INTRODUCTION
Edible mushrooms are excellent source of nutritious and unique umami-taste food. They are rich in carbohydrates, proteins, fibers, vitamins, minerals, and low-fat content (Beelman et al., 2019; Martinez-Medina et al., 2021). They also have interesting content of minerals such as potassium, calcium, phosphorus, magnesium, iron, manganese, zinc, sodium, copper, and selenium (Murugesan, 2017; Painuli et al., 2020). Apart from the nutritional values, edible mushrooms have also been exploited for a very long time as natural alternative remedy for various diseases. The therapeutic values and medicinal properties of edible mushrooms have found to stem from numerous biologically active compounds or metabolites (Ho et al., 2020; Lu et al., 2020; Painuli et al., 2020). Mushrooms have a wide variety of bioactive compounds, which have been shown to exhibit several biological activities including antimicrobial, cytotoxic, anti-inflammatory, antioxidant antidiabetic, anti-dyslipidemia, anti-hypertension, anti-obesity, antitumor, hepatoprotective, immunomodulatory, and antiviral (Dicks and Ellinger, 2020; Kupcova et al., 2018; Lu et al., 2020; Wang, 2020).
Several studies on the anticancer, antitumor, anti-proliferative, cytotoxic effects of mushrooms have been reported, which are strong evidence of high interest of many researchers around the world on mushrooms as natural sources of cytotoxic compounds. Chaitanya et al. (2019) enumerated the different cytotoxic compounds derived from mushrooms, including ganoderic acid, grifolin, ergosterol, trametenolic acid, inotodiol, lanostanes, ergosterol peroxide, hispidin, psilocin, psilocybin, bufotenin, aeruginascin, linolenic acid, eryngeolysin, ostreolysin, lecitins, laccases, beta-1,3-glucan, and lentinan. Mushroom metabolites such as lentinan, antrodan, hispolon, and macrocybin isolated from Lentinus edodes, Antrodia cinnamomea, Phellinus linteus, Macrocybe titans, have been found to be responsible for the cytotoxicity in breast cancer, prostate, nasopharyngeal, renal, and lung cancers, respectively (Ho et al., 2017; Li et al., 2018; Peng et al., 2015; Vilariño et al., 2020; Yun et al., 2019). Moreover, Bekci et al. (2019) reported the anticancer activities of Amanita caesarea, Sparassis crispa, Lepista nuda, Auricularia auricula, Tricholoma terreum, and Lentinus tigrinus against PC-3 and DU-14 prostate cancer cell lines. The antineoplastic effectiveness of Antrodia camphorata, Coriolus sinensis, Coriolus versicolor, Ganoderma lucidum, Ganoderma frondosa, and L. edodes in human clinical trials for breast cancer has been also demonstrated (Wong et al., 2020). In addition, our team elucidated the remarkable cytotoxicity of Gymnopilus purpureosquamulosus compared with ethanolic extracts from other wild Philippine mushrooms against hematologic malignant cells through activation of the stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) signaling pathway (Dulay et al., 2021).
Lentinus species, belong to Family Polyporaceae, are wild wood-rotting basidiomycetous mushrooms that grows solitary or in cluster on dead log and trunk of a tree. Their fruiting bodies have small to large, white to brown, convex at first to nearly flat, smooth to scaly, ?eshy ?rm to tough pileus with sawtooth-edge gills. The pileus is attached to centrally to eccentrically short, tough, scaly stipe. Their spore print is white to yellowish. Lentinus species are nutritious and medicinal mushroom. Lentinus tigrinus, for instance, contain carbohydrates, proteins, sugars, fiber, lipids and minerals, and show antioxidant, antibacterial and anti-hyperglycemic activities (Dulay et al., 2014, 2017). They are widely distributed in the different areas in the Philippines, mostly during the months of May to October (Arenas et al., 2015; De Leon et al., 2013; De Castro and Dulay, 2015; Dulay and Maglasang, 2017; Dulay et al., 2020). Recently, the fruiting bodies of different species of Lentinus were collected from the different areas of Luzon Island including Quezon Province, Camarines Sur, Ilocos Sur, Cagayan, Zambales, Tarlac and Nueva Ecija, and were successfully isolated through tissue culture. Mycelial biomasses of the different Lentinus isolates were mass produced using their optimal submerged culture conditions.
Herein, we reported the cytotoxicity of ethanolic extracts of Lentinus mycelia against human colorectal carcinoma (HCT-116), hepatocellular carcinoma (HepG2), and normal kidney epithelial (HK-2) cell lines using MTS proliferation assay.
MATERIALS AND METHODS
Mushroom culture
Pure cultures of the different isolates of Lentinus species were acquired from the culture collections of the Center for Tropical Mushroom Research and Development, and Tuklas Lunas Development Center, Department of Biological Sciences, College of Science, Central Luzon State University, Science City of Muñoz, Nueva Ecija, Philippines. The identity based on the morphological and molecular characteristics, culture code, and place of origin of the different Lentinus isolates are summarized in Table 1. Agar blocks of mycelia were sub-cultured on potato dextrose agar plates and incubated at 28°C for 7 days to allow mycelial growth. These cultures were used as source of inoculant for mass production of mycelial biomass in submerged culture.
