INTRODUCTION
Neurological disorders (NDs) ranked top 50 causes of disability—adjusted life years (DALYs), and it is the most common cause of death worldwide. From the global burden of estimation of disease, the reason for the prominent cause of disability is neurological diseases. Nearly one-third of people in the total population are affected by neurological disorders, and from 1990 to 2019, the death rate has also increased by 39%, and DALYs declined by 15% (Thakur et al., 2016). Neurodegenerative diseases (NDDs) are reported as the most challenging disease and a heterogeneous disorder from all the NDs. This is described by the gradual development in degeneration of the function and structure of a nervous system that can either be in central nervous system (CNS) or peripheral nervous system (PNS), followed by the death of the neuronal cell (Feigin et al., 2020; Kalia and Lang 2016). NDDs are referred to a greater extent as a severe global social health burden with the progression of the diagnosis of certain NDDs, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington disease, and prion disease. The pathology involved in these diseases is similar, which involves abnormal aggregates of amyloid protein that can cause selective damage in the neuron cells. Factors including inflammation, oxidation, and aggregates of proteins in the neurons can be the source of neuronal signal disruption that leads to chronic neurological disorders. The current treatment strategies for NDDs are intended for symptomatic relief, replacing certain neurotransmitters that may or may not affect the curative property in disease progression. The strategies used to manage NDDs are implicated in stopping or slowing the further degeneration of neuron cells using antioxidants, anti-inflammatory agents, and anti-amyloid drugs (Maiti and Dunbar 2018).
Resveratrol (Resv), a stilbene flavonoid (3, 4?, 5-trihydroxystilbene), because of its various exciting pharmacological action, has gained attention in the past few years. The stilbene compounds are referred to as phytoalexin as it is known for their resistance to fungal and microbial infections (Bavaresco et al., 1997). It can be obtained from various sources, including grapes, peanuts, wines, and berries. Stilbene compounds are presently available in larger quantities in injured, UV-treated, and deceased leaves (Langcake and McCarthy, 1979). Resv is a natural substance, and it can be used as a therapeutic compound for several diseases such as Sirtuin 1 (SIRT1) activator (Chung et al., 2010), anti-cancer agent as it directly promotes the apoptotic pathway in three stages of cancer disease by inhibiting the IGF-1R/Akt/Wnt pathway, (PI3K)/Akt pathway (Sarkar et al., 2009; Vanamala et al., 2010), anti-inflammatory agent by inhibiting the production of prostaglandins by inhibiting cyclo-oxygenases enzyme (Kundu et al., 2006), anti-hypertensive agent and prevent atherosclerosis by prohibiting the expression of vascular cell adhesion molecule (VCAM) (Stocker and Keaney, 2004), anti-platelet agent (Pace-Asciak et al., 1995), and also acts as a therapeutic agent for NDDs. Among several therapeutic effects of Resv, the neuroprotective effect of Resv has gained more attention. The Resv can protect the neuron cells by exhibiting several properties like antioxidant effect, antiamyloidogenic, antitauopathy, anti-inflammatory, Aβ (β-amyloid peptide)—clearance, mitochondrial dysfunction diminution. Also, it is involved in the anticancer activity in Glioma tumors (Bellaver et al., 2014; Min et al., 2010; Palle and Neerati, 2018; Vingtdeux et al., 2010).
With the knowledge of the present literature, we focus on the therapeutic mechanism of Resv involved in the treatment and prevention of neurodegenerative disorders like Alzheimer’s and PD. We aimed to offer an extensive overview of development concerning strategies involved in the nano carrier-based delivery of Resv to the brain and highlight its pharmacokinetics.
BIOAVAILABILITY OF RESVERATROL
Resv exhibits two isomeric forms, namely, trans-Resv and cis-Resv. The trans-isomer was reported as more stable compared to the cis-isomer. The better stability of trans-isomer is due to its stability in a light-protected environment for about 42 hours and in pH 1–7 for about 28 days. In contrast, the cis-isomer of Resv was reported stable only when it is completely shielded from light and at pH 7 (Trela and Waterhouse, 1996). The trans-form of Resv shows more biological activity than the cis form, which may be due to the non-planar conformation of the trans-isomer (Fulda, 2010; Rius et al., 2010). Hence, most studies have been performed (both pharmacological and drug delivery studies) using trans-isomer of Resv. To find out the bioavailability of Resv, several in vitro and in vivo studies have clarified the Pharmacokinetics of Resv.
Single dose and repeated doses for the absorption and bioavailability of Resv were reported in the literature. In the single dose of Resv, 25 mg was used, related to the average consumption of red wine. The Cmax was reported to be less than 10 ng/ml after 2 hours of oral administration, and the total plasma concentration of Resv metabolites was markedly high, reported between 400 and 500 ng/ml. From the study, the oral absorption was said to be around 75% based on the assessment of the radio-labeled dose of total Resv metabolites in urine excretion (Goldberg et al., 2003; Walle et al., 2004). The absorption of Resv is remarkably high because of the low solubility in aqueous media, and the consistent result has been reported in the study of absorption in the human intestine using Caco2 cell lines.
The transport of Resv is taken place by trans-epithelial diffusion (Delmas and Lin, 2011). The transport of resveratrol was independent of the time, and the absorption is nonlinear with the extensive formation of metabolites. It is reported that the metabolism of Resv may be the rate-limiting step for the transport of Resv to the systemic circulation. The Resv metabolites can be transported to the systemic circulation by an active transport mechanism (Walle, 2011). The repeated dose study has been done for enhancing the absorption and bioavailability of Resv to improve the efficacy of the treatment. The linearity was reported between plasma concentration and administered dose when the range was 25 to 5,000 mg. Since the high amount of 5,000 mg was administered, the peak level of plasma was reported only approximately 500 ng/ml, which may be due to poor aqueous solubility of Resv. The enhanced bioavailability might be achieved by saturation of metabolism by administering the dose repeatedly (Almeida et al., 2009; Boocock et al., 2007).
Studies have also been carried out using rats to quantify trans-Resv in the tissues, including the brain. After 30 minutes of administering polyphenol intravenously, Glucuronide conjugate increased from 33% to 79%, Resv decreased from 59% to 12%, and sulfate conjugate does not have a stable peak as it might have undergone extensive urinary excretion. Therefore, the author reported that the glucuronide conjugate concentration in plasma shows 4.9-folds more than sulfate conjugate. It implied that the leading metabolic pathway of Resv in the rat was glucuronidation. The trans-Resv extractions from the different tissues were done by the liquid extraction method. From this study, at 90 minutes from the time of administration of trans-Resv 15 mg/kg intravenously, the quantity detected in the kidney and lungs were higher than the tissue extracts of the brain and testes. The amount (Juan et al., 2010) is determined as represented in Table 1.
In contrast, the trans-Resv and its sulfate and glucuronide conjugation were comparatively low in the testes and brain. This may be due to eliminating xenobiotics by using ATP binding cassette (ABC) transporters from various organs (Dauchy et al., 2008). Despite the in vitro study that reported the conjugation of Resv with glucuronide in the brain (Sabolovic et al., 2007), the excretion of the glucuronide conjugate can occur by breast cancer-resistant protein (BCRP) and multidrug resistant protein 2 transporters. In another study, diffusion efficiency and bioavailability of Resv were determined by observing the concentration in the brain by administering it through different routes for CNS tumors. From this study, the systemic application of Resv can only reach the brain when an excessive quantity is administered. These reports suggest that Resv can cross blood–brain barrier (BBB), but the systemic route of administration did not achieve the curative level. They have administered Resv by different routes intraperitoneal, intrathecal, and external carotid artery (ECA) to achieve a therapeutic level of bioavailability in the brain. From the results, the LP route achieved a 5-fold higher drug peak concentration in the entire brain. Also, the brain’s bioavailability achieved by the LP route shows 8.5–38.5 times more than the IP route of administration when the dose was 6% lower than the IP injection. The author suggests that the bioavailability of the drug reaching the intracranial part of the brain was considerably higher in LP than in ECA injection (Shu et al., 2015).
 | Table 1. Quantity of resveratrol in different organs after 30 minutes of Intravenous administration. [Click here to view] |
Phase I clinical trials (Boocock et al., 2007) were conducted with a single dose of oral administration of Resv in the healthy volunteers to ensure safety and efficacy profile. In this study, after oral ingestion of Resv in different amounts of 0.5, 1.0, 2.5, and 5.0 g, the drug appears to be absorbed rapidly into the systemic circulation and achieved plasma peak concentration in 0.83 to 1.5 hours. The plasma peak concentration of the source compound from the administered dose ranges between 73 and 539 ng/ml. The concentration of metabolites increased by 20-folds than that of the parent compound, and the major metabolites were found to be two monoglucuronide conjugate and Resv 3-sulfate conjugate. Resv 3-sulfate conjugate shows higher concentrations between 1,135 and 4,294 ng/ml in all three major metabolites, almost 2-folds higher than monoglucuronide conjugates. In addition, Resv and metabolites have shown rapid excretion. Nearly 77% of the compound and its metabolites are excreted in 4 hours after the administration of 500 mg dose, and the author suggested that the repeated dose could enhance the bioavailability of Resv. Almeida et al. (2009) investigated the safety and pharmacokinetics of oral-multiple-dose regimens of four different doses in healthy volunteers, and the doses were administered every 4 hours. The author has reported that there was no severe adverse reaction throughout the study. The minimum quantity required to show desired pharmacological action was more than 5 µm/l, and after the repeated dose of Resv, the plasma concentration required for therapeutic activity was not achieved. The plasma peak concentration of Resv for four different doses (25, 50, 100, and 150 mg) was 3.89, 7.39, 23.1, and 63.8 ng/ml, and the half-life was improved from 1 to 2 hours followed by single administration to 2–5 hours followed by the repeated dose. The author concluded that the Resv tolerance in in vivo was in the desired range. However, the periodic dosing regimen was low, followed by the plasma concentration even in high amounts of Resv.
Sawda et al. (2017) carried out phase II clinical to study the safety and high dose tolerability of Resv. The author investigated it with randomized 119 participants, double-blind, placebo-controlled in patients with mild-to-moderate AD. The 119 patients were randomized for placebo or Resv (pure synthetic) started with 500 mg once a day orally with dose intensification by 500 mg once in 13 weeks until 2,000 mg was reached per day. From the study of 52 weeks, the oral dose of 2,000 mg of Resv was safe and had good tolerability in the patients with mild-to-moderate AD (Sawda et al., 2017). The bioavailability of resveratrol in the brain using various nanocarriers has been summarized in Table 2.
