INTRODUCTION
The pediatric population comprises a special group in drug dosing. The US pediatric population is approximately 20% of the general population (U.S. Census Bureau, n.d). However, prior to the last decade before the US Food and Drug Administration (FDA) and European Medicine Agency passed the regulatory legislation enabling drug development in pediatrics, drugs safety and efficacy testing were not usually carried out in the pediatric population before marketing authorization and commercial distribution (Auby, 2008; Ziswoski et al., 2010). The general tendency for the low level of safety and efficacy testing in children has been attributed primarily to the ethical and moral obligations of submitting children for studies, the difficulties of taking samples by invasive procedures, and the limited numbers and volume of samples that could be obtained from a child (Ziswoski et al., 2010). These have resulted in a general lack of rich clinical pharmacology data in the pediatric population (Caparelli and Williams, 2007). In addition, the development of dosing strategies in children is generally more complex than in adults due to the much greater variation in pediatric physical and physiologic characteristics such as body weight (BW), age, ongoing growth and different patterns of disease progression, and maturation of organs, especially the organs of metabolism and excretion as well as the enzyme systems within them (Bartelink et al., 2006). Moreover, many biomarkers of disease and physiologic processes have age-dependent ranges. Although it has been clearly asserted that children are not small adults (Giacoia and Mattison, 2005), advances in knowledge of pediatric pharmacology indicate that some extrapolations can be made with a significant level of accuracy in drug dosing of children based on adult clinical information. Based on this assumption, the US FDA published the Guidance for Industry: General Considerations for Pediatric Pharmacokinetic Studies for Drugs and Biological Products (DHHS, 1998). Since, for ethical and logistic reasons, characterization of drug efficacy and safety in adults almost always precedes pharmacometric studies in children, the design of studies in children could benefit from the existing wealth of preclinical and clinical data in adults.
Literature information suggests that physiologic distribution and elimination pathways show commonalities across ages even though their relative contribution to the overall pharmacokinetics of drugs may differ (Caparelli and Williams, 2007). Similarly, enzyme presence and activity show a high degree of similarity across age groups. However, despite the existence of overall homology for a set of enzymes, some differences may lead to unexpected developmental changes in the pharmacokinetics of drugs (Caparelli and Williams, 2007). In the absence of pediatric-specific pharmacodynamic models, exposure-response surfaces, and models linking biomarkers to pediatric outcomes, the application of pharmacometrics appears to be the only feasible and most rational way of optimizing drug use in pediatrics (Trivedi et al., 2013). The procedure involves estimation of the impact of different levels of covariates on pharmacokinetics and pharmacodynamics with the potential application in dosing strategies. The large ranges of important dosing parameters such as age, BW, and surface area in children imply that these covariates would impact pharmacokinetics and pharmacodynamics in children more than in adults.
The use of combination therapy of two or more drugs has been found to provide significant viral suppression and inhibit drug resistance (Riska et al., 1990). Owing to these benefits, several fixed-dose combination therapies have been developed recently (Blum et al., 1988; vanLeeuwen et al., 1992). However, the biggest challenge for anti-HIV therapy in children is the lack of age-appropriate formulations that can be easily and accurately administered to the children population. There are over 20 antiretroviral drugs licensed worldwide for the treatment of adults, but many do not have appropriate formulations for children. The pediatric formulations currently in use are either poorly palatable liquids or tablets that cannot be crushed (Chokephaibulkit et al., 2011; French et al., 2002; Kayitare et al., 2009; Marier et al., 2007; Monif et al., 2009; Pujari et al., 2004). In addition, mother-to-child transmission of drug-resistant viruses or development of drug resistance due to prenatal treatment can also be a concern in developing formulations for children. There is therefore an urgent necessity to address three major challenges in pediatric antiretroviral therapy: determining the flexible and adaptable dosage form, establishing the parameters for dosage determination, and adopting appropriate strategies for stemming mother-to-child transmission.
Esseku et al. (2013) previously developed fixed-dose formulations of three drugs: 3TC, AZT, and ZDV. The fixed-dose combination of these drugs was bioequivalent to the coadministered single entities of reference products (Chokephaibulkit et al., 2011). The outcome of earlier clinical studies in pediatrics indicated significantly new information and pharmacokinetic differences between children and adults (Leeder et al., 2010). Several physiologic processes that affect the absorption, distribution, metabolism, and excretion of drugs differ significantly among neonates, children, and adults. For example, the activity of cytochrome P450 (CYP 450) is less in neonates but higher in children when compared to adults (Anderson, 2002). This variation in the activity of CYP 450 should be considered when developing formulations and doses for pediatric populations. However, there is significantly less clinical data available with regard to the pediatric population.
