INTRODUCTION
Tuberculosis (TB) is the most contagious disease and is one of the top 10 causes of death worldwide. The causative organism Mycobacterium tuberculosis, expelled in the form of air droplets, affects the respiratory system including other parts of the body. Globally, 10 million new cases were reported in the year 2019 (Global tuberculosis report, 2020).
Sputum-positive mycobacterial infection altering the blood parameters emerged as increased neutrophils count, elevated erythrocyte sedimentation rate (ESR), reduced lymphocytes, monocytes, red blood cell (RBC) count, and hemoglobin (HGB) level. The delay in the conversion of sputum positive to negative and changes in the blood parameters pre- and post-therapy indicates valuable information in assessing the clinical status of the diseases in TB. Findings such as body weight, leukocyte count, HGB, thrombocytes, and sedimentation rate of red cells were observed to be the indicators for satisfactory outcomes. The abnormalities in the blood picture are considered indicators for the diagnosis of TB (Bozóky et al., 1997). Patients showed improved hematological parameters on treating with antitubercular drugs compared prior to the treatment initiation. This improvement was observed in the HGB, packed cell volume (PCV), RBC, and its indices (Mirlohi et al., 2016). The normocytic normochromic anemia was the usual finding in pulmonary TB. Inconsistency in counts in the blood parameters was observed in the leucocytes, neutrophils, lymphocytes, and monocytes. Increase in platelet (PLT) count and ESR was more prominent in pulmonary TB. The altered hematological parameters normalized on the initiation of antitubercular medication (Singh et al., 2001).
Diabetes and human immunodeficiency virus (HIV) are the exogenous factors that increase the risk of exposure to the bacillary load. The endogenous factors lead to the progression of the infection in diabetes mellitus (DM) by diminishing the T-cell-mediated immune responses. The active TB and altered biochemical pathways result in recurrent TB episodes and a higher mortality rate with deteriorated health outcomes. Impaired host defense mechanisms, altered biochemical activities, and persistent hyperglycemia lead to the development of disease complications, increasing the risk to infections (Revised National Tuberculosis Control Program, 2017). Patients with TB–DM and DM with other chest disease complaints identified significant differences with an increase in PLT count, ESR, loss of weight, and decreased HGB levels in TB–DM patients (Khalil and Ramadan, 2016). HGB was low in pulmonary TB subjects compared to nonpulmonary TB patients and high incidences of diabetics than HIV infection. Hence, identification of other medical conditions is essential while screening patients during their initial diagnosis of TB (Jamil et al., 2019). The other endocrine system much influenced by TB is the thyroid gland which plays a vital role in regulation of body metabolism and erythropoiesis. Anemia, deficiency of iron levels, and reduced HGB levels are the chief clinical presentations in thyroidal illness. The thyroidal imbalance influences the hemogram parameters mainly with RBC, HGB, white blood cell (WBC), mean corpuscular volume (MCV), and anisocytosis (Ahmed and Mohammed, 2020). TB afflicts the circulatory system, lungs, and increases the mortality in presence of comorbid conditions. The evaluation of complete blood picture helps in managing the patients for improved health outcomes. A study was conducted to evaluate the changes in the hemogram between pulmonary TB and its combination with other clinical conditions. The changes in RBC, HGB, WBC, ESR, PLT, and loss of weight contribute to the progression of TB. Significant alterations in monocyte, eosinophil counts, and ESR were observed in comorbidities. The findings suggest the need to correct the unusual blood picture (Javed et al., 2018).
Pulmonary TB is the most common opportunistic infection in HIV. An improved health outcome from TB infection in HIV-positive patients was comparable to those of HIV noninfected patients (Agbor et al., 2015). Patients with HIV and TB revealed reduced WBC, lymphocyte, neutrophil, basophil and eosinophil count. The coinfection of pulmonary TB–HIV deteriorates the hemogram parameters and clinical condition. Majority of the pulmonary TB patients were observed to be anemic, with an increase in PLT count and ESR (Abbas et al., 2020).