PHARMACOLOGICAL ASPECTS OF RESVERATROL IN ALZHEIMER’S AND PARKINSON’S DISEASE
As the Resv possesses multiple target strategies, it acquired significant attention. Due to its numerous target strategies, it regulates various pathways related to disease pathology. Furthermore, it plays a role in overcoming the resistance connected to specific drug targets. Since Resv possesses multiple targeting strategies, the interaction has a lower affinity than single-target drugs. Due to its lower affinity than single-target drugs, it has lesser side effects. Resv, a non-flavonoid compound, is well known for its pharmacological properties, including antioxidant, anti-inflammatory, neuroprotective, antiviral, anticancer, and antiphoto aging effects. NDDs are a significant cause of global disease burden with increasing population and average lifespan. Globally, there is an increase in the prevalence of the neurodegenerative disease. Alzheimer’s Association report states that in the United States, about 5.8 million individuals are leading their lives with AD. This disease is reported as the primary neurodegenerative disease, and it is more common among older people. Almost 96% of the total Alzheimer’s population falls above 65 years of age. From the study by the PD foundation, more than 10 million people are affected by PD, and it is reported as the second most common neurodegenerative disease.
 | Table 2. Bioavailability of resveratrol in the brain followed by intranasal administration of nanocarriers. [Click here to view] |
Alzheimer’s is referred to as AD; it is a disease with neurological reasons that ends in the loss of cortical and hippocampal neurons. It can be referred to as the gradual destruction of cognitive functions followed by disinhibition, disorientation, and aphasia. AD is most common in older adults with neurodegeneration, and the disease progression is mainly due to misfolding of tau and β-amyloid peptides (Hardy, 2006). Neuritic plaques and neuro-fibrillary tangles alter the peptides (Wang et al., 2017a). These altered peptides in the brain cause generation of reactive oxygen species (ROS), neuro-inflammations, and neuronal death (Heneka et al., 2015; Wyss-Coray and Rogers, 2012).
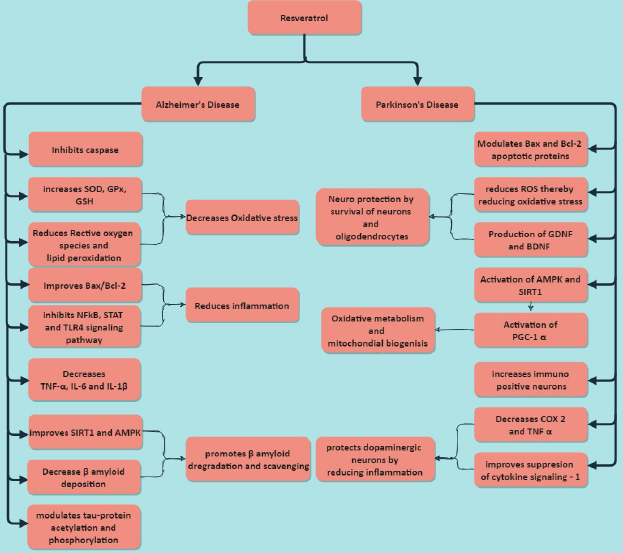
Resv shows its activity in various aspects that helps in the management of disease, including reducing oxidative stress by ROS prohibition, reducing glutathione levels, and elevating enzymes such as glutathione and superoxide dismutase. The decline in oxidative stress can also be claimed by decreased lipid peroxide. Resv involves in the management of neuro-inflammation by reducing inflammatory cytokines including IL6, IL-1β, and TNF-α. Also, the inhibition of microglial signaling by the transcription factor (NF-kB) manages the neuro-inflammation by decreasing the formation of enzymes such as COX-2, NO-synthase (Kim et al., 2006). Resv increases anti-apoptotic Bcl-2 and decreases the expression of pro-apoptotic protein Bax. In general, the Bcl-2 proteins family has contradictory functions in apoptosis regulation. The regulation of cell death or survival can be determined by interpretation of the relative ratio of antiapoptotic and pro-apoptotic protein. Resv plays a role in biological aging by activating the SIRT1 enzyme that prevents oxidative stress and cell apoptosis, thereby acting as an antiaging agent. Resv also has a pharmacological activity on other NDDs because of its well-known motor and cognition-based enhancements along with its antioxidant, anti-inflammatory, and antiapoptotic properties. This polyphenol eradicating the root cause of disease induces scavenging and degradation of accumulated and aggregated amyloid-β. Several studies reported that Resv could prevent misfolding of tau protein and showed reduced tau-protein levels in rat models (Al-Bishri et al., 2017).
Additionally, it modulates hyper-phosphorylation and inhibits aggregation (He et al., 2017; Pasinetti et al., 2014). Another study also concluded that Resv could protect tau protein’s misfolding by stimulating de-phosphorylation that relates to inhibiting CaMKII, GSK-3β, and activating PP2A (phosphatase-2 protein) (Jhang et al., 2017). The modulation in the misfolding of proteins has been reported in the phase-2 clinical trial that there is a suppression of amyloid-β levels in both plasma and cerebrospinal fluid (CSF).
PD is the second greatest common neurological movement disorder that affects almost 1% of the global population over 65 years of age (de rijk et al., 1997). It can be distinguished by rigidity, bradykinesia, and tremor. The primary pathology of the disease is characterized by a particular loss of dopaminergic neurons and the existence of Lewy bodies. Resv has the ability against the primary pathology of disease due to its cytoprotective activity. Resv can protect neurons by activating AMP activated protein kinase (AMPK) and SIRT1 which regulates the clearance of damaged mitochondria or misfolded proteins. On the other side, SIRT1 and AMPK are activators of the gene PGC-1α that manage oxidative stress and enable the biogenesis of mitochondria (Ferretta et al., 2014; Wu et al., 2011). Resv promotes the survival of neuron cells by regulating apoptotic and pro-apoptotic proteins such as Bax and Bcl-2 that release neurotrophic factors including Brain, glial-cell, and astroglia-derived neurotrophic factors (Brain derived Neurotrophic factor, Glial cell derived neurotrophic factor, and Astroglia derived neurotrophic factor) on dopamine neurons (Bournival et al., 2009; Zhang et al., 2012). Furthermore, Resv is also involved in managing secondary factors, including oxidative stress by restoring antioxidant defense, and dopamine in the striatal region was indicated by normalizing denervated neurons (Khan et al., 2010). The neuro-inflammation and pro-inflammatory cytokines play an essential role in the formation and progression of PD. It is regulated by reducing the levels of TNF-α, IL-6, IL-1β (inflammatory cytokines), and COX-2 levels (Degan et al., 2018; Jin et al., 2008; Lofrumento et al., 2014). Therefore, Resv holds immense ability to manage Parkinson’s and AD (Fig. 1).
NANOTECHNOLOGY-BASED STRATEGY FOR RESVERATROL DELIVERY IN NEUROLOGICAL DISORDERS
Resv has an aqueous solubility of 50 µg/ml, and it has good solubility in organic-based solvents such as Dimethyl sulfoxide, dimethylformamide, and ethanol, and it has PKa value of 11.4, 9.8, and 8.8 (López-Nicolás and García-Carmona, 2008; Robinson et al., 2015). It has a partition coefficient of 3.0. The chemical structure of Resv has been illustrated in Figure 2. The rate of transfer of mass across the biological membrane depends upon the aqueous solubility of the compound. Based on the dissolution of the compound and its absorption, the drug can be categorized in the Biopharmaceutical Classification System (BCS) classification. Because of the poor aqueous solubility and high absorption of Resv, it is classified under Class II BCS classification. The limited bioavailability of the drug is due to its poor solubility (Amidon et al., 1995; Amri et al., 2012; Das et al., 2008), and thereby, its clinical uses are restricted (Chan and Stewart, 1996; Hurst et al., 2007). Handling the various technologies and techniques can improve the bioavailability of Class II BCS drugs (Krishnaiah, 2010; Kumar et al., 2013). Administration of drugs in nanocarrier using surfactant can enhance the solubility (Aliabadi and Lavasanifar, 2006; Rangel-Yagui et al., 2005; Vinarov et al., 2018). Also, the simple techniques including micronization, co-solvency, precipitation, or evaporation techniques can be used for enhancing the solubility of a poorly soluble drug (Buckley et al., 2013; Kalepu and Nekkanti, 2015; Savjani et al., 2012; van Hoogevest et al., 2011). Noticeably, the utilization of compounds in nanotechnology has offered an essential tool for enhancing solubility and bioavailability of drugs and its capability to targeting the drug to the site of action, including the brain. Also, the drug administered in nanotechnology can minimize the dose-dependent adverse and toxic reaction (Farokhzad and Langer, 2009; Fonseca-Santos et al., 2015; Park, 2007, 2013). Resv incorporated in nano carrier-based formulations (Jung et al., 2015; Lu et al., 2009; Pangeni et al., 2014; Siddiqui et al., 2015; Summerlin et al., 2015) showed enhanced solubility and biological effects, including anti-inflammation, antioxidant, neuroprotective, anti-viral, anti-cancer, and anti-photoaging effects (Alarcón De La Lastra and Villegas, 2007; Chan et al., 2015; Csiszar, 2011; Fujimura et al., 2016; Quadros Gomes et al., 2018; Varoni et al., 2016). Additionally, Resv has a beneficial effect on NDDs (Andrade et al., 2018; Bastianetto et al., 2015; Farooqui, 2012; Singh et al., 2013).
 | Figure 1. Mechanism of action of resveratrol in AD and PD. [Click here to view] |
 | Figure 2. Chemical structure of resveratrol. [Click here to view] |
For the prevention of foreign molecules entering, the human body is made up of biological barriers. The barrier between the CNS and PNS is considered a vital element. The blood–brain barrier (BBB) and the CSF play a crucial role in the protection of the brain from the numerous foreign molecules, including pathogens, and protect its unique nature (Engelhardt and Liebner, 2014). The BBB acts as a junction between CNS tissues and systemic circulation. Hence, it limits the foreign molecule (including therapeutic drugs) from entering the brain and promotes the passage of essential hormones and nutrients (Patel and Frey, 2015). So, nearly all the drugs with high molecular weight and most of those with small molecular weight cannot enter CNS followed by systemic or oral administration (Pardridge, 2016). Still, there are two fundamental mechanisms through which therapeutic substance nter into the CNS, viz., Active and passive transport through endothelial cells of BBB. But these cells make it impossible for passive transport of therapeutic molecules by tight and rigid junctions (Rankovic, 2014).
The BBB is composed of endothelial cells linked with pericytes and astrocytes, and it acts as a barrier that is responsible for the prevention of almost many drugs, large molecules, and peptides to enter the brain (Abbott et al., 2006; Maussang et al., 2016; Pardridge, 2005). Several layouts other than pericytes and astrocytes are also responsible the for impermeability of BBB which is preventing the entry of infectious substance and toxic compounds to the brain including tight junction, high expression of proteins which has a role in efflux transport like multidrug-resistance protein-1, P-glycoprotein (P-gp), degrading enzymes, and BCRP (Abbott et al., 2010; Gabathuler, 2010). Despite its biological activity, it is considered a significant barrier in managing successful neurological disorders. It is expected that the novel approach and the novel formulations could enhance the penetration of drug molecules through BBB in brain delivery.
The strategy for new formulations is to attain effective treatment for patients with minimal side effects, managing pharmacokinetic profile accuracy, drug release control, and delivering the drug to a specifically targeted cell (Anselmo and Mitragotri, 2014; Bae and Park, 2011; Dong, 2018; Tiwari et al., 2012). Formulation based on nanotechnology systems with particle size 1–1,000 nm was used to treat various diseases (Park, 2007) comprising diabetes, cancer, cardiovascular disease, microbial, and inflammatory diseases (Patra et al., 2018). These nanotechnology systems exhibit reduced toxicity and adverse effects with economical price and better therapeutic value (Jain et al., 2015). It is comprised of various biodegradable ingredients that include lipids, synthetic or natural polymers, and metals. These biodegradable materials are called nanocarriers (Khodabandehloo et al., 2016; Suri et al., 2007). The various nanocarriers and their formulation strategy in the development of Resv nanocarriers are given in Figure 3.