Generally, the first dose of the drug for an infant is calculated based on the established dose in adults by four methods: age-based categories, normalization of dose to BW, body surface area (BSA), and the allometric method. Each of these methods has its own limitations. Variability and progressive changes in body composition of a developing child imply potentials for significant differences in the processes of dosage form-dependent drug release for absorption, distribution, metabolism, and excretion known as liberation, absorption, distribution, metabolism, and excretion (LADME). Differences in the rate and extent of LADME will in turn impact the pharmacodynamics and therapeutic outcomes of the drug. Hence, knowledge of physiology-based pharmacokinetics with the help of a rationally determined allometric equation will significantly improve the quality of drug dosing in pediatrics. Although significant progress has been made since 1994 when the US FDA enacted the FDA Modernization Act (FDAMA) (Breitkreutz, 2008) encouraging pharmaceutical manufacturers to conduct pediatric clinical trials, there is still a long way before sufficient information would be available for routine clinical use. In the alternative, pharmacokinetic modeling has been recognized as a useful tool for predicting the pharmacokinetic parameters in children based on the data obtained from clinical trials in adults. It appears that such information is still not available in the literature for antiretroviral therapies. This study was therefore designed to simulate the pediatric parameters from the clinical trial data in healthy adults administered with a pediatric fixed-dose combination of lamivudine (3TC)/zidovudine (AZT)/nevirapine (NVP) at the levels of 30/60/50 mg per 5 ml, respectively, which were presented as granules for reconstitution or fast disintegrating tablets (Esseku et al., 2013). Existing pharmaceutical information on the drugs and projected physical and physiologic information in virtual pediatric patient populations would be used as input data into the pharmacokinetic modeling and simulation software.
MATERIALS AND METHODS
Pharmacokinetic parameters of lamivudine, nevirapine, and zidovudine were determined from a meta-analysis of the plasma-time profiles obtained from an open-label, randomized, two-way crossover study conducted on 24 healthy adults as previously reported by Esseku et al. (2013). Drug physicochemical and molecular properties extracted from various literature, DrugBank, and other databases (Table 1) were entered into the GastroPlus® pharmacokinetic modeling and simulation software. For the respective drugs, Cp-versus-time profiles were entered into the model finding menu, and the drug dose, dosage form, and individual subject’s BW were populated in the appropriate menu. The model solution was sought for one-, two-, and three-compartment models, respectively, using the Akaike information criterion and Schwarz criterion for indicating the best fitting model. Hooke and Jeeves’s pattern search was selected, and the objective weighting was 1/Yhat. From the optimum solutions determined in GastroPlus®, the pharmacokinetic parameters [specifically clearance (CL)/F and Vc/F] associated with the solutions were extracted, plugged into an MS Excel spreadsheet, and used to generate the expected volume of distribution (Vd) and CL for a given age (months, after gestation), BW, and BSA (m2) of virtual subjects, up to 12 years. A set of regression equations was generated by regressing CL/F against BW, postgestational age (PGA), and BSA, respectively. Similarly, Vc/F (and V2/F and CL2/F for drugs that showed a two-compartment model in GastroPlus®) were in turn regressed against BW, PGA, and BSA, respectively.
 | Table 1. Sources of variation in pharmacokinetics (PK) between children and adult. [Click here to view] |
RESULTS AND DISCUSSION
At the end of 2020, about 1.7 million children around the world are living with HIV/AIDS, most cases of which were acquired through infection via mother-to-child transmission (available at https://www.unaids.org/en/keywords/children) (accessed January 03, 2022). Also, in 2020 there were 150,000 new HIV infections in children, mostly due to a lack of access to HIV testing and medication, especially in pregnant and breastfeeding women (available at https://www.unaids.org/en/keywords/children) (accessed January 03, 2022). Highly active antiretroviral treatment consisting of a combination of nonnucleoside reverse transcriptase, nucleoside reverse transcriptase, and protease inhibitors has been proven to suppress the replication of the HIV in the body. Some of the World Health Organization’s recommended treatments consist of zidovudine (for infants 6 weeks–12 months) and nevirapine (0–12 months). However, these drugs either are not available in suitable dosage formulations or have not been studied at a considerable level in pediatric patients. The introduction of the FDAMA and various legislative initiatives encouraging drug manufacturers to evaluate the existing data that could support pediatric drug labeling resulted in pediatric studies on over 300 drugs and biological products. The studies showed some important pharmacokinetic differences between children and adults (Leeder et al., 2010). The outcome of the studies reinforced the previously held notion that drug dosing in this population requires critical consideration of the differences in the pharmacokinetics in relation to the pharmacology of a specific drug across the age range from a preterm newborn infant, newborn infant (birth–28 days), and infant (28 days–23 months) through a young child (2–5 years) to older child (6–11 years) and adolescent (12–18 years). It also emphasized the need for the provision of age-appropriate dosage forms in order to enhance product acceptance by children and provide the needed flexibility for a more accurate dosage determination for the pediatric population.
