INTRODUCTION
Aeromonas hydrophila is a Gram-negative and opportunistic bacterial pathogen that infects freshwater fish. Aeromonas hydrophila causes motile aeromonad septicemia (MAS) disease and causes losses in freshwater fish farming (Olga et al., 2020). Fish farmers in Kalimantan, Indonesia, usually use antibiotics (oxytetracycline, chloramphenicol, erythromycin, streptomycin, prefuran, enrofloxacin, and neomycin) to overcome MAS disease (Aisiah et al., 2011). However, antibiotics in conquering illnesses brought about by bacterial contaminations can have an impact on the environment and well-being. Antibiotics not only kill the disease microorganisms but also kill microalgae that could become regular nourishment for refined fish (Agostini et al., 2019) and could be dispensed with nontarget microscopic organisms, for example, probiotic microbes that help refined fish’s development (Verschuere et al., 2000). Moreover, the utilization of anti-infection agents in fish cultivation could leave buildups on fish meat (Okocha et al., 2018) and dirty the oceanic climate (Monteiro et al., 2018).
One of the safe and environment-friendly efforts to overcome A. hydrophila infection is by utilizing natural products widely grown around the community. Kalimantan forests contain many types of plants that can be used as natural medicine (Aisiah et al., 2019; Negara et al., 2017). One of the Kalimantan tidal swamp plants that could potentially be used to treat A. hydrophila infection is Acanthus ilicifolius, locally named Jeruju. A. ilicifolius plant belongs to the Acanthaceae family and is included as a mangrove plant.
Jeruju plants (A. ilicifolius) are found in wetland regions at stream estuaries as mangrove vegetation. Jeruju was delegated a rising sea-going plant and occupies estuary waters, with a low saltiness level (Irawanto et al., 2015). The Jeruju plant’s qualities show a stem encircled by smooth and sharp spines. The natural surroundings of Jeruju are related with wild plants and are infrequently found ashore. Jeruju has enormous serrated leaves, with tightened tips and sharp spines (Noor et al., 2006). In Indonesia, the Jeruju (A. ilicifolius) plant is spread across West Sumatra, Bekasi, Central Java, East Java, Bali, East Nusa Tenggara, West Kalimantan, East Kalimantan, South Kalimantan, North Maluku, Maluku, West Papua, and Papua (Fig. 1).
Jeruju is commonly found in the coastal areas of Kalimantan, forming shrubs in areas where salinity is relatively low (Saptiani et al., 2013). Ethnobotanical studies have reported that Jeruju has been used to restore energy after childbirth, medication for stomach pain, rheumatism, hypertension, flatulence, and worm medicine by the Malay community in Sungai Tekong, West Kalimantan, Indonesia (Ernianingsih et al., 2014; Ratnasari et al., 2017). The Jeruju leaves were used as a fever-reducing medicine (antipyretic) in Teluk Selong, South Kalimantan, Indonesia (Forestryana et al., 2018).
Literature studies have reported that the chemical compounds (like bioactive or secondary metabolite) in Jeruju (A. ilicifolius) function as a neuralgic, analgesic, anti-inflammatory, antioxidant, hepatoprotective, antileukemic, anticancer, antimicrobial, antiviral, antifungal, and natural insecticide (Irawanto et al., 2015). Jeruju leaf extract could inhibit pathogenic bacteria growth, such as Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa (Khajure and Rathod, 2010), Escherichia coli, Klebsiella pneumoniae, Salmonella typhi (Chundakkadu et al., 2011), Staphylococcus epidermis (Govindasamy and Arulpriya, 2013), Proteus vulgaris, Streptococcus pneumoniae (Ravikumar et al., 2012), Streptococcus viridans (Pringgenies et al., 2020), Vibrio cholerae (Thirunavukkarasu et al., 2011a), and Vibrio harveyi (Saptiani et al., 2013; Sreenivasa et al., 2015).
Until now, literature studies have not shown the use of Jeruju leaf extract (A. ilicifolius) in overcoming A. hydrophila infection that causes MAS disease. Therefore, this study aimed to examine the potential of Jeruju leaf extract (A. ilicifolius) as a natural product to inhibit the growth of A. hydrophila. In addition, this study also evaluated the phytochemical content, metabolomic profiling, antioxidant activity, and total phenol content of the ethanol extract of Jeruju leaves.
MATERIALS AND METHODS
Materials
The research was conducted from July to December 2020 at the Fish Pests and Diseases Laboratory, Faculty of Fisheries and Marine, Universitas Lambung Mangkurat, Banjarbaru, Indonesia. The Jeruju leaves (A. ilicifolius) were collected from the riverbank of Bunipah, Aluh-aluh District, Banjar Regency, South Kalimantan, Indonesia. The isolate of A. hydrophila was obtained from the Mandiangin Freshwater Aquaculture Center for Fisheries (BPBAT), Banjar, South Kalimantan, Indonesia. The materials used were tryptic soy agar (TSA, Merck), tryptic soy broth (TSB, Merck), glutamate starch phenol agar (GSP agar, Merck), agar (Merck), distilled water, ethanol (Merck), and 2,2-diphenyl-1-picrylhydrazyl (DPPH, Merck). The tools used were digital scales, Whatman filter paper No. 42, ovens (Tungtec Instruments TH-160F), rotary vacuum evaporators (IKA RV 10), petri dishes, and Becker glass.
Jeruju leaves’ extraction
The plant materials (Jeruju leaves) were cleaned, cut into small pieces, and dried in an oven at 40°C–50°C. The dried Jeruju leaves were crushed into a fine powder. A total of 100 g of fine powder of Jeruju leaves was macerated in 400 ml of ethanol for 24 hours. Ethanol is used as a solvent that is safe for the consumption for fish and humans. Ethanol is also a natural solvent for both food and natural medicine (Hikmawanti et al., 2021). Ethanol used in this study is of analytical grade (Merck) and safe for the environment, considering it will apply to a fish culture environment. Ethanol is a solvent used to extract compounds from natural materials with good results (Sultana et al., 2009). The extract obtained was then evaporated using a rotary vacuum evaporator. Then, the Jeruju extract was dried in a vacuum desiccator for 4–5 days. The Jeruju leaf extract was stored at 4°C before assaying.
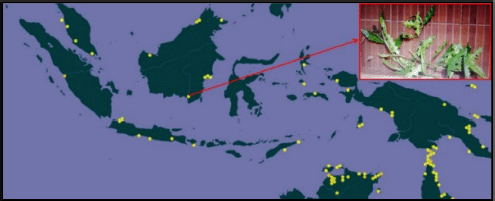 | Figure 1. Distribution of Jeruju (A. ilicifolius) in Indonesia. Source: Global Biodiversity Information Facility (2021). [Click here to view] |
Phytochemical screening
The ethanol extract of Jeruju leaves was subjected to qualitative screening of chemical components to determine the presence of alkaloids, anthraquinones, flavonoids, steroids, terpenoids, saponins, and phenols using conventional standard protocols, as described by Harborne (1998).
Metabolomic profiling
Metabolomic profiling of the Jeruju leaf extract was conducted using liquid chromatography and high-resolution mass spectrometry (LC-HRMS Shimadzu-8040, Japan) with an injection volume of 1 µl. LC-HRMS was equipped with an autosampler, binary pump, column compartment, and a diode array detector for scanning spectroscopy. Chromatographic separation was performed using a C-18 column, Shim Pack FC-ODS (2 mm ø × 150 mm, 3 µm). Two solvents were prepared including solvent A (H2O: MeOH, 8:2, with 0.1% formic acid) and solvent B (0.1% formic acid in acetonitrile). The two solvents were adjusted to 95:5 ratios, respectively, with an elution gradient of 0/0 at 0 minutes, 15/85 at 5 minutes, 20/80 at 20 minutes, and 90/10 at 24 minutes. Mass spectroscopy (MS) analysis was performed by electrospray ionization (ESI) with positive ions as the source. MS data were obtained through collision energy traps starting at 5.0 V. The ESI source parameters were regulated, including a capillary voltage of 3.0 kV, source temperature of 100°C, desolvation temperature of 350°C, sampling cone of 23 V, and desolvation gas flow of 6 l/hour. The chromatogram data obtained were compared with the data profile from the mzCloud Library system (Riyadi et al., 2020; 2021).
