INTRODUCTION
Cachexia is a multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass (with or without the loss of fat mass) that is unable to be reversed. It is caused by an underlying disease or diseases, and it is frequently (but not always) accompanied by anorexia (Fearon et al., 2011). The onset of cachexia is mainly due to an increased inflammatory status, muscle proteolysis, impaired carbohydrate, and protein and lipid metabolism. Inflammation plays the most essential role in the pathophysiology of cachexia, with an imbalance of pro- and anti-inflammatory cytokines now thought to be the cause. An excessive increase in IL-6 levels raises C-reactive protein which causes weight loss (Woo et al., 1999). Tumors release soluble substances that operate on skeletal muscle to cause wasting, in addition to tissues and organs (Baracos et al., 2018). Cachexia affects 50%–80% of cancer patients, and cachexia is responsible for 20% of cancer patient fatalities (Suzuki et al., 2013). Overactivation of various intracellular proteolytic systems, such as the ubiquitin-proteasome and autophagy, causes skeletal muscle atrophy. Anabolic capacity, autophagy, and myogenesis are engaged as a compensatory mechanism. This demonstrates that the right treatment can prevent tumor hosts from wasting due to malignancies (Penna et al., 2019). Cachexia in breast cancer patients is less addressed, as in advanced cases it decreases fat and abdominal muscle leading to cachexia, which further increases mortality (Nikita et al., 2016). Currently, there is no effective treatment which can completely reverses cachexia. However, the nutritional supplements or drugs that can modulate catabolic processes, cell injury, and inflammation are under consideration and might be a good approach to find the cure for cachexia (Rogers and Minteer, 2020).
Over the years, apart from modern medicine, researchers were successful in finding a remedy with alternative medicines, various herbs, and their combinations for various diseases. In the present study, HIM-CHX, which is a combination of oleo-gum resin of Boswellia serrata Roxb. ex Colebr, stem of Cissus quadrangularis L., and the roots of Withania somnifera (L.) Dunal, was evaluated in the experimental model of breast cancer-induced cachexia. The herbs used in HIM-CHX possess properties that ameliorate some of the pathophysiologic pathways that result in cachexia (Sharma et al., 2000). Boswellia serrata possesses anti-inflammatory (Aggarwal et al., 2011) and analgesic properties (Hamidpour et al., 2013); C. quadrangularis helps improve appetite (Mukherjee et al., 2016) and regulates inflammation (Shaikh et al., 2016); and W. somnifera possesses rejuvenating and anxiolytic properties (Singh et al., 2011). In this study, DMBA, which is a potent organ-specific carcinogen, was used for the development of mammary cancer-induced cachexia in rats. DMBA metabolizes to form dihydrodiol peroxide, a carcinogen which binds with adenine residues of DNA, resulting in mutagenesis and carcinogenesis. The histogenesis, morphological and biochemical features, and development of hyperplastic premalignant and malignant lesions with DMBA resemble that of human breast cancer (Wang and Zhang, 2017).
The efficacy of HIM-CHX in sarcopenia, which is one of the muscle-wasting diseases, has been established and published (Azeemuddin et al., 2019). The objective of this study is to evaluate its effect in other muscle-wasting diseases. Thus, HIM-CHX was evaluated for its efficacy in the experimental models of muscle atrophy, mammary cancer-induced cachexia, and longevity of cachectic animals.
MATERIALS AND METHODS
Chemicals
Isoflurane was bought from Neon, Mumbai, India; Cisplatin was bought from Fresenius Kabi India Pvt Ltd, Pune, Maharashtra, India; DMBA was bought from Sigma Aldrich, St. Louis, MO; C2C12 cells was bought from ATCC, USA; RPMI 1640 medium was bought from HiMedia Laboratories Pvt. Ltd. Mumbai, India; and all other chemicals were procured from Sigma Chemical Co., St. Louis, MO. Tumor necrosis factor (TNF)-α, interleukin (IL)-6, and nuclear factor kappa B (NF-κB) were analyzed using the enzyme-linked immunosorbent assay method and the assay kits were procured from Krishgen Biosystems, Mumbai, Maharashtra, India
Preparation and characterization of HIM-CHX
HIM-CHX is a code provided to the herbal blend of the alcoholic extract (95%) of B. serrata Roxb. ex Colebr. Oleo-gum resin, aqueous extract of C. quadrangularis L. stem, and hydroalcoholic extract of W. somnifera (L.) Dunal roots. The extracts were prepared in the R&D Center of Himalaya Wellness Company and the solvent for extraction was selected to extract more yields of herbal actives responsible for the desired activity. The finger printing and characterization of bioactive compounds of HIM-CHX were carried out using thin layer chromatography and liquid chromatography–mass spectrometry. The active compounds identified and characterized are α-boswellic acid and β-boswellic acid, 11-keto-β-boswellic acid (KBA), 3-acetyl-11-keto- β-boswellic acid (AKBA), 3-acetyl-β-boswellic acid, and 3-acetyl-α-boswellic acid, Withanolide L, E, F, H, J, K, and Withaferin A. The mass spectra and the analytical details are published in the journal of Experimental Gerontology (Azeemuddin et al., 2019).