Nanocarriers
Brain delivery of the drugs can be done in three ways, viz., intracerebroventricular (ICV) injection, oral, or intravenous (IV) injection, and Intranasal administration. Intravenous injection and oral administration are the primary routes that face challenges in targeting the brain due to BBB, leading to low bioavailability and low effectiveness (Agrawal et al., 2017). To overcome these bioavailability drawbacks, a novel tool (nanotechnology-based strategy) has been developed as a favorable carrier in the treatment of neurological disorders to transport the drug across BBB (Goldsmith et al., 2014; Kreuter, 2014; Patel et al., 2012; Srikanth and Kessler, 2012). CNS permeability can be improved by using surface-modified nanocarriers to bypass BBB through receptor-mediated endocytosis mechanism (Masserini, 2013; Wong et al., 2012). Hence, the application of nanocarriers in delivering resveratrol to the brain has significant importance. Here, we included an overview of several nanocarriers for the resveratrol delivery to the brain in managing Alzheimer’s and PD.
 | Figure 3. Formulation Strategies of Resveratrol to target the Brain [Click here to view] |
Liposomes
Liposomes are spherical vesicles comprised a phospholipid bilayer that has both hydrophilic and lipophilic properties. Liposomes are different in lamellarity and size that depends on the manufacturing procedures and phospholipids composition (Wang et al., 2017b). The delivery systems are known for successful brain delivery of specific proteins and growth factors (Rabanel et al., 2015). Liposomes have specific properties like delivering both lipophilic and hydrophilic drug candidates with less toxicity and biocompatibility (Barbara et al., 2017). Even though liposomes have lipophilic nature that helps in the transportation through biomembrane, the BBB stays as a primary obstacle that precludes the penetration of the molecule from entering the brain. These challenges can be overcome by using surface-modified liposomes to facilitate brain delivery through BBB by receptor-mediated endocytosis mechanism. Wang et al. (2018a and 2018b) carried out a study on the formulation and characterization of modified (magnetic) liposomes for enhancing the bioavailability of Resv in the brain. The author had used ferrous sulfate and ferrous chloride by co-precipitation method to formulate modified liposomes followed by the fabrication of resveratrol forming Fe3O4 modified resveratrol liposome. The results showed that slow and sustained release was observed in in vitro studies. Apart from this, magnetic oxide nano formulation owns enhanced Resv capacity and stability. The in vivo result revealed that the magnetic oxide Resv liposome crossed the BBB, followed by the improved drug availability in the brain using an external magnetic field. Hence, the author suggested that this formulation offers a promising platform for treating PD.
Wang et al. (2018a) formulated Resv loaded liposomes by using lecithin and cholesterol. It has been tested for its efficiency in rats, and it was reported that Resv liposomes exhibit way more efficiency than free Resv. Another author had formulated polymeric nanoparticles using polysorbate 80, dichloromethane, polyvinyl alcohol, and polylactide. The Resv loaded polymeric nanoparticle and free trans Resv were tested in mice against 1-methyl 4-phenyl- 1,2,3,6- tetrahydro pyridine (MPTP). The Resv loaded polylactide nanoparticles coated with tween 80 showed the promising effect to transport to the brain and in the management of PD (Wang et al., 2011). Based on the results, the liposome-based delivery would be more effective in treating NDDs. Upon oral or intravenous administration, it may have some challenges like rapid elimination, accumulation in liver and spleen, or may undergo degradation by gastrointestinal enzymes. Moreover, liposome-based formulations face challenges in mass manufacturing, sterilization techniques, and it has less storage stability (Liu et al., 2015; Noble et al., 2014).
Polymeric nanoparticle
Nanoparticle-based effective delivery of the drugs to the brain-related disorder provides alternative possibilities for enhanced brain bioavailability. It is a promising approach to overcome the challenges in delivering drugs for treating CNS diseases. These colloidal systems can encapsulate the drugs in both liquid and solid-state. This polymer-based nanoparticulate delivery provides various advantages include biocompatibility, improved absorption, low toxicity, and targeted delivery (Naahidi et al., 2013). Primary advantages for delivering the drug to the brain in the polymeric nanoparticle are rapid biodegradability and biocompatibility of the polymer used in the nanoparticle.
Lindner et al. (2015) carried out a study investigating the neuroprotective effect of poly (lactide) polysorbate 80 (PLA-PS80) coated Resv nanoparticle in mice models. They used a single emulsion solvent evaporation method to formulate nanoparticles using polysorbate 80 and polyvinyl alcohol. The PLA-PS80 nanoparticle has been used in MPTP-induced PD in mice. They have tested for both biochemical and behavioral changes and reported enhanced activity of resveratrol in the nanoparticulate system as it increases the brain bioavailability. The effectiveness of free Resv in protecting the neuron is limited from MPTP-induced PD as its pharmacokinetics is not favorable for brain delivery. The surface patterns of the nanoparticle can direct the nanoparticle distribution to the brain (Gelperina et al., 2010). Based on the knowledge, the fact shows that the PS80 coated nanoparticle improves brain bioavailability through receptor-mediated endocytosis mechanism followed by the P-gp inhibition (Göppert and Müller, 2005; Wohlfart et al., 2012). In BBB, the proteins associated with the low-density lipoprotein receptor are expressed (LRP1 & 2). The PS80 coating from the nanoparticle adsorbs the apolipoprotein from the blood that mimics the low-density lipoprotein receptor activity in BBB (Shamenkov et al., 2002). P-gp in the BBB limits the passing of exogenic molecules to the brain, and it is extensively available at BBB. The PS80 coated nanoparticle inhibits the P-gp efflux (Kreuter, 2005). These mechanisms can enhance the bioavailability in the brain by acting simultaneously. So, this study with PLA-PS80 enhances the neuroprotective effect of Resv by enhancing the Resv concentration in the brain. The polymeric nanoparticle may undergo prompt clearance from the systemic circulation due to the interaction among the reticuloendothelial system (RES) and the nanocarrier.
Solid lipid nanoparticle (SLN)
The use of lipid nanoparticles suggests an alternate strategy in treating CNS disorders for brain delivery as lipid nanoparticles prolong the elimination by escaping from the interaction with the RES. The mechanism associated with the transport of lipid nanoparticles to the brain would be by receptor-mediated endocytosis (Shah et al., 2015; Wen et al., 2017). The dispersion of spherical solid-state lipid particles in water or aqueous surface-active agents is referred to as SLN (Kaur et al., 2008). These nanoparticles can cross BBB because of their lipophilic property. Loureiro et al. (2017) had formulated and evaluated antibody functionalized solid lipid nanoparticles to overcome the challenges from rapid metabolism, elimination, and to improve the permeability of Resv to the brain. They have worked on the formulation of solid lipid nanoparticles encapsulated with Resv by high shear homogenization and ultrasonication method; they used 1, 2-distearoyl-sn-glycero-3-phosphoethanolamine-poly(ethylene glycol) (DSPE-PEG) and LissRhod-PE for the formulation. SLN assured its stability by size, Zeta Potential (ZP), polydispersity index (PDI), and surface morphology with an average particle size—176 nm and 94% of EE (encapsulation efficiency). To improve brain bioavailability, SLN was conjugated with an antibody (OX-26). The brain targeting efficiency of SLN was tested in in vitro model and reported that antibody functionalized SLN showed 4-fold enhanced transportation to the brain compared to SLN (Loureiro et al., 2017). The author has tested the formulation on endothelial cells that are similar to the human brain. This cellular model permits the prediction of drug permeability across BBB to the brain.
The result suggests that the OX-26 antibody functionalized enhances the SLN permeability through BBB than LB 509 antibody functionalized SLN and non-functionalized SLN. Another study has been carried out by Neves et al. (2016). They developed apolipoprotein functionalized Resv based SLN to enhance the brain bioavailability of Resv by the high shear homogenization technique. The permeability was studied in the cell monolayers of hCMEC/D3 by using free Resv and functionalized SLN. The functionalized SLN showed 1.8 times higher penetration than free Resv. This enhanced permeation may be due to the receptor-mediated endocytosis mechanism in BBB.
Polymeric micelle
Lu et al. (2009) formulated Resv loaded polymeric micelle to defend against oxidative stress induced by Aβ. The formulation was developed using Polycaprolactone as block copolymer for the hydrophobic core and PEG as hydrophilic shell, followed by lyophilization and drying. The size of the micelles was reported as less than 100 nm with a uniform spherical shape. The encapsulation efficiency was reported as having a high value of 89%. The polymeric micelles of Resv and free Resv were tested in PC12 cell lines for short-term effect, long-term effect, and 2 hours of pre-treatment for the protection against Aβ. The free Resv was effective and did not report any cytotoxicity in the short-term study, even at a higher concentration of 50 µm. Still, in the long-term study (48 hours), Resv has shown cytotoxicity at the concentration of 5–10 µm to PC12 cells. Also, in pre-treatment for 2 hours, Resv failed to protect the cells against Aβ. The same was treated with Resv loaded polymeric micelles. It was reported that it was protective, nontoxic, and positive against the activity of caspase 3 and oxidative stress against Aβ (Lu et al., 2009). Improved brain uptake of resveratrol-loaded polymeric micelles to the brain through intracellular delivery to treating NDDs offers an effective delivery based on the evidence presented in this segment. Additional study is required to validate this delivery system.
Nanocrystals
Xiong et al. (2020) had formulated the Resv nanocrystals to improve the bioavailability of Resv in the brain by the antisolvent precipitation method. The Resv nanocrystals were administered orally in the rats and showed better absorption than free Resv with an enhanced concentration in the brain and plasma. From the pharmacokinetic study in the rats, the maximum concentration of Resv (2.61 ± 0.21 µg/ml) was attained at 4 hours after oral administration of Resv nanocrystals (4 mg/kg). The brain bioavailability of Resv may be achieved by absorption into systemic circulation followed by the penetration through BBB to the brain (Chen et al., 2016). The hydroxypropyl methylcellulose in the formulation enhances the Resv nanocrystal penetration to the brain. Additionally, the elimination half-life of Resv nanocrystals was delayed in the brain compared to the plasma elimination half-life, which shows that the elimination of Resv in the plasma is more than that of the brain (Xiong et al., 2020).
Lipid core nanocapsules
Resv loaded- lipid core nanocapsule (LCNC) had formulated by Frozza et al. (2010) to enhance the bioavailability in the brain. LCNC was formulated by interfacial deposition of the polymer using poly (ε-caprolactone), capric triglyceride as a lipid, tween 80 as a surfactant, and span 60 as a stabilizer. The nanocapsules loaded with Resv tested their stability by performing particle size, ZP, and PDI. The Resv loaded LCNC administered in animals reported 6.6-, 3.4-, and 2.5-times higher liver, kidney, and brain concentrations, respectively, than those treated with free trans-Resv. The results from the in vivo study show that the Resv loaded LCNC could cross BBB to improve the concentration in the brain (Frozza et al., 2010). The other research has been reported in Frozza et al. (2013a) by using Resv loaded LCNC on Aβ-induced neuronal inflammation in hippocampal organotypic culture. They underwent both co-treatment and pre-treatment with Resv loaded LCNC for testing against reactive oxygen species and Aβ-induced cell death. The result revealed that pre-and co-treatment Resv loaded with LCNC can reduce Aβ triggered neuronal inflammation in hippocampal culture. Despite its pharmacological action, the free trans Resv showed efficient results only in higher concentrations, while Resv loaded LCNC showed significant neuronal protection in a lower concentration. Also, a higher antioxidant effect was reported by Resv loaded LCNC than free trans Resv. The author concluded in such a way that Resv-loaded LCNC with free Res could be a promising strategy in the prevention or delay of Aβ triggered neuronal inflammation (Frozza et al., 2013b). This study has been extended for testing the efficiency of Resv loaded LCNC against ICV (intra-Cerebro ventricular) injection in rats with Aβ1-42 induced memory dysfunction. The free trans Resv protects the rats from Aβ induced disturbances in cell signaling, microglial and astrocyte activation, and behavioral impairments in in vivo. The author has used Resv loaded LCNC to observe the brain’s concentration. The in vivo study reported that this formulation enhances the biodistribution of Resv in the brain (Frozza et al., 2013).
