INTRODUCTION
Marine-derived fungi have garnered considerable attention due to producing beneficial bioactive compounds (Cardoso et al., 2020; Jin et al., 2016). Recently, screening for bioactive compounds isolated from ascidians has gained popularity due to the fact that many of these compounds exhibit antioxidant and antibacterial activity (Casertano et al., 2020; Dou and Dong, 2019). Antimicrobial agents, which include antibiotics, antiviral agents, and antifungal agents, are critical components of the treatment of infectious diseases (Leekha et al., 2011). However, as a result of indiscriminate antimicrobial use, microorganisms have evolved resistance mechanisms (Wang et al., 2018). This has become a significant issue for public health in recent years. Hence, the research has been focused on identifying sources of natural antibiotics. In addition to antibiotics, antioxidants have garnered researchers’ attention in medicine due to their numerous health benefits (Dhalaria et al., 2020). Antioxidant and antibacterial activity has been demonstrated in several fungi associated with marine organisms such as ascidians (Da Luz Calado et al., 2021; Ramesh et al., 2021). It has been established that approximately 8% of the bioactive molecules isolated from ascidians are the result of symbiotic microorganisms (Casertano et al., 2020). Microorganisms associated with ascidians are a relatively untapped resource, particularly in Indonesia, despite the fact that Indonesia has one of the world’s largest seascapes. Certain ascidians found in North Sulawesi are unique to the region and are not necessarily found elsewhere, and their habitat contributes to the unique fungi associated with them. Certain compounds isolated from marine ascidians-derived fungi that are taxonomically related to or identical to terrestrial fungi have been identified, such as Aspergillus, Penicillium, Talaromyces, and Trichoderma species (Nicoletti and Vinale, 2018). Eudistoma sp. is one of the ascidian species which is known to be abundant and capable of producing bioactive compounds that have been widely used. The present study examined culturable fungi associated with Eudistoma sp. that were collected from the waters of Bunaken Island, North Sulawesi, Indonesia, for the presence of phytochemical, antioxidant, and antimicrobial activity.
 | Figure 1. Ascidian Eudistoma sp. collected from Bunaken waters. [Click here to view] |
MATERIALS AND METHODS
Sample material
Ascidian Eudistoma sp. (Fig.e 1) was obtained from the Bunaken waters of North Sulawesi, Indonesia, with coordinates 01°36′49.46″N, 124°46′03.17″S, at a depth of 7 m (Fig. 2). Following that, the sample was surface-sterilized three times with sterile seawater and stored in a plastic bag. Samples were immediately transported to the laboratory using a coolbox for the isolation of the symbiotic fungi.
Isolation, cultivation, and extraction of symbiotic fungi
Symbiotic fungi were isolated from the ascidian and cultivated using the method of Kjer et al. (2010) with slight modification by Sandrawati et al. (2020). The ascidian was cut into small fragments, and approximately 10 g was dissolved in sterile seawater to a volume of 100 ml in an Erlenmeyer. After gently shaking the Erlenmeyer flask, the solution was diluted to a concentration of 10−6. One milliliter of the sample was aseptically poured onto an sabouraud dextrose agar medium containing chloramphenicol in Petri dishes. After that, the plates were incubated for 5–7 days at a temperature ranging from 25°C to 27°C. Colonies exhibiting distinct forms were classified as distinct isolates. After that, each distinct colony was purified using the streak method to obtain pure isolates. Pure fungal isolates were cut into 1 × 1 cm squares and grown in a rice medium at room temperature for 4–6 weeks. When the entire rice medium was completely covered with fungal mycelia, the fungus had reached its maximum potential and was ready to harvest. The pure fungus was grown in a 250 ml Erlenmeyer bottle in a rice medium, each containing 10 g of rice in 10 ml of marine and distilled water with a ratio of 1:1 for 15 days in static conditions and at room temperature under daylight conditions. Following incubation, the fungal and rice medium substrate were extracted three times with EtOAc (100 ml) at the ratio of 1:1 for 24 hours and filtered. A rotary evaporator was used to evaporate the filtrate, yielding a thick extract of the fungus. The sample was then freeze-dried for 2 × 24 for further analysis.