The combination of HIM-CHX was selected based on the modern literature and ayurvedic wisdom. The core principle of ayurvedic medicine is to treat the disease in a holistic manner and to address the overall condition of the disease instead of targeting one mechanism or pathway. In traditional ayurvedic wisdom, the herbs selected for HIM-CHX are known to ameliorate the conditions associated with inflammation, appetite, stress, and muscle weakness, such as cachexia. They are also reported in the modern literature to interfere with various pathophysiological pathways which form the hallmark of cachexia. The proposed polyherbal combination HIM-CHX combines three medicinal ingredients which are additive to one another and are expected to function in a synergistic manner and holistically manage the disease.
Experimental animals
In-house bred female Wistar rats (12 weeks old) were procured from the central animal house facility (R&D, Himalaya Wellness Company, Bengaluru, Karnataka, India). The experimental protocols were approved by the Institutional Animal Ethics Committee of Himalaya Wellness Company (protocol no. 164/16) and the animals received care as per the guidelines prescribed by the Committee for the Purpose of Control and Supervision on Experiments on Animals, Government of India.
Effect of HIM-CHX on cisplatin-induced C2C12 cells atrophy
C2C12 (mouse skeletal muscle cells) myoblasts were used to evaluate the effect of HIM-CHX on muscle atrophy. The procedure was followed as per the research article published by Burattini et al. (2004). The morphological changes and the present state of differentiation of C2C12 myotubes were checked microscopically. After the initiation of the differentiation procedure, the wells were equally divided in to three groups of vehicle control, Cisplatin, and Cisplatin + HIM-CHX; the myotubes were treated with 50 μM Cisplatin for 24 hours to induce atrophy along with 50 μg/ml concentration of HIM-CHX to examine the counteract atrophy effect for the active ones. The myotubes length of the control, Cisplatin, and HIM-CHX-treated samples were measured by randomly counting at least 25 cells in the microscopic field view.
Evaluation of HIM-CHX in an experimental model of DMBA-induced cancer cachexia in rats
The experiment was of a curative design. Forty female Wistar rats (12 weeks old) were acclimatized for 1 week, after which they were administered with DMBA at a dose of 25 mg/kg b.wt. sc. near to the mammary glands to induce mammary carcinoma. The appearance and development of tumors was observed daily; the palpable tumor appeared after 12 weeks. Eight rats served as a normal control group and were injected with 0.1 ml Phosphate Buffer Saline (PBS). Based on the size of the tumor, 32 rats were selected and segregated into 4 groups of 8 each. The first group was the normal control; the second was the cachectic control (untreated); and groups 3–5 were treated with HIM-CHX for 4 weeks at doses of 125, 250, and 500 mg/kg body weight po, respectively. Body weight and feed intake were recorded every week. At the end of the treatment period, the animals were anesthetized using isoflurane and blood was collected from retro-orbital sinus and serum was separated for the estimation of TNF-α, IL-6, NF-κB and myostatin. After collecting blood, the animals were euthanized using excess isoflurane anesthesia; and the weights of gastrocnemius muscle, carcass, and tumor were measured. The gastrocnemius muscle of one limb was subjected for gene expression studies to evaluate insulin-like growth factor (IGF)-1, IL-15, and muscle ring finger (MuRF)-1 genes using real-time polymerase chain reaction (qRT-PCR model-CFX96TM real-time system, Bio Rad, USA) technique and the other limb for histopathological evaluation (Tepe et al., 2013).