Even though various routes like oral, IV, and IP are available to administer the drug-loaded nanocarriers to the brain, intranasal delivery plays a prominent role compared to the routes mentioned earlier because they can directly reach the target region produce the desired activity. For this purpose, we have emphasized the intranasal delivery of resveratrol for the management of AD and PD predominantly. The summary of the nanocarriers used in the formulation of Resv to manage AD and PD was given in Table 3.
Nasal delivery
The intranasal route of administration presents a promising and non-invasive approach for drug delivery directly to CNS through the olfactory region by bypassing BBB and CSFB (100,101). The nasal route can deliver the drug at a faster rate, and high absorption of the drug in the brain can be achieved with better patient compliance (Al Bakri et al., 2018; Johnson and Quay, 2005; Ozsoy and Gngör, 2011), but it has some limitations including rapid mucociliary clearance followed by less absorption through nasal epithelium (Marttin et al., 1998; Meredith et al., 2015). Several drug delivery systems include mucoadhesive systems or nanoscale formulations (Chaturvedi et al., 2011; Jiang et al., 2010; Mistry et al., 2009; Ugwoke et al., 2005) and chemical-based penetration enhancers present strategies that could be applied for overcoming these drawbacks for enhancing nasal absorption (Illum, 2003; Mittal et al., 2014; Sood et al., 2014). For the respiratory system, the interior structure of the nose or nasal cavity is the access point for the air inhalation and, by that, it builds up the front structure. The nose cavity is branched into binary with a septum in which the mucosal layer completely covers the cavity that provides immediate shelter from allergic and infectious pathogens to the body (Harkema et al., 2006).
 | Table 3. Resveratrol loaded nanocarriers in the management if AD and PD. [Click here to view] |
In addition, the nasal cavity is split into three sections, viz., the nasal vestibule (wider cavity of the nostril), respiratory section (passage for inhaled air), and olfactory section (having olfactory receptors) (Wang et al., 2018b). For the sense of smell, the receptors in the olfactory region are responsible. It has the olfactory neuron cells instead of having tiny cilia like another part of the nasal mucosa (Xi et al., 2015). The neuronal cells emerge from the olfactory bulb in the nasal cavity and terminate in the neuroepithelium. This neuroepithelium is the only area of CNS that has direct contact with the external environment with the unique characteristics of the regeneration potential. Therefore, this site of the olfactory region is suitable for the utilization of drugs to target CNS. Only the matters/particles with <200 nm have the favorable circumstances to transport through the olfactory neurons (Ahmad et al., 2017). The fate of nanocarriers can be influenced by the size of the particle and the carrier (composition) (Samaridou and Alonso, 2018), followed by the administration through intranasal delivery. Apart from the olfactory neurons, the mucosa in the nasal cavity has a supply of maxillary and trigeminal nerves. These nerves provide various sensations to the rest of the areas in the face. Still, the mechanism of nasal absorption to the CNS is not entirely known. Based upon the research carried out, there are two possible mechanisms of absorption of therapeutic agents. First, transport via neuronal path (olfactory or trigeminal neurons), and the second way comprises a lymphatic system and CSF (Mittal et al., 2014). The transport of therapeutic agents from the nasal cavity to the CNS either occurs by one or combined mechanisms. The olfactory neuronal pathway is considered as the primary mechanism of intranasal absorption of drugs to the CNS. Crossing the olfactory epithelium is the prerequisite for drug absorption by the olfactory nerves (Agrawal et al., 2018).
This transport of drugs is possible in three ways; para-cellular pathway, passive diffusion, and endocytosis mechanism by neurons. The hydrophilic drugs are transported by the para-cellular path, whereas passive diffusion is responsible for the absorption of lipophilic drugs. The molecular weight and hydrophobicity of the drug automatically alter the mechanism mentioned above (Bhise et al., 2008). Trigeminal nerves regulate sensory reports from the oral cavity, nasal cavity, and cornea. These trigeminal nerves innervate one end in the olfactory epithelium. The other end reached two different brain sites, viz., cerebrum, and near the pons, along with the frontal brain and the olfactory bulb to a lesser extent (Crowe et al., 2018). Accordingly, the trigeminal nerve pathway can also be the promising drug delivery pathway to the brain. Thorne et al. (2004) showed that a significant quantity of insulin-like growth factor-I was reached the brain via the trigeminal path. From the CSF of the brain in subarachnoid space, the lymphatic and CSF pathways are connected to the lymphatic system (nasal) into the perineural space through olfactory nerves. Yu et al. (2017) concluded that in the delivery of Evans blue to the brain, the lymphatic system acts as the bridge between nasal mucosa and mystacial pad. Johnston et al. (2004) showed evidence of radioactive substances in the cervical lymph node and nasal lymphatic system through the olfactory nerve channels injected into the CNS. Possibly, these pathways can help in the transportation of drugs from the nasal cavity to the CSF, and the perivascular area can distribute the drugs to other parts of the brain. Molecular weight, degree of ionization, and lipophilicity of the therapeutic molecule may influence the transportation and distribution of drugs into the CSF (Nau et al., 2010). Better distribution of the drug can be achieved by higher value lipophilicity. The individual input of these pathways to understand the transport of drugs from the nasal cavity to the brain is challenging. Still, the different studies with the radio-labeled molecule can support the perception of drug transport pathways from nose to brain. Although the drug enters the systemic circulation by oral or various route of administration, the drug should enter the brain by crossing BBB if not, there will be no pharmacological action that required for the management of disease (Gomes et al., 2016). The BBB has the transporters that allow the neuro-transmitters, nutrients, macromolecules, and amino acids to enter BBB by passing through endothelium (Abbott et al., 2010; Chen et al., 2004). The transport of drug through BBB can be achieved by triggering the transporter with functionalized nanocarriers with specific ligand-target for respective binding transporters (Abbott et al., 2010; Chen et al., 2004; Fonseca-Santos et al., 2015; Moura et al., 2019). The internalization of the drug-loaded nanocarrier can be achieved once the transporter identifies its ligand by promoting conformational change (Tamai and Tsuji, 2000). In the case of CNS disease, these transporters are either upregulated or downregulated. In AD, there is an upregulation of scavenger receptors (Eugenín et al., 2016; Wilkinson and El Khoury, 2012) and downregulation of low-density lipoprotein transporter (Helbecque and Amouyel, 2000) and glucose transporter (Gejl et al., 2017). In PD, there is an upregulation of glucose receptors (Aviles-Olmos et al., 2013). Functionalized nanocarriers and their targeting transporters showed promising results to target the brain (Moura et al., 2019).
In a study based on the nanostructured lipid carrier (NLC) in situ gel of Resv formulated by melt-emulsification probe sonication method, the author used Cetyl palmitate and capmul mono-diglyceride of medium chain fatty acids (MCM) (Solid lipid and oil) in a 1:1 ratio, using Acrysol K150 as a solubilizer and Poloxamer 188 and Tween 80 as the surfactant, reported the homogenous size with PDI less than 0.33 with singlet peak. The intranasal route was selected for administering for the management of AD, for which it resulted in five times enhanced permeation than the suspension form. The NLC based in situ gel showed significant progress in the memory function of rats from the study- amnesia induced model using scopolamine (Morris-water Maze’s test) (Rajput et al., 2018). This shows the effective use of nanocarriers for brain delivery.
Pangeni et al. (2014) has planned to formulate the Resv loaded d- α-tocopherol (vitamin E) Nanoemulsion (NE) to manage PD to brain delivery. They used the Spontaneous emulsification method to formulate the kinetically stabilized product. Nanoemulsion followed high-pressure homogenization; they used vitamin E and Sefsol in a 1:1 ratio as oil phase in o/w type nanoemulsion, Tween 80 and transcutol P as surfactant and co-surfactant, respectively. The PDI and ZP values confirmed nanoemulsion’s uniformity and stability, and its insignificant variation ensures a longer shelf life. The percentage of the cumulative release of drug for the post homogenized NE reported 1.5-folds higher release of the drug than pre-homogenized NE. A significant quantity of drugs had reached the brain through intranasal administration (non-invasive method). It enhances the brain bioavailability of NE due to the highly permeable and vascularized nasal area (Pardeshi et al., 2013; Pardeshi et al., 2013). It also enhanced the drug absorption facilitated by fewer enzymes and a significant surface area of about 150 cm2. In addition to that, intranasal delivery is a substitute approach that efficiently bypasses the first-pass metabolism and BBB (Haque et al., 2012; Mittal et al., 2014). Histopathological study revealed that there was a reduced degeneration in the animals that received Resv NE. In the Resv NE treated animal group, there are considerably higher levels of Glutathione and Superoxide dismutase, and there was a decreased level of malonaldehyde.
Another author had developed Resv and curcumin-based mucoadhesive lipidic hyaluronic acid nanoemulsion to manage the neurodegenerative disease through the intranasal route to the brain. NE was formulated by spontaneous emulsification method using Labrafac lipophile, Labrafac PG as oil phase, and Cremophor 40, tween 80 as surfactants. PDI and ZP ensured the formulation’s spherical morphology and stability. Based on the ZP and PDI value, the formulation was selected for hyaluronic acid encapsulation, conserving the antioxidant property and protecting it from deterioration. From the in vitro study results, nearly 60% of the drugs were released from the nanoemulsion by a diffusion-controlled release mechanism. Also, the ex vivo study confirmed that 2.86 mg/cm of Resv had been released in the nasal mucosa of the sheep.
 | Table 4. Intranasal delivery of resveratrol in managing AD and PD. [Click here to view] |
Furthermore, the formulated Mucoadhesive NE was reported to be safe on rat nasal tissues. In the in vivo study, the Resv aqueous solution showed limitation in bioavailability as it was influenced by BCRP-efflux pump (Scheepens et al., 2010). The pharmacokinetic analysis of the mucoadhesive formulation of Resv reveals that the brain bioavailability of Resv increased by seven folds (1,937.9 ng/ml). This study proposes a safe and effective method for brain targeting delivery. Still, further work is required in the future for clinical application (Nasr, 2016).
