INTRODUCTION
Hypertension is a challenging global problem and is increasing day by day in every community (NCD-RisC, 2021). It is reported that the deaths due to hypertension-related diseases are approximately 9.4 million annually (Guwatudde et al., 2015). There are different types of antihypertensive medicines to treat this problem. The dihydropyridine (DHP)-based calcium channel blockers (CCBs) are considered frontline agents in hypertension treatment and support cardiovascular benefits (Pitt et al., 2000; Whelton et al., 2018). These CCBs are safe as they do not require routine electrolyte or kidney function monitoring, nor do they cause diuresis (Caballero-Gonzalez et al, 2015; Li et al., 2014; Makani et al., 2011; Mann and Hilger et al., 2019; Whelton et al., 2018). Basically, CCBs work on calcium channel proteins. Calcium channel proteins consisting of calcium selective pores are found in different parts of the human plasma membrane, like cardiac muscle neurons, vascular smooth muscles, etc. (Catterall, 2011), and controls various physiological processes including cardiac muscle contraction and neurotransmitter release (Dolphin, 2016). Calcium ion movement through the CaV1.2 channel of hypertensive patients takes place more and CCBs control these movements (Johnson et al., 2020). Antihypertensive drugs prevent the entrance of calcium into the cell by blocking calcium channels, thereby exhibiting the antihypertensive effect. CCBs also shows renoprotective effects (Jadhav et al., 2021). 1,4-DHPs like amlodipine and nifedipine have been used as antihypertensive drugs successfully for the last several decades (De Luca et al., 2019; Karthick et al., 2022). Any molecule that can block CaV1.2 could be useful in the treatment of hypertension. It is generally known that DHPs have the ability to block calcium channels, and it has been demonstrated that even little alterations in the structure of the DHP ring can result in significant changes in pharmacological effects. The biological action of DHP calcium channel antagonists has been observed to be affected by substituents at different locations in the DHP ring (Jamalian et al., 2011). The 1,8-acridinedione scaffold showed a variety of biological activities that were improved (Moallem et al., 2015; Xiong et al., 2018). Few works reported that acridinedione moiety-based derivatives had lower calcium antagonist activity (Jamalian et al., 2011). It is also reported that acridinedione-based derivatives possess the vasorelaxant effect (Imenshahidi et al., 2012). Another research group investigated their effects on vascular potassium channels, and the potassium channel in particular has several general features analogous to the calcium channel (Berkan et al., 2002). As a matter of fact, the antagonist activity is optimized by different substituents at different locations of the molecule. Therefore, our aim is to modulate the antagonist activity of acridinedione by modifying the scaffold using different substituents. We consider the basic skeleton of amlodipine–nifidipine and the acridinedione structure and use the thiophene as the modifier substituent. Thiophene-based molecules have been used as hypertensive drugs (Ronald et al., 1988), and so our interest is to check and modulate the DHP-based scaffolds activity by introducing thiophene moiety. Further tuning was performed by substitution in the thiophene moiety. An “in silico” approach is considered to be one of the most demanding methods in modern approach to find the potential molecules for many reasons. This motivates us to work with molecular docking and simulation analysis to search for new effective drugs.
MATERIALS AND METHODS
Proteins and ligands selection
A good quality 3D structure is necessary for the development of calcium CCBs and complete 3D structure of the L-type calcium channel of humans is yet not available. Bacterial voltage-gated Ca2+ channels are probably the evolutionary ancestors of human calcium channels (Tang et al., 2016). So, in this study, we took the crystal structure of calcium channels of Arcobacterbutzleri (CavAb) having Protein Data Bank IDs 5KLB and 5KLS, respectively (https://www.rcsb.org/). As our target is to design novel DHPs having calcium channel blocking ability better than nifedipine and amlodipine, we designed a library of 25 DHP-based derivatives. 2D structures were drawn using ChemSketch (Advanced Chemistry Development, Inc., 2021) and, subsequently, geometrically optimized using MOPAC software (Stewart Computational Chemistry, 2021).
Molecular docking
25 new derivatives along with nifedipine and amlodipine were docked with the 2 CavAb proteins (5KLB and 5KLS) using AutoDock 4.2 programme in order to explore the protein–ligand interactions and to recognize the potent drug for the treatment of diseases. Proteins were prepared using AutoDock tools, downloaded from MGLTools (2021). During the docking simulation, the Lamarckian genetic algorithm was employed. BIOVIA Discovery Studio Visualizer (2021) and Chimera software’s were used to visualise docked complexes and also to study the docking interactions.