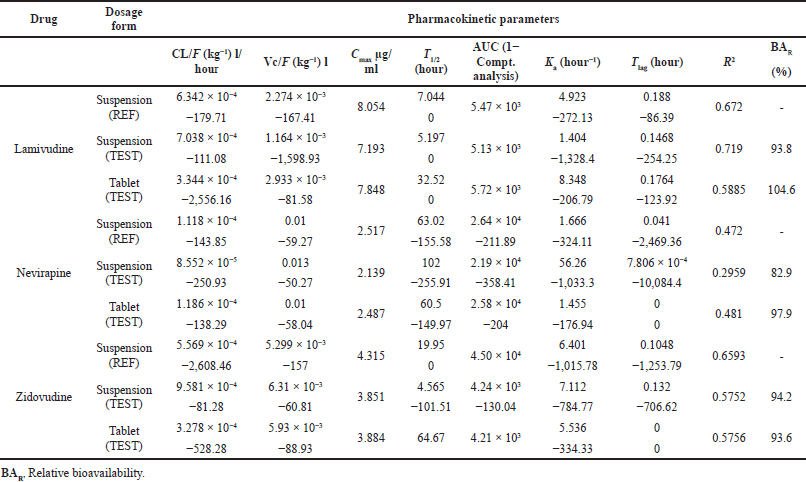
The superimposed Cp versus time of 12 volunteers who took the suspension dosage form containing lamivudine, nevirapine, and zidovudine are shown in Figures 1–3, respectively. The pharmacokinetic parameters of the three drugs are shown in Table 1. The area under the curve (AUC) from the noncompartmental analysis showed the relative bioavailability of the test suspension and test tablet to the reference suspension as 93.7% and 104.5% for lamivudine, 82.9% and 97.9% for nevirapine, and 94.2% and 93.6% for zidovudine, respectively (Table 2). The obtained bioavailability generally fell within the FDA acceptance criteria of −20 to +25% (i.e., 80 to 125%) of the reference formulation (DHHS, 1998). The 90% CI for Cmax, AUC0–t, and AUC0–∞ were 85.69%–102.54%, 96.13%–117.79%, and 82.63%–106.72%, respectively, for lamivudine; 83.33%–109.15%, 99.94%–124.54%, and 98.22%–122.43%, respectively, for zidovudine; and 87.71%–109.20%, 89.31%–106.26%, and 85.95%–102.82%, respectively, for nevirapine, indicating the bioequivalence of test to reference products.
The result of bioavailability/bioequivalence testing of the different fixed-dose combinations showed that the more soluble lamivudine (70 mg/ml, BCS Class III) and zidovudine (20 mg/ml; BCS Class 1) drugs generally followed the two-compartmental model while nevirapine (0.1 g/ml; BCS Class II) followed the one-compartmental model (Figs. 1–3).
Most literature models were determined in a relatively homogenous population of patients (Sabo et al., 2002). In order to capture the dependence of PK parameters on patient variables, the respective drug’s adult pharmacokinetic parameters as a function of BW were regressed on virtual patient parameters obtained from the literature including PGA, BW, and BSA in the form of linear, exponential, power, and polynomial models in order to determine the model that best captures the parameter dependence most accurately across the developmental age ranges.
