INTRODUCTION
Tri-Kesornmas (TK) is a traditional Thai medicine remedy that is comprised of three herbs in equal proportions. They are the stem bark from the coral bush (Jatropha multifida L., Euphorbiaceae), lotus stamen (Nelumbo nucifera Gaerth, Nymphaeaceae), and bael fruit (Aegle marmelos (L.) Correa, Rutaceae). It is commonly prescribed with other herbal medicines by traditional healers to aid patients in achieving optimal health. It is documented in several traditional medical books to restore health by overcoming imbalances of four basic elements, namely, earth, water, wind, and fire. According to the philosophy of traditional Thai medicine, these four elements must be in balance and work in harmony to maintain good health (Chokevivat and Chuthaputti, 2005). It is also commercially available as a dietary supplement to prevent age-related degeneration because of the presence of well-documented antioxidant ingredients, such as lotus stamen and bael fruit (Bhardwaj and Nandal, 2015; Chen et al., 2019; Jung et al., 2003). The formula has been integrated into the National Health Care System of Thailand since it was recorded in the National Drug List in 2013 to restore health in patients who are recovering from illnesses. It is recommended to infuse 1 g of the herb mixture in 200 ml of hot water each time and drink it four times per day.
Extensive studies have revealed that toxic oxygen species are produced from an imbalance between the production of reactive oxygen species (ROS) in tissues and the ability to remove them, causing oxidative stress and consequently stimulating chronic degenerative diseases and reducing lifespan in various aging models (Liochev, 2015; Oliveira et al., 2010). Antioxidants are believed to protect cells from these toxic oxygen species. Several studies have reported evidence that antioxidant supplementation can aid biological systems in the detoxification of toxic oxygen species. Therefore, it reduces oxidative stress and prolongs lifespan (Peng et al., 2014).
Considering that TK is a mixture of antioxidant herbs, the positive role for health provided by TK may arise from the ability to balance toxic radicals in the body. Therefore, this study aimed to investigate the potential oxidative stress resistance and lifespan extension ability of TK. In addition, oxidative stress is a factor that accelerates brain tissue injury in Alzheimer’s disease (AD) (Zhao and Zhao, 2013). Therefore, the activity of TK against oxidative injury caused by the accumulation of toxic proteins in the AD brain was also evaluated. The nematode Caenorhabditis elegans was used in this study. This model organism not only has a short lifespan and fast generation time but also shares several key genes with mammals, including humans. Furthermore, molecular and cellular pathways related to aging are evidently similar for both C. elegans and mammals (Prasad and Bondy, 2013). This similarity allows discoveries from studies of C. elegans to be deduced in humans.
MATERIALS AND METHODS
Plant material
Dry herbs obtained from the stem bark of the coral bush (J. multifida L., Euphorbiaceae), lotus stamen (N. nucifera Gaerth, Nymphaeaceae), and bael fruit (A. marmelos (L.) Correa, Rutaceae) were purchased from Vejpongosot Co., Ltd., Bangkok, Thailand. They were identified and deposited in the Herbarium of the Department of Pharmacognosy, Srinakharinwirot University, Thailand. Thai Herbal Pharmacopoeia 2019 (Ministry of Public Health, 2019) was used to determine the quality of the herbs. The outcomes of the herbal material evaluations were depicted in supporting documentation (Supplementary Figs. S1–3 and Supplementary Table S1).
Preparation of the extract
The herbs were separately washed and dried in a hot air oven at 50°C until dry before grinding to a fine powder. Equal proportions were blended. Deionized water (2,000 ml) was added to the herb mixture (100 g), and it was boiled for 5 minutes. Subsequently, the herb residue was removed by vacuum filtration. After filtration, the water was removed from the filtrate using a pilot-scale spray dryer (Büchi Labortechnik AG, Flawil, Switzerland). The spray dryer’s inlet temperature was 155°C, outlet temperature was 122°C, feed rate was 5 ml minute−1, airflow rate was 30 m3 hour−1, and spray flow rate was 473 l hour−1. After spray drying, the extract appeared as a pale orange fine powder, and the yield was 7.3% w/w.
Maintenance of C. elegans
The wild-type C. elegans strain N2 and the transgenic strain CL4176 were obtained from the Caenorhabditis Genetics Center (University of Minnesota). The worms were kept at 20°C (for N2) and 16°C (for CL4176) on a nematode growth media (NGM) plate with Escherichia coli strain OP50 as the food source.
Stock solutions for C. elegans assays
Stock solutions of TK were prepared using distilled water and diluted with the OP50 food source to achieve the desired final concentration for treatment.
Oxidative stress resistance assay
The synchronized larvae were exposed to various doses of TK extract at 20°C until they reached the L4 stage. Worms were placed in each well of a 96-well plate, which contained 40 μl of 100 mM paraquat (0.025 g of paraquat diluted in 1 ml of M9 buffer). The worms’ vitality was assessed hourly until no worms remained alive. When the worms did not respond to a mild touch with a platinum loop, they were declared dead (Possik and Pause, 2015).
