INTRODUCTION
Golden sea cucumbers, Stichopus sp., have been widely used as food and medicinal ingredients in the Middle East and Asia, including Indonesia (Sara et al., 2011). As a food ingredient, sea cucumbers have high nutritional value with good-quality protein consisting of a rich amino acid and low-fat profile, which is supported by minerals (Ceesay et al., 2019). Current studies have reported that the sea cucumber contains a lot of bioactive compounds that can be used as antitumor, anticancer, antioxidant, antifungal, anticoagulant, antihypertensive, antimicrobial, antithrombotic, anti-inflammatory, and antiangiogenic agents (Adriansyah et al., 2014; Bordbar et al., 2011; Hawa et al., 1999; Lu et al., 2010; Tian et al., 2005; Zhang et al., 2009). Several important bioactivity compounds that have been identified and isolated for this sea cucumber are fucosylated chondroitin sulfate, cerebrosides, sterols, gangliosides, omega-3 fatty acids, sterol glycosides, lectins, frondogenin, heparin, glycosides, and omega-6 (Bordbar et al., 2011; Findlay and Daljeet, 1984; Vieira and Mour˜ao, 1988).
Frondoside A, a triterpenoid glycoside compound, is one of the active chemical compounds contained in sea cucumbers that shows a potential to inhibit cancer cell growth through caspase activation in human pancreatic cells, leukemia, and breast cancer (Jin et al., 2009; Li et al., 2008; Marzouqi et al., 2011). Attoub et al. (2013) revealed that frondoside A exhibits the decline of growth, lymph node metastasis, and angiogenesis of LNM35 xenograft in athymic mice. Moreover, frondoside A had the capability to inhibit lung tumor growth. In another research finding, Zou et al. (2005) declared that cytotoxicity (ED50 0.96–5.0 μg/ml) against 10 human tumor cell lines was obtained from 6 triterpene glycosides isolated from sea cucumber. It is also known that the sea cucumber containing sulfated polysaccharides, fucoidans, has significant anticancer activity (Fitton et al., 2015; Malyarenko et al., 2017).
It was reported that the sea cucumber Stichopus sp. already contains sulfated polysaccharides, chondroitin sulfate, and sulfated fucan. Evaluation of anticancer activity demonstrated that sulfated fucan carried slight activity against migration of MDA-MB-231 cells in vitro and colony formation inhibition of those cell lines (Thinh et al., 2018). Based on our information, phytochemical screening, the total phenol and flavonoid content of Indonesian sea cucumber Stichopus sp. extract will differ from Stichopus and other species. Stichopus sp. which is available in Lampung (Indonesia) has never been studied before. For this reason, this study was designed to perform phytochemical screening as well as evaluate the cytotoxicity of sea cucumber extracts against MCF7 cancer cells and 3T3 normal cells. Furthermore, a molecular docking study was carried out to predict the interaction of the chemical constituents of Stichopus sp. with the MMP-9 enzyme. MMP-9 has been reported in many types of cancer and the inhibition of MMP 9 could be beneficial in the treatment of various cancer conditions (Paramvir et al., 2018).
MATERIALS AND METHODS
Chemicals and reagents
We used Indonesian (Lampung) sea cucumber Stichopus sp. All types of solvents and acids (pure analytical grade) were acquired from E. Merck. We obtained other chemicals from E. Merck.
Preparation of samples of Stichopus sp.
Sea cucumbers, Stichopus sp., were collected from Lampung, Sumatra Island, Indonesia. Fresh and healthy sea cucumbers were separated immediately and packed in polyethylene bags under an incubator in an ice bath. Samples were stored at a cold temperature before further processing. The sea cucumbers were washed with water until clean and then mashed using a blender until they formed a paste. The samples were then frozen at −70°C and then dried using a freeze dryer for 2 days. All Stichopus sp. powder samples were packed in closed glass bottles until the extraction process was carried out.
Extraction procedure
The stichopus sp. Powder (127.9 g) was macerated in methanol (2.5 l) until complete extraction and evaporated to obtain a crude methanol extract. The crude extract was partitioned in stages, starting from nonpolar to polar solvents. The solvents used were hexane, chloroform, ethyl acetate, butanol, acetone, and methanol. The six fractions obtained from this stratified partitioning process (hexane fraction, chloroform fraction, ethyl acetate fraction, butanol fraction, acetone fraction, and methanol fraction) were used for phytochemical screening and cytotoxicity assessment.