Evaluation of HIM-CHX on the longevity of DMBA-induced cancer (mammary tumor) cachectic rats
Twenty female Wistar rats (12 weeks) were acclimatized for 1 week. After acclimatization, the animals were administered with a single dose of DMBA (25 mg/kg sc), a laboratory carcinogen near to the mammary glands to induce mammary carcinoma. Animals were observed daily for the clinical signs and tumor development for 12 weeks. After confirmation of the tumor development and based on the tumor volume, 16 animals were selected and segregated into 2 groups of 8 each. The first group served as the control and was treated with water 10 ml/kg b.wt. po and the second group was treated with HIM-CHX at a dose of 500 mg/kg b.wt. po and observed for the total mortality of the animals (Reddy et al., 2012).
Statistical analysis
The values are expressed as the mean ± standard deviation. The results were statistically analyzed by one-way analysis of variance (ANOVA), followed by Dunnett’s multiple comparison test, The survival data were plotted on the Kaplan–Meier curve. The Prism GraphPad 6.07 software (GraphPad Software Inc., San Diego, CA) was used for statistical analysis. A p-value <0.05 was considered statistically significant.
RESULTS
HIM-CHX was subjected to efficacy studies after its characterization. The efficacy of HIM-CHX was first evaluated in the experimental model of cisplatin-induced muscle atrophy. The assay showed that the myotubes length of C2C12 (skeletal muscle cell) was greater in the HIM-CHX-treated group compared to the Cisplatin group. The length of the myotubes was significantly (p < 0.0001) longer in HIM-CHX-treated group compared to Cisplatin. This showed the beneficial effect of HIM-CHX in preventing or delaying muscle atrophy (Figs. 1 and 2).
In an experimental model of DMBA-induced cancer cachexia, the effect of HIM-CHX on body weight, feed intake, gastrocnemius muscle mass, carcass weight, proinflammatory cytokines, myokines, and proteolytic and protein regulators genes was evaluated. The obtained values were compared with the cachectic control. A significant increase in absolute body weight at 500 mg/kg (p < 0.05) and significant increase in food intake at 250 mg/kg (p < 0.05) and 500 mg/kg (p < 0.01) was observed in HIM-CHX’ treated rats (Figs. 3 and 4). A significant increase in the gastrocnemius muscle weight at 250 mg/kg (p < 0.05) and 500 mg/kg (p < 0.05) and carcass weight at 500 mg/kg (p < 0.05) was observed. A decrease in tumor weight was also observed but not found to be statistically significant. The elevated levels of TNF-α, IL-6, and myostatin were significantly decreased with all the three doses of HIM-CHX and a marginal decrease in NF-kB and creatine kinase level was also observed (Table 1). These findings suggest that HIM-CHX is efficient in decreasing the elevated proinflammatory cytokines and regulating the markers of muscle tissue damage, which are the typical pathological markers of cachexia.
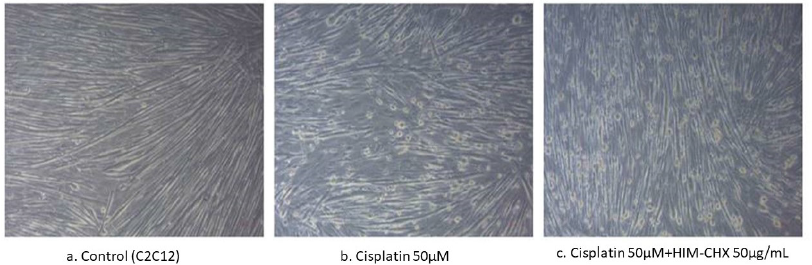 | Figure 1 Microscopy images of C2C12 cells (skeletal muscle cells) myotubes. (a) Control group exhibiting intact differentiated myotubes; (b) Cisplatin showing decreased myotube length; (c) HIM-CHX-treated group showing increased myotube length. [Click here to view] |
 | Figure 2. Effect of HIM-CHX on myotubes length in the experimental model of Cisplatin-induced muscle atrophy of C2C12 cells. [Click here to view] |
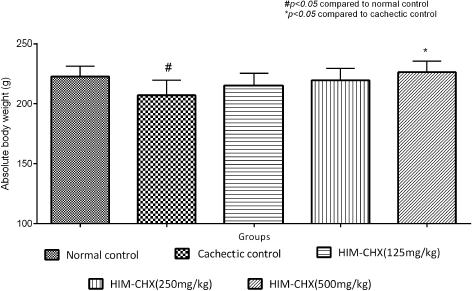 | Figure 3. Effect of HIM-CHX on absolute body weight (body weight–tumor weight) of DMBA-induced cancer cachectic rats. [Click here to view] |
In the gene expression studies of the gastrocnemius muscle, at 60°C annealing temperature, the cachectic rats had lower levels of IGF-1 and IL-15 and higher levels of MuRF-1 compared with normal control rats. The rats treated with HIM-CHX showed downregulation of MuRF-1 and upregulation of IGF-1 and IL-15 genes compared with the cachectic control (Fig. 5). A dose-dependent improvement in the muscle fibers was observed in the histopathological evaluation of gastrocnemius muscle of the animals treated with HIM-CHX, which showed its beneficial effect in muscle atrophy (Fig. 6).