Lifespan assay
The synchronized worms at the L4 stage were maintained in NGM plates seeded with live E. coli strain OP50 and supplemented with various concentrations of TK. When the worms reached the adult stage, they were transferred to new plates, which was considered day 1 of the nematode lifespan. Worms were transferred to freshly seeded plates every day during their reproductive period and every other day during the post-reproductive period. The number of dead and surviving worms was recorded daily until all worms were dead. Worms were scored as dead as previously described (Park et al., 2017).
Paralysis assay
Synchronized eggs of the transgenic C. elegans strain CL4176 were kept at 16°C on NGM plates seeded with live E. coli strain OP50 and supplied with various concentrations of TK extract. To stimulate amyloid-beta (Aβ) protein expression, the temperature was raised from 16°C to 25°C, starting at the 36th hour after egg laying and continuing until the completion of the paralysis assay. After the temperature increase, the scoring was done at hourly intervals for 24 hours, until the last worm fell paralyzed. Paralysis was diagnosed in worms that did not respond to light touch with a platinum loop (Luo et al., 2009).
Estimation of phenolic content
Twenty microliters of TK (5 mg ml−1) was mixed with 100 µl of Folin–Ciocalteu’s reagent. The solution was left for 5 minutes before mixing with 80 µl of a 7.5% sodium carbonate solution. The absorbance of triplicate samples was measured at 760 nm on a microplate reader (SpectraMax M3, Molecular Devices, USA) after 30 minutes of incubation in the dark at room temperature (Zhang et al., 2006). The phenolic content was estimated by comparison to a gallic acid calibration curve (y = 3.5359x + 0.0307, R2 = 0.9995), and the total phenolic content was expressed as gallic acid equivalents (GAE) in mg per 100 mg spray-dried powder.
Estimation of flavonoid content
One hundred microliters of TK (20 mg ml−1) was mixed with 100 µl of 2% aluminum chloride in ethanol. After 30 minutes of incubation at room temperature, the absorbance of triplicate samples was measured at 420 nm using a microplate reader (SpectraMax M3, Molecular Devices, USA) (P?kal and Pyrzynska, 2014). The flavonoid concentration was calculated by comparison to a rutin calibration curve (y = 3.6423x − 0.014, R2 = 1), and the results were reported as an equivalent amount to rutin (mg rutin/100 mg spray-dried powder).
Determination of protocatechuic acid using high-performance thin-layer chromatography (HPTLC)
The spray-dried powder of TK was dissolved in methanol (100 mg ml−1) with the aid of sonication in an ultrasound bath for 10 minutes at room temperature. The calibration range for standard protocatechuic acid (ChromaDex, USA) was 200–600 ng. TK and the standard were separately applied on an HPTLC plate precoated with silica gel 60 F254 (0.2 mm thickness, Merck) using a Linomat V applicator (CAMAG, Switzerland). The mobile phase consisted of toluene:ethyl acetate:formic acid (5:4:1, v/v/v). The plates were developed up to 85% of the total plate height in a CAMAG Twin Trough Glass Chamber (10 × 20 cm) and scanned at 254 nm using a TLC Scanner 4 linked to winCATS software. The peak areas and absorption spectra were documented. The UV absorption spectrum of the standard was overlaid with the bands in the sample track to validate the identity of the sample (Verma et al., 2016). The amount of protocatechuic acid in TK was calculated using the linear regression equation (y = 813.42x + 274.22, R2 = 0.9991) derived from the calibration curves. The standard curve of protocatechuic acid and HPTLC chromatograms were shown in supporting information (Supplementary Figs. S4–6 and Supplementary Tables S2–3).
Statistical analysis
The experiments of the C. elegans model were independently repeated three times. GraphPad Prism 5 software was used to plot and analyze the survival and paralysis curves. The significance of differences was determined using the one-way analysis of variance and Dunnett’s test. Statistical significance was defined as a value of p = 0.05. The contents of protocatechuic acid, total flavonoids, and total phenolic compounds are reported as the means ± SD.
RESULTS
Effect of TK on paraquat-induced oxidative stress in wild-type (N2) strain C. elegans
Paraquat was utilized to produce oxidative stress by creating ROS in wild-type (N2) worms. With a p-value of 0.0058, pre-treatment of the worms with TK at a dose of 10 μg ml−1 resulted in an increase in worm survival compared to the control (Fig. 1 and Table 1).
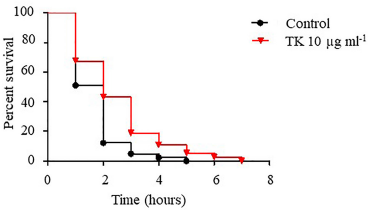 | Figure 1. The effect of TK on paraquat-induced oxidative stress in C. elegans wild-type (N2) strain. Worms were treated with either a control (OP50) or TK 10 µg ml−1 from the egg stage until they reached L4, at which point they were subjected to paraquat 100 mM. The survival rate was scored every hour until the last worm died. The survival graph was created with the GraphPad Prism software. A p-value < 0.05 was considered statistically significant. [Click here to view] |
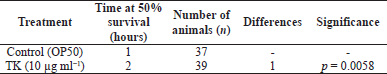 | Table 1. Effect of TK on paraquat-induced oxidative stress in C. elegans wild-type (N2) strain. [Click here to view] |
Effect of TK on lifespan extension in wild-eype (N2) strain C. elegans
With p values of 0.0392 and 0.0020, respectively, TK at doses of 10 and 100 μg ml−1 was capable of increasing the survival of wild-type (N2) worms when compared to the control. The median lifespan was 16 days, which was 6.7% longer than in the control group (Fig. 2 and Table 2).