Hao et al. (2016) formulated the Resv nanosuspension (RES-NS) based in situ gel for intranasal delivery to target the brain for CNS-related disorders. RES-NS was prepared by the antisolvent precipitation method using ethanol as solvent followed by lyophilization using mannitol as cryoprotectant. In the formulation of in situ gel deacetylated gellan gum (DGG) is used as an ion-sensitive polymer, glycerin as an isotonicity agent, and benzalkonium chloride as a preservative. The ZP and PDI assure the morphology and sensitivity of the formulation. 0.6% ion-sensitive polymer DGG showed promising gelling capability and suitable viscosity. A pharmacokinetic study revealed that the RES-NS in situ gel increased 2.88 times compared to Resv suspension in situ gel. DTE and DTP The concentration of Resv in the brain followed by intranasal delivery administration was 78.18% and 458.2% of DTE and DTP, respectively. The study shows that the Resv nanosuspension in situ gel can increase the resident nasal time and decrease nasal clearance (Hao et al., 2016).
Ahirrao and Shrotriya (2017) aimed to improve brain bioavailability by formulating Resv-cubosomes administered by the intranasal route. The cubosomes were prepared by the probe-sonication method using Lutrol F127 and glycerol monooleate. Here, lutrol F127 was used as a gelling agent and glycerol monooleate as monoglycerides. The optimized cubosomes assure its stability by particle size and PDI. The better entrapment efficiency was reported as 83.08% with ZP of -20.9mV, and 67% of the drug was released within 24h in the in vitro study. From the in vivo study, the intranasally (i.n) administered cubosomal in situ gel reported the higher concentration of Resv in the brain (564.49 ± 2.24 ng/ml) than drug solution administered intranasally (133.7 ±4.1 ng/ml) and drug solution administered orally (102.2 ± 9.8) and the author had concluded in a way that the cubosomal in situ formulation could be the better possible way to administer Resv to the brain intranasally (Ahirrao and Shrotriya, 2017).
Moreover, the novel strategy to deliver the Resv aerosol to the brain through intranasal delivery may offer a promising way to direct nose to brain transport. Aerosol inhalation offers the deposition of the drug on the nasal mucosa and the olfactory epithelium, followed by transportation to the olfactory region in the brain through the trigeminal or olfactory pathway (Khan et al., 2017). The study from another polyphenol reported that the aerosol formulation through the intranasal route enhanced the distribution of drugs in the brain than intravenous administration of the polyphenol (McClure et al., 2015). From this proof, intranasal delivery can be an efficient strategy to target the brain by bypassing the BBB that may offer better development in treating NDDs. Even though intranasal delivery offers promising benefits in brain delivery, there are some limitations including, mucociliary clearance, nasal toxicity, and drug degradation. These challenges can be overcome by administering a drug in nano formulation with appropriate delivery devices (Khan et al., 2017).The nanocarriers used in the formulation of Resv in managing AD and PD through the intranasal route were summarized in Table 4.
CONCLUSION
Despite having the drugs used in managing PD and AD, neither of them had reported reducing or preventing the neurodegeneration, which can only be used as a palliative treatment. Resveratrol is reported as a harmless phytochemical compound that has the potential efficacy in managing AD and PD. Resv reported various pharmacological effects in different diseases, but its bioavailability is limited due to its extensive formation of metabolites. The drug candidate used to treat CNS diseases should enter the brain by passing through BBB to exert its therapeutic effect. Resv can cross BBB, but its bioavailability is limited in low doses due to its metabolite formation with glucuronide and sulfate. The intranasal delivery of drugs for the administration to the brain is of growing interest. Suppose the Resv is administered in the solution form in the intranasal route, it will be eliminated from the nasal region due to its mucociliary clearance, resulting in low bioavailability in the brain. The in situ-based formulation delivers the Rev using nanocarriers. The gellan polysaccharides used in the in situ gel formulation are converted into gels by rapid gelation with cations present in the mucus that increase the resident nasal time of Resv. It favors the nano-carrier-based intranasal delivery.
The lipid-based nanosystem has reported pharmacological activity in NDDs. Since Resv is a hydrophobic candidate, it has a better ability to solubilize lipids that improve the bioavailability in the brain. Conversely, the physicochemical properties of Resv have not been established in preformulation studies, including the stability against enzyme, pH, and light. Also, when Resv is exposed to light, it can be transformed into cis-isomer from trans isomer of Resv in which cis-Resv has low pharmacological action compared to trans-Resv. The advantage of using the nanotechnology-based delivery system is that it protects the Resv against enzymes, light, and pH. Still, there was insufficient information regarding the capability of improving stability. However, many studies reported its pharmacological activity in cell lines. These animal models showed promising results with a nanotechnology-based delivery system, and there is a lack of reports in understanding the mechanism of action with the delivery systems. In the future, we believe that innovative technologies can improve the potential ability of Resv in managing NDDs.
ACKNOWLEDGMENT
The authors would like to thank the Department of Science and Technology-Fund for the improvement of Science and Technology Infrastructure in Universities and Higher Educational Institutions, New Delhi, for their infrastructure support to our department.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation
REFERENCES
Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci, 2006; 7(1):41–53. CrossRef
Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis [Internet], 2010; 37(1):13–25; doi:10.1016/j.nbd.2009.07.030 CrossRef
Agrawal M, Ajazuddin, Tripathi DK, Saraf S, Saraf S, Antimisiaris SG, Mourtas S, Hammarlund-Udenaes M, Alexander A. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. J Control Release [Internet], 2017; 260(April):61–77; doi:10.1016/j.jconrel.2017.05.019 CrossRef
Agrawal M, Saraf S, Saraf S, Antimisiaris SG, Chougule MB, Shoyele SA, Alexander A. Nose-to-brain drug delivery: an update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J Control Release [Internet], 2018; 281(April):139–77; doi:10.1016/j.jconrel.2018.05.011 CrossRef
Ahirrao M, Shrotriya S. In vitro and in vivo evaluation of cubosomal in situ nasal gel containing resveratrol for brain targeting. Drug Dev Ind Pharm [Internet], 2017; 43(10):1686–93; doi:10.1080/03639045.2017.1338721 CrossRef
Ahmad E, Feng Y, Qi J, Fan W, Ma Y, He H, Xia F, Dong X, Zhao W, Lu Y, Wu W. Evidence of nose-to-brain delivery of nanoemulsions: cargoes but not vehicles. Nanoscale, 2017; 9(3):1174–83. CrossRef
Al-Bishri WM, Hamza AH, Farran SK. Resveratrol treatment attenuates amyloid beta, tau protein and markers of oxidative stress, and inflammation in Alzheimer’s disease rat model. Int J Pharm Res Allied Sci [Internet], 2017; 6(3):71–8; Available via www.ijpras.com
Alarcón De La Lastra C, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem Soc Trans, 2007; 35(5):1156–60. CrossRef
Al Bakri W, Donovan MD, Cueto M, Wu Y, Orekie C, Yang Z. Overview of intranasally delivered peptides: key considerations for pharmaceutical development. Expert Opin Drug Deliv [Internet], 2018; 15(10):991–1005; doi:10.1080/17425247.2018.1517742 CrossRef
Aliabadi HM, Lavasanifar A. Polymeric micelles for drug delivery. Expert Opin Drug Deliv, 2006; 3(1):139–62. CrossRef
Almeida L, Vaz-da-Silva M, Falcão A, Soares E, Costa R, Loureiro AI, Fernandes-Lopes C, Rocha JF, Nunes T, Wright L, Soares-da-Silva P. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res, 2009; 53(Suppl. 1):7–15. CrossRef
Amidon GL, Lennernäs H, Shah VP, Crison JR. A Theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res, 1995; 12(3): 413–20. CrossRef
Amri A, Chaumeil JC, Sfar S, Charrueau C. Administration of resveratrol: what formulation solutions to bioavailability limitations? J Control Release [Internet], 2012; 158(2):182–93; doi:10.1016/j.jconrel.2011.09.083 CrossRef
Andrade S, Ramalho MJ, Pereira MDC, Loureiro JA. Resveratrol brain delivery for neurological disorders prevention and treatment. Front Pharmacol, 2018; 9(NOV):1–19. CrossRef
Anselmo AC, Mitragotri S. An overview of clinical and commercial impact of drug delivery systems. J Control Release [Internet], 2014; 190:15–28; doi:10.1016/j.jconrel.2014.03.053 CrossRef
Aviles-Olmos I, Limousin P, Lees A, Foltynie T. Parkinson’s disease, insulin resistance and novel agents of neuroprotection. Brain, 2013; 136(2):374–84. CrossRef
Bae YH, Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release [Internet], 2011; 153(3):198–205; doi:10.1016/j.jconrel.2011.06.001 CrossRef
Barbara R, Belletti D, Pederzoli F, Masoni M, Keller J, Ballestrazzi A, Vandelli MA, Tosi G, Grabrucker AM. Novel curcumin loaded nanoparticles engineered for blood-brain barrier crossing and able to disrupt Abeta aggregates. Int J Pharm [Internet], 2017; 526(1–2):413–24; doi:10.1016/j.ijpharm.2017.05.015 CrossRef
Bastianetto S, Ménard C, Quirion R. Neuroprotective action of resveratrol. Biochim Biophys Acta—Mol Basis Dis [Internet], 2015; 1852(6):1195–201; doi:10.1016/j.bbadis.2014.09.011 CrossRef
Bavaresco L, Petegolli D, Cantù E, Fregoni M, Chiusa G, Trevisan M. Elicitation and accumulation of stilbene phytoalexins in grapevine berries infected by Botrytis cinerea. Vitis, 1997; 36(2):77–83.