Molecular dynamic (MD) simulations
MD simulations were executed to investigate the stability of the protein–ligand complexes. Ligand and Receptor Molecular Dynamics (LARMD) server (2018) was used to study the dynamics of the protein–ligand complexes. We have used the default option of LARMD server (2018) to run 1 nanosecond MD simulations. PDB files of the protein–ligand-docked complexes were uploaded on the LARMD server (2018). The explicit water model was chosen to run the simulations. In the LARMD server (2018), AMBER16 program and force field AMBER ff14SB were used. In order to investigate the stability of the protein–ligand complexes, we inspected the following parameters, root mean square deviation (RMSD), root mean square fluctuation (RMSF), and radius of gyration (Rg) of the docked complexes. MM/PB (GB) SA binding energy calculations on the simulated paths were also performed.
Drug-likeness and Absorption, Distribution, Metabolism, Elimination, Toxicity (ADMET) properties
The drug-likeness and ADME properties were calculated using online server SWISSADME (2017) and to check the bioavailability score. Molinspiraton online server (Molinspiraton) was also used.
RESULTS AND DISCUSSION
1,4-DHPs (or acridinedione) act as hydrogen donors and could be potential pharmacophores. In the case of the Hantzsch ester, the partially hydrogenated N-heteroaromatic DHP nucleus or its fragments, i.e., the NH group, act as hydrogen donors, which are required for antioxidaive activity. DHPs have substantial hydrogen donating ability due to the presence of labile hydrogen atoms (mostly at positions 1,4-) in their molecules. DHPs ensure their reductant role by donating electrons and/or hydrogen, resulting in antioxidant activity and antiradical activity. The oxidation of DHPs results in the formation of heteroaromatic pyridine derivatives, which operate as antioxidative and antiradical agents (Velena et al., 2016). Antioxidants will protect the cell from oxidative injury by preventing and inhibiting the factors that cause it. An antioxidant’s capacity to scavenge free radicals is thought to be linked to its ability to donate hydrogen. Along with its β-blocking ability, Amlodipine besylate (AMB) may easily scavenge free radicals in the biological system. AMB minimises oxidative stress in biological system and hypertension in patients in this way (Safna Hussan et al., 2019).
The interaction of newly designed calcium CCBs with the proteins involved has been identified at the atomic level.
Molecular docking and binding mode analysis
Both 5KLB and 5KLS are CavAb proteins consisting of four identical chains forming a homotetramer. Four chains, containing 286 amino acid residues each, are similar to four homologous repeats I, II, III, and IV of mammalian CaV channels. Each chain comprises six transmembrane helices, S1–S6, and supporting helices, P1 and P2, having two functional domains: S1–S4 constitutes the voltage-sensing domain and S5 and S6 along with P1 and P2 constitute the ion-conducting pore domain.
The ion-selective pore is located in the homotetramer of the CavAb channel. The calcium CCBs nifedipine and amlodipine are widely used and hence serve as control molecules in this investigation. DHP is distinguished by its heterocyclic ring, which inhibits the flow of extracellular Ca+2 via L-type voltage-dependent calcium channels. The distinctive chemical structure of amlodipine was developed from the DHP (Safna Hussan et al., 2019). El-Moselhy (2012) employed nifedipine as a calcium antagonist reference. The interaction of various residues has been used to explain the affinity of nifedipine as a reference molecule to the DHP receptor (El-Moselhy, 2012).