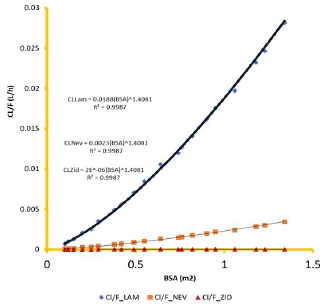
Clearance and Vd weighted on the bioavailability factor (F) per kg of subject BW was linearly correlated with the BW (R2 = 1.000, Figs. 4 and 5) and exponent, n =1.0 (Anderson, 2002; Esseku et al., 2013). CL and Vd obeyed a power function with BSA according to the equation
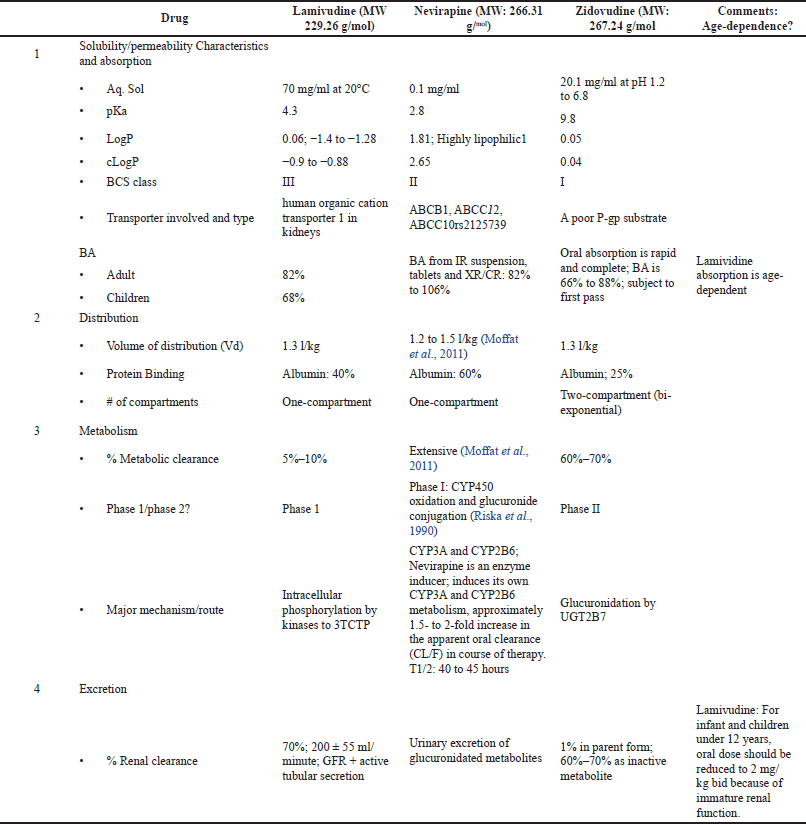
 | Figure 1. (a) Plasma concentration (Cp) versus time (t) profiles of subjects on lamivudine in three drug suspension formulations. (b) Plasma concentration (Cp) versus time (t) profiles of subjects on lamivudine oral disintegration tablets. [Click here to view] |
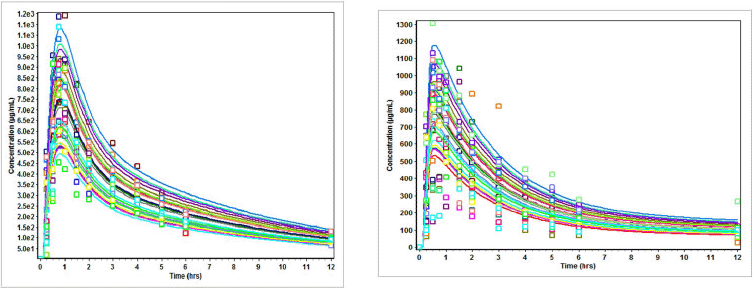
 | Figure 2. (a) Plasma concentration (Cp) versus time (t) profiles of subjects on nevirapine in three drug suspension formulations. (b) Plasma concentration (Cp) versus time (t) profiles of subjects on nevirapine oral disintegration tablets. [Click here to view] |
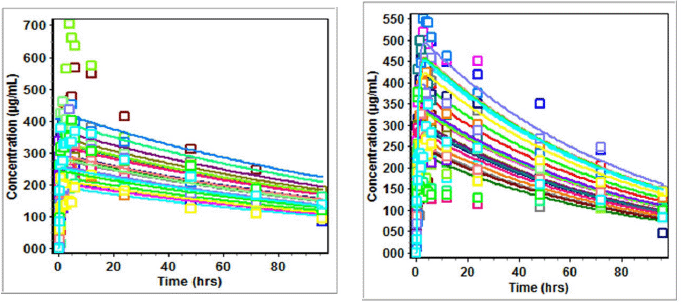
 | Figure 3. (a) Plasma concentration (Cp) versus time (t) profiles of subjects on zidovudine in three drug suspension formulations. (b) Plasma concentration (Cp) versus time (t) profiles of subjects on zidovudine oral disintegration tablets. [Click here to view] |
CL (or Vd) = a*(BSA)n, R2 ≥ 0.9987 (1)
The factor “a” in the equation is drug substance-dependent and is highest for lamivudine, a BCS-III drug, essentially renally cleared by the glomerular filtration rate (GFR) and active tubular secretion (vanLeeuwen, 1992), and lowest for zidovudine, a BCS I drug, essentially metabolized with only 1% eliminated in the parent form (Blum et al., 1988). Agreement between pediatric pharmacokinetic parameters derived using adult clinical data and the predicted parameter values in the virtual children population provided the regression profiles shown in Figures 6–8 for lamivudine, nevirapine, and zidovudine, respectively.