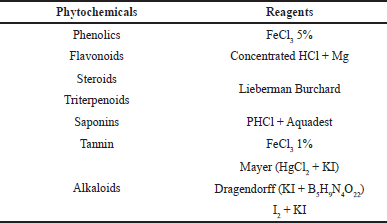 | Table 1. The reagents used for the analysis of phytochemical compounds of the fungal extract. [Click here to view] |
Phytochemical analysis
Phytochemical analyses were carried out following the procedure of Damongilala et al. (2021). The fungal ethyl acetate extract stock was prepared by weighing 100 mg of dry extract and adding it to a 10 ml volumetric flask with methanol p.a to obtain an extract with a concentration of 10 mg/ml (10,000 ppm). A stock solution with a concentration of 1 mg/ml (1,000 ppm) was made by taking 1 ml of the 10,000 ppm extract and adding it to a 10 ml volumetric flask with methanol p.a. Phytochemical compounds analyzed from fungal extracts and their reagents can be seen in Table 1.
Antioxidant activity assay
The antioxidant activities of the isolated fungus were assessed using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay, as described by Sanger et al. (2021) The samples and positive control DPPH were dissolved in methanol with final concentrations of 25, 50, 75, 100, and 125 ppm. DPPH was dissolved at concentrations of 0.05 mg/ml (0.5 μM) in anhydrous ethanol (EtOH). DPPH will bind the hydrogen donated by the antioxidants in the sample. A color shift from dark purple to light yellow indicated the binding reaction had taken place. After 100 minutes of reaction, the color change was observed using a UV-vis spectrophotometer (Shimadzu UV-Vis 1800, Japan) at an absorbance of 517 nm. The strength of the antioxidant activity of a compound is classified based on Blois (1958), which is very strong (< 50 μg/ml), strong (50–100 μg/ml), moderate (100–150 μg/ml), and weak (150–200 μg/ml).
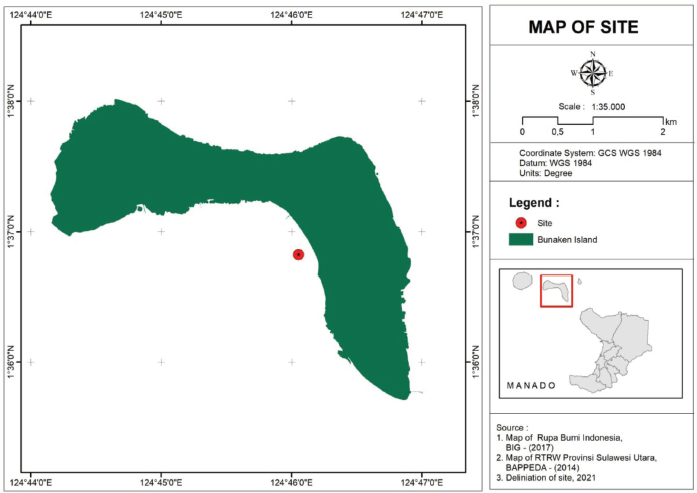 | Figure 2. Ascidian sampling location. [Click here to view] |
Antimicrobial activity assay
The antibacterial activity of the ethyl acetate fugal symbiont extract was determined in this study using the Kirby-Bauer disk diffusion method, which was slightly modified from Hudzicki (2009). The indicator microbes used in this study (Candida albicans, Staphylococcus aureus, Escherichia coli, Aeromonas hydrophila, and Salmonella sp.) were obtained from the Laboratory of Molecular Biology and Marine Pharmacy, Faculty of Fisheries and Marine Sciences, Sam Ratulangi University, Manado, Indonesia. These fungi and bacteria were cultured and maintained in liquid B1 media. Using B1 agar media, the antimicrobial activity of the ethyl acetate extract from fungal symbionts was tested. Each fungal extract with a concentration of 10 mg/ml (10,000 ppm), positive control (chloramphenicol 1,000 ppm), and negative control (methanol p.a) was dripped as much as 20 μl on each 6 mm diameter sterile paper disc and allowed to dry. This procedure was repeated three times for each indicator microbe. Disc papers were placed on previously prepared test media. Observations were made after incubation for 1 × 24 h and 2 × 24 hours. The resulting clear zone of inhibition was measured using a ruler, and the results were recorded in mm. The diameter of the inhibition zone is used to categorize the strength of antibacterial activity according to Davis and Stout (1971) as follows: very strong (≥20 mm), strong (10 to 20 mm), moderate (5 to <10 mm), and weak (≤5 mm).