Prediction of biological activity
The metabolomic profile detected from the ethanol extract of Jeruju leaves with LC-HRMS was predicted for biological activity using the PASS server (http://www.pharmaexpert.ru/passonline/index.ph). The PASS server is a software that is useful for predicting the biological activity of a compound (Rumengan et al., 2021). The predicted biological activity requires a structural formula in the form of canonical SMILE obtained from the National Center for Biotechnology Information (https://pubchem.ncbi.nlm.nih.gov/) (Aisiah et al., 2020).
Antibacterial activity assay
The antibacterial activity assay was conducted using the well-diffusion method on a petri dish with modifications (Balouiri et al., 2016; Tanod et al., 2019a). The well-diffusion method used two media layers, namely the base media and the seed media layer. The modification was carried out by adding TSA composition (2 g) with 2 g of bacto agar in 100 ml of distilled water as the base medium. The seedling layer was made from 70% TSA in 100 ml of distilled water, then put into a tube containing 9 ml of seed media and sterilized. Furthermore, 1 ml of A. hydrophila isolate was added to warm seedling with a density of 1 × 107 colony/ml (bacterial solution compared to the McFarland, HiMedia standard). The A. hydrophila isolates used pure culture from BPBAT Mandiangin. The seeding medium that was added with A. hydrophila was vortexed, and then poured onto the base media layer. After the media hardens slightly, a well hole was made at a certain distance, using a 5 mm diameter glass tube. Each well was filled with 50 µl of Jeruju leaves’ ethanol extracts with concentrations of 50, 100, 150, 200, and 300 mg/ml and incubated at 37°C for 24 hours. Cefadroxil and tetracycline (1 mg/ml each) were used as comparison controls. After that, the zone of inhibition was observed and measured. All experimental measurement data were carried out in three replications and expressed as mean ± standard deviation (n = 3).
Antibacterial activity was evaluated using the broth dilution method based on the guidelines (EUCAST, 2000; Wiegand et al., 2008) with modifications. Exactly 10 ml of TSB was inoculated with 100 µl of A. hydrophila (density 1 × 107 colony/ml), then incubated at 37°C for 24 hours. After that, 100 µl of the Jeruju leaves’ ethanol extract (200 mg/ml) was added. Aeromonas hydrophila culture with aquadest was used as the negative control, and cefadroxil and tetracycline (1 mg/ml each) were used as positive controls. The total colony count was carried out on GSP agar, based on the total plate count method, following the Indonesian National Standard No. 01-2332.3 of 2006 with modifications (Indonesian National Standardization Agency—BSN, 2006). Modifications made using TSB on broth media and solid media using GSP selective media. If the GSP is red, it indicates A. hydrophila is not growing, whereas if the GSP is yellow, it indicates A. hydrophila growth.
Antioxidant activity assay
Antioxidant activity was determined using the DPPH radical scavenging method (Molyneux, 2004; Tanod et al., 2019a). The ethanol extract of Jeruju leaves was added with ethanol so that the concentration was 100 µg/ml; then serial dilutions were made (6, 12, 24, 48, and 96 µg/ml). A 2 ml aliquot of each concentration’s extract solution was added to 2 ml of the 50 μM DPPH solution. The mixture was homogenized and left for 30 minutes in a dark room at room temperature. Then, the mixture measured the free radical scavenging at a wavelength of 517 nm with a spectrophotometer (UV-VIS spectrophotometer T90 + PG Instruments Ltd).
The absorbance value of the DPPH solution was also measured and determined by IC50 (half-maximal inhibitory concentration). Ascorbic acid was used as a positive control. IC50 was determined as the concentration of the extract solution required to scavenge 50% DPPH free radicals (Dewanto et al., 2021). The assay was carried out in three repetitions, and the measurement results were expressed with a standard deviation. The DPPH scavenging effects were calculated using the following equation:
Total phenol content assay
The ethanol extract of Jeruju leaves was evaluated for total phenol content according to the Folin–Ciocalteu method (Blainski et al., 2013; Lamuela-Raventós, 2017). Exactly 25 mg of Jeruju leaves’ ethanol extract was dissolved in 25 ml of ethanol:aquadest (1:1) solution. Then, 1 ml from the extract solution and 10 ml of distilled water + 1 ml of Folin–Ciocalteu reagent (homogenization) were added. After that, it was let to stand for 8 minutes and 3 ml of 20% Na2CO3 was added (which was let to stand for 2 hour at room temperature). Then, the absorption with a UV-Vis spectrophotometer at a wavelength of 750 nm was measured, which gave a blue color. In the same way, a gallic acid solution was prepared, i.e., 25 mg of gallic acid was dissolved in ethanol: water (1:1) to a volume of 25 ml. Then, the gallic acid solution in a series of dilutions of 5, 20, 40, 60, 80, and 100 g/ml was made. The standard curve of gallic acid was prepared with the concentration of gallic acid (µg/ml) against the absorbance value. Total phenolic was determined using the standard curve regression equation for gallic acid. The total phenol content was expressed in mg GAE/100 g dry extract (Muliadin et al., 2021; Riyadi et al., 2021b).
RESULTS AND DISCUSSION
Phytochemicals of Jeruju leaves’ ethanol extract
The Jeruju leaves were extracted using ethanol solvent to make the resulting extract more environment-friendly and safe to use for fish and humans. The extraction of 100 g of simplicia Jeruju leaves with 400 ml of ethanol solvent (analytical grade) obtained an extract weight of 21.30 g (extract yield of 21.30%). Phytochemical analysis was carried out to determine the type of natural products in the ethanol extract of Jeruju leaves. The phytochemicals’ screening of the ethanol extract of Jeruju leaves (A. ilicifolius) is presented in Table 1.
Table 1 shows the phytochemical screening of the ethanol extract of Jeruju leaves, indicating the presence of flavonoids, alkaloids, tannins, phenolics, steroids, and terpenoids. Jeruju was also reported to contain lignans (Kanchanapoom et al., 2001). Acanthus ilicifolius was also reported to contain alcohol, alkanes, fatty acids, lignans, steroids, and terpenoids (Wöstmann and Liebezeit, 2008). Acanthus ilicifolius collected from Kollam, Kerala, India, detected phytochemical components of saponins, tannins, terpenoids, flavonoids, alkaloids, and anthraquinones (Chundakkadu et al., 2011). The leaf extract of A. ilicifolius isolated 4-coumaric acid compounds, including coumarin compounds, lignans, flavonoids, and phenylethanoid (Ravikumar et al., 2012). Leaves of Jeruju collected from Sungai Tekong, Kubu Raya, West Kalimantan, Indonesia, detected phytochemical components of alkaloids, saponins, flavonoids, terpenoids, and phenol (Ernianingsih et al., 2014). Jeruju leaves collected from Kaligawe, Semarang, and the coastal area of Teluk Awur, Jepara, Central Java, Indonesia, were reported to contain saponins, quinone, and tannins compounds (Ardiantami et al., 2015). In A. ilicifolius, there were also found terpenes and flavonoids (Sreenivasa et al., 2015). Phytochemical studies of A. ilicifolius reported chemical constituents of triterpenoids, alkaloids, saponins glycosides, flavonoids, steroids, phenols, and coumarins (Bora et al., 2017). The methanol extract of A. ilicifolius leaves contained flavonoids, alkaloids, and phenols (Handayani et al., 2018). Jeruju also produces compounds of steroids, flavonoids, and tannins (Pringgenies et al., 2020).
The use of methanol, ethanol, and water solvents in plant extraction did not significantly affect the results of qualitative screening of phytochemical components. The statement is according to the results of Godwill et al. (2013), Chigayo et al. (2016), and Nurdyansyah and Widyastuti (2020). However, if quantitative screening of phytochemical components is carried out, the use of methanol showed higher amounts (especially for phenols, flavonoids, alkaloids, and terpenoids) than ethanol and water (Truong et al., 2019). So, it is indicated that methanol can extract better than ethanol and water.
Therefore, it strongly suspected that the difference in the phytochemical components in A. ilicifolius was due to environmental influences. The factors that influence differences in the production of chemical components are environmental conditions (Dewanto et al., 2019). Chemical components produced by organisms play a role in the defense system in maintaining life (Gallo et al., 2004), then organisms will produce more phytochemical components if they live in extreme environments. Liu et al. (2016) reported the difference of total tannin, flavonoid, rutin, and phenol in the same sample extract from different locations. Differences in phenol content were also reported in Mykhailenko et al. (2020); environmental factors affect the accumulation of phenolic compounds and their derivatives such as flavonoids, isoflavonoids, and xanthones in plants.