In the present research, we have compared the hematological parameters between male and female multidrug-resistant pulmonary TB (MDRPTB) and it was analyzed with medical conditions such as diabetes, HIV, and hypothyroidism. Few studies are available from the region of Telangana on the rate of infectivity among comorbid conditions in MDRPTB patients. This study is initiated to note the changes in hematologic parameters and is interpreted to assist with the diagnosis and management of the disease.
MATERIALS AND METHODS
Study site
Presuming for the outcome, prior approval and permission for the study were obtained from the Institutional Review Board (JCPN/112/2018). The prospective investigations were carried out from February, 2018 to December, 2020. The study was conducted in the Government TB and Chest hospital, Warangal, Telangana, India. The study participants elucidated about the protocol before obtaining their consent.
Inclusion and exclusion criteria
The enrollment criterion of subjects with drug-resistant TB patients’ suffering from the presence and absence of the comorbid conditions and proven positive report for the presence of mycobacterium was considered. Rapid molecular tests, such as cartridge-based nucleic acid amplification test, first line—line probe assay, second line—line probe assay, and culture tests were employed to recognize the drug resistance among patients. Few patients accounted for additional resistance amid treatment period were also recognized. The subjects excluded from the study include individuals less than 18 years and pregnant women.
Data collection and procurement of blood sample
Patients’ demographic data, history of clinical conditions, medication, and laboratory investigations for the diagnosis of MDRPTB were examined. 4 ml of blood was drawn from the patients to examine the hemogram parameters for any changes on the same day. The “Mindray BC 2800 Automated Hematology Analyzer” (Shenzhen Mindray, Bio-Medical Electronics Co., Ltd.) was used to evaluate the hemoglobin, RBC count, and its indices. The total WBC, differential count, PLT count, and its indices were are also estimated.
Categorization of patients
We reviewed patient details and confirmed the presence of comorbid conditions along with MDRPTB; the subjects were categorized into Group I with complications appropriated to multidrug resistance TB and Group II with MDRPTB and comorbid conditions. Furthermore, patients were classified into various disease categories with MDRPTB infection based on the comorbid conditions. Due to patients with multiple comorbidities, nerve and cardiac-related complications were developed in a limited number of cohorts, and hence not considered for the inferential statistical analysis. The hemogram of subjects with infection and other clinical conditions such as diabetes, retroviral disease (RVD/HIV), hypothyroid, and MDRPTB was interpreted.
Statistical analysis
The data were presented as number, percentage, and mean ± SD. Significant differences were considered when the probability value was p < 0.05. The intergroup differences were analyzed using analysis of variance (ANOVA) in GraphPad Prism V.5.
RESULTS
The study enrolled a total of 271 MDRPTB patients during the research period in which male (n = 195) outnumbered female patients (n = 76). Among 271 patients, majority of the patients were categorized as Group I (n = 199) and the remaining patients as Group II (n = 72) having comorbid complications. Considering the criteria of gender, presence or absence of other clinical conditions, the above two groups are sub-classified. The age was less in Group I subjects with MDRPTB and there was more loss of weight in contrast to Group II MDRPTB patients accompanied with clinical conditions. The distribution of age and weight of these patients is presented in Table 1.
Hemogram abnormalities between male and female patients in Groups I and II
The immune defense mechanism of leukocytosis to combat the invading bacterial population is noticed in both groups, but females had a lower incidence. Mycobacterium tuberculosis-activated neutrophils with a rise in count in male patients with statistical significance (p = 0.04*) were noticed. A decrease in lymphocytes count prompting the inadequate immune competency to combat the mycobacterial infection was reflected in male patients. Monocytes, the target cells of M. tuberculosis, were observed among two groups of male patients (p = 0.003**) with a rise in count. The rarely associated eosinophilia in TB showed its predominance in female patients of both groups. Basophils were present in a normal reference cell range. Majority of the patients in both groups and female in the comorbid group exhibited an increase in immature granulocytes. An upsurge of WBC and the differential count cells was recorded.