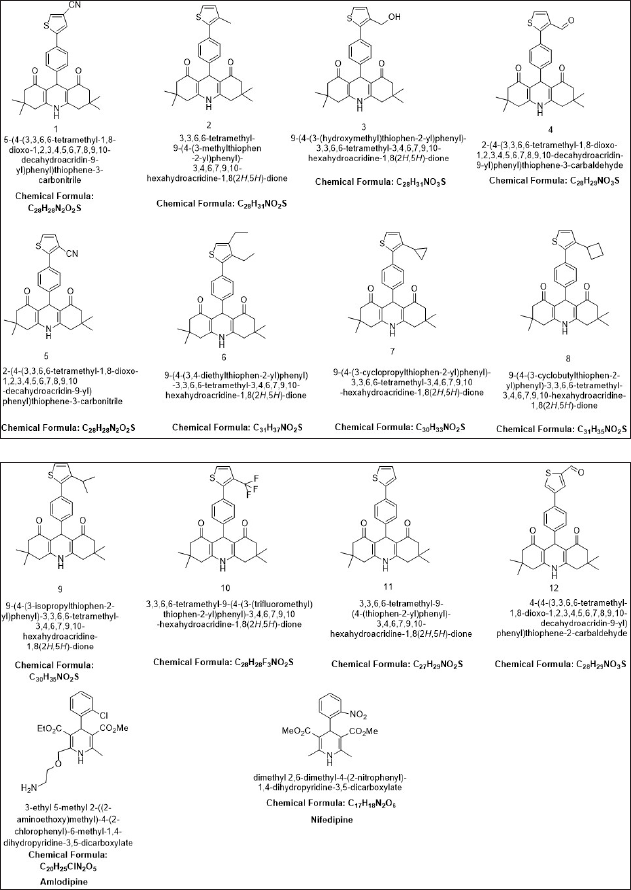 | Figure 1. Chemical structures of the thiophene-based derivatives. [Click here to view] |
Here, we are interested in designing calcium CCBs better than nifedipine and amlodipine. The calcium channel proteins were docked with 25 DHPs, as well as nifedipine and amlodipine. A molecular docking study was carried out on all of the newly developed DHPs derivatives; however, only 12 of them showed significantly greater binding affinity for the two target proteins (Figure 1 and Table 1) (Shityakov et al., 2014). The molecular docking analysis of the selected 12 designed derivatives along with control molecules with the proteins 5KLB and 5KLS showed that the majority of the compounds bind to 5KLB and 5KLS with favourable binding energies ranging between −10.05 and −6.86 kcal/mol. Among the compounds, compound 10 showed the lowest binding energy (−10.05 and −9.99 kcal/mol) with two target CavAb proteins. Safna Hussan et al. (2019) showed that the docking result of amlodipine based drug was of −7.2 kcal/mol (Safna Hussan et al., 2019). During the docking analysis, the binding pattern of compound 10 with the B-chain of protein 5KLB (Figure 2) showed the formation of three hydrogen bonds: a hydrogen bond between the O atom of keto group of the ligand and NH group of the Val158 with a distance of 2.67 Å and one of the F atoms of CF3 group formed two hydrogen bonds with the NH groups of both the residues Arg155 and Phe158 with a distance of 2.27 and 2.26 Å, respectively. Safna Hussan et al. (2019) reported similar results, and they found that amlodipine-based drugs interact with the active site amino acids of the protein via H-bonding (Safna Hussan et al., 2019). El-Moselhy (2012) revealed that the NH group of nifedipine forms a H-bond with the protein residue Tyr and hydrophobic interaction occurs between the aromatic ring and Tyr residue (El-Moselhy, 2012). A halogen (fluorine) interaction with residue Phe156, a pi–sigma interaction with Leu74, a pi–alkyl interaction with Arg155, and a large number of pi–alkyl interactions with residues Phe13, Arg15, Cys46, Pro47, Trp48, Trp79, Tyr224, His345, and Leu1176 of B-chain of 5KLB were also found. In the complex of compound 10 and protein 5KLS (Figure 3), there was a hydrogen bond in between the H atom of the NH group of the ligand and the O atom of the carbonyl group of Leu1176 (D chain) with a distance of 2.77 Å. Moreover, a halogen (fluorine) interaction with residue Met1174 (C chain), one pi–alkyl interaction with Leu1176 (B chain) and Leu1176 (C chain), three alkyl interactions with Leu1176 (B chain), Leu1176 (C chain), and Leu1176 (D chain), respectively, also existed. DHP derivatives with a bulky group, like –CF3, displayed a better binding affinity when compared to other substituents.
