INTRODUCTION
Breast cancer is a disease with a wide range of symptoms. Every year, around 1 million people are diagnosed with breast cancer worldwide, with approximately 170,000 of these being triple negative. The lack of expression of progesterone, human epidermal growth factor-2/neu, and estrogen receptors is a defining hallmark of triple negative breast cancer (TNBC). They represent 15%–20% of all breast cancer cases (Yadhav et al., 2015). TNBCs are high-grade, aggressive tumors that have a higher risk of distant metastasis and a lower disease-specific survival rate than other breast carcinomas (Al-Mahmood et al., 2018). Because the existing cancer treatments have failed to meet the requirements for effective cancer treatment, contemporary research has focused on the urgent need to produce a suitable therapy for the cure of cancer that is free of toxic adverse effects by systematic examination of the large pool of synthetic, biological, and natural products available (Falzone et al., 2018).
Plant-derived natural compounds have received a number of research studies because of their various pharmacological activities and cancer-prevention potential. The presence of flavonoids, alkaloids, and terpenes has cytotoxic effects, such as cell growth suppression, inducing apoptosis, and signal transduction regulation, while causing no major damage to normal cells (Kumar et al., 2015). Humans have employed medicinal herbs in folkloric medicine for years. Solanum nigrum, often known as Maku or black nightshade, grows as a weed in moist places in a wide range of soils. Traditional folk medicine has commonly investigated S. nigrum to treat a variety of diseases, including antiperiodic, antiphlogistic, diaphoretic, hepatitis, pain, inflammation, and fever (Jagadeeshan et al., 2017).
The steroidal glycoside degalactotigonin, discovered in this plant, showed exceptional cytotoxicity against a range of cell types. This chemical is believed to be the most cytotoxic steroidal glycoside discovered from Solanum nigrum to date. This compound has also been shown to inhibit the growth and metastasis of osteosarcoma (Tuan Anh et al., 2018). Even though conventional chemotherapy treatments are effective against malignant cancers, poor outcomes are observed. TNBC has a greater rate of pathologic complete response than other tumor types, according to studies examining chemotherapy in the neoadjuvant context; nevertheless, progression-free and overall survivals are paradoxically shorter. As a result, novel therapeutic techniques are required to effectively manage TNBC patients (Liedtke et al., 2008). Natural extracts and bioactive chemicals generated from traditional medicine plant species have a variety of potential therapeutic targets (Khazaei et al., 2017). Thus, the current study aims to understand the anticancer mechanism of degalactotigonin in triple negative breast cancer cell line (MDA MB 231).
METHODS AND MATERIALS
Cell culture and reagents
MDA MB 231 cell lines were purchased from the National Centre for Cell in Pune, India. The cells were incubated in a CO2 incubator, with a CO2 concentration of 5% and a humidity level of 95%. After achieving confluent growth, the cells were trypsinized with Trypsin-EDTA and seeded onto 6-well plates in the required quantities (106 cells/ml) for various investigations (Sumathi et al., 2018). The steroid compound degalactotigonin, isolated from Solanum nigrum, was commercially purchased from Natural remedies, Bangalore. The standard chemotherapeutic drug used for the experiments was etoposide (200 μM), which was purchased from Sigma-Aldrich. The MDA MB 231 cells were treated with/without degalactotigonin and with the respective standard anticancer drug etoposide (200 μM) and incubated for 24 hrs in 5% CO2 and in a 95% humidity atmosphere.
Cell viability by MTT assay
To regulate the optimal dose for inhibiting cell growth and inducing cytotoxicity, various dose and time responses were investigated. Therefore, the dosage and time point cytotoxicity studies were carried out for different treatment groups using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The drug’s cytotoxicity was assessed in vitro using MDA MB 231 cells using the MTT assay.
DNA fragmentation—agarose gel electrophoresis
Apoptotic DNA fragmentation is a hallmark of apoptosis, which is a sort of programmed cell death. Apoptotic DNA fragmentation is used as a marker of apoptosis and to identify apoptotic cells using the DNA laddering technique. Cells were seeded at a density of 1 × 106 cells/well in a 6-well plate and incubated for 24 hours at 37°C in a humidified 5% CO2 incubator. The wells were washed with sterile Fluorescein Isothiocyanate (PBS) and treated with DMSO, 200 μg/ml of etoposide sample, 8 μg/ml of degalactotigonin drug, and 8 μg/ml of degalactotigonin (DGT) drug along with 200 μg/ml of ET drug in a serum-free Dulbecco’s Modified Eagle Medium (DMEM), and incubated for 24 hours at 37°C in a humidified 5% CO2 incubator. Trypsinization was used to harvest the cells, which were then centrifuged at 10,000 rpm for 10 minutes. The supernatant was removed after centrifugation, and 500 μl of lysis buffer was added to the cell pellet, which was then incubated at room temperature for 1 hour. Then, 700 μl of phenol–chloroform–isoamyl alcohol was added, mixed by inversion, and centrifuged for 5 minutes at 10,000 rpm. The top (aquatic) phase was transferred to a new Eppendrof tube. Equal volumes of cold isopropanol were poured into the tubes and gently mixed by inversion. After centrifuging the tubes at 10,000 rpm for 5 minutes and discarding the supernatant, the pellet was air-dried for 30 minutes. The dried DNA was then dissolved in 50 μl of distilled water. Furthermore, the isolated DNA was measured using a UV spectrophotometer at 260 and 280 nm using optical density.