Mass production of mycelia
Mass production of mycelia was carried out by inoculating mycelia discs into culture bottles containing 30 ml of the best medium with the optimum pH, and incubated at the required temperature, illumination, and agitation conditions. Thirty replicates of mycelial cultures for each mushroom were done. After 7 days of incubation, the mycelia were harvested, air-dried at air-conditioned room for 3 days, and prepared for extraction.
 | Table 1. The different isolates of Lentinus collected from the different areas of Luzon. [Click here to view] |
Preparation of crude extracts
The ethanol extraction protocols as described by Dulay et al. (2014) and Boukes et al. (2017) were followed with minor modifications. Five grams of each powdered mushroom mycelia were soaked in 200 ml of 95% ethanol for 2 days and then filtered using Whatman # 1 filter paper. The filtrates were concentrated using a rotary evaporator and freeze-dried. The extract yield was recorded. Extracts were dissolved to a working concentration of 2,000 µg ml−1 of the extract in a final nontoxic concentration of 0.2% dimethyl sulfoxide (DMSO) (Buhian et al., 2018; Lu et al., 2010; Tan et al., 2018). These were vortexed for 30 minutes, centrifuged at 17,000 × g for 10 minutes, filter-sterilized using a 0.2 µM syringe filter and used for cytotoxicity test.
Cell lines
Human colorectal carcinoma (HCT-116), hepatocellular carcinoma (HepG2), and normal kidney epithelial (HK-2) cell lines of the American Type Culture Collection (ATCC, Manassas, VA) were acquired from the Cell-Based Assay Laboratory, Central Luzon State University. HCT-116 and HepG2 cells were grown in McCoy’s 5A medium and Eagle’s minimum essential medium (ATCC, Manassas, VA), respectively, supplemented with 10% fetal bovine serum and 1% Pen-Strep (penicillin + streptomycin). HK-2 cells were grown in keratinocyte serum free medium (ATCC, Manassas, VA) added with 0.05 mg ml−1 bovine pituitary extract, 5 ng ml−1 recombinant epidermal growth factor and 5 µg ml−1 gentamicin (Gibco, USA) in T75 flask (Corning, NY). Cell cultures were maintained under the following conditions: 5% CO2, 95% humidity and 37°C in an incubator until 90% confluent.
MTS proliferation assay
The cytotoxic effect of mushroom extracts was evaluated using CellTiter 96® Aqueous One Solution MTS Proliferation Assay Kit (Promega Co., Madison, WI). All cell lines at 90% confluence were harvested and the viable cell count was determined using trypan blue exclusion method. The cell density was adjusted to 4.5 × 104 viable cells ml−1 (Romero-Benavides et al.,2018). A 100 µl volume of viable cells was seeded into each well of a 96-well culture plate (Corning, NY), and subsequently incubated for 24 hours under 5% CO2, 95% humidity and 37°C conditions to allow attachment of cells and formation of monolayers. After incubation, 100 µl of each two-fold serial dilution of the prepared filter-sterilized working concentrations (2,000 µg ml−1) of mushroom extracts were added into each of the wells, resulting in final concentrations of 15.6, 31.25, 62.5, 125, 250, 500 and 1,000 µg ml−1. The cytotoxic compound 5-flurouracil (5-FU) (Sigma-Aldrich Co., St. Louis, MO) was used as the positive control in concentrations of 15.6, 31.25, 62.5, 125, 250, 500 and 1,000 µg ml−1, while media-DMSO dimethyl sulfoxide was used as a solvent vehicle control. Untreated wells served as the untreated negative controls. Each treatment was assayed in triplicate. Two assay trials were done in this sub-study. Assay plates were incubated under the same conditions. After 72 hours of treatment exposure, 10 µl of MTS tetrazolium solution (Promega Co., Madison, WI) were added and the set up was incubated for 30 minutes. The absorbance was measured at a wavelength of 492 nm using a microplate reader. Cytotoxicity indices were calculated using the equation, CI% = 100-{[(mean of treated absorbance values) / (mean of untreated absorbance values)] × 100}. Cytotoxicity graphs were constructed and the IC50 values were derived via Fit Spline/LOWESS in GraphPad Prism version 9.2.0 for Windows (GraphPad Software, San Diego, CA, www.graphpad.com).
