INTRODUCTION
In India, the prevalence rate for psychiatric disorders ranges from 9.5 to 370 per 1,000 individuals, and these differences in prevalence rates have also been documented in international studies (Polanczyk et al., 2015). Psychotropic medications are commonly prescribed to the elderly population; moreover, the major reported cause of death in this particular population was community-acquired pneumonia (CAP), whereas the use of antipsychotics (AP) has been associated with an increased incidence of pneumonia (Christodoulou and Kalaitzi, 2005). When comparing second-generation antipsychotics (SGA) to first-generation antipsychotics (FGA), SGA was associated with an increased incidence of pneumonia (Gau et al., 2010; Knol et al., 2008). Benzodiazepines (BZD) have also been associated with an increased incidence of CAP and death from it (Obiora et al., 2013). An elevated risk of respiratory-related morbidity and mortality was significant in case of selective serotonin reuptake inhibitors (SSRI) or selective norepinephrine reuptake inhibitors (SNRI) drugs (Vozoris et al., 2018).
The COVID-19 pandemic and the associated uncertainty could potentially increase the likelihood of mental illnesses and exacerbate health inequalities (Moreno et al., 2020). According to a recent study, a psychiatric epidemic is coexisting with the COVID-19 pandemic, necessitating the attention of the international health community (Hossain et al., 2020). Therefore, it is a crucial need to identify AP with minimal risk of causing respiratory infections and respiratory depression.
The meta-analysis by Dzahini et al. (2018) showed that exposure to AP, both FGA and SGA, was associated with an increased risk for pneumonia. However, the study did not take into consideration the use of any other psychotropic drug classes. However, this has been taken into account in our study. Our study assessed the association between the use of psychotropic agents and their effect on the respiratory function in patients with psychiatric disturbances and figured out the antipsychotic drug associated with minimal risk of pneumonia and respiratory depression. The rational and practical principles described here can also favor optimal management of psychotropic agents for COVID-19 patients, maintaining control of the underlying psychiatric condition, preventing the chances of aggravation of respiratory symptoms in these patients, mitigating the potentially aggravating effects of psychotherapy, reducing the length of hospital stay, and helping improve the patient’s quality of life.
MATERIALS AND METHODS
We followed the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) checklist to report the systematic review and meta-analysis of observational studies. The study protocol was registered with the International Prospective Register of Systematic Reviews number CRD42021234283.
Study eligibility and selection criteria
We included all case control studies, nested case control studies, retrospective and prospective cohort studies, case-crossover studies, and observational studies fulfilling the inclusion criterion. Studies available in English, observational studies reporting psychotropic drug usage, studies reporting original quantitative data associated with the relative incidence or mortality from respiratory failure, pneumonia or any other respiratory illness in patients prescribed with at least one psychotropic compared to those not prescribed any psychotropics were statistically analyzed (e.g., 95% confidence intervals, CIs). Studies reporting the use of psychotropic drugs in patients with bipolar disorder I and II, schizophrenia, major depressive disorder, cyclothymia, generalized anxiety disorder, panic attack, social anxiety, posttraumatic stress, substance abuse, and any other psychiatric disturbances were included. Participants included elderly, adults, and children with a psychotropic prescription. We did not restrict the participation to pregnancy and children. However, we excluded case reports, case series, reviews, and articles that did not report any kind of respiratory illness incidence and studies not available in English and articles with only abstract.
Intervention/Exposure
Psychotropic drugs included antipsychotic agents, mood stabilizers, benzodiazepines, SSRIs, tricyclic antidepressants and opioids.
Search strategy and selection of primary studies
We searched electronic databases, including PubMed, Scopus, Cochrane Central Register of Controlled Trials, Embase, LILACS, Google Scholar, MEDLINE, Science Direct, Web of Science, and DOAJ, for observational studies meeting the inclusion criterion. The search strategy combined terms of various respiratory illnesses, such as pneumonia, and various classes of psychotropic medication names. The searches were restricted to articles available only in English.
Retrieval of the articles was followed by quality screening by two independent reviewers. Studies with an inter-rater reliability of more than 90% were included in the analysis. Full-text articles of the included studies were obtained and screened. We discussed eligibility until consensus was achieved and was also reviewed by a third independent author, who acted as an arbiter. Statistical measures of association (odds ratio), relative risk, and attributable risk were extracted from the retrieved articles. Baseline demographics and clinical characteristics of patients were also extracted, if available, for subgroup analysis. The reference section of the relevant primary studies, systematic reviews, and guidelines were searched to identify any additional studies.
Study quality and data extraction
The potential risk of bias in each study was assessed by using the Newcastle–Ottawa Scale (NOS). It assessed the quality of methods used in the identification of groups, comparability (confounding bias), and ascertainment of the exposure in case control studies or outcome in cohort studies. Inter-rater agreement calculation was performed by using Rayyan QCRI. The data were extracted based on events in exposed group, events in unexposed group, total number of samples in exposed group, and total number of samples in the unexposed group.