Antimetastatic potential of degalactotigonin by scratch motility assay
A wound healing experiment was used to analyze the cell migration of MDA MB 231 cell lines. Cells were sown onto a six-well tissue culture dish in full medium and allowed to grow to 90% confluency. A plastic tip (1 mm) that touched the plate harmed cell monolayers, as shown in Xu and Deng’s (2006) study. After that, the wounded monolayers were washed four times with media to removed debris of the cell before being incubated in 1% Fetal Bovine Serum (FBS) medium. The cells were cultured for 48 hours after being treated with various quantities of degalactotigonin and commercial etoposide drug samples. The cells were seen using a mounted, inverted microscope camera (Rodriguez et al., 2005).
Colony formation potential of MDA MB 231 treated with degalactotigonin
The clonogenic test is the most effective technique for determining cell reproductive death. The clonogenic test, also known as the colony forming test, is an in vitro cell survival experiment that was established on a single cell’s capacity to develop into a colony. MDA MB 231 cells were seeded in six-well plates and incubated in triplicate overnight. After that, the cells were treated for 48 hours with different concentrations with 200 μg of the standard drug etoposide, 8 μM concentration of degalactotigonin, and a combination of DGT and etoposide (ET). A colony is defined as having at least 50 cells. The assay basically examines every cell in the population for the potential to multiply indefinitely, although it may also be used to assess the efficacy of various chemotherapeutic agents. Only a small percentage of seeded cells maintain the ability to form colonies. Cells were seeded out in suitable dilutions before or after treatment to develop colonies in 1–3 weeks. Colonies are treated with glutaraldehyde (6.0% v/v), stained with crystal violet (0.5% w/v), and counted using a stereomicroscope (Franken et al., 2006).
Identification of morphological changes by propidium iodide (PI) and Hoechst staining
PI staining was used to detect aberrant alterations in the nucleus of cells that had undergone apoptosis. To identify the cells that underwent apoptosis after DGT and regular pharmacological ET therapy, pre-adipocytes were seeded in a 6-well plate with coverslips. The cells were stained with Hoechst 33528 staining solution and incubated for 10 minutes (Crowley et al., 2018). MDA MB 231 tumor cells were treated with degalactotigonin and etoposide. Before being analyzed, the adherent cells were rinsed in 500 l of 1× PBS and fixed for 10 minutes in 4% paraformaldehyde. The cells were washed in 1× PBS after incubation and stained with propidium iodide (5 g/ml) and Hoechst 33258 staining for 10 minutes. Using a Leica DMIL LED and an integrated 3.2 Mega-Pixel Toupcam industrial digital camera 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (ICMOS HD) camera, a fluorescent microscope was used to analyze the cells that had undergone apoptosis and had condensed or fragmented nuclei.
Detection of cell apoptotic death by flow cytometry
The percentage of cells in early apoptosis and late apoptosis/necrosis was determined using flow cytometry. Triple negative breast cancer cells (MDA MB 231) were cultivated in flat-bottom, 6-well tissue culture plates at 5 × 104 cells per well and allowed to adhere overnight. For 24 hours, the incubated cells were treated with DGT and ET in various treatment groups. The cells were then extracted and stained for 15 minutes at room temperature with Annexin-V-FLUOS (Roche Diagnostics, Laval, QC) and PI (1 mg/ml) in detection buffer (10 mM HEPES, 140 mM NaCl, and 5 mM CaCl2) before being analyzed by flow cytometry.
Cell cycle analysis by flow cytometry
A cell cycle test based on DNA content is a common approach that uses flow cytometry to distinguish cells in different stages of the cell cycle. Every square centimeter was seeded with 1 × 106 MDA MB 231 cells, which were cultivated for 24 hours. The cells were treated with the steroidal substance degalactotigonin, the conventional medication etoposide, and a combination of DGT and ET after a 24-hour incubation period. The cells were extracted after a 24-hour incubation period, centrifuged twice with PBS, and fixed in 70% ethanol. Before being washed and stained in PBS with 0.02 mg/ml PI, 0.1% v/v Triton X-100, and 0.2 mg/ml DNase-free RNase A, the cells were maintained at 20°C for at least 24 hours. Cells were flow cytometrically examined after 30 minutes of incubation at room temperature in the dark, and the percentage of cells in each phase of the cell cycle was estimated using ModFitLT V2.0 software (BD Biosciences).