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A. Resveratrol increases antioxidant defenses and decreases pro-inflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol Vitr [Internet], 2014; 28(4):479–84; doi:10.1016/j.tiv.2014.01.006 CrossRef
Bhise S, Yadav A, Avachat A, Malayandi R. Bioavailability of intranasal drug delivery system. Asian J Pharm, 2008; 2(4):201. CrossRef
Boocock DJ, Faust GES, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev, 2007; 16(6):1246–52. CrossRef
Bournival J, Quessy P, Martinoli MG. Protective effects of resveratrol and quercetin against MPP+ -induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cell Mol Neurobiol, 2009; 29(8):1169–80. CrossRef
Buckley ST, Frank KJ, Fricker G, Brandl M. Biopharmaceutical classification of poorly soluble drugs with respect to “enabling formulations.” Eur J Pharm Sci, 2013; 50(1):8–16. CrossRef
Chaturvedi M, Kumar M, Pathak K. A review on mucoadhesive polymer used in nasal drug delivery system. J Adv Pharm Technol Res, 2011; 2(4):215–22. CrossRef
Chan OH, Stewart BH. Physicochemical and drug-delivery considerations for oral drug bioavailability. Drug Discov Today, 1996; 1(11):461–73. CrossRef
Chan CM, Huang CH, Li HJ, Hsiao CY, Su CC, Lee PL, Hung CF. Protective effects of Resveratrol against UVA-induced damage in ARPE19 cells. Int J Mol Sci, 2015; 16(3):5789–802. CrossRef
Chen Y, Dalwadi G, Benson HA. Drug delivery across the blood-brain barrier. Curr Drug Deliv, 2004; 1:361–76. CrossRef
Chen T, Li C, Li Y, Yi X, Lee SMY, Zheng Y. Oral delivery of a nanocrystal formulation of schisantherin a with improved bioavailability and brain delivery for the treatment of Parkinson’s disease. Mol Pharm, 2016; 13(11):3864–75. CrossRef
Chung S, Yao H, Caito S, Hwang J woong, Arunachalam G, Rahman I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch Biochem Biophys [Internet], 2010; 501(1):79–90; doi:10.1016/j.abb.2010.05.003 CrossRef
Crowe TP, Greenlee MHW, Kanthasamy AG, Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci [Internet], 2018; 195:44–52; doi:10.1016/j.lfs.2017.12.025 CrossRef
Csiszar A. Anti-inflammatory effects of Resveratrol: possible role in prevention of age-related cardiovascular disease. Ann N Y Acad Sci, 2011; 1215(1):117–22. CrossRef
Das S, Lin HS, Ho PC, Ng KY. The impact of aqueous solubility and dose on the pharmacokinetic profiles of resveratrol. Pharm Res, 2008; 25(11):2593–600. CrossRef
Dauchy S, Dutheil F, Weaver RJ, Chassoux F, Daumas-Duport C, Couraud PO, Scherrmann JM, De Waziers I, Declèves X. ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood-brain barrier. J Neurochem, 2008; 107(6):1518–28. CrossRef
Degan D, Ornello R, Tiseo C, Carolei A, Sacco S, Pistoia F. The role of inflammation in neurological disorders. Curr Pharm Des, 2018; 24(14):1485–501. CrossRef
Delmas D, Lin HY. Role of membrane dynamics processes and exogenous molecules in cellular resveratrol uptake: Consequences in bioavailability and activities. Mol Nutr Food Res, 2011; 55(8):1142–53. CrossRef
de rijk MC, Tzourio C, Breteler MMB, Dartigues JF, Amaducci L, Lopez-pousa S, Manubens-Bertran JM, Alpérovitch A, Rocca WA. Prevalence of parkinsonism and Parkinson’s disease in Europe: the EUROPARKINSON collaborative study. J Neurol Psychiatry, 1997; (62):10–5. CrossRef
Dong X. Current strategies for brain drug delivery. Theranostics, 2018; 8(6):1481–93. CrossRef
Engelhardt B, Liebner S. Novel insights into the development and maintenance of the blood-brain barrier. Cell Tissue Res, 2014; 355(3):687–99. CrossRef
Eugenín J, Vecchiola A, Murgas P, Arroyo P, Cornejo F, Von Bernhardi R. Expression Pattern of scavenger receptors and amyloid-β phagocytosis of astrocytes and microglia in culture are modified by acidosis: implications for Alzheimer’s disease. J Alzheimer’s Dis, 2016; 53(3):857–73. CrossRef
Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano, 2009; 3(1):16–20. CrossRef
Farooqui AA. Beneficial effects of resveratrol on neurological disorders. In: Farooqui Akhlaq A (ed.). Phytochemicals, signal transduction, and neurological disorders, pp 199–236, 2012; doi:10.1007/978-1-4614-3804-5_7 CrossRef
Feigin VL, Vos T, Nichols E, Owolabi MO, Carroll WM, Dichgans M, Deuschl G, Parmar P, Brainin M, Murray C. The global burden of neurological disorders: translating evidence into policy. Lancet Neurol, 2020; 19(3):255–65. CrossRef
Ferretta A, Gaballo A, Tanzarella P, Piccoli C, Capitanio N, Nico B, Annese T, Di Paola M, Dell’aquila C, De Mari M, Ferranini E, Bonifati V, Pacelli C, Cocco T. Effect of resveratrol on mitochondrial function: implications in parkin-associated familiar Parkinson’s disease. Biochim Biophys Acta—Mol Basis Dis [Internet], 2014; 1842(7):902–15; doi:10.1016/j.bbadis.2014.02.010 CrossRef
Fonseca-Santos B, Gremião MPD, Chorilli M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int J Nanomed, 2015; 10:4981–5003. CrossRef
Frozza RL, Bernardi A, Paese K, Hoppe JB, Da Silva T, Battastini AMO, Pohlmann AR, Guterres SS, Salbego C. Characterization of trans-resveratrol-loaded lipid-core nanocapsules and tissue distribution studies in rats. J Biomed Nanotechnol, 2010; 6(6):694–703. CrossRef
Frozza RL, Bernardi A, Hoppe JB, Meneghetti AB, Battastini AMO, Pohlmann AR, Guterres SS, Salbego C. Lipid-core nanocapsules improve the effects of Resveratrol against Aβ-induced neuroinflammation. J Biomed Nanotechnol. 2013a;9(12):2086–104. CrossRef
Frozza RL, Bernardi A, Hoppe JB, Meneghetti AB, Matté A, Battastini AMO, Pohlmann AR, Guterres SS, Salbego C. Neuroprotective effects of Resveratrol against Aβ administration in rats are improved by lipid-core nanocapsules. Mol Neurobiol, 2013b; 47(3):1066–80. CrossRef
Fujimura AT, Martinez RM, Pinho-Ribeiro FA, Lopes Dias Da Silva AM, Baracat MM, Georgetti SR, Verri WA Jr, Chorilli M, Casagrande R. Resveratrol-loaded liquid-crystalline system inhibits UVB-induced skin inflammation and oxidative stress in mice. J Nat Prod, 2016; 79(5):1329–38. CrossRef
Fulda S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov Today [Internet], 2010; 15(17–18):757–65; doi:10.1016/j.drudis.2010.07.005 CrossRef
Gabathuler R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol Dis [Internet], 2010; 37(1):48–57; doi:10.1016/j.nbd.2009.07.028 CrossRef
Gejl M, Brock B, Egefjord L, Vang K, Rungby J, Gjedde A. Blood-brain glucose transfer in Alzheimer’s disease: effect of GLP-1 analog treatment. Sci Rep, 2017; 7(1):1–10. CrossRef
Gelperina S, Maksimenko O, Khalansky A, Vanchugova L, Shipulo E, Abbasova K, Berdiev R, Wohlfart S, Chepurnova N, Kreuter J. Drug delivery to the brain using surfactant-coated poly(lactide-co-glycolide) nanoparticles: Influence of the formulation parameters. Eur J Pharm Biopharm [Internet], 2010; 74(2):157–63; doi:10.1016/j.ejpb.2009.09.003 CrossRef
Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem, 2003; 36(1):79–87. CrossRef
Goldsmith M, Abramovitz L, Peer D. Precision nanomedicine in neurodegenerative diseases. ACS Nano, 2014; 8(3):1958–65. CrossRef
Gomes MJ, Mendes B, Martins S, Sarmento B. Cell-based in vitro models for studying blood-brain barrier (BBB) permeability. Concepts Model Drug Permeability Stud Cell Tissue based Vitr Cult Model, 2016; 169–88. CrossRef
Göppert TM, Müller RH. Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: comparison of plasma protein adsorption patterns. J Drug Target, 2005; 13(3):179–87. CrossRef
Hao J, Zhao J, Zhang S, Tong T, Zhuang Q, Jin K, Chen W, Tang H. Fabrication of an ionic-sensitive in situ gel loaded with resveratrol nanosuspensions intended for direct nose-to-brain delivery. Colloids Surfaces B Biointerfaces [Internet], 2016; 147:376–86; doi:10.1016/j.colsurfb.2016.08.011 CrossRef
Haque S, Md S, Fazil M, Kumar M, Sahni JK, Ali J, Baboota S. Venlafaxine loaded chitosan NPs for brain targeting: Pharmacokinetic and pharmacodynamic evaluation. Carbohydr Polym [Internet], 2012; 89(1):72–9; doi:10.1016/j.carbpol.2012.02.051 CrossRef
Hardy J. Alzheimer’s disease: the amyloid cascade hypothesis—an update and reappraisal. J Alzheimer’s Dis, 2006; 9(SUPPL. 3):151–3. CrossRef
Harkema JR, Carey SA, Wagner JG. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol, 2006; 34(3):252–69. CrossRef
He X, Li Z, Rizak JD, Wu S, Wang Z, He R, Su M, Qin D, Wang J, Hu X. Resveratrol attenuates formaldehyde induced hyperphosphorylation of tau protein and cytotoxicity in N2a cells. Front Neurosci, 2017; 10(JAN):1–11. CrossRef
Helbecque N, Amouyel P. Very low density lipoprotein receptor in Alzheimer disease. Microsc Res Tech, 2000; 50:273–7. CrossRef
Heneka MT, Carson MJ, Khoury J El, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer’s disease. Lancet Neurol, 2015; 14(4):388–405. CrossRef
Hurst S, Loi C-M, Brodfuehrer J, El-Kattan A. Impact of physiological, physicochemical and biopharmaceutical factors in absorption and metabolism mechanisms on the drug oral bioavailability of rats and humans. Expert Opin Drug Metab Toxicol, 2007; 3(4):469–89. CrossRef
Illum L. Nasal drug delivery—possibilities, problems and solutions. J Control Release, 2003; 87(1–3):187–98. CrossRef
Jain K, Mehra N, Jain N. Nanotechnology in drug delivery: Safety and toxicity issues. Curr Pharm Des, 2015; 21(29):4252–61. CrossRef
Jhang KA, Park JS, Kim HS, Chong YH. Resveratrol ameliorates tau hyperphosphorylation at ser396 site and oxidative damage in rat hippocampal slices exposed to vanadate: implication of ERK1/2 and GSK-3β signaling cascades. J Agric Food Chem, 2017; 65(44):9626–34. CrossRef
Jiang L, Gao L, Wang X, Tang L, Ma J. The application of mucoadhesive polymers in nasal drug delivery. Drug Dev Ind Pharm, 2010; 36(3):323–36. CrossRef
Jin F, Wu Q, Lu YF, Gong QH, Shi JS. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. Eur J Pharmacol [Internet], 2008; 600(1–3):78–82; doi:10.1016/j.ejphar.2008.10.005 CrossRef
Johnson PH, Quay SC. 2005. Available via Johnson_Quay_NasalDeliveryJunctionTech_ExpOpinDrugDelivery_2005.pdf CrossRef
Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res, 2004; 1:1–13. CrossRef