RESULTS AND DISCUSSION
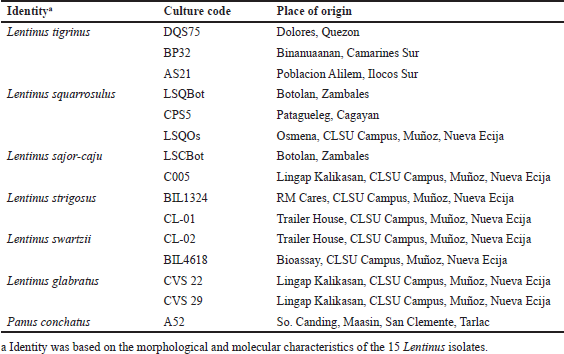
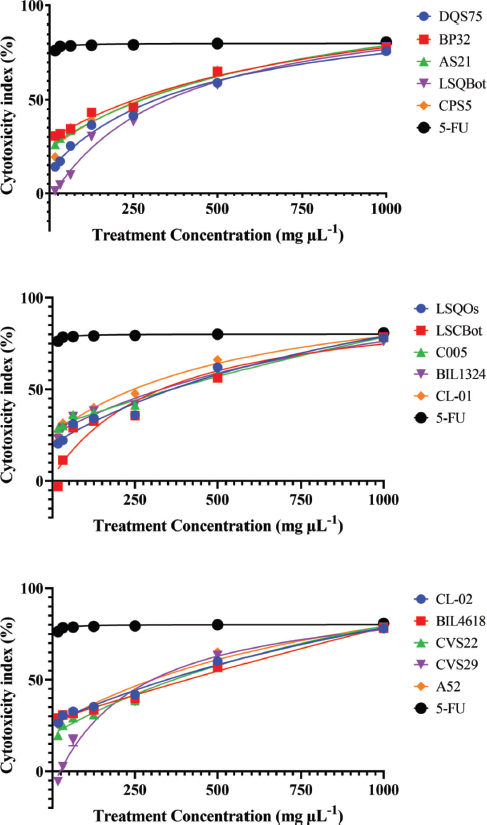
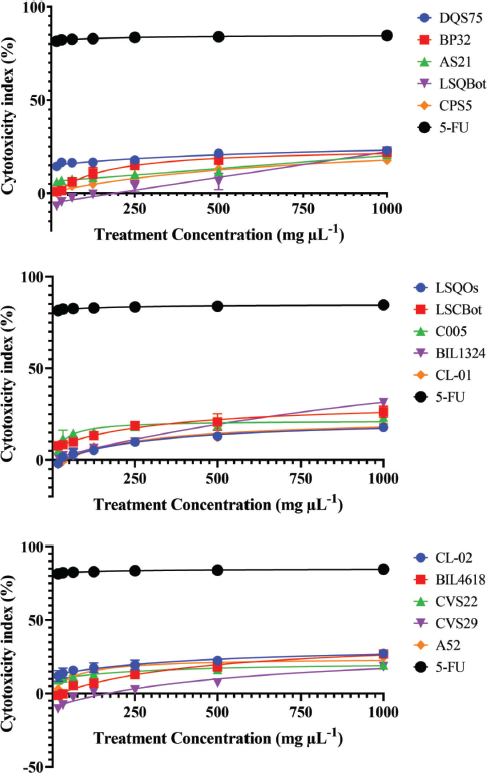
Mushrooms are the store houses of new anticancer compounds (Chaitanya et al., 2019). In the Philippines, the anticancer properties of many wild mushrooms remain to be unexplored. For this reason, this current study evaluated the cytotoxic effects of ethanolic extracts of the mycelia of 15 Lentinus isolates that were mass produced in their respective optimized submerged culture conditions against HCT-116 colon cancer and HepG2 hepatocellular carcinoma cell lines, and on normal HK-2 cell line using the MTS proliferation assay. This assay detects the reduction of MTS tetrazolium compound (Owen’s reagent) into a formazan product (yellow to violet) by nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) or nicotinamide adenine dinucleotide hydrogen (NADH) through the activity of the mitochondrial dehydrogenase in metabolically active cells (Berridge and Tan, 1993). The cytotoxicity index values for each extract were calculated based on the absorbance readings and then plotted against the extract concentrations. The cytotoxicity index plots showing the concentration-dependent cytotoxicity of the mushroom extracts for HCT-116, wherein the cytotoxicity indices increased with increasing extract concentrations (Fig. 1). The 15 mushroom extracts were found to be not toxic to the hepatocellular carcinoma (HepG2) and normal kidney epithelial (HK-2) cells, which demonstrated less than 50% cytotoxicity indices (Figs. 2 and 3).
The IC50 values of the mushroom extracts were determined using Fit Spline/LOWESS analysis of CI% values plotted against extract concentrations. Table 2 summarizes the IC50 values of the mushroom extracts and 5-FU afforded by the three cell lines. For HCT-116 colon cancer cells, the IC50 values of mushroom extracts ranged from 242.75 to 444.79 µg ml−1. Among the mushroom extracts, CL-01 extract was the most potent with the lowest IC50 value, whereas LSCBot extract registered the highest IC50 value. Statistical analysis revealed that the obtained IC50 values of mushroom extracts were significantly higher (p ? 0.01–0.0001) than that of the anticancer agent 5-FU (4.41 µg ml−1).
In the US National Cancer Institute plant screening program, a crude extract with an IC50 value ?20 µg ml−1 after 48–72 hours exposure is generally considered to have in vitro cytotoxic activity (Boik, 2001; Kuete et al., 2013). However, for mushrooms, Boukes et al. (2017) established that crude mushroom extracts with IC50 values of ?100 and 100–200 µg ml−1 are considered highly and moderately cytotoxic, respectively. They also added that crude extracts with IC50 values >100 µg ml−1 may cytotoxic compounds but at low concentrations. Accordingly, results of the present study suggest that all Lentinus extracts tested have cytotoxicity against HCT-116.
