INTRODUCTION
Colorectal cancer (CRC) continues to be a major cause of morbidity and mortality worldwide, being the third most incident and the second most mortal cancer in both sexes (Global Cancer Statistics, 2020). Current treatments for this disease incorporate combination chemotherapy mainly composed of 5-fluorouracil. Examples of these include FOLFIRI (5-FU/folinic acid/irinotecan), FOLFOXIRI (leucovorin/5-FU/oxaliplatin/irinotecan), and FOLFOX (5-FU/leucovorin/oxaliplatin). The use of these drugs is effective; however, they produce undesirable harmful effects associated with gastrointestinal and neurological disorders which many times cause limitations in the dose or discontinuation of anticancer therapy (McQuade et al., 2014; Pointet et al., 2017; Rejhova et al., 2018). Due to the increase in the statistics and the toxicity associated with conventional chemotherapy, extensive research is ongoing to develop new pharmaceutical agents with chemopreventive potential against colorectal cancer.
Genistein (Fig. 1) is a major metabolite of soy (a plant belonging to the Leguminosae family), which has displayed a wide range of biological activities such as antioxidant (Rahman Mazumder and Hongsprabhas, 2016), antiviral (LeCher et al., 2019), antiallergic (Mastuda et al., 2002), anti-inflammatory (Spagnuolo et al., 2018), antiestrogenic (Wang et al., 2006), and antibacterial (Satish et al., 2018). Furthermore, this compound has also exhibited anti-cancer activity (Ganai et al., 2015; Yang et al., 2016). Chemopreventive potential attributed to genistein and its related flavonoids is due to the capability to modulate signaling pathways and several epigenetic markers that contribute to develop human colorectal carcinogenesis. In fact, epidemiological studies have shown that genistein displays activity against colorectal cancer initiation and progression, in different cell lines (HCT-116, SW-480, and HT-29) (Chae et al., 2019; Tuli et al., 2019), through activation of apoptotic executioner molecules, such as the nuclear factor-kappa B (NF-κB), tumor necrosis factor (TNF-α) (Luo et al., 2014), B-cell lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), as well as by modulating the protease ability of caspase-3 (Zhou et al., 2017), inducing mitotic arrest for inhibiting Polo-like kinase 1 (Plk1) activity (Shin et al., 2017), and inhibiting the activity of cyclooxygenase?2 (COX?2) (Zhang et al., 2017).
On the other hand, aspirin, also known as acetylsalicylic acid (ASA) (Fig. 1), is a non-steroidal anti-inflammatory drug (NSAID), which has long been used as analgesic and anti-inflammatory agent (Alqahtani et al., 2018). This compound inhibits the COX activity; besides, its chemopreventive potential has been reported, particularly in colon cancer (Jiang et al., 2020). Epidemiological studies that include both men and women revealed that aspirin reduced the incidence of CRC by about 70%. This percentage increases with prolonged duration of aspirin intake (Avivi et al., 2012). Additionally, clinical studies have demonstrated a decrease in the mortality rate by up to 48% after continuous aspirin intake in diagnosed CRC patients (Avivi et al., 2012).
Nowadays, an emerging strategy for new drug discovery relies in the use of hybrid molecules which results from the covalent linking of two molecules with individual intrinsic pharmacological activity (Cardona et al., 2021). Hybrid molecules bear two distinct pharmacophores with different biological functions and may display dual activity, although both entities of the hybrid molecule are not necessarily acting on the same biological target (Cardona et al., 2018; de Oliveira Pedrosa et al., 20017).
In search for new alternatives to treat colorectal cancer, authors designed and synthesized a series of ASA-Genistein hybrids (Fig. 1), which were obtained via click reaction between different ASA-alkylazides and propargyl-genistein. In addition, it was conducted an oncoinformatics study to predict the binding affinity of promising hybrids through an exhaustive drug-likeness and in silico ADME/Tox modeling against six tumor-associated proteins targets in CRC, to propose a plausible biochemical mechanism by which these compounds exert a significant inhibitory effect on SW480 cell growth.
MATERIALS AND METHODS
Chemical synthesis
General remarks
Genistein (synthetic, >95%) was purchased from AK Scientific Chemicals (USA). Aspirin (≥99.0%, Acetylsalicylic acid) was purchased from Sigma-Aldrich (Germany). An ultrasound equipment (BRANSON) was used to assist reactions. 1H and 13C NMR spectra were obtained on a Varian equipment. As reference was used, the signals of the deuterated solvents and the chemical shifts (δ) were displayed in ppm. TMS was used as an internal standard. Coupling constants (J) are given in Hertz (Hz). HRMS was obtained using a Bruker Impact II UHR-Q-TOF mass spectrometry (Bruker Daltonik GmbH, Bremen Germany) in positive mode. For column chromatography and thin layer chromatography was used silica gel 60 (0.063–0.200 mesh, Merck, Whitehouse Station, NJ) and precoated silica gel plates (Merck 60 F254 0.2 mm).
General procedure for the synthesis of ASA-bromoalkyl (1a–h).
The titled compounds were synthesized following a procedure described in the literature (Lin et al., 2013): in a flat-bottomed flask of 25 ml equipped with a magnetic stirring bar, aspirin (1 mmol), N,N-Diisopropylethylamine (DIPEA) (1.5 mmol), and N,N-Dimethylformamide (DMF) (10 ml) were placed. The mixture was stirred for a period of 30 minutes. Then, 1, ω-dibromoalkane (1.2 mmol) was added to the reaction mixture and stirred for 3 hours. Water was added to reaction mixture and then it was extracted with ethyl acetate. Organic phase was dried on anhydrous sodium sulfate, filtered, concentrated under reduced pressure on a rotatory evaporator and the residue was purified by column chromatography over silica gel eluting with a mixture of hexane:ethyl acetate (ratio 8:2), to obtain bromoalkyl derivatives in yields ranging between 32% and 57%.
2-bromoethyl 2-acetoxybenzoate (1a): yellow oil, 51%. 1H-NMR (300 MHz, Chloroform-d) δ 8.11 (dd, J = 7.8, 1.8 Hz, 1H), 7.63 (ddd, J = 8.2, 7.8, 1.8 Hz, 1H), 7.38 (td, J = 7.7, 1.2 Hz, 1H), 7.17 (dd, J = 8.2, 1.2 Hz, 1H), 4.62 (t, J = 6.1 Hz, 2H), 3.65 (t, J = 6.1 Hz, 2H), 2.41 (s, 3H). 13C-NMR (75 MHz, Chloroform-d) δ 169.71 (C=O), 163.86 (C=O), 150.88, 134.31, 131.92, 126.16, 123.93, 122.63, 64.40, 28.64, 21.14.
 | Figure 1. Design of ASA-Genistein hybrids. [Click here to view] |
3-bromopropyl 2-acetoxybenzoate (1b): yellow oil, 57%. 1H-NMR (300 MHz, Chloroform-d) δ 8.05 (dd, J = 7.8, 1.8 Hz, 1H), 7.62 (td, J = 8.1, 1.8 Hz, 1H), 7.36 (td, J = 7.7, 1.2 Hz, 1H), 7.16 (dd, J = 8.1, 1.2 Hz, 1H), 4.47 (t, J = 6.0 Hz, 2H), 3.56 (t, J = 6.5 Hz, 2H), 2.43 (s, 3H), 2.38–2.25 (m, 2H). 13C-NMR (75 MHz, Chloroform-d) δ 169.75 (C=O), 164.26 (C=O), 150.74, 134.09, 131.71, 126.11, 123.91, 123.06, 62.83, 31.75, 29.42, 21.13.
4-bromobutyl 2-acetoxybenzoate (1c): yellow oil, 56%. 1H-NMR (300 MHz, Chloroform-d) δ 8.05 (dd, J = 7.8, 1.8 Hz, 1H), 7.61 (td, J = 8.1, 1.8 Hz, 1H), 7.36 (td, J = 7.8, 1.2 Hz, 1H), 7.15 (dd, J = 8.1, 1.2 Hz, 1H), 4.35 (t, J = 6.1 Hz, 2H), 3.51 (t, J = 6.3 Hz, 2H), 2.40 (s, 3H), 2.13–1.87 (m, 4H). 13C-NMR (75 MHz, Chloroform-d) δ 169.74 (C=O), 164.40 (C=O), 150.73, 133.98, 131.67, 126.09, 123.87, 123.21, 64.14, 33.15, 29.25, 27.36, 21.13.
5-bromopentyl 2-acetoxybenzoate (1d): yellow oil, 53%. 1H-NMR (300 MHz, Chloroform-d) δ 8.06 (dd, J = 7.8, 1.8 Hz, 1H), 7.60 (ddd, J = 8.1, 7.4, 1.8 Hz, 1H), 7.36 (td, J = 7.6, 1.3 Hz, 1H), 7.15 (dd, J = 8.1, 1.3 Hz, 1H), 4.33 (t, J = 6.6 Hz, 2H), 3.47 (t, J = 6.7 Hz, 2H), 2.40 (s, 3H), 2.03–1.90 (m, 2H), 1.88–1.76 (m, 2H), 1.73–1.56 (m, 2H). 13C-NMR (75 MHz, Chloroform-d) δ 169.73 (C=O), 164.46 (C=O), 150.71, 133.90, 131.70, 126.07, 123.84, 123.33, 64.81, 33.51, 32.31, 27.88, 24.67, 21.12.
6-bromohexyl 2-acetoxybenzoate (1e): yellow oil, 55%. 1H-NMR (300 MHz, Chloroform-d) δ 8.06 (dd, J = 7.8, 1.7 Hz, 1H), 7.60 (td, J = 8.1, 1.7 Hz, 1H), 7.36 (td, J = 7.7, 1.1 Hz, 1H), 7.15 (dd, J = 8.1, 1.1 Hz, 1H), 4.32 (t, J = 6.7 Hz, 2H), 3.46 (t, J = 6.7 Hz, 2H), 2.40 (s, 3H), 1.99–1.87 (m, 2H), 1.86–1.73 (m, 2H), 1.62–1.43 (m, 4H). 13C-NMR (75 MHz, Chloroform-d) δ 169.72 (C=O), 164.51 (C=O), 150.69, 133.86, 131.72, 126.06, 123.84, 123.40, 65.03, 33.79, 32.63, 28.54, 27.85, 25.23, 21.12.
8-bromooctyl 2-acetoxybenzoate (1f): yellow oil, 51%. 1H-NMR (300 MHz, Chloroform-d) δ 8.06 (dd, J = 7.8, 1.7 Hz, 1H), 7.60 (td, J = 8.1, 1.7 Hz, 1H), 7.36 (td, J = 7.8, 1.1 Hz, 1H), 7.14 (dd, J = 8.1, 1.1 Hz, 1H), 4.31 (t, J = 6.7 Hz, 2H), 3.45 (t, J = 6.8 Hz, 2H), 2.39 (s, 3H), 1.96–1.84 (m, 2H), 1.83–1.68 (m, 2H), 1.54–1.33 (m, 8H). 13C-NMR (75 MHz, Chloroform-d) δ 169.73 (C=O), 164.55 (C=O), 150.68, 133.82, 131.75, 126.05, 123.83, 123.46, 65.24, 34.05, 32.77, 29.09, 28.66, 28.63, 28.09, 25.89, 21.12.