Phytochemical screening
The stock solutions of all fractions were prepared by dissolving a 1 g sample in 100 ml of their solvent. These stock solutions were used for phytochemical screening following the Harborne (1998) and Kokate (1997) method. The phytochemical screening was accomplished to observe the presence of alkaloids, saponins, steroids, tannins, and terpenoids qualitatively. In addition, the Folin-Ciocalteu method was conducted to ascertain the total phenol compound content quantitatively, applying gallic acid as a standard (Chen et al., 2015).
Identification of the chemical compounds of Stichopus sp.
All the samples of Stichopus sp. fractions were weighed to as much as 10 mg to identify chemical compounds using quadrupole time-of-flight liquid chromatography-mass spectrometry (QTOF LCMS-MS) and gas chromatography-mass spectrometry (GC-MS) which are supported by the library database on the instrument.
Assessment of cytotoxicity
Cytotoxicity screening
Cell viability studies were conducted in breast cancer (MCF7) cell lines and fibroblast (NIH/3T3) cell lines as normal cells. The cell lines were grown in Dulbecco’s Modified Eagle Medium high glucose enriched with 10% (v/v) fetal bovine serum and 1% penicillin-streptomycin at 37°C in a CO2 incubator. Confluent cultures were harvested by trypsin and diluted in media to prepare a cell suspension. The centrifugation was applied to the cell suspension at 1,500 rpm for 5 minutes. A sample of cells was counted by using a hemocytometer. After calculating the cells, MCF7 and NIH/3T3 were seeded into 96-well plates at a density of 1 × 104 cells per well. The cell lines were cultured in a CO2 incubator for 24 hours.
Thiazolyl blue tetrazolium bromide (MTT) assays
The effect of Stichopus sp. extract on cell viability was determined using the MTT test. After the incubation period of MCF7 and NIH/3T3, the extracts dissolved in dimethyl sulfoxide (DMSO) (final concentrations of 50, 100, and 200 μg/ml) were placed into the well plates and put in a CO2 incubator for another 24 hours. Each sample was replicated three times. At the expiration of the 24 hours treatment period, supernatants were removed from the wells. Formerly, 90 μl of serum-free medium and 10 μl of MTT solution (5 mg/ml) were put into each well. The well plates were stored in a humidified 5% CO2 incubator for 3 hours at 37°C. Then, the medium and MTT solution were discarded, and 100 μl of DMSO was put into the well plates. The optical density (OD) was determined at 570 nm on a multiwell spectrophotometer (Varioskan Flash, Thermo Fisher Scientific), and the cell viability was demonstrated using the following formula:
By plotting cell survival (%) versus log concentration (μg/ml) in the GraphPad Prism software, the sample concentrations that decrease the viability of the intact cells by 50% (IC50) can be counted.
Morphological analysis
The cell lines treated with Stichopus sp. extract were observed by a light microscope (Olympus, Japan) to recognize morphological alterations of cells. The photographs were taken at ×20 magnification. This observation was accomplished to determine the changes (such as ballooning of the cells) induced by the samples.
Molecular docking of the chemical constituents of Stichopus sp.
Molecular docking was carried out using the AutoDock 4.2 software program. The interaction visualization of the molecular docking results was used by the Discovery Studio program. A molecular docking study was carried out to understand the interaction mode of cancer protein binding with ligands. The receptor used is an α-estrogen receptor taken from the Protein Data Bank (PDB) with PDB ID 4WZV, and external validation has been carried out (Liu et al., 2015).
RESULTS AND DISCUSSION
Study of phytochemical screening
The dried powder of Stichopus sp. sea cucumbers was obtained by drying the sea cucumber paste using a freeze dryer for 2 days. The dried samples of sea cucumbers were macerated using methanol, and the solvent was evaporated until the sea cucumber extract was obtained. The crude extract was partitioned in stages, starting from nonpolar to polar solvents. The solvents used were hexane, chloroform, ethyl acetate, butanol, acetone, and methanol. The six fractions obtained from this stratified partitioning process were used for phytochemical screening and cytotoxicity assessment. The stratified partition of Stichopus sp. sea cucumbers extract can be seen in Figure 1.
Based on the results of the phytochemical screening of the six fractions of Stichopus sp., it showed the presence of alkaloids, flavonoids, steroids, triterpenoids, and saponins. However, tannins were not present in Stichopus sp. fractions. Most fractions contain alkaloids. A triterpenoid, steroid, flavonoid, and alkaloid were detected in sea cucumber hexane and chloroform fractions. The ethyl acetate fraction showed the presence of alkaloid and triterpenoid compounds, butanol fraction contains alkaloids and flavonoids, acetone fraction contains saponins, and methanol fraction contains alkaloid compounds. The results of the phytochemical screening of the Stichopus sp. extract can be seen in Table 1.