On the other hand, the IC50 value could not be determined for all extract-treated HepG2 and HK-2 cells using the range of concentrations tested in the study. Hence, the IC50 values were reported as >1,000 µg ml−1, which was the highest concentration tested. In contrast, 5-FU exhibited high cytotoxic activity to HepG2 and HK-2 cells with IC50 values of 3.77 and 9.35 µg ml−1, respectively. The vehicle control, 0.2% dimethyl sulfoxide (DMSO) in media, showed no cytotoxic activities against the three cell lines (data were not shown).
 | Figure 1. Cytotoxicity index plots for HCT-116 human colorectal carcinoma cell line after 72-hour exposure to varying concentrations of ethanolic extracts of Lentinus isolates and the positive control 5-FU. DQS75, BP32, AS21—L. tigrinus; LSQBot, CPS5, LSQOs—L. squarrosulus; LSCBot, C005—L. sajor-caju; BIL1324, CL-01—L. strigosus; CL-02, BIL4618—L. swartzii; CVS22, CVS29—L. glabratus; A52—P. conchatus. [Click here to view] |
Although the ethanol extracts of Lentinus mycelia showed low cytotoxic activities on human colorectal carcinoma (HCT-116) as indicated by high IC50 values that ranged from 242.75 to 444.79 µg ml−1, these are comparable with the reported IC50 values of acetone extract (376 µg ml−1) and n-hexane extract (434 µg ml−1) of L. tigrinus against HepG2 cells (Sadi et al., 2015), and within the reported cytotoxic concentration range of 100–500 μg ml−1 of 79 kDa polysaccharide obtained from P. sajor-caju against the AGS human gastric carcinoma and HepG2 cell lines (Seedevi et al., 2019). Moreover, the IC50 values of the extracts in the current study are lower than those of the aqueous extracts of Lentinus sajor-caju (formerly known as Pleurotus sajor-caju), which demonstrated inhibitory activity against the proliferation of the human tumor cell lines laryngeal carcinoma (Hep-2) and cervical adenocarcinoma (HeLa) with IC50 values of 0.64% (or 6,400 µg ml−1) and 0.25% (or 2,500 µg ml−1), respectively (Finimundy et al., 2013).
 | Figure 2. Cytotoxicity index plots for HepG2 human hepatocellular carcinoma cell line after 72-hour exposure to varying concentrations of ethanolic extracts of Lentinus isolates and the positive control 5-FU. DQS75, BP32, AS21—L. tigrinus; LSQBot, CPS5, LSQOs—L. squarrosulus; LSCBot, C005—L. sajor-caju; BIL1324, CL-01—L. strigosus; CL-02, BIL4618—L. swartzii; CVS22, CVS29—L. glabratus; A52—P. conchatus. [Click here to view] |
 | Figure 3. Cytotoxicity index plots for HK-2 normal human kidney cell line after 72-hour exposure to varying concentrations of ethanolic extracts of Lentinus isolates and the positive control 5-FU. DQS75, BP32, AS21—L. tigrinus; LSQBot, CPS5, LSQOs—L. squarrosulus; LSCBot, C005—L. sajor-caju; BIL1324, CL-01—L. strigosus; CL-02, BIL4618—L. swartzii; CVS22, CVS29—L. glabratus; A52—P. conchatus. [Click here to view] |
On the other hand, the IC50 values obtained in the present study are higher when compared to the IC50 values of soluble protein fraction from L. tigrinus against MCF-7 (193.5 µg ml−1) and PC3 (33.6 µg ml−1) cancer cell lines (Mohammadnejad et al., 2019), and to the IC50 values of peptide extracted from the fruiting bodies of Lentinus squarrosulus against H460 (26.84 µg ml−1), H292 (2.80 µg ml−1) and H23 (18.84 µg ml−1) lung cancer cell lines (Prateep et al., 2017).
Moreover, Zajac et al. (2021) reported the anticancer activities of intracellular fractions from L. sajor-caju mycelia against colorectal cancer cell lines (HT-29, LS180 and SW948). Additionally, hypnophilin, a sesquiterpene isolated from Lentinus strigosus showed cytotoxicity against human skin melanoma (UACC-62) cells via induction of DNA fragmentation and morphological alterations, and calcium signaling (Pinto et al., 2013). However, no previous work has been done on the cytotoxicity evaluation of Lentinus swartzii, Lentinus glabratus and Panus conchatus extracts, which suggests furtherance of this study. In other basidiomycetes, ethanolic extracts of Fomitopsis lilacinogilva and Pycnoporus saguineus showed high cytotoxicity against HeLa, HT-29, MCF-7, MIA PaCa-2, and PC-3 cancer cell lines, whereas Gymnopilus junonius ethanol extract displayed high cytotoxic activities against HeLa and MIA PaCa-2 cells (Boukes et al., 2017).
