INTRODUCTION
The focal purpose of this review article is to provide ample information on the different clinical studies conducted in some countries in the Asian region on the utilization of the antiviral drug, Remdesivir, for coronavirus disease 2019 (COVID-19). COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 and has spread in most countries in 2020. Common symptoms of this disease include fever, cough, fatigue, and diarrhea. Certain medicinal agents are being studied to treat COVID-19. One of these medicinal agents is Remdesivir, which is an antiviral agent that is an adenosine nucleotide, which could impede the viral RNA polymerase enzyme. There were countries in the regions of Asia that conducted clinical studies on the use of Remdesivir as an antiviral therapy for COVID-19 infections. Asian countries such as Japan and South Korea permitted the employment of it as a medicinal agent for COVID-19 disease. In other countries in the region, Remdesivir is on phase 3 clinical trial. Doses of 100–200 mg/kg intravenous route could improve clinical outcomes of patients infected with COVID-19 in clinical trial settings. Thus, Remdesivir is a potential antiviral commodity that can be used to treat COVID-19 infections and opted to be wholly established.
Coronavirus disease 2019 (COVID-19) is a global medical problem that was detected in the last set of months of 2019. Another nomenclature of its cause is severe acute respiratory syndrome coronavirus 2 (SARS-COV-2). Its symptoms include fever, cough, myalgia, fatigue, and in some cases, diarrhea (Dewangan et al., 2020; Ludvigsson, 2020; Nandy et al., 2020; Rokade and Khandagale, 2020; Viner et al., 2020). It was declared a pandemic by the World Health Organization in March 2020. It widely spread across more than 200 countries in 2020. Healthcare workers and different government sectors of different countries are findings ways to manage this disease and to further prevent its transmission (Jain and Barhate, 2020; Musa et al., 2020; Xu et al., 2020;). Moreover, a number of vaccines are developed and are currently programmed to be available to the public soon. There are rare antiviral agents that were approved for the treatment of COVID-19 in some countries, such as Remdesivir (GS-5734), an adenosine nucleotide and antiviral agent that inhibits SARS-COV-2 RNA-dependent RNA polymerase. It was recently approved for the treatment of COVID-19 for infected adults and pediatric patients by the US Food and Drug Administration (Beigel et al., 2020; Yadav and Mohite, 2020a) . Here in Asia, there are some countries like Japan and Korea that have already approved the use of this antiviral agent against COVID-19 (Patil and Jain, 2020; Reddy Vegivinti et al., 2021; Yadav and Mohite, 2020). This research paper summarizes the findings through a systematic literature review of the different clinical studies in selected countries of Asia for the utilization of Remdesivir against COVID-19.
METHODS
The review was conducted by utilizing journal databases such as BMJ Global Health, ClinicalTrials.gov, Elsevier, Google Scholar, PubMed, and The Lancet. The search strategy was established for articles on each database without limitations on language and recent years (2019–2021) considered. The search keyword parameters include Remdesivir, GS-5734, NCOV-2019, COVID-19, Remdesivir clinical trials, or a combination of two from these keywords. The search started on February 1, 2021. Appropriate studies or articles were identified using specific criteria as follows: (1) articles that are focused on clinical trials on Remdesivir, (2) articles related to reports of different trials conducted or participated by a country within the Asian region, and (3) studies that analyze clinical outcomes of Remdesivir for COVID-19. Other considered characteristics for screening of these journal articles consist of the study design, intervention, sample size of participants, and, if any, adverse or side effects of the drug (Derouiche, 2020; Kishor et al., 2021; Penedones et al., 2019; Piscoya et al., 2021; Raymond et al., 2020). For the verification of these clinical studies, the author utilized ClinicalTrials.gov as a reference. There was no online review procedure for this study.
RESULTS AND DISCUSSION
After conducting a search on databases according to the criteria, 620 published articles were characterized. Among these, 320 were excluded due to duplication. There were 300 articles that underwent screening, and 240 of them did not meet the specifications of the criteria. Hence, 60 articles were carefully reviewed. In addition to that, eight more articles were excluded since they were out of scope or the clinical trials were conducted in non-Asian countries, another eight were removed due to insufficient details on clinical trials conducted for Remdesivir, and another six were removed due to the limited sternness of the articles. Thus, there were 40 eligible articles and information on clinical trials of Remdesivir for COVID-19 in the Asian region included in this review. This review of the clinical trials was conducted in selected countries in Asia, such as China, Hong Kong, Japan, Singapore, South Korea, and Taiwan. A number of trials involved a 200 mg intravenous initial dose of Remdesivir, which was administered on the first day of treatment following a succeeding 100 mg dose for 4 days or 9 days. There were 2 clinical trials that utilized 100 mg single dose infusion. The initial outcomes for each trial would include clinical improvements in oxygen degree of saturation, body temperature normalization, and decrease in percentage severity using an ordinal scale used by clinicians to assess the clinical status of the clinical trial participants. Adverse events were also noted by the authors, mortality, and reduction in viral load (Beigel et al., 2020; Bin Cao China-Japan Friendship Hospital, 2021).