According to Bartelink et al. (2006), BW provided the most predictive covariate for clearance, volume of distribution, and intercompartmental clearance and accounted for 65%, 75%, and 40% of the observed variability, respectively, in these parameters. The relationship between BW and clearance was best characterized using an allometric equation with a scaling exponent that changed with BW from 1.2 in neonates to 0.55 in young adults (Bartelink et al., 2006; Cella et al., 2012). For lamivudine and zidovudine, the renal system plays a crucial role in their elimination as either parent or metabolite form. Therefore, dosing should be carefully determined, especially during the postgestational and early childhood stages. Evidence abounds in the literature showing that, during the last months of gestation, GFR increases in parallel with gestational age until the 36th week due to an increase in both the number and size of nephrons and thereafter develops more slowly up to the time of birth. However, nevirapine CL/F has been shown to be insignificantly affected by patient demographics (age, gender) and only minimally affected by patient weight (Sabo et al., 2002).
 | Table 2. Pharmacokinetic parameters from 24 healthy subjects in 3 × 3 randomized crossover study. [Click here to view] |
The GFR is low in preterm infants at birth and varies with gestational age. The stage of renal development may be critical for lamivudine and nevirapine elimination in these neonates. Postnatal GFR develops slowly until 34 to 36 weeks of gestation. Thereafter, GFR increases rapidly, like postnatal GFR in term neonates, and GFR measured by inulin clearance at birth is almost 20 ml/minute/1.73 m2 in term neonates (van der Heijden et al., 1988). There is a large increase in GFR during the first 2 weeks after birth in term infants, which has been associated with a postnatal increment in gestational age, BW, and renal hemodynamic changes reaching 50 ml/minute/1.73 m2. Renal hemodynamics is independent of gestational age and BW (Otukesh et al., 2012). All these data support the need for pharmacokinetic monitoring of drug clearance in this population to ensure an optimal outcome and minimal adverse drug event.
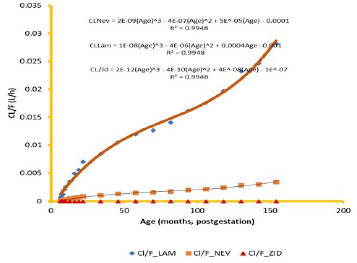
Regression of CL and Vd, respectively, on PGA of subjects followed a polynomial with the highest R2 at the third order according to the equations
CLNev = 2−09(Age)3 – 4−07(Age)2 + 5−05(Age) – 0.0001 (2)
(R² = 0.9948),
CLLam = 1−08(Age)3 – 4−06(Age)2 + 4−04 – 0.001 (3)
(R² = 0.9948),
CLZid = 2−12(Age)3 – 4−10(Age)2 + 4−08(Age) – 1−07 (4)
(R² = 0.9948).
Knowledge of PK of drugs is being used in the calculation of the first in-human dose (Jones et al., 2011; Rowland et al., 2011). Pending the availability of more extensive pharmacokinetic data of most drugs in pediatrics, allometric estimation of a child’s doses based on adult human clinical data provides a rational basis for age- and BW-dependent adjustments in antiretroviral medication, once the role of enzymes and transporters has been carefully considered. Thus, by selecting the most fitting equations (Figs. 4 and 5), more accurate age-appropriate doses can be determined for the pediatric population, from infant to age 12, using adult human PK data. The model fitness evaluated as a function of predicted versus observed Cp in virtual pediatric patients of postgestation to age 12 years is shown in Figures 6 and 7, with regression coefficients of 0.7084, 0.4822, and 0.5829, respectively, for lamivudine, nevirapine, and zidovudine. Allometric scaling is a well-established method for relating physiological or pharmacokinetic parameters to BW, and it is currently used for the prediction of human dose by extrapolation from preclinical animal studies and for dose estimation for other animal species. Fixed-exponent scaling from human adult pharmacokinetic data provides a more practical alternative to conventional interspecies scaling (Anderson and Holford, 2008; Holford et al., 2013; Tod et al., 2008). However, the validity of fixed exponents such as ? or ¾ has been questioned under changing physiologic or disease states, conditions of immature clearance mechanisms, extreme of ages, and immunocompromised patients (Holford, 1996; Johnson, 2008; Mahmood, 2006). The polynomial allometric equations developed in this study could provide more reliable indices of dosage determination across age ranges to assist with first-dose determination of these antiretrovirals in children. Once the first dose is administered, monitoring of the usual outcomes together with patient-specific pharmacokinetic parameters determination can be applied for optimization of therapeutic outcomes in the patient.