Few subjects experienced reduction in the red cell count in both groups. The iron-containing protein in the red cell HGB was deficient and noticed in the highest number of patients of Group I (89.44%) and Group II (91.66%). Visible differences in HGB levels in Group I were noticed among male and female patients and were statistically significant (p < 0.0001***). The infection influenced PCV levels in both groups with significant differences among male and female patients (p < 0.0001***). The hematocrit (HCT) value in TB patients significantly decreased during the intensive phase of TB treatment, and was below the reference range in female subjects of both groups. The magnitude of anemia among TB patients was assessed, and most of the patients implied normocytic normochromic anemia, followed by microcytic hypochromic anemia. Female subjects observed a lesser than usual range of MCV and MCHC, with statistical significance among two different genders. The difference in MCH in both Group I (n = 132) and Group II (n = 45) denoted prevalence of iron deficiency (p = 0.001**). The elevated red cell distribution width (RDW) indicated anisocytosis and was prominent in female patients. Similar to these incidences of red cell distribution width—coefficient of variance (RDWCV) measuring the variation in the size of red cells around MCV, anisocytosis was observed in female patients (p = 0.006**). Nucleated RBCs were noted in all the subjects and female patients presented an increased count. Altogether, the female gender was recognized with reduced levels of HGB, HCT, MCV, MCH, MCHC, RDWCV, and NRBC compared to male patients.
The increase in the PLT count that signifies the progression of infection with TB is observed in both genders. Platelet distribution width (PDW), mean platelet volume (MPV), platelet large cell ratio (PLCR), and plateletcrit (PCT) showed significant increases in all the indices of PLTs (Table 2). Our study suggested that active TB is associated mostly with very high ESR values in female patients.
WBC: White blood cell; IG: Immature granulocytes; RBC: Red blood cell; MCV: Mean corpuscular volume; MCH: Mean corpuscular hemoglobin; MCHC: Mean corpuscular hemoglobin concentration; RDW: Red blood cell distribution width; NRBC: Nucleated red blood cell; PDW: Platelet distribution width; MPV: Mean platelet volume; PLCR: Platelet large cell ratio; ESR: Erythrocyte sedimentation rate.
Hemogram profile in MDRPTB vs MDRPTB with comorbidities
The salient feature of increased WBC (p = 0.03*) was evident in Group I. The patients in Group II with RVD showed no major changes in the white cell count. Hyperinflammatory response of the neutrophils was noticed in the MDRPTB group while normal values were recorded in RVD patients. Tubercular bacilli reduced the lymphocytes in Group I and among hypothyroid patients with infection. Confirmed association with active TB disease and monocytes in increased counts was noticed in MDRPTB patients than in patients with other clinical conditions with infection (p = 0.0002***). The allergic outcomes of eosinophilia were illustrated in Group II and in patients with diabetic complications. Although basophils were not fluctuating from its normal values, it manifested with an increased count in Group I. The appearance of early stage immature granulocyte is an indication of the infection or inflammation. It was predominant in Group II and in hypothyroid patients (p = 0.009**).
The reduced RBC, HGB, and HCT were observed in Group II with comorbid conditions and RVD patients. The RVD group illustrated the values below normal with no statistical significance. The primary findings of Group 1 are attributed to microcytic anemia (p = 0.007**), iron deficiency (p = 0.01*), and hypochromic anemia. Both indices RDWSD (p = 0.02*) and RDWCV highlight the MDRPTB group for anisocytosis in red cells. The increase in NRBC was found in Group 1 and in hypothyroid patients.
PLT count and PCT showed an increment, while the indices PDW, MPV, and PLCR were reduced in the MDRPTB group. PDW is much reduced in hypothyroid patients. Raised ESR was noticed in majority of the patients. The subjects of Group 2 were noticed with elevated ESR than Group 1. Changes in the hemogram with reference to comorbid conditions in MDRPTB patients are illustrated in Table 3.
WBC: White blood cell; N: Neutrophil; L: Lymphocyte; M: Monocyte; E: Eosinophil; B: Basophil; IG: Immature granulocyte; RBC: Red blood cell; MCV: Mean corpuscular volume; MCH: Mean corpuscular hemoglobin; MCHC: Mean corpuscular hemoglobin concentration; RDW: Red blood cell distribution width; NRBC: Nucleated red blood cell; PDW: Platelet distribution width; MPV: Mean platelet volume; PLCR: Platelet large cell ratio; ESR: Erythrocyte sedimentation rate.