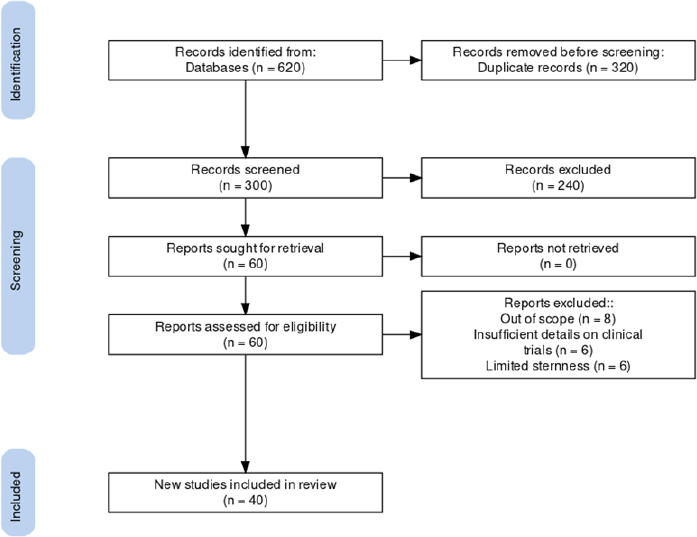 | Figure 1. PRISMA flow diagram of search screening (inclusion and exclusion) on the review. [Click here to view] |
In China, in February 2020, a randomized, placebo-controlled, multicenter phase 3 clinical trial was initiated with enrolled patients admitted to the hospital with confirmed COVID-19 infections. These patients were randomly assigned in a particular ratio to receive the administration of intravenous Remdesivir dosed at 200 mg on the first day and 100 mg on the succeeding days up to the 10th day. There were clinical improvements, such as on the oxygen saturation of the patients up to the 28th day (Reddy Vegivinti et al., 2021; Yang et al., 2021).
Ten hospitals in Hubei, China, were involved in a randomized, double-blind, placebo-controlled clinical trial for the use of Remdesivir with a single daily infusion of 100 mg dose. Enrolled patients were also allowed to be given interferons, corticosteroids, and antivirals, Lopinavir/Ritonavir. They found out that the said dose regimen of Remdesivir was tolerated and did not demonstrate significant clinical outcomes in seriously ill patients, but reductions in clinical immunopathology parameters were also noted. They recommended having a higher dose regimen of Remdesivir to enhance antiviral potency. The clinical study was tracked by Hangzhou Tigermed Consulting, a contract research organization (Chou et al., 2020; Wang et al., 2020).
The adaptive COVID-19 treatment trial, initiated in February 2020 up to April 2020, was conducted in 60 trial sites. Asian countries that participated in the said trial were Japan, Singapore, and South Korea. A 200 mg loading dose followed by a 100 mg daily dose up to 10 days was the regimen for Remdesivir. This is a placebo-controlled and randomized trial assigned in a 1:1 ratio. Good clinical outcomes of the antiviral therapy were observed in the 10-day course of Remdesivir. The data also indicated that the antiviral drug prevented the development of a higher stage of severe respiratory disease (Ikonne et al., 2021; Kichloo et al., 2021; Jo et al., 2021).