Statistical analysis
Heterogeneity was assessed by visual inspection of the forest plots and I2 statistics. An I2 value of 50% was considered significant. We primarily used odds ratio as the measure of association between psychotropic drugs usage and onset of respiratory insufficiency in patients. Publication bias was determined by using Egger’s test and funnel plot. Forest plots of both fixed-effects and random-effects models were used to determine the effect of psychotropic medications on respiratory function. Exploring the confounding factors contributing to heterogeneity, such as age group, comorbidities, gender, smoking status, and concomitant medications, to determine the effect of the variables on the onset of respiratory insufficiency in patients was planned; however, due to the limited number of studies under each comparison, such tests were undependable due to lower power and hence were not conducted. However, subgroup analysis was performed to identify AP with minimal risk of respiratory deficits. All statistical analyses were performed using R 4.0.3 version.
RESULTS
A total of 968,673 records were identified through the electronic database search using the keywords psychotropic, AP, respiratory failure, and pneumonia. After applying the exclusion criteria, 41 studies were included in the systematic review (see Fig. 1 and Table 1). Of the 41 studies included, 8 were excluded based on the NOS for risk of bias assessment. Thus, finally, 33 studies were included in the meta-analysis. Of these, 11 studies (n = 196,008) assessed the association between antipsychotic drug usage and risk of pneumonia; 11 studies (n = 1,797,641) assessed the association between benzodiazepines, non-benzodiazepines, and pneumonia; 2 studies (n = 88,580) assessed the association between antidepressant drug use and pneumonia risk; 4 studies (n = 25,392) assessed the association between prescribed opioid exposure and pneumonia, recurrent pneumonia due to antipsychotic exposure; 2 studies (n = 12,039) assessed the association between antipsychotic drug exposure and respiratory failure; and 2 studies (n = 24,737) assessed the association between benzodiazepine exposure and respiratory failure. Different methods were adopted by each study to identify the patient population which included medical records, health insurance database, general practice patient databases, and nursing home records.
 | Figure 1. PRISMA flowchart for inclusion of articles in the systematic review and meta-analysis. [Click here to view] |
Assessment of quality of studies
The quality of the nonrandomized study included in the systematic review/meta-analysis was evaluated using the NOS as recommended by the Cochrane Handbook. This scale assessed the quality of methods used in the selection of study groups, comparability of the groups, ascertainment of exposure in case control studies, and outcome in cohort studies. Each study was rated and awarded using a star scoring system, with a maximum of nine stars for good quality studies. The studies were independently reviewed by three reviewers and any discrepancy was resolved by discussions. The articles were reviewed using the web application Rayyan QCRI, a tool designed to collaborate and create systematic reviews (Ouzzani et al., 2016). The review was conducted for each objective: psychotropic agents induced pneumonia (34 articles) and psychotropic agents induced respiratory failure (7 articles). Based on the inclusion and exclusion criteria and Newcastle–Ottawa assessment, the list of studies to be included in the systematic review was finalized. Five articles were excluded based on the decisions of independent reviewers.
Assessment of publication bias
Funnel plots are visual tools for investigating publication bias. It shows a scattered plot of the different studies in meta-analysis with odds ratio given on the horizontal x-axis and standard error on the vertical y-axis. Funnel plots were used to assess the publication bias in psychotropic drug exposure versus no exposure (Fig. 2); SGA exposure versus other psychotropic drug exposure (Fig. 3); FGA exposure versus other psychotropic drug exposure (Fig. 4); and risk of respiratory failure associated with psychotropic drug exposure versus no exposure (Fig. 5).
Heterogeneity assessment
Heterogeneity of the studies was assessed by the visual representation of forest plots, and the fraction of variance was estimated using I2 statistics. Eleven articles contained data to compare the psychotropic drug exposed population (n = 350,728) with the nonexposed population (n = 2,460,405) to evaluate the risk of pneumonia following psychotropic drug exposure (Fig. 6). The risk of pneumonia was increased by psychotropic drug exposure (OR = 1.66; 95% CI 1.64–1.68; 11 studies). Five articles contained data to assess the risk of pneumonia associated with FGA exposure (n = 19,211) compared to other psychotropic drug exposure (n = 15,894) (Fig. 7). FGAs have a lower risk of pneumonia compared to other psychotropic drug exposures (OR = 0.93; 95% CI = 0.86–0.99; 5 studies). Six articles contained data to assess the risk of pneumonia associated with SGA exposure (n = 11,139) compared to other psychotropic drug exposures (n = 14,402) (Fig. 8). SGAs had an increased risk of pneumonia compared to other psychotropic drug exposures (OR = 1.12; 95% CI = 1.01–1.25; 6 studies). Risk of respiratory failure associated with psychotropic exposure was assessed by comparing the psychotropic drug exposed group (n = 9,618) to the nonexposed group (n = 16,219) with two articles (Fig. 9). Risk of respiratory failure was increased with exposure to psychotropic drugs (OR = 1.79; 95% CI = 1.61–2.00; 2 studies).