Effect of TK against Aβ toxicity (paralysis assay)
In C. elegans strain CL4176, TK at a concentration of 100 μg ml−1 considerably (p = 0.0387) delayed paralysis, whereas TK at a dose of 10 μg ml−1 did not (Fig. 3 and Table 3).
Chemical constituents in TK
A chromatogram developed via HPTLC revealed a major peak corresponding to protocatechuic acid at an Rf value of 0.34 (Fig. 4). The linear regression equation obtained from the calibration curves of standard protocatechuic acid was used to calculate the amount of protocatechuic acid in TK. The quantities of protocatechuic acid, total flavonoids, and total phenolic compounds are shown in Table 4.
DISCUSSION
TK is a traditional Thai remedy that comprises the stem bark of the coral bush, lotus stamen, and bael fruit. It has long been valued for bringing good health and assistance in healthy aging. It has been reported to exhibit antioxidant activity by scavenging DPPH radicals (Phuaklee and Itharat, 2015). In this study, TK was prepared by boiling in water, which is similar to traditional utilization. TK in its liquid form was further modified to a dry powder using the spray-drying method. The spray-dried powder of TK has been demonstrated to possess flavonoids and phenolic compounds. In addition, the content of protocatechuic acid, a major phenolic compound in TK, was determined.
 | Figure 2. The effect of TK on lifespan extension in C. elegans wild-type (N2) strain. This figure compares the effects of TK at concentrations of 10 and 100 mg ml−1 to the control. C. elegans synchronized eggs were maintained at 20°C in 60 × 10 mm culture dishes containing either OP50 (control) or TK 10 or 100 mg ml−1. The worms were fed either OP50 or TK from the egg stage onward. The survival was scored every day until the last worm died. Statistical significance was defined as a p-value < 0.05. [Click here to view] |
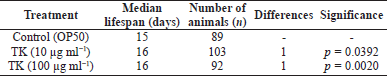 | Table 2. Effect of TK on lifespan extension in C. elegans wild-type (N2) strain. [Click here to view] |
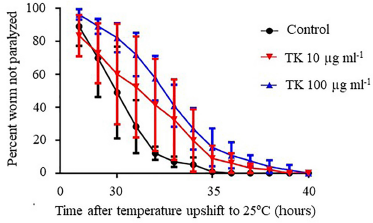 | Figure 3. The effect of TK on Aβ-induced paralysis in C. elegans strain CL4176. This figure compares the effects of TK at concentrations of 10 and 100 mg ml−1 to the control. Worms were given either OP50 (control) or TK 10 or 100 mg ml−1 from the egg stage onward. Synchronized eggs were maintained at 16°C on the 60 × 10 mm culture dishes containing either OP50 (control) or TK 10 or 100 mg ml−1. The hatched worms were reared at 16°C for 36 hours before being raised to 25°C to promote the expression of the Aβ transgene. The paralysis was scored at one-hour intervals. Data were presented as a percentage of the worms that were not paralyzed. Curves were obtained from three independent experiments. The error bars represent the standard deviation. [Click here to view] |
An oxidative stress resistance experiment was used to determine TK’s in vivo antioxidant activity. To create oxidative stress in C. elegans, paraquat, an herbicide that generates harmful hydrogen peroxide to raise intracellular superoxide levels, was utilized (Moran et al., 2010). The results showed that TK supported the recovery of the worms from oxidative stress.
Oxidative stress has been proposed to elicit the degradation of biomolecules, such as carbohydrates, lipids, nucleic acids, and proteins. In addition, it has been hypothesized to be a major factor that causes aging. It is obvious that the prevention of oxidative stress reduces the deterioration of these biomolecules and consequently delays aging and prolongs lifespan (Oliveira et al., 2010; Prasad and Bondy, 2013). In C. elegans, many studies have revealed that the lifespan extension effect is due to enhanced resistance to oxidative stress (Wan et al., 2014; Wang et al., 2020). Our findings demonstrated that TK at the concentration that showed oxidative stress resistance could lengthen the lifespan in adult C. elegans and that TK did not reach the toxic concentration, and lifespan extension was observed when the concentration was increased 10 times. Our observations agreed with the hypothesis that prevention of oxidative stress could delay aging.