RT-PCR gene expression analysis
Total RNA was extracted from the active cultures of control and treated with the previously stated concentration using the Trizol (Thermo Scientific, USA) technique, as recommended by the manufacturer. To remove the leftover medium and FBS, the treated cells were washed twice with ice cold PBS. To remove the dead cells, the washed PBS was centrifuged. In the same flask, the dead cells were reintroduced. Each flask was treated with 700 μl Trizol and left at room temperature for 10 minutes with continuous shaking. 1% of agarose gel with ethidium bromide was used to assess the purity of the RNA. The total RNA was quantified using a Labman UV-Vis spectrophotometer model 1200 and the usual 260/280 nm adsorption technique (Labman, India). DNAse treatment was used to extract the detectable genomic DNA, as directed by the manufacturer (Thermo scientific, USA). ABi First Strand cDNA Synthesis Kit (Thermo Scientific, USA) was used to convert DNase-treated total RNA 1 μg to cDNA using M-MLV reverse transcriptase in an ABi StepOnePlus thermal cycler, as directed by the manufacturer (Thermo Scientific, USA).
Statistical analysis
The significance of relationships was assessed using the GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA). One-way analysis of variance was used to examine differences. The significance level was chosen at p < 0.05, and the data were reported as mean standard error.
RESULTS
DGT exhibits enhanced cytotoxicity to TNBC cells
The MTT colorimetric assay determines mitochondrial function and cell survival. The TNBC cells were treated with various concentrations, namely 1, 2, 4, 8, 16, 24, and 32 μM of DGT, and different time intervals, namely 12, 24, 48, and 96 hours. The findings demonstrated that treating MDA MB 231 cells with various doses resulted in a concentration and time-dependent decrease of cell viability. The cell survival was found to be maximum 24 hours incubation time and IC50 value of DGT was found to be 8 μM. The results clearly revealed that DGT has a dose-dependent cytotoxic effect in MDA MB 231. Once the IC50 value of DGT was established, this dose was used to check the individual and combined effect of DGT with the standard drug etoposide. Figure 1 shows the dose- and time-dependent effects of degalactotigonin in triple negative breast cancer by MTT assay.
Cell survival assay (MTT) was carried out in different treatment groups. 8 μM degalactotigonin and 200 μM standard drug etoposide for 24-hour intervals were carried out to evaluate the effect of DGT and ET inhibiting the cell survival of MDA MB 231.
According to the data shown in Figure 2, the results revealed that the combination treatment with DGT and ET resulted significantly in maximum loss of cell viability of MDA MB 231.
Detection of apoptosis by DNA ladder assay
Apoptosis is observable as a ladder pattern of 180–200 bp on standard agarose gel electrophoresis related to DNA breakage by activation of a nuclear endonuclease. In order to check the mode of cancer cell death, we treated the MDA MB 231 cells with DGT at an IC50 of 8 μM for 24 hours and standard drug etoposide of 200 mg, and DNA was then isolated and separated by agarose gel electrophoresis. As shown in Figure 3, the results revealed that the combination of DGT and ET showed significant fragmentation. Therefore, these results strongly suggest that the combination of DGT and ET is an effective inducer of apoptosis in MDA MB 231.
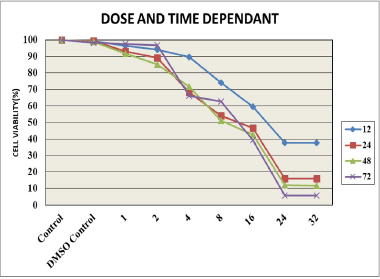 | Figure 1. Dose- and time-dependent effect of degalactotigonin. [Click here to view] |
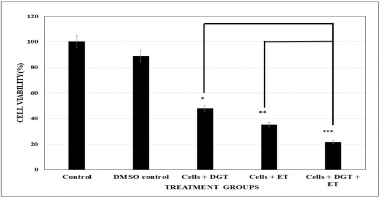 | Figure 2. Cytotoxicity studies of different treatment groups. Each data represents mean ± SD. ***Significant at 0.1% level; **Significant at 1% level; Significant at 5% level. [Click here to view] |
Figure 3 shows the DNA fragmentation in MDA MB 231 cells after treatment with etoposide and degalactotigonin.
Cell migration assay
Normal development, immunological response, and disease processes, such as cancer metastasis and inflammation, all require the ability of live cells to move. In cancer metastasis, migration is a crucial phase. We checked the TNBC cell migration treated with the standard drug etoposide, DGT, and a combination of ET and DGT. The results revealed that the percentage of cells migration was synergistically arrested by combinational treatment of DGT with ET, as shown in Figure 4.
Colony formation assay
The effect of degalactotigonin on the proliferation of TNBC cells was studied using a colony formation assay. MDA MB 231 cells were treated with the standard etoposide, degalactotigonin, and a combination of DGT and ET. Compared with the control cells, overexpression of the combination of DGT and ET significantly decreased the formation of colonies. Thus, our current findings reveal (Fig. 5) that DGT with ET show better results in inhibiting the growth of colonies from single cell and hence suggesting that DGT has a tumor suppressive role against TNBC.