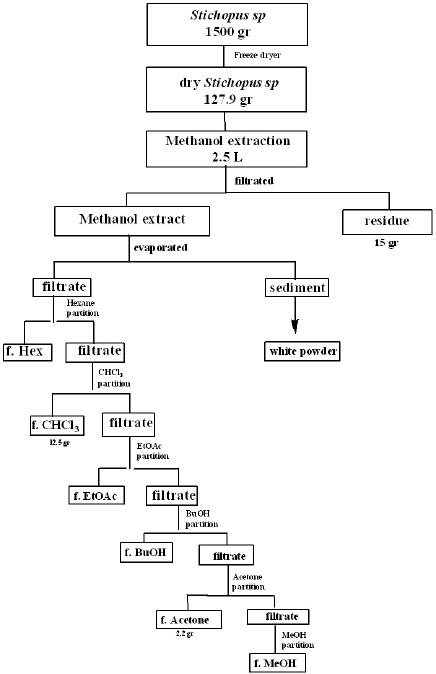 | Figure 1. Schematic of fractionation partition samples of Stichopus sp. [Click here to view] |
The total phenol content result of seven samples of Stichopus sp. demonstrated by the Folin-Ciocalteu method is presented in Table 1. Among the seven samples, the hexane fraction showed the highest amount of phenolic compounds (7.01 mg/g) followed by methanol (5.48 mg/g), butanol (4.33 mg/g), chloroform (3.77 mg/g), acetone (1.67 mg/g), and ethyl acetate (1.05 mg/g).
The chemical constituents contained in a fraction are biologically active substances. Almost all the active compounds present in the extract are components of secondary metabolites. These active compounds play important role in biological activity. The interaction of many active compounds in each fraction could be synergistic or antagonistic so that the existence of different active compounds in each fraction show different biological activity. This generates the uniqueness and attractiveness properties in fractions compared to drugs which generally contain only one active compound. In this study, phytochemical screening was carried out on the crude extract of sea cucumber Stichopus sp. using hexane, chloroform, ethyl acetate, butanol, acetone, and methanol as solvents. Screening results indicated that the Stichopus sp. fractions contained alkaloids, steroids, flavonoids, saponins, and triterpenoids.