 | Figure 4. Regression of drug clearance on BSA of subjects. [Click here to view] |
 | Figure 5. Regression of CL/F on age postgestation (6 months–12 years) of subjects. [Click here to view] |
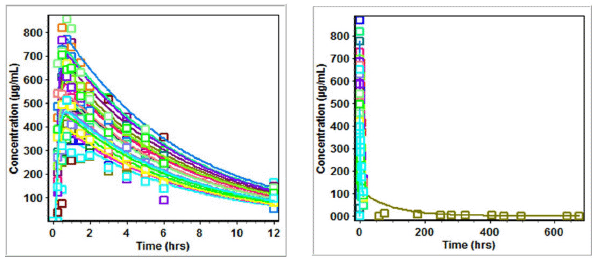
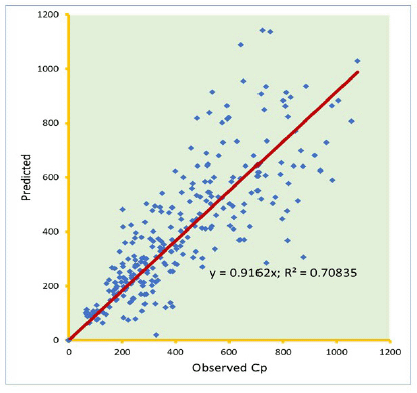 | Figure 6. Predicted versus observed Cp (µg/ml) of lamivudine on administration in three drug suspension formulations. [Click here to view] |
During the past decade, understanding of the effects of pharmacogenomics, allometry of metabolizing enzymes, and drug transporters has increased significantly. This information would provide the appropriate framework for pharmacokinetic adjustment of a dosage regimen in pediatrics following a more accurate determination of the starting dose using the pharmacokinetics-derived polynomial allometric equations reported in this study.
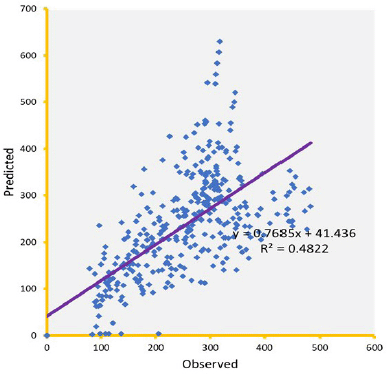 | Figure 7. Predicted versus observed Cp (µg/ml) of nevirapine on administration in three drug suspension formulations. [Click here to view] |
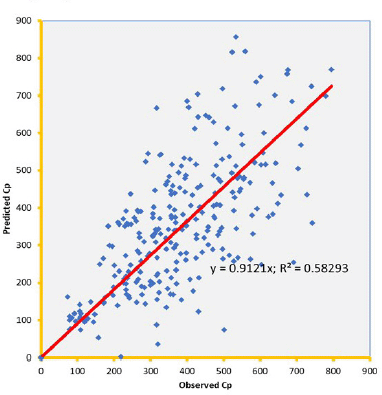 | Figure 8. Predicted versus observed Cp of zidovudine on administration in three drug suspension formulations. [Click here to view] |
CONCLUSION
Using adult human clinical data, we were able to generate equations for the determination of pediatric pharmacokinetic parameters for combination antiretroviral drugs lamivudine, nevirapine, and zidovudine. Model evaluations showed that drug clearance, a major parameter for dose and dosage regimen determination, and the volume of distribution were linearly dependent on BW and exponentially dependent on BSA and showed a polynomial relationship with PGA.
Although pharmacokinetic dose determination has been widely recognized as the hallmark of pediatric dosage determination, significant variability in patient characteristics due to the rapidly developing systems as well as the drug physical–chemical and pharmacokinetic properties implies a significant complication in first-dose determination. Unlike the fixed-exponent allometric equations which correlate clearance and Vd to BW or BSA, the polynomial allometric equations developed in this study could be used for rational determination of age-dependent first in-pediatric doses which will provide a more realistic basis for the subsequent patient monitoring and individualized pharmacokinetic adjustment.