Detection of DNA damage
PI staining and Hoechst staining were used to identify the apoptotic cell death and to detect the nuclear condensation, respectively. Our study reported that the treated MDA MB 231 cells were observed after staining with propidium iodide and Hoechst staining under a microscope. The results indicated that the nuclei of normal untreated cells appear spherical and equally stained, while the combination of DGT and ET demonstrated considerable cell death through apoptosis, such as cell shrinkage and nuclear condensation, as shown in Figures 6 and 7.
Mechanism of cell death and cell cycle analysis
Secondary necrosis occurs in cells that have undergone apoptosis in vitro. Control, DGT, and ET-treated flow cytometry were utilized to assess the presence of apoptotic and necrotic cell types in TNBC cells labeled with Annexin V-FITC/PI. Annexin V binds to phosphatidylserine, which is exposed on the exterior leaflet of the plasma membrane during the early stages of apoptosis, and FITC provides fluorescence for detection. Because it is a membrane impairment nucleic acid stain that is normally rejected by living cells, PI was utilized as a counter stain to detect necrotic cells. The results revealed in the current study that the MDA MB 231 cells treated with DGT and ET, as shown in Figures 8 and 9, have a higher percent of late apoptosis than early apoptosis. From these results, we suggest that the TNBC cells undergo apoptotic cell death.
Effects of degalactotigonin on cell cycle arrest in TNBC cells
Flow cytometry was used to define the influence of degalactotigonin on cell cycle processes, as well as the distribution index. The results showed that, when MDA MB 231 cells were treated with DGT and the standard drug etoposide for 24 hours, the G2/M phase was reduced compared to the control. In comparison to control cells, the percentage of the S phase in etoposide, DGT, and a combination of both treated cells was reduced. DGT had an impact on the accretion of cells in the G2/M phases, which was accompanied with cell cycle arrest, according to flow cytometry analysis. This result obscured the fact that the combination of DGT and ET promoted apoptosis in MDA MB 231 cells by arresting the cellular proliferation in the G0/G1 and S phases (Fig. 10).
 | Figure 3. Represents the DNA fragmentation in MDA MB 231 cells after treatment with ET and DGT. [Click here to view] |
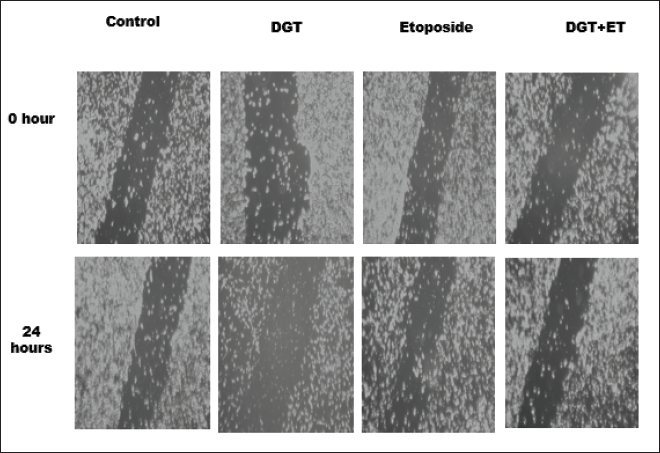 | Figure 4. The inhibition of cell migration. [Click here to view] |
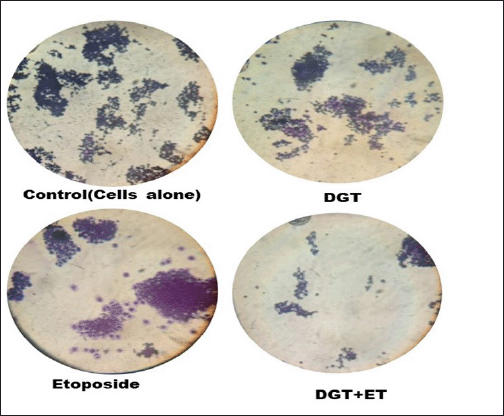 | Figure 5. Clonogenic assay showing colony formation of MDA-MB-231 cells treated with DGT and ET. [Click here to view] |
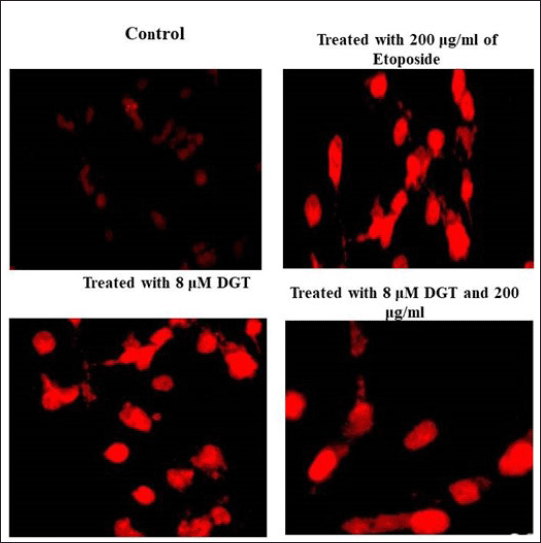 | Figure 6. Effect of DGT and ET on MDA MB 231 cells by propidium iodide (red staining). [Click here to view] |
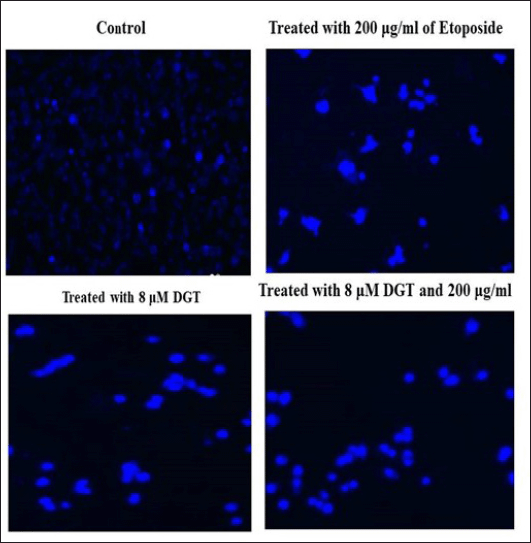 | Figure 7. Effect of DGT and ET on MDA MB 231 cells by Hoechst staining (blue staining). [Click here to view] |
Effect of degalactotigonin on upregulated genes of triple negative breast cancer cells