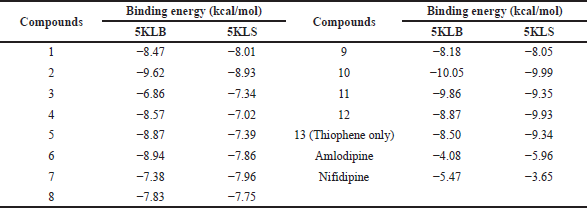 | Table 1. Results of the binding energy of the 12 DHP-based ligands docked with 2 CavAb proteins. [Click here to view] |
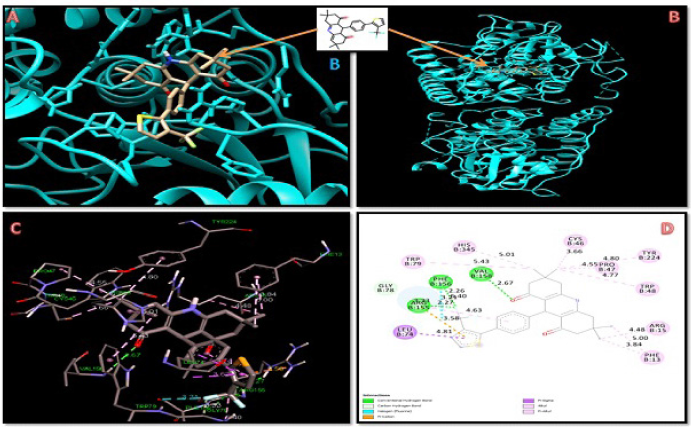 | Figure 2. Docking interaction of compound 10 with 5KLB protein. [Click here to view] |
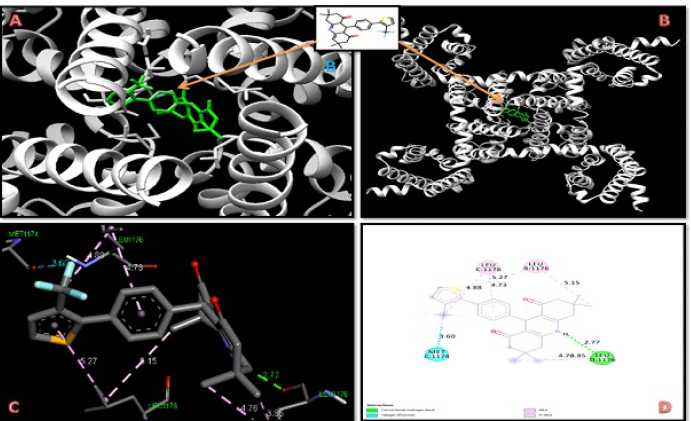 | Figure 3. Docking interaction of compound 10 with 5KLS protein. [Click here to view] |
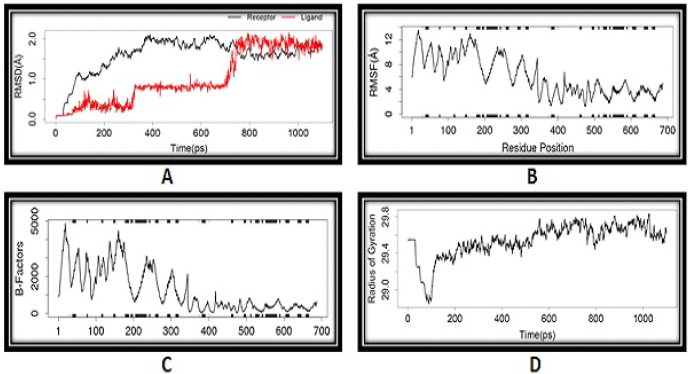 | Figure 4. MDs: (A) RMSD, (B) RMSF, (C) B-factor, and (D) Rg of compound 10 with 5KLB. [Click here to view] |
MD simulations
MD simulation is useful in understanding the conformational stability of docked complexes. Because compound 10 has the highest binding energy, we used MD simulation to investigate the stability of docked complexes with time. The docked complex of compound 10 was subjected to MD simulation utilising the LARMD server to achieve various geometric features such as RMSD, RMSF, Rg, and so on. The RMSD plots of the complexes of compound 10 with 5KLB and 5KLS are shown in Figures 4A and 5A. The higher the RMSD fluctuation of a ligand, the greater its conformational changes in the receptor. RMSD fluctuations of both complexes were found to remain either lesser than or around 2 Å. From the RMSD plots, it was found that the complex of compound 10 and 5KLB showed more favourable complexation compared to the complex of compound 10 and 5KLS. The time evolution plots of RMSF for all C-α atoms of the complexes are shown in Figures 4B and 5B. Compound 10–5KLB and compound 10–5KLS complexes showed regular RMSF fluctuation patterns between 2 and 14 Å and 2 and 20 Å, respectively. Such low fluctuations in the RMSF of both the complexes directed nonstop interactions between the receptor and compound 10. Structural integrity and compactness of protein structure are best understood by Rg. The Rg plots of both complexes are shown in Figures 4D and 5D. The Rg plots revealed that compound 10–5KLB complex showed minor fluctuations (Rg < 29.8 Å) compared to compound 10–5KLS complex (Rg < 33 Å). These results suggest that the compound 10–5KLB complex is more stable than the compound 10–5KLS complex.