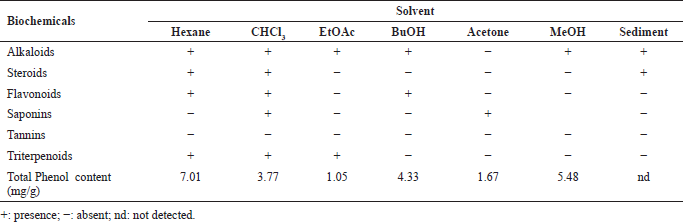 | Table 1. Phytochemical and total phenol screening analysis of Stichopus sp. fractions. [Click here to view] |
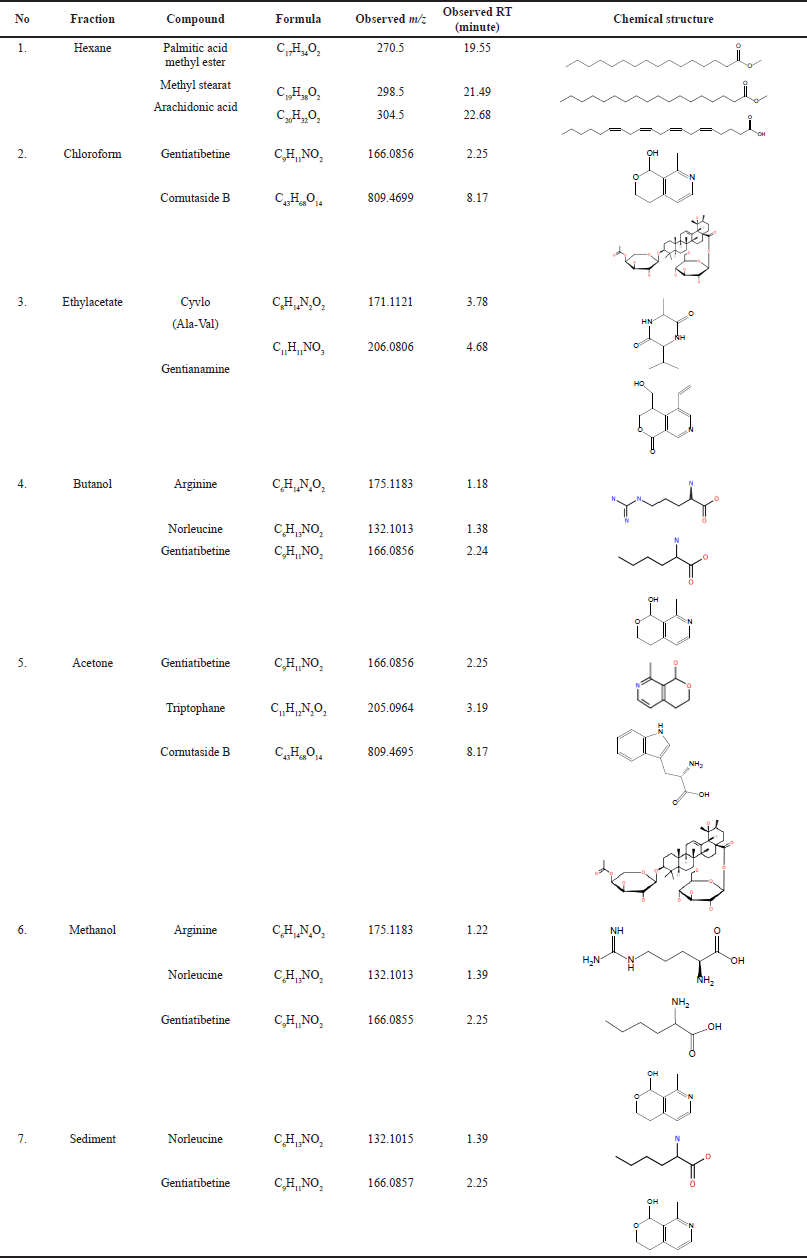 | Table 2. Chemical constituent of Stichopus sp. based on GCMS and QTOF-LCMS/MS. [Click here to view] |
All the samples of Stichopus sp. fractions were identified chemical compounds using QTOF LCMS-MS and GC-MS which are supported by the library database on the instrument. The chemical compounds that have been identified from the fractions of Stichopus sp. are hexane (palmitic acid methyl ester, methyl stearate, and arachidonic acid), chloroform (gentiatibetine, cornutaside B), ethyl acetate (cyclo (Ala-Val), gentianamine), butanol (arginine, norleucine, and gentiatibetine), acetone (gentiatibetine, tryptophan, and cornutaside B), and methanol (arginine, norleucine, and gentiatibetine) and sediment (norleucine, gentiatibetine). The chemical constituents of Stichopus sp. based on GC-MS and QTOF LCMS-MS were shown in Table 2.
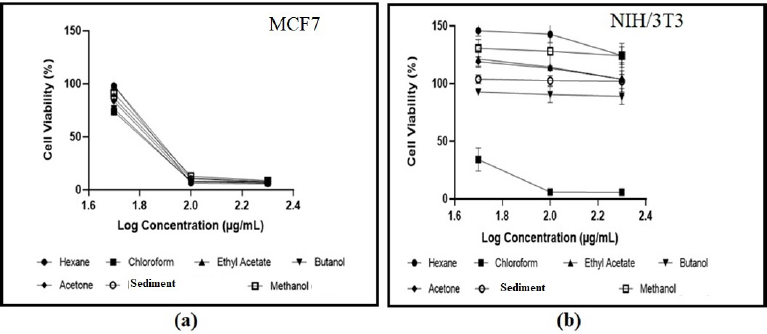 | Figure 2. Effect of Stichopus sp fractions at different concentrations on (a) MCF7 cell lines and (b) NIH/3T3 cell lines [Click here to view] |
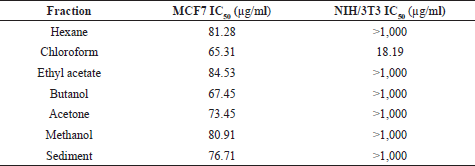 | Table 3. IC50 of samples of Stichopus sp. fractions. [Click here to view] |
In vitro assessment of cytotoxicity against MCF7 and 3T3 cell lines of Indonesian sea cucumber Stichopus sp.