9-bromononyl 2-acetoxybenzoate (1g): yellow oil, 40%. 1H-NMR (300 MHz, Chloroform-d) δ 8.06 (dd, J = 7.8, 1.6 Hz, 1H), 7.60 (td, J = 7.8, 1.6 Hz, 1H), 7.36 (tapp, J = 7.6 Hz, 1H), 7.14 (dapp, J = 8.1 Hz, 1H), 4.31 (t, J = 6.7 Hz, 2H), 3.45 (t, J = 6.8 Hz, 2H), 2.40 (s, 3H), 1.95–1.83 (m, 2H), 1.82–1.68 (m, 2H), 1.53–1.30 (m, 10H). 13C-NMR (75 MHz, Chloroform-d) δ 169.73 (C=O), 164.56 (C=O), 150.68, 133.80, 131.75, 126.04, 123.83, 123.48, 65.29, 34.09, 32.81, 29.33, 29.17, 28.70, 28.65, 28.15, 25.94, 21.11.
12-bromododecyl 2-acetoxybenzoate (1h): yellow oil, 32%. 1H-NMR (300 MHz, Chloroform-d) δ 8.07 (dd, J = 7.8, 1.7 Hz, 1H), 7.60 (td, J = 8.1, 1.7 Hz, 1H), 7.36 (td, J = 7.7, 1.1 Hz, 1H), 7.14 (dd, J = 8.1, 1.1 Hz, 1H), 4.31 (t, J = 6.8 Hz, 2H), 3.45 (t, J = 6.9 Hz, 2H), 2.40 (s, 3H), 1.95–1.83 (m, 2H), 1.82–1.68 (m, 2H), 1.52–1.27 (m, 16H). 13C-NMR (75 MHz, Chloroform-d) δ 169.74 (C=O), 164.58 (C=O), 150.67, 133.78, 131.77, 126.03, 123.82, 123.50, 65.35, 34.15, 32.86, 29.55, 29.53, 29.46, 29.29, 28.80, 28.67, 28.21, 25.99, 21.11.
General procedure for the synthesis of ASA-alkylazides (2a–h).
Intermediates 2a–h were synthesized following a procedure described in the literature (Ding et al., 2019): Compounds 1a–h (1 mmol), sodium azide (3 mmol), and DMF (5 ml) were placed in a 10 ml flat-bottomed flask. The mixture was sonicated for a period of 1 hour to 40°C. Water was added to reaction mixture and then it was extracted with hexane. Organic phase was dried on anhydrous sodium sulfate, filtered, concentrated under reduced pressure on a rotatory evaporator to obtain pure azides 2a–h in yields ranging between 72% and 96%.
2-azidoethyl 2-acetoxybenzoate (2a): light yellow oil, 77%, 1H-NMR (300 MHz, Chloroform-d) δ 8.09 (dd, J = 7.8, 1.8 Hz, 1H), 7.63 (ddd, J = 8.1, 7.6, 1.8 Hz, 1H), 7.37 (td, J = 7.8, 1.3 Hz, 1H), 7.16 (dd, J = 8.1, 1.3 Hz, 1H), 4.46 (t, J = 5.2 Hz, 2H), 3.62 (t, J = 5.2 Hz, 2H), 2.41 (s, 3H). 13C-NMR (75 MHz, Chloroform-d) δ 169.74 (C=O), 163.99 (C=O), 150.93, 134.33, 131.81, 126.18, 123.93, 122.55, 63.52, 49.86, 21.05.
3-azidopropyl 2-acetoxybenzoate (2b): light yellow oil, 74%, 1H-NMR (300 MHz, Chloroform-d) δ 8.05 (dd, J = 7.8, 1.8 Hz, 1H), 7.62 (td, J = 8.1, 1.8 Hz, 1H), 7.36 (td, J = 7.8, 1.2 Hz, 1H), 7.16 (dd, J = 8.1, 1.2 Hz, 1H), 4.41 (t, J = 6.2 Hz, 2H), 3.50 (t, J = 6.7 Hz, 2H), 2.39 (s, 3H), 2.11–2.01 (m, 2H). 13C-NMR (75 MHz, Chloroform-d) δ 169.74 (C=O), 164.26 (C=O), 150.75, 134.08, 131.64, 126.10, 123.89, 123.07, 61.95, 48.14, 28.25, 21.08.
4-azidobutyl 2-acetoxybenzoate (2c): light yellow oil, 81%, 1H-NMR (300 MHz, Chloroform-d) δ 8.05 (dd, J = 7.9, 1.8 Hz, 1H), 7.61 (td, J = 8,1, 1.8 Hz, 1H), 7.36 (td, J = 7.9, 1.2 Hz, 1H), 7.15 (dd, J = 8.1, 1.2 Hz, 1H), 4.35 (t, J = 6.3 Hz, 2H), 3.40 (t, J = 6.5 Hz, 2H), 2.40 (s, 3H), 1.95–1.83 (m, 2H), 1.83–1.68 (m, 2H). 13C-NMR (75 MHz, Chloroform-d) δ 169.73 (C=O), 164.39 (C=O), 150.73, 133.97, 131.66, 126.08, 123.86, 123.21, 64.41, 51.03, 25.99, 25.61, 21.10.
5-azidopentyl 2-acetoxybenzoate (2d): light yellow oil, 87%, 1H-NMR (300 MHz, Chloroform-d) δ 8.06 (dd, J = 7.8, 1.8 Hz, 1H), 7.67–7.54 (td, J = 8,1, 1.8 Hz, 1H), 7.36 (td, J = 7.8, 1.2 Hz, 1H), 7.15 (dd, J = 8.1, 1.2 Hz, 1H), 4.33 (t, J = 6.6 Hz, 2H), 3.34 (t, J = 6.7 Hz, 2H), 2.39 (s, 3H), 1.88–1.76 (m, 2H), 1.76–1.64 (m, 2H), 1.63–1.49 (m, 2H). 13C-NMR (75 MHz, Chloroform-d) δ 169.72 (C=O), 164.46 (C=O), 150.71, 133.90, 131.69, 126.07, 123.84, 123.33, 64.82, 51.27, 28.56, 28.27, 23.28, 21.10.
6-azidohexyl 2-acetoxybenzoate (2e): light yellow oil, 72%, 1H-NMR (300 MHz, Chloroform-d) δ 8.06 (dd, J = 7.9, 1.8 Hz, 1H), 7.60 (td, J = 7.9, 1.8 Hz, 1H), 7.36 (td, J = 8.1, 1.1 Hz, 1H), 7.15 (dd, J = 8.1, 1.1 Hz, 1H), 4.32 (t, J = 6.6 Hz, 2H), 3.32 (t, J = 6.8 Hz, 2H), 2.40 (s, 3H), 1.86–1.74 (m, 2H), 1.73–1.60 (m, 2H), 1.56–1.41 (m, 4H). 13C-NMR (75 MHz, Chloroform-d) δ 169.73 (C=O), 164.51 (C=O), 150.69, 133.86, 131.71, 126.06, 123.84, 123.40, 65.02, 51.35, 28.78, 28.57, 26.44, 25.61, 21.10.
8-azidooctyl 2-acetoxybenzoate (2f): light yellow oil, 96%, 1H-NMR (300 MHz, Chloroform-d) δ 8.06 (dd, J = 7.8, 1.8 Hz, 1H), 7.60 (td, J = 7.8, 1.8 Hz, 1H), 7.36 (td, J = 8.1, 1.2 Hz, 1H), 7.15 (dd, J = 8.1, 1.2 Hz, 1H), 4.31 (t, J = 6.7 Hz, 2H), 3.30 (t, J = 6.9 Hz, 2H), 2.40 (s, 3H), 1.85–1.72 (m, 2H), 1.71–1.57 (m, 2H), 1.53–1.33 (m, 8H). 13C-NMR (75 MHz, Chloroform-d) δ 169.73 (C=O), 164.55 (C=O), 150.68, 133.81, 131.74, 126.04, 123.83, 123.47, 65.24, 51.46, 29.14, 29.06, 28.83, 28.63, 26.66, 25.89, 21.11.
9-azidononyl 2-acetoxybenzoate (2g): light yellow oil, 94%, 1H-NMR (300 MHz, Chloroform-d) δ 8.06 (dd, J = 7.8, 1.8 Hz, 1H), 7.60 (ddd, J = 8.1, 7.4, 1.8 Hz, 1H), 7.35 (td, J = 8.1, 1.2 Hz, 1H), 7.14 (dd, J = 8.1, 1.2 Hz, 1H), 4.31 (t, J = 6.7 Hz, 2H), 3.29 (t, J = 6.9 Hz, 2H), 2.39 (s, 3H), 1.85–1.69 (m, 2H), 1.69–1.56 (m, 2H), 1.52–1.28 (m, 10H). 13C-NMR (75 MHz, Chloroform-d) δ 169.73 (C=O), 164.56 (C=O), 150.68, 133.80, 131.75, 126.03, 123.83, 123.49, 65.29, 51.47, 29.37, 29.17, 29.09, 28.85, 28.65, 26.70, 25.94, 21.10.
12-azidododecyl 2-acetoxybenzoate (2h): light yellow oil, 91%, 1H-NMR (300 MHz, Chloroform-d) δ 8.07 (dd, J = 7.8, 1.8 Hz, 1H), 7.60 (td, J = 7.8, 1.8 Hz, 1H), 7.36 (td, J = 8.1, 1.2 Hz, 1H), 7.15 (dd, J = 8.1, 1.2 Hz, 1H), 4.31 (t, J = 6.8 Hz, 2H), 3.30 (t, J = 7.0 Hz, 2H), 2.40 (s, 3H), 1.85–1.71 (m, 2H), 1.70–1.57 (m, 2H). 1.52–1.25 (m, 16). 13C-NMR (75 MHz, Chloroform-d) δ 169.73 (C=O), 164.58 (C=O), 150.67, 133.78, 131.77, 126.03, 123.83, 123.51, 65.35, 51.51, 29.53 (x3), 29.50, 29.29, 29.18, 28.87, 28.67, 26.74, 25.99, 21.10.
Synthesis of propargyl-genistein (3)
The intermediate 3 was synthesized following a procedure described in the literature (Ding et al., 2019): Genistein (1 mmol), DIPEA (2.5 mmol), and DMF (5 ml) were placed in a 10 ml flat-bottomed flask equipped with a magnetic stirring bar. The mixture was stirred for a period of 1 hour. Then, a mixture of propargyl bromide (1.5 mmol), KI (0.1 mmol), and DMF (1 ml) was added slowly to the reaction mixture and stirred for 24 hours. Water was added to reaction mixture and then it was extracted with ethyl acetate. Organic phase was dried on anhydrous sodium sulfate, filtered, concentrated under reduced pressure on a rotatory evaporator and the residue was purified by column chromatography over silica gel eluting with a mixture of hexane:ethyl acetate (ratio 85:15), to obtain compound 3 in 60% yield.