The differential genes involved in cell cycle arrest and apoptosis were selected from microarray data sets by the online software Gene Expression Omnibus. The upregulated and downregulated genes was scrutinized based on log FC values. From previous studies, we selected four upregulated genes, namely BUB1, BUB1B, CDC6, and Polo-like kinase (PLK)1 messenger RNA (mRNA). Budding uninhibited by benzimidazoles 1 (BUB1) gene is a mitotic checkpoint serine/threonine kinase that has been linked to a number of malignancies, including breast cancer, as an oncogene or tumor suppressor gene. CDC6 is a key component of the cell cycle regulation system that has also been linked to carcinogenic activity in several human malignancies. Polo-like kinase 1 (PLK1) is a serine/threonine kinase that regulates mitotic cell division and is overexpressed in several kinds of human malignancies, including breast cancer. Using quantitative RT-PCR analysis, we examined the effect upregulated triple negative genes treated with degalactotigonin. Figure 11A–D shows that there is a significant difference in upregulated genes BUB1, BUB1B, CDC6, and PLK1 mRNA levels treated with the compound degalactotigonin and the standard drug etoposide. BUB1 mRNA levels increased significantly in Triple Negative Breast Cancer cells, but were significantly changed in ET-treated cells. Degalactotigonin treatment also changed BUB1 expression retrograde, and a combination of DT and ET treatment caused significant changes in triple negative breast cancer cells. BUB1B mRNA expression in the control group was greater than in the ET and DGT-treated groups. Similarly, the results showed that the proposed DGT and ET exhibited a significant fold difference when compared to the control group, implying that BUB1B expression will be high in breast cancer. DGT and a combination of DGT and ET significantly reduced CDC6 mRNA expression. Combining DGT and ET has a positive effect on breast cancer control via regulating CDC6. The DGT has retrograded and altered the expression of PLK1 near normal as compared to the control, according to the quantitative RT-PCR analysis of the mRNA levels. Similarly, combining DGT and ET showed a significant fold difference, indicating that the combination yielded higher effects than either DGT or ET alone.
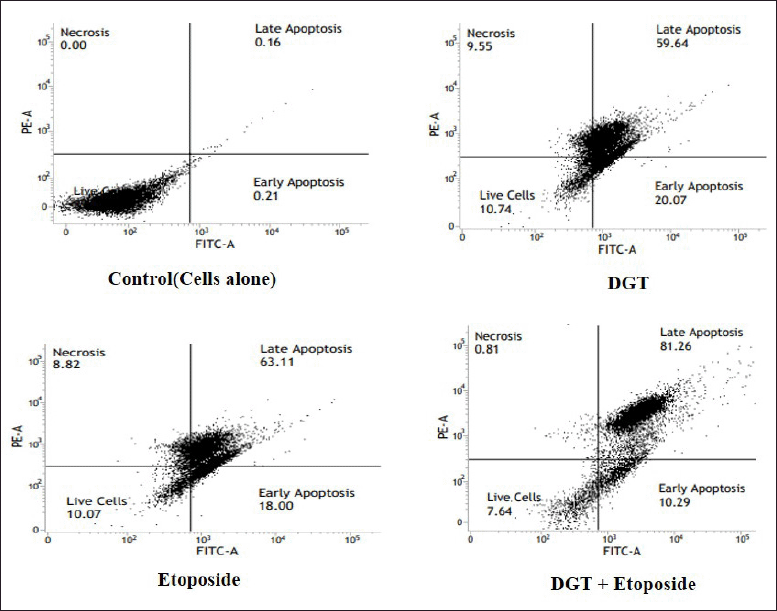 | Figure 8. Annexin V-FITC study of apoptotic and necrotic cell populations. [Click here to view] |
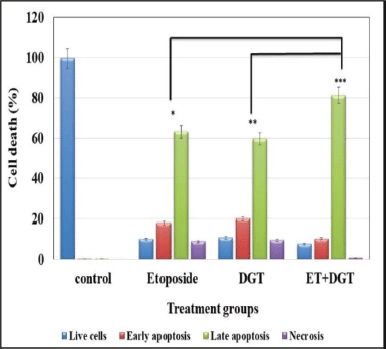 | Figure 9. PI flow cytometry study of apoptotic and necrotic cell populations. [Click here to view] |
Effect of degalactotigonin on downregulated genes of TNBC cells
CCL28 seems to be involved in a variety of additional malignancies. NRG1 is a HER3 receptor–ligand that forms heterodimers with other HER family receptors and modifies various signaling pathways, triggering growth, proliferation, reduced apoptosis, cellular migration, and angiogenesis, among other things. A variety of malignancies, including breast cancer, have been associated to PIGR. This study examined the levels of CCL28, NRG1, and PIGR mRNA in triple negative breast cancer. Figure 12A–C shows the levels of CCL28, NRG1, and PIGR mRNA in triple negative breast cancer treated with DGT and ET. The level of CCL28 mRNA in the DGT and ET groups had been changed to normal but in the DGT and ET groups, it was considerably increased. The control group had a lower level of CCL28 compared to the four groups. Similar results were identified in the expression of genes NRG1 and PIGR treated with DGT and etoposide.