The phytochemical components in Jeruju leaves, like flavonoids, alkaloids, tannins, phenols, steroids, and terpenoids, reported having antibacterial and antioxidant properties. The flavonoid action mechanism as an antibacterial damaged bacterial cell walls and membranes, binding to cell adhesions and deactivating enzymes (Cowan, 1999). Flavonoid compounds act as scavengers for free radicals that arise due to bacterial infection to protect cells from negative enzymatic reactions (Xie et al., 2015). Alkaloids are chemical components that act as scaffolds for antibacterial drugs (Cushnie et al., 2014). The alkaloid action mechanism as an antibacterial is by inhibiting reductase dihydrofolate and topoisomerase type I enzymes, which play a role in DNA synthesis (Kittakoop et al., 2014; Samoylenko et al., 2009). Alkaloids have also been reported as inhibiting bacterial virulence without affecting growth or survival (LaSarre and Federle, 2013). An N group in the alkaloid structure can be an antioxidant because it acts as a free radical scavenger (Neganova et al., 2012). Alkaloid components were reported to increase phenolic compounds’ performance to provide a more potent antioxidant effect in plant extracts (Gan et al., 2017).
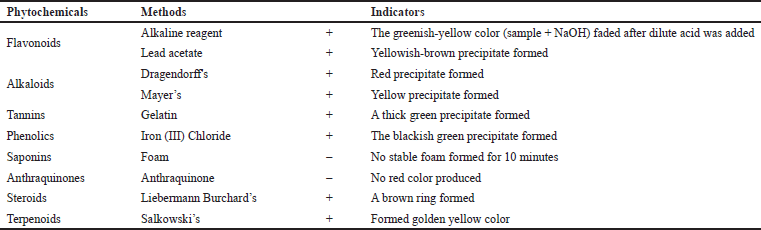 | Table 1. Phytochemical screening of Jeruju leaves’ ethanol extract (A. ilicifolius). [Click here to view] |
Tannin antibacterial mechanism was conducted by inactivating microbial adhesion cells and inhibiting iron availability for microorganisms. The antibacterial mechanism of tannins showed phenolic damage the bacterial cell wall of polypeptides (Akiyama et al., 2001; Ogbuagu, 2008). Tannins are known as antioxidants because they have the ?OH(hydroxyl) group attached to aromatic rings. Tannins effectively scavenged free radicals as an electron donor and a source of hydrogen atoms, active in metal chelation because of the ?OH groups and conjugated double bonds that allow the formation of electron delocalization (Dewanto et al., 2018). The phenol antibacterial mechanism deactivated proteins (enzymes) in the bacterial cell membrane. Phenol binds to proteins through hydrogen bonds resulting in a damaged protein structure where most of the cell wall and cytoplasmic membrane structures of bacteria contain protein and fat (Susanti et al., 2008). Phenolic components have the potential as antioxidants because they have –OH(hydroxyl) group on the aromatic ring (Agati et al., 2009). The hydroxyl group can donate H atoms to free radical compounds to reduce free radicals. Phenol hydroquinone and its derivatives act as oxidative inhibitors that bind to free radicals and react with reactive oxygen species (ROS) molecules to form more stable compounds (Harborne, 1998).
Steroids have been reported to fight Gram-positive and Gram-negative bacteria (Polat et al., 2011). Steroid components can disrupt the cell membrane of bacteria such as S. aureus, E. coli, and K. pneumoniae via the quaternary amine groups carried by the conjugate (Figueroa-Valverde et al., 2009). Steroids also exert cytotoxic effects on bacterial cells (Dogan et al., 2012). Steroid components were reported to play a role in enhancing endogenous antioxidants (Mooradian, 1993). The antibacterial mechanism of terpenoids is by damaging the bacterial cell membrane and dissolving the constituent membrane lipids (Cowan, 1999). The low concentrations of the terpenoid components only affect the enzymes involved in energy production, whereas the high concentrations of the terpenoids can lyse the membrane (Jasmine et al., 2011). Terpenoids have a relatively complex cyclic structure (consisting of alcohol, aldehyde, or carboxylic acid), so they have a hydroxyl group that could act as an antioxidant (Dewanto et al., 2019).
Metabolomic profiling of Jeruju leaves’ ethanol extract
Metabolomic profiles screening of the Jeruju leaves’ ethanol extract with LC-HRMS detected 95 peaks. The results of the mass spectrum of each peak, compared with the mass spectra in the mzCloud library database, showed 67 compound profiles. However, based on the mzCloud score, only 35 peaks (24 compounds) were confirmed with an accuracy level above 85. Screening of the metabolomic profiles of the Jeruju leaves’ ethanol extract with LCHRMS is presented in Table 2.
Table 2 shows that the list of metabolomic profiles was dominated by betaine (41.61%) and choline (40.27%). Betaine is an antioxidant substance that has been used in agriculture and health industry. Betaine is a precursor to S-adenosylmethionine, contributing to glutathione synthesis (endogenous antioxidant) (Jung et al., 2013). The mechanism of betaine as an antioxidant is by scavenging ROS in cells by regulating the endogenous nonenzymatic antioxidant defenses. In addition, betaine inhibits ROS formation by isolating cells from oxidative stress inducers (Zhang et al., 2016).
Choline was reported to reduce oxidant damage and regulate the antioxidant system in the immune system of Jian carp (Cyprinus carpio var. Jian), which was subjected to a challenge with A. hydrophila (Wu et al., 2014). Choline also reported increasing the antibacterial properties of gills and the relative level of gene expression for tight-jointed proteins, decreasing the inflammatory status, and regulating the mRNA level of the associated signaling molecule in grass carp gills (Ctenopharyngodon idella) (Zhao et al., 2016). Choline deficiency could cause oxidative damage due to changes in the transcription of antioxidant enzymes and signaling molecules Nrf-2/Keap-1 in the hepatopancreas and intestine (Wu et al., 2017).
Biological activity prediction with PASS server
Furthermore, the list of compounds in Table 2 predicted their potential biological activity using the PASS server. The predicted value of the compound’s biological activity in the ethanolic extract of Jeruju leaves was expressed as a probability to be active (Pa) (Fig. 2). The prediction was carried out as an inhibitor of cell wall biosynthesis, peptidoglycan membrane inhibitor, protein synthesis inhibitor, nucleic acid synthesis inhibitor, and free radical scavenger (Madigan et al., 2019).
Figure 2 shows the potential of Jeruju leaves’ ethanol extract as an antibacterial against the growth of A. hydrophila, closely related to its mechanism, which is thought to be a peptidoglycan glycosyltransferase enzyme inhibitor, DNA synthesis inhibitor, and free radical scavenger. Peptidoglycan glycosyltransferase enzyme plays a role in peptidoglycan biosynthesis in bacterial cell wall formation (Derouaux et al., 2013). By inhibiting the peptidoglycan glycosyltransferase enzyme action, bacteria cannot synthesize peptidoglycan; so, bacteria cannot maintain their shape and protect themselves from osmotic pressure.
Biological activity prediction of the Jeruju leaves’ ethanol extract as an inhibitor of the peptidoglycan glycosyltransferase enzyme, DNA synthesis inhibitor, and free radical scavenger was supported by the results phytochemical screening and metabolomic profiling with LC-HRMS. The flavonoid and phenol compounds in the extract are thought to deactivate and inhibit the peptidoglycan glycosyltransferase enzyme’s performance. In addition, alkaloid components could inhibit DNA synthesis by inhibiting the enzyme’s dihydrofolate reductase and topoisomerase type I (Kittakoop et al., 2014). Metabolomic profiles shown in Table 2, which have a hydroxyl group (–OH), an amine group (–NH2), and a cyclic nitrogen structure, are thought to act as free radical scavengers.