Binding free energy calculation through MM [generalized born surface area continuum solvation / Poisson–Boltzmann surface area continuum solvation (GBSA/PBSA)]
The binding free energy was also calculated on the simulated trajectories using both PBSA and GBSA methods in the LARMD server and the results are presented in Table 2. The deltaGB binding energy of the compound 10–5KLB complex was −19.42 kcal/mol and that of compound 10–5KLS was −20.94 kcal/mol. Electrostatic energy (ELE), van der Waals contribution, polar and nonpolar contributions to solvation (PBSOL or GBSOL) and entropy (TS) are taken into account for the estimation of free energy (deltaPB/ deltaGB). Complex of compound 10 with protein 5KLS has higher gas phase energy compared to complex of compound 10 and 5KLB. PBSOL to the binding free energy (deltaPB) of the complex of compound 10 and 5KLB was higher than that of complex of compound 10–5KLS (Table 2). 5KLS has higher deltaPB and deltaGB values when compared to 5KLB protein. Molecular mechanics/generalized born surface area (MM/GBSA)/PBSA calculation suggests that compound 10 is a potential candidate and may replace the first-line drugs.
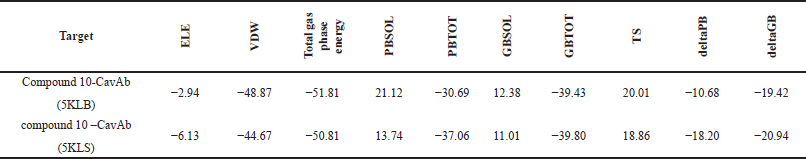 | Table 2. Binding free energy MM (GBSA/PBSA) of the best docked complexes of both the proteins. [Click here to view] |
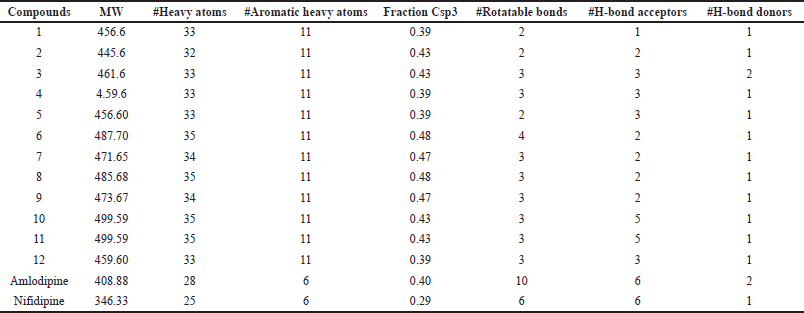 | Table 3. a. The ADME properties of compounds obtained from the online server SWISSADME. [Click here to view] |
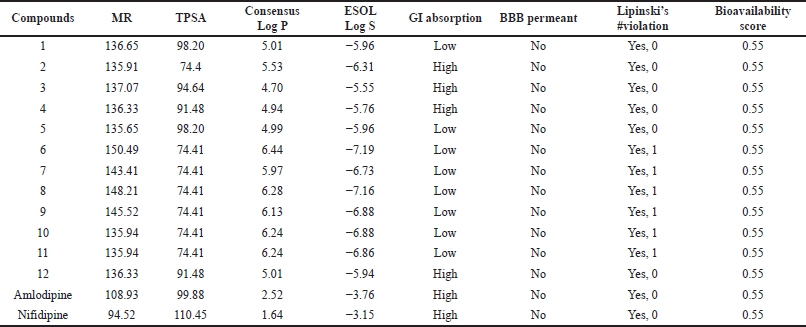 | Table 3. b. The ADME properties of compounds obtained from the online server SWISSADME. [Click here to view] |
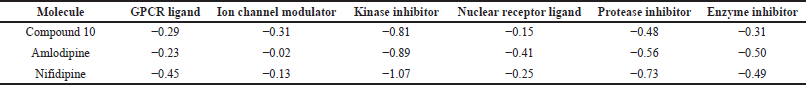 | Table 4. Bioactivity scores of the designed compounds according to Molinspiraton cheminformatics software. [Click here to view] |
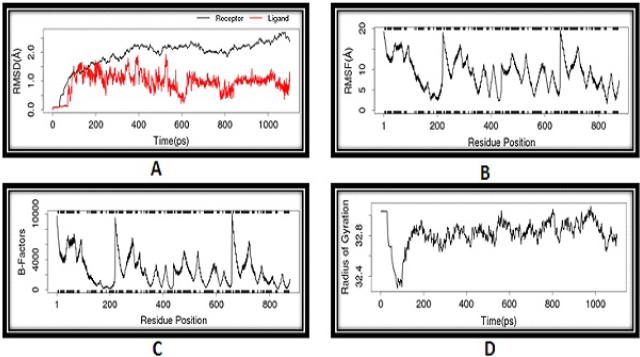 | Figure 5. Molecular dynamics: (A) RMSD, (B) RMSF, (C) B-factor and (D) Rg of compound 10 with 5KLS. [Click here to view] |
ADME properties analysis
The pharmacokinetic profile of a medicine is a critical component, and many therapeutic compounds are unable to enter the market because of their low profile. The features of a proposed therapeutic molecule should be appealing. The SWISSADME server was used to create the Lipinski rule of 5, as well as a few other important parameters (Daina et al., 2017). A therapeutic molecule will be active if the limiting value of two or more of these criteria is not breached, according to the Lipinski rule of 5. The profile has been presented in Table 3a and b and compared with the control molecules. Compound 10 follows the Lipinski rule with only one violation. Bioavailability score is a vital parameter and compound 10 possesses a score value of 0.55, which indicates that it has high-quality pharmacokinetic properties (Table 4). The same value was found in control molecules. The parameter topological polar surface area (TPSA) can be used to predict drug transport qualities. This metric is an excellent indicator of a drug’s