Molecular identification of the fungus
Fungi isolated from the ascidian were identified molecularly using the ITS1-4 marker following the protocol of Handayani et al. (2019, 2021). DNA isolation was carried out using the Quick-DNA Fungal/Bacterial Miniprep Kit (Zymo Research, D6005). Polymerase chain reaction amplification was performed using MyTaq HS Red Mix (Bioline; BIO-25048ITS1-4 primers were used to perform bidirectional sequencing).
RESULTS AND DISCUSSIONS
Marine-derived fungi are an abundant source of structurally novel natural products, which include a wide range of chemical compounds and pharmacological applications. Marine-derived fungi are those that are isolated in a marine environment. This is in contrast to the traditional definition of marine fungi, which are both obligatory and facultative inhabitants of the marine environment (Pang et al., 2016). The current study focuses on the exploration of a marine-derived fungus, or more precisely an ascidian-derived fungus obtained from the Bunaken seawaters of North Sulawesi, Indonesia, as a source of antimicrobials. As shown in Figure 1, the ascidian under study was the Eudistoma sp.
Molecular identification of the fungus
The pure fungus was isolated from the ascidian Eudistoma sp., as shown in Figure 3. The fungal strain was identified as Trichoderma asperellum based on DNA sequence amplification and sequencing, with sequence assembly 799 bp of the ITS1-4 region, and an identity percentage of 99.37%. Ascidian-derived fungi Trichoderma sp. is a potential natural resource containing a wide range of structurally novel natural products containing a wide range of abundant chemical substances with numerous applications in the field of pharmacology. So far, 78 different types of metabolites from this fungus have been identified, and the majority of these metabolites have therapeutic properties, allowing them to be used as a source of new drug discovery (Su et al., 2018). Up until now, there has been very little known about T. asperellum, which has been isolated from ascidians. However, a marine-derived fungus, T. asperellum, which produced six peptaibols known as asperelines A–F, was successfully isolated from the sediment of Antarctic Penguin Island (Ren et al., 2009). Additionally, T. asperellum cf44-2, an algal endophyte, also yielded seven previously unknown terpenes, including bisabolane, cyclonerane, and harziane derivatives (Song et al., 2018).
 | Figure 3. Pure culture of T. asperellum AFBN 4 isolated from the ascidian Eudistoma sp. [Click here to view] |
Phytochemical constituents analysis of fFungal EtOAc extract
The EtOAc extract of T. asperellum AFBN 4 was subjected to phytochemical analysis to determine the presence of alkaloids, triterpenoids, steroids, tannins, flavonoids, saponins, and phenolics. The phytochemical screening revealed the presence of secondary metabolites such as alkaloids, triterpenoids, tannins (1.11 mg/ml), flavonoids (3.76 mg/ml), and phenolics (5.98 mg/ml) in the fungal extract. The result is nearly identical to that discovered in previous studies, indicating that these compounds are present in the species Trichoderma sp (Ikram et al., 2019; Omomowo et al., 2020). The attractive chemical structures and notable biological activities of Trichoderma have attracted great attention (Zhang et al., 2021). As a result, numerous reviews have been published on various aspects of Trichoderma, not only for the chemical diversity of the metabolites, but also for the diverse bioactivities and possible applications (Zeilinger et al., 2016). Alkaloids trichoderamides A (136) and B (137) were produced by T. gamsii, an endophyte of Panax notoginseng (Ding et al., 2015). Trichoderma has been reported to produce the terpenoids trichothecenes (Shi et al., 2020; Yamazaki et al., 2020). In addition, Trichoderma has been reported to produce phenolic and flavonoid compounds as well (Yusnawan et al., 2020).