Juan ME, Maijó M, Planas JM. Quantification of trans-resveratrol and its metabolites in rat plasma and tissues by HPLC. J Pharm Biomed Anal, 2010; 51(2):391–8. CrossRef
Jung KH, Lee JH, Park JW, Quach CHT, Moon SH, Cho YS, Lee KH. Resveratrol-loaded polymeric nanoparticles suppress glucose metabolism and tumor growth in vitro and in vivo. Int J Pharm [Internet]; 2015; 478(1):251–7; http://dx.doi.org/10.1016/j.ijpharm.2014.11.049 CrossRef
Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B [Internet], 2015; 5(5):442–53; doi:10.1016/j.apsb.2015.07.003 CrossRef
Kalia LV., Lang AE. Parkinson disease in 2015: evolving basic, pathological and clinical concepts in PD. Nat Rev Neurol [Internet], 2016; 12(2):2–3; doi:10.1038/nrneurol.2015.249 CrossRef
Kaur IP, Bhandari R, Bhandari S, Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J Control Release, 2008; 127(2):97–109. CrossRef
Khan AR, Liu M, Khan MW, Zhai G. Progress in brain targeting drug delivery system by nasal route. J Control Release [Internet], 2017; 268(May):364–89; doi:10.1016/j.jconrel.2017.09.001 CrossRef
Khan MM, Ahmad A, Ishrat T, Khan MB, Hoda MN, Khuwaja G, Raza SS, Khan A, Javed H, Vaibhav K, Islam F. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson’s disease. Brain Res [Internet], 2010; 1328:139–51; doi:10.1016/j.brainres.2010.02.031 CrossRef
Khodabandehloo H, Zahednasab H, Hafez AA. Nano-carriers usage for drug delivery in cancer therapy. Int J Cancer Manag, 2016; 9(2). CrossRef
Kreuter J. Application of nanoparticles for the delivery of drugs to the brain. Int Congr Ser, 2005; 1277:85–94. CrossRef
Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Adv Drug Deliv Rev [Internet], 2014; 71:2–14; doi:10.1016/j.addr.2013.08.008 CrossRef
Kim YA, Lim SY, Rhee SH, Park KY, Kim CH, Choi BT, Lee SJ, Park YM, Choi YH. Resveratrol inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression in β-amyloid-treated C6 glioma cells. Int J Mol Med, 2006; 17(6):1069–75. CrossRef
Kotta S, Mubarak Aldawsari H, Badr-Eldin SM, Alhakamy NA, Md S. Coconut oil-based resveratrol nanoemulsion: optimization using response surface methodology, stability assessment and pharmacokinetic evaluation. Food Chem, 2021; 357:129721. CrossRef
Krishnaiah YS. Pharmaceutical technologies for enhancing oral bioavailability of poorly soluble drugs. J Bioequiv Availab, 2010; 02(02):28–36. CrossRef
Kumar S, Bhargava D, Thakkar A, Arora S. Drug carrier systems for solubility enhancement of BCS class II drugs: a critical review. Crit Rev Ther Drug Carrier Syst, 2013; 30(3):217–56. CrossRef
Kundu JK, Shin YK, Kim SH, Surh YJ. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-κB in mouse skin by blocking IκB kinase activity. Carcinogenesis, 2006; 27(7):1465–74. CrossRef
Langcake P, McCarthy WVM. The relationship of resveratrol production to infection of grapevine leaves by Botrytis cinerea. Vitis, 1979; 18(3):244–53. CrossRef
Lindner G da R, Santos DB, Colle D, Moreira ELG, Prediger RD, Farina M, Khalil NM, Mara Mainardes R. Improved neuroprotective effects of poly(lactide) nanoparticles in MPTP-induced Parkinsonism. Nanomedicine (Lond). 2015;10(7):1127–38. CrossRef
Liu W, Ye A, Liu W, Liu C, Han J, Singh H. Behaviour of liposomes loaded with bovine serum albumin during in vitro digestion. Food Chem [Internet], 2015; 175:16–24; doi:10.1016/j.foodchem.2014.11.108 CrossRef
Lofrumento DD, Nicolardi G, Cianciulli A, Nuccio F De, Pesa V La, Carofiglio V, Dragone T, Calvello R, Panaro MA. Neuroprotective effects of resveratrol in an MPTP mouse model of Parkinson’s-like disease: possible role of SOCS-1 in reducing pro-inflammatory responses. Innate Immun, 2014; 20(3):249–60. CrossRef
López-Nicolás JM, García-Carmona F. Aggregation state and pKa values of (E)-resveratrol as determined by fluorescence spectroscopy and UV-visible absorption. J Agric Food Chem,2008; 56(17):7600–5. CrossRef
Loureiro JA, Andrade S, Duarte A, Neves AR, Queiroz JF, Nunes C, Sevin E, Fenart L, Gosselet F, Coelho MA, Pereira MC. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules, 2017; 22(2):1–16. CrossRef
Lu X, Ji C, Xu H, Li X, Ding H, Ye M, Zhu Z, Ding D, Jiang X, Ding X, Guo X. Resveratrol-loaded polymeric micelles protect cells from Aβ-induced oxidative stress. Int J Pharm, 2009; 375(1–2):89–96. CrossRef
Maiti P, Dunbar GL. Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases. Int J Mol Sci, 2018; 19(6):1637. CrossRef
Masserini M. Nanoparticles for brain drug delivery. ISRN Biochem, 2013; 2013:1–18. CrossRef
Marttin E, Schipper NGM, Coos Verhoef J, Merkus FWHM. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv Drug Deliv Rev, 1998; 29(1–2):13–38. CrossRef
Maussang D, Rip J, van Kregten J, van den Heuvel A, van der Pol S, van der Boom B, Reijerkerk A, Chen L, de Boer M, Gaillard P, de Vries H. Glutathione conjugation dose-dependently increases brain-specific liposomal drug delivery in vitro and in vivo. Drug Discov Today Technol [Internet], 2016; 20:59–69; doi:10.1016/j.ddtec.2016.09.003 CrossRef
McClure R, Yanagisawa D, Stec D, Abdollahian D, Koktysh D, Xhillari D, Jaeger R, Stanwood G, Chekmenev E, Tooyama I, Gore JC, Pham W. Inhalable curcumin: offering the potential for translation to imaging and treatment of Alzheimer’s disease. J Alzheimer’s Dis, 2015; 44(1):283–95. CrossRef
Meredith ME, Salameh TS, Banks WA. Intranasal delivery of proteins and peptides in the treatment of neurodegenerative diseases. AAPS J, 2015; 17(4):780–7. CrossRef
Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, Meyers D, Cole PA, Ott M, Gan L. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron [Internet], 2010; 67(6):953–66. Available from: http://dx.doi.org/10.1016/j.neuron.2010.08.044 CrossRef
Mistry A, Stolnik S, Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm, 2009; 379(1–2):146–57. CrossRef
Mittal D, Ali A, Md S, Baboota S, Sahni JK, Ali J. Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv, 2014; 21(2):75–86. CrossRef
Moura RP, Martins C, Pinto S, Sousa F, Sarmento B. Blood-brain barrier receptors and transporters: an insight on their function and how to exploit them through nanotechnology. Expert Opin Drug Deliv [Internet], 2019; 16(3):271–85; doi:10.1080/17425247.2019.1583205 CrossRef
Naahidi S, Jafari M, Edalat F, Raymond K, Khademhosseini A, Chen P. Biocompatibility of engineered nanoparticles for drug delivery. J Control Release [Internet], 2013; 166(2):182–94; doi:10.1016/j.jconrel.2012.12.013 CrossRef
Nasr M. Development of an optimized hyaluronic acid-based lipidic nanoemulsion co-encapsulating two polyphenols for nose to brain delivery. Drug Deliv, 2016; 23(4):1444–52. CrossRef
Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev, 2010; 23(4):858–83. CrossRef
Noble GT, Stefanick JF, Ashley JD, Kiziltepe T, Bilgicer B. Ligand-targeted liposome design: Challenges and fundamental considerations. Trends Biotechnol [Internet], 2014; 32(1):32–45; doi:10.1016/j.tibtech.2013.09.007 CrossRef
Neves AR, Queiroz JF, Reis S. Brain?targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J Nanobiotechnol, 2016; 1–11. CrossRef
Ozsoy Y, Gngör S. Nasal route: an alternative approach for antiemetic drug delivery. Expert Opin Drug Deliv, 2011; 8(11):1439–53. CrossRef
Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: Implications for protection against coronary heart disease. Clin Chim Acta, 1995; 235(2):207–19. CrossRef
Palle S, Neerati P. Improved neuroprotective effect of resveratrol nanoparticles as evinced by abrogation of rotenone-induced behavioral deficits and oxidative and mitochondrial dysfunctions in rat model of Parkinson’s disease. Naunyn Schmiedebergs Arch Pharmacol, 2018; 391(4):445–53. CrossRef
Pangeni R, Sharma S, Mustafa G, Ali J, Baboota S. Vitamin e loaded resveratrol nanoemulsion for brain targeting for the treatment of Parkinson’s disease by reducing oxidative stress. Nanotechnology, 2014; 25(48). CrossRef
Pardeshi C V., Rajput P V., Belgamwar VS, Tekade AR, Surana SJ. Novel surface modified solid lipid nanoparticles as intranasal carriers for ropinirole hydrochloride: application of factorial design approach. Drug Deliv, 2013; 20(1):47–56. CrossRef
Pardeshi C V., Belgamwar VS, Tekade AR, Surana SJ. Novel surface modified polymer-lipid hybrid nanoparticles as intranasal carriers for ropinirole hydrochloride: in vitro, ex vivo and in vivo pharmacodynamic evaluation. J Mater Sci Mater Med, 2013; 24(9):2101–15. CrossRef
Pardridge WM. Drug and gene targeting to the brain via blood-brain barrier receptor-mediated transport systems. Int Congr Ser, 2005; 1277:49–62. CrossRef
Pardridge WM. CSF, blood-brain barrier, and brain drug delivery. Expert Opin Drug Deliv [Internet], 2016; 13(7):963–75; doi:10.1517/17425247.2016.1171315 CrossRef
Park K. Nanotechnology: what it can do for drug delivery. J Control Release, 2007; 120(1–2):1–3. CrossRef
Park K. Facing the truth about nanotechnology in drug delivery. ACS Nano, 2013; 7(9):7442–7. CrossRef
Pasinetti GM, Wang J, Ho L, Zhao W, Dubner L. Roles of Resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim Biophys Acta—Mol Basis Dis [Internet], 2014; 1852(6):1202–8; doi:10.1016/j.bbadis.2014.10.006 CrossRef
Patel JP, Frey BN. Disruption in the blood-brain barrier: the missing link between brain and body inflammation in bipolar disorder? Neural Plast, 2015; 2015:708306. CrossRef
Patel T, Zhou J, Piepmeier JM, Saltzman WM. Polymeric nanoparticles for drug delivery to the central nervous system. Adv Drug Deliv Rev [Internet], 2012; 64(7):701–5; doi:10.1016/j.addr.2011.12.006 CrossRef
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Kumara Swamy M, Sharma S, Habtemariam S, Shin HS. Nano based drug delivery systems: recent developments and future prospects 10 Technology 1007 Nanotechnology 03 Chemical Sciences 0306 Physical Chemistry (incl. Structural) 03 Chemical Sciences 0303 Macromolecular and Materials Chemistry 11 Medical and He. J Nanobiotechnology [Internet], 2018; 16(1):1–33; doi:10.1186/s12951-018-0392 CrossRef
-Quadros Gomes BA, Bastos Silva JP, Rodrigues Romeiro CF, dos Santos SM, Rodrigues CA, Gonçalves PR, Sakai JT, Mendes PFS, Varela ELP, Monteiro MC. Neuroprotective mechanisms of resveratrol in Alzheimer’s disease: role of SIRT1. Oxid Med Cell Longev, 2018; 2018:8152373. CrossRef