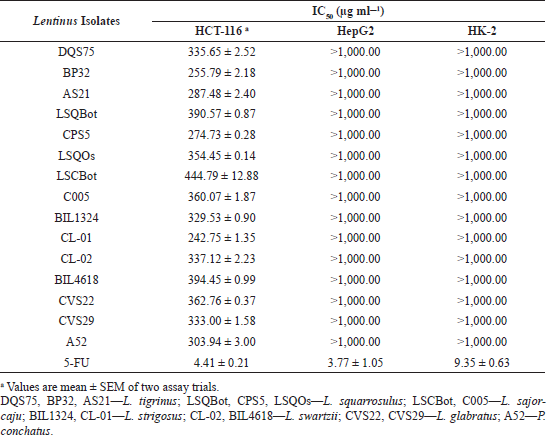 | Table 2. Inhibitory concentration 50% (IC50) values of the ethanolic extracts of 15 Lentinus isolates on the human cancer and normal cell lines tested. [Click here to view] |
Accordingly, the degree of cytotoxicity of Lentinus species may vary depending on the type of cancer cell lines, extracting solvent used, and isolated bioactive compounds tested. Therefore, the present work cannot rule out the cytotoxicity of ethanolic extracts of the 15 Lentinus mycelia against other cancer cell lines, which were not tested in this study. The use of other extracting solvent and the isolation of bioactive components of Lentinus mycelia for cytotoxicity assessment merits further investigation.
Among the chemical components of mushroom, polysaccharides have been widely studied for several biological activities including anticancer properties (Wang, 2020). Lentinan, for instance, an isolated component of highly distilled polysaccharide from L. edodes (shiitake), has been commonly utilized as anticancer drug against lung, stomach, ovarian, liver and colon cancer in Japan (Uddin Pk et al., 2020). Additionally, lentinan has been used as adjuvant therapy for treating pancreatic, gastric, colorectal, cardiac, nasopharyngeal, lung, ovarian, and cervical cancers and for non-Hodgkin’s lymphoma (Zhang et al., 2019). Aside from polysaccharides, the anticancer activity of wild medicinal mushrooms has been linked to the presence of phytochemicals, saponins, alkaloids, and phenolic compounds by interfering with particular signal transduction pathways, which play crucial role in the development and progression of cancer (De Silva et al., 2012; Lavi et al., 2012). Chemical composition analysis by silica gel chromatography and chemical structure analysis by nuclear magnetic resonance (NMR) spectroscopy of dichloromethane extract of the fruiting bodies of L. tigrinus yielded cerevisterol, stellasterol and ergosterol, which are reported to exhibit various bioactivities (Ragasa et al., 2018). Stellasterol was found to be one of the contributors of apoptosis and cell cycle arrest against the human cancer cell lines, MCF-7 and SH-SY5Y (Pereira et al., 2013).
Moreover, methanolic fractions (FR1, FR2 and FR3) of L. squarrosulus separated using reversed phase-high performance liquid chromatography exhibited significant antioxidant activity in cupric reducing antioxidant capacity assay. The FR3 fraction was found to be the most active and had free radical inhibitors such as uridine, ganoderic acid derivatives and flavonoids based on the liquid chromatography-mass spectrometry (Abdullah et al., 2018). Gas chromatography-mass spectrometry analysis of the ethanolic fraction of L. squarrosulus showed fatty acids and esters (39%), alkanes (23%), alkenes (13%), ketones (11%), alcohols (8%), aldehyde (3%) and others (3%), and identified 38 volatile compounds and the most prevailing were 9,12-octadecadienoic acid (Z, Z-), methyl ester (methyl linoleate) (24.21%), steroid ergosterol (16.98%), hexadecanoic acid methyl ester (methyl palmitate) (10.74%), methyl 2-oxo-1-pyrrolidine actetate (10.40%), ergosta-7, 22-dien-3-ol, (3.beta, 5.alpha 22E) (4.62%) and 9-octadecenoic acid, methyl ester (4.18%) (Reena Roy et al., 2020). Results of the above-cited recent studies established that Lentinus species can be source of bioactive compounds which possess bioactivities such as antimicrobial, antioxidant and anticancer properties.
It has been reported that there are several factors that affects the level and quality of chemical compositions of mushrooms, which could possibly explain the difference or inconsistency on the bioactivities of mushroom of same genus. Aside from geographical locations and climatic conditions, the chemical compositions may also vary depending on strains, nutrient value of the substrate, time of harvest, handling conditions and preparation techniques, biomass type, developmental stages, exposure to stress conditions such as UV radiation, extreme temperature, air pollution, wounding, parasites and infections (Barros et al., 2009; Eguchi et al., 2015; Dulay and Pamiloza, 2018; Manzi et al., 2001).
CONCLUSION
In conclusion, the cytotocixity screening showed that ethanolic extracts of mycelia of 15 Lentinus isolates had cytotoxicity against HCT-116 human colorectal carcinoma and non-cytotoxicity to HepG2 hepatocellular carcinoma and HK-2 normal kidney epithelial cell lines. The cytotoxicity of Lentinus species and other macrofungal species in the Philippines must be further studied using different extracting solvents and cancer cell lines, including their underlying mechanisms of action.