In the longevity study, the administration of DMBA (25 mg/kg sc) showed the development of mammary tumors after 12 weeks in Wistar rats. The survival of the cachectic animals treated with HIM-CHX at a dose of 500 mg/kg b.wt. showed a significant (p < 0.01) increase in the survival percentage compared to control animals (Fig. 7).
DISCUSSION
HIM-CHX was evaluated for efficacy studies in experimental models of cachexia. Cisplatin is a first-line drug used for the treatment of lung cancer with strong emetic activity. There is evidence that a high dose and prolonged use of cisplatin induce anorexia, loss of adipose tissue, and skeletal muscle (Wu et al., 2019). In this study, a higher concentration of cisplatin was used to induce atrophy of C2C12 cells which are skeletal muscle cells that differentiate and rapidly form myotubes and produce muscle proteins. Hence, C2C12 myoblasts cells are used to study muscle development and differentiation (Cole et al., 2018).
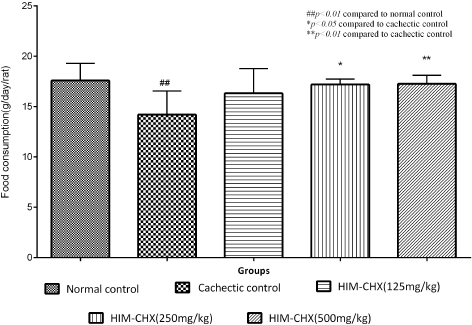 | Figure 4. Food intake of the DMBA-induced cancer cachectic rats after 4 weeks of treatment with HIM-CHX. [Click here to view] |
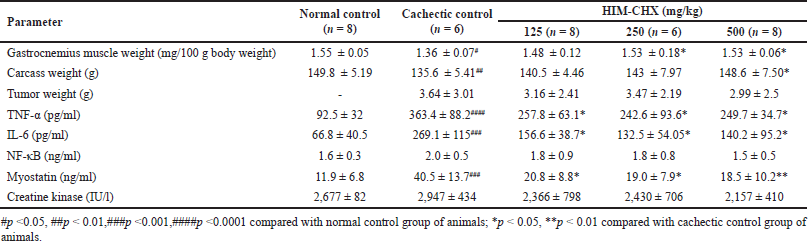 | Table 1. Effect of HIM-CHX on the muscle mass, proinflammatory cytokines, and myokines of cancer cachectic rats. [Click here to view] |
Cachexia is a muscle-wasting syndrome associated with most of the chronic diseases. The imbalances in pro- and anti-inflammatory cytokines, protein synthesis, and protein degradation are some of the important factors which lead to cachexia. The enhanced catabolism experienced by cancer patients with cachexia is mediated primarily by increases in pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, and interferon gamma (IFN-γ). Cachexia is also a product of tumor progression, as the tumor grows it activates an uncontrolled increase in pro-inflammatory cytokines that initiates cancer-related muscle-wasting during end-stage disease (Iwata, 2016). The extent of muscle tissue damage in cachexia is measured by estimating the levels of creatine kinase in the blood (Straughn and Karkar, 2019). The gene expression analysis showed that HIM-CHX regulated the protein degradation and protein synthesis genes in a way required for the improvement of cachexia.
Different types of cancer and chemotherapy are known to decrease the life expectancy of patients to a varying extent depending on the type of cancer. The increase in the life span of cancer patients is an indicator of recovery from cancer and its complication. The increase in the longevity of the cancer cachectic rats with the treatment of HIM-CHX indicates its overall beneficial effect on cancer and its complication especially cachexia, as the mortality of cancer patients due to cachexia is very high.