bioavailability. The considered compounds were found to have TPSA below 140 ?2. A low TPSA value indicates that compound 10 is better behaved than the control molecules (Imane et al., 2021). The number of bonds which can easily rotate indicates the conformational changes of the compounds and ultimately for the binding of receptors or channels, so it is a good parameter (Salma et al., 2017). A lower value of this parameter (<10) indicates the low conformational flexibility. Most of the molecules possess a lower range of rotatable bonds except for one of the control molecules. It is seen that all the designed molecules possess molecular weights (MWs) below 500. According to the Lipinski rule of 5, the limiting number of hydrogen bond donors (nOHNH) is less than or equal to 5. All the molecules possess only one hydrogen donor except compound 3 and amlodipine which have two donors. Hydrogen bond acceptors (nON) of all the molecules range from 1 to 6, which is below the limiting value (<10) of the Lipinski rule of 5. The Blood-brain barrier (BBB) parameter showed that the designed molecules have no issues. The absorption percentage (%Ab) was calculated using the following relationship (Husain et al., 2016; Zhao et al., 2002):
%Ab = 109 – (0.345 × TPSA).
The absorption percentage of compound 10 and control molecules are 83, 71, and 74, respectively, which indicates better oral bioavailability (Husain et al., 2016).
As a result of the foregoing findings, compound 10 possesses good therapeutic characteristics that meet the required criteria. So, this compound was thoroughly screened for bioactivity and compared to control molecules. Interaction with the living systems with these compounds were predicted by calculating the activity score toward G protein-coupled receptors (GPCR ligand), calcium CCB, transcription factors, kinase inhibitor, protease inhibitor, and enzyme inhibitor with the assistance of software Molinspiration (2002) score online. The bioactivity of the compounds is presented in Table 4; the drug-likeness score suggests that the compound has potential biological activity. So, the considered compound may be useful as a key compound for CCBs and transcription factors.
CONCLUSION
In this article, computational docking studies for two CavAb channel proteins (5KLB and 5KLS) were performed in order to screen the newly designed potential drugs. The docking results are also compared to the control molecules amlodipine and nifidipine. Compound 10 had the lowest binding energy with both the proteins (5KLS and 5KLB). MD simulations and MM/ (GBSA/ PBSA) calculations of the docked complexes of compound 10 with 5KLS and 5KLB showed that complexes were stable. In addition to that pharmacological in silico interpretations suggest that compound 10 may be projected for further in vivo and in vitro studies.
ACKNOWLEDGMENT
The authors would like to acknowledge the Chemistry Department, Raiganj University, West Bengal, India, for providing technical and library support during conducting this study.