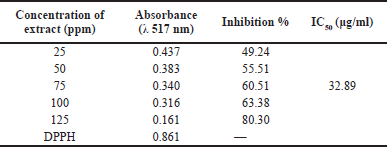 | Table 2. Antioxidant activity of fungal extracts using the DPPH method. [Click here to view] |
Antioxidant activity
The isolated fungal antioxidant activity was determined using the DPPH radical scavenging assay. This assay is frequently used to determine the presence of antioxidant activity within an organism. The IC50 value is used to determine the antioxidant activity of an extract using the DPPH method. This value represents the concentration of the extract that results in a 50% loss of DPPH activity. As presented in Table 2, the antioxidant activity of fungal extracts is 32.89 g/ml, which puts it in the category of a strong antioxidant. Trichoderma sp. has been known to produce antioxidant substances, as previously reported (Su et al., 2018; Zhang et al., 2017). The cosmetics and pharmaceutical industries can benefit from the radical scavenging properties of a variety of natural compounds derived from marine sources, according to numerous studies (Gogineni and Hamann, 2018; Wu et al., 2018). Trichoderma asperellum contains a variety of phytoconstituents, including alkaloids, triterpenoids, tannins, flavonoids, and phenolics. The antioxidant activity of phenolic compounds is significant (Shad et al., 2014). According to recent study, antioxidant properties are ascribed to the presence of polyphenols, flavonoids, alkaloids, terpenoids, and carotenoids (Farag et al., 2020; Larayetan et al., 2019). Antioxidants can neutralize reactive oxygen and nitrogen species, preventing the resurgence of a wide range of degenerative diseases such as cancer, autoimmune disorders, cardiovascular disease, and neurodegenerative disease (Vitale et al., 2020). As a result, antioxidants derived from T. asperellum can be studied further for potential use in medicinal applications, possibly in place of currently used synthetics.
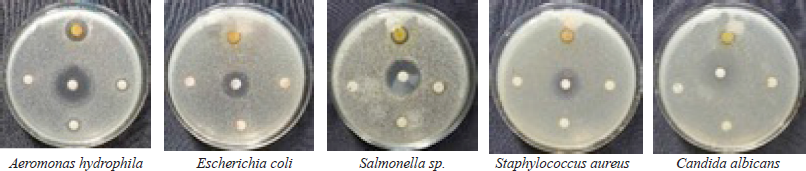 | Figure 4. Antimicrobial activity of T. asperellum AFBN against pathogenic microbes. [Click here to view] |
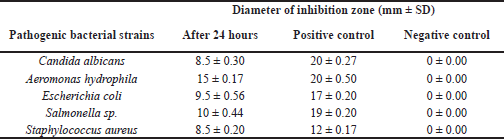 | Table 3. Diameter of inhibition zone of T. asperellum AFBN extract against several pathogenic microbes. [Click here to view] |
Antimicrobial activity
A large number of marine-derived fungi have antimicrobial properties (Sumilat et al., 2020) (Handayani et al., 2021; Sandrawati et al., 2020). The results of the antimicrobial activity analysis of T. asperellum AFBN are shown in Table 3 and Figure 4. According to Davis and Stout (1971), the antimicrobial activity of the fungal extract against C. albicans, E. coli, and S. aureus was moderate, while its activity against A. hydrophila and Salmonella sp. was classified as strong. Several previous studies have also reported that Trichoderma extract has antibacterial activity, including against methicillin-resistant S. aureus (Sadykova et al., 2015), S. aureus (Haryani et al., 2019; Leylaie and Zafari, 2018), C. albicans (Sadykova et al., 2015), E. coli (Haryani et al., 2019; Khethr et al., 2008; Leylaie and Zafari, 2018), and Salmonella typhi (Reena and Nagar, 2014). The antibacterial activity of the Trichoderma extract against A. hydrophila has not been previously reported. Additionally, Trichoderma sp. isolated from mangrove Ceriops tagal was also effective against Vibrio algionlyticus (Haryani et al., 2019). Similarly, Trichoderma reesei (CGF-11) exhibited antibacterial activity against a variety of phytopathogenic bacteria (Ikram et al., 2019).
CONCLUSION
Trichoderma asperellum AFBN has been isolated from ascidian Eudistoma sp. from Bunaken waters. This fungal EtOAc extract contained phytoconstituents such as alkaloids, triterpenoids, tannins, flavonoids, and phenolic compounds. Additionally, it also possesses significant antioxidant activity. The EtOAc extract of the fungus has broad-spectrum antibacterial activities. As a result, T. asperellum may be investigated further for potential commercialization in the health and pharmacoindustries.
ACKNOWLEDGMENTSS
Financial support for this study was provided by a grant from Sam Ratulangi University through the Higher Education Excellence Basic Research scheme (Contract No. 602/UN.12.13/LT/2020).