1H-NMR (300 MHz, Dimethyl sulfoxide (DMSO-d6) δ 12.97 (s, 1H), 9.63 (s, 25H), 8.43 (s, 1H), 7.39 (d, J = 8.6 Hz, 2H), 6.83 (d, J = 8.6 Hz, 2H), 6.72 (d, J = 2.2 Hz, 1H), 6.47 (d, J = 2.3 Hz, 1H), 4.96 (d, J = 2.3 Hz, 2H), 3.69 (t, J = 2.2 Hz, 1H). 13C-NMR (75 MHz, Chloroform-d) δ 181.04 (C=O), 163.22, 162.09, 157.79, 157.19, 152.99 (O-CH=C, chromone), 130.10, 123.92, 123.88, 121.56, 115.47, 106.56, 98.83, 93.30, 93.25, 77.22, 76.62 (alkyne), 56.14.
General procedure for the synthesis of ASA-Genistein hybrids (4a–h)
Hybrids 4a–h were synthesized following a procedure described in the literature (Shinde et al., 2019): Propargyl-genistein (1 mmol), ASA-alkylazides (2a–h) (1 mmol), and DMF (5 ml) were placed in a 10 ml flat-bottomed flask. The mixture was sonicated for 5 minutes to 40°C. Then, a mixture of ascorbic acid (0.5 mmol), copper acetate (0.5 mmol), DMF (1 ml), and water (1 ml) was added to the reaction mixture and sonicated for 1 hour to 40°C. Solution of HCl 10% was added to reaction mixture and then it was extracted with ethyl acetate. Organic phase was dried on anhydrous sodium sulfate, filtered, concentrated under reduced pressure on a rotatory evaporator and the residue was submitted to crystallization (MeOH:H2O, 1:1 ratio). Finally, the solid obtained was purified by preparative chromatography over silica gel eluting with a mixture of hexane/ethyl acetate of different ratios, to obtain compounds 4a–h in yields ranging between 43% and 94%.
3-(4-(((5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)ethyl 2-acetoxybenzoate (4a)
Yield 94%; pale yellow oil; 1H-NMR (300 MHz, DMSO-d6) δ 9.61 (s, OH), 8.40 (s, 1H, triazolyl), 8.37 (s, 1H, -O-CH=C-, chromone), 7.83 (d, J = 7.8 Hz, 1H), 7.67 (t, J = 8.1 Hz, 1H), 7.40 (d, J = 8.2 Hz, 2H), 7.37 (t, J = 7.8 Hz, 1H), 7.22 (d, J = 8.1 Hz, 1H), 6.84 (d, J = 8.2 Hz, 2H), 6.78 (d, J = 1.5 Hz, 1H), 6.49 (d, J = 1.5 Hz, 1H), 5.30 (s, 2H), 4.80 (t, J = 4.9 Hz, 2H), 4.66 (t, J = 4.9 Hz, 2H), 2.22 (s, 3H). 13C-NMR (75 MHz, DMSO-d6) δ 180.87 (C=O chromone), 169.56 (C=O) 164.28 (C=O), 163.82, 162.17, 157.94, 157.85, 154.92, 150.61 (-O-CH=C-,chromone), 134.99 (triazolyl), 131.97, 131.60, 130.63 (x2), 126.68, 125.82, 124.53, 122.98, 122.85, 121.48 (triazolyl), 115.54 (x2), 106.04, 99.06, 93.59, 63.70, 62.26, 49.05, 21.10. HRMS-ESI (m/z): 558.1509 [M+H]+, calcd. for C29H24N3O9 [M + H]+ 558.1507. HRMS-ESI m/z (% rel. abd.): 558.1509 (M+, 4), 516.1403 (100), 396.1189 (31), 368.1127 (7), 307.0603 (12), 271.0602 (73).
3-(4-(((5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)propyl 2-acetoxybenzoate (4b)
Yield 80%; pale yellow oil; 1H-NMR (300 MHz, DMSO-d6) δ 9.61 (s, OH), 8.42 (s, 1H, triazolyl), 8.34 (s, 1H, -O-CH=C-, chromone), 7.94 (dd, J = 7.8, 1.6 Hz, 1H), 7.69 (td, J = 8.0, 1.6 Hz, 1H), 7.43 (d, J = 7.8 Hz, 1H), 7.40 (d, J = 8.6 Hz, 2H), 7.25 (d, J = 8.0 Hz, 1H), 6.84 (d, J = 8.6 Hz, 2H), 6.80 (d, J = 2.2 Hz, 1H), 6.50 (d, J = 2.2 Hz, 1H), 5.28 (s, 2H), 4.55 (t, J = 6.9 Hz, 1H), 4.25 (t, J = 6.2 Hz, 2H), 2.52–2.50 (m, 2H), 2.29 (s, 3H). 13C-NMR (75 MHz, DMSO-d6) δ 180.89 (C=O chromone), 169.64 (C=O), 164.32 (C=O), 164.26, 162.19, 157.94, 157.88, 154.95, 150.52 (-O-CH=C-,chromone), 142.30 (triazolyl), 134.84, 131.79, 130.64 (x2), 126.72, 125.52, 124.47, 123.29, 122.99, 121.48 (triazolyl), 115.54 (x2), 106.05, 99.06, 93.62, 62.53, 62.31, 47.08, 29.29, 21.24. HRMS-ESI (m/z): 572.1664 [M+H]+ calcd. for C30H26N3O9 [M + H]+ 572.1663. HRMS-ESI m/z (% rel. abd.): 572.1664 (M+, 2), 530.1562 (100), 410.1347 (67), 307.0599 (8), 271.0603 (49), 232.0971 (38).
3-(4-(((5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)butyl 2-acetoxybenzoate (4c)
Yield 70%; pale yellow oil; 1H-NMR (300 MHz, DMSO-d6) δ 8.41 (s, 1H, triazolyl), 8.31 (s, 1H, -O-CH=C-, chromone), 7.94 (dd, J = 7.8, 1.6 Hz, 1H), 7.68 (td, J = 8.1, 1.6 Hz, 1H), 7.42 (d, J = 7.8 Hz, 1H), 7.40 (d, J = 8.6 Hz, 2H), 7.23 (d, J = 8.1 Hz, 1H), 6.84 (d, J = 8.6 Hz, 2H), 6.80 (d, J = 2.2 Hz, 1H), 6.51 (d, J = 2.2 Hz, 1H), 5.28 (s, 2H), 4.46 (t, J = 7.1 Hz, 2H), 4.25 (t, J = 6.5 Hz, 2H), 2.25 (s, 3H), 1.99–1.91 (m, 2H), 1.70–1.64 (m, 2H). 13C-NMR (75 MHz, DMSO-d6) δ 180.88 (C=O chromone), 169.59 (C=O), 164.47 (C=O), 164.33, 162.18, 157.94, 157.87, 154.93, 150.40, 142.22 (triazolyl), 134.73, 131.66, 130.63 (x2), 126.76, 125.38, 124.48, 123.57, 122.99, 121.48 (triazolyl), 115.54 (x2), 106.04, 99.07, 93.63, 64.66, 62.33, 49.47, 26.80, 25.66, 21.20. HRMS-ESI (m/z): 586.1820 [M+H]+ calcd. for C31H28N3O9 [M + H]+ 586.1827. HRMS-ESI m/z (% rel. abd.): 586.1820 (M+, 1), 544.1719 (100), 424.1506 (60), 406.1399 (29), 378.1335 (4), 307.0603 (5).
3-(4-(((5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)pentyl 2-acetoxybenzoate (4d)
Yield 86%; pale yellow oil; 1H-NMR (300 MHz, Chloroform-d) δ 12.85 (s, OH), 7.99 (d, J = 8.0 Hz, 1H), 7.84 (s, 1H, triazolyl), 7.65 (s, 1H, -O-CH=C-, chromone), 7.58 (t, J = 7.6 Hz, 1H), 7.35 (d, J = 8.1 Hz, 2H), 7.33 (d, J = 7.6 Hz, 1H), 7.12 (d, J = 8.0 Hz, 1H), 6.90 (d, J = 8.1 Hz, 2H), 6.49 (d, J = 2.0 Hz, 1H), 6.41 (d, J = 2.0 Hz, 1H), 5.21 (s, 2H), 4.41 (t, J = 7.1 Hz, 2H), 4.30 (t, J = 6.5 Hz, 2H), 2.36 (s, 3H), 2.05–1.98 (m,2H), 1.84-1.78 (m, 2H), 1.52–1.45 (m, 2H). 13C-NMR (75 MHz, Chloroform-d) δ 180.92 (C=O chromone), 169.94 (C=O), 164.49 (C=O), 163.93, 162.58, 157.86, 156.57, 152.87, 150.68, 142.85 (triazolyl), 134.05, 131.65, 130.20 (x2), 126.16, 123.84, 123.72, 123.19, 122.38 (triazolyl), 115.73 (x2), 106.56, 98.97, 93.07, 64.52, 63.73, 62.11, 50.38, 29.79, 28.03, 22.97, 21.14. HRMS-ESI (m/z): 600.1984 [M+H]+ calcd. for C32H30N3O9 [M + H]+ 600.1976. HRMS-ESI m/z (% rel. abd.): 600.1984 (M+, 1), 558.1881 (100), 438.1665 (58), 302.1384 (15), 271.0601 (28), 140.1076 (12).
3-(4-(((5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)hexyl 2-acetoxybenzoate (4e)
Yield 43%; pale yellow oil; 1H-NMR (300 MHz, Chloroform-d) δ 12.83 (s, OH), 7.99 (dd, J = 7.8, 1.5 Hz, 1H), 7.81 (s, 1H, triazolyl), 7.63 (s, 1H, -O-CH=C-, chromone), 7.55 (td, J = 7.8, 1.5 Hz, 1H), 7.32 (d, J = 8.0 Hz, 2H), 7.30 (d, J = 7.8 Hz, 1H), 7.10 (d, J = 8.0 Hz, 1H), 6.88 (d, J = 8.0 Hz, 2H), 6.48 (d, J = 2.2 Hz, 1H), 6.39 (d, J = 2.2 Hz, 1H), 5.21 (s, 2H), 4.37 (t, J = 7.2 Hz, 2H), 4.26 (t, J = 6.6 Hz, 2H), 2.34 (s, 3H), 1.99-1.91 (m, 2H), 1.80–1.70 (m, 2H), 1.51–1.43 (m, 2H), 1.43–1.36 (m, 2H). 13C-NMR (75 MHz, Chloroform-d) δ 180.91 (C=O chromone), 169.87 (C=O), 164.54 (C=O), 163.96, 156.27, 162.64, 157.89, 152.86, 150.67, 143.12 (triazolyl), 133.96, 131.70, 130.25 (x2), 126.12, 123.84, 123.73, 123.30, 122.78, 122.57 (triazolyl), 115.70 (x2), 106.59, 99.01, 93.11, 65.90, 64.87, 62.24, 30.15, 28.46, 26.15, 25.44, 21.13. HRMS-ESI (m/z): 614.2133 [M+H]+ calcd. for C33H32N3O9 [M + H]+ 614.2133. HRMS-ESI m/z (% rel. abd.): 614.2133 (M+, 1), 572.2027 (100), 452.1817 (48), 424.1754 (3), 274.1437 (36), 154.1225 (19).