The effects of seven samples on the viability of the cancerous MCF7 cell lines and normal NIH/3T3 cell lines evaluated by the MTT assay with various concentrations of the fractions (50, 100, and 200 μg/ml) were demonstrated in Figure 2. The fractions have different effects against the MCF7 cell lines and NIH/3T3 cell lines. The cell viability of MCF7 breast cancer cell lines treated with the fractions was found between 73% and 98% at a concentration of 50 μg/ml and showed a significant reduction at concentrations of 100 μg/ml (6%–12% cell viability) and 200 μg/ml (5%–8% cell viability). Nonetheless, almost all of the fractions on the NIH/3T3 cell lines exhibited a very high viability rate at 50 μg/ml (93%–146%), 100 μg/ml (90%–143%), and 200 μg/ml (87%–124%). The chloroform fraction has the lowest cell viability rate (34% cell viability at a concentration of 50 μg/ml and 5%–6% cell viability at concentrations of 100 and 200 μg/ml) against the NIH/3T3 cell lines compared to other fractions.
As shown in Table 3, all of the samples of Stichopus sp. fractions showed moderate cytotoxicity against the MCF7 cell lines with an IC50 value of 65–85 μg/ml. However, most fractions of Stichopus sp. exhibited no cytotoxicity against the NIH/ 3T3 cell achieving >1,000 μg/ml as an IC50 value. Interestingly, the chloroform fraction exhibited high cytotoxicity against NIH/3T3 with an IC50 value of 18.19 μg/ml. The criteria used to classify the cytotoxicity were determined as reported by the National Cancer Institute and the Geran protocol, IC50 < 20 μg/ml = high cytotoxicity, IC50 range between 21 and 200 μg/ml = moderate cytotoxicity, IC50 range between 201 and 500 μg/ml = weak cytotoxicity, and IC50 > 501 μg/ml = no cytotoxicity (Geran et al., 1972; Nguyen et al., 2020).
According to morphological observation at a concentration of 200 μg/ml, seven samples treated on MCF7 (Fig. 3a–g) promoted morphological changes (such as shrinking of cells and ballooning of cells) and presented signs that cells disengage from the surface, indicating cell death. In addition, Figure 4 shows the morphological untreated NIH/3T3 cell line, and Figure 4a–g exhibit treated NIH/3T3 cell lines. Six samples (Fig. 4a and c–g) provided no significant alteration compared to untreated NIH/3T3 cell lines (Fig. 4). There were a lot of cell attachments in those six samples’ figures. However, the chloroform fraction (Fig. 4b) treated on NIH/3T3 generated more cell detachment, more shrinking cells, and ballooning cells (rounded shape) compared to other samples. The rounded morphology is shown by arrowheads.
The results of the in vitro assessment of cytotoxicity against the MCF7 and 3T3 cell lines of Indonesian sea cucumber Stichopus sp. clearly revealed that all of the samples are toxic for cancerous MCF7 cell lines and most samples are not toxic for noncancerous NIH/3T3 cell lines. Impressively, the chloroform fraction presented higher cytotoxicity in NIH/3T3 cell lines than MCF7 cell lines. Existing phytochemicals play an important role in the cytotoxicity effect. The presence of flavonoid induced sensitivities of cancer cells to cell death (Das et al., 2010).