CONFLICT OF INTERESTS
None.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Blois MS. Antioxidant determinations by the use of a stable free radical. Nature, 1958; 181(4617):1199–200; doi:10.1038/1811199a0 CrossRef
Cardoso J, Nakayama DG, Sousa E, Pinto E. Marine-derived compounds and prospects for their antifungal application. Molecules, 2020; 25(24):5856; doi:10.3390/molecules25245856 CrossRef
Casertano M, Menna M, Imperatore C. The ascidian-derived metabolites with antimicrobial properties. Antibiotics, 2020; 9(8):1–30; doi:10.3390/antibiotics9080510 CrossRef
Da Luz Calado M, Silva J, Alves C, Susano P, Santos D, Alves J, Martins A, Gaspar H, Pedrosa R, Campos MJ. Marine endophytic fungi associated with Halopteris scoparia (Linnaeus) Sauvageau as producers of bioactive secondary metabolites with potential dermocosmetic application. PLoS One, 2021; 16:1–30; doi:10.1371/journal.pone.0250954 CrossRef
Damongilala JL, Wewengkang DS, Losung F, Ekawati Tallei T. Phytochemical and antioxidant activities of Eucheuma spinosum as natural functional food from North Sulawesi Waters, Indonesia. Pakistan J Biol Sci, 2021; 24:132–8; doi:10.3923/pjbs.2021.132.138 CrossRef
Davis WW, Stout TR. Disc plate method of microbiological antibiotic assay. I. Factors influencing variability and error. Appl Microbiol, 1971; 22(4):659–65; doi:10.1128/am.22.4.659-665.1971 CrossRef
Dhalaria R, Verma R, Kumar D, Puri S, Tapwal A, Kumar V, Nepovimova E, Kuca K. Bioactive compounds of edible fruits with their anti-aging properties: a comprehensive review to prolong human life. Antioxidants, 2020; 9(11):1–38; doi:10.3390/antiox9111123 CrossRef
Ding G, Chen L, Zhou C, Hong-Mei J, Liu YT, Chang X, Song B, Liu XZ, Gu YC, Zou ZM. Trichoderamides A and B, a pair of stereoisomers from the plant endophytic fungus Trichoderma gamsii. J Antibiot Res, 2015; 68(6):409–13; doi:10.1038/ja.2015 CrossRef
Dou X, Dong B. Origins and bioactivities of natural compounds derived from marine ascidians and their symbionts. Mar Drugs, 2019; 17(12):670; doi:10.3390/md17120670 CrossRef
Farag RS, Abdel-Latif MS, Abd El Baky HH, Tawfeek LS. Phytochemical screening and antioxidant activity of some medicinal plants’ crude juices. Biotechnol Rep, 2020; 28:e00536; doi:10.1016/j.btre.2020.e00536 CrossRef
Gogineni V, Hamann MT. Marine natural product peptides with therapeutic potential: chemistry, biosynthesis, and pharmacology. Biochim Biophys Acta, 2018; 1862(1):81–196; doi:10.1016/j.bbagen.2017.08.014 CrossRef
Handayani D, Ananda N, Artasasta MA, Ruslan R, Fadriyanti O, Tallei TE. Antimicrobial activity screening of endophytic fungi extracts isolated from brown algae Padina sp. J Appl Pharm Sci 2019; 9:9–13; doi:10.7324/JAPS.2019.90302 CrossRef
Handayani D, Artasasta MA, Mutia D, Atikah N, Tallei TE. Antimicrobial and cytotoxic activities screening of fungal secondary metabolites isolated from marine sponge Callyspongia sp. AACL Bioflux, 2021; 4(1):249–58.
Haryani Y, Hilma R, Delfira N, Martalinda T, Puspita F, Friska A, Juwita D, Farniga A, Ardi F. Potential antibacterial activity of endophytic fungi Penicillium sp. and Trichoderma sp. derived from mangrove Ceriops tagal (Perr.) C.B.Robb and Bruguiera sp. J Phys Conf Ser, 2019; 1351:12100; doi:10.1088/1742-6596/1351/1/012100 CrossRef
Hudzicki J. Kirby-Bauer disk diffusion susceptibility test protocol, 2009. Available via http://www.microbelibrary.org/component/resource/laboratory-test/3189-kirby-bauer-disk-diffusion-susceptibility-test-protocol (Accessed 10 September 2021).