 | Table 1. Summary of the studies included in the systematic review and meta-analysis. [Click here to view] |
Subgroup analysis
The risk of pneumonia for 10 individual drugs having ≥2 articles was calculated. All the SGAs, including risperidone (OR = 1.08; 95% CI = 1.00–1.17), olanzapine (OR = 1.30; 95% CI = 1.19–1.42), clozapine (OR = 1.85; 95% CI = 1.44–2.36), quetiapine (OR = 1.26; 95% CI = 1.15–1.38), zotepine (OR = 1.24; 95% CI = 0.92–1.66), and amisulpride (OR = 1.20; 95% CI = 1.01–1.42), have an increased risk of pneumonia following its usage compared to other psychotropics. However, aripiprazole had a lower risk (OR = 0.63; 95% CI = 0.48–0.81) (Fig. 12), but the results were statistically insignificant, with p = 0.4. FGAs, such as haloperidol (OR = 1.65; 95% CI = 1.14–2.38), have an increased risk of pneumonia; however, chlorpromazine (OR = 0.81; 95% CI = 0.60–1.10) (Fig. 10) and sulpiride (OR = 0.84; 95% CI = 0.70–1.01) (Fig. 11) had a lower risk of pneumonia compared to other psychotropic drug usage.
DISCUSSION
Respiratory disorders caused by psychotropic medications are of significant concern in the current era of the pandemic driven by SARS-CoV-2 infection (Bilbul et al., 2020). While few case control studies have linked antipsychotic therapy to severe breathing difficulties, acute respiratory distress, or acute respiratory failure, long-term use of SGA has been associated with an increased risk of pneumonia (Bilbul et al., 2020; Wang et al., 2017a). Our meta-analysis of observational studies determined significant association between psychotropic drug usage and onset of pneumonia (OR = 1.66; 95% CI = 1.62–1.70; p = 0.04). The results of our meta-analyses are on par with a previous meta-analysis that reported an 83% increased risk in pneumonia with antipsychotic usage (Haddad and Correll, 2018). However, in the subgroup analysis, we observed associations contrary to that reported. A 12% higher risk of pneumonia was noted with the use of SGAs, while the risk of pneumonia was found to be reduced by 7% with the use of FGAs. These results are contrary to those reported previously in the literature, which associated a relatively higher risk of pneumonia with SGAs to FGAs. Our study is the first meta-analysis to report a reduced risk of pneumonia with the use of FGAs.
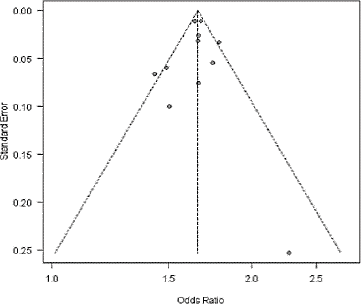 | Figure 2. Funnel plot of risk of pneumonia in psychotropic use versus no use. [Click here to view] |
 | Figure 3. Funnel plot of risk of pneumonia with SGAs versus other psychotropic drug use. [Click here to view] |
Potential pharmacological mechanisms
Extrapyramidal side effects may be a possible predisposing factor for pneumonia, especially aspiration pneumonia (Marik and Kaplan, 2003). The dopamine receptor blocking activity of FGAs is linked to the extrapyramidal symptoms (EPS) (Farah, 2005). The main EPS related to increased pneumonia risk was tardive dyskinesia (Rajamaki et al., 2020). Affinities of AP for neurotransmitter receptors, particularly the muscarinic-1 (M1) and histaminergic-1 (H1) receptors, may be associated with AP use and pneumonia (Hals et al., 1988). SGAs, such as clozapine, owing to their affinity for H1 receptor or anticholinergic action, are associated with increased pneumonia risk (Kuo et al., 2013). Xerostomia causing impaired oropharyngeal bolus transport was another possible mechanism (Cicala et al., 2019). Also, the effect of AP on cytokine production, difference in the antibody production causing immunosuppressive action, and association with immunological effects of the drug raised the risk of infections, including pneumonia (May et al., 2019; Pollmächer et al., 2000). Blockage of the D2 receptors can induce dystonia in the larynx, which in turn can precipitate difficulty in breathing and stridor in the patients (Lewis and O’Day, 2021). Previous case series evaluation has found an association between neuroleptic malignant syndrome and AP use (Wilson and Ridley, 2007). The risk for pneumonia was increased in the presence of several peripheral BZD receptors (Wang et al., 2017b). A possible mechanism to explain the risk involves normal pharyngeal function impairment, following muscle relaxation, resulting in aspiration pneumonia (Pitts, 2014). Both human and animal studies have shown that opioids suppress the immune system by macrophage, lymphocytes inhibition, altered cytokine production, and impaired migration of neutrophils and macrophages (Hamina et al., 2019). Opioids also cause sedation, which increase the risk for aspiration and can also result in respiratory depression (Dublin et al., 2011). SSRI and SNRI may have an adverse effect on the immune cell function and its count, resulting in lower infection threshold, thereby increasing the risk of respiratory depression (Vozoris et al 2018). They also exhibit immunosuppressant effect which increases the risk of pneumonia (Rajamaki et al., 2020).