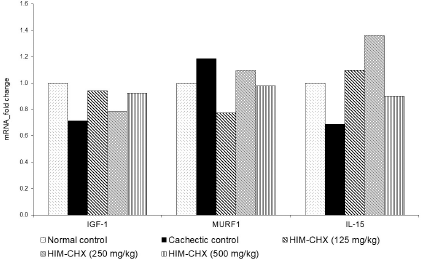 | Figure 5. Effect of HIM-CHX on gene expression studies of DMBA-induced cancer cachectic rats. [Click here to view] |
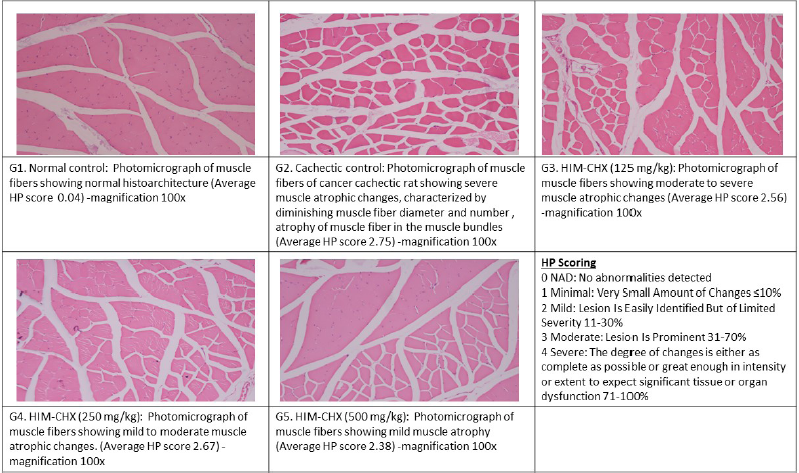 | Figure 6. Histopathological evaluation of the gastrocnemius muscle in DMBA-induced cachectic rats and HIM-CHX-treated rats. [Click here to view] |
HIM-CHX has decelerated most of the pathophysiologic changes of cancer-induced cachexia. This may be due to the presence of herbal actives like AKBA and Withaferin A, which possess the property to downregulate TNF-α, IL-1, IL-2, IL-4, IL-6, IFN-γ, and Nf-κB in chronic inflammatory conditions which are useful in ameliorating cachexia (Ammon, 2010). The probable mechanism of action of HIM-CHX on cachexia is by downregulating the overexpression of proinflammatory cytokines (TNF-α and IL-6); regulating myostatin, IGF-1, and IL-15; and downregulating MuRF-1, which is the ubiquitin ligase responsible for muscle atrophy. The effect of HIM-CHX on these signaling pathways resulted in the decrease in proinflammation and protein degradation and increase in protein synthesis, which finally decelerates the muscle-wasting process.
 | Figure 7. Effect of HIM-CHX on survival of DMBA-induced cachectic rats (Kaplan–Meier survival curve). [Click here to view] |
CONCLUSION
Treatment with HIM-CHX was found to be useful in ameliorating the muscle wasting in the selected experimental models, dose, and mode of administration. Thus, HIM-CHX may be used individually or in combination with nutritional supplements to decelerate or halt the muscle-wasting condition. It may also be recommended as an adjuvant to delay the complications of cancer and chemotherapy. However, further experimental and clinical studies are required to explore other mechanisms of action.
ACKNOWLEDGMENT
The authors acknowledge the Phytochemistry, Natural Product Innovations, and Preclinical Pharmacology and Toxicology Departments, R&D Center, Himalaya Wellness Company, for providing support during the study.
CONFLICT OF INTEREST
Some of the authors are the employees of Himalaya Wellness Company, Bangalore, Karnataka, India. The authors declare no other conflict of interest.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
FUNDING
No formal funding was received from any agency for this study.
REFERENCES
Aggarwal BB, Prasad S, Reuter S, Kannappan R, R Yadav V, Park B, Hye Kim J, C Gupta S, Phromnoi K, Sundaram C, Prasad S. Identification of novel anti-inflammatory agents from ayurvedic medicine for prevention of chronic diseases: reverse pharmacology and bedside to bench approach. Curr Drug Targets, 2011; 12(11):1595–653. CrossRef
Ammon HPT. Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine, 2010; 17(11):862–7. CrossRef
Azeemuddin MM, Rao CM, Rafiq M, Babu UV, Rangesh P. Pharmacological investigation of ‘HIM-CHX’: a herbal combination in the experimental muscle wasting condition. Exp Gerontol, 2019; 125:110663. CrossRef
Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers, 2018; 18 (4):17105. CrossRef
Burattini S, Ferri R, Battistelli M, Curci R, Luchetti F, Falcieri E. C2C12 murine myoblasts as a model of skeletal muscle development: morpho-functional characterization. Eur J Histochem, 2004; 48:223–34.