Ikram M, Ali N, Jan G, Hamayun M, Jan FG, Iqbal A. Novel antimicrobial and antioxidative activity by endophytic Penicillium roqueforti and Trichoderma reesei isolated from Solanum surattense. Acta Physiol Plant, 2019; 41:164; doi:10.1007/s11738-019-2957-z CrossRef
Jin L, Quan C, Hou X, Fan S. Potential pharmacological resources: natural bioactive compounds from marine-derived fungi. Mar Drugs, 2016; 14(4):76; doi:10.3390/md14040076 CrossRef
Khethr FBH, Ammar S, Saïdana D, Daami M, Chriaa J, Liouane K, Mahjoub MA, Helal AN, Mighri Z. Chemical composition, antibacterial and antifungal activities of Trichoderma sp. growing in Tunisia. Ann Microbiol, 2008; 58:303–8; doi:10.1007/BF03175334 CrossRef
Kjer J, Debbab A, Aly AH, Proksch P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat Protoc, 2010; 5:479–90; doi:10.1038/nprot.2009.233 CrossRef
Larayetan R, Ololade ZS, Ogunmola OO, Ladokun A. Phytochemical constituents, antioxidant, cytotoxicity, antimicrobial, antitrypanosomal, and antimalarial potentials of the crude extracts of Callistemon citrinus. Evid Based Comp Altern Med, 2019; 2019:5410923; doi:10.1155/2019/5410923 CrossRef
Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc, 2011; 86:156–67; doi:10.4065/mcp.2010.0639 CrossRef
Leylaie S, Zafari D. Antiproliferative and antimicrobial activities of secondary metabolites and phylogenetic study of endophytic Trichoderma species from Vinca plants. Front Microbiol, 2018; 9:1484; doi:10.3389/fmicb.2018.01484 CrossRef
Nicoletti R, Vinale F. Bioactive compounds from marine-derived Aspergillus, Penicillium, Talaromyces and Trichoderma species. Mar Drugs, 2018; 16:15–7; doi:10.3390/md16110408. CrossRef
Omomowo IO, Fadiji AE, Omomowo OI. Antifungal evaluation and phytochemical profile of Trichoderma harzianum and Glomus versiforme secondary metabolites on cowpea pathogens. Asian Jr Microbiol Biotech Env Sc, 2020; 22:265–72.
Pang KL, Overy DP, Jones EBG, da Luz Calado M, Burgaud G, Walker AK, Johnson JA, Kerr RG, Cha HJ, Bills GF. ‘Marine fungi’ and ‘marine-derived fungi’ in natural product chemistry research: Toward a new consensual definition. Fungal Biol Rev, 2016; 30:163–75; doi:10.1016/j.fbr.2016.08.001 CrossRef
Ramesh C, Tulasi BR, Raju M, Thakur N, Dufossé L. Marine natural products from Tunicates and their associated microbes. Mar Drugs, 2021; 19(6):308; doi:10.3390/md19060308 CrossRef
Reena P, Nagar K. Antimicrobial activity of Trichoderma harzianum against bacteria and fungi. Int J Curr Microbiol App Sci, 2014; 3(1):96–103.