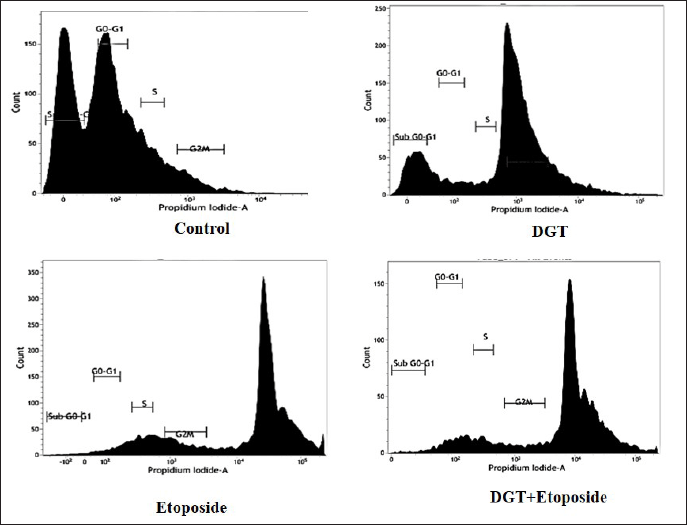 | Figure 10. Cell cycle analysis of DGT- and ET-treated MDA MB 231 cells. [Click here to view] |
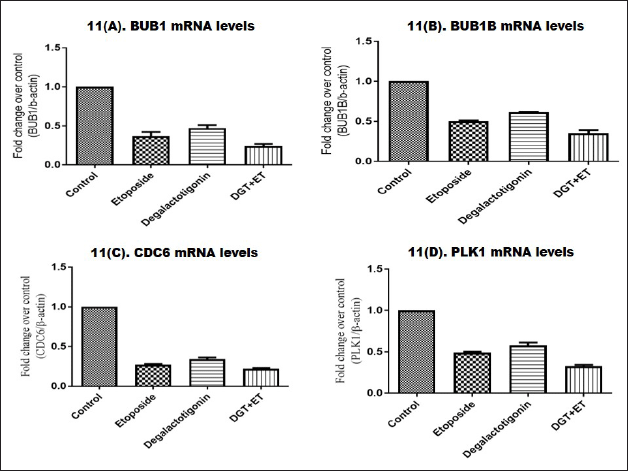 | Figure 11. Expressions of BUB1, BUB1B, CDC6, and PLK1 treated with DGT. [Click here to view] |
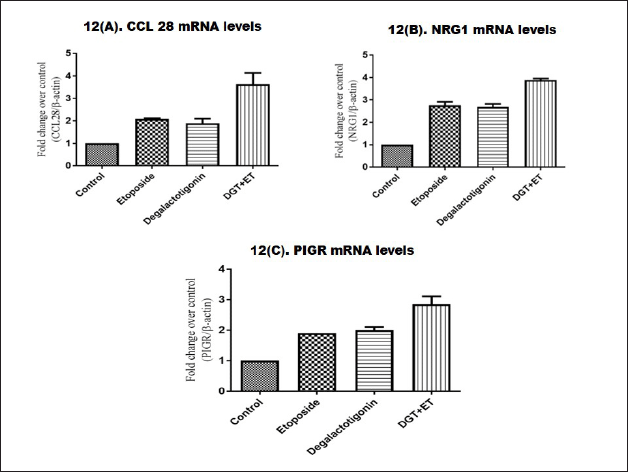 | Figure 12. Expressions of CCL28, NRG1, and PIGR treated with DGT. [Click here to view] |
DISCUSSION
The anticancer activity of degalactotigonin has not been well studied. In this paper, we studied the steroidal compound degalactotigonin and its combination with the standard chemotherapeutic drug etoposide on cell migration, cell viability, colony formation, and cell cycle development in TNBC cells (MDA MB 231). The IC50 value was determined based on cell viability rates. The optimum dose of 8 μm/ml degalactotigonin with an optimum period of 24 hours was optimized for further study. This indicates that the concentration of degalactotigonin increased the viability of MDA MB 231 cells. The effectiveness of degalactotigonin in MDA MB 231 cells was checked by cytotoxicity assay. The treatment groups are individual and combined effects of DGT with the standard drug etoposide. The findings revealed that DGT exposure to different treatment groups showed that the combination of DGT and etoposide resulted in the maximum loss of cell viability. A similar study discovered that when administered in low doses, the chemical Oblongofolin C was much more harmful to HeLa cells (Feng et al., 2012). When MCF7 cells were exposed to quinacrine, the cytotoxic effect was enhanced (Sabzichi et al., 2019).