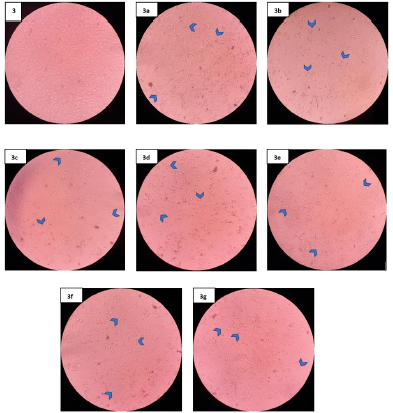 | Figure 3. Morphological observation of MCF7 cell lines untreated and treated with the fractions at concentration of 200 μg/mL. Untreated MCF7(3); Hexane fraction (3a); Chloroform fraction (3b); Ethyl Acetate fraction (3c); Butanol fraction (3d); Acetone fraction (3e); Sediment (3f); Methanol fraction (3g). The arrowheads show rounded morphology. [Click here to view] |
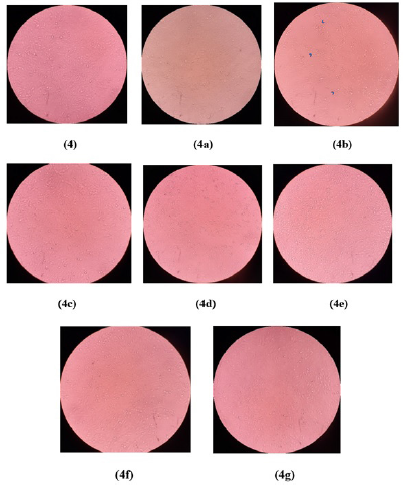 | Figure 4. Morphological observation of NIH/3T3 cell lines untreated and treated with the fractions at concentration of 200 μg/mL. Untreated NIH/3T3 (4); Hexane fraction (4a); Chloroform fraction (4b); Ethyl Acetate fraction (4c); Butanol fraction (4d); Acetone fraction (4e); Sediment (4f); Methanol fraction (4g). The arrowheads show rounded morphology. [Click here to view] |
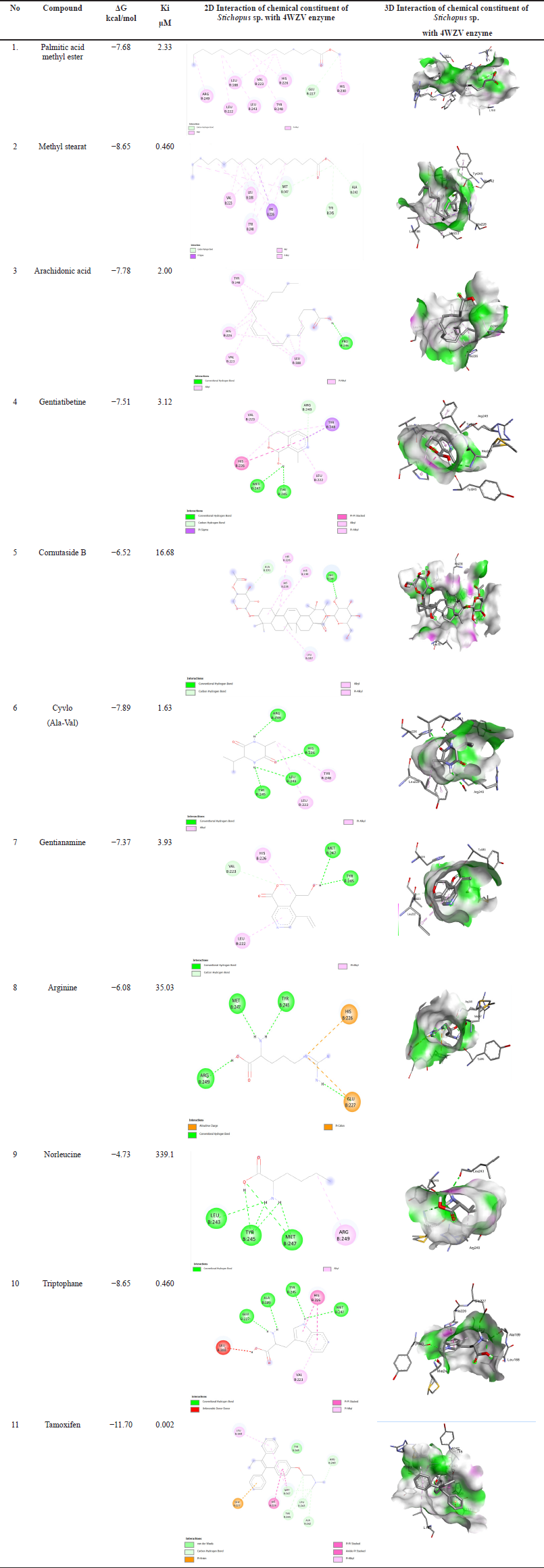 | Table 4. Molecular docking of chemical constituent of Stichopus sp. [Click here to view] |
Cepharanthine, liensinine, dauricine, and isoliensinine which are categorized as alkaloid compounds generate autophagy leading to cell death and showed cytotoxic effects specifically towards cancer cells (Law et al., 2014). Talib and Mahasneh (2010) reported that flavonoids with alkaloids in Onobis hirta showed superior activity to inhibit the growth of cancer cells.
In another study, Holothuria scabra and Cucumaria frondosa containing triterpene glycosides presented the capability to induce apoptosis in HepG2 cells and have better selective cytotoxicity as a novel antitumor drug (Wang et al., 2014). Furthermore, the sphingoid base isolated from Stichopus variegatus exhibited a high cytotoxicity effect, inhibited cancer cell growth, and also showed induction of the apoptosis pathway in human colon cancer cells (Janakiram et al., 2015).
Molecular docking study
Table 4 shows the interactions of the chemical constituents of Stichopus sp. with the amino acid residues involved in 4WZV. The chemical constituents of Stichopus sp. include palmitic acid methyl ester, methyl stearate, arachidonic acid, gentiatibetine, cornutaside B, cyclo (Ala-Val), gentianamine, arginine, norleucine, and tryptophan showing the interaction scores of Gibs energy values −7.68, −8.65, −7.78, −7.51, −6.52, −7.89, −7.57, −6.08, −4.73, and −8.65 kcal/mol). Meanwhile, the positive control compound used is tamoxifen, which is indeed used for cancer treatment. The score of Gibs energy interaction of tamoxifen was −11.70 kcal/mol. Unfortunately, for all of the chemical constituents identified in Stichopus sp., their inhibitory interaction activity in silico was still much lower than that of the tamoxifen as a positive control.