 | Figure 4. Funnel plot of risk of pneumonia with FGAs versus other psychotropic drug use. [Click here to view] |
 | Figure 5. Funnel plot of risk of respiratory failure with psychotropic drug use versus no use. [Click here to view] |
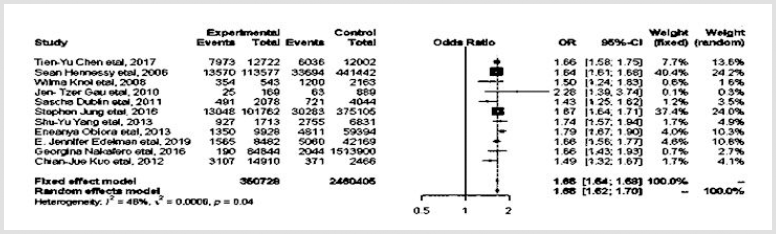 | Figure 6. Risk of pneumonia with psychotropic drug exposure versus no psychotropic drug exposure. [Click here to view] |
 | Figure 7. Risk of pneumonia with FGAs versus other psychotropic drug exposures. [Click here to view] |
 | Figure 8. Risk of pneumonia with SGAs versus other psychotropic drug exposures. [Click here to view] |
Clinical implications
AP, such as SGAs, BZD, opiates, and antidepressants, such as SSRI and SNRI, are associated with greater probability of developing pneumonia. Through our statistical subgroup analysis conducted, SGAs, such as risperidone, olanzapine, clozapine, quetiapine, zotepine, and amisulpride, have an increased risk of developing pneumonia; however, aripiprazole had a lower risk. Among the FGAs, haloperidol had an increased risk, whereas chlorpromazine and sulpiride had a lower risk of pneumonia. It was observed that the use of chlorpromazine among psychotropics was one of the most promising molecules showing inhibition of coronaviruses in the host cells (Plaze et al., 2020). Prescribed opioids, especially at doses which are high, and immunosuppressive opioids are associated with an increased risk of pneumonia (Edelman et al., 2019). A study (Cheng et al., 2018) reported that BZD has a dose-dependent association with the occurrence of pneumonia. BZRA, especially midazolam, was found to be related to an increased probability of occurrence of pneumonia, which reinforces the need for careful analysis of risk versus benefit before administration (Chen et al., 2017). SSRI and SNRI use was found to be associated with a statistically significant increase in the rate of respiratory-related morbidity and mortality, in a cohort of older adults with COPD (Vozoris et al., 2018). Respiratory infections, such as pneumonia, caused by AP and other psychotropic medications can cause unnecessary hospital admissions or prolong the duration of hospital stay in patients with psychiatric disorders (Stroup and Gray, 2018). In psychiatric patients with comorbid SARS-CoV-2 infection, aggravation of respiratory symptoms by use of antipsychotic medication can increase the need for ventilator support and risk of mortality (Wang et al., 2017a). Hence, it is vital to use AP appropriately in patients with SARS-CoV-2 infection and those at risk of other opportunistic infections (Ostuzzi et al., 2020). Our results demonstrate that FGAs should be the appropriate choice of AP in patients with schizophrenia with the following conditions: SARS-CoV-2 infection, prior history of recurrent pneumonia, immune status, and long-term use of corticosteroids.
 | Figure 9. Risk of respiratory failure with psychotropic drugs exposure versus no exposure. [Click here to view] |
 | Figure 10. Chlorpromazine. [Click here to view] |
 | Figure 11. Sulpiride. [Click here to view] |
 | Figure 12. Aripiprazole. [Click here to view] |
CONCLUSION
FGAs are particularly associated with a lower risk for pneumonia compared to exposure to other psychotropic drugs, whereas SGAs are more significantly associated with the development of pneumonia with respect to other psychotropic drug exposure. Thus, FGAs are relatively safer when compared to SGAs and can be used as a first-line choice for the treatment of schizophrenia in patients with underlying respiratory comorbidities. The exposure to psychotropics can also contribute to the development of respiratory failure as well. Among various AP, SGAs, including risperidone, olanzapine, clozapine, quetiapine, zotepine, and amisulpride, significantly contribute to the development of pneumonia. However, aripiprazole has a lower risk. FGAs, such as haloperidol, have an increased risk of developing pneumonia following its usage, whereas chlorpromazine and sulpiride have a lower risk of pneumonia compared to other psychotropic drug usage. The study focuses on the need to abandon collective considerations about AP with respect to the risk of pneumonia and also shift the focus toward relative risks for the development of various respiratory outcomes and the risk associated with individual psychotropic drugs as well. The subgroup analysis performed in the study may help clinicians to practice the use of AP with minimal risk of respiratory deficits.