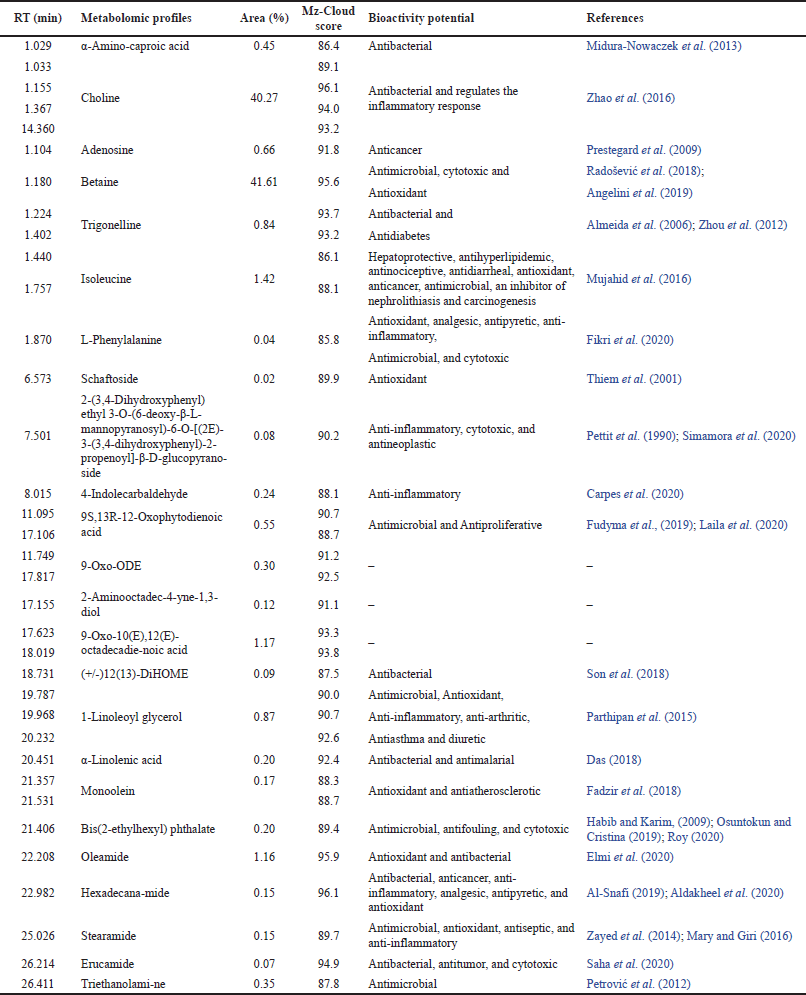 | Table 2. Metabolomic profiles of the Jeruju leaves’ ethanol extract with LC-HRMS. [Click here to view] |
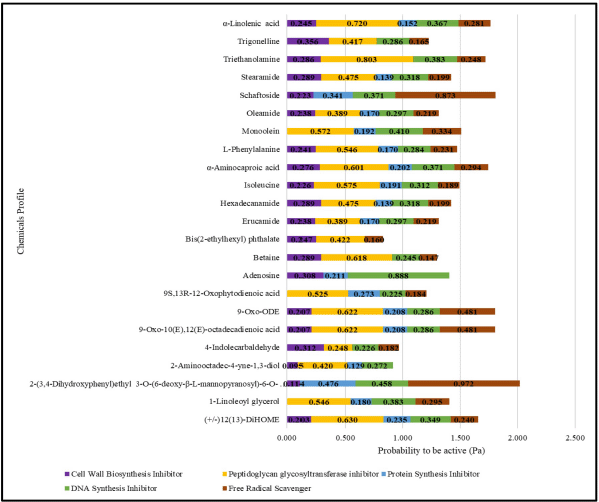 | Figure 2. Prediction of the biological activity of Jeruju leaves’ ethanol extract with PASS server. [Click here to view] |
Antibacterial activity of the Jeruju leaf extract
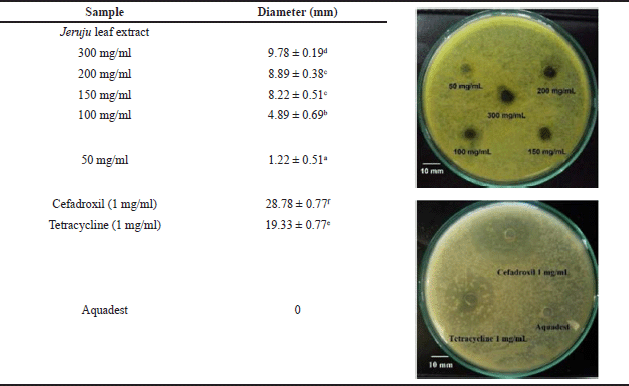
Antibacterial activity evaluation of the Jeruju leaves’ ethanol extract was carried out by observing the inhibition zone formed from A. hydrophila isolates. The measurement of the inhibition zone diameter of the Jeruju leaves’ ethanol extract (A. ilicifolius) compared to the control is shown in Table 3.
Table 3 shows the antibacterial activity, which increases depending on the extract concentration against the growth of A. hydrophila. The Jeruju leaves’ ethanol extract showed weak antibacterial activity against A. hydrophila with agar diffusion method (p < 0.05). According to the inhibition zone category by Paudel et al. (2014), there are four categories of antibacterial activity: very strong (inhibition zone ø > 20 mm), strong (inhibition zone, ø = 15–20 mm), moderate (inhibition zone ø = 10–15 mm), and weak (inhibition zone ø < 10 mm). As dominant profile components in Jeruju leaves, betaine and choline have mechanisms to increase endogen antioxidants. In addition, flavonoids, phenols, and alkaloids in Jeruju leaves have hydroxyl and amine groups, which are hydrophilic. Aeromonas hydrophila has a hydrophilic side, namely carboxyl, amino acid, and hydroxyl (Madigan et al., 2019). The hydrophilic side is a factor that determines the penetration, binding, and activity of antibacterial compounds (Sefa et al., 2020).
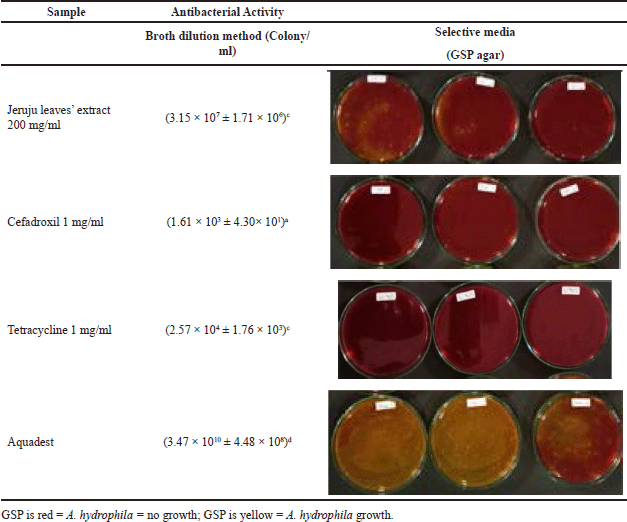
This study also observed the antibacterial power of the ethanol extract of Jeruju leaves in inhibiting the growth of A. hydrophila. Observations were made using the broth dilution method to count the quantitative number of A. hydrophila that could be inhibited. The results showed that Jeruju leaves’ ethanol extract (200 mg/ml) could suppress the number of A. hydrophila that grew on glutamate starch phenol agar (GSP agar) (p < 0.05) (Table 4). According to the media guidelines for Aeromonas Jeppesen (1995), A. hydrophila will degrade the red color of the GSP agar medium to yellow. The color change is because A. hydrophila degrades the starch in GSP agar by producing acid, causing the phenol red to turn yellow (Naviner et al., 2006).