ACKNOWLEDGMENTS
The authors are grateful for the GastroPlus PKTM license donation by Simulations Plus Inc., CA, and to Dr. Moji C. Adeyeye for providing the clinical metadata inputted into the platform.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
FUNDING
There is no funding to report.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
CONSENT TO PARTICIPATE
For this type of study, formal consent is not required.
ETHICAL APPROVAL
This article does not contain any studies with human participants or animals performed by any of the authors.
AUTHORS’ CONTRIBUTIONS
AA contributed to conceptualization and methodology. AA and RP contributed to formal analysis and investigation. RP and AA contributed to writing and original draft preparation and review and editing.
REFERENCES
Anderson GD. Children versus adults: pharmacokinetic and adverse-effect differences. Epilepsia, 2002; 43(Suppl 3):53–9. CrossRef
Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol, 2008; 48:303–32. CrossRef
Auby P. Pharmaceutical research in pediatric populations and the new EU Pediatric Legislation: an industry perspective. Child Adolesc Psychiatry Mental Health, 2008; 2:38; doi:10.1186/1753-2000-2-38 CrossRef
Bartelink IH, Rademaker CM, Schobben AF, van den Anker JN. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin Pharmacokinet, 2006; 45(11):1077–97. CrossRef
Breitkreutz J. European perspectives on pediatric formulations. Clin Ther, 2008; 30:2146–54. CrossRef
Blum MR, Liao SH, Good SS, De Miranda P. Pharmacokinetic and bioavailability of zidovudine in humans. Am J Med, 1988; 85:189–94.
Bureau, U. S. C. (2021, October 8). National population by characteristics: 2010–2020. Census.gov. Available via https://www.census.gov/programssurveys/popest/technical-documentation/research/evaluation-estimates/2020-evaluationestimates/2010s-national-detail.html (Accessed 16 May 2022).
Caparelli EV, Williams PJ. Pharmacometrics in pharmacotherapy and drug development: pediatric application. In: Ette EI, William PJ (eds.). Pharmacometrics: the science of quantitative pharmacology, John Willey & Sons, Inc., Hoboken, NY, 2007.
Cella M, Knibbe C, Danhof M, Della PO. What is the right dose for children? Br J Clin Pharmacol, 2012; 70:597–603. CrossRef
Chokephaibulkit K, Cressey TR, Capparelli E, Sirisanthana V, Muresan P, Hongsiriwon S, Ngampiyaskul C, Limwongse C, Wittawatmongkol O, Aurpibul L, Kabat B, Toye M, Smith ME, Eksaengsri A, McIntosh K, Yogev R, Team IP. Pharmacokinetics and safety of a new paediatric fixed-dose combination of zidovudine/lamivudine/nevirapine in HIV-infected children. Antiviral Ther, 2011; 16(8):1287–95. CrossRef
DHHS. Department of Health and Human Services, guidance for industry: general considerations for pediatric pharmacokinetic studies for drugs and biological products. US FDA, Rockfville, MD, 1998.