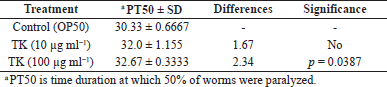 | Table 3. Effect of TK on Aβ-induced paralysis in C. elegans strain CL4176. [Click here to view] |
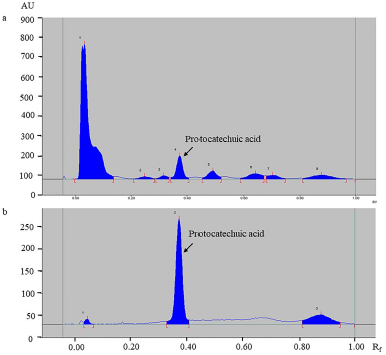 | Figure 4. HPTLC chromatogram of TK (a) and reference standard protocatechuic acid (b) scanned at 254 nm. [Click here to view] |
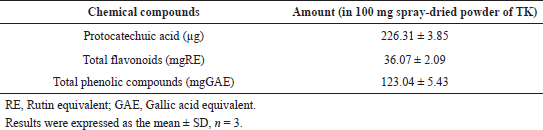 | Table 4. Chemical composition of TK. [Click here to view] |
Aging is a deleterious change in cell physiological function and is known as a risk factor for age-related disorders, such as neurodegenerative diseases (Liochev, 2015). Healthy aging is required for any person. AD is a common age-related neurodegenerative disease caused by the abnormal buildup of the toxic Aβ protein in the brain (Scheltens et al., 2016). Thus, a strategy that prevents Aβ accumulation in the brain could be useful for preventing AD. It is well documented that oxidative stress creates an environment that is favorable for the production of the Aβ protein (Cooper and Ma, 2017; Zuo et al., 2015). The potential of TK to slow the progression of age-related disorders, such as AD, was determined by a paralysis assay. In this assay, the test compound that possesses activity against Aβ toxicity delayed the paralysis of CL4176 C. elegans. CL4176 is a C. elegans strain that is genetically engineered to express the human Aβ protein in muscle cells. In the worm, expression of Aβ occurs when the temperature is upshifted from 16°C to 25°C. Consequently, it causes muscle paralysis (Luo et al., 2009). Our study revealed that TK at a concentration sufficient to prevent oxidative stress could not diminish the toxicity of Aβ in C. elegans, but a beneficial effect was observed at higher concentrations. Therefore, the depletion of Aβ toxicity may be attributed to the higher antioxidant activity.
The activities of TK are supported by the presence of protocatechuic acid as well as flavonoids and phenolic compounds. Protocatechuic acid isolated from Veronica peregrine has been proven to extend lifespan and increase resistance against osmotic, heat shock, and oxidative stress in C. elegans (Kim et al., 2014). In that study, protocatechuic acid at 100 µM (or 15.4 µg ml−1) and 200 µM (or 30.8 µg ml−1) has been reported to lengthen the lifespan of adult C. elegans. The content of protocatechuic acid in the 100 mg spray-dried powder of TK was 226.31 ± 3.85 µg, which was regarded to be high enough to exert the lifespan extension effect.
Flavonoids and phenolic compounds have been regarded as free-radical quenchers and have beneficial effects on oxidative stress resistance in C. elegans (Pallauf et al., 2017). The activities of TK were supported by its herbal components. For instance, lotus stamen has been reported to be a potential rich source of flavonoids and phenolic compounds and has been shown to boost stress resistance, lengthen lifespan, and prevent Aβ aggregation in C. elegans (Kampkötter et al., 2008; Regitz et al., 2014). A previous study on bael fruit in a C. elegans model revealed that bael fruit exhibited antioxidant activity under oxidative stress conditions and decreased Aβ toxicity via the DAF-16-mediated cell signaling system (Keowkase et al., 2020). DAF-16 regulates the expression of various genes related to stress responses (Murphy and Hu, 2013). Bael fruit contains phenolic compounds such as protocatechuic acid, ferulic acid, and gallic acid (Jagota and Rajadas, 2012). These compounds have been shown to prevent Aβ oligomerization, improve oxidative stress tolerance, and extend lifespan in C. elegans (Saul et al., 2011). There are few studies concerning the chemical constituents and bioactivity of the stem bark of the coral bush. However, the plant has been described to contain protocatechuic acid, and in vitro antioxidant activity has been reported (Hirota et al., 2012). In addition to reducing the toxicity of Aβ, bael fruit and lotus stamen have been reported to have anti-AD properties by exhibiting inhibitory activities against key enzymes involved in AD, such as acetylcholinesterase, butyrylcholinesterase, and β-secretase 1 (Tansin and Sitthithaworn, 2020; Temviriyanukul et al., 2020). These results confirmed that TK, which contains flavonoids, phenolic compounds, and protocatechuic acid, has a protective effect against oxidative stress. These chemicals are thought to protect cells from toxic oxygen species. As a result, they increase lifespan and protect cells from the oxidative damage produced by the harmful Aβ protein.
CONCLUSION
The results demonstrated for the first time that TK supports recovery from oxidative stress, extends lifespan, and reduces the toxicity of Aβ in C. elegans. Therefore, the results confirmed the benefit of TK in traditional medicine for the prevention of age-related degeneration and for supportive bodies to work in harmony to maintain good health. The recommended dose for the TK herbal mixture in the National Drug List is one gram of herb per one dose. Based on the yield obtained after boiling in water and spray drying, 73 mg of spray-dried powder was equivalent to the recommended dose. Because C. elegans shares several key genes with humans, the results discovered in C. elegans have been documented to be relevant to humans. Further studies should be performed to understand the mechanism of TK in C. elegans to extrapolate the molecular and cellular pathways of TK in humans. Moreover, various chemical constituents have been reported in TK, and these compounds may work additively or synergistically to achieve activities in C. elegans. To identify a potential active ingredient in addition to protocatechuic acid, active biological markers should be clarified.