ACKNOWLEDGMENT
The primary author would like to thank the Department of Science and Technology—Science Education Institute (DOST-SEI) in the Philippines for scholarship grant. This study was supported by the CLSU Tuklas Lunas Program funded by the Philippine Council for Health Research and Development, Department of Science and Technology. The authors would also like to express their sincerest gratitude to Ms. Dana Theresa De Leon and Mr. Rence Marrion Pineda for their technical assistance.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
FUNDING
Science Education Institute, and Philippine Council for Health Research and Development, Department of Science and Technology.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdullah S, Abdullah N, Sabaratnam V, Yap KC. CUPRAC assay-guided profiling of antioxidant compounds in methanol extract of Lentinus squarrosulus Mont. mycelium. Acta Agric Slov, 2018; 111(1):101–9. CrossRef
Arenas MC, Tadiosa ER, Alejandro GJD, Reyes RG. Macroscopic fungal flora of Mts. Palaypalay—Mataas na gulod protected landscape, Southern Luzon, Philippines. Asian J Biodivers, 2015; 6(1):1–22. CrossRef
Barros I, Duenas M, Ferreira ICFR, Baptista P, Santos-Buelga C. Phenolic acids determination by HPLC-DAD-ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem Toxicol. 2009; 47:1076–9. CrossRef
Beelman RB, Kalaras MD, Richie Jr. JP. Micronutrients and bioactive compounds in mushrooms: a recipe for healthy aging? Nutr Today, 2019; 54:16–22. CrossRef
Bekci H, Cam M, Cumaoglu A. Some wild edible mushroom anticancer activity against prostate cell lines. Proceedings, 2019; 40(1):40. CrossRef
Berridge MV, Tan AS. Characterization of the cellular reduction of 3-(4,5-dimethlthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys, 1993; 303:474–82. CrossRef
Boik J. Natural compounds in cancer therapy. Oregon Medical Press, Princeton, MI, 2001.
Boukes GJ, Koekemoer TC, van de Venter M, Govender S. Cytotoxicity of thirteen South African macrofungal species against five cancer cell lines. South Afr J Bot, 2017; 113:62–7. CrossRef
Buhian SPC, Oyong GG, Cabrera EC. Cytotoxicity of Peperomia pellucida (L.) HBK extracts on cancer cell lines and their effects on cfos and cjun genes. Philipp Agric Sci, 2019; 102(1):27–35.
Chaitanya MVNL, Jose A, Ramalingam P, Mandal SC, Narendra Kumar P. Multi-targeting cytotoxic drug leads from mushrooms. Asia Pac J Trop Med, 2019; 12(12):531–6. CrossRef
De Castro MEG, Dulay RMR. Macrofungi in multi-storey agroforestry systems in Mt. Makiling Forest Reserve, Los Banos, Laguna, Philippines. J Chem Biol Phys Sci, 2015; 5(2):1646–55.
De Leon AM, Luangsa-ard JJD, Karunarathna SC, Hyde KD, Reyes RG, dela Cruz TEE. Species listing, distribution, and molecular identification of macrofungi in six Aeta tribal communities in Central Luzon, Philippines. Mycosphere, 2013; 4(3):478–94. CrossRef
De Silva DD, Rapior S, Fons F, Bahkali AH, Hyde KD. Medicinal mushrooms in supportive cancer therapies: an approach to anti-cancer effects and putative mechanisms of action. Fungal Divers, 2012; 55:1–35. CrossRef
Dicks L, Ellinger S. Effect of the intake of oyster mushrooms (Pleurotus ostreatus) on cardiometabolic parameters—a systematic review of clinical trials. Nutrients, 2020; 12(4):1134. CrossRef
Dulay RM, Valdez BC, Li Y, Chakrabarti S, Dhillon B, Kalaw SP, Reyes RG, Cabrera EC. Cytotoxicity of Gymnopilus purpureosquamulosus extracts on hematologic malignant cells through activation of the SAPK/JNK signaling pathway. PLoS One, 2021; 16(5):e0252541. CrossRef
Dulay RMR, Arenas MC, Kalaw SP, Reyes RG, Cabrera EC. Proximate composition and functionality of the culinary-medicinal tiger sawgill mushroom, Lentinus tigrinus (Higher basidiomycetes), from the Philippines. Int J Med Mushrooms, 2014; 16(1):85–94. CrossRef
Dulay RMR, Carandang JS VI, Kalaw SP, Reyes RG. Distribution and species listing of wild macrofungi in Sitio Canding, Barangay Maasin, San Clemente, Tarlac Province, Philippines. J Appl Biol Biotechnol, 2020; 8(05):7–15.
Dulay RMR, Maglasang CC. Species listing of naturally occurring mushrooms in agroecosystem of Barangay Bambanaba, Cuyapo, Nueva Ecija, Philippines. Int J Biol Pharm All Sci, 2017; 6(8):1459–72.