Esseku F, Joshi A, Oyegbile Y, Edowhorhu G, Gbadero D, Adeyeye M. A randomized pahse I bioequivalence clinincal trial of a paediatric fixed-dose combination antiretroviral reconstitutable suspension in healthy adult volunteers. Antiviral Ther, 2013; 18(2):205–12. CrossRef
French M, Amin J, Roth N, Carr A, Law M, Emery S, Drummond F, Cooper D, OzCombo I. Randomized, open-label, comparative trial to evaluate the efficacy and safety of three antiretroviral drug combinations including two nucleoside analogues and nevirapine for previously untreated HIV-1 Infection: the OzCombo 2 study. HIV Clin Trials, 2002; 3(3):177–85. CrossRef
Giacoia GP, Mattison DR. Newborns and drug studies: the NICHD/FDA newborn drug development initiative. Clin Ther, 2005; 27(6):796–813. CrossRef
Holford NH. A size standard for pharmacokinetics. Clin Pharmacokinet, 1996; 30:329–32. CrossRef
Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci, 2013; 102:2941–52. CrossRef
Johnson TN. The problems in scaling adult drug doses to children. Arch Dis Child, 2008; 93:207–11. CrossRef
Jones HM, Gardner IB, Collard WT, Stanley PJ, Oxley P, Hosea NA, Plowchalk D, Gernhardt S, Lin J, Dickins M, Rahavendran SR, Jones BC, Watson KJ, Pertinez H, Kumar V, Cole S. Simulation of human intravenous and oral pharmacokinetics of 21 diverse compounds using physiologically based pharmacokinetic modelling. Clin Pharm, 2011; 50(5):331–47. CrossRef
Kayitare E, Vervaet C, Ntawukulilyayo JD, Seminega B, Bortel V, Remon JP. Development of fixed dose combination tablets containing zidovudine and lamivudine for paediatric applications. Int J Pharm, 2009; 370(1–2):41–6. CrossRef
Leeder JS, Keaarns GL, Spielberg, SP Anker JV. Understanding the relative roles of pharmacogenetics and ontogeny in pediatric drug development and regulatory science. J Clin Pharmacol, 2010; 50(12):1377–87. CrossRef
Mahmood I. Prediction of drug clearance in children from adults: a comparison of several allometric methods. Br J Clin Pharmacol, 2006; 61:545–57. CrossRef
Marier JF, Dimarco M, Guilbaud R, Dodard C, Morelli G, Tippabhotla SK, Singla AK, Thudi NR, Monif T. Pharmacokinetics of lamivudine, zidovudine, and nevirapine administered as a fixed-dose combination formulation versus coadministration of the individual products. J Clin Pharmacol, 2007; 47(11):1381–9. CrossRef
Moffat AC, Osselton MD, Widdop B, Watts. Clarke’s analysis of drugs and poisons: In: Pharmaceuticals, body fluids and postmortem material. Pharmaceutical Press, London, UK, 2011.
Monif T, Reyar S, Tiwari HK, Tippabhotla SK, Khuroo A, Thudi NR, Ahmed S, Raghuvanshi R. A single-dose, randomized, open-label, two-period crossover bioequivalence study comparing a fixed-dose pediatric combination of lamivudine and stavudine tablet for oral suspension with individual liquid formulations in healthy adult male volunteers. Arzneimittelforschung, 2009; 59(2):104–8. CrossRef
Otukesh H, Hoseini R, Rahimzadeh N, Hosseini S. Glomerular function in neonates. Br J Clin Pharmacol, 2012; 40(5):439–43.
Pujari SN, Patel AK, Naik E, Patel KK, Dravid A, Patel JK, Mane AA, Bhagat S. Effectiveness of generic fixed-dose combinations of highly active antiretroviral therapy for treatment of HIV infection in India. J Acquir Immune Defic Syndr, 2004; 37(5):1566–9. CrossRef
Riska P, Lamson M, MacGregor T, Sabo J, Hattox S, Pav J, Keirns J. Disposition and biotransformation of antiretroviral drug nevirapine in humans. Drug Metab Dispos, 1990; 27:895–901.
Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol, 2011; 51:45–73. CrossRef
Sabo JP, Lamson MJ, Leitz G, Yong C, MacGregor TR. Pharmacokinetics of nevirapine and lamivudine in patients with HIV-1 infection. AAPS Pharm Sci, 2002; 2:1–9. CrossRef
Todd M, Jullien V, Pons G. Facilitation of drug evaluationin children by population methods and modeling. Clin Pharmacokinet, 2008; 47:231–43. CrossRef
Trivedi A, Lee RE, Meibohm B. Applications of pharmacometrics in the clinical development and pharmacotherapy of anti-infectives [Internet]. Expert Rev Clin Pharmacol, 2013; 6. Available via http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3809903/ (Accessed 22 December 2015). CrossRef
U.S. Census Bureau. USAGov. (n.d.). Available via https://www.usa.gov/federal-agencies/u-s-census-bureau (Accessed 16 May 2022).
van der Heijden AJ, Grose WF, Ambagtsheer JJ, Provoost AP, Wolff ED, Sauer PJ. Glomerular filtration rate in the preterm infant: the relation to gestational and postnatal age. Eur J Pediatr, 1988; 148:24–8. CrossRef
vanLeeuwen R, Lange JM, Hussey EK, Donn KH, Hall ST, Harker AJ, Jonker P, Danner SA. The safety and pharmacokinetics of a reverse transcriptase inhibitor, 3TC, in patients with HIV infection: a phase 1 study. AIDS, 1992; 6:1471–5. CrossRef
Ziswoski J, Krause A, Dingemanse J. Drug development for pediatric populations: regulatory aspects. Pharmaceutics, 2010; 2:364–88. CrossRef