ACKNOWLEDGMENTS
The authors acknowledge the Research Unit for Drug Discovery and Development of the Faculty of Pharmacy, Srinakharinwirot University, for providing research facilities. The authors would like to express gratitude to Professor Dr. Edward Moreton of the University of Maryland at Baltimore School of Pharmacy for his insight and assistance with the English language presentation of the manuscript.
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.
FUNDING
This work was supported by the National Research Council of Thailand (Grant no. 060/2559).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
Not applicable.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Bhardwaj RL, Nandal U. Nutritional and therapeutic potential of bael (Aegle marmelos Corr.) fruit juice: a review. Nutr Food Sci, 2015; 45:895–919. CrossRef
Chen G, Zhu M, Guo M. Research advances in traditional and modern use of Nelumbo nucifera: phytochemicals, health promoting activities and beyond. Crit Rev Food Sci Nutr, 2019; 59:S189–209. CrossRef
Chokevivat V, Chuthaputti A. The role of Thai traditional medicine in health promotion. Bangkok, Thailand, 2005. Available via http://thaihealingalliance.com/wp-content/uploads/The_Role_ofThai_Traditional_Medicine_in_Health_Promotion_by_Vichai_Chokevivat_and_Anchalee_Chuthaputti.pdf (Accessed 02 October 2019).
Cooper EL, Ma MJ. Alzheimer disease: clues from traditional and complementary medicine. J Tradit Complement Med, 2017; 7(4):380–5. CrossRef
Hirota B, Miyazaki C, Mercali C, Verdam M, Kalegari M, Gemin C, Lordello A, Miguel M, Miguel O. C-glycosyl flavones and a comparative study of the antioxidant, hemolytic and toxic potential of Jatropha multifida leaves and bark. Int J Phytomed, 2012; 4(1):1–5.
Jagota S, Rajadas J. Effect of phenolic compounds against Aβ aggregation and Aβ-induced toxicity in transgenic C. elegans. Neurochem Res, 2012; 37(1):40–8. CrossRef
Jung H, Kim J, Chung H, Choi J. Antioxidant principles of Nelumbo nucifera stamens. Arch Pharm Res, 2003; 26(4):279–85. CrossRef
Kampkötter A, Timpel C, Zurawski R, Ruhl S, Chovolou Y, Proksch P, Wätjen W. Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comp Biochem Physiol B Biochem Mol Biol, 2008; 149(2):314–23. CrossRef
Keowkase R, Kijmankongkul N, Sangtian W, Poomborplab S, Santa-ardharnpreecha C, Weerapreeyakul N, Sitthithaworn W. Protective effect and mechanism of fruit extract of Aegle marmelos against amyloid-β toxicity in a transgenic Caenorhabditis elegans. Nat Prod Commun, 2020; 15(7):1–12. CrossRef
Kim Y, Seo H, Lee M, Kim K, Jeon H, Cha D. Protocatechuic acid extends lifespan and increases stress resistance in Caenorhabditis elegans. Arch Pharm Res, 2014; 37:245–52. CrossRef
Liochev S. Which is the most significant cause of aging? Antioxidants (Basel), 2015; 4(4):793–810. CrossRef
Luo Y, Wu Y, Brown M, Link CD. Caenorhabditis elegans model for initial screening and mechanistic evaluation of potential new drugs for aging and Alzheimer’s disease. In: Buccafusco JJ (ed.). 2nd edition. Methods of behavior analysis in neuroscience, Chapter 16, CRC Press/Taylor & Francis Boca Raton, FL, 2009. Available via https://www.ncbi.nlm.nih.gov/books/NBK5226/ (Accessed 20 September 2020).
Ministry of Public Health. Thai herbal pharmacopoeia. Ministry of Public Health, 2019. Available via https://bdn.go.th/thp/home (Accessed 20 September 2020).