CONFLICT OF INTERESTS
No potential conflict of interests was reported by the authors.
FUNDING
None.
AUTHORS’ CONTRIBUTION
All the authors in the manuscript are equally responsible for the technical information communicated to the journal. All the authors discussed the results and contributed to the final manuscript.
REFERENCES
Advanced Chemistry Development, Inc. (ACD/Labs), 2021. Available via https://www.acdlabs.com/resources/freeware/chemsketch/index.php
Berkan O, Sarac B, Simsek R, Yildirim S, Sarioglu Y, Safak C. Vasorelaxing properties of some phenylacridine type potassium channel openers in isolated rabbit thoracic arteries. Eur J Med Chem, 2002; 37:519–23. CrossRef
BIOVIA Discovery Studio Visualizer. A free, feature-rich molecular modeling application for viewing, sharing and analyzing protein and small molecule, 2021. Available via https://discover.3ds.com/discovery-studio-visualizer-download (Accessed 8 June 2021).
Caballero-Gonzalez FJ. Calcium channel blockers in the management of hypertension in the elderly. Cardiovasc Hematol Agents Med Chem, 2015; 12(3):160–5; doi:10.2174/1871525713666150310111554. CrossRef
Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol, 2011; 3(8):a003947. CrossRef
Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep, 2017; 7:42717. CrossRef
De Luca M, Ioele G, Ragno G. 1,4-dihydropyridine antihypertensive drugs: recent advances in photostabilization strategies. Pharmaceutics, 2019; 11(2):85. CrossRef
Dolphin AC. Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and pharmacology. J Physiol, 2016; 594(19):5369–90. CrossRef
El-Moselhy T. QSAR and docking studies of 1,4-dihydropyridine calcium antagonists with methylsulfonylimidazolyl substituent based on genetic function approximation. Chem Biol Interf, 2012; 2(3):172–82.
Guwatudde D, Mutungi G, Wesonga R, Kajjura R, Kasule H, Muwonge J. The epidemiology of hypertension in Uganda: findings from the National Non-Communicable Diseases Risk Factor Survey. PLoS One, 2015; 10(9):e0138991. CrossRef
Husain A, Ahmad A, Khan SA, Bhutani MAR, Al-Abbasi FA. Synthesis, molecular properties, toxicity and biological evaluation of some new substituted imidazolidine derivatives in search of potent anti-inflammatory agents. Saudi Pharm J, 2016; 24:104–14. CrossRef
Safna Hussan KP, Shahin Thayyil M, Rajan VK, Muraleedharan K. DFT studies on global parameters, antioxidant mechanism and molecular docking of amlodipine besylate. Comput Biol Chem, 2019; 80:46–53. CrossRef
Imane A, Faiçal H, Sohayb BB, Said G. In silico study the inhibition of angiotensin converting enzyme 2 receptor of COVID-19 by Ammoidesverticillata components harvested from Western Algeria. J Biomol Struct Dyn, 2021; 39(9):3263–76; doi:10.1080/07391102.2020.1763199
Imenshahidi M, Hadizadeh F, Firoozeh-Moghadam A, Shirinbak MSA, Gharedaghi MB. Synthesis and vasorelaxant effect of 9-aryl-1,8-acridinediones as potassium channel openers in isolated rat aorta. Iran J Pharm Res, 2012; 11(1):229–33.
Jadhav U, Mohanan PP, Almeida AF, Abraham G, Yunus Khan M, Gaurav K, Mane A, Vikas S, Jain M, Meel B. Effectiveness and effect on renal parameters of amlodipine vs. other dihydropyridine calcium channel blockers in patients with essential hypertension: retrospective observational study based on real-world evidence from electronic medical records. Cardiol Ther, 2021; 10:465–80. CrossRef
Jamalian A, Miri R, Firuzi O, Amini M, Moosavi-Movahedi AA, Shafiee A. Synthesis, cytotoxicity and calcium antagonist activity of novel imidazolyl derivatives of 1,8-acridinediones. J Iran Chem Soc, 2011; 8(4) 983–91. CrossRef
Johnson MT, Gudlur A, Zhang X, Xin P, Emrich SM, Yoast RE, Courjaret R, Nwokonko RM, Li W, Hempel N, Machaca K. L-type Ca2+ channel blockers promote vascular remodeling through activation of STIM proteins. PANAS, 2020; 117(29):17369–80. CrossRef
Karthick R, Velraj G, Pachamuthu MP, Karthikeyan S. Synthesis, spectroscopic, DFT, and molecular docking studies on 1,4-dihydropyridine derivative compounds: a combined experimental and theoretical study. J Mol Model, 2022; 28(1):5; doi:10.1007/s00894-021-04939-2 CrossRef
LARMD server. A web server for molecular dynamics simulations, 2018. Available via http://chemyang.ccnu.edu.cn/ccb/server/LARMD (Accessed 28 July 2021).