CONCLUSION
The study of phytochemical screening results indicated that the fractions of Stichopus sp. contained alkaloids, steroids, flavonoids, saponins, and triterpenoids. The chemical compounds identified in the Stichopus sp. fractions were palmitic acid methyl ester, methyl stearate, arachidonic acid, gentiatibetine, cornutaside B, cyclo (Ala-Val), gentianamine, arginine, norleucine, and tryptophan. The in vitro assessment of cytotoxicity against the MCF7 and 3T3 cell lines showed that all Stichopus sp. fractions are toxic for cancerous MCF7 cell lines and most fractions are not toxic for noncancerous NIH/3T3 cell lines (normal cell lines). The results of the molecular docking study showed moderate activity of the chemical constituents of Stichopus sp. compared to tamoxifen as a positive control.
ACKNOWLEDGMENTS
This project is a research collaboration between the Research Center for Chemistry – National Research and Innovation Agency (BRIN) and Edward Basilianus (PT Nucleus Farma). The authors are also very thankful for all the support and help from the National Research and Innovation Agency (BRIN) in facilitating this research.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Adriansyah H, Kamaludin MT, Theodorus, Sulastri H. Efek Hepatoprotektif Teripang Emas (Stichopus variegatus) pada Tikus Jantan Dewasa Galur Wistar yang Diinduksi Parasetamol Dosis Toksik. Majalah Kedokteran Sriwijaya, 2014; 46(2):136–43.
Attoub S, Arafat K, Ge´laude A, Al Sultan MA, Bracke M, Collin P, Takahashi T, Adrian TE, Wever OD. Frondoside A suppressive effects on lung cancer survival, tumor growth, angiogenesis, invasion, and metastasis. PLoS One, 2013; 8(1):e53087. CrossRef
Bordbar S, Anwar F, Saari N. High-value components and bioactives from sea cucumbers for functional foods. Mar Drugs, 2011; 9(10):1761–805. CrossRef
Ceesay A, Shamsudin MN, Paiko MA, Ismail IS, Nazarudin MF, Alipiah NM. Extraction and characterization of organ components of the malaysian sea cucumber Holothuria leucospilota yielded bioactives exhibiting diverse properties. Bio Med Res Int, 2019; 2019:1–16. CrossRef
Chen LY, Cheng CW, Liang JY. Effect of esterification condensation on the Folin–Ciocalteu method for the quantitative measurement of total phenols. Food Chemistry, 2015; 170:10–5. CrossRef
Das A, Banik NL, Ray SK. Flavonoid activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer, 2010; 116(1):164–76. CrossRef
Findlay JA, Daljeet A. Frondogenin, a new aglycone from the sea cucumber cucumaria frondosa. J Nat Prod, 1984; 47(2):320–4. CrossRef
Fitton H, Stringer DN, Karpiniec SS. Therapies from fucoidan: an update. Mar Drugs, 2015; 13:5920–46. CrossRef
Geran RI, Greenberg NH, MacDonald MM, Schumacher A, Abbot BJ. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chem Rep, 1972; 3:1–103.
Harborne JB. Phytochemical methods: a guide to modern techniques of plant analysis. 2nd edition, Chapman and Hall, London, UK, pp 54–84, 1998.
Hawa I, Zulaikah M, Jamaludin M, Abidin AAZ, Kaswandi MA, Ridzwan BH. The potential of the Coelomic fluid in sea cucumber as an antioxidant. Malaysia J Nutr, 1999; 5:55–9.
Janakiram B, Mohammed A, Rao CV. Sea cucumbers metabolites as potent anti-cancer agents. Mar Drugs, 2015; 13(5):2909–23. CrossRef
Jin JO, Shastina VV, Shin SW, Xu Q, Park JI, Rasskazov VA, Avilov SA, Fedorov SN, Stonik VA, Kwak JY. Differential effects of triterpene glycosides, frondoside A and cucumarioside A2-2 isolated from sea cucumbers on caspase activation and apoptosis of human leukemia cells. FEBS Lett, 2009; 583:697–702. CrossRef
Kokate KC. Practical pharmacognosy. 4th edition, Vallabh Prakashan, Delhi, India, 218p, 1997.