3-(4-(((5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)octyl 2-acetoxybenzoate (4f)
Yield 60%; pale yellow oil; 1H-NMR (300 MHz, Chloroform-d) δ 12.84 (s, OH), 8.00 (dd, J = 8.0, 1.0 Hz, 1H), 7.83 (s, 1H, triazolyl), 7.63 (s, 1H, -O-CH=C-, chromone), 7.58 – 7.53 (m, 1H), 7.34 (d, J = 8.0 Hz, 2H), 7.31 (t, J = 7.6 Hz, 1H), 7.10 (d, J = 8.0 Hz, 1H), 6.92 – 6.86 (m, 2H), 6.50 (d, 1.1 Hz, 1H), 6.42 (d, J = 2.0 Hz, 1H), 5.24 (s, 2H), 4.36 (t, J = 7.2 Hz, 2H), 4.25 (t, J = 6.7 Hz, 2H), 2.34 (s, 3H), 1.95-1.89 (m, 2H), 1.75–1.68 (m, 2H), 1.44–1.29 (m, 6H), 1.28–1.22 (m, 2H). 13C-NMR (75 MHz, Chloroform-d) δ 180.92 (C=O chromone), 169.89 (C=O), 164.59 (C=O), 163.94, 162.63, 157.89, 156.47, 152.84, 150.66, 142.84 (triazolyl), 133.88, 131.74, 130.21 (x2), 126.10, 123.83, 123.75, 123.40, 122.98, 122.47 (triazolyl), 115.73 (x2), 106.59, 99.02, 93.12, 65.20, 62.24, 50.63, 30.23, 29.74, 28.87, 28.58, 26.41, 25.83, 21.13. HRMS-ESI (m/z): 642.2447 [M+H]+ calcd. for C35H36N3O9 [M + H]+ 642.2446. HRMS-ESI m/z (% rel. abd.): 642.2447 (M+, 2), 600.2342 (100), 480.2132 (51), 330.1813 (29), 302.1752 (47), 271.0602 (15).
3-(4-(((5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)nonyl 2-acetoxybenzoate (4g)
Yield 50%; pale yellow oil; 1H-NMR (300 MHz, Chloroform-d) δ 12.86 (s, OH), 8.03 (dd, J = 7.8, 1.3 Hz, 1H), 7.85 (s, 1H, triazolyl), 7.65 (s, 1H, -O-CH=C-, chromone), 7.59–7.55 (m, 1H), 7.36 (d, J = 8.2 Hz, 2H), 7.33 (t, J = 7.8 Hz, 1H), 7.12 (d, J = 8.1 Hz, 1H), 6.91 (d, J = 8.2 Hz, 2H), 6.52 (d, J = 2.1 Hz, 1H), 6.44 (d, J = 2.1 Hz, 1H), 5.27 (s, 2H), 4.38 (t, J = 7.2 Hz, 2H), 4.27 (t, J = 6.7 Hz, 2H), 2.37 (s, 3H), 1.98-1.91 (m, 2H), 1.78–1.69 (m, 2H), 1.46 – 1.26 (m, 10H). 13C-NMR (75 MHz, Chloroform-d) δ 180.92 (C=O chromone), 169.92 (C=O), 164.61 (C=O), 163.92, 162.60, 157.88, 156.56, 152.85, 150.65, 142.82 (triazolyl), 133.87, 131.75, 130.19 (x2), 126.09, 123.82, 123.75, 123.41, 123.00, 122.38 (triazolyl), 115.75 (x2), 106.58, 99.02, 93.11, 65.28, 62.20, 50.66, 30.25, 29.25, 29.11, 28.90, 28.61, 26.45, 25.89, 21.13. HRMS-ESI (m/z): 656.2603 [M+H]+ calcd. for C36H38N3O9 [M + H]+ 656.2602. HRMS-ESI m/z (% rel. abd.): 656.2603 (M+, 2), 614.2494 (100), 494.2283 (49), 344.1969 (27), 316.1907 (46), 271.0600 (15).
3-(4-(((5-hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)dodecyl 2-acetoxybenzoate (4h)
Yield 77%; pale yellow oil; 1H-NMR (300 MHz, Chloroform-d) δ 12.84 (s, OH), 8.01 (d, J = 8.1 Hz, 1H), 7.82 (s, 1H, triazolyl), 7.64 (s, 1H, -O-CH=C-, chromone), 7.55 (t, J = 7.6 Hz, 1H), 7.34 (d, J = 7.8 Hz, 2H), 7.30 (t, J = 7.6 Hz, 1H), 7.09 (d, J = 8.1 Hz, 1H), 6.88 (m, 2H), 6.49 (d, J = 1.7 Hz, 1H), 6.41 (d, J = 1.7 Hz, 1H), 5.25 (s, 2H), 4.36 (t, J = 7.3 Hz, 2H), 4.25 (t, J = 6.8 Hz, 2H), 2.34 (s, 3H), 1.95-1.98 (m, 2H), 1.75–1.68 (m, 4H), 1.43 – 1.36 (m, 2H), 1.35–1.21 (m, 12H). 13C-NMR (75 MHz, Chloroform-d) δ 180.93 (C=O chromone), 169.91 (C=O), 164.66 (C=O), 163.92, 162.61, 157.88, 156.54, 152.86, 150.63, 142.40 (triazolyl), 133.85, 131.77, 130.18 (x2), 126.09, 123.82, 123.75, 123.45, 123.00, 122.38 (triazolyl), 115.76 (x2), 106.59, 99.05, 93.11, 65.40, 62.23, 50.77, 30.26, 29.51 (x3), 29.39, 29.27, 29.00, 28.65, 26.51, 25.96, 21.13. HRMS-ESI (m/z): 698.3072 [M+H]+ calcd. for C39H44N3O9 [M + H]+ 698.3072. HRMS-ESI m/z (% rel. abd.): 698.3072 (M+, 5), 656.2963 (100), 536.2752 (40), 386.2437 (26), 358.2376 (41), 307.0598 (4).
In vitro biological assays
Cell lines and culture medium
Biological experiments were performed using SW480 colon cancer cells, its metastatic derivative (SW620) and normal cells (CHO-K1) obtained from The European Collection of Authenticated Cell Cultures (ECACC, England). Cells were cultured in 25-cm2 flasks containing Dulbecco’s Modified Eagle Medium, supplemented with 10% horse serum, 1% non-essential amino acids, and 1% penicillin/streptomycin (Gibco Invitrogen, Carlsbad, USA). For all experiments, there was a reduction in horse serum (to 3%) and the medium was supplemented with insulin (10 mg/ml), transferrin (5 mg/ml), and selenium (5 mg/ml) (ITS-defined medium; Gibco, Invitrogen, Carlsbad, USA) (Herrera et al., 2018).
Growth inhibition (SRB)
The growth inhibition of the new hybrids based on ASA/Genistein and the standard drug (5-fluorouracil; 5-FU) was evaluated through sulforhodamine B (SRB) assay. Briefly, cells were seeded at 20.000 cells/well in 96-well tissue culture plates. Then, these were incubated at 37°C and 5% CO2. After a 24-hour adaptation period for the cells, they were treated with the vehicle (1% of DMSO) or increasing concentrations (0.01–300 μM) of the hybrids. After the treatment, it was added trichloroacetic acid (50% v/v; MERCK) and left for 1 hour at 4°C. Cells proteins were further stained with 0.4% SRB (w/v; Sigma-Aldrich, United States), and washed with 1% acetic acid. After air-drying, Tris-base (10 mM) was added to solubilize protein bound stain. A microplate reader (Mindray MR-96A) was used to read the absorbance at 492 nm (Pérez et al., 2014). All experiments were performed in triplicate.
Statistical analysis
All experiments were performed at least in triplicate. Data are reported as mean ± SE (standard error). Statistical analysis to demonstrate the differences were evaluated by one-way ANOVA followed by the Dunnett’s test. The results with p values lower than 0.05 were considered significant. GraphPad Prism version 8.0.1 for Windows was used to analyze all data (Graph Pad Software, San Diego, California, USA).
Molecular docking studies
Protein structure and setup
To propose a plausible mode of action for the active hybrids 4a and 4c, in this work, five apoptotic proteins and one pro-inflammatory protein were selected, which are closely associated with activation and progression of CRC. The crystal structures of Plk1 (PDB: 2OWB, ATP-binding site), Plk1 (PDB 4X9R, PBD domain), Bcl-2 (PDB: 4MAN), BAX (PDB: 4S0O), caspase–3 (PDB: 5I9B), TNF-α (PDB: 2AZ5), and COX-2 (PDB: 3LN1) were retrieved from the Protein Data Bank. Discovery Studio Visualizer was used to removed crystallographic water molecules and metal-ions in the 3D structure. AutoDockTools was used for parameterizing the structures of the selected proteins (Morris et al., 2009). Overall, to polar side chains hydrogens were added to facilitate the formation of bonds, and the assignment of appropriate atomic partial charges to the ligand was essential to obtain meaningful results from electrostatic calculations (Kollman and Gasteiger). AutoDock was used to save the resulting protein file in Protein Data Bank, Partial Charge (Q), & Atom Type (T) (PDBQT) format.
Ligand dataset preparation and optimization
Here, novel hybrids compounds 4a and 4c, and six inhibitors that have been used as marketed drugs or potent drug candidates: Volasertib (Plk1 ATP-site), PHA-680626 (Plk1 ATP-site), ABT-199 (Bcl-2), ICPM (Plk1 PBD-site), a inhibitor of TNF-α, and the anti-inflammatory drug celecoxib (COX–2). Aiming to rewrite the data files into pdb format, Discovery Studio Visualizer was used. AutoDockTools was used to parameterize the structures of the ligands. Then, full hydrogens were added, rotatable bonds were assigned, partial atomic charges computed (Kollman and Gasteiger), and the resulting structures saved to be used AutoDock. Moreover, AUTOTUTORS in AutoDockTools were used to define all probable flexible torsions of the ligand molecules in order to facilitate hybrids receptor binding during simulations (Morris et al., 1998, 2009).
Docking analysis
AutoDock Vina were used aiming to run docking simulations considering flexible ligand to a rigid target. Catalytic sites of each selected protein-target were chossen to run docking calculations. Then, the most favorable binding pose and affinity scores (in kcal/mol) were determinated. Assisted by AutoDock Vina 1.1.2 (Trott et al., 2010), docking calculations were accomplished into a grid box enclosing the catalytic domain of 38 × 38 × 38 Å with a grid spacing of 1Å, and exhaustiveness of 20. Finally, The PyMOL Molecular Graphics System 2.0 programs and DS Visualizer 2.5 (http://3dsbiovia.com/products/) were used to graphically inspect the binding docking conformations and generate the 2D ligand interaction diagrams, respectively.