ACKNOWLEDGMENT
The authors thank the management of Krupanidhi College for the support provided during the conduct of the study.
AUTHORS’ CONTRIBUTIONS
All the authors were involved in the literature review process, review designing, interpretation of the data and manuscript revision and shall be accountable for all the aspects of the study.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Almirall J, Bolíbar I, Balanzó X, González CA. Risk factors for community-acquired pneumonia in adults: a population-based case-control study. Eur Respir J, 1999; 13(2):349–55; doi:10.1183/09031936.99.13234999 CrossRef
Almirall J, Bolíbar I, Serra-Prat M, Roig J, Hospital I, Carandell E, Agustí M, Ayuso P, Estela A, Torres A; Community-Acquired Pneumonia in Catalan Countries (PACAP) Study Group. New evidence of risk factors for community-acquired pneumonia: a population-based study. Eur Respir J, 2008; 31(6):1274–84; doi:10.1183/09031936.00095807 CrossRef
Aparasu RR, Chatterjee S, Chen H. Risk of pneumonia in elderly nursing home residents using typical versus atypical antipsychotics. Ann Pharmacother, 2013; 47(4):464–74; doi:10.1345/aph.1R510 CrossRef
Barnett MJ, Perry PJ, Alexander B, Kaboli PJ. Risk of mortality associated with antipsychotic and other neuropsychiatric drugs in pneumonia patients. J Clin Psychopharmacol, 2006; 26(2):182–7; doi:10.1097/01.jcp.0000203598.43314.34 CrossRef
Bilbul M, Paparone P, Kim AM, Mutalik S, Ernst CL. Psychopharmacology of COVID-19. Psychosomatics, 2020; 61(5):411–27; doi:10.1016/j.psym.2020.05.006 CrossRef
Brunnström HR, Englund EM. Cause of death in patients with dementia disorders. Eur J Neurol, 2009; 16(4):488–92; doi:10.1111/j.1468-1331.2008.02503.x CrossRef
Cheng SY, Chen WY, Liu HC, Yang TW, Pan CH, Yang SY, Kuo CJ. Benzodiazepines and risk of pneumonia in schizophrenia: a nationwide case-control study. Psychopharmacology (Berl), 2018; 235(11):3329–38; doi:10.1007/s00213-018-5039-9 CrossRef
Chen SJ, Yeh CM, Chao TF, Liu CJ, Wang KL, Chen TJ, Chou P, Wang FD. The use of benzodiazepine receptor agonists and risk of respiratory failure in patients with chronic obstructive pulmonary disease: a nationwide population-based case-control study. Sleep, 2015; 38(7):1045–50. Published 2015 Jul 1. doi:10.5665/sleep.4808 CrossRef
Chen TY, Winkelman JW, Mao WC, Liu CL, Hsu CY, Wu CS. The use of benzodiazepine receptor agonists and the risk of hospitalization for pneumonia: a nationwide population-based nested case-control study. Chest, 2018; 153(1):161–71; doi:10.1016/j.chest.2017.07.030 CrossRef
Chen YH, Lin HC, Lin HC. Poor clinical outcomes among pneumonia patients with schizophrenia. Schizophr Bull, 2011; 37(5):1088–94; doi:10.1093/schbul/sbq019 CrossRef
Christodoulou C, Kalaitzi C. Antipsychotic drug-induced acute laryngeal dystonia: two case reports and a mini review. J Psychopharmacol, 2005; 19(3):307–11; doi:10.1177/0269881105051543 CrossRef
Cicala G, Barbieri MA, Spina E, de Leon J. A comprehensive review of swallowing difficulties and dysphagia associated with antipsychotics in adults. Expert Rev Clin Pharmacol, 2019; 12(3):219–34; doi:10.1080/17512433.2019.1577134 CrossRef
Ciranni MA, Kearney TE, Olson KR. Comparing acute toxicity of first- and second-generation antipsychotic drugs: a 10-year, retrospective cohort study. J Clin Psychiatry, 2009; 70(1):122–9; doi:10.4088/jcp.08m04315 CrossRef
Dublin S, Walker RL, Jackson ML, Nelson JC, Weiss NS, Von Korff M, Jackson LA. Use of opioids or benzodiazepines and risk of pneumonia in older adults: a population-based case-control study. J Am Geriatr Soc, 2011; 59(10):1899–907; doi:10.1111/j.1532-5415.2011.03586.x CrossRef
Dzahini O, Singh N, Taylor D, Haddad PM. Antipsychotic drug use and pneumonia: systematic review and meta-analysis. J Psychopharmacol, 2018; 32(11):1167–81; doi:10.1177/0269881118795333 CrossRef
Edelman EJ, Gordon KS, Crothers K, Akgün K, Bryant KJ, Becker WC, Gaither JR, Gibert CL, Gordon AJ, Marshall BDL, Rodriguez-Barradas MC, Samet JH, Justice AC, Tate JP, Fiellin DA. Association of prescribed opioids with increased risk of community-acquired pneumonia among patients with and without HIV. JAMA Intern Med, 2019; 179(3):297–304; doi:10.1001/jamainternmed.2018.6101 CrossRef