DISCUSSION
The changes in the hematological parameters play an important role and influence patients’ treatment outcomes. Treatment outcomes can be improved by monitoring the hematological markers and by careful evaluation and management of TB. The results reported here reinforce anemia and drug resistance influencing the various blood parameters. The results describe the changes in hematological parameters in both genders. The changes in hematological parameters were investigated in the MDRPTB infectious group and with medical conditions such as diabetes, RVD, and hypothyroid. Consistent with other studies, the present study noticed variations in hematological parameters in male and female subjects (Comstock, 2008), which has been an enduring observation in many of the research works. Considering the comorbid conditions, diabetics and HIV patients are at a great risk of drug-resistant TB and its relapse within 2 years (van den Hof et al., 2015). This signaled a treatment with regular intervals of monitoring patients. The sign of thyroidal abnormalities before or after initiation of the treatment is marked in TB patients with or without HIV (Ige et al., 2016). The current investigation also owns up that diabetics with drug resistance outweighed the patients with RVD and thyroid comorbid conditions. The patients with MDRPTB weighed lesser than the other group. Innate immunity contributes to activating the immune cells granulocytes and agranulocytes help in promoting phagocytosis and oxidative lysis of bacilli. Among granulocytes, neutrophils provide antimicrobial effects and help resolve infections (Nordenfelt and Tapper, 2011). Neutrophils also exhibit a disparity in the gene expressions in both genders (Patin et al., 2018). An elevated WBC count was detected in male patients and in MDRPTB subjects. Reduced WBC count in peripheral blood in immunodeficient HIV patients was noticed (Moore and Schneider, 2013). The coexisting condition of HIV contributing to the reduced WBC count is signaled in the present study. Immature granulocytes upsurge in Group 2 subjects, particularly in hypothyroid with MDRPTB patients, was prominent. The increased eosinophil counts occur in adult women and adults of both genders with respiratory infections. This count normalizes on the initiation of antitubercular regimens (Kolobovnikova et al., 2012). Raised eosinophil count was predominantly observed in female subjects of both groups and in the group with comorbidities. Studies report that the higher the leucocyte number, the smaller the basophil count (Fossati, 2009). A marginally increased basophil count was noticed in MDRPTB along with an increase in WBC count.
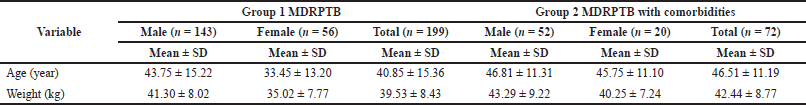 | Table 1. Age and weight of MDRPTB patients. [Click here to view] |
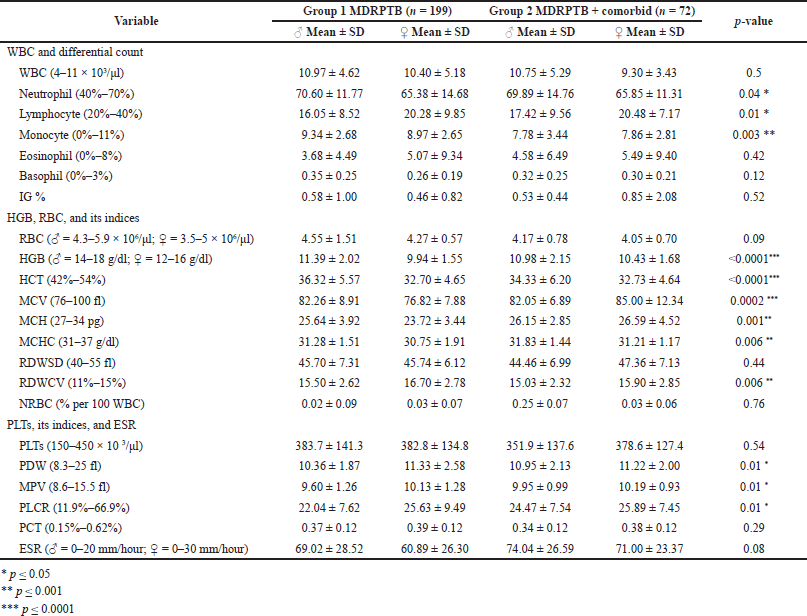 | Table 2. Comparison of hemogram between MDRPTB and comorbid patients. Statistical analysis carried out using ANOVA, followed by Tukey’s multiple comparison test. [Click here to view] |
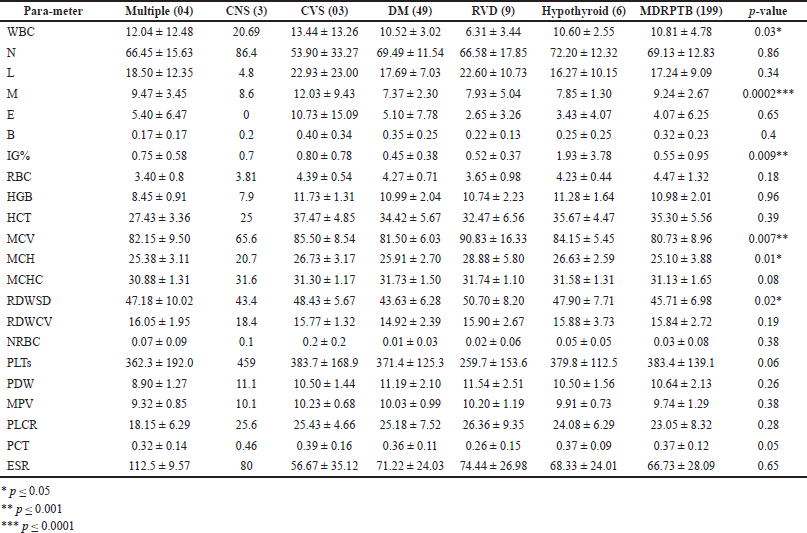 | Table 3. Changes in the hemogram with reference to comorbid conditions in MDRPTB patients. Statistical analysis was carried out using ANOVA, followed by Tukey’s multiple comparison test. [Click here to view] |
