(2):75–80. CrossRef
Chang MC, Park DH. Incidence of post-traumatic stress disorder after coronavirus disease. Healthcare (Basel, Switzerland), 2020; 8(4):373. CrossRef
Chaumont H, San-Galli A, Martino F, Couratier C, Joguet G, Carles M, Roze E, Lannuzel A. Mixed central and peripheral nervous system disorders in severe SARS-CoV-2 infection. J Neurol, 2020; 267(11):3121–7. CrossRef
Daher A, Balfanz P, Cornelissen C, Müller A, Bergs I, Marx N, Müller-Wieland D, Hartmann B, Dreher M, Müller T. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir Med, 2020; 174:106197. CrossRef
De Lorenzo R, Conte C, Lanzani C, Benedetti F, Roveri L, Mazza MG, Brioni E, Giacalone G, Canti V, Sofia V, D’Amico M, Di Napoli D, Ambrosio A, Scarpellini P, Castagna A, Landoni G, Zangrillo A, Bosi E, Tresoldi M, Ciceri F, Rovere-Querini P. Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS One, 2020; 15(10):e0239570. CrossRef
Disser NP, De Micheli AJ, Schonk MM, Konnaris MA, Piacentini AN, Edon DL, Toresdahl BG, Rodeo SA, Casey EK, Mendias CL. Musculoskeletal consequences of COVID-19. J Bone Joint Surg Am, 2020; 102(14):1197–204. CrossRef
Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med, 2020; 8:21. CrossRef
Fiani B, Covarrubias C, Desai A, Sekhon M, Jarrah R. A Contemporary review of neurological sequelae of COVID-19. Front Neurol, 2020; 11:640. CrossRef
Frija-Masson J, Debray MP, Gilbert M, Lescure FX, Travert F, Borie R, Khalil A, Crestani B, d’Ortho MP, Bancal C. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J, 2020; 56(2):2001754. CrossRef
Fumagalli A, Misuraca C, Bianchi A, Borsa N, Limonta S, Maggiolini S, Bonardi DR, Corsonello A, Di Rosa M, Soraci L, Lattanzio F, Colombo D. Pulmonary function in patients surviving to COVID-19 pneumonia. Infection, 2020; 28(7):1–5. CrossRef
Goërtz Y, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FVC, Houben-Wilke S, Burtin C, Posthuma R, Franssen FME, van Loon N, Hajian B, Spies Y, Vijlbrief H, van ‘t Hul AJ, Janssen DJA, Spruit MA. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res, 2020; 6(4):00542–2020. CrossRef
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DS, Du B, Zeng G, Yuen K, Chen R, Tang C, Wang T, Chen P, Xiang J, Li S, Wang J, Liang Z, Peng Y, Wei L, Liu Y, Hu Y, Peng P, Wang J, Liu J, Chen Z, Li G, Zheng Z, Qiu S, Luo J, Ye C, Zhu S, Zhong N, China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med, 2020; 382(18):1708–20. CrossRef
Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res, 2020; 7(1):11. CrossRef
Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, Walshaw C, Kemp S, Corrado J, Singh R, Collins T, O’Connor RJ, Sivan M. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol, 2020; 93(2):1013–22. CrossRef
Helding L, Carroll TL, Nix J, Johns MM, LeBorgne WD, Meyer D. COVID-19 after effects: concerns for singers. J Voice, 2020; S0892-1997(20)30281-2.
Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, Liu W, Zeng H, Tao Q, Xia L. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging, 2020a; 13:2330–9. CrossRef
Huang Y, Tan C, Wu J, Chen M, Wang Z, Luo L, Zhou X, Liu X, Huang X, Yuan S, Chen C. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res, 2020b; 21(1):163. CrossRef
Jamiolkowski D, Mühleisen B, Müller S, Navarini AA, Tzankov A, Roider E. SARS-CoV-2 PCR testing of skin for COVID-19 diagnostics: a case report. Lancet (London, England), 2020; 396(10251):598–9. CrossRef
Kamal M, Omirah MO, Hussein A, Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract, 2021; 75(3):e13746. CrossRef
Kamal M, Abo Omirah M, Hussein A, Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract, 2021; 75(3):e13746. CrossRef
Keefe JB, Cellai M. Characterization of prolonged COVID-19 symptoms and patient comorbidities in an outpatient telemedicine cohort. medRxiv, 2020. 2020:2020.07.05.20146886. CrossRef
Koumpa FS, Forde CT, Manjaly JG. Sudden irreversible hearing loss post COVID-19. BMJ Case Rep, 2020; 13:e238419. CrossRef
Lahiri D, Ardila A. COVID-19 pandemic: a neurological perspective. Cureus, 2020; 12(4):e7889. CrossRef
Le MQ, Rosales R, Shapiro LT, Huang LY. The down side of prone positioning: the case of a covid-19 survivor. Am J Phys Med Rehabil, 2020; 99(10):870–2. CrossRef
Lim ST, Janaway B, Costello H, Trip A, Price G. Persistent psychotic symptoms following COVID-19 infection. BJPsych Open, 2020; 6(5):e105. CrossRef
Liu BM, Yang QQ, Zhao LY, Xie W, Si XY. Epidemiological characteristics of COVID-19 patients in convalescence period. Epidemiol Infect, 2020; 3(148):e108. CrossRef
Mazza MG, Lorenzo RD, Conte C, Poletti S, Vai B, Bollettini I, Melloni EMT, Furlan R, Ciceri F, Rovere-Querini P, COVID-19 BioB Outpatient Clinic Study group, Benedetti F. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun, 2020; 89(2020):594–600. CrossRef
Mitrani RD, Dabas N, Goldberger JJ. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm, 2020; 17(11):1984–90. CrossRef
Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, Lei C, Chen R, Zhong N, Li S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J, 2020; 55(6):2001217. CrossRef
Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab, 2020; 318:736–41. CrossRef
Novak P. Post COVID-19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: a case report. eNeurologicalSci, 2020; 21(2020):100276. CrossRef
Park WB, Jun KI, Kim G, Choi JP, Rhee JY, Cheon S, Lee CH, Park JS, Kim Y, Joh JS, Chin BS, Choe PG, Bang JH, Park SW, Kim NJ, Lim DG, Kim YS, Oh MD, Shin HS. Correlation between pneumonia severity and pulmonary complications in Middle East respiratory syndrome. J Korean Med Sci, 2018; 33(24):e169. CrossRef
Peleg Y, Kudose S, D’Agati V, Siddall E, Ahmad S, Kisselev S, Gharavi A, Canetta P. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep, 2020; 5(6):940–5. CrossRef
Perego E, Callard F, Stras L, Melville-Jóhannesson B, Pope R, Alwan NA. Why the patient-made term “long covid” is needed. Wellcome Open Res, 2020; 5:224. CrossRef
Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E.. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol, 2020; 5(11):1265–73. CrossRef
Ros-Castelló V, Quereda C, López-Sendón J, Corral I. Post-hypoxic myoclonus after COVID-19. Mov Disord Clin Pract, 2020; 7(8):983–4. CrossRef
Sarma D, Bilello LA. A case report of acute transverse myelitis following novel coronavirus infection. Clin Pract Cases Emerg Med, 2020; 4(3):321–3. CrossRef