Farah A. Atypicality of atypical antipsychotics. Prim Care Companion J Clin Psychiatry, 2005; 7(6):268–74; doi:10.4088/pcc.v07n0602 CrossRef
Gau JT, Acharya U, Khan S, Heh V, Mody L, Kao TC. Pharmacotherapy and the risk for community-acquired pneumonia. BMC Geriatr, 2010; 10:45. Published 2010 Jul 6. doi:10.1186/1471-2318-10-45 CrossRef
Haddad PM, Correll CU. The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses. Ther Adv Psychopharmacol, 2018; 8(11):303–18. Published 2018 Oct 8. doi:10.1177/2045125318781475 CrossRef
Hals PA, Hall H, Dahl SG. Muscarinic cholinergic and histamine H1 receptor binding of phenothiazine drug metabolites. Life Sci, 1988; 43(5):405–12; doi:10.1016/0024-3205(88)90519-x CrossRef
Hamina A, Taipale H, Karttunen N, Tanskanen A, Tiihonen J, Tolppanen AM, Hartikainen S. Hospital-treated pneumonia associated with opioid use among community dwellers with Alzheimer’s disease. J Alzheimers Dis, 2019; 69(3):807–16; doi:10.3233/JAD-181295 CrossRef
Hennessy S, Bilker WB, Leonard CE, Chittams J, Palumbo CM, Karlawish JH, Yang YX, Lautenbach E, Baine WB, Metlay JP. Observed association between antidepressant use and pneumonia risk was confounded by comorbidity measures. J Clin Epidemiol, 2007; 60(9):911–8; doi:10.1016/j.jclinepi.2006.11.022 CrossRef
Hossain MM, Tasnim S, Sultana A, Faizah F, Mazumder H, Zou L, McKyer ELJ, Ahmed HU, Ma P. Epidemiology of mental health problems in COVID-19: a review. F1000Res, 2020; 9:636. Published 2020 Jun 23. doi:10.12688/f1000research.24457.1 CrossRef
Hung GC, Liu HC, Yang SY, Pan CH, Liao YT, Chen CC, Kuo CJ. Antipsychotic reexposure and recurrent pneumonia in schizophrenia: a nested case-control study. J Clin Psychiatry, 2016; 77(1):60–6; doi:10.4088/JCP.14m09301 CrossRef
Huybrechts KF, Gerhard T, Crystal S, Olfson M, Avorn J, Levin R, Lucas JA, Schneeweiss S. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: population based cohort study. BMJ, 2012; 344:e977. Published 2012 Feb 23. doi:10.1136/bmj.e977 CrossRef
Huybrechts KF, Rothman KJ, Silliman RA, Brookhart MA, Schneeweiss S. Risk of death and hospital admission for major medical events after initiation of psychotropic medications in older adults admitted to nursing homes. CMAJ, 2011; 183(7):E411–9; doi:10.1503/cmaj.101406 CrossRef
Huybrechts KF, Schneeweiss S, Gerhard T, Olfson M, Avorn J, Levin R, Lucas JA, Crystal S. Comparative safety of antipsychotic medications in nursing home residents. J Am Geriatr Soc, 2012; 60(3):420–9; doi:10.1111/j.1532-5415.2011.03853.x CrossRef
Jackson JW, VanderWeele TJ, Blacker D, Schneeweiss S. Mediators of first- versus second-generation antipsychotic-related mortality in older adults. Epidemiology, 2015; 26(5):700–9; doi:10.1097/EDE.0000000000000321 CrossRef
Jung S, Spence MM, Escasa NM, Lee EA, Hui RL, Gibbs NE. The risk of pneumonia in older adults using nonbenzodiazepine hypnotics. J Manag Care Spec Pharm, 2016; 22(8):932–8; doi:10.18553/jmcp.2016.22.8.932 CrossRef
Kales HC, Valenstein M, Kim HM, McCarthy JF, Ganoczy D, Cunningham F, Blow FC. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry, 2007; 164(10):1568–623. doi:10.1176/appi.ajp.2007.06101710 CrossRef
Kim DH, Huybrechts KF, Patorno E, Marcantonio ER, Park Y, Levin R, Abdurrob A, Bateman BT. Adverse events associated with antipsychotic use in hospitalized older adults after cardiac surgery. J Am Geriatr Soc, 2017; 65(6):1229–37; doi:10.1111/jgs.14768 CrossRef
Knol W, van Marum RJ, Jansen PA, Souverein PC, Schobben AF, Egberts AC. Antipsychotic drug use and risk of pneumonia in elderly people. J Am Geriatr Soc, 2008; 56(4):661–6; doi:10.1111/j.1532-5415.2007.01625.x CrossRef
Kuo CJ, Yang SY, Liao YT, Liao YT, Chen WJ, Lee WC, Shau WY, Chang YT, Tsai SY, Chen CC. Second-generation antipsychotic medications and risk of pneumonia in schizophrenia. Schizophr Bull, 2013; 39(3):648–57; doi:10.1093/schbul/sbr202 CrossRef
Lewis K, O’Day CS. Dystonic reactions. In: StatPearls. StatPearls Publishing, Treasure Island, FL, 2021.