The agranulocytes link adaptive and innate immunity involved in recognizing the antigen-presenting cells and invade the bacterial cells found reduced, which is a characteristic of bacterial infection. Neutrophils revive, activate, and prioritize the recruitment of antigen-presenting cells that identify the pathogen in inflammatory responses. These communicate the monocytes to the site of injury through the cascades of chemotactic signals. In turn, the monocytes mediate the innate and adaptive immunity through antigen presentation to lymphocytes (Chertov et al., 1997). The increase in monocyte count and reduced lymphocyte characterized in TB patients indicates the progression of the infection (Serbina et al., 2008). The present study observed a similarity with these mononuclear leucocytes in male patients and in MDRPTB group. Previous studies reported reduced monocyte count in diabetes along with TB than TB alone (Gomez et al., 2013). Monocyte count increased in MDRPTB subjects. The coinfection of TB and HIV resulted in reduced neutrophil counts, monocytes by tubercle bacilli, and depleted lymphocytes by HIV virus (Pathak et al., 2010). The mainstream study showed reduced neutrophils, normal lymphocytes, and monocytes in RVD with MDRPTB.
The presence of iron augments the growth of M. tuberculosis observed in an in vitro study. The suppression of bacterial growth in the presence of iron-limiting chelators in the media emphasizes the need of iron in TB patients (Olakanmi et al., 2002). The prevalent anemia was noticed in most patients; however, the severity was identified in females with MDRPTB. Most of the patients revealed normocytic normochromic anemia, followed by microcytic hypochromic anemia (Beard, 2000), which is consistent with the present study. Microcytic hypochromic anemia was prominent in female patients and in the MDRPTB group in the current study. The evidence of the reduced MCH represents iron deficiency anemia, red cell production, or changes in inflammatory markers. The low iron content modulates the potent immune responses favoring the advancement of TB infection (de Mendonça et al., 2021). The reduced MCH values were pronounced in female patients with MDRPTB and in the group of MDRPTB patients. The improved anemic conditions normalize the RDW and it correlates with ameliorated MCV. Increased RDW relates to malabsorption and nutritional deficiency. Impaired iron metabolism reduces the red cell life span leading to the progression of infection (Berkowitz, 1991). Anisocytosis of RBCs, increase in monocyte count, and existence of NRBCs prompt complications in erythropoiesis (Buoro et al., 2016). NRBCs are considered prematurely released red cells and are rarely present in adults. Its presence signifies the existence of anemic conditions, shortness of breath, and reduced clinical improvement (Constantino and Cogionis, 2000). Anisocytosis in red cells was well noticed in female patients with MDRPTB. The present study observed NRBC in a limited number of patients. The reduced HGB, HCT, indices of red cells MCV, MCH, and MCHC, and increased anisocytosis around microcytic cells (RDWCV) in female patients in the MDRPTB group were manifested. Patients with diabetes and TB reported an increase in HGB, ESR, and reduced lymphocyte count compared with TB (Alisjahbana et al., 2007). The comorbidities in TB infection demonstrated a reduced level of RBC, HGB, HCT, and anisocytosis with an increase in MCV, MCH, and MCHC levels.