Antioxidant activity and total phenol content of the Jeruju leaf extract
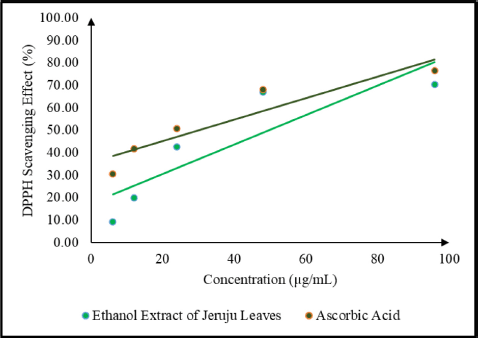
This study also evaluated the antioxidant activity of the Jeruju leaf extract using the DPPH radical scavenging method. DPPH was a stable free radical and can accept electrons or hydrogen radicals to form a stable diamagnetic molecule (Tanod et al., 2019b). Antioxidant activity indicates chemical components’ ability to inhibit oxidation reactions, expressed as the percentage of DPPH radical scavenging. The percentage of DPPH radical scavenging for Jeruju leaf extract and ascorbic acid as a control is shown in Figure 3.
 | Table 3. Diameter of the inhibition zone from Jeruju leaves’ ethanol extracts against A. hydrophila. [Click here to view] |
 | Table 4. Antibacterial activity of Jeruju leaves’ ethanol extract against A. hydrophila after 24 hours. [Click here to view] |
 | Figure 3. DPPH scavenging effect of Jeruju leaves’ ethanol extract compared with ascorbic acid. [Click here to view] |
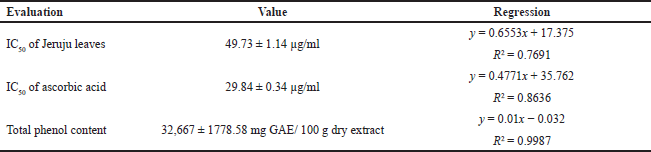 | Table 5. IC50 and total phenol content of Jeruju leaves’ ethanol extract (A. ilicifolius). [Click here to view] |
Figure 3 shows an increase in the effect of DPPH radical scavenging, along with the increase in the concentration of Jeruju leaf extract. The antioxidant activity indicates the ability of an extract to scavenge free radicals (Tanod et al., 2019a). The ethanol extract of Jeruju leaf is thought to donate H atoms/electrons to interact with DPPH radicals. This study evaluated the IC50 value and total phenol content of the Jeruju leaves’ ethanol extract (A. ilicifolius) using the Folin–Ciocalteu method (Table 5). The Jeruju leaves’ ethanol extract showed very strong potential as an antioxidant. Antioxidant activity was evaluated according to Blois (1958) and Riyadi et al.’s (2019) studies, namely very strong (IC50 < 50 µg/ml), strong (IC50 between 50 and 100 µg/ml), moderate (IC50 between 100 and 150 µg/ml) and weak (IC50 between 150 and 200 µg/ml).
Previous research has also evaluated the percentage of DPPH radical scavenging from Jeruju leaf extract (A. ilicifolius). The percentage of DPPH radical scavenging from the ethanol extract of A. ilicifolius, collected from Poondiyankuppam, northeast coast of India, ranged from 50.55 ± 2.88 to 86.87 ± 5.04%, which was compared with DPPH radical scavenging of ascorbic acid ranging from 65.12 ± 5.40 to 90.32 ± 5.12% (concentration = 0.1–2 mg/ml, with a DPPH concentration of 0.1 mM). In addition, it was also reported that the total phenol content of the ethanolic extract of A. ilicifolius was 257 mg GAE/g (Thirunavukkarasu et al., 2011b).
The ethanol extract of A. ilicifolius leaves collected from Alapakkam, Tamil Nadu, India, detected flavonoid and phenol components, with DPPH scavenging activity ranging from 20.59 to 76.79%, which was compared with DPPH scavenging activity of ascorbic acid ranging from 91.18 to 96.43% (concentration = 200–1000 µg, with a DPPH concentration of 0.1 mM). This study also reported that the ethanol extract of A. ilicifolius leaves’ total phenol content was 17.22 mg/10 ml extract (Vani and Manikandan, 2018).
Previous studies also reported IC50 of A. ilicifolius leaves’ ethanol extract of 78.90 ± 1.87 µg/ml and ascorbic acid of 10.08 ± 1.79 µg/ml (DPPH concentration = 0.2 mM), and total phenol content of 128.86 ± 0.01 mg GAE/g dry weight (Biswas et al., 2019). The methanol extract of A. ilicifolius leaves collected from Wonorejo, East Java, Indonesia, reported alkaloid components, flavonoids, glycosides, polyphenols, steroids, and tannins. In addition, it also reported an IC50 value of 17.51 µg/ml of the methanol extract of A. ilicifolius leaves, with a DPPH concentration of 0.06 mM (Andriani et al., 2020).
CONCLUSION
These research findings provide a potential activity for Jeruju (A. ilicifolius) leaves’ ethanol extract as antioxidant and antibacterial for inhibiting A. hydrophila growth. The antibacterial action mechanism of Jeruju leaf extract is thought to be closely related to its antioxidant properties. Metabolomic profile structure indicates the alkaloid and flavonoid components that play a role in the antioxidant and antibacterial activity of the Jeruju extract. Further studies on an ethanol extract of Jeruju leaves in vivo on fish infected with A. hydrophila need to be carried out to observe the Jeruju leaf extract’s toxicity and stability.
ACKNOWLEDGMENTS
The authors acknowledge the Rector, Dean of Faculty of Fisheries and Marine, Head of Institute for Research and Community Service, Universitas Lambung Mangkurat, and Head of Mandiangin Freshwater Aquaculture Center for Fisheries (BPBAT) for providing research facilities.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FUNDING
PNBP funds funded this research through the Compulsory Research Lecturer Program with DIPA funding of 2020 (No.023.17.2.6777518/2020), Universitas Lambung Mangkurat, Indonesia.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Agati G, Stefano G, Biricolti S, Tattini, M. Mesophyll distribution of “antioxidant” flavonoid glycosides in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann Bot, 2009; 104(5):853–61; doi:10.1093/aob/mcp177 CrossRef
Agostini VO, Lopes LFDP, Macedo AJ, Muxagata E. A review on the effects of antimicrobials use in cultures of planktonic organisms: a procedure for ecological experiments. Lat Am J Aquat Res, 2019; 47(3):394–415. CrossRef
Aisiah S, Muhammad, Anita. The use of Piper betle Linn extract on Aeromonas hydrophila to obstruct and the toxicity to Pangasius hypophthalmus. Fish Sci, 2011; 1(2):190–201. CrossRef
Aisiah S, Olga, Tanod WA, Salosso Y, Bambang, Riyadi PH. Computational analysis of ethyl acetate extract of Nauclea subdita (Korth.) Steud. leaves as peptidoglycan glycosyltransferase inhibitor in Aeromonas hydrophila. IOP Conf Ser: Earth Environ Sci, 2020; 584:012022. CrossRef
Aisiah S, Prajitno A, Maftuch M, Yuniarti A. The potential of bangkal leaf (Nauclea subdita [Korth.] Steud.) extract as antibacterial in catfish Pangasius hypophthalmus culture. AACL Bioflux, 2019; 12(6):2093–102.
Akiyama H, Fujii K, Yamasaki O, Oono T, Iwatsuki K. Antibacterial action of several tannins against Staphylococcus aureus. J Antimicrob Chemoth, 2001; 48(4):487–91. CrossRef
Al-Snafi AE. A review on Lagerstroemia indica: a potential medicinal plant. IOSR J Pharm, 2019; 9(6):36–42.
Aldakheel RK, Rehman S, Almessiere MA, Khan FA, Gondal MA, Mostafa A, Baykal A. Bactericidal and in vitro cytotoxicity of Moringa oleifera seed extract and its elemental analysis using laser-induced breakdown spectroscopy. Pharmaceuticals, 2020; 13(8):1–18. CrossRef
Almeida AAP, Farah A, Silva DAM, Nunan EA, Gloria MBA. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J Agric Food Chem, 2006; 54(23):8738–43. CrossRef
Andriani D, Revianti S, Prananingrum W. Identification of compounds isolated from a methanolic extract of Acanthus ilicifolius leaves and evaluation of their antifungal and antioxidant activity. Biodiversitas, 2020; 21(6):2521–5. CrossRef
Angelini P, Girometta C, Tirillini B, Moretti S, Covino S, Cipriani M, D’Ellena E, Angeles G, Federici E, Savino E, Cruciani G, Venanzoni R. A comparative study of the antimicrobial and antioxidant activities of Inonotus hispidus fruit and their mycelia extracts. Int J Food Prop, 2019; 22(1):768–83. CrossRef
Ardiantami AS, Sarjito, Prayitno SB. The effect of kind dose jeruju (Acanthus ilicifolius Linn.) leaf extract immersion on survival rate of mud crab (Scylla serrata) infected by Vibrio harveyi. J Aquac Manag Technol, 2015; 4(4):159–66.
Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal, 2016; 6:71–79. CrossRef
Biswas R, Rahman SMM, Didarul Islam KM, Billah MM, Aunjum A, Nurunnabi TR, Kundu SK, Emdadul Islam M. Antioxidant, anti-inflammatory, and anticoagulation properties of Aegiceras corniculatum and Acanthus ilicifolius. Pharm Biomed Res, 2019; 5(3):35–44. CrossRef
Blainski A, Lopes GC, De Mello JCP. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules, 2013; 18(6): 6852–65. CrossRef
Blois M. Antioxidant determinations by the use of a stable free radical. Nature, 1958; 181(4617):1199–200. CrossRef
Bora R, Adhikari PP, Das AK, Raaman N, Sharma GD. Ethnomedicinal, phytochemical, and pharmacological aspects of genus Acanthus. Int J Pharm Pharm Sci, 2017; 9(12):18–25. CrossRef
Carpes RdeM, Corrêa Fernandes D, Coelho MGP, Creed JC, Fleury BG, Garden SJ, Felzenszwalb I. Anti-inflammatory potential of invasive sun corals (Scleractinia: Tubastraea spp.) from Brazil: alternative use for management? J Pharm Pharmacol, 2020; 72(4):633–47. CrossRef
Chigayo K, Mojapelo PEL, Mnyakeni-Moleele S, Misihairabgwi JM. Phytochemical and antioxidant properties of different solvent extracts of Kirkia wilmsii tubers. Asian Pac J Trop Biomed, 2016; 6(12):1037–43. CrossRef
Chundakkadu AP, Sathish KM, Santhoshkumar TR, Eppurathu VS. Phytochemical analysis and in vitro screening for biological activities of Acanthus ilicifolius. J Pharm Res, 2011; 4(7):1977–81.
Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev, 1999; 12(4):564–82. CrossRef
Cushnie TPT, Cushnie B, Lamb AJ. Alkaloids: an overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int J Antimicrob Agents, 2014; 44(5):377–86. CrossRef
Das UN. Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: a review. J Adv Res, 2018; 11: 57–66. CrossRef
Derouaux A, Sauvage E, Terrak M. Peptidoglycan glycosyltransferase substrate mimics as templates for the design of new antibacterial drugs. Front Immunol, 2013; 49(78):1–6. CrossRef
Dewanto DK, Finarti F, Hermawan R, Ndobe S, Riyadi PH, Tanod WA. Antioxidant activity of soft corals extracts from Palu Bay, Central Sulawesi, Indonesia. JPBKP: Jurnal Pascapanen Bioteknologi Kelautan dan Perikanan, 2019; 14(2):163–78. CrossRef
Dewanto DK, Hermawan R, Muliadin, Riyadi PH, Aisiah S, Tanod WA. GC-MS profile of Rhizophora apiculata leaf extract from the coast of Tomini bay, Central Sulawesi with antibacterial and antioxidant activity. Jurnal Kelautan: Ind J Mar Sci Tech, 2021; 14(1):30–42. CrossRef
Dewanto DK, Tanod WA, Finarti F, Renol R. Screening of antiradical activity from some Central Sulawesi mangroves. Pharmaciana, 2018; 8(1):155–68. CrossRef
Dogan A, Otlu S, Buyuk F, Aksu P, Tazegul E, Erdag D. Effects of cysteamine, putrescine and cystemine-putrescine combination on some bacterium. Kafkas Univ Vet Fak, 2012; 18(6):1015–9.
Elmi A, Spina R, Risler A, Philippot S, Mérito A, Duval RE, Abdoul-latif FM, Laurain-Mattar D. Evaluation of antioxidant and antibacterial activities, cytotoxicity of Acacia seyal Del bark extracts and isolated compounds. Molecules, 2020; 25(2392): 1–15. CrossRef
Ernianingsih WS, Mukarlina, Rizalinda. Ethnopharmacology of Mangroves Acanthus ilicifolius L., Acrostichum speciosum L. and Xylocarpus rumphii Mabb. in Sungai Tekong Village, Sungai Kakap District, Kubu Raya Regency. Protobiont, 2014; 3(2):252–8.
EUCAST. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin Microbiol Infect, 2000; 9(8):9–15. CrossRef
Fadzir UA, Mokhtar KI, Mustafa BE, Darnis DS. Evaluation of bioactive compounds on different extracts of Linum usitatissimum and its antimicrobial properties against selected oral pathogens. Makara J Health Res, 2018; 22(3):121–7.
Figueroa-Valverde L, Díaz-Cedillo F, López-Ramos M, Díaz-Ku E. Synthesis, characterization and antibacterial activity of danazol-pregnenolone conjugate. Asian J Chem, 2009; 21(8):6209–20. CrossRef
Fikri F, Thohawi M, Purnama E. Pharmacology and phytochemistry overview on Sauropus androgynous. Syst Rev Pharm, 2020; 11(6):124–8.
Forestryana D, Arnida, Yunus R. Pharmacognostic study of jeruju from Teluk Selong Martapura South Borneo. Borneo J. Pharmascientech, 2018; 2(2):103–12. CrossRef
Fudyma JD, Lyon J, Aminitabrizi R, Gieschen H, Chu RK, Hoyt DW, Kyle JE, Toyoda J, Tolic N, Heyman HM, Hess NJ, Metz TO, Tfaily MM. Untargeted metabolomic profiling of Sphagnum fallax reveals novel antimicrobial metabolites. Plant Direct, 2019; 3(11):e00179. CrossRef
Gallo ML, Seldes AM, Cabrera GM. Antibiotic long-chain and α,β-unsaturated aldehydes from the culture of the marine fungus Cladosporium sp. Biochem Syst Ecol, 2004; 32(6):545–51. CrossRef
Gan J, Feng Y, He Z, Li X, Zhang H. Correlations between antioxidant activity and alkaloids and phenols of maca (Lepidium meyenii). J Food Qual, 2017; 2017:1–10.
Global Biodiversity Information facility. 2021. Available via https://www.gbif.org/species/6359819 (Accessed 27 June 2021).
Godwill EA, Paul N, Chidubem NJ, Innocent OT, Chinenye EB. Comparative qualitative analysis of the phytochemical load of water, methanol, ethyl acetate and hexane extracts of six selected medicinal plants. Int J Pharmacogn Phytochem Res, 2013; 5(3):164–7. CrossRef
Govindasamy C, Arulpriya M. Antimicrobial activity of Acanthus ilicifolius: skin infection pathogens. Asian Pac J Trop Dis, 2013; 3(3):180–3. CrossRef
Habib MR, Karim MR. Antimicrobial and cytotoxic activity of di-(2-ethylhexyl) phthalate and anhy-drosophoradiol-3-acetate isolated from Calotropis gigantea (Linn.) flower. Mycobiology, 2009; 37(1):31–6. CrossRef
Handayani S, Najib A, Wati NP. Antioxidant activity assay daruju leaves extract (Acanthus ilicifolius L.) using 1,1-diphenyil-2-picrylhidrazil (DPPH) free radical stabling method. Jurnal Fitofarmaka Indonesia, 2018; 5(2):299–308.
Harborne JB. Phytochemical methods: a guide to modern techniques of plant analysis, Chapman and Hall in association with Methuen, Inc., New York, vol. 3, 1998. CrossRef
Hikmawanti NPE, Fatmawati S, Asri AW. The effect of ethanol concentrations as the extraction solvent on antioxidant activity of katuk (Sauropus androgynus (L.) Merr.) leaves extracts. IOP Conf Ser: Eahikrth Environ Sci, 2021; 755:012060.
Indonesian National Standardization Agency ? BSN. 2006. Microbiology test method part 3: determination of total plate count (TPC) in fishery products. Indonesian National Standard (SNI) No. 01-2332.3. Badan Standardisasi, Nasional Indonesia, Jakarta, Indonesia.
Irawanto R, Ariyanti EE, Hendrian R. Seaholly (Acanthus ilicifolius): seed, germination and uses. In: Proceedings of the National Seminar on the Indonesian Biodiversity Society, Yogyakarta, Indonesia, vol. 1, pp 1011–8, 2015.
Jasmine R, Selvakumar B, Daisy P. Investigating the mechanism of action of terpenoids and the effect of interfering substances on an Indian medicinal plant extract demonstrating antibacterial activity. Int J Pharm Stud Res, 2011; 2(2):19–24.