Maeda T, Babazono A, Nishi T, Yasui M. Quantification of adverse effects of regular use of triazolam on clinical outcomes for older people with insomnia: a retrospective cohort study. Int J Geriatr Psychiatry, 2016; 31(2):186–94; doi:10.1002/gps.4310 CrossRef
Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest, 2003; 124(1):328–36; doi:10.1378/chest.124.1.328 CrossRef
May M, Beauchemin M, Vary C, Barlow D, Houseknecht KL. The antipsychotic medication, risperidone, causes global immunosuppression in healthy mice. PLoS One, 2019; 14(6):e0218937. Published 2019 Jun 26. doi:10.1371/journal.pone.0218937 CrossRef
Mehta S, Pulungan Z, Jones BT, Teigland C. Comparative safety of atypical antipsychotics and the risk of pneumonia in the elderly. Pharmacoepidemiol Drug Saf, 2015; 24(12):1271–80; doi:10.1002/pds.3882 CrossRef
Moreno C, Wykes T, Galderisi S, Nordentoft M, Crossley N, Jones N, Cannon M, Correll CU, Byrne L, Carr S, Chen EYH, Gorwood P, Johnson S, Kärkkäinen H, Krystal JH, Lee J, Lieberman J, López-Jaramillo C, Männikkö M, Phillips MR, Uchida H, Vieta E, Vita A, Arango C. How mental health care should change as a consequence of the COVID-19 pandemic [published correction appears in Lancet Psychiatry. 2021 Jul;8(7):e16]. Lancet Psychiatry, 2020; 7(9):813–24; doi:10.1016/S2215-0366(20)30307-2 CrossRef
Nakafero G, Sanders RD, Nguyen-Van-Tam JS, Myles PR. The association between benzodiazepines and influenza-like illness-related pneumonia and mortality: a survival analysis using UK Primary Care data. Pharmacoepidemiol Drug Saf, 2016; 25(11):1263–73; doi:10.1002/pds.4028 CrossRef
Obiora E, Hubbard R, Sanders RD, Myles PR. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax, 2013; 68(2):163–70; doi:10.1136/thoraxjnl-2012-202374 CrossRef
Ostuzzi G, Papola D, Gastaldon C, Schoretsanitis G, Bertolini F, Amaddeo F, Cuomo A, Emsley R, Fagiolini A, Imperadore G, Kishimoto T, Michencigh G, Nosé M, Purgato M, Dursun S, Stubbs B, Taylor D, Thornicroft G, Ward PB, Hiemke C, Correll CU, Barbui C. Safety of psychotropic medications in people with COVID-19: evidence review and practical recommendations [published correction appears in BMC Med. 2020 Sep 12;18(1):291]. BMC Med, 2020; 18(1):215. Published 2020 Jul 15. doi:10.1186/s12916-020-01685-9 CrossRef
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev, 2016; 5(1):210. Published 2016 Dec 5. doi:10.1186/s13643-016-0384-4 CrossRef
Pitts T. Airway protective mechanisms. Lung, 2014; 192(1):27–31; doi:10.1007/s00408-013-9540-y CrossRef
Plaze M, Attali D, Petit AC, Blatzer M, Simon-Loriere E, Vinckier F, Cachia A, Chrétien F, Gaillard R. Repurposing chlorpromazine to treat COVID-19: the reCoVery study. Encephale, 2020; 46(3):169–72; doi:10.1016/j.encep.2020.05.006 CrossRef
Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry, 2015; 56(3):345–65; doi:10.1111/jcpp.12381 CrossRef
Pollmächer T, Haack M, Schuld A, Kraus T, Hinze-Selch D. Effects of antipsychotic drugs on cytokine networks. J Psychiatr Res, 2000; 34(6):369–82; doi:10.1016/s0022-3956(00)000327 CrossRef
Pratt N, Roughead EE, Ramsay E, Salter A, Ryan P. Risk of hospitalization for hip fracture and pneumonia associated with antipsychotic prescribing in the elderly: a self-controlled case-series analysis in an Australian health care claims database. Drug Saf, 2011; 34(7):567–75; doi:10.2165/11588470-000000000-00000 CrossRef