Moran J, Ortiz-Ortiz M, Ruiz-Mesa L, Fuentes J. Nitric oxide in paraquat-mediated toxicity: a review. J Biochem Mol Toxicol, 2010; 24(6):402–9. CrossRef
Murphy C, Hu P. Insulin/insulin-like growth factor signaling in C. elegans, 2013. Available via http://www.wormbook.org (Accessed 08 February 2020). CrossRef
Oliveira B, Nogueira-Machado J, Chaves M. The role of oxidative stress in the aging process. Sci World J, 2010; 10:1121–8. CrossRef
Pallauf K, Duckstein N, Rimbach G. A literature review of flavonoids and lifespan in model organisms. P Nutri Soc, 2017; 76(2):145–62. CrossRef
Park H, Jung Y, Lee SV. Survival assays using Caenorhabditis elegans. Mol Cells, 2017; 40(2):90–9. CrossRef
P?kal A, Pyrzynska K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal Methods, 2014; 7:1776–82. CrossRef
Peng C, Wang X, Chen J, Jiao R, Wang L, Li Y, Zuo Y, Liu Y, Lei L, Ma KY, Huang Y, Chen Z. Biology of ageing and role of dietary antioxidants. Biomed Res Int, 2014; 2014:831841; doi:10.1155/2014/831841 CrossRef
Phuaklee P, Itharat A. Biological activities and total phenolic content of Tregaysornmas formula Planta Med, 2015; 81(11):pe4. CrossRef
Possik E, Pause A. Measuring oxidative stress resistance of Caenorhabditis elegans in 96-well microtiter plates. J Vis Exp, 2015; 99:1–8; doi:10. 3791/ 52746 CrossRef
Prasad KN, Bondy SC. Evaluation of role of oxidative stress on aging in Caenorhabditis elegans: a brief review. Curr Aging Sci, 2013; 6(3):215–9. CrossRef
Regitz C, Dußling LM, Wenzel U. Amyloid-beta (Aβ1-42)-induced paralysis in Caenorhabditis elegans is inhibited by the polyphenol quercetin through activation of protein degradation pathways. Mol Nutr Food Res, 2014; 58(10):1931–40. CrossRef
Saul N, Pietsch K, Sturzenbaum SR, Menzel R, Steinberg C. Diversity of polyphenol action in Caenorhabditis elegans: between toxicity and longevity. J Nat Prod, 2011; 74(8):1713–20. CrossRef
Scheltens P, Blennow K, Breteler M, de Strooper B, Frisoni G, Salloway S, Van der Flier W. Alzheimer’s disease. Lancet, 2016; 388(10043):505–17. CrossRef