The increase in PLT and its indices, PCT and PDW, in TB relates to the severity of lung infection and inflammation carried out in culture-positive patients (?ahin et al., 2012). The inflammatory mediator interleukin-6 enhances the production of PLTs as they involve in the strategies of the immune system (Unsal, 2005). Male patients recorded increased PCT (Kartloglu et al., 2001). The present study observed increased PLT counts and PCT in MDRPTB patients in gender categorization and in clinical conditions. The indices of PLT, PDW, MPV, and PLCR, increased in female subjects and in subjects with a combination of comorbidities than subjects with MDRPTB alone.
The ESR was elevated in female compared to male patients diagnosed with TB (Sulochana et al., 2018). The other study found an increased sedimentation of red cells in HIV and TB than in HIV and nontuberculous mycobacterial infection (Cai et al., 2017). Furthermore, the elevated ESR in male and in MDRPTB patients with comorbidities was there upon recorded. There was no increase in immune cells in patients with comorbid conditions of diabetes with TB (Al-Attiyah and Mustafa, 2009). Some studies illustrated a difference in the immune responses in allied clinical conditions with TB (Al-Attiyah and Mustafa, 2009; Tsukaguchi et al., 1999). The present study is similar to some previous studies.
CONCLUSION
Our study envisions differences in the hematological parameters between male and female patients. Microcytic hypochromic anemia, reduced HCT, presence of anisocytosis, increased eosinophils, PLTs, and its indices suggest monitoring female patients in specific. Medical issues in combination with MDRPTB determine prominent changes in leucocytes, monocytes, MCV, MCH, and anisocytosis. This study contributes the management of the hematological abnormalities resulting from anti-TB drug treatment, which should be addressed throughout the course of the therapy.
ACKNOWLEDGMENTS
The authors sincerely thank Dr. Sravan Kumar, Superintendent of Govt. Chest and TB hospital, Warangal, Telangana, India, for permission to utilize the hospital facilities. The authors are grateful to Dr. S. Vasudeva Murthy, Principal, Jayamukhi College of Pharmacy, for his illimitable support, motivation, and critical analysis of the research work. They are also grateful to the participants in accomplishing the research.
LIST OF ABBREVIATIONS
ESR: Erythrocyte sedimentation rate; HCT: Hematocrit; HGB: Hemoglobin; PCT: Plateletcrit; PDW: Platelet distribution width; PLCR: Platelet large cell ratio; PLT: Platelet count; RBC: Red blood cell; RDW: Red blood cell distribution width; RDW-CV: Red blood cell distribution width—coefficient of variation; RDW-SD: Red blood cell distribution width—standard deviation; RVD: Retroviral disease; TB: Tuberculosis; MCHC: Mean corpuscular hemoglobin concentration; MCH: Mean corpuscular hemoglobin; MCV: Mean corpuscular volume; MDR-TB: Multidrug-resistant TB; MPV: Mean platelet volume; NRBC: Nucleated red blood cell; WBC: White blood cell
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abbas B, Mustapha Dogara M, Muhammad Sani N, Mohammed Gumel A, Lawal D. Assessment of haematological parameters of pulmonary tuberculosis patients with and without HIV infection attending two secondary health facilities in Jigawa State. Int J Microbiol Biotechnol, 2020; 5(3):103–9. CrossRef