Theorical drug-likeness studies
In silico drug-likeness studies along with further ADMET (absorption, distribution, metabolism, excretion, and toxicity) indices present an array of opportunities which help in accelerating the discovery of new anticancer candidates. In this scenario, for active hybrids 4a and 4c, eleven key biopharmaceutical parameters were estimated by using the opensource platforms Molinspiration software (for: Topographical polar surface area (TPSA), molecular weight (MW), and rotatable bonds descriptors), the Virtual Computational Chemistry Laboratory (ALOGPS 2.1) for Log Po/w descriptor, and the Pre-ADMET 2.0 cheminformatic tool for estimation of key pharmacokinetic indices such as LogKHSA (human serum albumin), apparent predicted intestinal permeability models (App. Caco-2 and MDCK), and Gastrointestinal uptake and absorption (% GI). Note that, these important biopharmaceutical parameters govern oral exposure, as well as permeability, absorption, motion, and action of potential drug candidates. SwissADME (Daina et al., 2017), a free online software, was used to find out alerts for pan-assay interference compounds (PAINS). The OSIRIS Property Explorer (free open source) was used to evaluate the overall drug-score and the most common toxicity human parameters, such as mutagenicity, tumorigenicity, irritant effect, and possible injuries that can affect the reproductive system. Finally, MetStabOn, a powerful online server for the qualitative prediction of metabolic stability (Podlewska et al., 2018), was used to estimate the metabolic stability for the top-two hybrids 4a and 4c.
RESULTS AND DISCUSSION
Chemistry
The synthesis of hybrids of this study began with the obtention of the ASA-bromoalkyl 1a–h by means of the Williamson etherification of aspirin with 1, ω-dibromoalkanes (ω = 3, 4, 5, 8, 9, and 12) with yields ranging between 32% and 57%. Compounds 1a–h were treated with sodium azide leading to the formation of the ASA-alkylazides 2a–h in 72%–96% yields (Ding et al., 2019). On the other hand, reaction of genistein with propargyl bromide led to propargyl-genistein (3) with 60% yield. Finally, click reaction between azide alkylaspirin 2a-h and the compound 3 led to the formation of hybrids 4a–h in 43%–94% yields (Shinde et al., 2019) (Scheme 1).
 | Scheme 1. Synthesis of ASA-Genistein hybrids. [Click here to view] |
The structures of all compounds have been established by a combined study of High resolution electrospray ionization mass spectrometry (HRMS-ESI) (m/z), 1H-NMR and 13C-NMR spectra. HRMS-ESI (m/z) spectra showed characteristic [M+H]+ peaks corresponding to their molecular weights. The assignments of all the signals to individual H or C-atoms have been performed based on typical δ-values and J-constants. The 1H-NMR spectra of hybrids dissolved in DMSO or CDCl3 showed around 8.41 ppm or 7.83 ppm, respectively, a signal corresponding to triazolyl ring. The chromone (-O-CH=C-) signal was observed around 8.30 ppm on DMSO or 7.65 ppm when was using CDCl3. The acetyl group of aspirin was observed around 2.25 ppm on DMSO or 2.34 ppm on CDCl3. 13C-NMR spectra of hybrids showed around 180.8, 169.6, and 164.5 ppm signals corresponding to the carbonyl groups (C=O). The triazolyl ring exhibited signals around 142.2 and 121.4 ppm.
Biological activity
Effect of ASA-Genistein hybrids SW480 and CHO-K1 cell viability
To assess the effect of the new hybrids on cell viability, these were tested against malignant SW480 cells and normal CHO-K1 cells. As shown in Figure 2, when treated SW480 cells were microscopically observed, they exhibited a disturbed morphology after the treatment with hybrids 4a and 4c, showing changes in size and shape, while the vehicle control (DMSO) showed normal and healthy shape. The severity of morphological changes increased in a time- and dose-dependent manner. Moreover, treatment with both hybrids caused a clear reduction in cell count compared to the control, suggesting an increase toward cell death or a cell cycle arrest.
The evaluated hybrids induced a time- and concentration-dependent activity. Among the results, hybrids 4a and 4c caused the highest inhibition on cell viability with increasing concentrations. This activity increased with the time, since there was not activity at 24 hours (data not shown) but it was observed a significant activity, regarding vehicle control, after 48 hours treatment (Fig. 3). It is important to notice that these hybrid compounds increased activity and selectivity through time, which is consistent with previous reports with the parental compound aspirin where different authors also suggest that aspirin acts in a time-dependent manner (Lai et al., 2008; Satya et al., 2012; Zhang et al., 2018). This was also reflected in the IC50 values (50% inhibitory concentration) summarized in Table 1, where hybrids 4a and 4c did not display inhibition at 24 hours but after 48 hours treatment the IC50 values on the malignant SW480 cells were 449.8 ± 25.98 and 635.8 ± 92.99, respectively, besides, compound 4c was selective against this malignant cell line without exhibiting inhibition on healthy CHO-K1 cells unlike the parental compound genistein (IC50 =48.44 μM ± 4.13 and 52.62 μM ± 8.31 for CHO-K1 and SW480, respectively), the conventional chemotherapeutic agent, 5FU (IC50 = 173.2 μM ± 14.61 and 174.3 μM ± 19.1 for CHO-K1 and SW480, respectively), which were even more toxic for the non-malignant cell line, and aspirin alone which did not display activity in these cells. The other synthesized hybrids (4d, 4e, 4f, and 4h) did not exhibit activity in SW480 cells at the conditions evaluated. Besides, none of the compounds was active against SW620 cells (data not shown). We hypothesized that the lack of activity of these hybrid compounds could be attributed to the big size of the molecule, which makes it difficult to cross the cell membrane. These biological activities provided an approach to the mechanisms of action associated with the most active ASA/Genistein hybrids (4a and 4c) on SW480, which was also strengthened and expanded with the docking results, providing valuable evidence about the potential targets for these molecules in colorectal cancer.
 | Figure 2. Representative images of SW480 cells 48 hours after treatment with the highest concentration evaluated (300 μM; Magnification: 40×). [Click here to view] |
 | Figure 3. Effect of ASA-Genistein hybrids (4a, 4b, 4c, and 4g) on cell viability of SW480 cells after 48 hours treatment with 0.01, 10, and 300 μM. Data are presented as the mean ± SE of at least three independent experiments (****p < 0.0001). Vehicle control (DMSO 1%) was assumed as 100% of viability. [Click here to view] |
 | Table 1. Cytotoxic effect of ASA-Genistein hybrids against SW480 and CHO-K1 cell lines at 48h. [Click here to view] |
 | Table 2. Molecular docking scores of the top-ranked hybrids 4a and 4c (kcal/mol) based on AutoDock Vina. [Click here to view] |
Multi-target docking studies
Hybrids 4a and 4c and its interaction with the PLK-1 ATP binding domain
To provide better insights into the mechanisms by which the most active ASA/Genistein hybrids 4a and 4c elicit inhibitory effects on SW480 cell growth, an extensive multi-target docking and multi-site based virtual screening were performed by using multiple proteins associated with activation and progression of colorectal cancer. To achieve this purpose, authors selected five druggable proteins targets (Plk1, Bcl-2, BAX, Caspase-3, and TNF-α) which play a vital role in activation and progression of CRC. Besides, considering that ASA was included in the design of the compounds, this work investigated a likely interaction of ASA/Genistein hybrids 4a and 4c with the pro-inflammatory COX-2 protein which have demonstrated to induce cancer stem cell (CSC)-like activity, promoting proliferation, invasion, angiogenesis, inflammation, resistance, and metastasis of cancer cells (Hashemi et al., 2019). Thus, promising 4a and 4c were docked into each catalytic domain of X-ray crystal structure of target proteins by employing grid-based ligand docking with AutoDock Vina, and affinity scores along the best binding pose (Table 2).
Bcl−2: B-cell lymphoma−2, BAX: Bcl-2 associated X protein, TNF-α: tumor necrosis factor alpha, Plk1: Human Polo-like kinase 1, COX-2: cyclooxygenase-2. Celecoxib is a selective COX-2 inhibitor, Volasertib is an FDA-approved drug reported as potent and selective inhibitor of PLK1-ATP binding site (enzyme IC50 = 0.87 nM, EC50 = 11–37 nM on a panel of cancer cell lines). ABT-199, Bcl-2-Navitoclax analog: potent and selective Bcl-2 inhibitor (EC50 = 4 nM). PHA-680626: pyrrolo-pyrazole PLK1-ATP binding site inhibitor IC50 = ~0.53 μM. PBD: Human Polo-box domain. ICPM: Imidazolium-containing phosphopeptide macrocycle, a PBD inhibitor (IC50 = 15 nM).
Interestingly, among these targeted proteins, hybrids 4a and 4c displayed a high-affinity binding for Plk1 (Human Polo-like kinase 1) as well as for the Bcl-2/Bax system with a docking score in the range of −10.2 to −9.3 kcal/mol. Moreover, docking action of 4a and 4c against the pro-inflammatory COX-2 enzyme (above 10.0 kcal/mol) suggests these compounds could act into the catalytic center of the enzyme to alter its catalytic properties. Therefore, authors hypothesized these facts would play a key role in SW480 growth inhibition caused by the hybrids.
 | Figure 4. (A). Superposition of the best docked conformations of hybrids 4a (blue) and 4c (green), and crystallographic binding mode of inhibitor PHA-680626 (red) within the Plk1 ATP-binding domain. (B). 2D protein-ligand plot after docking procedure for 4a. (C). 2D ligand–receptor interaction diagram for 4c. Catalytic residues on Plk1 ATP-binding domain are colored in cyan. [Click here to view] |
Polo-like kinase 1 (Plk1) is a key regulator enzyme for cell mitosis and it is overexpressed in many cancers, particularly CRC. Targeting Plk1, specifically the Plk1-ATP binding domain, could be an effective therapeutic strategy to combat colorectal malignancies (Takahashi et al., 2003). As reported, ATP-catalytic domain of Plk1 is constituted by key binding residues which play a critical role in its biochemical function, including Arg57, Leu59, Cys67, Ala80, Lys82, Glu101, His105, Val114, Leu130, Glu131, Leu132, Cys133, Arg135, Arg136, Lys178, Asn181, and Phe183 (Kothe et al., 2007). So, docking of promising 4a and 4c into the ATP-binding pocket of Plk1 reveals several features that might be important for their cytotoxic effects displaying remarkable binding free energies of −10.2 and −10.1 kcal/mol, respectively. As can be seen in Figure 4A, docking results revealed that these hit-compounds were well accommodated inside the binding cavity and oriented in a very similar binding mode to the X-ray pose of the inhibitor PHA-680626, besides, they exhibited key interactions with those critical residues essential for the development of Plk1 (Fig. 4B and C). Thus, lead compounds show to interact with Plk1 through three hydrogen bonds between the catalytic triad Glu131, Cys133, and Lys82 and the features of chromone and triazole. Chromone ring of 4a and 4c showed two π-contacts with Phe183 and Leu59 residues. Moreover, Asp194 (a key residue for the binding of ATP at the active site) forms one π-anion interaction with compound 4c. In addition, numerous hydrophobic interactions were mediated by Gly62, Ala65, Leu130, Leu132, Arg134, Ser137, Glu140, Gly193, Lys178, Gly180, Asp194, and Phe195 which potentially confer stability during the binding occurrence. These computational findings suggest that inhibition of function human Polo-like kinase 1 (Plk1) could become a primary mode of action for active hybrids 4a (blue) and 4c (green). This presumably could explain the cytotoxic effect of these molecules on human adenocarcinoma cells (SW480).