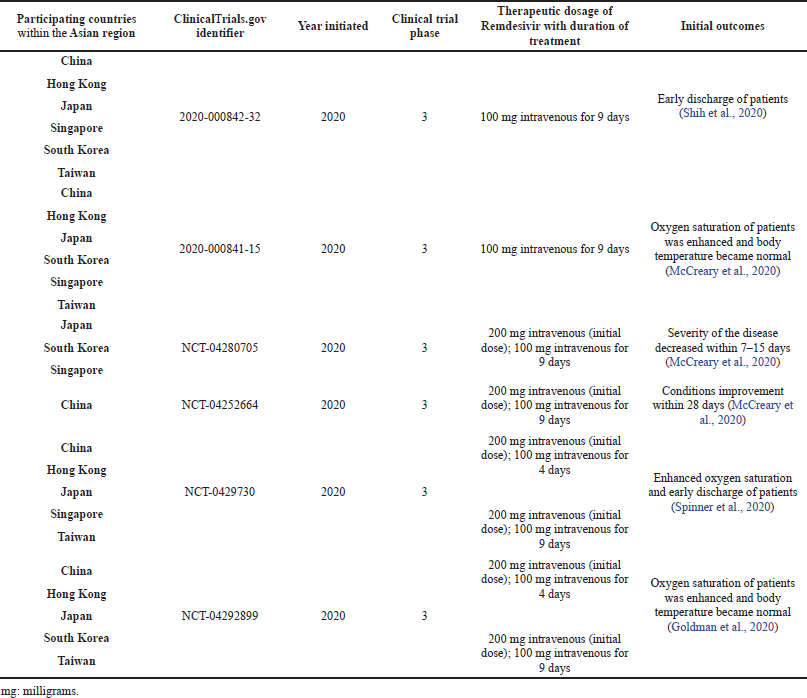 | Table 1. Different clinical studies on the use of Remdesivir for COVID-19 participated by Asian countries. [Click here to view] |
In India, the process of application of Remdesivir for its compassionate use is already ongoing. There are two Indian pharmaceutical companies pursued for a clinical study waiver from their health authorities (Dawood, 2021; Devi and Rosy, 2021; Jain and Barhate, 2021; Singh et al., 2020).
A solidarity randomized clinical trial having the registration number ISRCTN83971151 was participated by some Asian countries such as India, Indonesia, Iran, Israel, Lebanon, Malaysia, Philippines, Qatar, Saudi Arabia, and Thailand. In March 2020, it underwent recruitment, and the mode of intervention was standard care and doses of Remdesivir for 10 days. Additional treatment agents such as Chloroquine or Hydroxychloroquine, Lopinavir + Ritonavir, and Beta-Interferon were included (Pan et al., 2021; Pimentel et al., 2020).
In the Philippines, Remdesivir was already granted a permit for compassionate use in hospitals. Ages 12 and above can be administered with this antiviral agent in severe cases of infections of COVID-19 (FDA, 2021).
Remdesivir has exhibited efficacy in both in vitro and in vivo models against coronaviruses. Recently, through compassionate utilization, this drug has adjunct evidence for producing some improvement in the clinical condition of COVID-19 patients (Eastman et al., 2020; Grein et al., 2020).
CONCLUSION
There are still limited clinical investigations that would mend provisions for the full utilization of Remdesivir to treat COVID-19, specifically in Asia. Conversely, a number of phase 3 clinical studies have been completed, and mostly trials are ongoing. There were some pertinent data that were not yet being reported. The profile for adverse effects of Remdesivir is yet to be established. Nevertheless, the results on these phase 3 clinical trials initiated and participated by selected countries in the Asian region proved the significant clinical improvements of conditions of the patients with the intravenous administration of Remdesivir. Hence, this antiviral drug has the potential to be one of the standards for treating COVID-19 and remains to be fully established.
ACKNOWLEDGMENTS
The authors would like to acknowledge San Pedro College, Davao City, Philippines, and Centro Escolar University for their support on this endeavor.
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest regarding this review article.
FUNDING
None.
ETHICAL APPROVAL
This study does not involve any utilization of test animals or human subjects.
AUTHORS’ CONTRIBUTIONS
The authors made significant contributions to the concept, design, data, interpretation, and revisions of this article. They are eligible to be an author based on the guidelines set by the esteemed International Committee of Medical Journal Editors (ICMJE).
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19 – final report. N Engl J Med, 2020; 383(19):1813–26. CrossRef
Chou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet, 2020; 395:1054–62. CrossRef
Dawood AA. Using Remdesivir and Dexamethasone for treatment of SARS-CoV-2 shortens the patient’s stay in the hospital. Asian J Pharm, 2021; 11(2):138–40. CrossRef
Derouiche S. Current review on herbal pharmaceutical improve immune responses against COVID-19 infection. Res J Pharma Dosage Forms Tech, 2020; 12(3):181–4. CrossRef
Devi KN, Rosy JS. ECMO therapy for COVID-19. Asian J Nurs Educ and Res, 2021; 11(2):299–301.