The mode of cell death was checked by DNA fragmentation by agarose gel electrophoresis assay and the results strongly suggest that the combination of DGT and ET is a potent inducer of apoptosis against MDA MB 231 (Weng et al., 2015). It was revealed that treating human oral cancer SCC-4 cells to gallic acid results in a laddering pattern, demonstrating that gallic acid triggers DNA fragmentation. After 24 hours of incubation, the HepG2 cells treated with AB2 showed considerable fragmentation. The capability of living cells to migrate is essential for normal development, immunological reaction, and related disorders, including cancer metastasis and infection. Seeing that TNBC cells treated with DGT had migrated, the results revealed that the percentage of cells migration was synergistically arrested by the combinational treatment of DGT with ET. MDA-MB-231 cells were treated with strictinin, which suppresses cell migration and proliferation in a drug-like approach (Fultang et al., 2019).
The colony formation assay helps to determine cell death at a reproductive stage. The findings reveal that DGT with ET shows better results in inhibiting the growth of colonies from single cell and hence suggesting that DGT has a tumor-suppressive role against TNBC. Similar results demonstrates that the ability of the semi-synthetic Cu (II)–cardamonin compound to kill MDA-MB-231 and PAN-1 malignant cells while inhibiting recolonization demonstrates its cytotoxicity (Hossan et al., 2021). When a unique natural alkaloid Jerantinine B compound was treated with the MCF-7 cell line, it prevented colony formation (Qazzaz et al., 2016).
Propidium iodide and Hoechst staining help to measure the apoptotic cell death and to detect the nuclear condensation. The results reveal that the combination of DGT and ET showed significant cell death by apoptosis, such as cell contraction and nuclear condensation. The mechanism of cell death was identified by propidium iodide using the flow cytometry technique. The results showed that the percentage of apoptotic cells was higher in the group treated with the combination of ET and DGT, compared to the control and other groups, and also indicated it arrested the cells at early and late apoptotic stages. The outcome of DGT on cell cycle arrest by flow cytometry showed that in the control group, the cells were equally distributed in all phases, but in DGT, ET, and combination of DGT and ET, a change in arrested cells towards a Sub-G1 population, which resulted in late apoptotic cell death, was noted. The impact of D. innoxia ethanolic extract on the cell cycle of LoVo cells was studied using flow cytometry. The G2/M phase was interrupted, indicating a cell cycle halt or delay at this point (Al-Zharani et al., 2021). The presence of sub-diploid DNA content in a population of cells indicates that DNA fragmentation occurs during apoptosis. The cell cycle was arrested in G2M phase because this phase is the check point in the cell cycle and allows the cells to not initiate mitosis that leads to DNA damage or incomplete replication. The effect of degalactotigonin on upregulated and downregulated genes of Triple Negative Breast Cancer cells was evaluated by RT-PCR. The results revealed that the effect of DGT on unregulated and downregulated genes are regulated based on the fold change values. It is clear that the combination of DGT and ET exhibited a significant role in regulating the expression of upregulated genes, i.e., BUB1, BUB1B, CDC6, and PLK1, and downregulated genes, i.e., CCL28, NRG1, and PIGR.