 | Figure 5. (A). Superposition of the best docked conformations of hybrids 4a (blue) and 4c (green) within the Bcl-2 active site. (B). Superposition of the best docked conformations of hybrids 4a (blue) and 4c (green) within the Bax dimer interface. (C). 2D protein-ligand plot interaction for 4a and 4c into the Bcl-2 active site. (D). 2D ligand–receptor interaction diagram for 4a and 4c within the Bax dimer interface. Catalytic residues are colored in cyan. [Click here to view] |
Hybrids 4a and 4c and the interaction with the Bcl-2/Bax system and COX-2 receptor
In addition to binding human Polo-like kinase 1 (Plk1), docking studies would suggest that hybrids 4a and 4c contribute to inhibit SW480 cell growth by modulating a second signaling pathway critically involved in colorectal cancer (CRC) development. As depicted in Table 2, hybrids 4a and 4c displayed a high-affinity binding for the human Bcl-2/Bax system in about -10 kcal/mol. Proapoptotic BAX and antiapoptotic Bcl-2 proteins are key regulators of apoptosis, which play an important role in resistance and expansion of neoplastic cells, particularly in human colorectal carcinomas (Nehls et al., 2007). Therapeutic strategies targeting the human Bcl-2/Bax system has emerged as an attractive alternative in patients with colorectal carcinomas and they are considered as a rational starting point to develop molecules that selectively trigger apoptosis of cancer cells. Figure 5A–D shows the most stable binding pose of hybrids 4a and 4c within the Bcl-2 catalytic domain (−10.1 and −9.9 kcal/mol, respectively) as well as inside the Bax active site (−9.8 and −9.3 kcal/mol, respectively). As illustrated in Figures 2A–D, docking studies revealed that 4a and 4c have a strong interaction with residues of the Bcl-2/Bax system, fitting well into the binding pocket of the targeted receptors. For Bcl-2 protein, several amino acid residues have demonstrated to be essential for its function, among them, the role of Asp100, Phe101, Tyr105, Arg107, Asp108, Glu111, Met112, Arg143, Val145, Ala146, and Tyr199 in Bcl-2 active site has already been studied to develop more selective and effective molecules able to modulate Bcl-2 (Souers et al., 2013). On the other hand, human BAX protein exists in an inactive and dimeric conformation which is disrupted to release BAX monomers that are primed for a potent activation and apoptosis induction. The design of compounds able to disrupt the interactions of these swapped dimer structures could promote apoptosis in malignant cells (Garner et al., 2016). Activation site, which can be used to dissociate the BAX dimers, comprises ten key amino acid residues: Leu25, Leu26, Gln28, Gln 32, Ala34, Asp48, Asp102, Glu131, Ile133, and Arg134.
 | Figure 6. (A). Superposition of the best docked conformations of hybrids 4a (blue) and 4c (green), and X-ray crystallographic pose of celecoxib (red) within the COX-2 active site. (B). 2D protein-ligand plot interaction for 4a into the COX-2 active site. (C). 2D ligand–receptor interaction diagram for 4c within the COX-2 active domain. Catalytic residues are colored in cyan. [Click here to view] |
According to outlined above, this work found that hybrids 4a and 4c exhibit numerous intermolecular interactions with those essential residues for function of human Bcl-2/Bax system (Fig. 5C and D). Based on docking findings, titled compounds 4a and 4c bind to Bcl-2 receptor through H-bonds with key Tyr199, π-contacts with Phe101, Tyr105, Tyr199, π-anion interaction with charged residue Asp100, establishing several hydrophobic interactions which play an important role in stabilizing the binding event. Here, docking studies suggest that promising hybrids 4a and 4c could trigger apoptotic cell death in SW480 cells not only through inhibition of human Polo-like kinase 1 (Plk1), but also by modulation of the Bcl-2/Bax system.
Emerging evidence have proved that COX-2 protein actively participates in proliferation, invasion, and adhesion of malignant cells, particularly in colorectal cancer. Therefore, novel candidates targeting COX-2 could suppresses tumor cell growth and invasion/migration and may be useful as an auxiliary therapeutic opportunity in colorectal cancer (CRC) patients (Huang et al., 2017; Ferrández et al., 2003; Sheng et al., 2020; Sinicrope et al., 2004). The COX-2 binding surface has been well-established. Thirteen residues form a large and deep pocket/cavity on the binding site of COX-2: Gln178, Val335, Leu338, Ser339, Gly340, Tyr341, Phe367, Ala502, Ile503, Phe504, Val509, Ala513, and Leu517 (Wang et al., 2010). Interestingly, when 4a and 4c were docked into the active site of COX-2 (PDB: 3LN1), showed a high binding affinity in about −10 kcal/mol which was comparable to that anti-inflammatory drug celecoxib (−9.6 kcal/mol). The sampling provided by AutoDock showed that 4a and 4c were well-accommodated in the active site of COX-2, as can be seen from Figure 6A. In fact, the best docking conformations obtained for hybrids 4a and 4c were in good agreement to that X-ray pose of celecoxib. Furthermore, an exhaustive analysis of interaction fingerprint performance in DS Visualizer (Fig. 6B and C) revealed that the plausible binding modes of compounds 4a and 4c form key hydrogen bond interactions with Gln178, Ile503, Phe504 playing an important role in the COX-2 function. Moreover, the molecular docking of 4a and 4c in the COX-2 shows interactions by π-contacts with the residuals comprising the catalytic region of the enzyme, such as Val335 and Val509. Finally, 4a and 4c formed numerous hydrophobic interactions at the active site of COX-2, which likely would play a crucial role in complex stabilizing upon ligand binding. Based on these findings, authors hypothesized that a probable modulation of COX-2 due to the action of ASA/Genistein hybrids 4a and 4c would be an important contributing factor to enhance the cytotoxic effect associated to these compounds against SW480 cells.
Taken altogether, docking results suggest that active hybrids 4a and 4c could trigger apoptotic SW480 cell death by attenuating the activity of the Polo-like kinase 1 (Plk1), which has a critical role in human colorectal carcinoma growing, thereby offering key insights into the cytotoxic mechanism of novel 4a and 4c. Moreover, authors believe that the active hybrids 4a and 4c could be also acting via multitarget recognition of the pro-apoptotic Bcl-2/Bax system and COX-2, thereby could explain their cytotoxic effect on SW480 colorectal cancer cells. In this regard, hybrids 4a and 4c could represent a new class of multi-potent therapeutic candidates for colorectal cancer treatment. However, these results clearly suggest that additional combined experimental and computational studies should be conducted to validate our docking statements.
Pharmacokinetic studies and in silico ADME/Tox modeling
Lipinski’s rule and drug-likeness evaluation
Early theorical calculations of drug-likeness parameters have the most profound influence during novel anti-cancer hits development, concerning to their pharmacokinetic quality. Accordingly, pharmacokinetic properties based on the structure of promising oncology compounds could help to find more effective and safer candidates for further preclinical testing. To assess the drug-likeness of the most active synthesized compounds 4a and 4c, in silico pharmacokinetics studies were conducted by using free web tools for cheminformatics analysis, and results are summarized in Table 3. Thus, ten biopharmaceutical and pharmacokinetic descriptors such as MW, H-bond donor/acceptor, number of rotatable bonds, TPSA, octanol/water partition coefficient (log Po/w), gastrointestinal absorption (GI), permeability values across traditional Caco-2 and MDCK cells models and the binding-serum albumin (as LogKHSA) were estimated for the titled hybrids, and data are shown in Table 3.
Interestingly, favorable pharmacokinetics indices were founded for the hybrids compared to 95% of US-marketed drugs. According to Lipinski’s guidelines (1997), the top-two hybrids 4a and 4c displayed an optimal physicochemical profile (no more than one violation is acceptable), which suggests these compounds are likely to be orally active anti-cancer candidates. Despite those high degrees of lipophilicity (expressed as logPo/w) that were estimated for hybrids 4a and 4c (3.025 and 5.597, respectively), both fit well within the optimal range for lipid-based formulations (–2.0 to 6.0) (Ditzinger et al., 2019).
 | Table 3. Lipinski’s rule and pharmacokinetic indices for the active hybrids 4a and 4c. [Click here to view] |
In addition, active hybrids 4a and 4c exhibited optimal human intestinal absorption (%GI) numbers greater than 50%, this means that upon oral administration, the target hybrids could have a greater chance to be absorbed throughout the gastrointestinal segments. This parameter was also checked through calculations by using transmembrane permeation across traditional Caco-2 and MDCK cells lines. Currently, these cells lines are the most widely used in vitro model at investigating of permeability of drugs through intestinal barrier in the development of oral pharmaceutical products (Broccatelli et al., 2016; Pham-The et al., 2018; Press et al., 2008). Thus, hybrids 4a and 4c showed to have good permeability with values in the range of 13 to 66 nm/s, demonstrating that these compounds could be moderately absorbed into the gastrointestinal tract.
For hybrids 4a and 4c, another key biopharmaceutical parameter, named Polar Surface Area (TPSA), which is a good predictor of drug–membrane interactions and permeation across biological membranes was also investigated. Thus, TPSA measurements for hybrids 4a and 4c showed acceptable therapeutic values of 175.154 and 180.608 Å2, respectively, which in turn suggests that these compounds would penetrate tumor cells more efficiently.
In addition, the ability of the hybrids to bind to human serum albumin (calculated as logKHSA) was investigated, which is the most significant parameter for distribution and transport of drug and drug-like molecules in the systemic circulation. For therapeutic uses, LogKHSA values in the range of −1.5 to 1.5 are recommended for potential drugs (Colmenarejo et al., 2003; Zhivkova et al., 2015). It was then estimated that compounds 4a and 4c display affinities with HSA within the recommended therapeutic values, finding logKHSA numbers between 0.305 and 0.504, respectively. Finally, the pan-assay interference compounds (PAINS) filter which provide early alerts for potential toxicity as part of successful drug discovery, showed that novel hybrids can be regarded as valid development starting points in cancer drug discovery.
Taken altogether, merging ASA and Genistein sub-units into a unique structural core provide active compounds with optimal pharmacokinetic profile, making this scaffold promissory to develop novel drug-candidates against colorectal cancer.
OSIRIS drug-likeness score and toxicity calculations
Prediction of drug-relevant properties and overall toxicity of bioactive ASA-Genistein hybrids 4a and 4c were carried out by using the OSIRIS property explorer (a free web-based program), which is a powerful web server to predict physicochemical and toxicological molecular properties (Table 4). Calculations were used to estimate the risks of side effects, such as mutagenicity, tumorigenic risk, irritant, and reproductive effects, as well as drug-like index to quantify the drug-like “degree.” The toxicity risk predictions locate substructures within a molecule that could enhance the risks of adverse drug reactions on a cell, organ, or organisms. Notably, the toxicity risk assessment suggested that hybrids 4a and 4c would display low or null toxicity regarding mutagenicity, tumorigenicity, irritant effect, but unfortunately some molecular features in the chemical structure of the synthesized compounds were identified as irritating on reproductive system.