Law BYK, Chan WK, Xu SW, Wang JR, Bai LP, Liu L, Wong VK. Natural small-molecule enhancers of autophagy induce autophagic cell death in apoptosis-defective cells. Sci Rep, 2014; 4:1–14. CrossRef
Li X, Roginsky AB, Ding XZ, Woodward C, Collin P, Newman RA, Bell, Jr RH, Adrian TE. Review of the apoptosis pathways in pancreatic cancer and the anti-apoptotic effects of the novel sea cucumber compound, Frondoside A. Ann NY Acad Sci, 2008; 1138:181–98. CrossRef
Liu L, Ju R, Xie H, Zeng F, Zhang C, Zhao W, Lu W. Efficacy and mechanism of a compound epirubicin plus quinine injection for the treatment of drug-resistant breast cancer. J Chinese Pharm Sci, 2015; 24(9):563–71. CrossRef
Lu Y, Zhang BY, Dong Q, Wang BL, Sun XB. The effects of Stichopus japonicus acid mucopolysaccharide on the apoptosis of the human hepatocellular carcinoma cell line HepG2. Am J Med Sci, 2010; 339:141–4. CrossRef
Malyarenko OS, Ermakova SP. Fucoidans: anticancer activity and molecular mechanisms of action. In: Venkatesan J, Anil S (eds.). Seaweed polysaccharides: isolation, biological and biomedical applications, Elsevier, Amsterdam, Netherlands, pp , 2017. CrossRef
Marzouqi NA, Iratni R, Nemmar A, Arafat K, Ahmed AM, Al Sultan MA, Yasin J, Collin P, Mester J, Adrian TE, Attoub S. Frondoside A inhibits human breast cancer cell survival, migration, invasion and the growth of breast tumor xenografts. Eur J Pharmacol, 2011; 668:25–34. CrossRef
Nguyen NH, Ta QTH, Pham QT, Luong TNH, Phung VT, Duong TH,Vo VG. Anticancer activity of novel plant extracts and compounds from adenosma bracteosum (Bonati) in human lung and liver cancer cells. Molecules, 2020; 25:1–16. CrossRef
Paramvir S, Ajmer S, Grewal DP, Viney L. Synthesis and evaluation of a series of caffeic acid derivatives as anticancer agents. Future J Pharm Sci, 2018; 4:124–30. CrossRef
Sara B, Farooq A, Nazamid S. High value components and bioactives from sea cucumbers for functional foods-a review. J Mar Drugs, 2011; 9:1761–805. CrossRef
Talib WH, Mahasneh AM. Antiproliferative activity of plant extracts used against cancer in traditional medicine. Sci Pharm, 2010; 78(1):33–45.
Thinh PD, Ly BM, Usoltseva RV, Shevchenko, NM, Rasin, AB, Anastyuk SD, Malyarenko OS, Zvyagintseva TN, San PT, Ermakova SP. A novel sulfated fucan from Vietnamese sea cucumber Stichopus variegatus: isolation, structure and anticancer activity in vitro. Int J Biol Macromolecule, 2018; 117:1101–9. CrossRef
Tian F, Zhang X, Tong Y, Yi Y, Zhang S, Li L, Sun P, Lin L, Ding J. A new sulfated saponin from sea cucumber, exhibits anti-angiogenic and anti-tumor activities in vivo and in vitro. Cancer Biol and Ther, 2005; 4:874–82. CrossRef
Vieira RP, Mour˜ao PA. Occurrence of a unique fucosebranched chondroitin sulfate in the body wall of a sea cucumber. J Biol Chem, 1988; 263(34):18176–83. CrossRef
Wang J, Han H, Chen X, Yi Y, Sun H. Cytotoxic and apoptosis-inducing activity of triterpene glycosides from Holothuria scabra and Cucumaria frondosa against HepG2 Cells. Mar Drugs, 2014; 12:4274–90. CrossRef
Zhang W, Lu Y, Xu B, Wu J, Zhang L, Gao M, Zheng S, Wang A, Zhang C, Chen L, Lei N. Acidic mucopolysaccharide from Holothuria leucospilota has antitumor effect by inhibiting angiogenesis and tumor cell invasion in vivo and in vitro. Cancer Biol Ther, 2009; 8:1489–99. CrossRef
Zou LZ, Yi Y, Wu H, Yao X, Du L, Jiuhong W, Liaw CC, Lee KH. Intercedensides D-I, Cytotoxic triterpene glycosides from the sea cucumber Mensamaria intercedens. J Nat Prod, 2005; 68:540–6. CrossRef