Agbor A, Bigna J, Plottel C, Billong S, Tejiokem M, Ekali G, Noubiap JJ, Toby R, Abessolo H, Koulla-Shiro S. Characteristics of patients co-infected with HIV at the time of inpatient tuberculosis treatment initiation in Yaoundé, Cameroon: a tertiary care hospital-based cross-sectional study. Arch Public Health, 2015; 73(1):24. CrossRef
Ahmed S, Mohammed A. Effects of thyroid dysfunction on hematological parameters: case controlled study. Ann Med Surg, 2020; 57:52–5. CrossRef
Al-Attiyah R, Mustafa A. Mycobacterial antigen-induced T helper type 1 (Th1) and Th2 reactivity of peripheral blood mononuclear cells from diabetic and non-diabetic tuberculosis patients and Mycobacterium bovisbacilli Calmette-Guérin (BCG)-vaccinated healthy subjects. Clin Exper Immunol, 2009; 158(1):64–73. CrossRef
Alisjahbana B, Sahiratmadja E, Nelwan E, Purwa A, Ahmad Y, Ottenhoff TH, Nelwan RH, Parwati I, van der Meer JW, van Crevel R. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin Infect Dis, 2007; 45(4):428–35. CrossRef
Beard J. Iron requirements in adolescent females. J Nutr 2000; 130(2):440S–2S. CrossRef
Berkowitz F. Hemolysis and infection: categories and mechanisms of their interrelationship. Clin Infect Dis, 1991; 13(6):1151–62. CrossRef
Bozóky G, Ruby E, Góhér I, Tóth J, Mohos A. Hematologic abnormalities in pulmonary tuberculosis. Orv Hetil, 1997; 138(17):1053–6.
Buoro S, Manenti B, Seghezzi M. Which clinical significance has automatic detection of very low levels of nucleated red blood cells in the peripheral blood? Ann Translat Med 2016; 4(11):230–30. CrossRef
Cai R, Yu F, Tao Z, Qian X, Chen J, Lu H. Routinely detected indicators in plasma have a predictive effect on the identification of HIV-infected patients with non-tuberculous mycobacterial and tuberculous infections. Infect Dis Povert 2017; 6(1):162. CrossRef
Chertov O, Ueda H, Xu L, Tani K, Murphy W, Wang J, Howard OM, Sayers TJ, Oppenheim JJ. Identification of human neutrophil-derived Cathepsin G and Azurocidin/CAP37 as chemoattractants for mononuclear cells and neutrophils. J Exper Med 1997; 186(5):739–47. CrossRef
Comstock G. Tuberculosis studies in Muscogee County, Georgia 1: I. community-wide tuberculosis research. Am J Epidemiol, 2008; 168(7):687–91. CrossRef
Constantino B, Cogionis B. Nucleated RBCs—significance in the peripheral blood film. Lab Med, 2000; 31(4):223–9. CrossRef
de Mendonça E, Schmaltz C, Sant’Anna F, Vizzoni A, Mendes-de-Almeida D, de Oliveira R, Rolla VC. Anemia in tuberculosis cases: a biomarker of severity? PLoS One, 2021; 16(2):e0245458. CrossRef
Fossati C. The basophil cells of the blood in tuberculosis. Acta Med Scand, 2009; 138(6):457–9. CrossRef
Global tuberculosis report 2020. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO.
Gomez DI, Twahirwa M, Schlesinger LS, Restrepo BI. Reduced Mycobacterium tuberculosis association with monocytes from diabetes patients that have poor glucose control. Tuberculosis, 2013; 93(2):192–7. CrossRef
Ige O, Akinlade K, Rahamon S, Edem V, Arinola O. Thyroid function in multidrug-resistant tuberculosis patients with or without human immunodeficiency virus (HIV) infection before commencement of MDR-TB drug regimen. Afr Health Sci, 2016; 16(2):596–602. CrossRef
Jamil A, Gohar A, Haider Ali. Screening of diabetes and HIV infection in newly diagnosed pulmonary tuberculosis patients. J Med Physiol Biophys, 2019; 55:92–6.