Dewangan V, Sahu R, Satapathy Y, Roy A. The exploring of current development status and the unusual symptoms of coronavirus pandemic (COVID-19). Res J Pharmacol Pharmacodyn, 2020; 12(4):172–6. CrossRef
Eastman R, Roth J, Brimacombe K, Simeonov A, Shen M, Patnaik S, Hall MD. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent Sci, 2020; 6(5):672–83. CrossRef
FDA. Clarification on the approval and use of Remdesivir. 2021. Available via https://www.fda.gov.ph/wp-content/uploads/2021/05/FDA-Advisory-No.2021-0759.pdf
Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D’Arminio Monforte A, Ismail S, Kato H, Lapadula G, L’Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate use of Remdesivir for patients with severe COVID-19. N Engl J Med, 2020; 382(24):2327–36. CrossRef
Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn MY, Nahass RG, Chen YS, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Muñoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A. Remdesivir for 5 or 10 days in patients with severe COVID-19. N Engl J Med, 2020; 383(19):1827–37. CrossRef
Ikonne E, Ikpeazu V, Ugbogu O, Emmanuel O, Nwakuche I, Iweala E, Ugbogu E. A review on the clinical trials of repurposing therapeutic drugs, mechanisms and preventive measures against SARS- CoV-2. Drug Metab Pers Ther, 2021. Epub ahead of print. PMID: 33818026. CrossRef
Jain M, Barhate S. Corona viruses are a family of viruses that range from the common cold to MERS corona virus: a review. Asian J Res Pharm Sci, 2020; 10(3):204–10. CrossRef
Jain M, Barhate S. Favipiravir has been investigated for the treatment of life-threatening pathogens such as Ebola virus, Lassa virus, and now COVID-19: a review. Asian J Pharm Res, 2021; 11(1); 39–42. CrossRef
Jo Y, Jamieson L, Edoka I, Long L, Silal S, Pulliam JRC, Moultrie H, Sanne I, Meyer-Rath G, Nichols BE. Cost-effectiveness of Remdesivir and Dexamethasone for COVID-19 treatment in South Africa. Open Forum Infect Dis, 2021; 8(3):ofab040. CrossRef
Kichloo A, Albosta M, Kumar A, Aljadah M, Mohamed M, El-Amir Z, Wani F, Jamal S, Singh J, Kichloo A. Emerging therapeutics in the management of COVID-19. World J Virol, 2021; 10(1):1–29. CrossRef
Kishor R, Rajendra B, Ramhari B, Siddheshwar, S. Review paper on ayush system of medicine against COVID-19. J Pharmacogn Phytochem, 2021; 13(2):103–6.
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr, 2020; 109(6):1088–95. CrossRef
Rokade M, Khandagale P. Coronavirus disease: a review of a new threat to public health. Asian J Pharm Res, 2020; 10(3):241–4. CrossRef
McCreary EK, Angus DC. Efficacy of Remdesivir in COVID 19. JAMA, 2020; 324(11):1041–2. CrossRef
Musa A, Pendi K, Hashemi A, Warbasse E, Kouyoumjian S, Yousif J, Blodget E, Stevens S, Aly B, Baron DA. Remdesivir for the treatment of COVID-19: a systematic review of the literature. West J Emerg Med, 2020; 21(4):737–41. CrossRef
Nandy K, Salunke A, Pathak SK, Pandey A, Doctor C, Puj K, Sharma M, Jain A, Warikoo V. Coronavirus disease (COVID-19): a systematic review and meta-analysis to evaluate the impact of various comorbidities on serious events. Diabetes Metab Syndr, 2020; 14(5):1017–25. CrossRef
Pan H, Peto R, Henao-Restrepo AM, Preziosi M, Sathiyamoorthy V, Abdool Karim Q, Alejandria MM, Hernández García C, Kieny MP, Malekzadeh R, Murthy S, Reddy KS, Roses Periago M, Abi Hanna P, Ader F, Al-Bader AM, Alhasawi A, Allum E, Alotaibi A, Alvarez-Moreno CA, Appadoo S, Asiri A, Aukrust P, Barratt-Due A, Bellani S, Branca M, Cappel-Porter HBC, Cerrato N, Chow TS, Como N, Eustace J, García PJ, Godbole S, Gotuzzo E, Griskevicius L, Hamra R, Hassan M, Hassany M, Hutton D, Irmansyah I, Jancoriene L, Kirwan J, Kumar S, Lennon P, Lopardo G, Lydon P, Magrini N, Maguire T, Manevska S, Manuel O, McGinty S, Medina MT, Mesa Rubio ML, Miranda-Montoya MC, Nel J, Nunes EP, Perola M, Portolés A, Rasmin MR, Raza A, Rees H, Reges PPS, Rogers CA, Salami K, Salvadori MI, Sinani N, Sterne JAC, Stevanovikj M, Tacconelli E, Tikkinen KAO, Trelle S, Zaid H, Røttingen JA, Swaminathan S. Repurposed antiviral drugs for COVID-19 - interim WHO solidarity trial results. N Engl J Med, 2021; 384(6):497–511. CrossRef