Tansin P, Sitthithaworn W. Acetylcholinesterase inhibitor activities of Aegle marmelos fruit beverage. Thai Pharmaceut Health Sci J, 2020; 15(4):223–7.
Temviriyanukul P, Sritalahareuthai V, Promyos N, Thangsiri S, Pruesapan K, Srinuanchai W, Nuchuchua O, Siriwan D, On-nom N, Suttisansanee U. The effect of sacred lotus (Nelumbo nucifera) and its mixtures on phenolic profiles, antioxidant activities, and inhibitions of the key enzymes relevant to Alzheimer’s disease. Molecules, 2020; 25(16):3713. CrossRef
Verma S, Gupta A, Ramana M, Rawat A. High-performance thin-layer chromatographic analysis for the simultaneous quantification of gallic acid, vanillic acid, protocatechuic acid, and quercetin in the methanolic fraction of Limonia acidissima L. fruits. JPC-J Planar Chromat, 2016; 29:356–60. CrossRef
Wan F, Zhi D, Liu D, Xian J, Li M, Abulizi A, Ju W, Li H. Lifespan extension in Caenorhabiditis elegans by several traditional Chinese medicine formulas. Biogerontology. 2014; 15(4):377–87. CrossRef
Wang J, Deng N, Wang H, Li T, Chen L, Zheng B, Liu R. Effects of orange extracts on longevity, healthspan, and stress resistance in Caenorhabditis elegans. Molecules, 2020; 25(2):351. CrossRef
Zhang Q, Zhang J, Shen J, Silva A, Dennis D, Barrow C. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. In: Anderson R, Brodie J, Onsøyen E, Critchley AT (eds.). Eighteenth International Seaweed Symposium. Developments in applied phycology, Springer, Dordrecht, Netherlands, 2006, vol. 1; doi:10.1007/978-1-4020-5670-3_27
Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxidative Med Cell Longev, 2013; 2013:316523; doi:10.1155/2013/316523 CrossRef
Zuo L, Hemmelgarn BT, Chuang C, Best T. The role of oxidative stress-induced epigenetic alterations in amyloid-β production in Alzheimer’s disease. Oxid Med Cell Longev, 2015; 2015:604658; doi:10.1155/2015/604658 CrossRef
SUPPLEMENTARY DATA
CHARACTERIZATION OF THE HERBAL DRUGS
1. Microscopic study of the herbal drugs