CONCLUSION
Finally, the current study analyzed the cytotoxic, metastatic, apoptosis, and cell cycle activities of the steroidal substance DGT and the conventional medication ET in MDA MB 231 cells. Against MDA MB 231, the combination of DGT and ET demonstrated substantial cytotoxic action. Furthermore, the combination of DGT and ET inhibited cancer cell colony formation, migration ability, modifying the cell cycle arrest and the mitochondria-mediated intrinsic apoptotic pathway. As a conclusion, our findings provide additional knowledge about degalactotigonin’s antitumor mechanism and suggests that it might be exploited as a biologically active drug in the inhibition and treatment of human Triple Negative Breast Cancer. Future in vivo investigations are proposed on this groundwork in order to further develop the compound as a potential therapeutic drug for TNBC.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
his study does not involve experiments on animals or
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Al-Mahmood S, Sapiezynski J, Garbuzenko OB, Minko T. Metastatic and triple-negative breast cancer: challenges and treatment options. Drug Deliv Transl Res, 2018; 8(5):1483–507. CrossRef
Al-Zharani M, Nasr FA, Alqahtani AS, Cordero MAW, Alotaibi AA, Bepari A, Alarifi S, Daoud A, Barnawi IO, Daradka HM. In vitro cytotoxic evaluation and apoptotic effects of daturainnoxia grown in Saudi Arabia and phytochemical analysis. Appl Sci, 2021; 11(6):1–12. CrossRef
Crowley LC, Marfell BJ, Waterhouse NJ. Analyzing cell death by nuclear staining with Hoechst 33342. Cold Spring Harb Protoc, 2016; 2016(9); doi:10.1101/pdb.prot087205, 2018; 778-781 CrossRef
Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol, 2018; doi:10.3389/fphar.2018.01300 CrossRef
Feng C, Zhou L-Y, Yu T, Xu G, Tian HL, Xu JJ, Xu HX, Luo KQ. A new anticancer compound, Oblongifolin C, inhibits tumor growth and promotes apoptosis in HeLa cells through Bax activation. Int J Cancer, 2012; 131:1445–54. CrossRef
Franken NAP, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc, 2006; 1(5):2315–9. CrossRef
Fultang N, Illendula A, Chen B, Wu C, Jonnalagadda S, Baird N, Klase Z, Peethambaran B. Strictinin, a novel ROR1-inhibitor, represses triple negative breast cancer survival and migration via modulation of PI3K/AKT/GSK3ß activity. PLoS One, 2019; 14(5):1–15. CrossRef
Hossan MS, Break MKB, Bradshaw TD, Collins HM, Wiart C, Khoo TJ, Alafnan A. Novel semi-synthetic Cu (II)–cardamonin complex exerts potent anticancer activity against triple-negative breast and pancreatic cancer cells via inhibition of the Akt signaling pathway. Molecules, 2021; 26(8):2166. CrossRef
Jagadeeshan S, David D, Jisha S, Manjula S, Asha Nair S. Solanum nigrum Unripe fruit fraction attenuates Adriamycin resistance by down regulating multi-drug resistance protein (Mdr)-1 through Jak-STAT pathway. BMC Complement Altern Med, 2017; 17(1):370. CrossRef
Khazaei S, Hamid RA, Ramachandran V, Esa NM, Pandurangan AK, Danazadeh F, Ismail P. Cytotoxicity and proapoptotic effects of Allium atroviolaceum flower extract by modulating cell cycle arrest and caspase-Dependant and p53 independent pathway in breast cancer cell lines. Evid Based Complement Altern Med, 2017; 2017:1468957; doi:10.1155/2017/1468957 CrossRef
Kumar PS, Singh Y, Nangare DD, Bhagat K, Kumar M, Taware PB. Influence of growth stage specific water stress on the yield, physic-chemical quality and functional characteristics of tomato grown in shallow basaltic soils. Sci Hortic, 2015; 197:261–71. CrossRef
Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients w ith triple-negative breast cancer. J Clin Oncol, 2008; 26(8):1275–81. CrossRef
Qazzaz ME, Raja VJ, Lim KH, Kam TS, Lee JB, Gershkovich P, Bradshaw TD. In vitro anticancer properties and biological evaluation of novel natural alkaloid jerantinine B. Cancer Lett, 2016; 370(2):185–97. CrossRef
Rodriguez LG, Wu X, Guan JL. Wound-healing assay. Methods Mol Biol, 2005; 294:23–9.
Sabzichi M, Ramezani M, Mohammadian J, Ghorbani M, Mardomi, A, Najafipour F and Mehdizadeh, A. The synergistic impact of quinacrine on cell cycle and anti-invasiveness behaviors of doxorubicin in MDA-MB-231 breast cancer cells. Process Biochem, 2019; 81:175–81. CrossRef
Sumathi S, Poornima A, Muthukumari D, Padma PR. Cytotoxicity and in-silico studies of anethole in triple negative breast cancer cells. Int J Pharm Sci Res, 2018; 9(8):3414–9.
Tuan Anh HL, Tran PT, Thao DT, Trang DT, Dang NH, Van Cuong P, Kiem PV, Minh CV, Lee JH. Degalactotigonin, a steroidal glycoside from solanum nigrum, induces apoptosis and cell cycle arrest via inhibiting the EGFR signaling pathways in pancreatic cancer cells. Bio Med Res Int, 2018; 3120972:1–8. CrossRef
Weng SW, Hsu SC, Liu HC, Ji BC, Lien JC, Yu FS, Liu KC, Lai KC, Lin JP, Chung JG. Gallic acid, induces dna damage and inhibits dna repair-associated protein expression in human oral cancer SCC-4 cells. Anticancer Res, 2015; 35:2077–84.
Xu L, Deng X. Protein kinase Ciota promotes nicotine-induced migration and invasion of cancer cells via phosphorylation of micro- and m-calpains. J Biol Chem, 2006; 281(7):4457–66. CrossRef
Yadav BS, Chanana P, Jhamb S. Biomarkers in triple negative breast cancer: a review. World J Clin Oncol, 2015; 6(6):252–63. CrossRef