Rabanel JM, Faivre J, Paka GD, Ramassamy C, Hildgen P, Banquy X. Effect of polymer architecture on curcumin encapsulation and release from PEGylated polymer nanoparticles: toward a drug delivery nano-platform to the CNS. Eur J Pharm Biopharm, 2015; 96:409–20. CrossRef
Rajput A, Bariya A, Allam A, Othman S, Butani SB. In situ nanostructured hydrogel of resveratrol for brain targeting: in vitro-in vivo characterization. Drug Deliv Transl Res, 2018; 8(5):1460–70. CrossRef
Rangel-Yagui C de O, Pessoa A, Tavares LC. Micellar solubilization of drugs. J Pharm Pharm Sci, 2005; 8(2):147–63. CrossRef
Rankovic Z. CNS drug design: balancing physicochemical properties for optimal brain exposure. J Med Chem, 2015; 58(6):2584–608. CrossRef
Rius C, Abu-Taha M, Hermenegildo C, Piqueras L, Cerda-Nicolas J-M, Issekutz AC, Estañ L, Cortijo J, Morcillo EJ, Orallo F, Sanz M-J. Trans-but not cis-resveratrol impairs angiotensin-II–mediated vascular inflammation through inhibition of NF-κB activation and peroxisome proliferator-activated receptor-γ upregulation. J Immunol, 2010; 185(6):3718–27. CrossRef
Robinson K, Mock C, Liang D. Pre-formulation studies of resveratrol. Drug Dev Ind Pharm, 2015; 41(9):1464–9. CrossRef
Sabolovic N, Heurtaux T, Humbert AC, Krisa S, Magdalou J. Cis- and trans-resveratrol are glucuronidated in rat brain, olfactory mucosa and cultured astrocytes. Pharmacology, 2007; 80(2–3):185–92. CrossRef
Samaridou E, Alonso MJ. Nose-to-brain peptide delivery—the potential of nanotechnology. Bioorganic Med Chem [Internet], 2018; 26(10):2888–905; doi:10.1016/j.bmc.2017.11.001 CrossRef
Sarkar FH, Li Y, Wang Z, Kong D. Cellular signaling perturbation by natural products. Cell Signal [Internet], 2009; 21(11):1541–7; doi:10.1016/j.cellsig.2009.03.009 CrossRef
Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm, 2012; 2012:195727. CrossRef
Sawda C, Moussa C, Turner RS. Resveratrol for alzheimer’s disease. Ann N Y Acad Sci, 2017; 1403(1):142–9. CrossRef
Scheepens A, Tan K, Paxton JW. Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes Nutr, 2010; 5(1):75–87. CrossRef
Shah B, Khunt D, Bhatt H, Misra M, Padh H. Application of quality by design approach for intranasal delivery of rivastigmine loaded solid lipid nanoparticles: effect on formulation and characterization parameters. Eur J Pharm Sci [Internet], 2015; 78:54–66; doi.org/10.1016/j.ejps.2015.07.002 CrossRef
Shamenkov D, Petrov V, Ramge P, Cychutek K, Koch-brandt C, Alyautdin R. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood–brain barrier. J Drug Target. 2002; 10(4):317–25. CrossRef
Shu XH, Wang LL, Li H, Song X, Shi S, Gu JY, Wu ML, Chen XY, Kong QY, Liu J. Diffusion efficiency and bioavailability of resveratrol administered to rat brain by different routes: therapeutic implications. Neurotherapeutics, 2015; 12(2):491–501. CrossRef
Siddiqui IA, Sanna V, Ahmad N, Sechi M, Mukhtar H. Resveratrol nanoformulation for cancer prevention and therapy. Ann N Y Acad Sci, 2015; 1348(1):20–31. CrossRef
Singh N, Agrawal M, Doré S. Neuroprotective properties and mechanisms of resveratrol in in vitro and in vivo experimental cerebral stroke models. ACS Chem Neurosci, 2013; 4(8):1151–62. CrossRef
Sood S, Jain K, Gowthamarajan K. Optimization of curcumin nanoemulsion for intranasal delivery using design of experiment and its toxicity assessment. Colloids Surfaces B Biointerfaces [Internet], 2014; 113:330–7; doi:10.1016/j.colsurfb.2013.09.030 CrossRef
Stocker R, Keaney JF. Role of oxidative modifications in atherosclerosis. Physiol Rev, 2004; 84(4):1381–478. CrossRef
Srikanth M, Kessler JA. Nanotechnology—novel therapeutics for CNS disorders. Nat Rev Neurol [Internet], 2012; 8(6):307–18; doi:10.1038/nrneurol.2012.76 CrossRef
Summerlin N, Soo E, Thakur S, Qu Z, Jambhrunkar S, Popat A. Resveratrol nanoformulations: Challenges and opportunities. Int J Pharm [Internet]; 2015; 479(2):282–90; doi:10.1016/j.ijpharm.2015.01.003 CrossRef
Suri SS, Fenniri H, Singh B. Nanotechnology-based drug delivery systems. J Occup Med Toxicol, 2007; 2(1):1–6. CrossRef
Tamai I, Tsuji A. Transporter-mediated permeation of drugs across the blood-brain barrier. J Pharm Sci, 2000; 89(11):1371–88. CrossRef
Thakur K, Albanese E, Giannakopoulos, Jette N, Linde M, Prince M, Steiner T, Dua T. Neurological disorders. Dis control priorities, 3rd edition, vol. 4, Ment Neurol Subst Use Disord, pp 87–107, 2016. CrossRef
Thorne RG, Pronk GJ, Padmanabhan V, Frey WH. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience, 2004; 127(2):481–96. CrossRef
Tiwari G, Tiwari R, Bannerjee S, Bhati L, Pandey S, Pandey P, Bannerjee SK. Drug delivery systems: an updated review. Int J Pharm Investig, 2012; 2(1):2. CrossRef
Trela BC, Waterhouse AL. Resveratrol: isomeric molar absorptivities and stability. J Agric Food Chem, 1996; 44(5):1253–7. CrossRef
Ugwoke MI, Agu RU, Verbeke N, Kinget R. Nasal mucoadhesive drug delivery: background, applications, trends and future perspectives. Adv Drug Deliv Rev, 2005; 57(11):1640–65. CrossRef
Vanamala J, Reddivari L, Radhakrishnan S, Tarver C. Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer, 2010;10:238. CrossRef
van Hoogevest P, Liu X, Fahr A. Drug delivery strategies for poorly water-soluble drugs: the industrial perspective. Expert Opin Drug Deliv, 2011; 8(11):1481–500. CrossRef
Varoni EM, Lo Faro AF, Sharifi-Rad J, Iriti M. Anticancer molecular mechanisms of resveratrol. Front Nutr, 2016; 3(April). CrossRef
Vijayakumar MR, Kosuru R, Singh SK, Prasad CB, Narayan G, Muthu MS, Singh S. resveratrol loaded PLGA:D-α-tocopheryl polyethylene glycol 1000 succinate blend nanoparticles for brain cancer therapy. RSC Adv, 2016a; 6(78):74254–68. CrossRef
Vijayakumar MR, Vajanthri KY, Balavigneswaran CK, Mahto SK, Mishra N, Muthu MS, Singh S. pharmacokinetics, biodistribution, in vitro cytotoxicity and biocompatibility of Vitamin E TPGS coated trans resveratrol liposomes. Colloids Surfaces B Biointerfaces [Internet], 2016b; 145:479–91; doi:10.1016/j.colsurfb.2016.05.037 CrossRef
Vinarov Z, Katev V, Radeva D, Tcholakova S, Denkov ND. Micellar solubilization of poorly water-soluble drugs: effect of surfactant and solubilizate molecular structure. Drug Dev Ind Pharm [Internet], 2018; 44(4):677–86; doi:10.1080/03639045.2017.1408642 CrossRef
Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, Marambaud P. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-β peptide metabolism. J Biol Chem, 2010; 285(12):9100–13. CrossRef
Walle T. Bioavailability of Resveratrol. Ann N Y Acad Sci, 2011; 1215(1):9–15. CrossRef
Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos, 2004; 32(12):1377–82. CrossRef
Wang Y, Xu H, Fu Q, Ma R, Xiang J. Protective effect of resveratrol derived from Polygonum cuspidatum and its liposomal form on nigral cells in Parkinsonian rats. J Neurol Sci [Internet], 2011; 304(1–2):29–34; doi:10.1016/j.jns.2011.02.025 CrossRef
Wang J, Gu BJ, Masters CL, Wang YJ. A systemic view of Alzheimer disease—insights from amyloid-β metabolism beyond the brain. Nat Rev Neurol, 2017a; 13(10):612–23. CrossRef
Wang Y, Ying X, Xu H, Yan H, Li X, Tang H. The functional curcumin liposomes induce apoptosis in C6 glioblastoma cells and C6 glioblastoma stem cells in vitro and in animals. Int J Nanomedicine, 2017b; 12:1369–84. CrossRef
Wang M, Li L, Zhang X, Liu Y, Zhu R, Liu L, Fang Y, Gao Z, Gao D. Magnetic resveratrol liposomes as a new theranostic platform for magnetic resonance imaging guided Parkinson’s disease targeting therapy. ACS Sustain Chem Eng, 2018a; 6(12):17124–33. CrossRef
Wang Q, Zhang Y, Wong CH, Edwin Chan HY, Zuo Z. Demonstration of direct nose-to-brain transport of unbound HIV-1 replication inhibitor DB213 via intranasal administration by pharmacokinetic modeling. AAPS J, 2018b; 20(1). CrossRef
Wen MM, El-Salamouni NS, El-Refaie WM, Hazzah HA, Ali MM, Tosi G, Farid RM, Blanco-Prieto MJ, Billa N, Hanafy AS. Nanotechnology-based drug delivery systems for Alzheimer’s disease management: technical, industrial, and clinical challenges. J Control Release [Internet], 2017; 245:95–107; doi:10.1016/j.jconrel.2016.11.025 CrossRef
Wilkinson K, El Khoury J. Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer’s disease. Int J Alzheimers Dis, 2012; 2012:489456. CrossRef
Wohlfart S, Gelperina S, Kreuter J. Transport of drugs across the blood-brain barrier by nanoparticles. J Control Release [Internet], 2012; 161(2):264–73; doi:10.1016/j.jconrel.2011.08.017 CrossRef
Wong HL, Wu XY, Bendayan R. Nanotechnological advances for the delivery of CNS therapeutics. Adv Drug Deliv Rev [Internet], 2012; 64(7):686–700; doi:10.1016/j.addr.2011.10.007 CrossRef
Wu Y, Li X, Zhu JX, Xie W, Le W, Fan Z, Jankovic J, Pan T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson’s disease. NeuroSignals, 2011; 19(3):163–74. CrossRef
Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease—a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med, 2012; 2(1):1–24. CrossRef
Xi J, Zhang Z, Si XA. Improving intranasal delivery of neurological nanomedicine to the olfactory region using magnetophoretic guidance of microsphere carriers. Int J Nanomed, 2015; 10:1211–22. CrossRef
Xiong S, Liu W, Zhou Y, Mo Y, Liu Y, Chen X, Huafeng P, Dongsheng Y, Qi W, Tongkai C. Enhancement of oral bioavailability and anti-Parkinsonian efficacy of resveratrol through a nanocrystal formulation. Asian J Pharm Sci [Internet], 2020; 15(4):518–28; doi:10.1016/j.ajps.2019.04.003 CrossRef
Yu XC, Yang JJ, Jin BH, Xu HL, Zhang HY, Xiao J, Lu CT, Zhao YZ, Yang W. A strategy for bypassing the blood-brain barrier: facial intradermal brain-targeted delivery via the trigeminal nerve. J Control Release [Internet], 2017; 258(January):22–33; doi:10.1016/j.jconrel.2017.05.001 CrossRef
Zhang F, Wang YY, Liu H, Lu YF, Wu Q, Liu J, Shi JS. Resveratrol produces neurotrophic effects on cultured dopaminergic neurons through prompting astroglial BDNF and GDNF release. Evidence Based Complement Alternat Med, 2012; 2012:937605. CrossRef