Jeppesen C. Media for Aeromonas spp., Plesiomonas shigelloides and Pseudomonas spp. from food and environment. In: Corry JEL, Curtis GDW, Baird RM (eds.). Culture media for food microbiology, Elsevier Science B.V., Amsterdam, Netherlands, vol. 34, pp 111–27, 1995. CrossRef
Jung YS, Kim SJ, Kwon DY, Ahn CW, Kim YS, Choi DW, Kim YC. Alleviation of alcoholic liver injury by betaine involves an enhancement of antioxidant defense via regulation of sulfur amino acid metabolism. Food Chem Toxicol, 2013; 62: 292–8. CrossRef
Kanchanapoom T, Kamel MS, Kasai R, Yamasaki K, Picheansoonthon C, Hiraga Y. Lignan glucosides from Acanthus ilicifolius. Phytochemistry, 2001; 56(4):369–72. CrossRef
Khajure PV, Rathod JL. Antimicrobial activity of extracts of Acanthus ilicifolius extracted from the mangroves of Karwar Coast Karnataka. Recent Res Sci Technol, 2010; 2(6):98–9.
Kittakoop P, Mahidol C, Ruchirawat S. Alkaloids as important scaffolds in therapeutic drugs for the treatments of cancer, tuberculosis, and smoking cessation. Curr Top Med Chem, 2014; 14(2):239–52. CrossRef
Laila F, Fardiaz D, Yuliana ND, Damanik MRM, Nur Annisa Dewi F. Methanol extract of Coleus amboinicus (Lour) exhibited antiproliferative activity and induced programmed cell death in colon cancer cell WiDr. Int J Food Sci, 2020; 2020:1–12. CrossRef
Lamuela-Raventós RM. 2017. Folin-Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In: Apak R, Capanoglu E, Shahidi F (eds.). Measurement of antioxidant activity and capacity: recent trends and applications. John Wiley & Sons Ltd, Hoboken, NJ, pp 107–15. CrossRef
LaSarre B, Federle MJ. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev, 2013; 77(1):73–111. CrossRef
Liu W, Yin D, Li N, Hou X, Wang D, Li D, Liu J. Influence of environmental factors on the active substance production and antioxidant activity in Potentilla fruticosa L. and its quality assessment. Sci Rep, 2016; 6:28591. CrossRef
Madigan MT, Bender KS, Buckley DH, Sattley WM, Stahl DA. Brock biology of microorganisms, Pearson Education Limited, London, UK, vol. 15, 2019.
Mary APF, Giri RS. Phytochemical screening and GC-MS analysis in ethanolic leaf extracts of Ageratum conyzoides (L.). World J Pharm Res, 2016; 5(7):1019–29.
Midura-Nowaczek K, Purwin M, Markowska A, Drozdowska D, Bruzgo M. Effect of short peptides containing lysine and ε-Aminocaproic acid on fibrinolytic activity of plasmin and topoisomerase II action on supercoiled DNA. Acta Pol Pharm, 2013; 70(3):431–4.
Molyneux P. The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Tech, 2004; 26:211–9.
Monteiro SH, Andrade GCRM, Garcia F, Pilarski F. Antibiotic residues and resistant bacteria in aquaculture. Pharm Chem J, 2018; 5(4):127–47.
Mooradian AD. Antioxidant properties of steroids. J Steroid Biochem Mol Biol, 1993; 45(6):509–11. CrossRef
Mujahid M, Ansari VA, Sirbaiya AK, Kumar R, Usmani A. An insight of pharmacognostic and phytopharmacology study of Adenanthera pavonina. J Chem Pharm Res, 2016; 8(2):586–96.
Muliadin, Dewanto DK, Hermawan R, Riyadi PH, Tanod WA. Soft coral Sinularia gibberosa extracts origin Palu bay, Central Sulawesi with antibacterial and antioxidant activity. Saintek Perikanan: Indo J Fish Sci Tech, 2021; 17(1):47–57.
Mykhailenko O, Gudžinskas Z, Kovalyov V, Desenko V, Ivanauskas L, Bezruk I , Georgiyants V. Effect of ecological factors on the accumulation of phenolic compounds in Iris species from Latvia, Lithuania and Ukraine. Phytochem Anal, 31(5):545–63. CrossRef
Naviner M, Giraud E, Le Bris H, Armand F, Mangion C, Ganière JP. Seasonal variability of intestinal microbiota in rainbow trout (Oncorhynchus mykiss), with a particular attention to Aeromonas spp. as candidate indicator of antimicrobial resistance. Rev Med Vet (Toulouse), 2006; 157(12):597–602.
Neganova ME, Afanas’Eva SV, Klochkov SG, Shevtsova EF. Mechanisms of antioxidant effect of natural sesquiterpene lactone and alkaloid derivatives. Bull Exp Biol Med, 2012; 152(6):720–2. CrossRef
Negara CK, Murjani, Basyid A. Effect of kelakai extract (Stenochlaena palustris) against hemoglobin of white rat (Rattus norvegicus). Borneo J Pharmascientech, 2017; 1(1):10–7.
Noor YR, Khazali M, Suryadiputra IN. Introduction to mangroves in Indonesia. Wetlands International Indonesia Programme, Bogor, Indonesia, vol. 2, 2006.
Nurdyansyah F, Widyastuti DA. Comparison of antioxidant activity of ethanolic, methanolic, nhexan, and aqueous extract of Parkia speciosa peel based on half -maximal inhibitory concentration through free radical inhibition. Adv Sustain Sci Eng Technol, 2020; 2(2):0200207-01–07. CrossRef
Ogbuagu MN. The nutritive and anti-nutritive compositions of calabash (Crescentia cujete) fruit pulp. J Anim Vet Adv, 2008; 7(9):1069–72.
Okocha RC, Olatoye IO, Adedeji OB. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Heath Rev, 2018; 39(21):1–22. CrossRef
Olga O, Aisiah S, Tanod WA, Risjani Y, Nursyam H, Maftuch M. Immunogenization of heat-killed vaccine candidate from Aeromonas hydrophila in catfish (Pangasius hypophthalamus) using strain of Banjar, South Kalimantan, Indonesia. Egypt J Aquat Biol Fish, 2020; 24(4):1–13. CrossRef
Osuntokun OT, Cristina GM. Bio-guided isolation, chemical purification, identification, antimicrobial and synergistic efficacy of extracted essential oils from stem bark extract of Spondias mombin (Linn). Int J Mol Biol, 2019; 4(4):135–43. CrossRef
Parthipan B, Suky MGT, Mohan VR. GC-MS analysis of phytocomponents in Pleiospermium alatum (Wall. ex Wight & Arn.) swingle, (Rutaceae). J Pharmacogn Phytochem, 2015; 4(1):216–22.