Ren J, Xue C, Tian L, Xu M, Chen J, Deng Z, Proksch P, Lin W. Asperelines A−F, Peptaibols from the marine-ferived fungus Trichoderma asperellum. J Nat Prod, 2009; 72:1036–44; doi:10.1021/np900190w CrossRef
Sadykova VS, Kurakov AV, Kuvarina AE, Rogozhin EA. Antimicrobial activity of fungi strains of Trichoderma from Middle Siberia. Appl Biochem Microbiol, 2015; 51:355–61; doi:10.1134/S000368381503014X CrossRef
Sandrawati N, Hati SP, Yunita F, Putra AE, Ismed F, Tallei TE, Hertiani T, Handayani D. Antimicrobial and cytotoxic activities of marine sponge-derived fungal extracts isolated from Dactylospongia sp. J Appl Pharm Sci, 2020; 10:28–33; doi:10.7324/JAPS.2020.104005 CrossRef
Sanger G, Rarung LK, Wonggo D, Dotulong V, Damongilala LJ, Tallei TE. Cytotoxic activity of seaweeds from North Sulawesi marine waters against cervical cancer. J Appl Pharm Sci, 2021; 11(09):66–73; doi:10.7324/japs.2021.110908 CrossRef
Shad AA, Ahmad S, Ullah R, AbdEl-Salam NM, Fouad H, Ur Rehman N, Hussain H, Saeed W. Phytochemical and biological activities of four wild medicinal plants. Sci World J, 2014; 2014:857363; doi:10.1155/2014/857363 CrossRef
Shi ZZ, Liu XH, Li XN, Ji NY. Antifungal and antimicroalgal Trichothecene sesquiterpenes from the marine algicolous fungus Trichoderma brevicompactum A-DL-9-2. J Agric Food Chem, 2020; 68:15440–8; doi:10.1021/acs.jafc.0c05586 CrossRef
Song YP, Liu XH, Shi ZZ, Miao FP, Fang ST, Ji NY. Bisabolane, cyclonerane, and harziane derivatives from the marine-alga-endophytic fungus Trichoderma asperellum cf44-2. Phytochemistry, 2018; 152:45–52; doi:10.1016/j.phytochem.2018.04.017 CrossRef
Su D, Ding L, He S. Marine-derived Trichoderma species as a promising source of bioactive secondary metabolites. Mini Rev Med Chem, 2018; 18:1702–13; doi:10.2174/1389557518666180727130826 CrossRef
Sumilat DA, Ginting EL, Pollo GAV, Adam AA, Tallei TE. Antimicrobial activities of Rhopalaea-associated fungus Aspergillus flavus strain mfabu9. Pakistan J Biol Sci, 2020; 23:911–6; doi:10.3923/pjbs.2020.911.916 CrossRef
Vitale GA, Coppola D, Palma Esposito F, Buonocore C, Ausuri J, Tortorella E, de Pascale D. Antioxidant molecules from marine fungi: methodologies and perspectives. Antioxidants, 2020; 9:1183; doi:10.3390/antiox9121183 CrossRef
Wang W, Arshad MI, Khurshid M, Rasool MH, Nisar MA, Aslam MA, Qamar MU, Salamat MK. Antibiotic resistance?: a rundown of a global crisis. Infect Drug Resist, 2018:1645–58. CrossRef
Wu ZH, Liu D, Xu Y, Chen JL, Lin WH. Antioxidant xanthones and anthraquinones isolated from a marine-derived fungus Aspergillus versicolor. Chin J Nat Med, 2018; 16:219–24; doi:10.1016/S1875-5364(18)30050-5 CrossRef
Yamazaki H, Yagi A, Takahashi O, Yamaguchi Y, Saito A, Namikoshi M, Uchida R. Antifungal trichothecene sesquiterpenes obtained from the culture broth of marine-derived Trichoderma cf. brevicompactum and their structure-activity relationship. Bioorg Med Chem Lett, 2020; 30(17):127375. CrossRef
Yusnawan E, Inayati A, Baliadi Y. Screening of antagonistic fungi against web blight disease and identification of volatile metabolites produced by Trichoderma. IOP Conf Ser Earth Environ Sci, 2020; 456:12060; doi:10.1088/1755-1315/456/1/012060 CrossRef
Zeilinger S, Gruber S, Bansal R, Mukherjee PK. Secondary metabolism in Trichoderma – chemistry meets genomics. Fungal Biol Rev, 2016; 30:74–90; doi:10.1016/j.fbr.2016.05.001 CrossRef
Zhang JC, Chen GY, Li XZ, Hu M, Wang BY, Ruan BH, Zhou H, Zhao LX, Zhou J, Ding ZT, Yang YB. Phytotoxic, antibacterial, and antioxidant activities of mycotoxins and other metabolites from Trichoderma sp. Nat Prod Res, 2017; 31:2745–52; doi:10.1080/14786419.2017.1295235 CrossRef
Zhang JL, Tang WL, Huang QR, Li YZ, Wei ML, Jiang LL, Liu C, Yu X, Zhu HW, Chen GZ, Zhang XX. Trichoderma: a treasure house of structurally diverse secondary metabolites with medicinal importance. Front Microbiol, 2021; 12:2037; doi:10.3389/fmicb.2021.723828 CrossRef