1.1 Aegle marmelos fruit
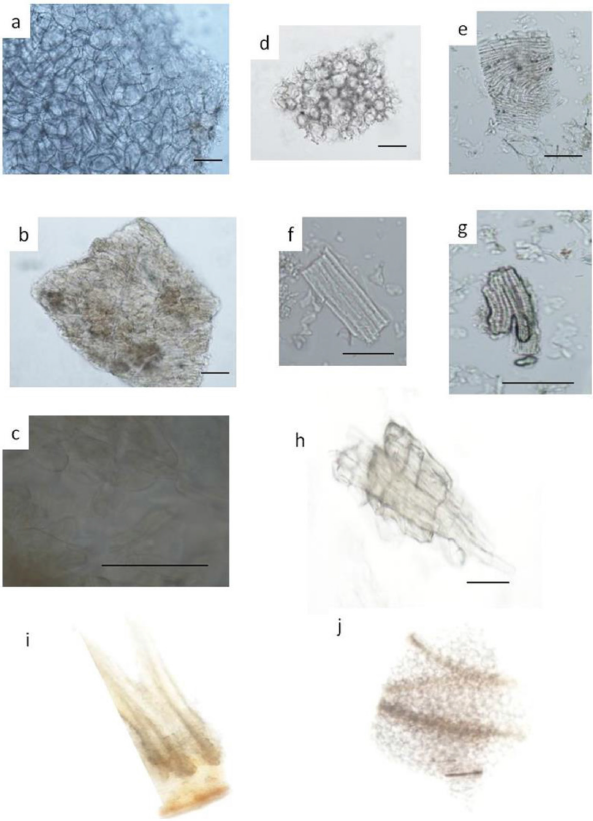 | Supplementary Figure S1. Aegle marmelos fruit: a) parenchyma b; c) inner epidermis of testa; d) outer epidermis of testa; e) epidermis, f) fiber; g) Sclereid; h) epicarp; i) epidermis and parenchyma; j) parenchyma and vascular bundle. Bar represent 0.1 mm. [Click here to view] |
1.2. Nelumbo nucifera stamen
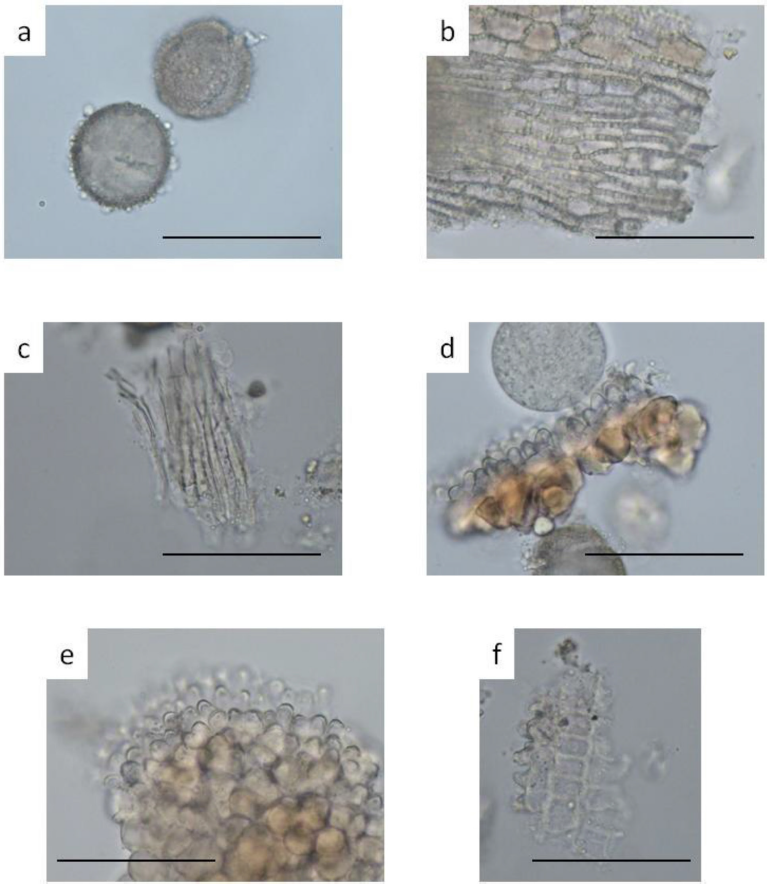 | Supplementary Figure S2. Nelumbo nucifera stamen: a) pollen grain; b) sclereid of the filament; c) parenchyma of the filament; d) endothecium of the anther sac in surface view; e) endothecium of the anther sac in surface view; f) endothecium of the anther sac. Bar represent 0.1 mm. [Click here to view] |
1.3. Jatropha multifida stem bark
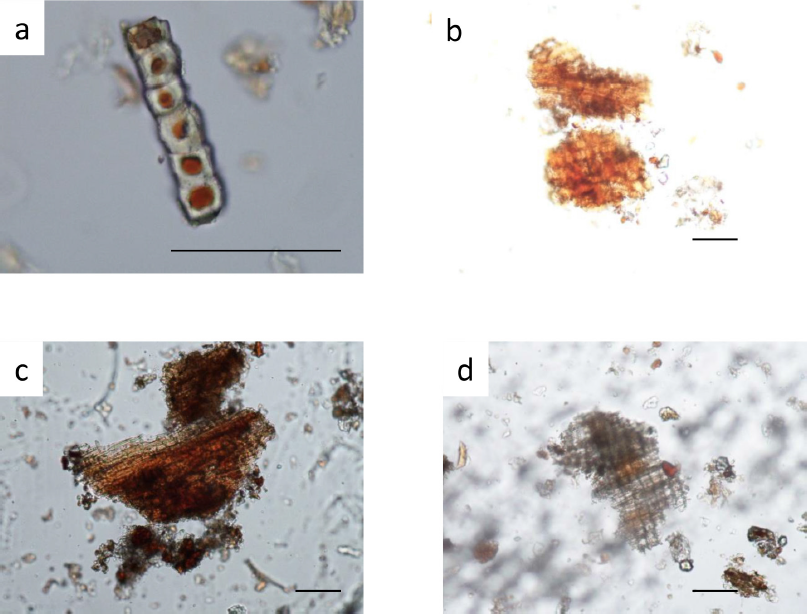 | Supplementary Figure S3. Jatropha multifida stem bark: a) sclereids; b) parenchyma; c) layer of cork and cortex of bark; d) cork in surface view. Bar represent 0.1 mm. [Click here to view] |
1. Physicochemical study
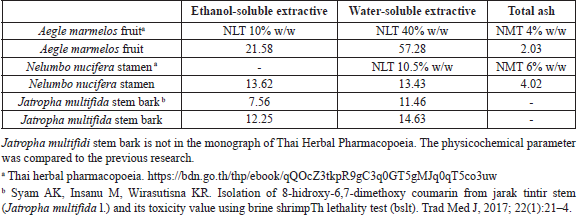 | Supplementary Table S1. Physicochemical properties of herbal drugs. [Click here to view] |
Determination of protocatechuic acid content using high performance thin layer chromatography (HPTLC)
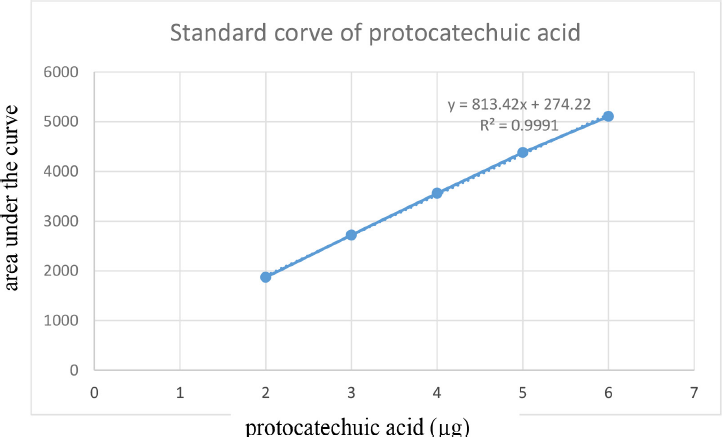 | Supplementary Figure S4. Standard curve of protocatechuic acid. [Click here to view] |
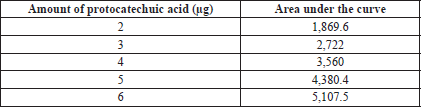 | Supplementary Table S2. Amount of protocatechuic acid and its area under the curve. [Click here to view] |
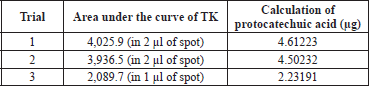 | Supplementary Table S3. Calculation of protocatechuic acid content in TK. [Click here to view] |
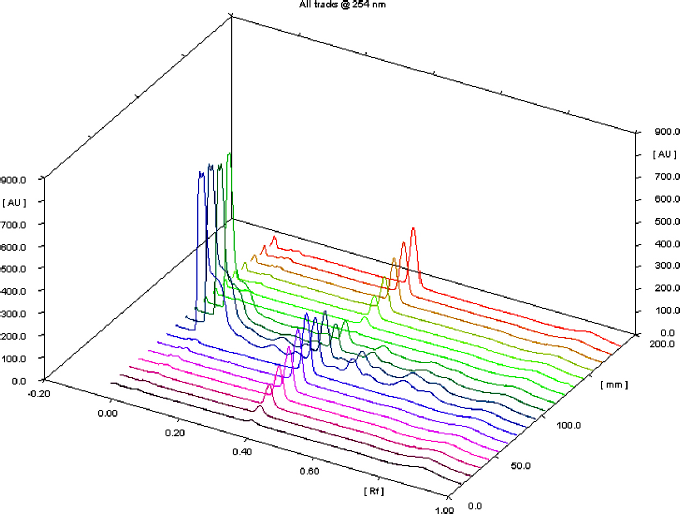 | Supplementary Figure S5. Three-dimensional chromatogram of the standard protocatechuic acid at various concentrations of TK. [Click here to view] |
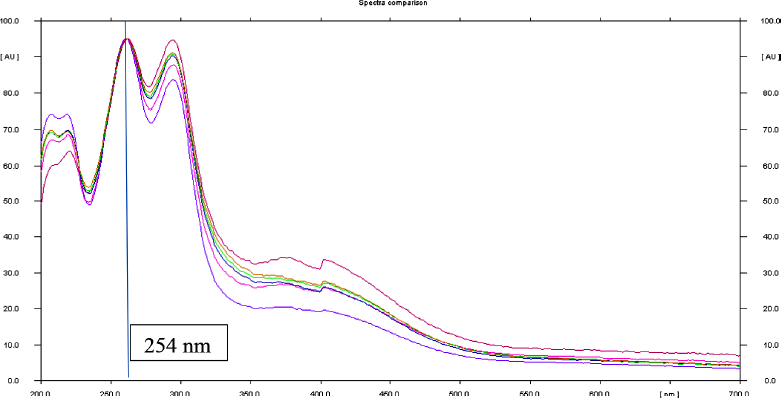 | Supplementary Figure S6. Overlay UV absorption spectra of the standard protocatechuic acid and TK. [Click here to view] |