Savastano A, Crincoli E, Savastano MC, Younis S, Gambini G, De Vico U, Cozzupoli GM, Culiersi C, Rizzo S, Gemelli Against Covid-Post-Acute Care Study Group. Peripapillary retinal vascular involvement in early post-COVID-19 patients. J Clin Med, 2020; 9(9):2895. CrossRef
Shoar S, Khavandi S, Tabibzadeh E, Vaez A, Oskouei AK, Hosseini F, Naderan M, Shoar N. A late COVID-19 complication: male sexual dysfunction. Prehosp Disaster Med, 2020; 35(6):688–9. CrossRef
Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, Pujol JC, Klaser K, Antonelli M, Canas LS, Molteni E, Modat M, Jorge Cardoso M, May A, Ganesh S, Davies R, Nguyen LH, Drew DA, Astley CM, Joshi AD, Merino J, Tsereteli N, Fall T, Gomez MF, Duncan EL, Menni C, Williams FMK, Franks PW, Chan AT, Wolf J, Ourselin S, Spector T, Steves CJ. Attributes and predictors of Long-COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. medRxiv, 2020. 2020:2020.10.19.20214494. CrossRef
Tenforde MW, Kim SS, Lindsell CJ, Billig Rose E, Shapiro NI, Files DC, Gibbs KW, Erickson HL, Steingrub JS, Smithline HA, Gong MN, Aboodi MS, Exline MC, Henning DJ, Wilson JG, Khan A, Qadir N, Brown SM, Peltan ID, Rice TW, Hager DN, Ginde AA, Stubblefield WB, Patel MM, Self WH, Feldstein LR, IVY Network Investigators, CDC COVID-19 Response Team, IVY Network Investigators. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a Multistate Health Care Systems Network—United States, March-June 2020. MMWR Morb Mortal Wkly Rep, 2020; 69(30):993–8. CrossRef
To KK, Hung IF, Ip JD, Chu AW, Chan WM, Tam AR, Fong CH, Yuan S, Tsoi HW, Ng AC, Lee LL, Wan P, Tso EY, To WK, Tsang DN, Chan KH, Huang JD, Kok KH, Cheng VC, Yuen KY. Coronavirus disease 2019 (COVID-19) re-infection by a phylogenetically distinct severe acute respiratory syndrome coronavirus 2 strain confirmed by whole genome sequencing. Clin Infect Dis, 2021; 73(9):e2946–51. CrossRef
Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain, Behav Immun, 2020; 87:34–9. CrossRef
Vaira LA, Hopkins C, Petrocelli M, Lechien JR, Chiesa-Estomba CM, Salzano G, Cucurullo M, Salzano FA, Saussez S, Boscolo-Rizzo P, Biglioli F, De Riu G. Smell and taste recovery in coronavirus disease 2019 patients: a 60-day objective and prospective study. J Laryngol Otol, 2020; 134(8):703–9. CrossRef
Vitti-Ruela BV, Dokkedal-Silva V, Rosa DS, Tufik S, Andersen ML. Possible sequelae in post-SARS-CoV-2 patients: effects on sleep and general health condition. Sleep Breath, 2020; 22:1–2. CrossRef
Wong AW, Shah AS, Johnston JC, Carlsten C, Ryerson CJ. Patient-reported outcome measures after COVID-19: a prospective cohort study. Eur Respir J, 2020; 56(5):2003276. CrossRef
Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, Dong W. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect, 2020; 27(1):89–95. CrossRef
Yelin D, Wirtheim E, Vetter P, Kalil AC, Bruchfeld J, Runold M, Guaraldi G, Mussini C, Gudiol C, Pujol M, Bandera A, Scudeller L, Paul M, Kaiser L, Leibovici L. Long-term consequences of COVID-19: research needs. Lancet Infect Dis, 2020; 20(10):1115–7. CrossRef
Yu C, Lei Q, Li W, Wang X, Liu W, Fan X, Li W. Clinical characteristics, associated factors, and predicting COVID-19 mortality risk: a retrospective study in Wuhan, China. Am J Prev Med, 2020; 59:168–75. CrossRef
Zhao Y, Shang Y, Song W, Li Q, Xie H, Xu Q, Jia JL, Li LM, Mao HL, Zhou XM, Luo H, Gao YF, Xu AG. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. Eclin Med, 2020; 25:100463. CrossRef
Zoghi A, Ramezani M, Roozbeh M, Darazam IA, Sahraian MA. A case of possible atypical demyelinating event of the central nervous system following COVID-19. Mult Scler Relat Disord, 2020; 44:102324. CrossRef