Paudel B, Bhattarai HD, Kim IC, Lee H, Sofronov R, Ivanova L, Poryadina L, Yim JH. Estimation of antioxidant, antimicrobial activity and brine shrimp toxicity of plants collected from Oymyakon region of the republic of Sakha (Yakutia), Russia Biol Res, 2014; 47(1):1–6. CrossRef
Petrovi? ZD, ?omi? L, Stefanovi? O, Simijonovi? D, Petrovi? VP. Antimicrobial activity of the ionic liquids triethanolamine acetate and diethanolamine chloride, and their corresponding Pd(II) complexes. J Mol Liq, 2012; 170:61–5. CrossRef
Pettit GR, Numata A, Takemura T, Ode RH, Narula AS, Schmidt JM, Cragg GM, Pase CP. Antineoplastic agents, 107. Isolation of acteoside and isoacteoside from Castilleja linariaefolia. J Nat Prod, 1990; 53(2):456–8. CrossRef
Polat ZA, Savage PB, Genberg C. In vitro amoebicidal activity of a ceragenin, cationic steroid antibiotic-13, against Acanthamoeba castellanii and its cytotoxic potential. J Ocul Pharmacol Ther, 2011; 27(1):1–5. CrossRef
Prestegard SK, Oftedal L, Coyne RT, Nygaard G, Skjærven KH, Knutsen G, Døskeland SO, Herfindal L. Marine benthic diatoms contain compounds able to induce leukemia cell death and modulate blood platelet activity. Mar Drugs, 2009;7(4):605–23. CrossRef
Pringgenies D, Setyati WA, Wibowo DS, Djunaedi A. Antibacterial activity of Acanthus ilicifolius extract against multi drug resistant bacteria. Jurnal Kelautan Tropis, 2020; 23(2):145–56. CrossRef
Radoševi? K, ?anak I, Pani? M, Markov K, Bubalo MC, Frece J, Sr?ek VG, Redovnikovi? IR. Antimicrobial, cytotoxic and antioxidative evaluation of natural deep eutectic solvents. Environ Sci Pollut Res, 2018; 25(14):14188–96. CrossRef
Ratnasari, Fahrizal, Dirhamsyah M. The utilization of mangrove vegetation in Padang Tikar Island, Batu Ampar District, Kubu Raya Regency. Jurnal Tengkawang, 2017; 7(2):110–5. CrossRef
Ravikumar S, Raja M, Gnanadesigan M. Antibacterial potential of benzoate and phenylethanoid derivatives isolated from Acanthus ilicifolius L. leaf extracts. Nat Prod Res, 2012; 26(23):2270–3. CrossRef
Riyadi PH, Romadhon, Anggo AD, Suharto S, Tanod WA, Aryani A. Anti-inflammatory potential from tilapia (Oreochromis niloticus) viscera hydrolysate with bioinformatics analysis (prediction of activity spectra for substances – PASS). IOP Conf Ser: Earth Environ Sci, 2021a; 750:012044. CrossRef
Riyadi PH, Tanod WA, Dewanto DK, Herawati VE, Susanto E, Aisiah S. Chemical profiles and antioxidant properties of Bruguiera gymnorrhiza fruit extracts from Central Sulawesi, Indonesia. Food Res, 2021b; 5(Suppl. 3):37–47. CrossRef
Riyadi PH, Tanod WA, Wahyudi D, Susanto E, Fahmi AS, Aisiah S. Potential of tilapia (Oreochromis niloticus) viscera bioactive peptides as antiviral for SARS-CoV-2 (COVID 19). IOP Conf Ser: Earth Environ Sci, 2020; 584:012004. CrossRef
Riyadi PH, Wahyudi D, Tanod WA. Effects of dichloromethane Sarcophyton spp. extract on the lipopolysaccharide-induced expression of nuclear factor-kappa B and inducible nitric oxide synthase in mice. Vet World, 2019; 12(12):1897–902. CrossRef
Roy RN. Bioactive natural derivatives of phthalate ester. Crit Rev Biotechnol, 2020; 40(7):913–929. CrossRef
Rumengan AP, Mandiangan ES, Tanod WA, Paransa DSJ, Paruntu CP, Mantiri DMH. Identification of pigment profiles and antioxidant activity of Rhizophora mucronata mangrove leaves origin Lembeh, North Sulawesi, Indonesia. Biodiversitas, 2021; 22(7):2805–16. CrossRef
Saha K, Proma RZ, Khan N. Phytochemical screening of plant extracts and GC-MS analysis of n-hexane extract of the leaves of Cassia alata Linn. J Phytopharmacol, 2020; 9(5):342–7. CrossRef
Samoylenko V, Khan SI, Jacob MR, Tekwani BL, Walker LA, Hufford CD, Muhammad I. Bioactive (+)-manzamine A and (+)-8-hydroxymanzamine A tertiary bases and salts from Acanthostrongylophora ingens and their preparations. Nat Prod Commun, 2009; 4(2):185–92. CrossRef
Saptiani G, Prayitno SB, Anggoro S. Antibacteria potential of jeruju (Acanthus ilicifolius) leaf extracts on the in vitro growth of the Vibrio harveyi. Ind J Vet Sci, 2013; 7(1): 17–20. CrossRef
Sefa C, Albayrak TA, Sevim A, Ozel AE, Sigirci BD. Synthesis, antimicrobial activity, molecular docking and ADMET study of a caprolactam- glycine cluster. J Biomol Struct Dyn, 2020; 2020:1–20.
Simamora A, Santoso AW, Timotius KH, Rahayu I. Antioxidant activity, enzyme inhibition potentials, and phytochemical profiling of Premna serratifolia L. leaf extracts. Int J Food Sci, 2020; 2020:1–11. CrossRef
Son SY, Lee S, Singh D, Lee NR, Lee DY, Lee CH. Comprehensive secondary metabolite profiling toward delineating the solid and submerged-state fermentation of Aspergillus oryzae KCCM 12698. Front Microbiol, 2018; 9(1076):1–12. CrossRef
Sreenivasa RM, Teja G, Sirisha IR, Yedukondala RP. Screening of antimicrobial activity of mangrove plant Acanthus ilicifolius on shrimp and fish pathogens. Asian J Plant Sci Res, 2015; 5(5):1–3.
Sultana B, Anwar F, Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules, 2009; 14: 2167–80. CrossRef
Susanti A, Rimayanti, Sukmanandi M. Antibacterial activity of the ethanol extract Pluchea indica less leaves against Escherichia coli by in vitro. Veterinaria Medika, 2008; 1(1):29–32.
Tanod WA, Dewanto DK, Ndobe S, Riyadi PH, Putra MY. Screening of antibacterial and antioxidant activity of soft corals Sinularia sp. and Sarcophyton sp. from Palu Bay Central Sulawesi, Indonesia. Squalen: Bull Mar Fish Postharvest Biotech, 2019a; 14(2):73–83. CrossRef
Tanod WA, Yanuhar U, Maftuch, Wahyudi D, Risjani Y. DPPH scavenging property of bioactives from soft corals origin Palu Bay, Central Sulawesi, Indonesia. IOP Conf Ser: Earth Environ Sci, 2019b; 236:012121. CrossRef
Thiem B, Wesolowska M, Skrzypczak L, Budzianowski J. Phenolic compounds in two Solidago L. species from in vitro culture. Acta Pol Pharm, 2001; 58(4):277–81.
Thirunavukkarasu P, Ramanathan T, Ramkumar L. Hemolytic and antimicrobial effect in the leaves of Acanthus ilicifolius. J Pharmacol Toxicol, 2011a; 6(2):196–200. CrossRef
Thirunavukkarasu P, Ramanathan T, Shanmugapriya R, Umamaheswari G, Renugadevi G. Antioxidant and free radical scavenging effect of Acanthus ilicifolius. Res J Appl Sci, 2011b; 6(3):218–22. CrossRef
Truong DH, Nguyen DH, Ta NTA, Bui AV, Do TH, Nguyen HC. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J Food Qual, 2019; 8178294:1–9. CrossRef
Vani M, Manikandan T. Phytochemical analysis and antioxidant activity of Acanthus ilicifolius. Int J Curr Res, 2018; 10(12):76891–6.
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W. Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev, 2000; 64(4):655–71. CrossRef
Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc, 2008; 3(2):163–75. CrossRef
Wöstmann R, Liebezeit G. Chemical composition of the mangrove holly Acanthus ilicifolius (Acanthaceae) - review and additional data. Senckenbergiana Maritima, 2008; 38(1):31–7. CrossRef
Wu P, Jiang W, Liu Y, Chen G, Jiang J, Li S. Effect of choline on antioxidant defenses and gene expressions of Nrf2 signaling molecule in the spleen and head kidney of juvenile Jian carp (Cyprinus carpio var . Jian). Fish Shellfish Immunol, 2014; 38(2):374–82. CrossRef
Wu P, Liu Y, Jiang W, Jiang J, Zhao J, Zhang Y, Zhou X, Feng L. A comparative study on antioxidant system in fish hepatopancreas and intestine affected by choline deficiency: different change patterns of varied antioxidant enzyme genes and Nrf2 signaling factors. PloS One, 2017; 12(1):e0169888. CrossRef
Xie Y, Yang W, Tang F, Chen X, Ren L. Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Curr Med Chem, 2015; 22(1):132–49. CrossRef
Zayed MZ, Ahmad FB, Ho WS, Pang SL. GC-MS analysis of phytochemical constituents in leaf extracts of Neolamarckia cadamba (rubiaceae) from Malaysia. Int J Pharm Pharm Sci, 2014; 6(9):123–7.
Zhang M, Zhang H, Li H, Lai F, Li X, Tang Y, Min T, Wu H. Antioxidant mechanism of betaine without free radical scavenging ability. J Agric Food Chem, 2016; 64(42):7921–30. CrossRef
Zhao HF, Jiang WD, Liu Y, Jiang J, Wu P, Kuang SY, Tang L, Tang WN, Zhang YA, Zhou XQ, Feng L. Dietary choline regulates antibacterial activity, inflammatory response and barrier function in the gills of grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol, 2016; 52:139–50. CrossRef
Zhou J, Chan L, Zhou S. Trigonelline: a plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr Med Chem, 2012; 19(21):3523–31. CrossRef