Dulay RMR, Miranda LA, Malasaga JS, Kalaw SP, Reyes RG, Hou CT. Antioxidant and antibacterial activities of acetonitrile and hexane extracts of Lentinus tigrinus and Pleurotus djamour. Biocatal Agric Biotechnol, 2017; 9:141–4. CrossRef
Dulay RMR, Pamiloza DG. Proximate composition and bioactivities of hairy sawgill mushroom, Lentinus strigosus (BIL 1324) from the Philippines. Int J Biol Pharm All Sci, 2018; 7(3):361–9. CrossRef
Eguchi F, Kalaw SP, Dulay RMR, Miyasawa N, Yoshimoto H, Seyama T, Reyes RG. Nutrient composition and functional activity of different stages in the fruiting body development of Philippine paddy straw mushroom, Volvariella volvacea (Bull.:Fr.) Sing. Adv Environ Biol, 2015; 9(22):54–65.
Finimundy TC, Abreu RMV, Bonetto N, Scariot FJ, Dillon AJP, Echeverrigaray S, Barros L, Ferreira ICFR, Henriques JAP, Roesch-Ely M. Apoptosis induction by Pleurotus sajor-caju (Fr.) Singer extracts on colorectal cancer cell lines. Food Chem Toxicol, 2018; 112:383. CrossRef
Ho HY, Ho YC, Hsieh MJ, Yang SF, Chuang CY, Lin CW, Hsin CH. Hispolon suppresses migration and invasion of human nasopharyngeal carcinoma cells by inhibiting the urokinase-plasminogen activator through modulation of the Akt signaling pathway. Environ Toxicol, 2017; 32:645–55. CrossRef
Ho LH, Zulkifli NA, Tan TC. Edible mushroom: nutritional properties, potential nutraceutical values, and its utilisation in food product development, an introduction to mushroom, Ajit Kumar Passari and Sergio Sánchez. IntechOpen Limited 5 Princes Gate Court, London, SW7 2QJ, United Kingdom, 2020. CrossRef
Kuete V, Seo EJ, Krusche B, Oswald M, Wiench B, Schroder S, Greten HJ, Lee IS, Efferth T. Cytotoxicity and pharmacogenomics of medicinal plants from traditional Korean medicine. Evid-Based Complement Alternat Med, 2013; 2013:14. CrossRef
Kupcova K, Stefanova I, Plavcova Z, Hosek J, Hrouzek P, Kubec R. Antimicrobial, cytotoxic, anti-inflammatory, and antioxidant activity of culinary processed shiitake medicinal mushroom (Lentinus edodes, Agaricomycetes) and its major sulfur sensory-active compound lenthionine. Int J Med Mushrooms, 2018; 20(2):165–75. CrossRef
Lavi I, Nimri L, Levinson D, Peri I, Hadar Y, Schwartz B. Glucans from the edible mushroom Pleurotus pulmonarius inhibit colitis-associated colon carcinogenesis in mice. J Gastroenterol, 2012; 47:504–18. CrossRef
Li W, Wang J, Hu H, Li Q, Liu Y, Wang K. Functional polysaccharide Lentinan suppresses human breast cancer growth via inducing autophagy and caspase-7-mediated apoptosis. J Funct Foods, 2018; 45:75. CrossRef
Lu MK, Shih YW, Chang Chien TT, Fang LH, Huang HC, Chen PS. α-Solanine inhibits human melanoma cell migration and invasion by reducing matrix metalloproteinase-2/9 activities. Biol Pharma Bull, 2010; 33(10):1685–91. CrossRef
Lu X, Brennan MA, Narciso J, Guan W, Zhang J, Yuan L, Serventi L, Brennan CS. Correlations between the phenolic and fibre composition of mushrooms and the glycaemic and textural characteristics of mushroom enriched extruded products. LWT: Food Sci Technol, 2020; 118:108730. CrossRef
Manzi P, Aguzzi A, Pizzoferrato L. Nutritional value of mushrooms widely consumed in Italy. Food Chem, 2001; 73:321–5. CrossRef
Martinez-Medina GA, Chavez-Gonzalez ML, Verma DK, Prado-Barragan LA, Martínez-Hernandez JL, Flores-Gallegos AC, Thakur M, Srivastav PP, Aguilar CN. Bio-funcional components in mushrooms, a health opportunity: ergothionine and huitlacohe as recent trends. J Funct Foods, 2021; 77:104326. CrossRef
Mohammadnejad S, Pourianfar HR, Drakhshan A, Jabaleh I, Potent antiproliferative and proapoptotic effects of a soluble protein fraction from culinary medicinal mushroom Lentinus tigrinus on cancer cells. J Food Measure Character, 2019; 2019:1–10. CrossRef
Murugesan S. Sustainable food security: edible and medicinal mushroom. In: Sustainable agriculture towards food security, Springer, Singapore Pte Ltd., pp 185–96, 2017. CrossRef
Painuli S, Semwal P, Egbuna C. Mushroom: nutraceutical, mineral, proximate constituents and bioactive component. In: Egbuna C, Dable Tupas G (eds.). Functional foods and nutraceuticals, Springer Nature Switzerland AG, 2020. CrossRef