Cole CL, Kleckner IR, Jatoi A, Schwarz E, Dunne RF. The role of systemic inflammation in cancer-associated muscle wasting and rationale for exercise as a therapeutic intervention. JCSM Clin Rep, 2018; 3(2):1–9. CrossRef
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol, 2011; 12(5):489–95. CrossRef
Hamidpour R, Hamidpour S, Hamidpour M, Shahlari M. Frankincense (R? Xi?ng; Boswellia species): from the selection of traditional applications to the novel phytotherapy for the prevention and treatment of serious diseases. J Tradit Complement Med, 2013; 3:221–6. CrossRef
Iwata Y, Suzuki N, Ohtake H, Kamauchi S, Hashimoto N, Kiyono T, Wakabayashi S. Cancer cachexia causes skeletal muscle damage via transient receptor potential vanilloid 2-independent mechanisms, unlike muscular dystrophy. J Cachexia Sarcopenia Muscle, 2016; 7(3):366–76. CrossRef
Mukherjee T, Saha N, Palbag S. Ethnopharmacology, phytochemistry and pharmacology of ayurvedic plant Hadjod - Cissus quadrangularis (L.). Int J Res Ayurveda Pharm, 2016; 7(4):78-82. CrossRef
Consul N, Guo X, Coker C, Lopez-Pintado S, Hibshoosh H, Zhao B, Kalinsky K, Acharyya S. Monitoring metastasis and cachexia in a patient with breast cancer: a case study. Clin Med Insights Oncol, 2016; 10:83–94. CrossRef
Penna F, Ballarò R, Beltrà M, De Lucia S, García Castillo L, Costelli P. The skeletal muscle as an active player against cancer cachexia. Front Physiol, 2019; 10:41. CrossRef
Reddy NS, Nirmala P, Chidambaram N, Kumar PA. Quercetin in dimethyl benzanthracene induced breast cancer in rats. Am J Pharmacol Toxicol, 2012; 7(2):68–72. CrossRef
Rogers JB, Minteer JF. Cachexia. StatPearls, Treasure Island, FL [Internet], 2020.
Shaikh RU, Pund MM, Gacche RN. Evaluation of anti-inflammatory activity of selected medicinal plants used in Indian traditional medication system in vitro as well as in vivo. J Tradit Complement Med, 2016; 6(4):355–61. CrossRef
Sharma PC, Yelne MB, Dennis TJ, Joshi A, Billore K V. Database on medicinal plants used in Ayurveda. Central Council for Research in Ayurveda & Siddha, Deptt. of ISM & H, Min. of Health & Family Welfare, Govt. of India, India, vol. 418, p 430, 2000.
Singh N, Bhalla M, de Jager P, Gilca M. An overview on Ashwagandha: a rasayana (Rejuvenator) of ayurveda. African J Tradit Complement Altern Med, 2011; 8 (5S):208–230. CrossRef
Straughn AR, Kakar SS. Withaferin A ameliorates ovarian cancer-induced cachexia and proinflammatory signaling. J Ovarian Res, 2019; b12(1):115. CrossRef
Suzuki H, Asakawa A, Amitani H, Nakamura N, Inui A. Cancer cachexia—pathophysiology and management. J Gastroenterol, 2013; 48(5):574–94. CrossRef
Tepe B, Tuncer E, Saraydän SU, Özer H, ?en M, Karadayi K, Inan DS, Karadayi S, Polat Z, Akpulat A, Duman M. Antitumoral effects of Allium sivasicum on breast cancer in vitro and in vivo. Mol Biol Rep, 2013; 40(1):597–604. CrossRef
Wang Z, Zhang X. Chemopreventive activity of Honokiol against 7, 12—dimethylbenz[a]anthracene-induced mammary cancer in female sprague dawley rats. Front Pharmacol, 2017; 17(6):676–95. CrossRef
Woo J, Ho SC, Yu AL. Walking speed and stride length predicts 36 months dependency, mortality, and institutionalization in Chinese aged 70 and older. J Am Geriatr Soc, 1999; 47(10):1257–60. CrossRef
Wu CT, Liao JM, Ko JL, Lee YL, Chang HY, Wu CH, Ou CC. D-Methionine ameliorates cisplatin-induced muscle atrophy via inhibition of muscle degradation pathway. Integr Cancer Ther, 2019; 18:1534735419828832. CrossRef