Javed I, Javed M, Mahmood Z, Riaz M, Iqbal R, Rasul A. Hematological profiling of tuberculosis-infected and co-morbid patients: a study carried out in central Punjab, Pakistan. Eur J Inflamm, 2018; 16:205873921881868. CrossRef
Kartloglu Z, Cerrahoglu K, Okutan O, Ozturk A, Aydilek R. Parameters of blood coagulation in patients with pulmonary tuberculosis. Inter J Intern Med, 2001; 2(2):1–4.
Khalil N, Ramadan R. Study of risk factors for pulmonary tuberculosis among diabetes mellitus patients. Egypt J Chest Dis Tubercul, 2016; 65(4):817–23. CrossRef
Kolobovnikova Y, Urazova O, Novitskii V, Mikheeva K, Goncharov M. Molecular mechanisms of formation of blood eosinophilia under pulmonary tuberculosis. Ann Russ Acad Med Sci, 2012; 67(5):58–62. CrossRef
Mirlohi M, Ekrami A, Shirali S, Ghobeishavi M, Pourmotahari F. Hematological and liver toxicity of anti-tuberculosis drugs. Electron Phys, 2016; 8(9):3005–10. CrossRef
Moore J, Schneider S. Acute human immunodeficiency virus (HIV) infection presenting with fever, elevated amylase/lipase, and hematologic abnormalities. J Emerg Med, 2013; 44(5):e341–4.
National framework for joint TB-Diabetes collaborative activities. Revised National Tuberculosis Control Program (RNTCP). National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS). Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India, 2017.
Nordenfelt P, Tapper H. Phagosome dynamics during phagocytosis by neutrophils. J Leuk Biol, 2011; 90(2):271–84. CrossRef
Olakanmi O, Schlesinger L, Ahmed A, Britigan B. Intraphagosomal Mycobacterium tuberculosis acquires iron from both extracellular transferrin and intracellular iron pools. J Biol Chem, 2002; 277(51):49727–34. CrossRef
Patin E, Hasan M, Bergstedt J, Rouilly V, Libri V, Urrutia A, Alanio C, Scepanovic P, Hammer C, Jönsson F, Beitz B, Quach H, Lim YW, Hunkapiller J, Zepeda M, Green C, Piasecka B, Leloup C, Rogge L, Huetz F, Peguillet I, Lantz O, Fontes M, Di Santo JP, Thomas S, Fellay J, Duffy D, Quintana-Murci L, Albert ML; Milieu Intérieur Consortium. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat Immunol, 2018; 19(6):645. CrossRef
Pathak S, Wentzel-Larsen T, Asjo B. Effects of in vitro HIV-1 infection on mycobacterial growth in peripheral blood monocyte-derived macrophages. Infect Immun, 2010; 78:4022–32. CrossRef
?ahin F, Yazar E, Y?ld?z P. Prominent features of platelet count, plateletcrit, mean platelet volume and platelet distribution width in pulmonary tuberculosis. Multidisciplin Respir Med, 2012; 7:38. CrossRef
Serbina N, Jia T, Hohl T, Pamer E. Monocyte-mediated defense against microbial pathogens. Ann Rev Immunol, 2008; 26(1):421–52. CrossRef
Singh KJ, Ahulwalia G, Sharma SK, Saxena R, Chaudhary VP, Anant M. Significance of hematological manifestations in patients with tuberculosis. J Asso Physicians Ind, 2001; 49:788–94.
Sulochana S, Subhashini V, Srinivasan C. Pulmonary tuberculosis—a prospective analysis of hematological changes. Asian J Pharma Clin Res, 2018; 11(4):169–72. CrossRef
Tsukaguchi K, de Lange B, Boom W. Differential regulation of IFN-γ, TNF-α, and IL-10 production by CD4+ αβTCR+ T Cells and Vδ2+ γδ T cells in response to monocytes infected with Mycobacterium tuberculosis-H37Ra. Cell Immunol, 1999; 194(1):12–20. CrossRef
Unsal E. Potential role of interleukin-6 in reactive thrombocytosis and acute phase response in pulmonary tuberculosis. Postgrad Med J, 2005; 81(959):604–7. CrossRef
van den Hof S, Tursynbayeva A, Abildaev T, Adenov M, Pak S, Ismailov S. HIV and multidrug-resistant tuberculosis: overlapping risk factors. Eur Respir J, 2015; 45(2):567–9. CrossRef