Peng CC, Lin YT, Chen KC, Chyau CC, Peng RY. Antrodan, a β-glucan obtained from Antrodia cinnamomea mycelia, is beneficial to benign prostate hyperplasia. Food Funct, 2015; 6:635–45. CrossRef
Pereira DM, Correia-da-Silva G, Valentão P, Teixeira N, Andrade PB. Palmitic acid and ergosta-7,22-dien-3-ol contribute to the apoptotic effect and cell cycle arrest of an extract from Marthasterias glacialis L. in neuroblastoma cells. Marine Drugs, 2013; 12(1):54–68. CrossRef
Pinto MCX, Cota BB, Rodrigues MA, Leite MF, de Souza-Fagundes EM. The cytotoxic and proapoptotic activities of hypnophilin are associated with calcium signalling in UACC-62 cells. J Biochem Mol Toxicol, 2013; 27(11):479–85. CrossRef
Prateep A, Sumkhemthong S, Suksomtip M, Chanvorachote P, Chaotham C. Peptides extracted from edible mushroom: Lentinus squarrosulus induces apoptosis in human lung cancer cells. Pharm Biol, 2017; 55(1):1792–9. CrossRef
Ragasa CY, Tan MCS, De Castro MEG, De Los Reyes MM, Oyong GG, Shen CC. Sterols from Lentinu tigrinus. Pharma J, 2018; 10(6):1079–81. CrossRef
Reena Roy D, Kandagalla S, Krishnappa M. Exploring the ethnomycological potential of Lentinus squarrosulus Mont. through GC–MS and chemoinformatics tools. Mycology, 2020; 11:78–89. CrossRef
Romero-Benavides JC, Ortega-Torres GC, Villacis J, Vivanco-Jaramillo SL, Galarza-Urgilés KI, Bailon-Moscoso N. Phytochemical study and evaluation of the cytotoxic properties of methanolic extract from Baccharis obtusifolia. Int J Med Chem, 2018; 2018:8908435. CrossRef
Sadi G, Emsen B, Kaya A, Kocabas A, Çinar S, Kartal DI. Cytotoxicity of some edible mushrooms extracts over liver hepatocellular carcinoma cells in conjunction with their antioxidant and antibacterial properties. Pharma Mag, 2015; 11 Suppl S1:6–18. CrossRef
Seedevi P, Ganesan AR, Mohan K, Raguraman V, Sivakumar M, Sivasankar P, Loganathan S, Rajamalar P, Vairamani S, Shanmugam A. Chemical structure and biological properties of a polysaccharide isolated from Pleurotus sajor-caju. RSC Adv, 2019; 9:20472. CrossRef
Tan MS, Oyong GG, Shen CC, Ragasa CY. Cytotoxic activities of the dichloromethane extracts from Andrographis paniculata (Burm. f.) Nees. J Nat Sci Biol Med, 2018; 9:201–6. CrossRef
Uddin Pk M, Pervin R, Jahan J, Talukder RI, Ahmed S., Rahman M. Mushroom Polysaccharides: Chemistry and Anticancer Potentials, An introduction to mushroom, Ajit Kumar Passari and Sergio Sánchez. IntechOpen Limited 5 Princes Gate Court, London, SW7 2QJ, United Kingdom, 2020. CrossRef
Vilariño M, García-Sanmartín J, Ochoa-Callejero L, López-Rodríguez A, Blanco-Urgoiti J, Martíne A. Macrocybin, a natural mushroom triglyceride, reduces tumor growth in vitro and in vivo through caveolin-mediated interference with the actin cytoskeleton. Molecules, 2020; 25:6010. CrossRef
Wang CA. A review on the potential reuse of functional polysaccharides extracted from the by-products of mushroom processing. Food Bioproc Technol, 2020; 13:217–28. CrossRef
Wong JH, Ng TB, Chan HHL, Liu Q, Man GCW, Zhang CZ, Guan S, Ng CCW, Fang EF, Wang H, Liu F, Ye X, Rolka K, Naude R, Zhao S, Sha O, Li C, Xia L. Mushroom extracts and compounds with suppressive action on breast cancer: evidence from studies using cultured cancer cells, tumor-bearing animals, and clinical trials. Appl Microbiol Biotechnol, 2020; 104(11):4675–703. CrossRef
Yun JM, Min KJ, Kwon TK. Involvement of up-regulation of death receptors and bim in hispolon-mediated TNF-related apoptosis-inducing ligand sensitization in human renal carcinoma. J Cancer Prevent, 2019; 24:155–62. CrossRef
Zajac A, Piet M, Stefaniuk D, Chojnacki M, Jakubowicz-Gil J, Paduch R, Matuszewska A, Jaszek M. Pro-health and anticancer activity of fungal fractions isolated from milk-supplemented cultures of Lentinus (Pleurotus) sajor-caju. Biomolecules, 2021; 11:1089. CrossRef
Zhang M, Zhang Y, Zhang L, Tian Q. Chapter thirteen -mushroom polysaccharide lentinan for treating different types of cancers: a review of 12 years clinical studies in china. In: Zhang L (ed.). Progress in molecular biology and translational science, Academic Press, Cambridge, MA, pp 297–328, vol. 163, 2019. CrossRef