On the other hand, drug-likeness model score used by the OSIRIS web tool suggests the potential therapeutic properties for top-hybrids 4a and 4c in comparison with marketed drugs. The OSIRIS database contains 5300 substructure fragments, with their associated drug-likeness scores, which have been identified in 15000 commercially available chemical compounds and 3300 US-traded drugs. For every fragment, after correction and removal of highly redundant fragments, a drug-likeness score is assigned based on the frequency of occurrence of the fragment. The diagram of the distribution of drug-likeness values for the marketed drugs versus the commercially available chemicals shows that about 80% of traded drugs have positive values of the drug-likeness score while the commercial compounds (non-drug related compounds) have negative values. Hence, a positive number for the drug-likeness score indicates that the compound is made up mostly of building blocks (fragments) that are commonly found in marketed drugs. As depicted in Table 4, hybrids compounds 4a and 4c display positive drug-likeness values of 0.77 and 0.67, respectively. These findings suggest that these compounds could be safe drug candidates, or that their structures could be used as models for scaffold-based drug discovery in colorectal cancer chemotherapy.
Data obtained by the OSIRIS web tool suggest favorable outcomes of safety and drug-likeness for the most bioactive ASA-Genistein hybrids 4a and 4c, which in turn means that these compounds have great chance to advance through the earlier preclinical testing steps as well as serve as attractive candidates for future drug development treatments focused on the initiation, progression, and migration linked with colorectal cancer.
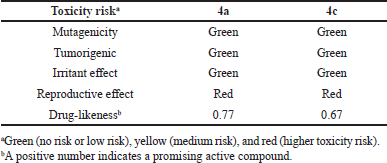 | Table 4. Drug-likeness score and toxicity risk calculations of hybrids 4a and 4c based on OSIRIS property explorer. [Click here to view] |
In vitro assessment of metabolic stability
From the therapeutic view, metabolism studies of a potential drug-candidate represent one of the most important pharmacokinetic and biochemical challenges to consider during the early stages of drug discovery (Zhang et al., 2018). In this context, metabolic stability is a key pharmacokinetic parameter which often provides a valuable information about bioavailability, half-life, intrinsic clearance, and volume of distribution of a potential clinical drug candidate (S?oczy?ska et al., 2019). Thus, metabolic stability data of a drug-like molecule may offer key evidence about its susceptibility to be bio-transformed. However, in vitro and in vivo metabolic stability studies often requires laborious manual manipulations, automated, and robust assays that tend to increase the cost- and time-effectiveness of the product development cycle. In contrast, chemoinformatics tools provide a low-cost, high-throughput, and accurate approach for a rapid screening of metabolic stability of large compound library identify as potential drug-candidates. MetStabOn is a powerful web server for the qualitative prediction of metabolic stability based on the application of machine learning methods and separate models for human, rat, and mouse. MetStabOn not only expresses the metabolic stability of a hit-compound as half-lifetime and clearance, but values are assigned within three metabolic stability classes [expressed as T1/2, low (≤ 0.6 hours), medium (0.6–2.32 hours), or high (>2.32 hours)], which are based on in vitro assays performed on liver microsomes referring to human, rat, and mouse experiments (Podlewska et al., 2018). Herein, MetStabOn was used to estimate the metabolic stability for top-two hybrids 4a and 4c, aiming to provide early insight into the first-pass metabolism and bioavailability. The predicted metabolic stabilities values for hybrids 4a and 4c are summarized in Table 5.
Interestingly, these compounds exhibited favorable numerical values of metabolic stability in human, rat, and mouse models with half-lifetimes values belonging to medium (0.6–2.32 hours) and high (>2.32 hours) stability class. More importantly, referring to human model, in which human liver microsomes were used, the obtained T1/2 stability values for ASA-Genistein hybrids 4a and 4c varied from 1.001 to 1.033 hours, indicating that these compounds would have a medium metabolic stability. Similarly, the predictions of metabolic stability with mouse model produced on T1/2 liver microsomes data revealed that hybrids 4a and 4c may have median values of half-lifetimes. In view of the above findings regarding to metabolic stability, novel ASA-Genistein hybrids 4a and 4c apparently would have low hepatic first-pass metabolism and sufficient time to trigger the desired therapeutic response.
 | Table 5. Predicted metabolic stabilities for hybrids 4a and 4c [Click here to view] |
CONCLUSION
In this work, different hybrids based on ASA and genistein were synthesized for the first time. Besides, the chemopreventive potential of these molecules using a 2D in vitro model of colorectal cancer was evaluated. Our findings suggest that hybrids based on ASA and genistein could involve selective growth inhibition, suggesting an increase toward cell death or a cell cycle arrest, leading to changes in size and shape after treatment, with better and selective results than parental compounds and the conventional chemotherapeutic (5-FU), highlighting the potential of the strategy based on molecular hybridization. Additionally, docking studies show markedly binding affinities when active 4a and 4c were docked against Polo-like kinase 1 protein in about −10 kcal/mol, as well as against Bcl-2/Bax system and COX-2 (in the range of −10.1 to −9.3 kcal/mol), suggesting that these compounds apparently may induce synergistic cytotoxic effect in SW480 cells due to multi-target interactions with these proteins. Finally, in silico drug-likeness evaluation was accessed for active hybrids 4a and 4c, and results suggest that these compounds have acceptable pharmacokinetics indices to be considered drug-like scaffolds in medicinal chemistry. All these biological and molecular modeling data obtained from this study suggest that ASA/Genistein hybrids (4a and 4c) could be valuable therapeutic scaffolds for further studies to identify new alternatives for the treatment of colorectal cancer.
ACKNOWLEDGMENT
This research was funded by the University of Antioquia, MINCIENCIAS, MINEDUCATION, MINCIT and ICETEX, through the Program Ecosistema Científico Cod. FP44842-211-2018 project number 58537. The authors wish to thank Dr. Sabina Podlewska for their work in estimated metabolic stability (Institute of Pharmacology, Polish Academy of Sciences, Department of Medicinal Chemistry).
AUTHOR CONTRIBUTIONS
L.G.: synthesis and characterization of hybrid molecules. G.M.: evaluation of biological activities and formal analysis. A.H.-R.: conceptualization, methodology, validation, evaluation of biological activities and formal Analysis, writing-original Draft, writing-review & editing. A.F-Y: in silico study and writing-original Draft. W.C.-G.: conceptualization, methodology, resources, writing-original draft, writing-review & editing, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
FUNDING
This research was funded by the University of Antioquia, MINCIENCIAS, MINEDUCATION, MINCIT and ICETEX, through the Program Ecosistema Científico Cod. FP44842-211-2018 project number 58537.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVAL
This study does not involve the use of animals or human subjects.
REFERENCES
Alqahtani Z, Jamali F. Clinical outcomes of aspirin interaction with other non-steroidal anti-inflammatory drugs: a systematic review. J Pharm Pharm Sci, 2018; 21(1s):29854; doi: 10.18433/jpps29854. CrossRef
Avivi D, Moshkowitz M, Detering E, Arber N. The role of low-dose aspirin in the prevention of colorectal cancer. Expert Opin Ther Targets 2012; 16(Suppl 1):S51–62; doi:10.1517/14728222.2011.647810. CrossRef
Broccatelli F, Salphati L, Plise E, Cheong J, Gobbi A, Lee ML, Aliagas I. Predicting passive permeability of drug-like molecules from chemical structure: where are we? Mol Pharm, 2016; 13(12):4199–208; doi:10.1021/acs.molpharmaceut.6b00836 CrossRef
Cardona-G W, Herrera-R A, Castrillón-L W, Ramírez-Malule H. Chemistry and anticancer activity of hybrid molecules and derivatives based on 5-fluorouracil. Curr Med Chem, 2021; 28:1–53; doi: 10.2174/0929867328666210211164314 CrossRef
Cardona-G W, Yepes AF, Herrera-R A. Hybrid Molecules: promising compounds for the development of new treatments against Leishmaniasis and Chagas disease. Curr Med Chem, 2018; 25:3615–57; doi:10.2174/0929867325666180309111428. CrossRef
Chae HS, Xu R, Won JY, Chin YW, Yim H. Molecular targets of genistein and its related flavonoids to exert anticancer effects. Int J Mol Sci, 2019; 20(10):2420–9; doi:10.3390/ijms20102420. CrossRef
Colmenarejo G. In silico prediction of drug-binding strengths to human serum albumin. Med Res Rev, 2003; 23(3):275–301; doi:10.2174/1381612821666150302113710. CrossRef
Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep, 2017; 7:1–13; doi:10.1038/srep42717. CrossRef
Ding Y, Li S, Ge W, Liu Z, Zhang X, Wang M, Chen T, Chen Y, Zhang Q. Design and synthesis of parthenolide and 5-fluorouracil conjugates as potential anticancer agents against drug resistant hepatocellular carcinoma. Eur J Med Chem, 2019; 183:111706; doi:10.1016/j.ejmech.2019.111706 CrossRef
de Oliveira Pedrosa M, Duarte da Cruz RM, de Oliveira Viana J, de Moura RO, Ishiki HM, Barbosa Filho JM, Diniz MF, Scotti MT, Scotti L, Bezerra Mendonca FJ. Hybrid compounds as direct multitarget ligands: a review. Curr Top Med Chem, 2017; 17:1044–79; doi:10.2174/1568026616666160927160620. CrossRef
Ditzinger F, Price DJ, Ilie AR, Köhl NJ, Jankovic S, Tsakiridou G, Aleandri S, Kalantzi L, Holm R, Nair A, Saal C, Griffin B, Kuentz M. Lipophilicity and hydrophobicity considerations in bio-enabling oral formulations approaches—a PEARRL review. J Pharm Pharmacol, 2019; 71:464–82; doi:10.1111/jphp.12984. CrossRef
Ferrández A, Prescott S, Burt RW. COX-2 and colorectal cancer. Curr Pharm Des, 2003; 9(27):2229–51; doi:10.2174/1381612033454036 CrossRef
Ganai AA, Farooqi H. Bioactivity of genistein: a review of in vitro and in vivo studies. Biomed Pharmacother, 2015; 76:30–8; doi:10.1016/j.biopha.2015.10.026 CrossRef
Garner TP, Reyna DE, Priyadarshi A, Chen HC, Li S, Wu Y, Ganesan YT, Malashkevich VN, Cheng EH, Gavathiotis E. An autoinhibiteddimeric form of BAX regulates the BAX activation pathway. Mol Cell, 2016; 63(3):485–97; doi:10.1016/j.molcel.2016.06.010 CrossRef
Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin, 2021; 0:1–41. Available via https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660 (Accessed June 2021).