Rafaniello C, Lombardo F, Ferrajolo C, Sportiello L, Parretta E, Formica R, Potenza S, Rinaldi B, Irpino A, Raschetti R, Vanacore N, Rossi F, Capuano A. Predictors of mortality in atypical antipsychotic-treated community-dwelling elderly patients with behavioural and psychological symptoms of dementia: a prospective population-based cohort study from Italy. Eur J Clin Pharmacol, 2014; 70(2):187–95; doi:10.1007/s00228-013-1588-3 CrossRef
Rajamaki B, Hartikainen S, Tolppanen AM. Psychotropic drug-associated pneumonia in older adults. Drugs Aging, 2020; 37(4):241–61; doi:10.1007/s40266-020-00754-1 CrossRef
Rohde C, Siskind D, de Leon J, Nielsen J. Antipsychotic medication exposure, clozapine, and pneumonia: results from a self-controlled study. Acta Psychiatr Scand, 2020; 142(2):78–86; doi:10.1111/acps.13142 CrossRef
Setoguchi S, Wang PS, Alan Brookhart M, Canning CF, Kaci L, Schneeweiss S. Potential causes of higher mortality in elderly users of conventional and atypical antipsychotic medications. J Am Geriatr Soc, 2008; 56(9):1644–50; doi:10.1111/j.1532-5415.2008.01839.x CrossRef
Star K, Bate A, Meyboom RH, Edwards IR. Pneumonia following antipsychotic prescriptions in electronic health records: a patient safety concern? Br J Gen Pract, 2010; 60(579):e385–94; doi:10.3399/bjgp10X532396 CrossRef
Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry, 2018; 17(3):341–56; doi:10.1002/wps.20567 CrossRef
Taipale H, Tolppanen AM, Koponen M, Tanskanen A, Lavikainen P, Sund R, Tiihonen J, Hartikainen S. Risk of pneumonia associated with incident benzodiazepine use among community-dwelling adults with Alzheimer disease. CMAJ, 2017; 189(14):E519–29; doi:10.1503/cmaj.160126 CrossRef
Tolppanen AM, Koponen M, Tanskanen A, Lavikainen P, Sund R, Tiihonen J, Hartikainen S, Taipale H. Antipsychotic use and risk of hospitalization or death due to pneumonia in persons with and those without Alzheimer disease. Chest, 2016; 150(6):1233–41; doi:10.1016/j.chest.2016.06.004 CrossRef
Trifirò G, Gambassi G, Sen EF, Stephenson AL, O’Donnell DE, Gershon AS, Gill SS, Rochon PA. Association of community-acquired pneumonia with antipsychotic drug use in elderly patients: a nested case-control study. Ann Intern Med, 2010; 152(7):418-W140; doi:10.7326/0003-4819-152-7-201004060-00006 CrossRef
Vozoris NT, Wang X, Austin PC, et al. Serotonergic antidepressant use and morbidity and mortality among older adults with COPD. Eur Respir J, 2018; 52(1):1800475. Published 2018 Jul 27. doi:10.1183/13993003.00475-2018 CrossRef
Wang MT, Lin CW, Tsai CL, Wang YH, Lai JH, Yeh CB, Huang YL, Hsu YJ. Use of antipsychotics and the risk of acute respiratory failure among adults: a disease risk score-matched nested case-control study. Br J Clin Pharmacol, 2020; 86(11):2204–16; doi:10.1111/bcp.14321 CrossRef
Wang MT, Tsai CL, Lin CW, Yeh CB, Wang YH, Lin HL. Association between antipsychotic agents and risk of acute respiratory failure in patients with chronic obstructive pulmonary disease. JAMA Psychiatry, 2017; 74(3):252–60; doi:10.1001/jamapsychiatry.2016.3793 CrossRef
Wang MT, Wang YH, Chang HA, Tsai CL, Yang YS, Lin CW, Kuo CC, Hsu YJ. Benzodiazepine and Z-drug use and risk of pneumonia in patients with chronic kidney disease: a population-based nested case-control study. PLoS One, 2017; 12(7):e0179472. Published 2017 Jul 10. doi:10.1371/journal.pone.0179472 CrossRef
Wilson H, Ridley S. Antipsychotic drugs and the acute respiratory distress syndrome. Br J Anaesth, 2007; 99(2):301–2; doi:10.1093/bja/aem195 CrossRef
Yang SY, Liao YT, Liu HC, Chen WJ, Chen CC, Kuo CJ. Antipsychotic drugs, mood stabilizers, and risk of pneumonia in bipolar disorder: a nationwide case-control study. J Clin Psychiatry, 2013; 74(1):e79–86; doi:10.4088/JCP.12m07938 CrossRef