Li EC, Heran BS, Wright JM. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst Rev, 2014; (8):CD009096; doi:10.1002/14651858. CD009096.pub2 CrossRef
Makani H, Bangalore S, Romero J. Peripheral edema associated with calcium channel blockers: incidence and withdrawal rate: a meta-analysis of randomized trials. J Hypertens, 2011; 29(7):1270–80; doi:10.1097/HJH. 0b013e3283472643. CrossRef
Mann JFE, Hilgers KF. Use of thiazide diuretics in patients with primary (essential) hypertension, 2017. Available via https://www.uptodate.com/contents/use-of-thiazide-diuretics-in-patients-with-primary-essential-hypertension (Accessed 28 July 2021).
MGLTools. A graphical user interface for AutoDock 4.2, 2021. Available via https://ccsb.scripps.edu/mgltools/downloads/ (Accessed 10 June 2021).
Moallem SA, Dehghani N, Mehri S, Shahsavand SH, Alibolandi M, Hadizadeh F. Synthesis of novel 1,8-acridinediones derivatives: Investigation of MDR reversibility on breast cancer cell lines T47D and tamoxifen-resistant T47D. Res Pharm Sci, 2015; 10(3):214–21.
Molinspiration. Calculation of molecular properties and bioactivity score, 2002. Available via https://www.molinspiration.com/cgi-bin/properties (Accessed 15 June 2021).
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participant. Lancet, 2021; 398:957–80.
Pitt B, Byington RP, Furberg CD. PREVENT investigators. Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. Circulation, 2000; 102(13):1503–10; doi:10.1161/01.CIR.102. 13.1503 CrossRef
Ronald KR, Jeffery BP, Richard AR, James JM, Robert F, Joan AK, David AB, Alfonso T. Thiophene systems. Thienopyrimidinedione derivatives as potential antihypertensive agents. J Med Chem, 1988; 31:1786–93. CrossRef
Salma A, Sherbeni E, Tarek, F, Moselhy E. Computational evaluation of the druggability and biological activity of Iodo-1,4-dihydropyridine derivatives. Chem Res J, 2017; 2(5):131–41.
Shityakov S, Sohajda T, Puskas I, Roewer N, Forster C, Broscheit JA. Ionization states, cellular toxicity and molecular modelling studies of midazolam complexed with trimethyl-β-cyclodextrin. Molecules, 2014; 19(10):16861–76. CrossRef
Stewart Computational Chemistry, 2021. Available via http://openmopac.net/
SwissADME. A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules, 2017. Available via http://www.swissadme.ch (Accessed 15 June 2021).
Tang L, Gamal El-Din T, Swanson T. Structural basis for inhibition of a voltage-gated Ca2+ channel by Ca2+ antagonist drugs. Nature, 2016; 537:117–21. CrossRef
Velena A, Zarkovic N, Troselj K, Bisenieks E, Krauze A, Poikans J, Duburs G. 1,4-dihydropyridine derivatives: dihydronicotinamide analogues—model compounds targeting oxidative stress. Oxid Med Cell Longev , 2016; 2016:35; doi:10.1155/2016/1892412 CrossRef
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 CC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension, 2018; 71(6):1269–324; doi:10.1161/HYP.0000000000000066 CrossRef
Xiong H, Han J, Wang J, Lu W, Wang C, Chen Y, Lian F, Zhang N, Liu YC, Zhang C, Ding H, Jiang H, Lu W, Luo C, Zhou B. Discovery of 1,8-acridinedione derivatives as novel GCN5 inhibitors via high throughput screening. Eur J Med Chem, 2018; 151:740–51; doi: 10.1016/j.ejmech.2018.02.005 CrossRef
Zhao Y, Abraham MH, Le J, Hersey A. Luscombe CN, Beck G, Sherborne B, Cooper I. Rate-limited steps of human oral absorption and QSAR studies. Pharm Res, 2002; 19(10):1446–57. CrossRef