Hashemi Goradel N, Najafi M, Salehi E, Farhood B, Mortezaee K. Cyclooxygenase-2 in cancer: a review. J Cell Physiol, 2019; 234(5):5683–99; doi:10.1002/jcp.27411 CrossRef
Herrera-RA, Castrillón W, Otero E, Ruiz E, Carda M, Agut R, Naranjo T, Moreno G, Maldonado ME, Cardona-G W. Synthesis and antiproliferative activity of 3- and 7-styrylcoumarins. Med Chem Res, 2018; 27:1893–905. CrossRef
Huang WW, Hsieh KP, Huang RY, Yang YH. Role of cyclooxygenase-2 inhibitors in the survival outcome of colorectal cancer patients: a population-based cohort study. Kaohsiung J Med Sci, 2017; 33(6):308–14; doi:10.1016/j.kjms.2017.03.004 CrossRef
Jiang W, Yan Y, Chen M, Luo G, Hao J, Pan J, Hu S, Guo P, Li W, Wang R, Zuo Y, Sun Y, Sui S, Yu W, Pan Z, Zou K, Zheng Z, Deng W, Wu X, Guo W. Aspirin enhances the sensitivity of colon cancer cells to cisplatin by abrogating the binding of NF-κB to the COX-2 promoter. Aging (Albany NY), 2020; 12(1):611–27; doi: 10.18632/aging.102644 CrossRef
Kothe M, Kohls D, Low S, Coli R, Cheng AC, Jacques SL, Johnson TL, Lewis C, Loh C, Nonomiya J, Sheils AL, Verdries KA, Wynn TA, Kuhn C, Ding YH. Structure of the catalytic domain of human polo-like kinase 1. Biochemistry, 2007; 46(20):5960–71; doi:10.1021/bi602474j CrossRef
Lai MY, Huang JA, Liang ZH, Jiang HX, Tang GD. Mechanisms underlying aspirin-mediated growth inhibition and apoptosis induction of cyclooxygenase-2 negative colon cancer cell line SW480. World J Gastroenterol 2008;14(26):4227-4233; doi: 10.3748/wjg.14.4227 CrossRef
LeCher JC, Diep N, Krug PW, Hilliard JK. Genistein has antiviral activity against Herpes B virus and acts synergistically with antiviral treatments to reduce effective dose. Viruses, 2019; 11(6):499; doi:10.3390/v11060499 CrossRef
Lin LP, Wu FH, Liang JY. The first examples of ilexgenin A hybrids as a new class of multi-potent, anti-platelet agents. Chin Chem Lett, 2013; 24:723–6. CrossRef
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev, 1997; 23:3–25; doi:10.1016/s0169-409x(00)00129-0 CrossRef
Luo Y, Wang SX, Zhou ZQ, Wang Z, Zhang YG, Zhang Y, Zhao P. Apoptotic effect of genistein on human colon cancer cells via inhibiting the nuclear factor-kappa B (NF-κB) pathway. Tumour Biol, 2014; 35(11):11483–8; doi:10.1007/s13277-014-2487-7 CrossRef
Mastuda H, Morikawa T, Ueda K, Managi H, Yoshikawa M. Structural requirements of flavonoids for inhibition of antigen-Induced degranulation, TNF-α and IL-4 production from RBL-2H3 cells. Bioorg Med Chem, 2002; 10:3123–8; doi:10.1016/S0968-0896(02)00227-4 CrossRef
McQuade RM, Bornstein JC, Nurgali K. Anti-colorectal cancer chemotherapy-induced diarrhoea: current treatments and side effects. Int J Clin Med, 2014; 5:393–406; doi:10.4236/ijcm.2014.57054 CrossRef
Morris GM, Goodshell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. Docking using a Lamarckian genetic algorithm and empirical binding free energy function. J Comput Chem, 1998; 19:1639–62; doi:10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B CrossRef
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem, 2009; 30(16):2785–91; doi:10.1002/jcc.21256 CrossRef
Nehls O, Okech T, Hsieh CJ, Enzinger T, Sarbia M, Borchard F, Gruenagel HH, Gaco V, Hass HG, Arkenau HT, Hartmann JT, Porschen R, Gregor M, Klump B. Studies on p53, BAX and Bcl-2 protein expression and microsatellite instability in stage III (UICC) colon cancer treated by adjuvant chemotherapy: major prognostic impact of proapoptotic BAX. Br J Cancer, 2007; 96(9):1409–18; doi:10.1038/sj.bjc.6603728. CrossRef
Rahman Mazumder MA, Hongsprabhas P. Genistein as antioxidant and antibrowning agents in in vivo and in vitro: a review. Biomed Pharmacother, 2016; 82:379–92; doi: 10.1016/j.biopha.2016.05.023 CrossRef
Rejhova A, Opattova A, Cumov A, Slíva D, Vodicka P. Natural compounds and combination therapy in colorectal cancer treatment. Eur J Med Chem, 2018; 144:582–94; doi:10.1016/j.ejmech.2017.12.039 CrossRef
Satish A, Farha Syeda S, Urooj A. Quantification of flavonoids by UPLC-MS and its antibacterial activity from Brassica oleracea var. Capitata L. GSC Biol Pharm Sci, 2018; 05(01):109–14; doi:10.30574/gscbps.2018.5.1.0105 CrossRef
Satya P, Indira J, Gayathri C, Vijayalekshmi N, Syng-Ook L, Stephen S. Aspirin inhibits colon cancer cell and tumor growth and downregulates specificity protein (Sp) transcription factors. PLoS One, 2012; 7(10):e48208. Published online 2012 Oct 26; doi: 10.1371/journal.pone.0048208 CrossRef
Sheng J, Sun H, Yu FB, Li B, Zhang Y, Zhu YT. The role of cyclooxygenase-2 in colorectal cancer. Int J Med Sci, 2020; 17(8):1095–101; doi:10.7150/ijms.44439 CrossRef
Shin SB, Woo SU, Chin YW, Jang YJ, Yim H. Sensitivity of TP53-mutated cancer cells to the phytoestrogen genistein is associated with direct inhibition of Plk1 activity. J Cell Physiol, 2017; 232(10):2818–28; doi:10.1002/jcp.25680 CrossRef
Shinde V, Mhaske PC, Singh A, Sarkar D, Mahulikar P. Synthesis and biological evaluation of new 4-(4-(1-benzyl-1H-1,2,3-triazol-4-yl)phenyl)-2-phenyl thiazole derivatives. J Heterocyclic Chem, 2019; 56:3093–101; doi:10.1002/jhet.3708. CrossRef
Sinicrope FA, Gill S. Role of cyclooxygenase-2 in colorectal cancer. Cancer Metastasis Rev, 2004; 23(1–2):63–75; doi:10.1023/a:1025863029529. CrossRef
S?oczy?ska K, Gunia-Krzy?ak A, Koczurkiewicz P, Wójcik-Pszczo?a K, ?elaszczyk D, Popió? J, P?kala E. Metabolic stability and its role in the discovery of new chemical entities. Acta Pharm, 2019; 69(3):345–61; doi:10.2478/acph-2019-0024. CrossRef
Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med, 2013; 19(2):202–8; doi:10.1038/nm.3048 CrossRef
Spagnuolo C, Moccia S, Russo GL. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur J Med Chem, 2018; 153:105–15; doi: 10.1016/j.ejmech.2017.09.001 CrossRef
Pérez JM, Maldonado ME, Rojano B, Alzate F, Sáez J, Cardona W. Comparative antioxidant, antiproliferative and apoptotic effects of Ilex laurina and Ilex paraguariensis on colon cancer cells. Trop J Pharm Res, 2014; 13:1279–86. CrossRef
Pham-The H, Cabrera-Pérez MÁ, Nam NH, Castillo-Garit JA, Rasulev B, Le-Thi-Thu H, Casañola-Martin GM. In silico assessment of ADME properties: advances in Caco-2 cell monolayer permeability modeling. Curr Top Med Chem, 2018; 18(26):2209–29; doi:10.2174/1568026619666181130140350. CrossRef
Podlewska S, Kafel R. MetStabOn-online platform for metabolic stability predictions. Int J Mol Sci, 2018; 19:1040–56; doi:10.3390/ijms19041040 CrossRef
Pointet AL, Taieb J. Cáncer de colon. EMC—Tratado De Med, 2017; 21:1–7; doi:10.1016/S1636-5410(16)81792-4 CrossRef
Press B, Di Grandi D. Permeability for intestinal absorption: Caco-2 assay and related issues. Curr Drug Metab, 2008; 9(9):893–900; doi:10.2174/138920008786485119 CrossRef
Takahashi T, Sano B, Nagata T, Kato H, Sugiyama Y, Kunieda K, Kimura M, Okano Y, Saji S. Polo-like kinase 1 (PLK1) is overexpressed in primary colorectal cancers. Cancer Sci, 2003; 94(2):148–52; doi:10.1111/j.1349-7006.2003.tb01411.x CrossRef
Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem, 2010; 31(2):455–61; doi:10.1002/jcc.21334 CrossRef
Tuli HS, Tuorkey MJ, Thakral F, Sak K, Kumar M, Sharma AK, Sharma U, Jain A, Aggarwal V, Bishayee A. Molecular mechanisms of action of genistein in cancer: recent advances. Front Pharmacol, 2019; 10:1336–41; doi:10.3389/fphar.2019.01336 CrossRef
Wang XY, Clubbs EA, Bomser JA. Genistein modulates prostate epithelial cell proliferation via estrogen-and extracellular signal-regulated kinase-dependent pathways. J Nutr Biochem, 2006; 17:204–10; doi:10.1016/j.jnutbio.2005.07.005. CrossRef
Wang JL, Limburg D, Graneto MJ, Springer J, Hamper JR, Liao S, Pawlitz JL, Kurumbail RG, Maziasz T, Talley JJ, Kiefer JR, Carter J. The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: the second clinical candidate having a shorter and favorable human half-life. Bioorg Med Chem Lett, 2010; 20(23):7159–63; doi:10.1016/j.bmcl.2010.07.054 CrossRef
Yang Y, Zang A, Jia Y, Shang Y, Zhang Z, Ge K, Zhang J, Fan W, Wang B. Genistein inhibits A549 human lung cancer cell proliferation via miR-27a and MET signaling. Oncol Lett, 2016; 12(3):2189–93; doi:10.3892/ol.2016.4817 CrossRef
Zhang Z, Chen FL, Shang L. Advances in antitumor effects of NSAIDs. Cancer Manag Res, 2018; 10:4631–40; doi:10.2147/CMAR.S175212 CrossRef
Zhang Zh, Tang W. Drug metabolism in drug discovery and development. Acta Pharm Sin B, 2018; 8(5):721–32; doi:10.1016/j.apsb.2018.04.003. CrossRef
Zhivkova ZD. Studies on drug-human serum albumin binding: the current state of the matter. Curr Pharm Des, 2015; 21(14):1817–30; doi:10.2174/1381612821666150302113710 CrossRef
Zhang J, Su H, Li Q, Li J, Zhao Q. Genistein decreases A549 cell viability via inhibition of the PI3K/AKT/HIF?1α/VEGF and NF?κB/COX?2 signaling pathways. Mol Med Rep, 2017; 15(4):2296–302; doi:10.3892/mmr.2017.6260 CrossRef
Zhou P, Wang C, Hu Z, Chen W, Qi W, Li A. Genistein induces apoptosis of colon cancer cells by reversal of epithelial-to-mesenchymal via a Notch1/NF-κB/slug/E-cadherin pathway. BMC Cancer, 2017; 17(1):813–22; doi:10.1186/s12885-017-3829-9 CrossRef