Patil P, Jain, R. Theoretical study and treatment of novel COVID-19. Res J Pharmacol Pharmacodyn, 2020; 12(2):71–2. CrossRef
Penedones A, Alves C, Batel-Marques F. Recommendations to conduct and report systematic reviews in medical literature: a scoping review. BMC Med Res Methodol, 2019; 19:234. CrossRef
Pimentel J, Laurie C, Cockcroft A, Andersson N. Clinical studies assessing the efficacy, effectiveness and safety of remdesivir in management of COVID-19: a scoping review. Br J Clin Pharmacol, 2020; 87:1–22. CrossRef
Piscoya A, Ng-Sueng LF, Parra del Riego A, Cerna-Viacava R, Pasupuleti V, Roman YM, Thota P, White CM, Hernandez AV. Efficacy and harms of remdesivir for the treatment of COVID-19: a systematic review ad meta-analysis. PLoS One, 2021; 15(12):e0243705. CrossRef
Report from Bin Cao, China-Japan Friendship Hospital. A phase 3 randomized, double-blind, placebo-controlled multicenter study to evaluate the efficacy and safety of Remdesivir in hospitalized adult patients with mild and moderate COVID-19, 2020. Available via https://clinicaltrials.gov/ct2/show/NCT04252664 (Accessed 11 March 2021)
Raymond AS, Sato J, Kishioka Y, Teixeira T, Hasslboeck C, Kweder SL. Remdesivir emergency approvals: a comparison of the U.S., Japanese, and EU systems. Expert Rev Clin Pharmacol, 2020; 13(10):1095–101. CrossRef
Shih W, Yao C, Xie T. Data monitoring for Chinese clinical trials of Remdesivir in treating patients with COVID-19 during the pandemic crisis. Ther Innov Regul Sci, 2020; 54:1236–55. CrossRef
Singh D, Wasan H, Mathur A, Gupta YK. Indian perspective of remdesivir: a promising COVID-19 drug. Indian J Pharmacol, 2020; 52(3):227–8. CrossRef
Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, Ogbuagu O, Malhotra P, Mullane KM, Castagna A, Chai LYA, Roestenberg M, Tsang OTY, Bernasconi E, Le Turnier P, Chang SC, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wang H, Gaggar A, Brainard DM, McPhail MJ, Bhagani S, Ahn MY, Sanyal AJ, Huhn G, Marty FM. Effect of Remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA, 2020; 324(11):1048–50. CrossRef
Viner RM, Ward JL, Hudson LD, Ashe M, Patel SV, Hargreaves D, Whittaker E. Systematic review of reviews of symptoms and signs of COVID-19 in children and adolescents. Arch Dis Child, 2020. CrossRef
Reddy Vegivinti CT, Pederson JM, Saravu K, Gupta N, Barrett A, Davis AR, Kallmes KM, Evanson KW. Remdesivir therapy in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Ann Med, 2021; 62:43–8. CrossRef
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y. Remdesivir in adults with severe COVID-19: a randomized, double-blind, placebo-controlled, multicenter trial. Lancet, 2020; 295(10236):1569–78. CrossRef
Xu J, Ma XP, Long B, Miao W, Wu D, Ning. A systematic review of etiology, epidemiology, clinical manifestations, image findings, and medication of 2019 corona virus disease-19 in Wuhan, China. Medicine, 2020; 99(42):e22688. CrossRef
Yadav A, Mohite S. A novel approach for treatment of COVID-19 with convalescent plasma. Res J Pharma Dosage Forms Tech, 2020a; 12(3):227–30. CrossRef
Yadav A, Mohite S. A review on severe acute respiratory infection (SARI) and its clinical management in suspect/ confirmed novel coronavirus (nCoV) cases. Res J Pharma Dosage Forms Tech 2020b; 12(3):178–80. CrossRef
Yang C, Wei Y, Chang H, Chang P, Tsai C, Chen Y, Hsueh P. Remdesivir use in the coronavirus disease 2019 pandemic: a mini-review. J Microbiol Immunol, 2021; 54(1):27–36. CrossRef